JAM-C blockade may be useful for CNV suppression by inhibiting macrophage transmigration, RPE cell migration, and monolayer RPE barrier malfunction. These data reveal a novel function of JAM-C and demonstrate that JAM-C may be a compelling target for CNV therapy.
Abstract
Purpose.
To identify the expression of junctional adhesion molecule-C (JAM-C) in choroidal neovascularization (CNV) and evaluate the effect of JAM-C targeting on CNV formation and on cellular functions relevant to CNV in vitro, such as macrophage transmigration, human retinal pigment epithelial (hRPE) cell migration, and monolayer RPE permeability.
Methods.
JAM-C expression in CNV was analyzed by real-time PCR, immunoblot analysis, and immunofluorescence staining. CNV area and blood vessel leakage were quantified using isolectin B4 staining and fluorescein angiography, respectively, 1 week after laser treatment. Macrophage infiltration within the CNV area was measured by immunofluorescence, and transmigration through monolayer RPE was analyzed using a transepithelial migration assay. After JAM-C shRNA transfection, human RPE cell migration was quantified using a transwell assay, and monolayer RPE permeability was determined by measuring the apical-to-basolateral movements of sodium fluorescein.
Results.
JAM-C expression was upregulated during CNV formation after laser treatment in a time-dependent manner. However, no change in JAM-C expression was found in the retina up to 14 days after laser treatment. JAM-C targeting by intravitreal injection of JAM-C Fc chimera inhibited CNV, blood vessel leakage, and macrophage infiltration. JAM-C Fc chimera inhibited basolateral-to-apical transmigration in vitro through a monolayer of hRPE of macrophages from patients with wet AMD. In addition, shRNA-mediated JAM-C knockdown inhibited hRPE cell migration and hRPE permeability.
Conclusions.
JAM-C blockade may prove useful for CNV suppression by inhibiting macrophage transmigration, RPE cell migration, and monolayer RPE barrier malfunction.
Choroidal neovascularization (CNV) is a common pathologic feature in numerous ocular diseases, such as wet age-related macular degeneration (AMD), pathologic myopia, angioid streaks, ocular histoplasmosis, uveitis, eye trauma, and ocular tumors. Patients may have CNV, which is a major cause of blindness worldwide, regardless of their age and sex. Angiogenesis is one of the basic mechanisms of CNV formation1 and is therefore a major therapeutic target in CNV.2 Photodynamic therapy and intravitreal injection of vascular endothelial growth factor (VEGF) antagonists have been widely used in the clinic; almost all the therapeutic options for CNV focus on the suppression of neovascularization. Although some available treatments may limit CNV progression to a certain extent, a portion of CNV patients still experience clinical deterioration. In fact, anti–VEGF treatment is reported to inhibit vascular growth in pathologic angiogenesis in only approximately 50% of patients.3 Furthermore, many CNV patients do not fit the enrollment criteria for the current treatments. Thus, new antiangiogenic therapeutic methods for CNV are eagerly awaited.
Integrity of the blood retinal barrier (BRB) is critical for retinal homeostasis. Some adhesion molecules expressed in the junctions of endothelial/epithelial cells have been studied in ocular angiogenesis animal models, such as vascular endothelial cadherin (VE-cadherin)4 and vascular adhesion protein-1 (VAP-1).5 Inhibition of excessive infiltration of inflammatory cells by targeting of adhesion molecules is an appealing route toward new CNV therapies. The junctional adhesion molecules (JAMs) regulate leukocyte infiltration and vascular permeability during inflammation.6,7 Among the members of the JAM family, JAM-C has been shown to be expressed abundantly in the neural retina and retinal pigment epithelial (RPE) cells.8 Furthermore, a JAM-C neutralizing antibody has been shown to inhibit tumor angiogenesis and growth,9 whereas a soluble JAM-C Fc chimera reduced pathologic angiogenesis in the model of oxygen-induced retinopathy of prematurity10 and tumor metastasis to the lung.11 On the other hand, recent studies showed that JAM-C also exists in a soluble form, which can induce angiogenesis in vitro and in vivo.12 However, thus far, there are no reports of JAM-C expression in CNV nor of its role during CNV formation.
In this study, we found that JAM-C expression is upregulated in CNV after laser treatment. Intravitreal injection of JAM-C antagonists suppressed CNV and decreased retinal blood vessel leakage and macrophage infiltration. JAM-C knockdown by shRNA inhibited RPE cell migration and decreased monolayer RPE permeability. JAM-C inhibition may therefore have therapeutic applications in CNV treatment.
Materials and Methods
Human RPE Cells and Macrophages
A healthy donor eye from an adult was obtained from the Xi'an Central Eye Bank (Xi'an, China) and was used for experiments in accordance with applicable laws and with the tenets of the Declaration of Helsinki. As described previously,13 the anterior segment of the eye, vitreous, and retina were removed. Then the remaining eyecup was washed with phosphate-buffered saline (PBS); this was followed by the addition of 0.025% trypsin-EDTA and incubation for 30 minutes at 37°C. The RPE cells were gently scraped and seeded in DMEM with 15% fetal bovine serum (FBS) in a cell culture dish. After proliferation, the RPE cells were re-trypsinized and cultured in minimum essential medium with 10% FBS, nonessential amino acids, penicillin (100 U/mL), and streptomycin (100 U/mL). Only cells between passages 4 to 8 were used in the study.
