Abstract
G-protein-coupled receptors (GPCRs) are a large family of remarkably versatile membrane proteins that are attractive therapeutic targets because of their involvement in a vast range of normal physiological processes and pathological diseases. Upon activation, intracellular domains of GPCRs mediate signaling to G-proteins, but these domains have yet to be effectively exploited as drug targets. Cell-penetrating lipidated peptides called pepducins target specific intracellular loops of GPCRs and have recently emerged as effective allosteric modulators of GPCR activity. The lipid moiety facilitates translocation across the plasma membrane, where pepducins then specifically modulate signaling of their cognate receptor. To date, pepducins and related lipopeptides have been shown to specifically modulate the activity of diverse GPCRs and other membrane proteins, including protease-activated receptors (PAR1, PAR2, and PAR4), chemokine receptors (CXCR1, CXCR2, and CXCR4), sphingosine 1-phosphate receptor-3 (S1P3), the melanocortin-4 receptor, the Smoothened receptor, formyl peptide receptor-2 (FPR2), the relaxin receptor (LGR7), G-proteins (Gαq/11/o/13), muscarinic acetylcholine receptor and vanilloid (TRPV1) channels, and the GPIIb integrin. This minireview describes recent advances made using pepducin technology in targeting diverse GPCRs and the use of pepducins in identifying potential novel drug targets.
Keywords: Chemokines, Drug Design, G-protein-coupled Receptors (GPCRs), G-proteins, Peptides, Agonist, Allosteric Modulator, Antagonist, Pepducin Technology, Protease-activated Receptors
G-protein-coupled Receptor Protein Families and Their Intracellular Domains
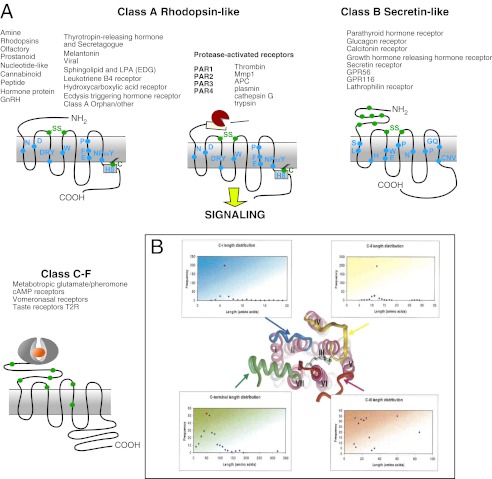
G-protein-coupled receptors (GPCRs)2 represent a large family of cell-surface receptors that are activated by an array of ligands, including odors, hormones, peptides, and large proteins (Fig. 1). Despite significant sequence and functional diversity between members of the GPCR family, all GPCRs have a conserved molecular architecture of seven transmembrane domains. The GPCR superfamily, with an estimated number of >1000 members (1, 2), is divided into six main subfamilies (Classes A–F) based on signature conserved residues and ligand interactions (Fig. 1A) (3, 4). The Class A subfamily represents the largest group, and many Class A receptors are major drug targets (5). Class A receptors are further divided into subgroups on the basis of ligand specificity, such as the protease-activated receptors (PARs). To date, four PARs have been identified: PAR1, PAR2, PAR3, and PAR4. PARs are activated when cleaved by proteases at the N-terminal extracellular domain (6). Proteolytic cleavage exposes a new N terminus that binds to the body of the receptor in an intramolecular mode that activates G-protein signaling.
FIGURE 1.
Schematic representation of Classes A–F of GPCRs. A, six main families (Class A, rhodopsin-like; Class B, secretin-like; and Classes C–F, which include the Frizzled and Smoothened receptors) and their ligands are schematically shown for each class. The conserved residues within each class of family are marked within the seven transmembrane domains. These drawings have been modified from the original source (4). GnRH, gonadotropin-releasing hormone; LPA, lysophosphatidic acid; EDG, endothelial differentiation gene; APC, activated protein C; T2R, type 2 taste receptor. B, sequence length analysis of the intracellular loops of Class A GPCRs. The horizontal axis represents the number of amino acids in each loop domain. The vertical axis shows the number of receptors out of a total of 270 (modified from Ref. 19).
Aside from a number of conserved motifs, there is modest sequence homology across family classes, especially in the extracellular and intracellular loops. Functionally important conserved residues in transmembrane regions within each receptor class have been identified (7, 8). In Class A receptors, the conserved (D/E)RY motif, located at the interface between TM3 and intracellular loop 2, is involved in formation of salt bridges that maintain the receptor in the inactive conformation. In rhodopsin, this salt bridge formation, also referred to as an “ionic lock,” occurs between Arg-135 in TM3 and Glu-247 in TM6. Another essential motif is the NPXXY sequence in TM7, which connects ligand binding with the intracellular eighth helix and G-protein activation. A specific cholesterol-binding site was observed in proximity to a conserved tryptophan residue from TM4 present in a large number of Class A members, suggesting a potential functional role in receptor binding and thermal stability (9). Another important conserved motif in many Class A receptors is an intramolecular disulfide bond between extracellular cysteines in the first and second extracellular loops. The cytoplasmic surface of rhodopsin is shown in Fig. 1B and identifies three cytoplasmic loops (C1, C2, and C3) and a C-terminal tail (C4). Within the C-terminal domain is the highly conserved eighth helix (H8) previously identified in rhodopsin (9) and other Class A receptors, including PAR1 and PAR2 (10). H8 is anchored to the membrane by palmitoylation of C-terminal cysteine residues. Swift et al. (10) identified important interactions between TM7, H8, and the first intracellular loop and showed that these interactions transfer the signal from PAR1 to G-protein in a coordinated manner and may be conserved in other Class A family members.
In recent years, elucidation of the crystal structures of rhodopsin (9), opsin both alone and in combination with the C-terminal peptide from the Gα protein transducin (11, 12), β2- and β1-adrenergic receptors (13, 14), the A2A adenosine receptor (15, 16), the dopamine D3 receptor (17), and the chemokine CXCR4 (18) has provided new insights into the common features of the overall structures, but significant differences in extracellular/transmembrane ligand binding make each receptor unique. The length of the individual intracellular loops of GPCRs has a relatively narrow variance among family members. In general, C1 is the shortest in length, with relatively conserved length between family members, whereas the highest degree of variability is found in C3 and C4 (Fig. 1B) (19, 20). Although mutagenesis studies demonstrated that the C3 loop of GPCRs largely mediates coupling between the receptor and G-protein (21), pepducins targeting all three intracellular loops and the C-terminal tail have shown in vitro and/or in vivo efficacy, suggesting that all intracellular domains may be important for intracellular signal transduction.
