Background: Regulation of neuronal progenitor cell migration is critical for proper brain lamination.
Results: G protein-coupled receptor signaling promotes cell migration, lamellipodium formation, and Rac activation by phosphorylating a microtubule-associated protein, doublecortin.
Conclusion: Doublecortin is released from microtubules and induces actin reorganization in a phosphorylation-dependent manner.
Significance: This is the first evidence for the coordinated regulation of microtubule and actin dynamics.
Keywords: Actin, Cell Migration, G Proteins, Microtubules, Neuroprogenitor Cell, Phosphorylation, Protein Kinase A (PKA), Rac
Abstract
Doublecortin (DCX) is a microtubule- associated protein that is specifically expressed in neuronal cells. Genetic mutation of DCX causes lissencephaly disease. Although the abnormal cortical lamination in lissencephaly is thought to be attributable to neuronal cell migration defects, the regulatory mechanisms governing interactions between DCX and cytoskeleton in the migration of neuronal progenitor cells remain obscure. In this study we found that the Gs and protein kinase A (PKA) signal elicited by pituitary adenylate cyclase-activating polypeptide promotes neuronal progenitor cells migration. Stimulation of Gs-PKA signaling prevented microtubule bundling and induced the dissociation of DCX from microtubules in cells. PKA phosphorylated DCX at Ser-47, and the phospho-mimicking mutant DCX-S47E promoted cell migration. Activation of PKA and DCX-S47E induced lamellipodium formation. Pituitary adenylate cyclase-activating polypeptide and DCX-S47E stimulated the activation of Rac1, and DCX-S47E interacted with Asef2, a guanine nucleotide exchange factor for Rac1. Our data reveal a dual reciprocal role for DCX phosphorylation in the regulation of microtubule and actin dynamics that is indispensable for proper brain lamination.
Introduction
During brain development, proliferation, migration, and maturation of neuronal progenitor cells (NPCs)4 are strictly regulated by a number of signaling pathways. In particular, coordinated migration of NPCs patterns the cortical brain layers (1, 2). NPCs migrate radially to the cortical plate (CP) along the radial glial fiber from the ventricular zone (VZ), in which NPCs proliferate with multipotency and self-renewal. This process is called radial migration. NPCs extend their leading process in the direction of migration, which is followed by nucleokinesis. Nucleokinesis in migrating cells depends on microtubule dynamics, which is regulated by several signaling molecules (3, 4). Genetic disorders causing defects of radial migration have been reported, and various signaling molecules have been suggested to be involved in these defects (5, 6). However, the detailed mechanisms governing these signaling molecules and their regulation of brain development are poorly understood.
G protein-coupled receptors (GPCRs), the largest family of seven-transmembrane receptors, transduce extracellular signals into intracellular signaling events. GPCRs are involved in a variety of physiological processes, such as proliferation, differentiation, and migration. Some GPCRs have been suggested to regulate cell migration during brain development; for example, CXCR4, a chemokine GPCR for stroma cell-derived factor-1, regulates tangential migration (7). Mutations of the orphan GPCR GPR56 are found in patients with brain cortex malformation, suggesting that human GPR56 is essential for proper lamina formation in the developing brain (8). Although several reports indicate that GPCR signaling is involved in NPC migration, the mechanism by which GPCR signaling regulates migration has not been clarified. Previously, we demonstrated that the endothelin receptor type B transduces the signal for the inhibition of NPC migration through the Gq and JNK pathway (9). Moreover, we revealed that GPR56 signaling inhibits migration through the G12/13 and Rho pathway (10). These results suggest that distinct G proteins regulate NPC migration through individual pathways. However, the stimulatory pathway mediated through G proteins in neuronal progenitor cells remains elusive.
Doublecortin (DCX) is a microtubule-associated protein (MAP) that is involved in microtubule stabilization, bundling, and nucleation (11–13). DCX has two homologous DCX domains (amino acids 47–135 and 174–259) that are necessary for interaction with microtubules (14). DCX was identified as a gene responsible for double cortex and X-linked lissencephaly (15). Hemizygous DCX missense mutations of two clusters were found in the DCX domains (16). Knockdown of DCX using short hairpin RNAs (shRNAs) in utero caused the impairment of radial migration (17). These results strongly suggest that DCX acts as a key regulator of NPC migration for proper brain lamination (18).
DCX is known to be phosphorylated at various Ser/Thr residues by kinases such as Cdk5 (19), JNK (20, 21), Rho kinase (22), glycogen synthase kinase 3β (23), protein kinase A (PKA), and MARK/Par1 (24). Phosphorylation of DCX by PKA or MARK/Par1 at Ser-47, which is located in the first DCX domain, decreases the binding affinity of DCX for microtubules. Interestingly, mutation of Ser-47 to Arg was found in a lissencephaly patient (15). In addition, the mutation of Ser-47 to Glu, which acts as a phospho-mimic mutation, promotes the interaction of DCX with an actin-binding protein, neurabin2/spinophilin (25). These results highlighted DCX as a candidate downstream molecule in the G protein-signaling pathways that may act as a key regulator in orchestrating microtubule and actin dynamics. Lamellipodia are formed by actin assembly and induced by activation of a Rho family small GTPase, Rac. Lamellipodia at the leading edge are essential for cell migration. However, the mechanism of coordinated regulation of microtubule and actin dynamics, which is important for cell migration in response to GPCR stimulation, remains unclear.
Here, we first utilized a slice culture system of embryonic cerebral cortex to investigate the action of the G protein-signaling pathway in the developing mouse brain. We revealed that Gs-PKA signaling promotes radial migration. Moreover, we found that pituitary adenylate cyclase-activating protein (PACAP) is a candidate endogenous positive regulator of the radial migration. Next, we focused on DCX as a downstream molecule of Gs signaling. PACAP and Gs-PKA signals induced the phosphorylation of DCX at Ser-47 and promoted neuronal cell migration. Unexpectedly, DCX phosphorylated by PKA stimulated lamellipodium formation in NPCs in a Rac-dependent manner. Our data indicate that phosphorylated DCX is released from microtubules and then interacts with a Rac guanine nucleotide exchange factor (GEF). These results indicate a dual reciprocal role for DCX phosphorylation in the regulation of microtubule and actin dynamics for proper brain lamination.
EXPERIMENTAL PROCEDURES
Materials
Mouse epidermal growth factor (EGF) and DNase I were purchased from Roche Diagnostics. Human basic fibroblast growth factor (bFGF) was obtained from Peprotech EC. B27 supplement and trypsin were purchased from Invitrogen. Endothelin-1, PACAP1–38 (PACAP), and PACAP6–38 (PACAP antagonist) were purchased from the Peptide Institute. All other reagents were purchased from Sigma unless otherwise indicated.
Recombinant Adenovirus
Adenoviruses expressing green fluorescent protein (GFP) and C-terminal peptides of Gαs, Gαq, Gαi2, and Gα12 were prepared as described previously (26) and kindly provided by Dr. Kurose (Kyushu University). Infection of cells by adenovirus was monitored by GFP fluorescence.
Cortical Slice Culture and GFP-labeled Cell Migration
Mouse brain slices were prepared from E16.5 brains as described previously (9). Slices were fixed in 4% paraformaldehyde. Images were captured using AxioVision software, and the relative migrating distances of GFP-labeled cells from VZ (0%) to CP (100%) were assessed using Scion Image to estimate fluorescence intensity in the slice. Fluorescence intensity less than half-maximum was designated as background, and the weighted average of the distance between VZ and CP was calculated (Dav = Σ(F × D)/ΣF). The mean value was calculated for each slice.
Cell Culture and Transfection
HEK293T and COS7 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (JRH Biosciences), 100 units/ml penicillin, and 100 μg/ml streptomycin at 37 °C and 5% CO2. Cortical neural progenitor cells were isolated from E11.5 mouse brains as described previously (10). Cells were maintained in DMEM/F-12 medium supplemented with B27, 1 mg/ml BSA, 2 μg/ml heparin, 20 ng/ml EGF, and 20 ng/ml basic FGF at 37 °C and 5% CO2. HEK293T cells and COS7 cells were transfected by the calcium phosphate method. The Neon transfection system (Invitrogen) was used to transfect NPCs.