In the case of macrophages, peripheral blood monocytes were collected from healthy adults and wet AMD patients matched for age and sex. Then the cells were purified, cultured, and differentiated. Briefly, peripheral blood monocytes were isolated from leukopheresed buffy coat fractions after density gradient centrifugation using aqueous medium (Ficoll-Paque; Amersham Biosciences, Piscataway, NJ) according to the manufacturer's protocol. Anti–human CD14 (monocyte marker) microbeads (Miltenyi Biotec, Cambridge, MA) were used for positive selection/purification of monocytes with a magnetic cell separation instrument (AutoMACS; Miltenyi Biotec). The purity of the CD14+ cell population was greater than 90%. Complete medium (Biosource, Rockville, MD) consisting of RPMI 1640 supplemented with penicillin (100 U/mL), streptomycin (100 U/mL), l-glutamine (2 mM), and 10% FBS was used for monocyte culture. Macrophage-colony stimulating factor (M-CSF; PeproTech, Rocky Hill, NJ) was used to differentiate the monocytes into macrophages for 6 days.
Laser-Induced CNV Model and Fluorescein Angiography
All animal experiments were approved by the Animal Care and Use Committee at the Fourth Military Medical University (FMMU) and were performed according to FMMU guidelines and regulations. Eight- to 10-week-old female C57Bl6 mice were used for experiments. Four laser spots were made (75-μm spot size, 75 ms, 90-mW power; OcuLight Infrared Laser System 810 nm; IRIDEX Corporation, Mountain View, CA) in the area surrounding the optic disc in the eye. For JAM-C mRNA or protein expression analysis, eyeballs were removed at each time point and dissected for samples of the choroid-RPE complex (with CNVs) and neural retina tissue. For JAM-C Fc chimera treatment, intravitreal injections of mouse JAM-C Fc chimera (1 μg/eye; R&D Systems, Minneapolis, MN) were performed immediately after laser treatment and repeated at day 4. CNV area was analyzed 1 week after laser treatment using isolectin B4 staining. Fluorescein angiography for blood vessel leakage analysis was performed as described.14,15 Briefly, 1 week after laser treatment, mice were anesthetized. FITC-dextran (100 μL; MW 40 kDa, 20 mg/mL; Sigma, St. Louis, MO) in PBS was injected intravenously through the tail vein during a period of 10 seconds. After 20 minutes, the eyes were enucleated, and angiograms were taken using a fluorescence microscope. Angiograms were graded as follows: score 0, no dextran leakage; score 1, slight leakage; score 2, moderate leakage; score 3, intensive leakage. Two examiners graded the leakage of the CNV lesions in a masked fashion, and the average score was presented. If both scores for a particular lesion differed greatly, the sample was regraded and discussed until a similar result was reached.
Real-time PCR
Total RNA was isolated using an RNA purification kit (RNeasy Mini-kit; Qiagen, Valencia, CA) according to the manufacturer's instructions. cDNA was synthesized from 3 μg total RNA (SuperMix kit; Invitrogen, Frederick, MD) and was used for real-time PCR (ABI Prism 7500 HT Sequence Detection System; Applied Biosystems, Foster City, CA). All experiments were performed in triplicate and were repeated at least twice. Primers used are described in Table 1.
Table 1.
Primer Sets Used for Real-time PCR
| Gene | Species | Amplicon Size (bp) | Sequences |
|---|---|---|---|
| JAM-C | Human | 123 | Forward: GAGACTCAGCCCTTTATCGC |
| Reverse: CCTTCGGCACTCTACAGACA | |||
| Jam-c | Mouse | 113 | Forward: TCCTGGAGAATGTGTTTGGA |
| Reverse: GCATCTCTTGGGAAGGAGAG | |||
| ACTB | Human | 137 | Forward: TGGACTTCGAGCAAGAGATG |
| Reverse: GAAGGAAGGCTGGAAGAGTG | |||
| Actb | Mouse | 133 | Forward: GGTCATCACTATTGGCAACG |
| Reverse: ACGGATGTCAACGTCACACT |
Immunoblot Analysis
All samples were lysed using RIPA buffer (Sigma-Aldrich, St. Louis, MO) supplemented with complete protease inhibitors (Roche, Basel, Switzerland) on ice. The lysate was centrifuged at 14,000 rpm for 15 minutes, the supernatant was collected, and the protein concentration was quantified using a BCA protein assay (Pierce, Rockford, IL). Proteins were separated on a 10% NuPAGE polyacrylamide gel (Invitrogen) under nonreducing conditions and transferred to polyvinylidene difluoride membranes. Membranes were probed with a rabbit anti–JAM-C antibody (Invitrogen). An anti–rabbit horseradish peroxidase–conjugated antibody (Pierce) was used as the secondary antibody. An enhanced chemiluminescence kit (SuperSignal Pico ECL; Pierce) was used to detect the signal using an imaging system (LAS-3000 Imager; Fujifilm, Tokyo, Japan).
