Background: Ferritins, cytoplasmic protein nanocages, with internal and cytoplasmic pores terminating trans-cage ion channels, reversibly concentrate iron and scavenge oxidants.
Results: Changing ferritin conserved channel residues altered Fe2+ exit, channel flexibility, protein-crowding sensitivity, ion binding, and N-terminal folding.
Conclusion: Eukaryotic ferritin N termini form cytoplasmic gates stabilized by hydrogen bonds and ionic bonds.
Significance: Shared structure and function of ferritin with membrane ion channels includes cytoplasmic, N-terminal gates.
Keywords: Ferritin, Gating, Ion Channels, Iron, Protein Folding, Nanocage
Abstract
Ferritin protein nanocages, self-assembled from four-α-helix bundle subunits, use Fe2+ and oxygen to synthesize encapsulated, ferric oxide minerals. Ferritin minerals are iron concentrates stored for cell growth. Ferritins are also antioxidants, scavenging Fenton chemistry reactants. Channels for iron entry and exit consist of helical hairpin segments surrounding the 3-fold symmetry axes of the ferritin nanocages. We now report structural differences caused by amino acid substitutions in the Fe2+ ion entry and exit channels and at the cytoplasmic pores, from high resolution (1.3–1.8 Å) protein crystal structures of the eukaryotic model ferritin, frog M. Mutations that eliminate conserved ionic or hydrophobic interactions between Arg-72 and Asp-122 and between Leu-110 and Leu-134 increase flexibility in the ion channels, cytoplasmic pores, and/or the N-terminal extensions of the helix bundles. Decreased ion binding in the channels and changes in ordered water are also observed. Protein structural changes coincide with increased Fe2+ exit from dissolved, ferric minerals inside ferritin protein cages; Fe2+ exit from ferritin cages depends on a complex, surface-limited process to reduce and dissolve the ferric mineral. High concentrations of bovine serum albumin or lysozyme (protein crowders) to mimic the cytoplasm restored Fe2+ exit in the variants to wild type. The data suggest that fluctuations in pore structure control gating. The newly identified role of the ferritin subunit N-terminal extensions in gating Fe2+ exit from the cytoplasmic pores strengthens the structural and functional analogies between ferritin ion channels in the water-soluble protein assembly and membrane protein ion channels gated by cytoplasmic N-terminal peptides.
Introduction
Membrane channel proteins and ferritin nanocages move ions across organic barriers. In ferritins, ferrous ions move through the protein cage to and from the cytoplasm and destinations within the cage (1, 2). After entering the protein cage through the ion channels (Fig. 1) (3, 4), the Fe2+ ions are guided to iron and oxygen oxidoreductase sites that are buried in the four-helix bundle subunits of eukaryotic ferritins. The diferric oxo or hydroxo products of catalytic coupling navigate through the protein cage, forming mineral nuclei (5). Nanomineral nuclei grow in the large, central ferritin cavity that is 60% of the cage volume. Only a few of the thousands of iron atoms in ferritin are bound directly to the protein itself at any one time. Thousands of other iron atoms are in a solid, Fe2O3·H2O mineral sequestered in the capacious central cavity. Iron is recovered from ferritin in vivo in response to signals of iron requirements for cell division, repair of oxidative damage, or specialized functions, such as hemoglobin or nitrogenase synthesis. The phase transitions that form the solid, ferric oxo mineral inside soluble, ferritin protein cages and the regulated reduction and dissolution of the caged ferric oxo mineral that provides iron ions for cytoplasmic metabolism are not well understood. Ferritin is central to biology, however. Illustrations of the importance of ferritins include presence in all forms of life, lethality of gene deletion in mouse embryos (6), coordinated regulation of DNA with other antioxidant response proteins (1, 7, 8), and, in animals, iron-regulated noncoding mRNA structures that coordinate the synthesis of iron trafficking and storing proteins (9, 10).
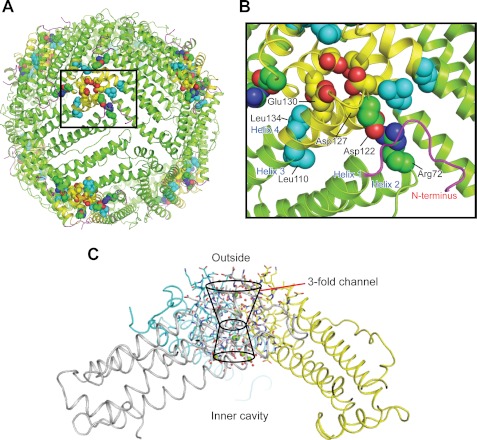
FIGURE 1.
Conserved interactions rigidify the ion entry and exit channels at the 3-fold symmetry axes of ferritin protein cages. A, the ferritin protein cage of a eukaryotic ferritin (24 subunits), the frog M ferritin model (PDB code 3KA3). Boxed region, the ion entry and exit channel. B, space-filling representations of selected residues important in ferritin ion channel function. C, a single ferritin ion channel at the junction of three subunits. The cross-section of helix 3-loop-helix 4 from one of the three subunits that form the Fe2+ entry and exit channels is shown with residues involved in ion channel activity. Asp-127 and Glu-130 affect Fe2+ entry in solution studies (4, 52, 53). Arg-72, Leu-110, Asp-122, and Leu-134 affect Fe2+ exit in solution studies (11, 12, 18). Yellow, helix-turn-helix of one of the three subunits; green, the major section of the four-helix bundle that contains the active site and nucleation channel; magenta, the N-terminal peptide.
Flexible pores (11, 12) at the ferritin cytoplasmic surface and at the entry to the ferritin mineral growth cavity, connected by Fe2+ entry and exit channels, separate the reducing environment of the cell and the caged, ferric mineral (Fig. 1). Fe2+ entry and exit channels in eukaryotic ferritins are ∼15 Å long (3, 5). They are composed of helices and loops from three subunits juxtaposed around the 3-fold axes of ferritin cage structures (Fig. 1). There are eight such channels in 24-subunit ferritins and four in 12-subunit miniferritins (also called Dps proteins). Ion channels in membranes, by comparison, range in length from 10 to 90 Å. The quaternary structure and function of ferritins are remarkably conserved, despite variations in sequence (up to 80%), in the size of the subunit N- and C-terminal extensions and in oxidoreductase mechanisms (1, 2, 13).
To minimize leaking of Fe2+ from reactions of the ferric mineral with cytoplasmic reductants, ferritin protein pore gates are largely closed until iron is required by the cells. The cytoplasmic signals and carrier proteins for Fe2+ release from ferritin minerals are not known, but physiological “gating” of ferritin is observed in vivo. An illustration is the recovery of iron from tissue ferritin to synthesize new red blood cells after hemorrhage (14, 15). In solution, opening and closing ferritin pore gates are observed as changes in the reduction and dissolution of ferritin minerals and Fe2+ exit from the protein cage; Fe2+ exit is triggered by natural reductants NADH plus FMN (16). Chaotropes, low heat, substitution of specific conserved, channel amino acids, and tight binding peptides, selected from combinatorial libraries, all alter ferritin mineral reduction and dissolution and Fe2+ exit (12, 17, 18). Selectivity of ferritin channel residue function is indicated by the absence of an effect of M-E130A and M-D127A channel residue substitutions on Fe2+ exit but large (>97%) losses of oxidoreductase activity (4). In contrast, the H-R72D substitution causes large effects on Fe2+ exit with no detectable effect on oxidoreductase activity (18).