The four intracellular loops of GPCRs all directly interact with heterotrimeric G-proteins that are composed of α, β, and γ subunits (22). A number of Gα subunits exist and are divided into Gαi, Gα12/13, Gαq, and Gαs subtypes (23). Ligand binding to GPCRs mediates a large conformational change in TM3 and TM6, leading to disruption of the ionic lock and alterations in the cytoplasmic loops, most notably C2 and C3, which in turn promote activation of G-proteins by exchange of GDP for GTP on the Gα subunit (24). Receptor-Gα contact sites have been studied in several inactive and active receptors, suggesting that the receptor-Gα protein complex is preformed prior to receptor activation (25).
Pepducin Technology
Pepducin technology, as first described in a report by Covic et al. (26), is an entirely novel approach to modulate GPCR activity that exploits interaction of GPCRs with G-proteins at the cytosolic plasma membrane interface. Pepducins are composed of a lipid moiety, such as palmitate, myristate, or lithocholic acid, attached to a peptide that corresponds to an amino acid segment from one of the cytoplasmic loops (C1, C2, or C3) or the C-terminal tail (C4) of the target GPCR. Depending on the peptide sequence, pepducins have been shown to act as agonists, antagonists, or modulators of GPCR activity (Tables 1 and 2). As evidenced by recent studies, libraries of pepducins can be rapidly designed to a GPCR of interest and readily tested for agonist or antagonist activity by high-throughput screening (27, 28). Pepducin agonists provide a means to identify the functional activity of orphan receptors or GPCRs that previously proved refractory to examination, whereas highly specific pepducin antagonists have been used to discriminate the activity of closely related receptors (29). Furthermore, pepducins have favorable biological properties and are effective modulators of GPCR activity in vivo in many animal models (29–32), including non-human primates (28).
TABLE 1.
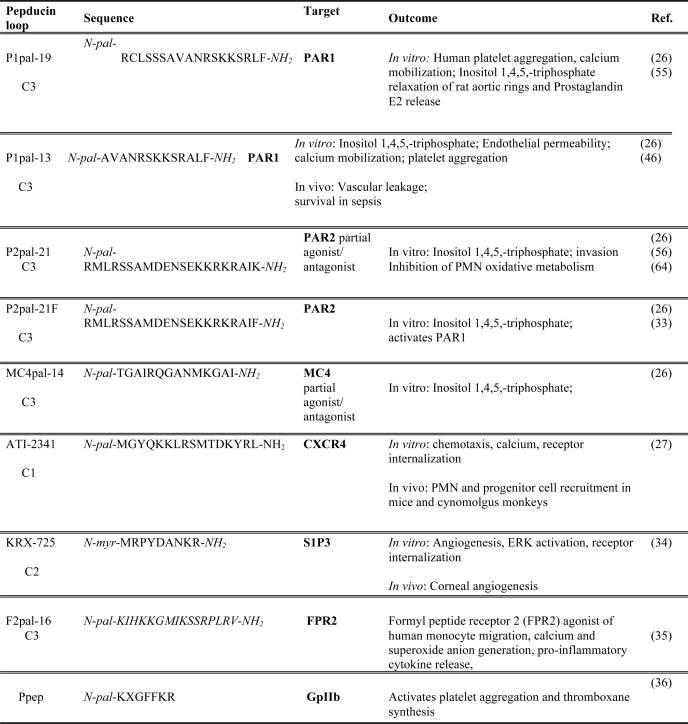
Pepducin and other agonist activity
pal, palmitoyl.
TABLE 2.
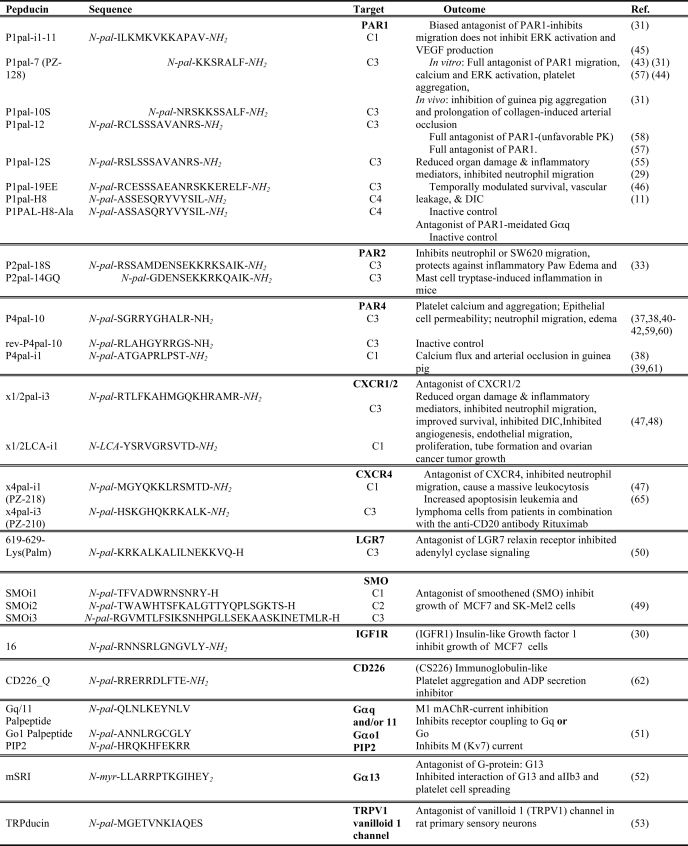
Pepducin antagonist and other target activity
pal, palmitoyl; LCA, lithocholic acid; LN, norleucine.
Biochemical and pharmacologic studies indicated that the lipid moiety of pepducins facilitates translocation across the lipid bilayer and serves to anchor the pepducin in the cytosolic face of the plasma membrane in the vicinity of the target receptor, thereby increasing the effective molarity (33). Unequivocal confirmation that palmitoylated peptides traverse across the lipid bilayer of platelets was provided by Wielders et al. (34), who used a FRET-based assay with fluorescently NBD-labeled phosphatidylserine (NBD-PS) as a donor molecule and rhodamine-labeled PAR1-targeted pepducin (Rho-P1pal-12) as an acceptor (supplemental Fig. 1, A and B). Rho-P1pal-12 quenched NBD-PS fluorescence, indicating that P1pal-12 translocates across the plasma membrane to come into close proximity with PS localized on the inner leaflet of the plasma membrane. More recently, it was shown by confocal microscopy that fluorescently labeled pepducin antagonists targeted to the Smoothened receptor concentrate on the plasma membrane and subsequently intracellular membranes (35). Together, these data demonstrate that lipidation mediates delivery of peptide across the plasma membrane and anchors pepducins in intracellular membranes.