Construction of Plasmids
Total RNA was extracted from E16.5 mouse brains using TRIzol reagent (Invitrogen). The RNA was reverse-transcribed using oligo(dT20) and Superscript ΙΙΙ (Invitrogen). DCX cDNA was amplified by PCR using primers 5′-GAAGATCTATGGAACTTGATTTTGGACATTTTG-3′ and 5′-CTTCAAGCTTTCACATGGAATCGCCAAG-3′. Full-length DCX cDNA was inserted into plasmids including pCMV5 containing an N-terminal FLAG tag, pmCherry-C1 (Clontech), pEGFP-C1 (Clontech), and the pCold-GST vector (27), which was generously provided by Dr. Kojima (Osaka University). Point mutants of DCX at Ser-47 were generated using a QuikChange II site-directed mutagenesis kit (Stratagene) and the following primers: S47A, 5′-GAACCTTGCAGGCATTAGCTAATGAGAAGAAGGCCAAG-3′; S47E, 5′-GAACCTTGCAGGCATTAGAGAATGAGAAGAAGGCCAAG-3′; and S47R, 5′-GAACCTTGCAGGCATTAAGAAATGAGAAGAAGGCCAAG-3′.
Production of Adenoviruses
To construct adenoviral plasmids harboring shRNA against DCX, annealed oligonucleotides for murine DCX were inserted into the Mlu1/Hind3 sites of the pRNAT-H1.1/Adeno vector (GenScript), an adenoviral siRNA shuttle vector. Recombination of the adenoviral vector was performed using the AdEasy XL Adenoviral Vector System (Stratagene). The recombined adenovirus vector was amplified with XL10-Gold Ultracompetent cells (Stratagene). The purified adenovirus vector was digested with Pac1 and transfected into HEK293 cells to produce recombinant adenovirus particles. The sequences of each shRNA adenoviral vector were: sh-RNA-1, 5′-CGCGTCCGGAGTGCGCTACATTTATATTTTCAAGAGAAATATAAATGTAGCGCACTCCTTTTTA-3′; sh-RNA-4, 5′-CGCGTCCGTACGTTTCTACCGCAATGTTTTCAAGAGAAACATTGCGGTAGAAACGTACTTTTTA-3′; sh-3′-UTR-DCX (17), 5′-CGCGTCCGCTCAAGTGACCAACAAGGCTATAGACACAATAGCCTTGTTGGTCACTTGAGCTTTTTA-3′.
Migration Assay
NPCs were infected with an adenovirus producing shRNA against the 3′-UTR of DCX. Forty-eight hours later, the cells were infected with adenoviruses harboring DCX cDNA (wild-type and mutant forms lacking the 3′-UTR). Neurospheres infected for a total of 72 h were trypsinized, and 1 × 105 cells were placed on an 8-μm-pore-size transwell (BD Biosciences) coated with 100 μg/ml poly-d-lysine, together with the reagents indicated under “Results,” and incubated for 16 h. Cells on the top side of the filters were removed using a cotton bud, and the filters were then fixed and stained with Giemsa solution. The number of stained cells on the bottom surface of the filters was counted.
Microtubule Fractionation Assay
To investigate the co-localization of wild-type and mutant DCX with microtubules, we prepared free tubulin and microtubule fractions using the sequential extraction method as described previously (28). Cells were washed with PM2G buffer (0.1 m PIPES (pH 6.9), 15% glycerol, 5 mm MgCl2, 2 mm EGTA) and were permeabilized with PM2G buffer containing 0.5% Nonidet P-40 at 37 °C for 15 min. After centrifugation at 700 × g for 5 min at room temperature, the supernatants were collected as the free tubulin fraction. The precipitates were incubated in PM2G buffer containing 0.5% Nonidet P-40 and 50 mm CaCl2 at 37 °C for 15 min. Finally, the microtubule fraction was collected as precipitates by centrifugation at 700 × g for 5 min.
Time-lapse Imaging
Time-lapse images of living cells were captured using AxioObserver (Carl Zeiss) equipped with an incubation chamber. NPCs were cultured in Leibovitz medium (Invitrogen) with a B27 supplement and 1 mg/ml BSA on a glass-bottomed dish (Greiner bio-one) coated with 100 μm poly-d-lysine and 10 μm laminin. Cells were analyzed every 10 s for 5 min with a 100 × objective lens. Imaging data were stored as files in AxioVision (Carl Zeiss).
Tubulin Polymerization Assay
Tubulin polymerization was performed in 50 μl at 37 °C using the Tubulin Polymerization Assay kit (Cytoskeleton). Purified DCX protein (2.5 μg for each sample) was added to a solution including 2 μg/μl tubulin and fluorescent dye. Fluorescence intensity was measured every 60 s for 1 h according to the manufacturer's instructions.
Immunostaining
COS7 cells expressing wild-type or mutant DCX were grown on a glass coverslip (Matsunami) until 30% confluent and fixed with 4% paraformaldehyde in PBS for 10 min. Fixed cells were washed 3 times with PBS for 5 min, and permeabilization and blocking were performed with blocking buffer (0.1% Triton X-100, 10% FBS in PBS) for 1 h. Cells were incubated with a primary antibody diluted in blocking buffer for 2 h and then washed with PBS three times for 5 min. A monoclonal antibody against β-tubulin (T4026, Sigma) was used at 1:250 dilution. The cells were then stained with goat anti-mouse IgG conjugated with Alexa Fluor 594 at 1:1000 dilution for 2 h. The cells were then washed with PBS, and each coverslip was mounted with PermaFluor Mounting Medium (Thermo Scientific). Cells were observed using AxioObserver (Carl Zeiss) with a 63 × objective lens.
Immunoblot Analysis
Cells were lysed with lysis buffer (20 mm HEPES-NaOH (pH 7.5), 0.5% Nonidet P-40, 1 mm EGTA, 3 mm MgCl2, 30 mm NaCl, 1 mm phenylmethylsulfonyl fluoride (PMSF), and 1 μg/ml leupeptin). Protein concentration of each lysate was quantified using the Bradford protein assay and adjusted to 1 mg/ml. The lysates (10 μg protein/lane) were separated by SDS-PAGE and transferred onto an Immobilon PVDF membrane (Millipore). Membranes were probed with primary antibodies against DCX (C-18, Santa Cruz Biotechnology), β-tubulin (Sigma), β-actin (Nacalai Tesque), and FLAG epitope (Sigma). Band intensity was quantified using multigauge software (Fujifilm).
Expression and Purification of Recombinant Proteins
To express recombinant proteins of wild-type and mutant DCX, their cDNAs were inserted into modified pCold-I-GST plasmids (27). Protein expression of His-GST-tagged DCX in the BL21 (DE3) codon plus strain was induced by 0.1 mm IPTG at 16 °C for 24 h. Proteins were extracted in PBS with sonication. Soluble proteins were loaded onto Glutathione-Sepharose CL-4B (GE Healthcare) and gently rotated for 2 h. The beads were then washed with a wash buffer (20 mm Tris-HCl (pH 8.0), 300 mm NaCl, 1 mm EDTA, 1 mm DTT, 1 μg/ml leupeptin, 1 μg/ml aprotinin, and 1 mm PMSF), and bound proteins were eluted with 40 mm glutathione in 50 mm Tris-HCl (pH 8.0), 150 mm NaCl, 1 mm EDTA, 1 mm DTT, 1 μg/ml leupeptin, 1 μg/ml aprotinin, and 1 mm PMSF. Eluted DCX proteins were dialyzed against the elution buffer without glutathione. In some experiments, untagged DCX proteins were used. Each recombinant His-GST-DCX was cleaved by PreScission Protease (GE Healthcare). The released His-GST and the protease were removed by Glutathione-Sepharose CL-4B and nickel-nitrilotriacetic acid-agarose (Qiagen), respectively.
Pulldown Assay for Rac1 Activation
Rac1 activation was analyzed by a modification of a protocol described previously (29). NPCs were treated with 5 μm PACAP antagonist or 10 μm KT5720 for 30 min before 50 nm PACAP stimulation for 1 h. Cells were washed with PBS and then lysed with GST-FISH buffer (50 mm Tris-HCl (pH 7.5), 100 mm NaCl, 2 mm MgCl2, 1% Nonidet P-40, 10% glycerol, 1 μg/ml leupeptin, and 1 mm PMSF). When HEK293T cells were used for the assay, they were transfected with plasmids encoding wild-type or mutant DCX. Two days after transfection, lysates were prepared and incubated with 1 μg of GST-PAK-CRIB or GST protein bound to glutathione-Sepharose CL-4B (GE Healthcare) for 1.5 h at 4 °C. Beads were washed with GST-FISH buffer three times, and the proteins were eluted with Laemmli sample buffer and analyzed by immunoblotting with anti-Rac1 antibody.