To identify the cytoplasmic gates that control mineral dissolution and Fe2+ exit from ferritin, we compared the effects in high resolution protein crystal structures of amino acid substitutions close to the cytoplasmic pores (M-R72D, and M-D122R) deep inside the Fe2+ iron entry and exit channels (M-L134P). A previous, lower resolution protein crystal structure of frog H-L134P ferritin (11) indicated extensive helix unfolding in the ion channels. However, despite the 85% sequence identity between the H and M ferritin subunits, wild-type H ferritin from frogs is difficult to express. Suitable crystals for high resolution x-ray diffraction have never been obtained, and there is much less mechanistic information than for frog M (1).
Our results reveal the structural and functional importance of the interaction network between the subunit bundle, N-terminal extension residues Gln-6 and Asn-7, Arg-72 (helix 2), and Asp-122 (helix 3–4 loop) for gating Fe2+ exit. Studies of M-R72D, M-D122R, and M-L134P, each in different parts of ferritin ion channels, showed distinct differences in localized channel flexibility (crystallographic B′ values), channel metal ion binding, and Fe2+ exit. Protein crowders restored normal Fe2+ exit in the variants. The significance of identifying the N-terminal extensions of the subunits as the Fe2+ exit gates on the cytoplasmic surface of ferritin ion channels is 2-fold. First, the gates are potential sites for regulating iron release from ferritin. Second, our results emphasize mechanistic similarities between ion channels in the water-soluble ferritin cage and N-terminal-gated ion channels in membranes (19–22).
EXPERIMENTAL PROCEDURES
Protein Preparation
The frog M ferritin Fe2+ ion channel mutants, M-R72D, M-D122R, and M-L134P, were generated by the QuikChange method (Stratagene). The template was a pET-3a vector encoding wild-type frog M ferritin (12). The sequences of all expression vectors were confirmed by DNA sequencing. Recombinant proteins were expressed in Escherichia coli BL21(DE3) pLysS (Stratagene) (12). For R72D and D122R, betaine and sorbitol were used in the culture medium to minimize incorrect folding and to increase yields (23). Expressed proteins were purified as described (12, 24). The final buffer for the proteins was 100 mm MOPS (pH 7.0) and 100 mm NaCl. Protein concentrations were determined by the Bradford method (Bio-Rad protein assay kit) with bovine serum albumin as a standard.
Iron Release
Recombinant ferritins, isolated as the self-assembled cages with <4 irons per cage, were mineralized by adding solutions of freshly prepared 50 mm FeSO4 in 1 mm HCl to the buffered protein solutions. The final protein concentrations were 1.0 μm protein cages and 480 μm iron, except in the protein crowding experiments, where the protein cages were 2.08 μm, and iron was 1.0 mm. After mixing, the solutions were incubated for 2 h at room temperature and then overnight at 4 °C to complete the iron mineralization reaction (11).
Fe2+ exit from caged ferritin minerals was initiated by reducing the ferritin mineral with added NADH and FMN and trapping the reduced and dissolved Fe2+ as the pink Fe2+-(2,2′-bipyridyl)3 complex outside the protein cage (16). The intermediate steps in ferric reduction on the mineral surface and Fe2+ transit from the mineral surface through the ion channels to the outside of the cage are not characterized currently. Fe2+ exit from the protein cage was measured as the absorbance of Fe2+-(2,2′-bipyridyl)3 at the λmax of A522 nm, as described previously (11). Briefly, to 500 μl of mineralized ferritin, 25 °C, 500 μl of a mixture of 2.5 mm FMN, 2.5 mm NADH, and 2.5 mm bipyridyl, all in the final protein buffer, was added to the protein solution. Initial rates, which can only be computed for the linear phase that accounts for only 7% of the iron in ferritin, used the extinction coefficient at 522 nm of 8,340 m−1 cm−1 for the Fe2+-(2,2′-bipyridyl)3 complex. The time (min) required for 25 and 50% of the mineralized ferric iron (t25 and t50) to be dissolved and exit the cage as Fe2+ was also determined. Data analyses used Igor Pro software, version 5.0.5.A (WaveMetrics). Averaged data were from 5–8 independent experiments, using at least two different preparations of each protein. Errors are the S.D. Statistical analyses used Student's t test.
Protein Crystallization
Crystals of wild-type M ferritin and the modified ferritins (M-R72D, M-D122R, and M-L134P) were obtained by mixing 2 μl of protein solution (5–20 mg/ml) with an equal volume of the precipitant solution, 2.0 m MgCl2 and 100 mm Bicine6 (pH 9.0) (3). Cubic crystals formed within 1–2 days at 4 °C. The crystals were transferred to the precipitant solution containing 20% (v/v) ethylene glycol as a cryoprotectant for at least 5 min before flash-freezing in liquid nitrogen.
X-ray Data Collection and Structure Refinement
X-ray diffraction data were collected at 11,111 eV on Beamline 8.3.1 at the Advanced Light Source at the Lawrence Berkeley National Laboratory. The crystals were maintained at 100 K during data collection. Data were processed using the programs ELVES, MOSFLM, and SCALA (25–27). Data collection statistics are summarized in Table 1.
TABLE 1.
Statistics for x-ray data collection and model refinement
| Frog M ferritin variant |
|||
|---|---|---|---|
| R72D | D122R | L134P | |
| Data collection | |||
| Wavelength (Å) | 1.11587 | 1.11587 | 1.11587 |
| Space group | F432 | F432 | F432 |
| Unit cell edge (a = b = c) (Å) | 183.560 | 183.434 | 183.775 |
| Resolution range (Å) | 1.65-106.00 | 1.45-91.67 | 1.35-64.96 |
| (1.65-1.74)a | (1.45-1.53) | (1.35-1.42) | |
| No. of unique reflections | 32,417 | 47,217 | 58,567 |
| Completeness (%) | 100 (100) | 100 (100) | 100 (100) |
| Multiplicity | 20.1 | 17.3 | 13.1 |
| Rmerge | 0.102 (1.187) | 0.088 (1.006) | 0.071 (0.807) |
| I/σ | 22.6 (2.2) | 20.4 (2.2) | 19.5 (2.3) |
| Refinement parameters | |||
| R | 0.165 | 0.155 | 0.135 |
| Rfree | 0.195 | 0.182 | 0.161 |
| Root mean square deviation from ideal values | |||
| Bond lengths (Å) | 0.034 | 0.032 | 0.030 |
| Bond angles (degrees) | 2.484 | 2.516 | 2.447 |
| No. of atoms | |||
| Solvent (H2O) | 271 | 170 | 228 |
| Mg(II) | 8 | 7 | 10 |
| Cl− | 9 | 3 | 7 |
| Average B values (Å2)b | 20.4 | 18.0 | 15.9 |
a Values in parentheses are for the highest resolution shell.
b For all protein atoms. The average B value for wild-type protein (PDB code 3KA3) is 14.6 Å2.