Pharmacokinetic and pharmacodynamic studies revealed the favorable drug-like properties of pepducins. To examine the pharmacokinetics of PAR1 pepducins, Cisowski et al. (36) injected the C3 loop antagonist pepducin P1pal-7 intravenously and subcutaneously in CF-1 mice and collected blood at regular intervals for up to 16 h. Mass spectrometry analysis indicated that the plasma levels of P1pal-7 reached >10 μm shortly after intravenous injection of 3 mg/kg P1pal-7 and decayed to 0.06 μm by 16 h (supplemental Fig. 1C). After subcutaneous injection of 10 mg/kg P1pal-7, peak pepducin plasma levels reached 1.1 μm at 1 h, which persisted for 4 h, followed by elimination to 0.2 μm by 6 h. Residual P1pal-7 levels were 10 nm at 16 h after injection. Interestingly, the N-terminal C3 loop pepducin of PAR1, P1pal-10S, had a much shorter half-life compared with P1pal-7, whereas the C1 loop-targeted pepducin P1pal-i1-11 exhibited sustained plasma levels of >1 μm for up to 16 h. Therefore, proteolysis by peptidases and rate of elimination may be a function of amino acid sequence composition and may be altered by introducing non-cleavable residues. These studies demonstrate that pepducins can be administered intravenously and subcutaneously, with distinct pharmacokinetic profiles depending on route of administration and pepducin sequence.
We also previously found that parenterally delivered pepducins become widely distributed throughout animal tissues (37). Intravenous and subcutaneous injections of low-dose radioactively labeled P4pal-10 pepducin resulted in pepducin detected in liver, kidneys, lungs, and spleen, whereas a high dose resulted in an appearance in kidneys, lungs, liver, and spleen, with lower levels detected in heart, blood, muscle, and fat, but not in brain. This pattern is consistent with a biodistribution to highly vascularized tissues. Consistent with previous reports with other peptides, pepducins do not appear to readily penetrate the blood-brain barrier.
Targeting Intracellular Domains of GPCRs Using Pepducin Technology: Agonist Approach
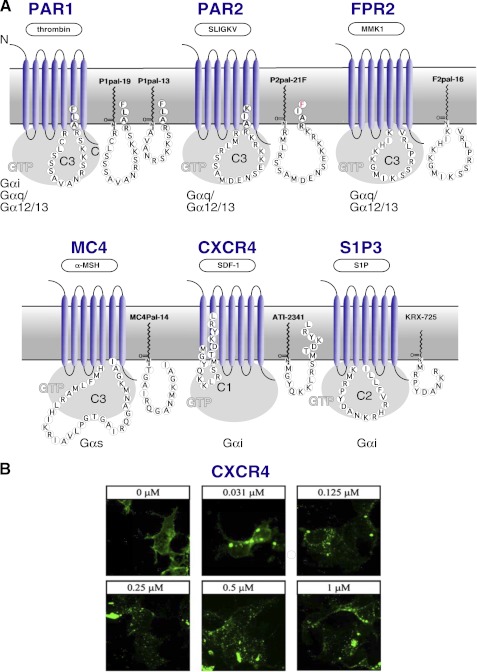
Classically, GPCRs are activated by extracellular ligands. Intracellular pepducin agonists have been generated against more than a dozen Class A receptors, including PAR1 (26), PAR2 (26, 38), melanocortin-4 (MC4) (26), CXCR4 (28), S1P3 (39), and FPR2 (formyl peptide receptor-2) (40), and against the GPIIb integrin (41) as shown in Fig. 2A and Table 1. These pepducins were derived from all three intracellular loops (C1, C2, and C3) and could activate receptors that couple to all four G-protein subfamilies, including Gαi, Gαq, Gα12/13, and Gαs.
FIGURE 2.
Pepducin agonist activity. A, schematic representation of GPCRs (PAR1, PAR2, FPR2, MC4, CXCR4, and S1P3) with agonist pepducin activity. The composition of membrane-tethered pepducins is shown next to each receptor. α-MSH, α-melanocyte-stimulating hormone; S1P, sphingosine 1-phosphate. B, ATI-2341 induces internalization of CXCR4. HEK-293 cells transiently transfected with a CXCR4-enhanced GFP fusion construct were stimulated with the indicated concentrations of ATI-2341 for 30 min at 37 °C. Enhanced GFP fluorescence was visualized directly using a Zeiss Axiovert inverted microscope (modified from Ref. 28).
The PAR1 C3 loop-targeted pepducin P1pal-19 mediated calcium mobilization and platelet aggregation and also acted as a full agonist of the homologous PAR2 receptor. An N-terminally truncated analog of 19-mer P1pal-19 pepducin, P1pal-13 (13-mer), retained almost complete PAR1 agonist activity but did not cross-activate PAR2, demonstrating that selectivity for the intended target GPCR can be obtained by modification of the parent pepducin. P2pal-21, a pepducin targeted to the full-length wild-type C3 loop of PAR2, had a partial PAR2 agonist activity of 18% by inositol triphosphate production in COS-7 cells compared with the exogenous PAR2 ligand SLIGKV. Mutation of the C-terminal positively charged lysine residue of P2pal-21 to phenylalanine, generating P2pal-21F, converted the pepducin to a full agonist (26). P2pal-21F had full PAR2 agonist activity in human colorectal carcinoma cells exogenously expressing PAR2 as determined by calcium flux, whereas P2pal-21 gave a weaker agonist signal (38). These data suggest that mutations in the peptide sequence can generate more selective and efficacious pepducin agonists.
To confirm that pepducin technology can be applied to diverse GPCRs, pepducins were synthesized for the Gαs-coupled MC4 obesity receptor. MC4pal-14 pepducin acted as a potent agonist of MC4 as measured by cAMP production (26). Pepducins were also recently employed to target CXCR4, a member of the chemokine receptor family. Under normal physiological conditions, CXCR4 and its natural ligand, SDF-1, regulate hematopoiesis and homing and retention of hematopoietic stem and progenitor cells in bone marrow. Tchernychev et al. (28) generated a CXCR4-targeted pepducin library to identify lipopeptide compounds with agonist activity. The most potent of these compounds, ATI-2341, mediated calcium mobilization, inhibited cAMP production and induced receptor internalization in a dose-dependent manner (Fig. 2B), similar to SDF-1. Furthermore, intravenous administration of ATI-2341 to mice and non-human primates elicited release of hematopoietic stem/progenitor cells from bone marrow, suggesting that these agents may be efficacious for stem cell mobilization.