Immunoprecipitation Assay
HEK293T cells were transfected with plasmids harboring myc-Asef2 and FLAG-tagged wild-type and mutant DCX. After 48 h, cells were collected, and lysates were prepared with lysis buffer. Cell lysates were used for immunoprecipitation with 1 μg of anti-FLAG antibody and protein-G-Sepharose (GE Healthcare) for 1.5 h at 4 °C. Beads were washed with lysis buffer six times, and immunoprecipitated proteins were eluted with 200 μg/ml FLAG peptide. Eluted proteins were analyzed by immunoblotting with M2 and 9E10 antibodies.
RESULTS
Gs-PKA Signaling Promotes Neuronal Migration
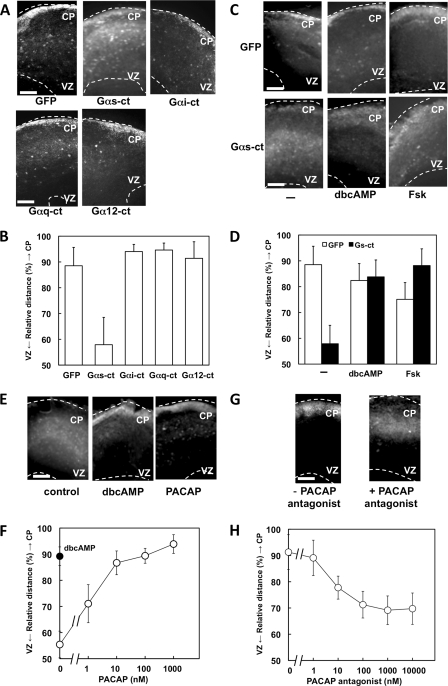
We utilized an E16.5 cerebral cortex slice culture system to investigate the action of GPCR-mediated signals in the developing mouse brain. Cells in the VZ were infected with recombinant adenoviruses expressing GFP to monitor their radial migration (9). Infected cells subsequently migrated from the VZ to the CP for 3–4 days in culture. To evaluate the roles of G protein signaling in radial migration, we examined the effect of C-terminal peptides of the G protein α subunit (Gα-ct) with GFP (Fig. 1, A and B). Each G protein α subunit can selectively inhibit receptor-G protein coupling (26). Infection by adenoviruses harboring Gαs-ct inhibited radial migration, whereas Gαi-ct, Gαq-ct, and Gα12-ct did not. Cyclic AMP (cAMP) is a common intracellular second messenger downstream of Gαs. To confirm the involvement of cAMP in the regulation of radial migration, we examined the effect of dibutyryl-cAMP (dbcAMP) and of forskolin (Fsk), which is an activator of adenylyl cyclase. The inhibitory effect of Gαs-ct on radial migration was attenuated by both dbcAMP and Fsk (Fig. 1, C and D), and PKA inhibitor KT5720 also inhibited radial migration (supplemental Fig. 1). These results indicated that Gs, cAMP, and PKA promote radial migration during brain development.
FIGURE 1.
Gs signaling promotes radial migration. A, cortical slices were prepared from E16.5 mouse telencephalon. Cells in the VZ were infected with adenoviruses harboring GFP alone or GFP plus either Gαs-ct, Gαi-ct, Gαq-ct, or Gα12-ct. Slices were cultured for 4 days in vitro. White dotted lines mark pial (upper) and ventricular (lower) surfaces. B, the intensity of GFP fluorescence was measured, and the relative distance from VZ to CP of GFP-positive cells was calculated as described under “Experimental Procedures.” Data are the mean ± S.D. of at least five slices from three independent experiments. C and D, the inhibitory effect of Gαs-ct on the radial migration is attenuated by dbcAMP and Fsk. Cells in the VZ were infected with adenoviruses harboring GFP alone or GFP plus Gαs-ct. Slices were cultured for 4 days in vitro without or with 1 mm dbcAMP or 1 μm Fsk. E, PACAP promotes neuronal migration in slice culture. Cells in the VZ were infected with an adenovirus harboring GFP. Slices were cultured for 2 days in vitro without or with 1 mm dbcAMP or 100 nm PACAP. F, dose-dependent stimulation of radial migration by PACAP is shown. The filled circle indicates the effect of 1 mm dbcAMP. G and H, the PACAP antagonist PACAP6–38 inhibits the radial migration induced by endogenous ligand(s). Slices were cultured for 4 days in vitro without or with 1 μm PACAP6–38 (G). PACAP6–38 inhibits the radial migration in a dose-dependent manner (H). Scale bars, 250 μm.
The next goal prompted by these results was to identify the endogenous Gs-coupled receptor ligand that is involved in radial migration. Among the many ligands for Gs-coupled receptor, pituitary adenylyl cyclase-activating peptide (PACAP) is known to act as an anti-mitogenic signal in the developing cerebral cortex (30). Moreover, in the embryonic brain, the PACAP receptor PAC1 is expressed at very high levels in the VZ, whereas PACAP is expressed in the post-mitotic parenchyma (31). GFP-labeled cells in 2 day in vitro slices mainly stayed in the intermediate zone. As expected, PACAP promoted neuronal progenitor migration dose-dependently (Fig. 1, E and F). Moreover, the PACAP antagonist inhibited radial migration in slice cultures at 4 days in vitro (Fig. 1, G and H). These results indicated that G protein-coupled receptor signaling positively regulates NPC migration through Gs and PKA and that PACAP is an endogenous positive regulator of radial migration.
DCX Mediates Gs/PKA-induced Neuronal Progenitor Cell Migration
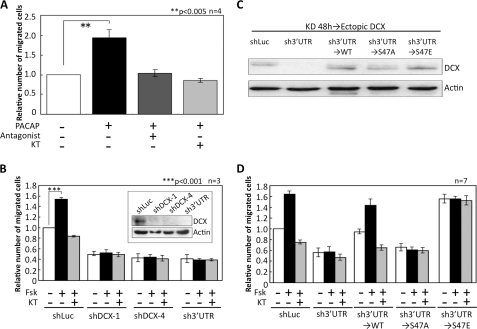
The positive effect of PACAP on neuronal cell migration was also observed in a Boyden chamber assay with primary-cultured NPCs. PACAP increased cell migration to approximately twice that for unstimulated cells (Fig. 2A). This effect was completely blocked by the PACAP antagonist and KT5720. Next, we focused on DCX. It has been reported that DCX is phosphorylated by PKA at Ser-47 in vitro (24) and that DCX knockdown using the RNAi in utero electroporation system suppresses radial migration (17). To investigate whether DCX is involved in the promotion of NPC migration induced by PACAP/Gs/PKA signaling, we first infected NPCs with adenoviruses harboring three shRNAs. Knockdown of endogenous DCX by all three shRNAs abolished the promotion of NPC migration induced by forskolin (Fig. 2B), suggesting that DCX plays an essential role in the stimulatory effect of PKA on NPC migration.
FIGURE 2.
Gs-PKA signaling promotes neuronal cell migration. A, neuronal progenitor cells in a Boyden chamber were stimulated with 10 nm PACAP and either 1 μm PACAP6–38 (Antagonist) or 1 μm KT5720 (KT). After 16 h, the number of cells that had migrated into the lower chamber was counted. **, p < 0.005, Student's t test. The S.D. is shown as a bar. n = 4. B, endogenous DCX in neuronal progenitor cells was down-regulated by three shRNAs. Short hairpin Luciferase (shLuc) was used as the control. The number of migrated cells was counted 16 h after treatment with 1 μm Fsk or 1 μm KT5720 (KT). ***, p < 0.001, Student's t test. n = 4. C and D, endogenous DCX in neuronal progenitor cells was down-regulated by sh3′UTR-DCX. After knockdown, cells were infected again with adenoviruses harboring DCX-WT and mutants. Cells were treated with 1 μm forskolin alone or with 1 μm KT5720, and the number of migrated cells was counted after 16 h. n = 7.
To examine the role of phosphorylation at Ser-47, we constructed the DCX-S47A mutant in which serine at position 47 was substituted by alanine. Wild-type DCX (DCX-WT) and DCX-S47A were expressed in NPCs, and the phosphorylation of DCX by PACAP was investigated. Two-dimensional electrophoresis revealed that PACAP stimulation increased the amount of acidic wild-type DCX in NPCs. In contrast to DCX-WT, DCX-S47A did not exhibit a mobility shift under treatment with PACAP (supplemental Fig. 2).