Initial models were obtained by molecular replacement with EPMR (28). The search model was a single subunit of wild-type frog M ferritin (PDB code 1MFR) (29), excluding Mg2+ ions and water molecules. The structures were refined using the program REFMAC5 (30). Structural models were visualized and manually modified based on the 2mFo − DFc and mFo − DFc electron density maps by using the program COOT (31, 32). Water molecules were positioned initially using ARP/wARP (33). Positions of Mg2+ ions were deduced from relatively strong electron density and octahedral coordination. Atomic temperature factors were refined isotropically for R72D ferritin and anisotropically for the D122R and L134P variants. Model quality was evaluated using the WHAT IF web server (34). Structure figures were prepared using PyMOL (35).
The degree of protein flexibility was assessed using the B values of the Cα atoms. Because B values are influenced by parameters that differ between structures, the B values were normalized for each model. The normalized B value (B′ value) of each Cα atom was calculated using the equation, B′ = (B − 〈B〉)/σ(B), where 〈B〉 and σ(B) are the mean and the S.D. of the B values for the Cα atoms of the protein, respectively (36).
Protein Crowding
Effects of protein crowding (37, 38) (incubation of ferritin (480 irons/cage) with bovine serum albumin (250 mg/ml) or lysozyme (125 mg/ml) for 24 h at 4 °C) were measured as changes in Fe2+ exit using the methods described above. Data analyses used OriginPro software, version 8.0.
RESULTS
Amino Acid Substitution Selectively Alters Localized Channel Flexibility
Ferritin protein cages, which are 85% α-helical, are unusually stable, resisting 6 m urea or temperatures of 80 °C. Thus, the localized unfolding of regions of the protein cages, when first observed, was unexpected (11, 12, 39). The effects of localized unfolding of ferritin protein on Fe2+ exit identified the channels around the 3-fold axes as the sites of Fe2+ exit. In wild-type ferritin, the exit pores are unusually sensitive to small changes in the aqueous solvent, such as physiological urea concentrations (1 mm), and temperatures far below the protein cage Tm (12).
To obtain structural information on the effects of changing channel amino acids that regulate Fe2+ exit, we used the frog M ferritin model for several reasons. First, we obtained high resolution (1.3–1.7 Å) protein crystal structures not available for frog H ferritins. Second, mechanistic information of oxidoreduction and mineral growth for frog M is much more extensive than for frog H or human H ferritin, in part because of the spectroscopically favorable kinetics of the diferric peroxo intermediate (1). Finally, the amino acid substitutions made in the frog M protein are at residues conserved in eukaryotic ferritins and in many bacterial ferritins.
Comparisons of the crystal structures of wild-type frog M ferritin and the M-R72D, M-D122R, and M-L134P variants reveal that localized structural changes are associated with each of the amino acid substitutions (Fig. 2). In addition, we calculated the normalized B values (B′ values) of the models to correct for the effects of differences in crystallographic resolution (36) and to assess changes in main-chain flexibility. As judged by increases in the normalized B values of the Cα atoms, the M-D122R and M-L134P substitutions increased the disorder of the main chain around the 3-fold pore, including the ion channel residues 110–134 (Fig. 2E).
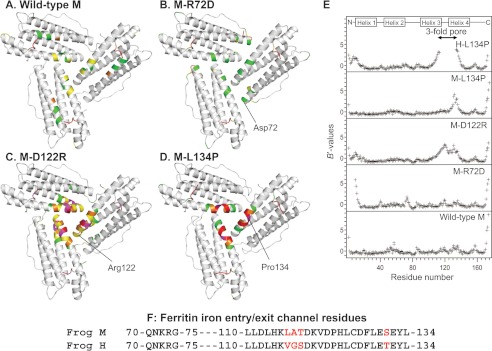
FIGURE 2.
Changing ion channel residues selectively affects main-chain flexibility. A–D, view from the internal surface of ferritin protein cages around the ion channel exits. The same sections of three helices form the ion channels around the 3-fold symmetry axes of the cage. The colors indicate the distribution of normalized B value (B′ value) for Cα atoms: white, B′ < 0.5; green, 0.5 < B′ < 1.0; yellow, 1.0 < B′ < 1.5; magenta, 1.5 < B′ < 2.0, red, 2.0 < B′. A, wild-type frog M (PDB code 3KA3); B, frog M-L134P (PDB 3SHX); C, frog M-D122R (PDB code 3SH6); D, frog M-R72D (PDB code 3SE1). E, B′ values of the Cα atoms for frog M ferritins and frog H-L134P (PDB code 1BG7) from (11); wild-type H and M ferritin isoforms share 85% sequence identity. F, amino acid sequence comparisons. The 3-fold channel sequences frog M and H ferritins are compared. Red type, difference in H and M ion channel sequence.
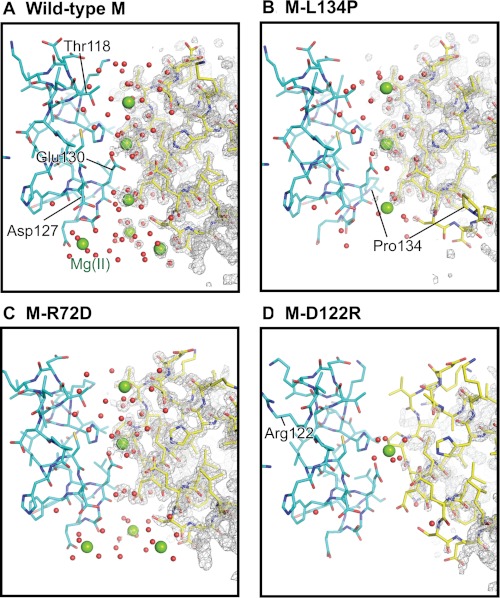
M-D122R is a substitution of the aspartate residue in the short loop that links helices 3 and 4 of each of the three subunits that compose a single ferritin Fe2+ channel (Fig. 1). When the ion pair between Asp-122 and Arg-72 is disrupted in M-D122R, the atomic coordinates for the ion channel and N terminus are almost identical to that of wild type. However, the electron density in and around the channel (residues 102–138) is lower than in wild-type or in M-L134P. B′ values for the backbone Cα atoms in the M-D122R structure are higher throughout the entire channel, including the N terminus (residues 5–13) and around the entrances to the external pores (Fig. 2, C and E).
The M-R72D substitution disrupts the conserved ion pair between Arg-72, near the end of helix 2, and Asp-122 in the helix 3–4 loop (Fig. 1B). The B′ values throughout the four-helix bundle of the M-R72D model are similar to those in the wild-type ferritin structure (Fig. 2B). Mg2+ from the crystallization solution (2.0 m MgCl2) mediates an interaction between Asp-72 and Asp-122 (supplemental Fig. S1) and probably compensates for the negative charge of the carboxylate group introduced at Asp-72. However, the N-terminal residues of M-R72D are extensively disordered, unique among the protein structures studied here (Figs. 2E and 3). In addition to the disorder of eight N-terminal residues, residues 9–13 in the N-terminal extension of the helix bundle have large B′ values. When the M-R72D and M-wild-type ferritin structures are superimposed, the carboxylate side chain of Asp-122 overlaps the ring of Tyr-8 in the N-terminal peptide. This steric incompatibility accounts for the distributed conformational changes observed between M-wild-type and M-R72D ferritins (supplemental Fig. S1).