Immunofluorescence
Cryosections were obtained from frozen mouse eyes 7 days after laser treatment. Slides were washed, fixed in 4% PFA for 10 minutes, and permeabilized with 0.1% Triton X-100 in PBS for 5 minutes. Slides were blocked with 1% BSA and 5% goat serum in PBS for 1 hour at room temperature. Rabbit anti–JAM-C (Invitrogen) or rat anti–Mac3 (Becton-Dickinson, Franklin Lakes, NJ) antibody was applied, followed by appropriately conjugated secondary antibodies. Thereafter, slides were washed and mounted in medium (DAPI Fluoromount G; SouthernBiotech, Birmingham, Alabama). Fluorescence images were captured using a laser scanning microscope (Zeiss, Thornwood, NY).
Transepithelial Migration Assay
The transepithelial migration assay was performed as previously described.16 Briefly, RPE cells were plated on the bottom of inverted cell culture wells (Corning Costar, Lowell, MA) in the lower chamber and left upside down for 24 hours. Then the culture wells were returned to let the cells grow for 3 weeks with the hRPE apical membrane facing the lower chamber. Migration assay medium (serum-free RPMI in the absence or presence of 50 ng/mL MCP-1) was added to the lower compartment of the cell culture system. Human macrophages were added to the upper compartment, in the presence of MCP-1, IgG Fc, or JAM-C Fc. After incubation for 3 hours at 37°C, the number of transmigrated cells in the lower (apical membrane facing) compartment was analyzed by manually counting the cells using a Neubauer chamber.
Transfection
Human RPE cells (passages 4–8) were grown on culture dishes precoated with 0.2% gelatin (Sigma-Aldrich, St. Louis, MO). For transfection, human JAM-C shRNA or nontargeting control vector (Open Biosystems, Lafayette, CO) were applied at a concentration of 100 nM using reagent (Oligofectamine; Invitrogen) in medium (OptiMEM; Invitrogen). Cells were cultured for 72 hours after transfection. The knockdown efficiency of the shRNA was analyzed using real-time PCR and immunoblot analysis.
RPE Cell Transwell Migration and Proliferation Assay
Human RPE cells were placed on the upper layer of a permeable membrane coated with collagen (Sigma-Aldrich, St. Louis, MO), and the solution containing the test reagent was placed under the membrane. After 18 hours, the cells that migrated through the membrane were stained using a hematoxylin and eosin staining method and were counted. Conditioned medium of macrophages from patients with wet AMD was used to culture the RPE cells. The migration rate was calculated as the ratio of transfected versus nontransfected RPE cells. For proliferation assays, the cells were starved in serum-free medium overnight before the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; Invitrogen) assay was performed, as previously described.17
Permeability Assay
The permeability of the monolayer RPE was determined by measuring the apical-to-basolateral movements of sodium fluorescein. At the 14th day of cell culture, wet AMD macrophage-conditioned medium, VEGF, or histamine was added to the RPE cells on the microporous filters apically and basolaterally for 1 hour. Then fluorescein dye was added to the apical compartment of the filters. Thirty minutes after the addition of the test medium or molecules, 20 μL medium was collected from the basolateral side of the wells. The concentration of sodium fluorescein was quantified (Versa Fluor Fluorometer; Bio-Rad, Hercules, CA).
Statistical Analysis
Data were analyzed using ANOVA assuming equal variances. P < 0.01 was considered statistically significant. Data were represented as the mean ± SEM. Each test was performed in triplicate.
Results
Increased JAM-C Expression in the Lesion Area during CNV Formation
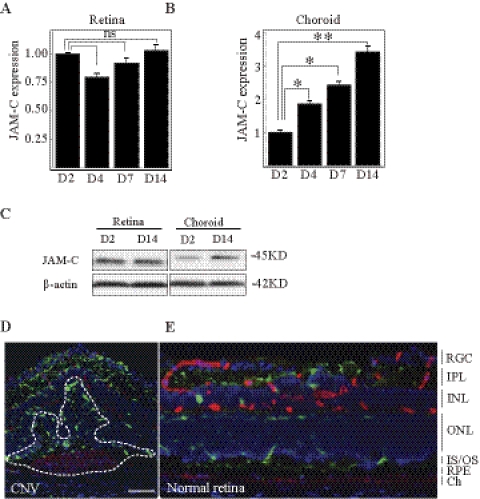
To investigate the potential role of JAM-C in CNV formation, we performed real-time PCR analysis to verify its expression pattern in a laser-induced CNV model. The data showed that JAM-C expression was upregulated in the lesion area (choroid-RPE complex with CNV) during CNV formation in a time-dependent manner, with no changes in the neural retina (Figs. 1A, 1B; n = 8). The upregulated expression of JAM-C in the CNV lesion was confirmed by immunoblot analysis at the protein level (Fig. 1C). Moreover, immunofluorescence staining showed abundant JAM-C expression within the CNV area (Fig. 1D, lined area showing the CNV lesion), whereas JAM-C was detected mainly in the RPE layer, inner and outer segments, and inner plexiform layer of the normal retina (Fig. 1E). The elevated expression of JAM-C in the lesion area during CNV formation suggested a role of JAM-C in CNV.