We then examined the effect of unphosphorylated mutant (DCX-S47A) and phospho-mimic mutant (DCX-S47E) DCXs on neuronal cell migration. Cells were first infected with an adenovirus to express shRNA against the 3′-untranslated region (UTR) of DCX mRNA for knockdown. Forty-eight hours after infection, cells were infected again with adenoviruses harboring shRNA-insensitive DCX-WT, DCX-S47A, and DCX-S47E. To avoid any nonphysiological effect in cells due to overexpression of DCX, we optimized the conditions of adenoviral infection to express the ectopic DCX proteins at levels comparable with those of endogenous DCX (Fig. 2C). Similar to the results of Fig. 2B, forskolin promoted the migration of cells infected with shLuciferase (control), and this effect was eliminated by the PKA inhibitor and shRNA against DCX (Fig. 2D). Expression of ectopic DCX-WT rescued the inhibitory effect of DCX knockdown. On the other hand, unphosphorylated DCX-S47A failed to rescue the inhibition of cell migration by shRNA against DCX. Strikingly, the ectopic expression of DCX-S47E stimulated cell migration without forskolin, and forskolin did not enhance this stimulation. Moreover, the stimulation by DCX-S47E was not inhibited by the PKA inhibitor. On the basis of these results, we propose that phosphorylation of DCX at Ser-47 by PKA is essential for neuronal progenitor cell migration induced by Gs signaling during brain development.
PACAP/PKA Signal Decreases Affinity of DCX for Microtubules in Cells
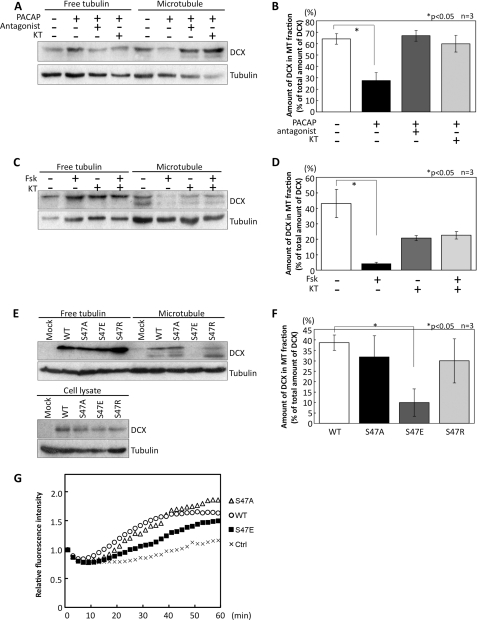
Phosphorylation of DCX at Ser-47 by PKA/MARK has been reported to decrease the affinity of DCX for microtubules in vitro (24). However, it remains unclear whether the Gs/PKA signal regulates this process in cells. To analyze the binding affinity of DCX for microtubules, we prepared free tubulin and microtubule fractions from NPCs treated with PACAP. Stimulation with PACAP decreased the amount of DCX protein in the microtubule fraction. This reduction was canceled by treatment with the PACAP antagonist and the PKA inhibitor (Fig. 3, A and B). Treatment with forskolin produced a similar result (Fig. 3, C and D). Forskolin decreased the amount of DCX in the microtubule fraction, and this diminution was attenuated by PKA inhibitor. Because forskolin is a potent activator of adenylyl cyclase, the inhibitory effect of forskolin was greater than that of PACAP. Although PKA inhibitor alone appeared to reduce the amount of DCX in the microtubule fraction, the difference between control and PKA inhibitor treatment was not significant (Fig. 3D). Furthermore, we examined the affinity of DCX and its mutants for microtubules in HEK293T cells. In addition to DCX-WT, DCX-S47A, and DCX-S47E, we constructed another point mutation, DCX-S47R, that has been found in a lissencephaly patient (15). HEK293T cells were transfected with the wild-type and these mutant constructs, and fractionation was then performed. The amounts of DCX-WT, DCX-S47A, and DCX-S47R proteins in the microtubule fractions were comparable. On the other hand, the amount of DCX-S47E protein in the microtubule fraction was lower than that of DCX-WT (Fig. 3, E and F). These results indicated that Gs signaling weakens the binding affinity of DCX for the microtubule by phosphorylating DCX at Ser-47 in cells.
FIGURE 3.
PACAP/PKA signaling decreases the affinity of DCX for microtubules. A, NPCs were pretreated with 1 μm PACAP6–38 (PACAP antagonist) or 10 μm KT5720 (KT) for 30 min and then stimulated with 10 nm PACAP for 1 h. Control cells were pretreated with 1% DMSO for 30 min, and PBS was added. Free tubulin and microtubule fractions were prepared, and the amount of DCX and tubulin in each fraction was then detected by immunoblot. B, quantitative analysis of data in A is shown. The relative amount of DCX in the microtubule fraction was normalized to the total amount of DCX, which was evaluated by the summation of DCX in both free tubulin and microtubule fractions. *, p < 0.05, Student's t test. The S.D. is shown as a bar. n = 3. C, NPCs were pretreated with 10 μm KT5720 for 30 min and then treated with 1 μm Fsk for 1 h. Control cells were treated with 1% DMSO for 1.5 h. DCX and tubulin were detected using their antibodies. D, quantitative analysis of data in C is shown. *, p < 0.05, Student's t test. E, free tubulin and microtubule fractions were prepared from HEK293T cells transfected with FLAG-DCX and its mutants. DCX and tubulin in fractions were detected by anti-DCX and anti-tubulin antibodies. F, quantitative analysis of data in E is shown. *, p < 0.05, Student's t test. G, shown is the effect of DCX and its mutants on tubulin polymerization. DCX and its mutants were added into a mixture solution including tubulin and fluorescent dye. The intensity of fluorescence was measured. Relative fluorescence intensity was calculated as fluorescence intensity at each time point/fluorescence intensity at the starting point.
In addition, we investigated whether Ser-47-phosphorylated DCX might have a reduced capacity for microtubule assembly. To analyze the effects of DCX and its mutants on tubulin polymerization, a tubulin reconstitution assay was performed using a fluorescent dye that shows enhanced fluorescence when bound to microtubules (Fig. 3G). Recombinant DCX-WT, DCX-S47A, and DCX-S47E proteins were purified from bacteria. We added them to the assay solutions and evaluated tubulin polymerization activities by measuring fluorescence intensity. DCX-WT and DCX-S47A increased the fluorescence intensity within 20 min of the start of the incubation. However, DCX-S47E exhibited lower activity in facilitating tubulin polymerization. This result suggested that phosphorylation at Ser-47 by PKA reduces the ability of DCX to accelerate tubulin polymerization.
Phosphorylation of DCX at Ser-47 Decreases Microtubule Bundling Activity
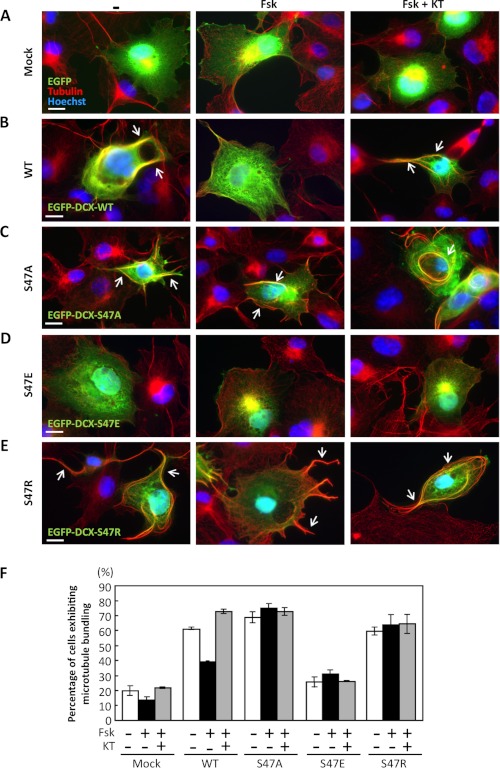
Previous studies have shown that overexpression of DCX induces microtubule bundling (14, 32), but whether PKA signaling regulates this activity of DCX is still unknown. To observe bundling activity, COS7 cells were transfected with EGFP-fused wild-type or mutant DCX. The cells were treated with forskolin and KT5720 and then analyzed by immunostaining with β-tubulin antibody. As expected, overexpression of EGFP-DCX-WT in COS7 induced microtubule bundling (Fig. 4B). Forskolin treatment inhibited the effect of DCX-WT, and this inhibition was canceled by treatment with KT5720 (Fig. 4B). Ectopic expression of DCX-S47A and DCX-S47R also induced microtubule bundling; however, forskolin failed to affect microtubule bundling (Fig. 4, C and E). As predicted, overexpression of DCX-S47E did not induce microtubule bundling (Fig. 4D). These observations indicate that phosphorylation of DCX at Ser-47 decreases the bundling activity of DCX for microtubules in cells.