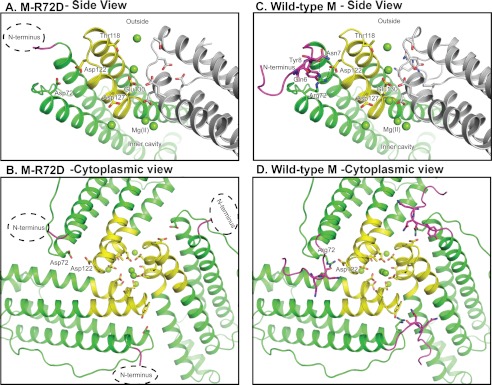
FIGURE 3.
Structural interactions of N-terminal pore gates with ion entry and exit channel residues in ferritin protein cages. A and B, M-R72D ferritin. A hydrated Mg2+ is bound by Asp-72 and Asp-122, and the alignment of the channel ions is changed compared with the wild-type structure. C and D, wild-type frog M ferritin binds Mg2+ in the crystals at Thr-118 and in line in the channel.
The M-L134P substitution in helix 4 disrupts the interactions with the conserved Leu-110 in helix 3 (18). Main-chain fluctuations increase in ion channel residues 125–135 of the M-L134P variant compared with wild-type M (Fig. 2, D and E). Similarly, the 3-fold pore residues 114–133 are extensively disordered in the L134P mutant of frog H ferritin, which also shows disorder in the N-terminal extension residues 1–12 (Fig. 2E).
Disorder in N-terminal Extensions of Ferritin Subunit Helix Bundles in M-R72D Ferritin Increases Fe2+ Exit and Identifies Cytoplasmic Gates
Fe2+ exit from ferritins is the result of a complex, surface-limited process where electrons originating from reduced flavin in solution outside the protein cage convert Fe3+ ions on the surface of the protein-caged mineral to Fe2+. Reduction is followed by hydration of the oxo bridges between each iron atom in the mineral and migration of dissolved Fe2+ from the mineral surface to and through the ion channels to the outside of the protein cage. Where and how the electrons are transferred from the flavin to the ferric ions in the mineral, what is the transit path of the Fe2+ ions to the inner pore of the ion channels, and where the Fe2+ reacts with chelators, such as bipyridyl, are unknown. Although important questions, they have been experimentally intractable to date. However, monitoring formation of the Fe2+-bipyridyl complex outside the ferritin protein cage (11, 12, 39) has been a useful way to understand the role of the protein cage in regulating the stability of the ferric mineral and preventing the leakage of Fe2+ from ferritin minerals that could occur from reactions with cytoplasmic reductants (1, 2).
When the N-terminal sequence is disordered by loss of interactions with the helix bundle in M-R72D ferritin, Fe2+ exit, after adding reductants such as NADH and FMN, is much more rapid (Fig. 4 and Table 2). The influence of protein cage structure on Fe2+ exit is only apparent after the first 30 s, when ∼7% of the iron has complexed with bipyridyl (Table 2). Differences in Fe2+ exit among wild-type and variant ferritins persist for more than 1 h after the addition of reductant (Fig. 4). A functional role of the ferritin N-terminal amino acids explains the conservation of sequence and structural interactions observed among many ferritins.
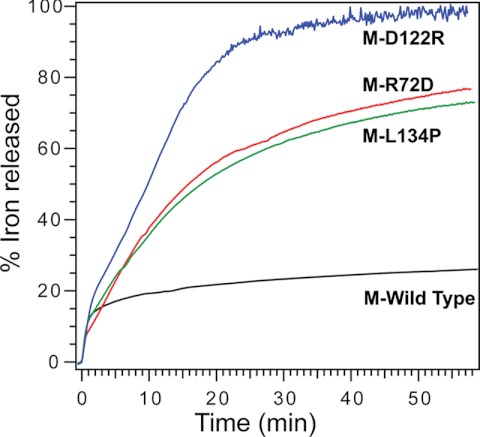
FIGURE 4.
Effects of changing ferritin ion exit and entry channel residues on Fe2+ exit from eukaryotic ferritin protein cages. Progress curves for the surface-limited reduction and dissolution of protein-caged ferric minerals and Fe2+ exit from the protein cages of wild-type, M-R72D, M-R122D, and M-L134P ferritins. The Fe2+ release reaction was initiated by mixing solutions of mineralized ferritin with a solution of 2.5 mm FMN, NADH, and bipyridyl and monitoring Fe2+-(bipyridyl)3 outside the protein cages. (numerical rates are shown in supplemental Table S2). Data are representative of two independent protein preparations, with 3–4 repetitions for each. Black, M-WT; green, M-L134P; red, M-R72D; blue, M-D122R.
TABLE 2.
Ion channel residues that influence ferritin iron mineral dissolution and Fe2+ exit
| Protein | Via (7% of mineral) (0–30 s) | Time of 25% mineral dissociation | Time of 50% mineral dissociation |
|---|---|---|---|
| irons/min | min | min | |
| Wild type | 78 ± 8.2 | 43.0 ± 7.1b | >60.0b |
| R72D | 77 ± 7.8 | 7.4 ± 1.0 | 17 ± 3.5 |
| L134P | 75 ± 7.3 | 7.0 ± 1.2 | 19 ± 3.8 |
| D122R | 63 ± 6.6 | 5.8 ± 0.8 | 12 ± 2.4c |
a Initial rates of Fe2+ exit can only be computed for the first 30 s, when the progress curve is linear; only 7% of the Fe2+ iron exits during this period. Changing ferritin cage ion channel structure has no effect on the initial rate; such iron probably represents iron bound in the protein cage rather than dissolved from the bulk mineral. Mineral dissolution, a surface-limited process, and Fe2+ exit continue for minutes to hours. Note that substitutions at other conserved channel residues, exemplified by Glu-130 and Asp-127, have no affect on Fe2+ exit (4).
b Significantly slower (p < 0.01) than any of the ferritins with substitutions at conserved ion channel residues.
c Fe2+ exit is significantly faster in M-D122R at 60 min than for M-R72D (p < 0.05) and M-L134P.
In M-L134P ferritin, Fe2+ exit is comparable with that in the M-R72D protein, although the increased channel flexibility is deep in the channel rather than at the cytoplasmic pore (Fig. 4). Fe2+ exit in M-L134P, however, is slower than H-L134P, where Fe2+ exit is 75% complete within 5 min (11). The faster Fe2+ exit for the same amino acid substitution in the H ferritin isoforms, compared with M, reflects the weaker helix-helix interactions in H ferritin (Fig. 2).
Among the M ferritin variants studied here, the M-D122R substitution has the largest effect on NADH and FMN-triggered Fe2+ exit (Fig. 4). The Fe2+ exit progress curve is similar to frog H-L134P (11). When changes in the N-terminal extension of the subunit helix bundle are included, the overall change in channel flexibility of M-D122R approaches that of H-L134P (Fig. 2E).