Figure 1.
Upregulated JAM-C expression during CNV formation. (A, B) Real-time PCR showed upregulated expression of JAM-C in the lesion area (choroid-RPE complex with CNV, n = 8) during CNV formation in a time-dependent manner. No such change was found in the retina. Gene expression relative to day 2 (D2). (C) Western blot analysis confirmed the upregulated expression of JAM-C at the protein level. (D, E) Immunofluorescence staining showed abundant JAM-C expression (green) within the CNV area (dotted line). JAM-C was detected primarily in the RPE layer, inner and outer segments, and inner plexiform layer of normal retina. Blue: nuclei stained by 4′,6-diamidino-2-phenylindole (DAPI); red: ColIV+ (vascular marker). IPL, inner plexiform layer; INL/ONL, inner and outer nuclear layers; IS/OS, inner and outer segments; Ch, choroid. Scale bar, 50 μm. *P < 0.01; **P < 0.001.
JAM-C Blockade Inhibits CNV Formation and Blood Vessel Leakage
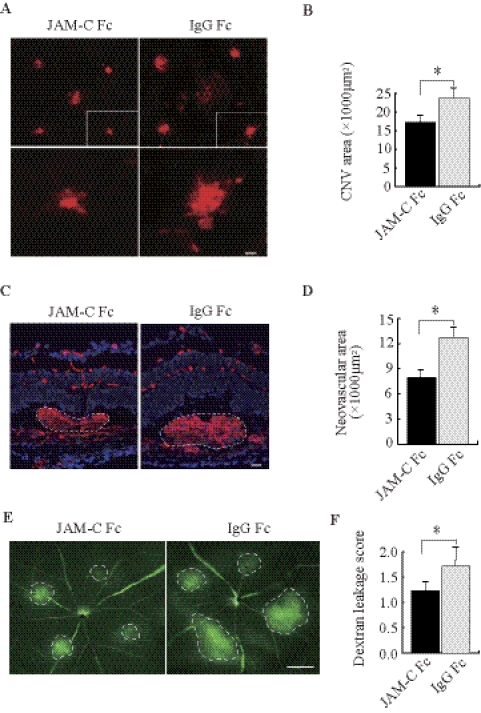
We next tested whether JAM-C targeting could inhibit CNV. Intravitreal injection of JAM-C Fc chimera (1 μg/eye) was performed immediately after laser treatment with a repeat injection at day 4. This treatment reduced the CNV area significantly compared with control Fc (Figs. 2A, 2B; n = 8; P < 0.01). Furthermore, histologic analysis displayed a decreased CNV neovascular area on treatment with JAM-C Fc chimera (Figs. 2C, 2D; n = 8; P < 0.01). Moreover, fluorescein angiography showed that JAM-C Fc chimera treatment decreased blood vessel leakage in the CNV 1 week after laser treatment (Figs. 2E, 2F; n = 10; P < 0.01).
Figure 2.
JAM-C blockade inhibits CNV formation and blood vessel leakage. (A, B) Intravitreal injection of JAM-C Fc chimera reduced CNV areas 1 week after laser treatment using isolectin B4 staining (n = 8). (C, D) Treatment with JAM-C Fc chimera decreased the neovascular tissue area (dotted lines, n = 8). (E, F) Fluorescein angiography showed reduced blood vessel leakage (dotted lines, n = 10) in the JAM-C Fc chimera–treated CNVs 1 week after laser treatment. (C, blue) Nuclei stained by 4′,6-diamidino-2-phenylindole (DAPI). Scale bars: 50 μm (A, C); 250 μm (E). *P < 0.01.
JAM-C Blockade Inhibits Macrophage Infiltration and Transmigration
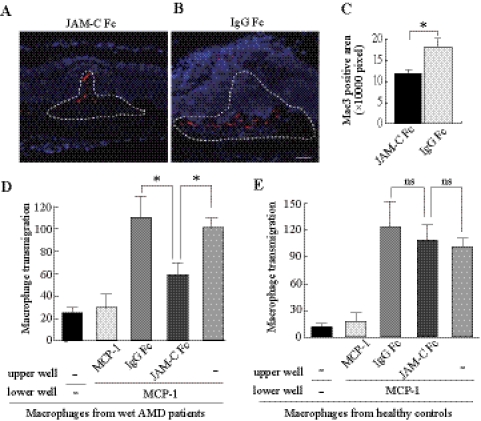
It is known that macrophages are activated and may aggregate within the lesion area during CNV development. We found that JAM-C Fc chimera treatment reduced Mac3+ staining (a macrophage marker) within the CNV area (Figs. 3A–C; n = 8; P < 0.01), demonstrating that JAM-C inhibition suppressed inflammation in vivo. In addition, we found that JAM-C Fc chimera inhibited the basolateral-to-apical transmigration of macrophages derived from patients with wet AMD through the monolayer hRPE (Fig. 3D; n = 5; P < 0.01). However, the same effect was not observed in macrophages derived from healthy controls (Fig. 3E; n = 5). These data thus suggest that JAM-C plays a role in macrophage transmigration and infiltration under pathologic conditions.