FIGURE 4.
PKA signaling decreases DCX-dependent microtubule bundling. A–E, COS7 cells were transfected with EGFP (A), EGFP-DCX-WT (B), EGFP-DCX-S47A (C), EGFP-DCX-S47E (D), or EGFP-DCX-S47R (E) (green). After 2 days cells were treated with 1 μm Fsk either alone or with 10 μm KT5720 (KT). Control cells were treated with 1% DMSO for 1.5 h. Tubulin was immunostained with an anti-tubulin antibody (red). Arrows indicate microtubule bundling. Scale bars, 10 μm. F, the number of cells displaying microtubule bundling was counted, and the percentage of cells exhibiting microtubule bundling among GFP-positive cells was calculated.
Effect of DCX Phosphorylation on Actin Behavior in Neuronal Progenitor Cells
Cytoskeletal molecules such as actin and tubulin are polymerized and depolymerized dynamically in migrating cells (33), and it is well known that actin filaments and microtubules organize cell polarity along the axis of filaments (34). At the leading edge of NPCs, lamellipodia are induced by activated Rac1, a Rho family small GTPase (35). Rho GTPases contribute to cell migration by regulating cytoskeletal dynamics and adhesion assembly, which generate traction force (36).
To investigate the dynamics and function of DCX in migrating cells, NPCs were transfected with mCherry-DCX and GFP-α-tubulin. The mCherry-DCX fusion proteins DCX-WT, DCX-S47A, and DCX-S47R mainly co-localized with GFP-α-tubulin (data not shown). Surprisingly, mCherry-DCX-S47E was observed not only on microtubules, albeit weakly, but also in the cytoplasm, and it induced membrane ruffling at the periphery (data not shown).
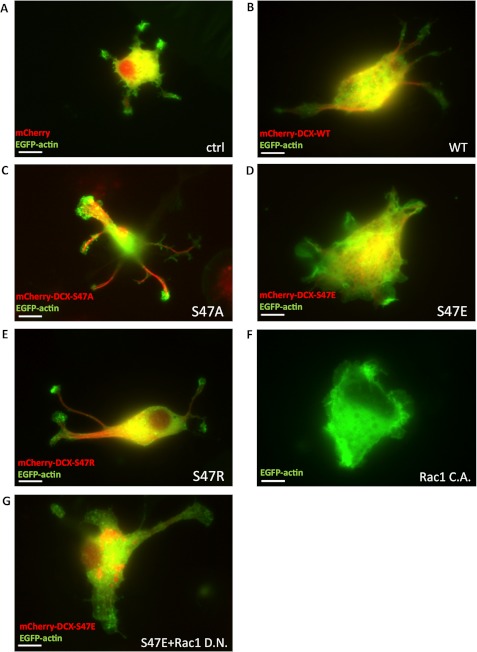
Membrane ruffling is well known to coincide with lamellipodium formation. To clarify whether phosphorylated DCX is involved in actin dynamics, NPCs were transfected with mCherry-DCX and EGFP-actin. In control cells expressing mCherry, small lamellipodia were observed at the periphery (Fig. 5A), and in cells expressing mCherry-DCX-WT, mCherry-DCX- S47A, and mCherry-DCX-S47R, lamellipodia were observed mainly at the protrusion edges (Fig. 5, B, C, and E). Strikingly, the expression of mCherry-DCX-S47E induced drastic lamellipodium formation on the whole cell membrane without protrusions, as seen in the cells expressing active Rac1 (Fig. 5, D and F). PACAP stimulation also induced lamellipodia (supplemental Fig. 3). Lamellipodia induced by DCX-S47E were inhibited by dominant negative Rac1 (Fig. 5G) but not by treatment with taxol and colchicine in COS7 cells (supplemental Fig. 4), suggesting that microtubule dynamics is not involved in DCX-S47E-induced lamellipodium formation. These results suggest that phosphorylated DCX causes lamellipodium formation mediated through a canonical Rac signaling pathway.
FIGURE 5.
Phospho-mimic mutant induces lamellipodium formation in NPCs. A–E, NPCs were transfected with EGFP-actin and mCherry (A), mCherry-DCX-WT (B), mCherry-DCX-S47A (C), mCherry-DCX-S47E (D), or mCherry-DCX-S47R (E). The dynamics of EGFP-actin and mCherry-DCX were observed by fluorescence microscopy. F, NPCs were transfected with EGFP-actin and a constitutively active (C.A.) form of Rac1. G, NPCs were transfected with EGFP-actin, mCherry-DCX-S47E, and a dominant negative (D.N.) form of Rac1. Scale bars, 10 μm.
Gs-PKA Signal and Phospho-mimic Mutant of DCX Activate Rac1
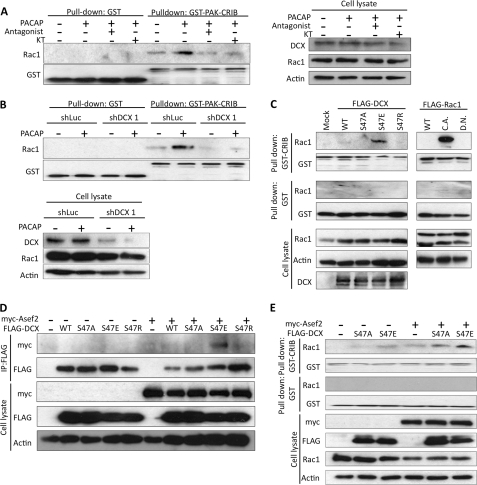
The inhibition of lamellipodium formation by dominant negative Rac (Fig. 5G) suggested that the Rac1-mediated signaling pathway lies downstream of DCX. Hence, we investigated whether phosphorylation of DCX by Gs-PKA signaling can stimulate Rac1 activation. To test this, NPCs were stimulated with PACAP, and then a GTP-bound form of Rac1 was analyzed by a pulldown assay using GST-PAK-CRIB. PACAP caused the accumulation of GTP-bound Rac1, and this accumulation was inhibited by PACAP antagonist and KT5720 (Fig. 6A). Knockdown of DCX also inhibited Rac1 activation induced by PACAP (Fig. 6B). Next, to determine whether a phospho-mimic mutant of DCX is able to stimulate Rac1, HEK293T cells were transfected with DCX and its mutants. Overexpression of DCX-WT resulted in a slight accumulation of GTP-bound Rac1. The DCX-S47E mutant caused a remarkable increase of GTP-bound Rac1 relative to that observed with DCX-S47A and DCX-S47R (Fig. 6C). These results indicated that Gs-PKA signaling induces the phosphorylation of DCX at Ser-47 and sequentially activates Rac1.
FIGURE 6.
Phosphorylation of DCX by PKA induces Rac1 activation, and DCX-S47E interacts with Asef2. A, NPCs were stimulated with 10 nm PACAP for 1 h after pretreatment with 1 μm PACAP antagonist and 10 μm KT5720 for 30 min. Control cells were pretreated with 1% DMSO for 30 min and then incubated for 1 h with PBS instead of PACAP. GTP-bound Rac1 was pulled down from cell lysates with GST-PAK-CRIB. B, NPCs were infected with adenoviruses harboring shLuc and shDCX1. Cells were stimulated with PACAP, and a pulldown assay was performed. Control cells were treated with PBS. C, HEK293T cells were transfected with a constitutively active (C.A.) or dominant negative (D.N.) form of FLAG-Rac1 and FLAG-DCX mutants. Forty-eight hours after transfection, GTP-bound Rac1 was pulled down with GST-PAK-CRIB. D, HEK293T cells were transfected with myc-Asef2 and FLAG-DCX. FLAG-DCX was immunoprecipitated (IP) with anti-FLAG antibody, and co-immunoprecipitated Asef2 was detected by anti-myc antibody. E, HEK293T cells were transfected with myc-Asef2 and FLAG-DCX. GTP-bound Rac1 was precipitated with GST-PAK-CRIB. GST was used as a control.