Changes in External Environment Can Rescue Ferritins with Altered Ion Channels or Pore Gates
The sensitivity of Fe2+ exit in wild-type ferritin proteins to small molecules added to the buffer (e.g. physiological urea (1–10 mm) (12, 17, 18) or binding peptides (12, 17, 18)) suggested that ferritin pores and channels might be sensitive to large macromolecules in solution that mimic cytoplasmic crowding. Two globular proteins with opposite overall charge, bovine serum albumin and egg white lysozyme, are frequently used at high concentrations to create protein crowding (37, 38). Could they decrease ion channel fluctuations in ferritin (Fig. 2)?
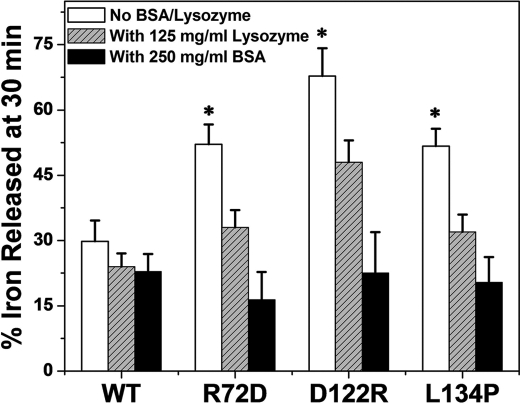
Fe2+ exit was monitored for solutions of ferritin M-R72D, M-D122R, and M-L134P and wild type after incubation of the mineralized ferritins with 3.8 mm BSA (250 mg/ml) or 8.7 mm lysozyme (125 mg/ml). The excluded volumes, calculated using the radii in solution (40), were 1.6 or 0.33 × 10−25 m3/protein molecule, respectively, which increases the solvent excluded by 37% (BSA) or 17% (lysozyme).
Wild-type M ferritin was relatively resistant to the high concentrations of BSA or lysozyme (Fig. 5). In contrast, Fe2+ exit from the variant ferritins, M-D122R, M-R72D, and M-L134P, was returned to wild-type values (Fig. 5) by protein crowding. To eliminate the possibility that NADH or FMN binding to BSA decreased available reductant, we determined the effect of doubling the reductant concentration. If NADH or FMN had become limiting in the presence of BSA, adding excess reductant should increase Fe2+ exit. However, when the concentration of NADH and FMN was doubled, Fe2+ exit from variant M-R72D was still like wild-type protein (supplemental Fig. S2). Lysozyme, used at only 125 mg/ml because of solubility, had a smaller effect on the variant proteins than BSA at 250 mg/ml. (Fig. 5). The differences could be due to difference in viscosity because the viscosity of the lysozyme solution was about one-third that of BSA solutions, or the smaller effect of lysozyme could relate to the smaller amount of solvent excluded: 17% for lysozyme compared with 37% for BSA. Finally, the smaller effect of lysozyme could be due to specific BSA or lysozyme interactions with ferritin or to a combination of specific binding effects, viscosity, and excluded solvent. When the selective effects of heptapeptides (17) or stoichiometric concentrations of urea (12) on ferritin pores are considered with the quantitatively different effects of lysozyme and BSA, the existence of specific cytoplasmic regulators or chaperones that regulate ferritin Fe2+ exit is tenable.
FIGURE 5.
High concentrations of protein crowders restore wild-type ferritin ion channel function disrupted by substitutions of conserved, channel amino acids. Fe2+ exit (Fe2+-bipyridyl complex formation), a process dependent on the surface-limited reduction and dissolution of caged ferritin minerals, was measured in the presence and absence of high concentrations of unrelated protein, BSA (3.8 mm) or lysozyme (8.7 mm), that mimic the crowded cytoplasm. The percentage free volume excluded (40) was 17% for lysozyme and 37% for BSA. Fe2+ exit was triggered with NADH and FMN and monitored as Fe2+-(bipyridyl)3 outside the cage (11, 12). The mean and S.D. (error bars) from 5–8 independent experiments with two different protein preparations are shown. *, significantly different (p < 0.0001) from wild-type ferritin.
Mutations Alter Metal Ion Binding in Ferritin Ion Channels
The highest resolution crystals in a screen with multiple conditions were grown in the presence of 2 m MgCl2 (3). Three hydrated Mg2+ ions are aligned in the crystals in a row inside wild-type ferritin ion channels (Fig. 6A). Mg2+ is also a convenient divalent cation model for air-sensitive Fe2+ ions (1). Mg2+ binds at a number of ferritin sites known to be Fe2+ sites, but the affinity is relatively low because Mg2+ is displaced by Co2+ and does not inhibit Fe2+ and O2 reactions (3, 42).
FIGURE 6.
Effects of changing conserved ferritin Fe2+ exit residues on channel ion binding. There are four Mg2+ sites per ion channel (sites A–D, from outside to inside (supplemental Table S1)). A, wild type; full occupancy at sites A–D. B, M-L134P; zero occupancy at site D; partial occupancy at sites A, C, and D; and full occupancy at site B, the channel constriction. C, M-R72D (N-terminal gate “open”); similar to wild-type ferritin, except there is no binding at site C. D, M-D122R; zero occupancy at site A, C, or D and full occupancy at site B, the channel constriction. The changes in Mg2+ ion occupancies track channel main-chain flexibility (Fig. 2) and effects on Fe2+ exit in mineralized ferritin (Fig. 3). Green spheres, hydrated Mg2+; red dots, ordered water; gray mesh, 2Fo − Fc electron density.
The Mg2+ ions in the ferritin channels span the distance from the cytoplasmic pore to the channel exits into the central cavity. This arrangement of Mg2+ ions in ferritin ion channels (3) is reminiscent of K+ ions in a voltage-dependent K+ channel crystallized at high [K+] (41). However, K+ channels differ in containing only protein loops contrasting with ferritin channel helices and loops, by using backbone carbonyls to bind metal ions, contrasting with ferritin side-chain carboxylates, and in being anhydrous, contrasting with ordered water in ferritin. One of the Mg2+ ions is bound by a cluster of three Glu-130 residues at the channel constriction (Fig. 1C). Glu-130 is required for diferric peroxo formation during normal catalysis (4). Three Mg2+ ions cluster at the ferritin ion channel exits linked through coordinated H2O to three conserved Asp-127 residues. Asp-127 is also required for normal diferric peroxo formation, but D127A can be partly rescued with increased [Fe2+] (4). The Mg2+ sites in the ion channels are fully occupied in the crystals of wild-type M ferritin (3), (supplemental Table S1).
The number of Mg2+ binding sites and the site occupancies decrease in the ferritin variants compared with wild-type ferritin (Fig. 6 and supplemental Table S1). Effects are smallest in M-R72D followed by M-L134P and M-D122R. The R72D substitution, which destabilizes the cytoplasmic N-terminal peptide gates (Figs. 2 and 3), results in replacement of Mg2+ at Thr-118 (site A, supplemental Table S1) by Mg2+ at Asp-72. There is no metal ion binding at channel site C, Asp-127, Glu-130, and Ser-131 (Fig. 6 and supplemental Fig. S1). The differences in metal binding may reflect changes in electrostatics (43) caused by the negatively charged Asp in place of positively charged Arg at position 72.