Figure 3.
JAM-C blockade inhibits macrophage infiltration and transmigration. (A–C) Immunofluorescence staining showed that JAM-C Fc chimera treatment reduced Mac3+ staining (red, macrophage marker) within the CNV areas (dotted lines, n = 8). (D) JAM-C Fc chimera inhibited the basolateral-to-apical transepithelial migration of macrophages derived from patients with wet AMD toward MCP-1 (n = 5). Transmigrated macrophages are shown as a percentage of the control (last column). (E) JAM-C Fc chimera had no significant effect on the basolateral-to-apical transepithelial migration of macrophages derived from healthy subjects toward MCP-1 (n = 5). Scale bar, 50 μm. Blue: nuclei stained by 4′,6-diamidino-2-phenylindole (DAPI). *P < 0.01; ns, not significant.
JAM-C Knockdown Inhibits hRPE Cell Migration but Not Proliferation
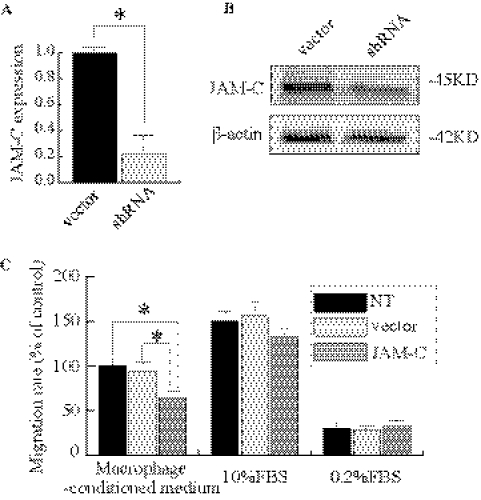
The hRPE is a key player in the pathogenesis of CNV. JAM-C has been shown to be expressed in the RPE.16 We therefore investigated whether JAM-C knockdown influences RPE cell migration and proliferation. On JAM-C shRNA treatment, hRPE JAM-C expression was reduced to approximately 20% of control, as shown by real-time PCR (Fig. 4A). This result was further confirmed by immunoblot analysis (Fig. 4B). Transwell migration assays showed that JAM-C knockdown inhibited hRPE cell migration compared with that of the vector-treated cells or nontreated cells when the wet AMD macrophage-conditioned medium was used as a chemoattractant. There was no significant difference among the three groups when the 10% FBS or 0.2% FBS medium was used (Fig. 4C; n = 3; P < 0.01). In contrast, JAM-C knockdown had no effect on RPE cell proliferation (data not shown).
Figure 4.
JAM-C knockdown inhibits hRPE cell migration. (A) Real-time PCR shows that in hRPE cells, JAM-C expression is reduced to approximately 20% of normal level after JAM-C shRNA transfection. (B) Immunoblot analysis shows that JAM-C protein expression decreases after JAM-C shRNA transfection in hRPE cells. (C) JAM-C knockdown inhibited hRPE cell migration compared with vector-treated cells or nontreated cells when using conditioned medium from the macrophages derived as a chemoattractant from patients with wet AMD. Migrated RPE cells were shown as a percentage of the control (first column). There was no significant difference among the three groups when 10% FBS or 0.2% FBS medium was used as a chemoattractant (n = 3). *P < 0.01.
JAM-C Knockdown Decreases the Permeability of Monolayer hRPE
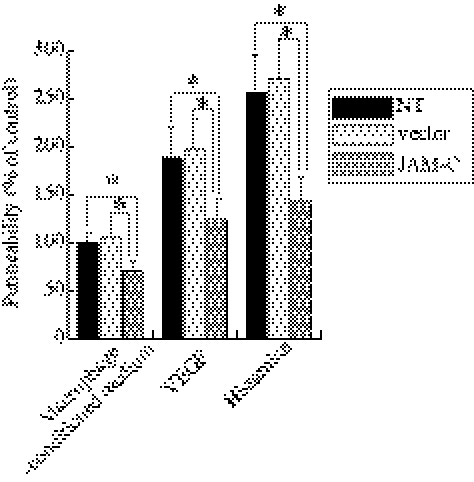
The RPE maintains the outer BRB and prevents certain substances from entering the retina. We therefore investigated whether JAM-C knockdown influences the permeability of the RPE barrier. To stimulate permeability, we used conditioned medium from wet AMD macrophages, VEGF, or histamine. We found that the concentration of fluorescein dye in the basal compartment of JAM-C shRNA–transfected cells was lower than that of the other two control groups 30 minutes after the addition of the fluorescein dye (Fig. 5; n = 3; P < 0.01), demonstrating that JAM-C knockdown decreased the permeability of the hRPE cell monolayer.