We then examined whether DCX interacts with a Rac1-specific GEF and activates Rac1. Adenomatous polyposis coli (APC)-stimulated guanine nucleotide exchange factor 2 (Asef2; also known as SPATA13 or FLJ31208) is a GEF for Rac1 and Cdc42 (37, 38). Asef2 mRNA is abundant in cerebral cortex and olfactory bulb in E14.5 mouse (39); hence, we focused on Asef2. We transfected HEK293T cells with plasmids harboring myc-Asef2 and FLAG-DCX. Strikingly, immunoprecipitation analysis using anti-FLAG antibody revealed that DCX-S47E, but not DCX-S47A or DCX-S47R, interacted with Asef2 (Fig. 6D). In addition, co-expression of Asef2 with DCX-S47E enhanced the accumulation of the GTP-bound form of Rac1 (Fig. 6E).
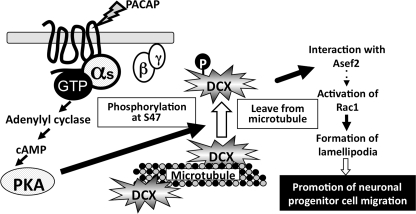
The results from our study lead us to propose a model that explains the involvement of DCX in NPC migration mediated by Gs-PKA signaling (Fig. 7). Phosphorylation of DCX at Ser-47 by PKA induces the dissociation of DCX from microtubules and its interaction with Asef2, leading to the activation of Rac1 and lamellipodium formation.
FIGURE 7.
Model for the positive regulation of NPC migration by PACAP/Gs/PKA signal-induced DCX phosphorylation. DCX is phosphorylated by PKA, and the phosphorylated DCX is released from microtubules and interacts with Asef2. The resultant activation of Rac1 induces lamellipodia, which promote NPC migration.
DISCUSSION
Radial migration of NPCs is controlled by signals that stimulate and inhibit motility. Inhibitory signals mediated through GPCRs have been demonstrated; for example, GPR56 can inhibit NPC migration by activating the G12/13 and Rho pathway (10, 40), and signaling from the endothelin receptor to JNK through Gq also inhibits NPC migration (9). However, no stimulatory signal mediated through GPCR has been reported. In this study we demonstrated that PACAP promotes radial migration mediated through Gs and PKA activation (Fig. 1). PACAP expression is prominent at the CP of the cortex (41), whereas its specific receptor, PAC1, is highly expressed in the VZ (42). These expression patterns raise the possibility that PACAP-producing cells contribute to generating a concentration gradient of PACAP from CP to VZ and that PAC1-expressing NPCs are thus attracted to the CP. This possibility was supported by the inhibitory effect of the PACAP antagonist, as shown in Fig. 1H. In contrast to our results, previous reports showed that PACAP inhibits the migration of immature granule neurons in the Purkinje cell layer at the postnatal stage (43, 44). On the other hand, it has been reported that a high concentration of PACAP induces Gq signaling (45), and Gq signaling induced by the endothelin receptor inhibits the migration of NPCs (9). These findings suggest that G protein signaling under PACAP stimulation in migrating NPCs may switch from the Gs signal to the Gq signal in a PACAP-concentration-dependent manner. This signal shift may contribute to the cessation of migration of neuronal cells reaching the CP to prevent overmigration. Thus, spatial regulation should occur during brain development.
The dynamics of cytoskeletal molecules are tightly regulated during cell migration. Here, we propose a novel mechanism whereby Gs-PKA signaling promotes NPC migration. Our results showed that PKA-induced phosphorylation of DCX at Ser-47 confers the ability to induce lamellipodia with actin filament reorganization (Fig. 5) and decrease microtubule bundling (Fig. 4). These findings indicate that one molecule can regulate two distinct cytoskeletons reciprocally in a phosphorylation-dependent manner. This highlights the DCX S47R point mutation in a lissencephaly patient. DCX-S47R, as well as DCX-S47A, has the ability to promote microtubule formation and bundling (Fig. 2, G and E), but is insensitive to PACAP and forskolin stimulation. This observation again indicates that phosphorylation at Ser-47 by PKA is indispensable for proper six-layer lamination during brain development.
We showed that PACAP stimulation and DCX-S47E expression promoted neuronal progenitor cell migration (Fig. 2, A and D). Unexpectedly, DCX-S47E expression in HEK293T cells also promoted cell migration (data not shown). Consistent with this finding, Rac activation in HEK293T cells was induced by DCX-S47E (Fig. 6E), and Asef2 was expressed in HEK293T cells (data not shown). These observations suggested that neuronal and non-neuronal cells harbor similar machinery to stimulate cell migration. Positive and negative regulation of cell migration by the cAMP/PKA pathway in several types of cells has been shown (46). DCX appears to function as a critical regulator to accelerate cell migration induced by the cAMP/PKA pathway in NPCs.
It is noteworthy that DCX and doublecortin-like kinase (Dclk) double-knock-out mice showed a defect of axon outgrowth and neuronal migration (47). In the peripheral domain (P-domain) of the growth cone, the actin meshwork is highly concentrated at the leading edge, and lamellipodium formation mediated by Rac1 signaling is frequently observed at the growth cone. In lamellipodia, the accumulation of several adhesion molecules, including L1 and neural cell adhesion molecule (NCAM), contributes to providing the driving force for neuronal cell morphological change and movement (48). In contrast to actin filaments, microtubules accumulate in the central region (C-domain) of the growth cone, which is distinguishable from the peripheral domain. However, it was observed that some of the ends of the microtubule bundles spread and invade the peripheral domain (49). Phosphorylation at Ser-47 decreases the affinity of DCX for microtubules in cells (Fig. 3). It has been reported that DCX-S47E interacts with neurabin2, an actin-binding protein that acts as a scaffold and is involved in the regulation of many signaling pathways (25, 50). An in situ experiment indicated that DCX co-localizes with neurabin2 in the E15.5 mouse brain (51). Taken together with the findings in previous reports, our data strongly suggest that PACAP-induced PKA activation results in the release of DCX from microtubules and that this released DCX associates with actin filaments through neurabin2 at the growth cone. cAMP-dependent neurite extension and axon formation are well known (52, 53), but the detailed mechanism remains unclear. The PKA-regulated switching mechanism of DCX function between microtubules and actin filaments may be involved in neuronal migration and morphological change in response to extracellular cues.
We showed that DCX-S47E remarkably induced the formation of lamellipodia throughout the cell and that lamellipodia were inhibited by dominant negative Rac1 (Fig. 5). These results suggest that DCX-S47E acts upstream of Rac1 and regulates the activity of a GEF for Rac1. As shown in Fig. 6, PACAP induced the activation of Rac1, and this activation required DCX. DCX-S47E could interact with Asef2, a GEF protein for Rac1 (Fig. 6D) that is expressed in the developing brain at E14.5 (39). We observed that a dominant negative Cdc42 inhibited DCX-S47E-induced lamellipodium formation (data not shown). Because Asef2 was identified as a GEF for Rac1/Cdc42, the inhibitory effect of dominant negative Cdc42 is consistent with the idea that Asef2 acts downstream of phosphorylated DCX. In a previous study, Asef1/2 was shown to be stimulated by APC and to participate in colorectal cancer metastasis. APC is a microtubule-associated protein and is known as a tumor suppressor. APC binds directly to Asef2 through both the APC binding domain and the SH3 domain of Asef2 (37, 38). Interestingly, Asef2 also binds to neurabin2, although neurabin2 fails to enhance its GEF activity (54), suggesting that interaction of DCX and neurabin2 may serve as a platform for the linking between actin filaments and a GEF. Regulation of actin and tubulin dynamics is necessary for neuronal migration (33), and the two cytoskeletal systems must be strictly coordinated through cross-talk in migrating cells (55). Although many molecules contribute to cross-talk between actin filaments and microtubules, extracellular signal-induced regulation of cytoskeletons and migration has not been fully clarified. Our finding provides a new insight, namely, that a microtubule-associated protein interacts with a GEF for Rac1 and regulates actin dynamics in migrating cells.
How phosphorylated DCX regulates the GEF activity of Asef2 remains an open question. In an attempt to investigate the interaction and activation of Asef2 with DCX, we constructed a series of truncation and deletion mutants of Asef2. However, to date we have not been able to map the sites involved in the interaction and activation of Asef2. Several difficulties were encountered in expressing the mutants in cells. The physiological significance of Asef2 in neuronal migration thus remains to be elucidated. It is possible that a DCX-neurabin2-Asef2 complex functions coordinately with the two cytoskeleton systems in brain development.