Disrupting ion channel structure by the M-L134P or M-D122R substitutions (Fig. 2) decreases metal occupancies in the channel more than in the M-R72D protein. In M-L134P, site occupancy decreased at two of three Mg2+ positions in the ion channel and eliminated the cluster of metal ion binding sites around the three Asp-127 residues in the ion channel exits. When refined with full occupancy, the two sites in L134P resulted in excess electron difference density. Maps calculated with occupancy manually set to 50% no longer showed excess electron density and gave reasonable B values; occupancy refinement is not reliable at this resolution due to correlation between B values. Mg2+ binding at the channel constriction was unchanged in M-L134P or M-D122R. Ordered water in the channels decreased in both M-L134P and M-D122R, especially at the cytoplasmic pores (Fig. 6). The largest effect on ion metal binding in the ferritin ion channels occurs in the M-D122R variant. Changes in B′ values occur at both the N terminus and the ion channel (Fig. 2E). Mg2+ only binds at one site in the channel, the channel constriction (Fig. 6D). The largest effects of the amino acid substitutions that change main-chain structure are on Fe2+ exit (Fig. 4) in contrast to ferritins with substitutions at channel residues Glu-130 and Asp-127, which alter Fe2+ entry and oxidoreduction with no effect on Fe2+ exit (4).
DISCUSSION
Ion entry and exit channels in ferritin protein cages share structural and functional features with membrane ion channels: the use of charged residues on surfaces of protein helices to move metal ions from one environment to another across a largely hydrophobic barrier. The lines of metal ions in the channels detected in protein crystals of both a K+ channel (41) and ferritin protein cages (3) (Fig. 6A) are a visual demonstration of the similarities between ion channels in soluble ferritin protein cages and ion channels in membrane proteins. In integral membrane ion channels, the cell membrane creates the barrier to ion flow. In ferritin, the protein cage itself separates cytoplasmic iron and iron in the mineral growth cavity. The complexity of the ferritin protein cage, which was long thought to be just a “shell” around the mineral, continues to emerge (1, 2, 5, 13) as does the complexity of membranes and membrane channels (44–46). Three main conclusions from the ferritin structure and function studies reported here are as follows: 1) the N-terminal segment is a critical part of the cytoplasmic gate for Fe2+ exit; 2) flexible ion channel regions are stabilized by specific interactions and by external crowding effects of proteins like BSA; and 3) decreased binding of metal ions and increased flexibility at specific channel sites increases reduction and dissolution of the caged ferritin mineral, a surface-limited process, and Fe2+ exit from the protein cage.
The N termini in eukaryotic ferritins protrude from the four-α-helix subunit bundles and interact with residues around the ion channel cytoplasmic pores (3, 47, 48) (Figs. 2 and 4). Proximity of the N termini to the cytoplasmic pores was observed in ferritin protein structures as long ago as 1978 (49), but the functional significance remained unknown until now. The correlation of N-terminal structural changes in the M-R72D variant with increased Fe2+ exit makes clear the gating function of the N-terminal segment. Conserved residues Gln-6 and Asn-7 form hydrogen bonds to Arg-72 in helix 2 (Fig. 3). Arg-72 in turn is linked through a salt bridge to Asp-122 in the loop between helices 3 and 4, around the pores. This network of interactions stabilizes the channel and pores in a closed position. Disruption of the network unfolds the pore gates (the N-terminal subunit extension) (Figs. 2 and 3), and Fe2+ exit increases (Fig. 4).
The network residues Gln-6, Asn-7, Arg-72, and Asp-122 are present in all eukaryotic ferritins. They are absent in the 12 subunit miniferritins (Dps proteins) of archaea and bacteria, where the N-terminal subunit extensions vary widely in sequence and in some cases form DNA binding sites (13). The same residues control both Fe2+ entry and Fe2+ exit in the smaller ferritins (13). Miniferritins are selectively regulated by stress and expressed under different physiological conditions than 24-subunit ferritins present in the same bacteria (2, 50). H2O2 is often the preferred oxidant in miniferritin mineral synthesis. The multipurpose activity of eukaryotic ferritins, both in cellular iron nutrition and recovery from oxidant damage, requires responses to multiple signals, which may explain the complex ion channel and pore structures.
Ion channel structure, channel metal ion binding, and Fe2+ exit are differentially changed by each of the amino acid substitutions that we characterized here. Because the channels and pores are composed of three subunits, each substitution changes three amino acids in close proximity. Asp-122 in the short loop between helices 3 and 4 plays a major role in directing Fe2+ out of the protein cage. Not only does Asp-122 minimize fluctuations of the cytoplasmic gate, indicated by decreased Fe2+ exit (Fig. 4); it increases metal ion binding in the channel (Fig. 6). M-D122R is catalytically active despite the altered metal binding in the channel. Mg2+ occupancy at the channel constriction (site B, supplemental Table S1) is unchanged by the M-D122R substitution (Fig. 6 and supplemental Table S1).
The hydrophobic properties of Leu-134, deep inside the Fe2+ channels, also influence metal ion binding. Without Leu-134, no metal ions bind at the interior channel exits around the three Asp-127 residues. In contrast, three metal ions bind at this site in wild type in crystals of the wild-type protein (Fig. 6). The M-L134P substitution also decreases the occupancies of sites A and C (supplemental Table S1). Substitution of the master residue, Arg-72, in the cytoplasmic gate stabilizing network (Fig. 2) has relatively small effects on metal ion distribution in the channel. The different contributions of Arg-72, Asp-122, and Leu-134 to ferritin ion channel function are reflected in the difference among ion channel structural fluctuations, metal ion binding, and Fe2+ exit. The ability of high concentrations of external proteins in solutions to restore normal channel Fe2+ exit in variant M ferritins emphasizes the sensitivity of ferritin ion channels to the external environment.
The increase in Fe2+ exit when metal occupancy in the channel decreases illustrates the complexity of the production and exit of Fe2+ from ferritin. If Fe2+ exit simply depended on electrostatics and ion binding in the channels, decreased ion binding in M-L134P, for example, would not increase Fe2+ exit. However, Fe2+ exit also depends on electrons flowing from the external reductant to the protein-caged mineral. Changes in channel structure that facilitate electron flow and mineral reduction will increase Fe2+ exit. Such changes could involve direct contact of reductant with the mineral or enhanced electron transfer. As a result of changed channel structure, hydrophobic or positively charged amino acids can influence Fe2+ exit even if metal ion binding in the channel decreases. Contrasting with the complex channels in eukaryotic ferritins, substitution of channel carboxylate residues in miniferritins affects both Fe2+ entry and Fe2+ exit, suggesting that in these smaller ferritins, channel electrostatics dominate control of both Fe2+ entry and exit (13).
Ferritin expression in animals, where there are no separate H2O2-consuming miniferritin genes known, is part of a metabolic feedback loop. The ferritin substrates, iron and oxidants, activate ferritin gene expression, both DNA and mRNA, to increase ferritin protein synthesis. Ferritin protein, by consuming iron and oxidant to synthesize iron mineral, shuts down the feedback loop (51). The complex network of intracage protein-protein interactions in ferritin that stabilize the cytoplasmic pore gates suggests that the gates may be targets for cytoplasmic ligands that control ferritin pore function and iron utilization. The specific features of genetic regulation and structure in eukaryotic ferritins may reflect the relatively long life spans and/or high dioxygen gradients of many animal cells.