Figure 5.
JAM-C knockdown decreases monolayer hRPE permeability. The concentration of the fluorescein dye in the basal compartment of JAM-C shRNA–transfected RPE cells was lower than in those of the two control groups 30 minutes after the addition of the fluorescein dye in a permeability assay. The response was similar in each of the following media used in the experiment: macrophage-conditioned medium in patients with wet AMD, VEGF, or histamine. Permeability is shown as a percentage of the control (first column). n = 3. *P < 0.01.
Discussion
CNV prevention and therapy are challenging because of the tissue's special location, the involvement of multiple cellular components, and the complexity of its pathogenesis. Comprehensive understanding of this disease to search for therapeutic targets is an important way to meet this challenge. CNV is a nonspecific response to injury and repair in various pathologic states of the eye.18,19 Inflammation is an important part of CNV. Therefore, in addition to confronting angiogenesis directly, we can consider other therapeutic measures.20 CNV development can be divided into the initial stage (macrophage aggregation), the active phase of inflammation (cytokine secretion), and the stable phage of inflammation (fibrosis).21 During all the stages, macrophages play a vital role.22 Resident and recruited macrophages in the neural retina infiltrate the lesion. Monocytes are attracted from the choriocapillaris along the outer surface of Bruch's membrane and are locally differentiated to macrophages.2 The recruited macrophages contribute to CNV formation through vascular growth factor secretion,23 complement activation,24 vascular endothelial cells, and Bruch's membrane injury.25,26 Indeed, macrophage depletion inhibited experimental CNV.27,28 Based on these studies, searching for key traffic factors regulating macrophage infiltration and retinal barrier function represents a promising route to CNV therapy.
JAM is a member of the immunoglobulin superfamily involved in the composition and maintenance of tight junctions (TJs) and the establishment of endothelial and epithelial cell polarity.29 Recent studies have elegantly demonstrated that JAM plays an important role in monocyte migration during angiogenesis and inflammation.6 Among JAM family members, JAM-C is highly expressed in the retina and RPE,8 suggesting it has a function in the eye. JAM-C expression is found both at the apical junctional complexes and in the long apical processes of Müller and RPE cells, which extend into the subretinal space and ensheathe the photoreceptors.8 In our study, we found that JAM-C was detected primarily in the RPE layer, inner and outer segments, and inner plexiform layer of the retina. To examine JAM-C expression during CNV formation, we performed a laser-induced CNV model in mice and found that JAM-C expression was upregulated during CNV development. Hence, it was important to investigate the role of JAM-C in CNV formation.
JAM-C is a key “gatekeeper” molecule expressed at the TJs of the BRB,8,30 which is broken down during CNV formation. Given that we found that JAM-C expression increased during CNV, JAM-C may contribute to CNV pathogenesis. We functionally blocked JAM-C by intravitreal injection of a JAM-C Fc chimera and analyzed CNV size. Histologic analysis showed that JAM-C targeting reduced the CNV area 7 days after laser treatment. Moreover, blood vessel leakage in the CNV decreased accordingly. Interestingly, it has been shown that blocking the function of JAM-C significantly reduced angiogenesis associated with the inflammation and permeability of blood vessels in the hypoxia-induced retinal neovascularization mouse model.10 Thus, our data support that JAM-C may be a potential target molecule for CNV therapy.
Macrophages aggregate into the lesion area during the initial stage of CNV formation. For this purpose, macrophages must transmigrate through the TJs of BRB. We tested whether JAM-C blockade can suppress this process and found that intravitreal injection of the JAM-C Fc chimera reduced macrophage accumulation within the CNV area. We confirmed this observation using a macrophage basolateral-to-apical transmigration assay. A previous study reported that the inhibition of JAM-C decreased the apical-to-basal transmigration of granulocytes but not of monocytes.16 The monocytes used in their experiment came from healthy donors. In our study, the macrophages were derived from healthy donors or patients with wet AMD. However, the inhibitory effect of JAM-C could only be seen in the macrophages derived from patients with wet AMD but not in the macrophages from healthy controls. One possible explanation for this phenomenon may be that the expression pattern of molecules and receptors is different in the macrophages derived from CNV patients and healthy controls. Another explanation may be that the response to JAM-C deprivation may be different between the two types of macrophages.
In addition to being producing homodimers,31 JAM-C can form heterodimers with JAM-B and Mac-1.6 It has not been reported whether macrophages express JAM-B as well as JAM-C. However, JAM-C expressed at the TJs can heterodimerize with Mac-1 expressed at the macrophage surface to facilitate transmigration.32,33 One study has shown that a JAM-C–neutralizing antibody can inhibit neovascularization and tumor growth both in vivo and in vitro. The neutralizing antibody does not affect endothelial cell proliferation and apoptosis; one way to inhibit tumor angiogenesis is to reduce macrophage accumulation.9 Thus, based on our data, JAM-C blockade may suppress CNV, at least partially, by reducing macrophage infiltration.