DCX is phosphorylated not only by PKA but also by various protein kinases. Phosphorylation of DCX at Ser-297 by Cdk5 decreased the binding affinity of DCX for microtubules in the neurite shaft, and this phosphorylation was canceled by spinophilin-protein phosphatase1 (PP1) complex. Dephosphorylation at Ser-297 is necessary for axon growth (56). We noted that overexpression of DCX-WT, DCX-S47A, and DCX-S47R caused the extension of neurites rather than of lamellipodia (Fig. 5). Moreover, we found that multiple axons were formed at day 5 in vitro when DCX-WT, DCX-S47A, and DCX-S47R were overexpressed in hippocampal neurons (data not shown). These results indicate that stabilization of microtubules by unphosphorylated DCX is important for axon growth. Furthermore, lissencephaly1 (Lis1), another microtubule-associated protein, and DCX cooperatively regulate the function of dynein, which mediates nucleus-centrosome coupling during nucleokinesis. Stabilization of microtubules by DCX is important for nuclear movement (57). Therefore, understanding the cross-talk among various kinase-mediated regulations of DCX is an important future challenge.
Supplementary Material
Acknowledgments
We thank H. Kurose (Kyushu University) for the gift of the adenovirus, C. Kojima (Osaka University) for the gift of pCold-GST vector, I. Smith (Nara Institute of Science and Technology) for reviewing the manuscript, and other members of our laboratory for helpful discussions.
This work was supported by Ministry of Education, Culture, Sports, Science, and Technology of Japan Research Grants 21370056 and 21590094 and by the Mitsubishi Foundation.

This article contains supplemental Figs. 1–4.
- NPC
- neural progenitor cell
- DCX
- doublecortin
- VZ
- ventricular zone
- CP
- cortical plate
- GPCR
- G protein-coupled receptor
- PACAP
- pituitary adenylate cyclase-activating peptide
- GEF
- guanine nucleotide exchange factor
- dbcAMP
- dibutyryl cyclic AMP
- Asef2
- APC-stimulated guanine nucleotide exchange factor 2
- APC
- adenomatous polyposis coli
- Fsk
- forskolin
- EGFP
- enhanced GFP.
REFERENCES
- 1. Nadarajah B., Parnavelas J. G. (2002) Modes of neuronal migration in the developing cerebral cortex. Nat. Rev. Neurosci. 3, 423–432 [DOI] [PubMed] [Google Scholar]
- 2. Bielas S., Higginbotham H., Koizumi H., Tanaka T., Gleeson J. G. (2004) Cortical neuronal migration mutants suggest separate but intersecting pathways. Annu. Rev. Cell Dev. Biol. 20, 593–618 [DOI] [PubMed] [Google Scholar]
- 3. Lambert de Rouvroit C., Goffinet A. M. (2001) Neuronal migration. Mech. Dev. 105, 47–56 [DOI] [PubMed] [Google Scholar]
- 4. Solecki D. J., Model L., Gaetz J., Kapoor T. M., Hatten M. E. (2004) Par6α signaling controls glial-guided neuronal migration. Nat. Neurosci. 7, 1195–1203 [DOI] [PubMed] [Google Scholar]
- 5. Feng Y., Walsh C. A. (2001) Protein-protein interactions, cytoskeletal regulation and neuronal migration. Nat. Rev. Neurosci. 2, 408–416 [DOI] [PubMed] [Google Scholar]
- 6. Kerjan G., Gleeson J. G. (2007) Genetic mechanisms underlying abnormal neuronal migration in classical lissencephaly. Trends Genet. 23, 623–630 [DOI] [PubMed] [Google Scholar]
- 7. Borrell V., Marín O. (2006) Meninges control tangential migration of hem-derived Cajal-Retzius cells via CXCL12/CXCR4 signaling. Nat. Neurosci. 9, 1284–1293 [DOI] [PubMed] [Google Scholar]
- 8. Piao X., Hill R. S., Bodell A., Chang B. S., Basel-Vanagaite L., Straussberg R., Dobyns W. B., Qasrawi B., Winter R. M., Innes A. M., Voit T., Ross M. E., Michaud J. L., Déscarie J. C., Barkovich A. J., Walsh C. A. (2004) G protein-coupled receptor-dependent development of human frontal cortex. Science 303, 2033–2036 [DOI] [PubMed] [Google Scholar]
- 9. Mizuno N., Kokubu H., Sato M., Nishimura A., Yamauchi J., Kurose H., Itoh H. (2005) G protein-coupled receptor signaling through Gq and JNK negatively regulates neural progenitor cell migration. Proc. Natl. Acad. Sci. U.S.A. 102, 12365–12370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Iguchi T., Sakata K., Yoshizaki K., Tago K., Mizuno N., Itoh H. (2008) Orphan G protein-coupled receptor GPR56 regulates neural progenitor cell migration via a Gα12/13 and Rho pathway. J. Biol. Chem. 283, 14469–14478 [DOI] [PubMed] [Google Scholar]
- 11. Horesh D., Sapir T., Francis F., Wolf S. G., Caspi M., Elbaum M., Chelly J., Reiner O. (1999) Doublecortin, a stabilizer of microtubules. Hum. Mol. Genet. 8, 1599–1610 [DOI] [PubMed] [Google Scholar]
- 12. Gleeson J. G., Lin P. T., Flanagan L. A., Walsh C. A. (1999) Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron 23, 257–271 [DOI] [PubMed] [Google Scholar]
- 13. Moores C. A., Perderiset M., Kappeler C., Kain S., Drummond D., Perkins S. J., Chelly J., Cross R., Houdusse A., Francis F. (2006) Distinct roles of doublecortin modulating the microtubule cytoskeleton. EMBO J. 25, 4448–4457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim M. H., Cierpicki T., Derewenda U., Krowarsch D., Feng Y., Devedjiev Y., Dauter Z., Walsh C. A., Otlewski J., Bushweller J. H., Derewenda Z. S. (2003) The DCX-domain tandems of doublecortin and doublecortin-like kinase. Nat. Struct. Biol. 10, 324–333 [DOI] [PubMed] [Google Scholar]
- 15. Gleeson J. G., Allen K. M., Fox J. W., Lamperti E. D., Berkovic S., Scheffer I., Cooper E. C., Dobyns W. B., Minnerath S. R., Ross M. E., Walsh C. A. (1998) Doublecortin, a brain-specific gene mutated in human X-linked lissencephaly and double cortex syndrome, encodes a putative signaling protein. Cell 92, 63–72 [DOI] [PubMed] [Google Scholar]
- 16. Taylor K. R., Holzer A. K., Bazan J. F., Walsh C. A., Gleeson J. G. (2000) Patient mutations in doublecortin define a repeated tubulin binding domain. J. Biol. Chem. 275, 34442–34450 [DOI] [PubMed] [Google Scholar]
- 17. Bai J., Ramos R. L., Ackman J. B., Thomas A. M., Lee R. V., LoTurco J. J. (2003) RNAi reveals doublecortin is required for radial migration in rat neocortex. Nat. Neurosci. 6, 1277–1283 [DOI] [PubMed] [Google Scholar]
- 18. LoTurco J. J., Bai J. (2006) The multipolar stage and disruptions in neuronal migration. Trends Neurosci. 29, 407–413 [DOI] [PubMed] [Google Scholar]
- 19. Tanaka T., Serneo F. F., Tseng H. C., Kulkarni A. B., Tsai L. H., Gleeson J. G. (2004) Cdk5 phosphorylation of doublecortin Ser-297 regulates its effect on neuronal migration. Neuron 41, 215–227 [DOI] [PubMed] [Google Scholar]
- 20. Gdalyahu A., Ghosh I., Levy T., Sapir T., Sapoznik S., Fishler Y., Azoulai D., Reiner O. (2004) DCX, a new mediator of the JNK pathway. EMBO J. 23, 823–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jin J., Suzuki H., Hirai S., Mikoshiba K., Ohshima T. (2010) JNK phosphorylates Ser-332 of doublecortin and regulates its function in neurite extension and neuronal migration. Dev. Neurobiol. 70, 929–942 [DOI] [PubMed] [Google Scholar]
- 22. Amano M., Tsumura Y., Taki K., Harada H., Mori K., Nishioka T., Kato K., Suzuki T., Nishioka Y., Iwamatsu A., Kaibuchi K. (2010) A proteomic approach for comprehensively screening substrates of protein kinases such as Rho kinase. PLoS One 5, e8704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bilimoria P. M., de la Torre-Ubieta L., Ikeuchi Y., Becker E. B., Reiner O., Bonni A. (2010) A JIP3-regulated GSK3β/DCX signaling pathway restricts axon branching. J. Neurosci. 30, 16766–16776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schaar B. T., Kinoshita K., McConnell S. K. (2004) Doublecortin microtubule affinity is regulated by a balance of kinase and phosphatase activity at the leading edge of migrating neurons. Neuron 41, 203–213 [DOI] [PubMed] [Google Scholar]