Agonist pepducins were also generated for S1P3, a receptor involved in pro-angiogenic signaling. The pepducin KRX-725, derived from the C2 loop of S1P3, mimics the activity of the natural ligand sphingosine 1-phosphate. KRX-725 induced internalization of S1P3 and activated ERK1/2 signaling. Furthermore, KRX-725 alone mediated neovascularization in ex vivo and in vivo angiogenesis assays and also acted in synergy with pro-angiogenic factors (39). Agonist pepducins to FPR2 activated chemotaxis and calcium release of human monocytes (40). Together, these data demonstrate that GPCR-specific pepducin agonists can be rapidly engineered and validated and may be therapeutically relevant for a multitude of applications in which activation of GPCR activity is indicated, including stem cell mobilization and induction of angiogenesis in pathological conditions, such as peripheral arterial disease and cardiac ischemia. It remains to be determined whether long-term use of agonist pepducins may potentially desensitize their cognate receptor and act as functional inhibitors.
Pepducin Antagonist Activity
After the initial development of agonist pepducins, antagonist pepducins were subsequently described for the thrombin receptors PAR1 and PAR4 and most recently for the tryptase/trypsin PAR2 receptor (Table 2). Early work on the PAR1 C3 loop pepducin antagonist P1pal-12 demonstrated high specificity of this pepducin for PAR1 (30). Treatment of human platelets with P1pal-12 decreased aggregation by up to 95% in response to the exogenous PAR1 peptide ligand SFLLRN but had no effect on aggregation in response to agonists for the PAR4, thromboxane, ADP, collagen, and GPIb/IX/V receptors (30). Similarly, a PAR4 C3 loop-based antagonist pepducin, P4pal-10, completely blocked AYPGKF (PAR4 agonist)-induced aggregation and partially blocked PAR1 activation at higher concentrations but did not affect the ADP, thromboxane, and GPIb/IX/V receptors (30). Furthermore, P4pal-10 completely abrogated human neutrophil migration to thrombin while having no effect on migration to IP-10, SDF-1, and sphingosine 1-phosphate, and this pepducin, but not the reverse-sequence pepducin rev-P4pal-10, inhibited human platelet aggregation to PAR4 agonists (42). In vivo, P4pal-10 delayed thrombin generation and platelet accumulation at the site of injury in carotid artery (34) and protected against systemic thrombus formation in response to AYPGKF plus epinephrine (30). P4pal-10 was also used to elucidate the functional roles of PAR4 in joint inflammation, irritable bowel syndrome, and ulcerative colitis (43–45). P4pal-10 ameliorated the clinical and physiological signs of acute joint inflammation and pain in a mouse model of rheumatoid arthritis, indicating that PAR4 activation leads to proinflammatory changes (43). Dabek et al. (44) employed P4pal-10 to demonstrate that increased colonic epithelial permeability induced in response to ulcerative colitis fecal supernatants can be blocked by PAR4 inhibition.
To distinguish between the activities of the thrombin receptors PAR1 and PAR4, which can form a heterodimer on human platelets, Leger et al. (29) generated a specific PAR4 C1 loop pepducin, P4pal-i1, which has no antagonist activity for PAR1. This study demonstrated that PAR1 and PAR4 can independently mediate platelet aggregation and that combined antagonism of these receptors is more effective in preventing acute arterial thrombosis in guinea pig models.
PAR1 plays a well documented role in the invasive and metastatic processes of breast, ovarian, and lung cancers. To examine the integral role of PAR1 in breast cancer progression, Boire et al. (46) inoculated PAR1-expressing human breast cancer cells into mammary fat pads of nude mice and treated the animals with the PAR1 C3 loop antagonist pepducin P1pal-7. P1pal-7 decreased tumor volume by >60% compared with vehicle-treated mice. In a subsequent study, P1pal-7 in combination with Taxotere chemotherapy decreased the size of breast tumor xenografts by 95% and significantly attenuated metastasis of breast cancer cells to the lungs of mice (47). Furthermore, P1pal-7 inhibited migration of primary human lung carcinoma cells in vitro and reduced tumor growth by 75% in mouse lung cancer xenograft models (36). This effect on tumor growth was comparable with that of Avastin, the clinically used VEGF inhibitor, suggesting that targeting PAR1 may be as effective as targeting VEGF in certain cancers. P1pal-7 has also been successfully used to study the involvement of PAR1 in angiogenesis in mouse models of ovarian cancer, in which P1pal-7 treatment almost completely abrogated angiogenesis of peritoneal ovarian cancer (48).
PAR2 has proven largely recalcitrant to inhibition by small-molecule approaches, and specific and effective pharmacologic blockade has not been demonstrated until recently. Sevigny et al. (38) used molecular modeling of a PAR2 homodimer and mutational analysis of the third intracellular loop to identify key residues that control constitutive activity. This structurally guided approach led to the generation of the P2pal-18S pepducin, which completely suppressed PAR2-mediated signaling in vitro and effectively blocked PAR2-dependent inflammatory responses in mouse models.
Using the PAR1 agonist pepducin P1pal-13 and the antagonist pepducin P1pal-12S, Kaneider et al. (32) made the discovery that PAR1 switches from having a vascular-disruptive to vascular-protective function in sepsis in animals. This was evidenced by the observation that treatment of septic mice with P1pal-12S at early time points, but not at late time points, improved survival and prevented disseminated intravascular coagulation (DIC), a common complication in sepsis. However, treatment with the agonist pepducin P1pal-13 at late time points also improved survival and prevented DIC by inhibiting endothelial barrier permeability by activating the PAR1-PAR2 heterodimer (32). These protective effects were completely lost in PAR1- and PAR2-deficient mice.
Treatment of septic mice with CXCR1/2 pepducins (Table 2) designed against the C1 and C3 loops of CXCR1 and CXCR2 (x1/2pal-i3 and x1/2LCA-i1, respectively) blocked IL-8-dependent neutrophil activity, improved survival, and reversed organ failure and DIC (31). These CXCR1/2 pepducins also blocked IL-8-driven angiogenesis in ovarian cancer (31, 49). Similarly, CXCR4 C1 and C3 loop antagonist pepducins x4pal-i1 (PZ-218) and x4pal-i3 (PZ-210) blocked SDF-1-dependent neutrophil chemotaxis and caused leukocytosis, consistent with the role of CXCR4 in neutrophil homeostasis (28, 31). CXCR4 pepducins prolonged survival in combination with the anti-CD20 antibody rituximab in a mouse model of disseminated lymphoma (50).
Remsberg et al. (51) generated a series of lipidated peptides corresponding to N- and C-terminal truncations of the three intracellular loops of Smoothened and identified potent inhibitors of breast (MCF-7) and melanoma (SK-MEL-2) cell growth derived from the second intracellular loop. Synthesis of metabolically stable retro-inverso derivatives using all d-amino acids improved potency by >10-fold over the parent compound. Shpakov et al. (52) synthesized the C-terminal portion of the third cytoplasmic loop of the type 1 relaxin receptor (LGR7) linked to palmitate, which resulted in activation of adenylyl cyclase activity and effectively inhibited relaxin-mediated adenylyl cyclase activity.