In addition to forming the BRB, RPE cells play important roles in CNV formation. Therefore, we investigated RPE cell migration and proliferation and monolayer RPE permeability after JAM-C shRNA treatment. Compared with the nontransfected or vector-transfected group, JAM-C knockdown inhibited RPE cell migration toward the conditioned medium from cultured macrophages of patients with wet AMD but did not affect the migration of RPE cells cultured in 10% FBS or 0.2% FBS medium. In addition, JAM-C knockdown did not affect RPE cell proliferation. Based on our data, JAM-C may be involved in the pathogenic process of CNV formation. On the other hand, we found that JAM-C blockade helped keep monolayer RPE barrier function normal under challenge (e.g., VEGF, histamine). This may be another reason JAM-C targeting suppressed CNV. However, the detailed mechanisms require further investigation.
During CNV formation, various challenges (e.g., ischemia, hypoxia, oxidative damage, infection) stimulate the production of proinflammatory and chemotactic factors and the activation of local and blood-derived macrophages. These may lead to changes in JAM-C expression, which may result in BRB malfunction. With JAM-C facilitating macrophage transmigration continuously and further increasing the permeability of monolayer RPE, the BRB breaks down, and RPE cells begin to migrate and participate in CNV formation. Thus, JAM-C function and macrophage over-transmigration blockade may be useful for the early prevention and treatment of CNV. Recently, JAM-C was found to have a soluble form and to induce human microvascular endothelial cell tube formation in vitro and angiogenesis in vivo.12 These results confirm that JAM-C targeting may be a new route for CNV treatment. Together, we conclude that JAM-C may be involved, at least in part, in CNV formation by influencing BRB function and macrophage infiltration and that JAM-C inhibition may have therapeutic application in CNV treatment.
Acknowledgments
The authors thank the IOVS Volunteer Editor Program and Timothy Corson for editorial assistance.
Footnotes
Supported by National Basic Research Program of China Grant 973 Program/2011CB510200 and in part by National Natural Science Foundation of China Grants 30872818 and 81070748.
Disclosure: X. Hou, None; D. Hu, None; Y. Wang, None; Z. Tang, None; F. Zhang, None; T. Chavakis, None; Y. Li, None; X. Li, None
References
- 1. Green WR, Wilson DJ. Choroidal neovascularization. Ophthalmology. 1986;93:1169–1176 [DOI] [PubMed] [Google Scholar]
- 2. Grossniklaus HE, Green WR. Choroidal neovascularization. Am J Ophthalmol. 2004;137:496–503 [DOI] [PubMed] [Google Scholar]
- 3. Aiello LP, Pierce EA, Foley ED, et al. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Pro Natl Acad Sci U S A. 1995;92:10457–10461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Navaratna D, Maestas J, McGuire PG, Das A. Suppression of retinal neovascularization with an antagonist to vascular endothelial cadherin. Arch Ophthalmol. 2008;126:1082–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Noda K, She H, Nakazawa T, et al. Vascular adhesion protein-1 blockade suppresses choroidal neovascularization. FASEB J. 2008;22:2928–2935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bradfield PF, Nourshargh S, Aurrand-Lions M, Imhof BA. JAM family and related proteins in leukocyte migration (Vestweber series). Arterioscler Thromb Vasc Biol. 2007;27:2104–2112 [DOI] [PubMed] [Google Scholar]
- 7. Orlova VV, Chavakis T. Regulation of vascular endothelial permeability by junctional adhesion molecules (JAM). Thromb Haemost. 2007;98:327–332 [PubMed] [Google Scholar]
- 8. Daniele LL, Adams RH, Durante DE, Pugh EN, Jr, Philp NJ. Novel distribution of junctional adhesion molecule-C in the neural retina and retinal pigment epithelium. J Comp Neurol. 2007;505:166–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lamagna C, Hodivala-Dilke KM, Imhof BA, Aurrand-Lions M. Antibody against junctional adhesion molecule-C inhibits angiogenesis and tumor growth. Cancer Res. 2005;65:5703–5710 [DOI] [PubMed] [Google Scholar]
- 10. Orlova VV, Economopoulou M, Lupu F, Santoso S, Chavakis T. Junctional adhesion molecule-C regulates vascular endothelial permeability by modulating VE-cadherin-mediated cell-cell contacts. J Exp Med. 2006;203:2703–2714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Langer HF, Orlova VV, Xie C, et al. A novel function of junctional adhesion molecule-C in mediating melanoma cell metastasis. Cancer Res. 2011;71:4096–4105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rabquer BJ, Amin MA, Teegala N, et al. Junctional adhesion molecule-C is a soluble mediator of angiogenesis. J Immunol. 2010;185:1777–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hou X, Han QH, Hu D, et al. Mechanical force enhances MMP-2 activation via p38 signaling pathway in human retinal pigment epithelial cells. Graefe's Arch Clin Exp Ophthalmol. 2009;247:1477–1486 [DOI] [PubMed] [Google Scholar]