Local, structural fluctuations in eukaryotic ferritin iron ion channels, including unfolding of the N-terminal cytoplasmic pore gates observed here in high resolution protein crystal structures, have little impact on overall ferritin cage structure. Complementary solution data on the differential stability of ferritin pores and ion channels and the cage include exquisite channel sensitivity to urea (>6,000 times more sensitive than the cage) and heat (channel Tm > 28 °C lower than the cage) (12). Even short peptides (e.g. tight binding heptapeptides selected from a large combinatorial library) change Fe2+ exit, possibly mimicking cytoplasmic gating ligands (17, 39). The biological ligands that control ferritin cytoplasmic gating and Fe2+ exit in vivo, which have eluded identification efforts to date, will have a large impact on understanding iron protein synthesis and on chelation therapies for treating hypertransfusion iron overload. Even now, however, the emerging structural and functional analogies between the cytoplasmic gates and ion channels in membrane channel proteins (19–22) and in water-soluble ferritin protein cage, provide novel opportunities for ion channel exploration.
Supplementary Material
Acknowledgments
We thank Drs. George Meigs and James Holton for assistance during the data collection on Beamline 8.3.1 at the Advanced Light Source of Lawrence Berkeley National Laboratory.
This work was supported, in whole or in part, by National Institutes of Health Grants DK20251 (to E. C. T., R. K. B., B. O., and T. T.), GM48958, and GM70962 (to H.-L. N. and T. A.). This work was also supported by the Children's Hospital Oakland Research Institute Foundation (to E. C. T.) and a Japan Society for the Promotion of Science postdoctoral fellowship for research abroad (to T. T.).
The atomic coordinates and structure factors (codes 3SE1, 3SH6, and 3SHX) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).

This article contains supplemental Table S1 and Figs. S1 and S2.
- Bicine
- N,N-bis(2-hydroxyethyl)glycine
- PDB
- Protein Data Bank.
REFERENCES
- 1. Theil E. C. (2011) Ferritin protein nanocages use ion channels, catalytic sites, and nucleation channels to manage iron/oxygen chemistry. Curr. Opin. Chem. Biol. 15, 304–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Le Brun N. E., Crow A., Murphy M. E., Mauk A. G., Moore G. R. (2010) Iron core mineralization in prokaryotic ferritins. Biochim. Biophys. Acta 1800, 732–744 [DOI] [PubMed] [Google Scholar]
- 3. Tosha T., Ng H. L., Bhattasali O., Alber T., Theil E. C. (2010) Moving metal ions through ferritin-protein nanocages from 3-fold pores to catalytic sites. J. Am. Chem. Soc. 132, 14562–14569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Haldar S., Bevers L. E., Tosha T., Theil E. C. (2011) Moving iron through ferritin protein nanocages depends on residues throughout each four α-helix bundle subunit. J. Biol. Chem. 286, 25620–25627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Turano P., Lalli D., Felli I. C., Theil E. C., Bertini I. (2010) NMR reveals pathway for ferric mineral precursors to the central cavity of ferritin. Proc. Natl. Acad. Sci. U.S.A. 107, 545–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ferreira C., Bucchini D., Martin M. E., Levi S., Arosio P., Grandchamp B., Beaumont C. (2000) Early embryonic lethality of H ferritin gene deletion in mice. J. Biol. Chem. 275, 3021–3024 [DOI] [PubMed] [Google Scholar]
- 7. Briat J. F., Ravet K., Arnaud N., Duc C., Boucherez J., Touraine B., Cellier F., Gaymard F. (2010) New insights into ferritin synthesis and function highlight a link between iron homeostasis and oxidative stress in plants. Ann. Bot. 105, 811–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hailemariam K., Iwasaki K., Huang B. W., Sakamoto K., Tsuji Y. (2010) Transcriptional regulation of ferritin and antioxidant genes by HIPK2 under genotoxic stress. J. Cell Sci. 123, 3863–3871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goss D. J., Theil E. C. (2011) Iron-responsive mRNAs. A family of Fe2+-sensitive riboregulators. Acc. Chem. Res. 44, 1320–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hentze M. W., Muckenthaler M. U., Galy B., Camaschella C. (2010) Two to tango. Regulation of Mammalian iron metabolism. Cell 142, 24–38 [DOI] [PubMed] [Google Scholar]
- 11. Takagi H., Shi D., Ha Y., Allewell N. M., Theil E. C. (1998) Localized unfolding at the junction of three ferritin subunits. A mechanism for iron release? J. Biol. Chem. 273, 18685–18688 [DOI] [PubMed] [Google Scholar]
- 12. Liu X., Jin W., Theil E. C. (2003) Opening protein pores with chaotropes enhances Fe reduction and chelation of Fe from the ferritin biomineral. Proc. Natl. Acad. Sci. U.S.A. 100, 3653–3658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chiancone E., Ceci P. (2010) The multifaceted capacity of Dps proteins to combat bacterial stress conditions. Detoxification of iron and hydrogen peroxide and DNA binding. Biochim. Biophys. Acta 1800, 798–805 [DOI] [PubMed] [Google Scholar]
- 14. Baynes R. D., Bothwell T. H. (1990) Iron deficiency. Annu. Rev. Nutr. 10, 133–148 [DOI] [PubMed] [Google Scholar]
- 15. Milman N. (2012) Postpartum anemia II. Prevention and treatment. Ann. Hematol. 91, 143–154 [DOI] [PubMed] [Google Scholar]
- 16. Jones T., Spencer R., Walsh C. (1978) Mechanism and kinetics of iron release from ferritin by dihydroflavins and dihydroflavin analogues. Biochemistry 17, 4011–4017 [DOI] [PubMed] [Google Scholar]
- 17. Liu X. S., Patterson L. D., Miller M. J., Theil E. C. (2007) Peptides selected for the protein nanocage pores change the rate of iron recovery from the ferritin mineral. J. Biol. Chem. 282, 31821–31825 [DOI] [PubMed] [Google Scholar]
- 18. Jin W., Takagi H., Pancorbo B., Theil E. C. (2001) “Opening” the ferritin pore for iron release by mutation of conserved amino acids at interhelix and loop sites. Biochemistry 40, 7525–7532 [DOI] [PubMed] [Google Scholar]
- 19. Inanobe A., Matsuura T., Nakagawa A., Kurachi Y. (2011) Inverse agonist-like action of cadmium on G-protein-gated inward-rectifier K+ channels. Biochem. Biophys. Res. Commun. 407, 366–371 [DOI] [PubMed] [Google Scholar]