Non-GPCR Lipidated Peptides
Although this minireview has focused on the development of pepducin modulators of GPCRs, lipidated peptide agonists and antagonists of other classes of cell-surface receptors have also been developed. Edwards et al. (27) employed a bioinformatic/high-throughput screening technique to identify lipidated peptide modulators of platelet function targeted to putative signaling-rich juxtamembrane regions of receptors. Palmitoylated peptides derived from the insulin-like growth factor-1 receptor juxtamembrane region were highly specific inhibitors of insulin-like growth factor-1-mediated Akt activation and breast cancer cell growth (35). Robbins et al. (53) targeted G-proteins rather than GPCRs and synthesized palmitoylated peptides corresponding to the C terminus of Gq/11 to inhibit the muscarinic acetylcholine receptor. They demonstrated selectivity between Gq/11- and Go-targeted palmitoylated peptides. Gong et al. (54) used a myristoylated C-terminal peptide fragment of G13 (55) to inhibit signaling from the platelet receptor GPIIb (αIIbβ3) to associated G13. Valente et al. (56) demonstrated that cell-penetrating lipidated peptides are selective antagonists of the TRPV1 channel by targeting the intracellular C-terminal transient receptor domain that is predicted to interact with the highly conserved receptor internal gate. These studies show that lipidated peptide antagonists represent a promising approach to target a diverse range of cell-surface receptors, including adhesion and tyrosine kinase receptors and ion channels, and can be designed using bioinformatic approaches or rational design.
Mechanism of Action
Although several studies have demonstrated that translocation across the lipid bilayer and pepducin activity are dependent on the anchoring lipid moiety, the mechanism of action by which pepducins modulate GPCR signaling is not completely understood. Soluble peptides corresponding to the C-terminal part of the third cytoplasmic loop of the β2-adrenergic receptor have been shown to activate Gs protein under cell-free conditions (57), suggesting the possibility that the peptide sequence of pepducins may activate G-proteins in a similar manner. However, this does not account for the specificity of pepducins for their respective cognate receptor or for antagonist activity. In this regard, it was shown that PAR1 and CXCR4 agonist pepducins require expression of a functional receptor to mediate activity, suggesting that pepducins targeting GPCRs do not have the ability to directly modify G-proteins (26, 28). In vivo experiments employing PAR1 (32) and PAR2 (38) knock-out mice confirmed that pepducin activity is dependent on receptor expression. As specificity is a function of the sequences and structures of the target versus off-target receptors, certain intracellular loop sequences may have off-target effects, especially among close family members. For instance, there is nearly 100% identity between the intracellular loops of CXCR1 and CXCR2, and these pepducins completely inhibit both receptors (31).
It is well recognized that GPCRs assume heterogeneous conformations from resting state to full activation and that the ionic lock between TM3 and TM6 is destabilized to selectively activate G-protein. The crystal structure of opsin (11, 12), the first example of an activated GPCR, indicates that the cytoplasmic loops move upon receptor activation. Therefore, it is highly possible that pepducins may stabilize the GPCR-G-protein complex in specific on- or off-conformation, possibly by mimicking a receptor dimer (38). The mechanism of pepducin-mediated GPCR activation is of great interest because activation of diverse GPCRs can be triggered by pepducins corresponding to any of the intracellular loops of target GPCRs, including the shortest C1 loop. Biochemical and pharmacologic approaches suggest that the site of interaction between C3 pepducin agonists for PAR1 and C3 pepducin antagonists for PAR2 appears to be the H8 (helix 8) region located in the cytoplasmic tails of PAR1 and PAR2 (26, 38). Direct binding between an allosteric agonist pepducin and its target receptor CXCR4 has also been recently demonstrated by cross-linking studies (58).
Many GPCRs likely exist as homo- or heterodimers and possibly as larger oligomeric complexes, which may have differing specificity and affinity for ligand and G-protein binding, adding to the complexity of physiological signaling. One possible mechanism of action of pepducins may involve modulation of GPCR signaling by mimicking the associations between intracellular loops of GPCRs that normally participate in homo- or heterodimer interactions. For example, Leger et al. (29, 59) demonstrated that PAR1 and PAR4 form stable heterodimers in human platelets and act in synergy to mediate thrombosis. Interestingly, the C3-derived pepducin of PAR4 could cross-inhibit PAR1 only when the receptors were coexpressed, whereas the C1-derived pepducin of PAR1 could not (29, 30). This suggests that targeting distinct loops of the GPCRs can inhibit either one receptor or both within the heterodimer.
New studies have identified pepducins that exhibit biased agonism or antagonism of discrete signaling pathways within a cell, which holds great promise for drug development. Cisowski et al. (36) delineated divergent signaling pathways dependent on the C1 versus C3 loops of PAR1 in lung adenocarcinoma cell lines. Pepducins targeting the PAR1 C3 loop completely inhibited cell motility, calcium mobilization, and ERK1/2 activation. In contrast, the PAR1 C1 loop pepducin inhibited cell motility but had no effect on ERK1/2 activation and only partially inhibited calcium mobilization.
Conclusion and Perspectives: Pepducins as Therapeutics
This minireview outlines the development of the new class of cell-penetrating pepducin agonists and antagonists of GPCRs and other cell-surface proteins. Studies indicate that these lipopeptides can be rapidly designed and engineered to specifically target their cognate GPCRs, which have otherwise proved refractory to small-molecule approaches. Further knowledge of the three-dimensional structure of GPCRs and interactions with pepducins and G-proteins will undoubtedly facilitate the development of even more potent pepducin allosteric modulators. Modification of the lipid moiety, amino acid side chains, and polarity of the peptide sequence may not only increase potency but could also affect the biodistribution, tissue targeting, and biological half-life of pepducins. In conclusion, pepducins represent a promising new class of compounds for the study of GPCRs and their pharmacology and may be developed as potential therapeutics in a variety of diseases.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grant CA104406 (to L. C.) and Grants CA122992, HL64701, and HL57905 (to A. K.). This work was also supported by Susan Komen Grants BCTR0706763 (to L. C.) and BCTR0601348 (to A. K.) and United States Department of Defense Grant BC045321 (to L. C.).

This article contains supplemental Fig. 1.
- GPCR
- G-protein-coupled receptor
- PAR
- protease-activated receptor
- TM
- transmembrane domain
- H8
- eighth helix
- NBD
- 12-(N-methyl-N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl))
- PS
- phosphatidylserine
- MC4
- melanocortin-4
- DIC
- disseminated intravascular coagulation.