- 14. Hou X, Kumar A, Lee C, et al. PDGF-CC blockade inhibits pathological angiogenesis by acting on multiple cellular and molecular targets. Proc Natl Acad Sci U S A. 2010;107:12216–12221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dong X, Wang YS, Dou GR, et al. Influence of Dll4 via HIF-1alpha-VEGF signaling on the angiogenesis of choroidal neovascularization under hypoxic conditions. PLoS One. 2011;6:e18481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Economopoulou M, Hammer J, Wang F, Fariss R, Maminishkis A, Miller SS. Expression, localization, and function of junctional adhesion molecule-C (JAM-C) in human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2009;50:1454–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang F, Tang Z, Hou X, et al. VEGF-B is dispensable for blood vessel growth but critical for their survival, and VEGF-B targeting inhibits pathological angiogenesis. Proc Natl Acad Sci U S A. 2009;106:6152–6157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grossniklaus HE, Martinez JA, Brown VB, et al. Immunohistochemical and histochemical properties of surgically excised subretinal neovascular membranes in age-related macular degeneration. Am J Ophthalmol. 1992;114:464–472 [DOI] [PubMed] [Google Scholar]
- 19. Grossniklaus HE, Hutchinson AK, Capone A, Jr, Woolfson J, Lambert HM. Clinicopathologic features of surgically excised choroidal neovascular membranes. Ophthalmology. 1994;101:1099–1111 [DOI] [PubMed] [Google Scholar]
- 20. Kent D, Sheridan C. Choroidal neovascularization: a wound healing perspective. Mol Vis. 2003;9:747–755 [PubMed] [Google Scholar]
- 21. Grossniklaus HE, Ling JX, Wallace TM, et al. Macrophage and retinal pigment epithelium expression of angiogenic cytokines in choroidal neovascularization. Mol Vis. 2002;8:119–126 [PubMed] [Google Scholar]
- 22. Tsutsumi C, Sonoda KH, Egashira K, et al. The critical role of ocular-infiltrating macrophages in the development of choroidal neovascularization. J Leukoc Biol. 2003;74:25–32 [DOI] [PubMed] [Google Scholar]
- 23. Szekanecz Z, Koch AE. Macrophages and their products in rheumatoid arthritis. Curr Opin Rheumatol. 2007;19:289–295 [DOI] [PubMed] [Google Scholar]
- 24. Gasque P. Complement: a unique innate immune sensor for danger signals. Mol Immunol. 2004;41:1089–1098 [DOI] [PubMed] [Google Scholar]
- 25. Radi ZA, Kehrli ME, Jr, Ackermann MR. Cell adhesion molecules, leukocyte trafficking, and strategies to reduce leukocyte infiltration. J Vet Intern Med. 2001;15:516–529 [DOI] [PubMed] [Google Scholar]
- 26. Sarks JP, Sarks SH, Killingsworth MC. Morphology of early choroidal neovascularisation in age-related macular degeneration: correlation with activity. Eye. 1997;11(pt 4):515–522 [DOI] [PubMed] [Google Scholar]
- 27. Sakurai E, Anand A, Ambati BK, van Rooijen N, Ambati J. Macrophage depletion inhibits experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:3578–3585 [DOI] [PubMed] [Google Scholar]
- 28. Espinosa-Heidmann DG, Suner IJ, Hernandez EP, Monroy D, Csaky KG, Cousins SW. Macrophage depletion diminishes lesion size and severity in experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:3586–3592 [DOI] [PubMed] [Google Scholar]
- 29. Mandell KJ, Parkos CA. The JAM family of proteins. Adv Drug Deliv Rev. 2005;57:857–867 [DOI] [PubMed] [Google Scholar]
- 30. Mandell KJ, Berglin L, Severson EA, Edelhauser HF, Parkos CA. Expression of JAM-A in the human corneal endothelium and retinal pigment epithelium: localization and evidence for role in barrier function. Invest Ophthalmol Vis Sci. 2007;48:3928–3936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Santoso S, Orlova VV, Song K, Sachs UJ, Andrei-Selmer CL, Chavakis T. The homophilic binding of junctional adhesion molecule-C mediates tumor cell-endothelial cell interactions. J Biol Chem. 2005;280:36326–36333 [DOI] [PubMed] [Google Scholar]
- 32. Santoso S, Sachs UJ, Kroll H, et al. The junctional adhesion molecule 3 (JAM-3) on human platelets is a counterreceptor for the leukocyte integrin Mac-1. J Exp Med. 2002;196:679–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chavakis T, Keiper T, Matz-Westphal R, et al. The junctional adhesion molecule-C promotes neutrophil transendothelial migration in vitro and in vivo. J Biol Chem. 2004;279:55602–55608 [DOI] [PubMed] [Google Scholar]