- 20. Oz S., Tsemakhovich V., Christel C. J., Lee A., Dascal N. (2011) CaBP1 regulates voltage-dependent inactivation and activation of CaV1.2 (L-type) calcium channels. J. Biol. Chem. 286, 13945–13953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rosenhouse-Dantsker A., Logothetis D. E., Levitan I. (2011) Cholesterol sensitivity of KIR2.1 is controlled by a belt of residues around the cytosolic pore. Biophys. J. 100, 381–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Soveral G., Prista C., Moura T. F., Loureiro-Dias M. C. (2010) Yeast water channels. An overview of orthodox aquaporins. Biol. Cell 103, 35–54 [DOI] [PubMed] [Google Scholar]
- 23. Waldo G. S., Theil E. C. (1993) Formation of iron(III)-tyrosinate is the fastest reaction observed in ferritin. Biochemistry 32, 13262–13269 [DOI] [PubMed] [Google Scholar]
- 24. Tosha T., Hasan M. R., Theil E. C. (2008) The ferritin Fe2 site at the diiron catalytic center controls the reaction with O2 in the rapid mineralization pathway. Proc. Natl. Acad. Sci. U.S.A. 105, 18182–18187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Holton J., Alber T. (2004) Automated protein crystal structure determination using ELVES. Proc. Natl. Acad. Sci. U.S.A. 101, 1537–1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Evans P. (2006) Scaling and assessment of data quality. Acta Crystallogr. D Biol. Crystallogr. 62, 72–82 [DOI] [PubMed] [Google Scholar]
- 27. Leslie A. G. (2006) The integration of macromolecular diffraction data. Acta Crystallogr. D Biol. Crystallogr. 62, 48–57 [DOI] [PubMed] [Google Scholar]
- 28. Kissinger C. R., Gehlhaar D. K., Fogel D. B. (1999) Rapid automated molecular replacement by evolutionary search. Acta Crystallogr. D Biol. Crystallogr. 55, 484–491 [DOI] [PubMed] [Google Scholar]
- 29. Ha Y., Theil E. C., Allewell N. M. (1997) Preliminary analysis of amphibian red cell M ferritin in a novel tetragonal unit cell. Acta Crystallogr. D Biol. Crystallogr. 53, 513–523 [DOI] [PubMed] [Google Scholar]
- 30. Murshudov G. N., Vagin A. A., Dodson E. J. (1997) Refinement of macromolecular structures by the maximum likelihood method. Acta Crystallogr. D Biol. Crystallogr. 53, 240–255 [DOI] [PubMed] [Google Scholar]
- 31. Duong-Ly K. C., Nanda V., Degrado W. F., Howard K. P. (2005) The conformation of the pore region of the M2 proton channel depends on lipid bilayer environment. Protein Sci. 14, 856–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Emsley P., Cowtan K. (2004) Coot. Model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 33. Perrakis A., Harkiolaki M., Wilson K. S., Lamzin V. S. (2001) ARP/wARP and molecular replacement. Acta Crystallogr. D Biol. Crystallogr. 57, 1445–1450 [DOI] [PubMed] [Google Scholar]
- 34. Hooft R. W., Vriend G., Sander C., Abola E. E. (1996) Errors in protein structures. Nature 381, 272. [DOI] [PubMed] [Google Scholar]
- 35. Delano W. (2010) The PyMol Molecular Graphics System, version 1.3r1, Schrodinger LLC, New York [Google Scholar]
- 36. Yuan Z., Zhao J., Wang Z. X. (2003) Flexibility analysis of enzyme active sites by crystallographic temperature factors. Protein Eng. 16, 109–114 [DOI] [PubMed] [Google Scholar]
- 37. Makowski L., Rodi D. J., Mandava S., Minh D. D., Gore D. B., Fischetti R. F. (2008) Molecular crowding inhibits intramolecular breathing motions in proteins. J. Mol. Biol. 375, 529–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li C., Pielak G. J. (2009) Using NMR to distinguish viscosity effects from nonspecific protein binding under crowded conditions. J. Am. Chem. Soc. 131, 1368–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu X., Theil E. C. (2005) Ferritins. Dynamic management of biological iron and oxygen chemistry. Acc. Chem. Res. 38, 167–175 [DOI] [PubMed] [Google Scholar]
- 40. Uversky V. N. (1993) Use of fast protein size-exclusion liquid chromatography to study the unfolding of proteins which denature through the molten globule. Biochemistry 32, 13288–13298 [DOI] [PubMed] [Google Scholar]
- 41. Valiyaveetil F. I., Leonetti M., Muir T. W., Mackinnon R. (2006) Ion selectivity in a semisynthetic K+ channel locked in the conductive conformation. Science 314, 1004–1007 [DOI] [PubMed] [Google Scholar]
- 42. Ha Y., Shi D., Small G. W., Theil E. C., Allewell N. M. (1999) Crystal structure of bullfrog M ferritin at 2.8 Å resolution. Analysis of subunit interactions and the binuclear metal center. J. Biol. Inorg. Chem. 4, 243–256 [DOI] [PubMed] [Google Scholar]
- 43. Takahashi T., Kuyucak S. (2003) Functional properties of 3-fold and 4-fold channels in ferritin deduced from electrostatic calculations. Biophys. J. 84, 2256–2263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lingwood D., Simons K. (2010) Lipid rafts as a membrane-organizing principle. Science 327, 46–50 [DOI] [PubMed] [Google Scholar]
- 45. Lundmark R., Carlsson S. R. (2010) Driving membrane curvature in clathrin-dependent and clathrin-independent endocytosis. Semin. Cell Dev. Biol. 21, 363–370 [DOI] [PubMed] [Google Scholar]
- 46. Doherty G. J., McMahon H. T. (2009) Mechanisms of endocytosis. Annu. Rev. Biochem. 78, 857–902 [DOI] [PubMed] [Google Scholar]
- 47. Lawson D. M., Artymiuk P. J., Yewdall S. J., Smith J. M., Livingstone J. C., Treffry A., Luzzago A., Levi S., Arosio P., Cesareni G. (1991) Solving the structure of human H ferritin by genetically engineering intermolecular crystal contacts. Nature 349, 541–544 [DOI] [PubMed] [Google Scholar]
- 48. Masuda T., Goto F., Yoshihara T., Mikami B. (2010) Crystal structure of plant ferritin reveals a novel metal binding site that functions as a transit site for metal transfer in ferritin. J. Biol. Chem. 285, 4049–4059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Banyard S. H., Stammers D. K., Harrison P. M. (1978) Electron density map of apoferritin at 2.8 Å resolution. Nature 271, 282–284 [DOI] [PubMed] [Google Scholar]
- 50. Cornelis P., Wei Q., Andrews S. C., Vinckx T. (2011) Iron homeostasis and management of oxidative stress response in bacteria. Metallomics 3, 540–549 [DOI] [PubMed] [Google Scholar]
- 51. Theil E. C., Goss D. J. (2009) Living with iron (and oxygen). Questions and answers about iron homeostasis. Chem. Rev. 109, 4568–4579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bauminger E. R., Harrison P. M., Hechel D., Hodson N. W., Nowik I., Treffry A., Yewdall S. J. (1993) Iron(II) oxidation and early intermediates of iron core formation in recombinant human H-chain ferritin. Biochem. J. 296, 709–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Levi S., Santambrogio P., Corsi B., Cozzi A., Arosio P. (1996) Evidence that residues exposed on the 3-fold channels have active roles in the mechanism of ferritin iron incorporation. Biochem. J. 317, 467–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.