REFERENCES
- 1. Takeda S., Kadowaki S., Haga T., Takaesu H., Mitaku S. (2002) Identification of G-protein-coupled receptor genes from the human genome sequence. FEBS Lett. 520, 97–101 [DOI] [PubMed] [Google Scholar]
- 2. Fredriksson R., Lagerström M. C., Lundin L. G., Schiöth H. B. (2003) The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol. Pharmacol. 63, 1256–1272 [DOI] [PubMed] [Google Scholar]
- 3. Kolakowski L. F., Jr. (1994) GCRDb: a G-protein-coupled receptor database. Receptors Channels 2, 1–7 [PubMed] [Google Scholar]
- 4. Bockaert J., Pin J. P. (1999) Molecular tinkering of G-protein-coupled receptors: an evolutionary success. EMBO J. 18, 1723–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lagerström M. C., Schiöth H. B. (2008) Structural diversity of G-protein-coupled receptors and significance for drug discovery. Nat. Rev. Drug. Discov. 7, 339–357 [DOI] [PubMed] [Google Scholar]
- 6. Vu T. K., Hung D. T., Wheaton V. I., Coughlin S. R. (1991) Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell 64, 1057–1068 [DOI] [PubMed] [Google Scholar]
- 7. Probst W. C., Snyder L. A., Schuster D. I., Brosius J., Sealfon S. C. (1992) Sequence alignment of the G-protein-coupled receptor superfamily. DNA Cell Biol. 11, 1–20 [DOI] [PubMed] [Google Scholar]
- 8. Wess J. (1998) Molecular basis of receptor/G-protein coupling selectivity. Pharmacol. Ther. 80, 231–264 [DOI] [PubMed] [Google Scholar]
- 9. Palczewski K., Kumasaka T., Hori T., Behnke C. A., Motoshima H., Fox B. A., Le Trong I., Teller D. C., Okada T., Stenkamp R. E., Yamamoto M., Miyano M. (2000) Crystal structure of rhodopsin: a G-protein-coupled receptor. Science 289, 739–745 [DOI] [PubMed] [Google Scholar]
- 10. Swift S., Leger A. J., Talavera J., Zhang L., Bohm A., Kuliopulos A. (2006) Role of the PAR1 receptor 8th helix in signaling: the 7-8-1 receptor activation mechanism. J. Biol. Chem. 281, 4109–4116 [DOI] [PubMed] [Google Scholar]
- 11. Park J. H., Scheerer P., Hofmann K. P., Choe H. W., Ernst O. P. (2008) Crystal structure of the ligand-free G-protein-coupled receptor opsin. Nature 454, 183–187 [DOI] [PubMed] [Google Scholar]
- 12. Scheerer P., Park J. H., Hildebrand P. W., Kim Y. J., Krauss N., Choe H. W., Hofmann K. P., Ernst O. P. (2008) Crystal structure of opsin in its G-protein-interacting conformation. Nature 455, 497–502 [DOI] [PubMed] [Google Scholar]
- 13. Cherezov V., Rosenbaum D. M., Hanson M. A., Rasmussen S. G., Thian F. S., Kobilka T. S., Choi H. J., Kuhn P., Weis W. I., Kobilka B. K., Stevens R. C. (2007) High-resolution crystal structure of an engineered human β2-adrenergic G-protein-coupled receptor. Science 318, 1258–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Warne T., Serrano-Vega M. J., Baker J. G., Moukhametzianov R., Edwards P. C., Henderson R., Leslie A. G., Tate C. G., Schertler G. F. (2008) Structure of a β1-adrenergic G-protein-coupled receptor. Nature 454, 486–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jaakola V. P., Griffith M. T., Hanson M. A., Cherezov V., Chien E. Y., Lane J. R., Ijzerman A. P., Stevens R. C. (2008) The 2.6 angstrom crystal structure of a human A2A adenosine receptor bound to an antagonist. Science 322, 1211–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hanson M. A., Cherezov V., Griffith M. T., Roth C. B., Jaakola V. P., Chien E. Y., Velasquez J., Kuhn P., Stevens R. C. (2008) A specific cholesterol-binding site is established by the 2.8 Å structure of the human β2-adrenergic receptor. Structure 16, 897–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chien E. Y., Liu W., Zhao Q., Katritch V., Han G. W., Hanson M. A., Shi L., Newman A. H., Javitch J. A., Cherezov V., Stevens R. C. (2010) Structure of the human dopamine D3 receptor in complex with a D2/D3 selective antagonist. Science 330, 1091–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu B., Chien E. Y., Mol C. D., Fenalti G., Liu W., Katritch V., Abagyan R., Brooun A., Wells P., Bi F. C., Hamel D. J., Kuhn P., Handel T. M., Cherezov V., Stevens R. C. (2010) Structures of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide antagonists. Science 330, 1066–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mirzadegan T., Benkö G., Filipek S., Palczewski K. (2003) Sequence analyses of G-protein-coupled receptors: similarities to rhodopsin. Biochemistry 42, 2759–2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Otaki J. M., Firestein S. (2001) Length analyses of mammalian G-protein-coupled receptors. J. Theor. Biol. 211, 77–100 [DOI] [PubMed] [Google Scholar]
- 21. Kjelsberg M. A., Cotecchia S., Ostrowski J., Caron M. G., Lefkowitz R. J. (1992) Constitutive activation of the α1B-adrenergic receptor by all amino acid substitutions at a single site. Evidence for a region that constrains receptor activation. J. Biol. Chem. 267, 1430–1433 [PubMed] [Google Scholar]
- 22. Lambright D. G., Sondek J., Bohm A., Skiba N. P., Hamm H. E., Sigler P. B. (1996) The 2.0 Å crystal structure of a heterotrimeric G-protein. Nature 379, 311–319 [DOI] [PubMed] [Google Scholar]
- 23. Offermanns S. (2003) G-proteins as transducers in transmembrane signaling. Prog. Biophys. Mol. Biol. 83, 101–130 [DOI] [PubMed] [Google Scholar]
- 24. Gether U., Kobilka B. K. (1998) G-protein-coupled receptors. II. Mechanism of agonist activation. J. Biol. Chem. 273, 17979–17982 [DOI] [PubMed] [Google Scholar]
- 25. Hu J., Wang Y., Zhang X., Lloyd J. R., Li J. H., Karpiak J., Costanzi S., Wess J. (2010) Structural basis of G-protein-coupled receptor/G-protein interactions. Nat. Chem. Biol. 6, 541–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Covic L., Gresser A. L., Talavera J., Swift S., Kuliopulos A. (2002) Activation and inhibition of G-protein-coupled receptors by cell-penetrating membrane-tethered peptides. Proc. Natl. Acad. Sci. U.S.A. 99, 643–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Edwards R. J., Moran N., Devocelle M., Kiernan A., Meade G., Signac W., Foy M., Park S. D., Dunne E., Kenny D., Shields D. C. (2007) Bioinformatic discovery of novel bioactive peptides. Nat. Chem. Biol. 3, 108–112 [DOI] [PubMed] [Google Scholar]
- 28. Tchernychev B., Ren Y., Sachdev P., Janz J. M., Haggis L., O'Shea A., McBride E., Looby R., Deng Q., McMurry T., Kazmi M. A., Sakmar T. P., Hunt S., 3rd, Carlson K. E. (2010) Discovery of a CXCR4 agonist pepducin that mobilizes bone marrow hematopoietic cells. Proc. Natl. Acad. Sci. U.S.A. 107, 22255–22259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Leger A. J., Jacques S. L., Badar J., Kaneider N. C., Derian C. K., Andrade-Gordon P., Covic L., Kuliopulos A. (2006) Blocking the protease-activated receptor 1-4 heterodimer in platelet-mediated thrombosis. Circulation 113, 1244–1254 [DOI] [PubMed] [Google Scholar]
- 30. Covic L., Misra M., Badar J., Singh C., Kuliopulos A. (2002) Pepducin-based intervention of thrombin-receptor signaling and systemic platelet activation. Nat. Med. 8, 1161–1165 [DOI] [PubMed] [Google Scholar]
- 31. Kaneider N. C., Agarwal A., Leger A. J., Kuliopulos A. (2005) Reversing systemic inflammatory response syndrome with chemokine receptor pepducins. Nat. Med. 11, 661–665 [DOI] [PubMed] [Google Scholar]
- 32. Kaneider N. C., Leger A. J., Agarwal A., Nguyen N., Perides G., Derian C., Covic L., Kuliopulos A. (2007) “Role reversal” for the receptor PAR1 in sepsis-induced vascular damage. Nat. Immunol. 8, 1303–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Covic L., Tchernychev B., Jacques S., Kuliopulos A. (2007) in Handbook of Cell-penetrating Peptides (Langel U., ed) 2nd Ed., pp. 245–257, Taylor & Francis, New York [Google Scholar]
- 34. Wielders S. J., Bennaghmouch A., Reutelingsperger C. P., Bevers E. M., Lindhout T. (2007) Anticoagulant and antithrombotic properties of intracellular protease-activated receptor antagonists. J. Thromb. Haemost. 5, 571–576 [DOI] [PubMed] [Google Scholar]
- 35. Johannessen L., Remsberg J., Gaponenko V., Adams K. M., Barchi J. J., Jr., Tarasov S. G., Jiang S., Tarasova N. I. (2011) Peptide structure stabilization by membrane anchoring and its general applicability to the development of potent cell-permeable inhibitors. ChemBioChem 12, 914–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cisowski J., O'Callaghan K., Yang J., Kuliopulos A., Nguyen N., Deng Q., Yang E., Fogel M., Tressel S., Foley C., Agarwal A., Hunt S. W., 3rd, McMurry T., Brinckerhoff L., Covic L. (2011) Targeting protease-activated receptor-1 with cell-penetrating pepducins in lung cancer. Am J. Pathol. 179, 513–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tressel S. L., Koukos G., Tchernychev B., Jacques S. L., Covic L., Kuliopulos A. (2011) Pharmacology, biodistribution, and efficacy of GPCR-based pepducins in disease models. Methods Mol. Biol. 683, 259–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sevigny L. M., Zhang P., Bohm A., Lazarides K., Perides G., Covic L., Kuliopulos A. (2011) Interdicting protease-activated receptor-2-driven inflammation with cell-penetrating pepducins. Proc. Natl. Acad. Sci. U.S.A. 108, 8491–8496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Licht T., Tsirulnikov L., Reuveni H., Yarnitzky T., Ben-Sasson S. A. (2003) Induction of pro-angiogenic signaling by a synthetic peptide derived from the second intracellular loop of S1P3 (EDG3). Blood 102, 2099–2107 [DOI] [PubMed] [Google Scholar]
- 40. Lee H. Y., Kim S. D., Shim J. W., Kim H. J., Kwon J. Y., Kim J. M., Baek S. H., Park J. S., Bae Y. S. (2010) Activation of human monocytes by a formyl peptide receptor-2-derived pepducin. FEBS Lett. 584, 4102–4108 [DOI] [PubMed] [Google Scholar]
- 41. Stephens G., O'Luanaigh N., Reilly D., Harriott P., Walker B., Fitzgerald D., Moran N. (1998) A sequence within the cytoplasmic tail of GPIIb independently activates platelet aggregation and thromboxane synthesis. J. Biol. Chem. 273, 20317–20322 [DOI] [PubMed] [Google Scholar]
- 42. Hollenberg M. D., Saifeddine M., Sandhu S., Houle S., Vergnolle N. (2004) Proteinase-activated receptor-4: evaluation of tethered ligand-derived peptides as probes for receptor function and as inflammatory agonists in vivo. Br. J. Pharmacol. 143, 443–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McDougall J. J., Zhang C., Cellars L., Joubert E., Dixon C. M., Vergnolle N. (2009) Triggering of proteinase-activated receptor-4 leads to joint pain and inflammation in mice. Arthritis Rheum. 60, 728–737 [DOI] [PubMed] [Google Scholar]
- 44. Dabek M., Ferrier L., Roka R., Gecse K., Annahazi A., Moreau J., Escourrou J., Cartier C., Chaumaz G., Leveque M., Ait-Belgnaoui A., Wittmann T., Theodorou V., Bueno L. (2009) Luminal cathepsin g and protease-activated receptor 4: a duet involved in alterations of the colonic epithelial barrier in ulcerative colitis. Am J. Pathol. 175, 207–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Annaházi A., Gecse K., Dabek M., Ait-Belgnaoui A., Rosztóczy A., Róka R., Molnár T., Theodorou V., Wittmann T., Bueno L., Eutamene H. (2009) Fecal proteases from diarrheic IBS and ulcerative colitis patients exert opposite effect on visceral sensitivity in mice. Pain 144, 209–217 [DOI] [PubMed] [Google Scholar]
- 46. Boire A., Covic L., Agarwal A., Jacques S., Sharifi S., Kuliopulos A. (2005) PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell 120, 303–313 [DOI] [PubMed] [Google Scholar]
- 47. Yang E., Boire A., Agarwal A., Nguyen N., O'Callaghan K., Tu P., Kuliopulos A., Covic L. (2009) Blockade of PAR1 signaling with cell-penetrating pepducins inhibits Akt survival pathways in breast cancer cells and suppresses tumor survival and metastasis. Cancer Res. 69, 6223–6231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Agarwal A., Covic L., Sevigny L. M., Kaneider N. C., Lazarides K., Azabdaftari G., Sharifi S., Kuliopulos A. (2008) Targeting a metalloprotease-PAR1 signaling system with cell-penetrating pepducins inhibits angiogenesis, ascites, and progression of ovarian cancer. Mol. Cancer Ther. 7, 2746–2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Agarwal A., Tressel S. L., Kaimal R., Balla M., Lam F. H., Covic L., Kuliopulos A. (2010) Identification of a metalloprotease-chemokine signaling system in the ovarian cancer microenvironment: implications for anti-angiogenic therapy. Cancer Res. 70, 5880–5890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. O'Callaghan K., Lee L., Nguyen N., Hsieh M. Y., Kaneider N. C., Klein A. K., Sprague K., Van Etten R. A., Kuliopulos A., Covic L. (2012) Targeting CXCR4 with cell-penetrating pepducins in lymphoma and lymphocytic leukemia. Blood 119, 1717–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Remsberg J. R., Lou H., Tarasov S. G., Dean M., Tarasova N. I. (2007) Structural analogs of Smoothened intracellular loops as potent inhibitors of Hedgehog pathway and cancer cell growth. J. Med. Chem. 50, 4534–4538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shpakov A. O., Gur'yanov I. A., Kuznetsova L. A., Plesneva S. A., Shpakova E. A., Vlasov G. P., Pertseva M. N. (2007) Studies of the molecular mechanisms of action of relaxin on the adenylyl cyclase signaling system using synthetic peptides derived from the LGR7 relaxin receptor. Neurosci. Behav. Physiol. 37, 705–714 [DOI] [PubMed] [Google Scholar]
- 53. Robbins J., Marsh S. J., Brown D. A. (2006) Probing the regulation of M (Kv7) potassium channels in intact neurons with membrane-targeted peptides. J. Neurosci. 26, 7950–7961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gong H., Shen B., Flevaris P., Chow C., Lam S. C., Voyno-Yasenetskaya T. A., Kozasa T., Du X. (2010) G-protein subunit Gα13 binds to integrin αIIbβ3 and mediates integrin “outside-in” signaling. Science 327, 340–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Huang J. S., Dong L., Kozasa T., Le Breton G. C. (2007) Signaling through Gα13 switch region I is essential for protease-activated receptor-1-mediated human platelet shape change, aggregation, and secretion. J. Biol. Chem. 282, 10210–10222 [DOI] [PubMed] [Google Scholar]
- 56. Valente P., Fernández-Carvajal A., Camprubí-Robles M., Gomis A., Quirce S., Viana F., Fernández-Ballester G., González-Ros J. M., Belmonte C., Planells-Cases R., Ferrer-Montiel A. (2011) Membrane-tethered peptides patterned after the TRP domain (TRPducins) selectively inhibit TRPV1 channel activity. FASEB J. 25, 1628–1640 [DOI] [PubMed] [Google Scholar]
- 57. Okamoto T., Murayama Y., Hayashi Y., Inagaki M., Ogata E., Nishimoto I. (1991) Identification of a Gs activator region of the β2-adrenergic receptor that is autoregulated via protein kinase A-dependent phosphorylation. Cell 67, 723–730 [DOI] [PubMed] [Google Scholar]
- 58. Janz J. M., Ren Y., Looby R., Kazmi M. A., Sachdev P., Grunbeck A., Haggis L., Chinnapen D., Lin A. Y., Seibert C., McMurry T., Carlson K. E., Muir T. W., Hunt S., 3rd, Sakmar T. P. (2011) Direct interaction between an allosteric agonist pepducin and the chemokine receptor CXCR4. J. Am. Chem. Soc. 133, 15878–15881 [DOI] [PubMed] [Google Scholar]
- 59. Leger A. J., Covic L., Kuliopulos A. (2006) Protease-activated receptors in cardiovascular diseases. Circulation 114, 1070–1077 [DOI] [PubMed] [Google Scholar]
- 60. Kubo S., Ishiki T., Doe I., Sekiguchi F., Nishikawa H., Kawai K., Matsui H., Kawabata A. (2006) Distinct activity of peptide mimetic intracellular ligands (pepducins) for proteinase-activated receptor-1 in multiple cells/tissues. Ann. N.Y. Acad. Sci. 1091, 445–459 [DOI] [PubMed] [Google Scholar]
- 61. Kaufmann R., Oettel C., Horn A., Halbhuber K. J., Eitner A., Krieg R., Katenkamp K., Henklein P., Westermann M., Böhmer F. D., Ramachandran R., Saifeddine M., Hollenberg M. D., Settmacher U. (2009) Met receptor tyrosine kinase transactivation is involved in proteinase-activated receptor-2-mediated hepatocellular carcinoma cell invasion. Carcinogenesis 30, 1487–1496 [DOI] [PubMed] [Google Scholar]
- 62. Sroussi H. Y., Lu Y., Villines D., Sun Y. (2012) The down-regulation of neutrophil oxidative metabolism by S100A8 and S100A9: implication of the protease-activated receptor-2. Mol. Immunol. 50, 42–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Trivedi V., Boire A., Tchernychev B., Kaneider N. C., Leger A. J., O'Callaghan K., Covic L., Kuliopulos A. (2009) Platelet matrix metalloprotease-1 mediates thrombogenesis by activating PAR1 at a cryptic ligand site. Cell 137, 332–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Keuren J. F., Wielders S. J., Ulrichts H., Hackeng T., Heemskerk J. W., Deckmyn H., Bevers E. M., Lindhout T. (2005) Synergistic effect of thrombin on collagen-induced platelet procoagulant activity is mediated through protease-activated receptor-1. Arterioscler. Thromb. Vasc. Biol. 25, 1499–1505 [DOI] [PubMed] [Google Scholar]
- 65. Houle S., Papez M. D., Ferazzini M., Hollenberg M. D., Vergnolle N. (2005) Neutrophils and the kallikrein-kinin system in proteinase-activated receptor-4-mediated inflammation in rodents. Br. J. Pharmacol. 146, 670–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Slofstra S. H., Bijlsma M. F., Groot A. P., Reitsma P. H., Lindhout T., ten Cate H., Spek C. A. (2007) Protease-activated receptor-4 inhibition protects from multiorgan failure in a murine model of systemic inflammation. Blood 110, 3176–3182 [DOI] [PubMed] [Google Scholar]
- 67. Grenegård M., Vretenbrant-Oberg K., Nylander M., Désilets S., Lindström E. G., Larsson A., Ramström I., Ramström S., Lindahl T. L. (2008) The ATP-gated P2X1 receptor plays a pivotal role in activation of aspirin-treated platelets by thrombin and epinephrine. J. Biol. Chem. 283, 18493–18504 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.