- 25. Tsukada M., Prokscha A., Ungewickell E., Eichele G. (2005) Doublecortin association with actin filaments is regulated by neurabin II. J. Biol. Chem. 280, 11361–11368 [DOI] [PubMed] [Google Scholar]
- 26. Maruyama Y., Nishida M., Sugimoto Y., Tanabe S., Turner J. H., Kozasa T., Wada T., Nagao T., Kurose H. (2002) Gα12/13 mediates α1-adrenergic receptor-induced cardiac hypertrophy. Circ. Res. 91, 961–969 [DOI] [PubMed] [Google Scholar]
- 27. Hayashi K., Kojima C. (2008) pCold-GST vector. A novel cold-shock vector containing GST tag for soluble protein production. Protein Expr. Purif. 62, 120–127 [DOI] [PubMed] [Google Scholar]
- 28. Solomon F. (1986) Direct identification of microtubule-associated proteins by selective extraction of cultured cells. Methods Enzymol. 134, 139–147 [DOI] [PubMed] [Google Scholar]
- 29. Woodcock S. A., Jones R. C., Edmondson R. D., Malliri A. (2009) A modified tandem affinity purification technique identifies that 14-3-3 proteins interact with Tiam1, an interaction which controls Tiam1 stability. J. Proteome Res. 8, 5629–5641 [DOI] [PubMed] [Google Scholar]
- 30. Suh J., Lu N., Nicot A., Tatsuno I., DiCicco-Bloom E. (2001) PACAP is an anti-mitogenic signal in developing cerebral cortex. Nat. Neurosci. 4, 123–124 [DOI] [PubMed] [Google Scholar]
- 31. Jaworski D. M., Proctor M. D. (2000) Developmental regulation of pituitary adenylate cyclase-activating polypeptide and PAC(1) receptor mRNA expression in the rat central nervous system. Brain Res. Dev. Brain Res. 120, 27–39 [DOI] [PubMed] [Google Scholar]
- 32. Tsukada M., Prokscha A., Eichele G. (2006) Neurabin II mediates doublecortin-dephosphorylation on actin filaments. Biochem. Biophys. Res. Commun. 343, 839–847 [DOI] [PubMed] [Google Scholar]
- 33. Heng J. I., Chariot A., Nguyen L. (2010) Molecular layers underlying cytoskeletal remodeling during cortical development. Trends Neurosci. 33, 38–47 [DOI] [PubMed] [Google Scholar]
- 34. Zhou F. Q., Cohan C. S. (2004) How actin filaments and microtubules steer growth cones to their targets. J. Neurobiol. 58, 84–91 [DOI] [PubMed] [Google Scholar]
- 35. Nobes C. D., Hall A. (1995) Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 81, 53–62 [DOI] [PubMed] [Google Scholar]
- 36. Parsons J. T., Horwitz A. R., Schwartz M. (2010) Cell adhesion. Integrating cytoskeletal dynamics and cellular tension. Nat. Rev. Mol. Cell Biol. 11, 633–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kawasaki Y., Sagara M., Shibata Y., Shirouzu M., Yokoyama S., Akiyama T. (2007) Identification and characterization of Asef2, a guanine-nucleotide exchange factor specific for Rac1 and Cdc42. Oncogene 26, 7620–7627 [DOI] [PubMed] [Google Scholar]
- 38. Hamann M. J., Lubking C. M., Luchini D. N., Billadeau D. D. (2007) Asef2 functions as a Cdc42 exchange factor and is stimulated by the release of an autoinhibitory module from a concealed C-terminal activation element. Mol. Cell. Biol. 27, 1380–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yoshizawa M., Sone M., Matsuo N., Nagase T., Ohara O., Nabeshima Y., Hoshino M. (2003) Dynamic and coordinated expression profile of dbl-family guanine nucleotide exchange factors in the developing mouse brain. Gene Expr. Patterns 3, 375–381 [DOI] [PubMed] [Google Scholar]
- 40. Luo R., Jeong S. J., Jin Z., Strokes N., Li S., Piao X. (2011) G protein-coupled receptor 56 and collagen III, a receptor-ligand pair, regulates cortical development and lamination. Proc. Natl. Acad. Sci. U.S.A. 108, 12925–12930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Skoglösa Y., Takei N., Lindholm D. (1999) Distribution of pituitary adenylate cyclase activating polypeptide mRNA in the developing rat brain. Mol. Brain Res. 65, 1–13 [DOI] [PubMed] [Google Scholar]
- 42. Hashimoto H., Nogi H., Mori K., Ohishi H., Shigemoto R., Yamamoto K., Matsuda T., Mizuno N., Nagata S., Baba A. (1996) Distribution of the mRNA for a pituitary adenylate cyclase-activating polypeptide receptor in the rat brain. An in situ hybridization study. J. Comp. Neurol. 371, 567–577 [DOI] [PubMed] [Google Scholar]
- 43. Falluel-Morel A., Vaudry D., Aubert N., Galas L., Benard M., Basille M., Fontaine M., Fournier A., Vaudry H., Gonzalez B. J. (2005) Pituitary adenylate cyclase-activating polypeptide prevents the effects of ceramides on migration, neurite outgrowth, and cytoskeleton remodeling. Proc. Natl. Acad. Sci. U.S.A. 102, 2637–2642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cameron D. B., Galas L., Jiang Y., Raoult E., Vaudry D., Komuro H. (2007) Cerebellar cortical-layer-specific control of neuronal migration by pituitary adenylate cyclase-activating polypeptide. Neuroscience 146, 697–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Spengler D., Waeber C., Pantaloni C., Holsboer F., Bockaert J., Seeburg P. H., Journot L. (1993) Differential signal transduction by five splice variants of the PACAP receptor. Nature 365, 170–175 [DOI] [PubMed] [Google Scholar]
- 46. Howe A. K. (2004) Regulation of actin-based cell migration by cAMP/PKA. Biochim. Biophys. Acta 1692, 159–174 [DOI] [PubMed] [Google Scholar]
- 47. Deuel T. A., Liu J. S., Corbo J. C., Yoo S. Y., Rorke-Adams L. B., Walsh C. A. (2006) Genetic interactions between doublecortin and doublecortin-like kinase in neuronal migration and axon outgrowth. Neuron 49, 41–53 [DOI] [PubMed] [Google Scholar]
- 48. Maness P. F., Schachner M. (2007) Neural recognition molecules of the immunoglobulin superfamily. Signaling transducers of axon guidance and neuronal migration. Nat. Neurosci. 10, 19–26 [DOI] [PubMed] [Google Scholar]
- 49. Zhou F. Q., Waterman-Storer C. M., Cohan C. S. (2002) Focal loss of actin bundles causes microtubule redistribution and growth cone turning. J. Cell Biol. 157, 839–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sarrouilhe D., di Tommaso A., Métayé T., Ladeveze V. (2006) Spinophilin. From partners to functions. Biochimie 88, 1099–1113 [DOI] [PubMed] [Google Scholar]
- 51. Shmueli A., Gdalyahu A., Sapoznik S., Sapir T., Tsukada M., Reiner O. (2006) Site-specific dephosphorylation of doublecortin (DCX) by protein phosphatase 1 (PP1). Mol. Cell. Neurosci. 32, 15–26 [DOI] [PubMed] [Google Scholar]
- 52. Shmueli O., Gdalyahu A., Sorokina K., Nevo E., Avivi A., Reiner O. (2001) DCX in PC12 cells. CREB-mediated transcription and neurite outgrowth. Hum. Mol. Genet. 10, 1061–1070 [DOI] [PubMed] [Google Scholar]
- 53. Shelly M., Lim B. K., Cancedda L., Heilshorn S. C., Gao H., Poo M. M. (2010) Local and long-range reciprocal regulation of cAMP and cGMP in axon/dendrite formation. Science 327, 547–552 [DOI] [PubMed] [Google Scholar]
- 54. Sagara M., Kawasaki Y., Iemura S. I., Natsume T., Takai Y., Akiyama T. (2009) Asef2 and Neurabin2 cooperatively regulate actin cytoskeletal organization and are involved in HGF-induced cell migration. Oncogene 28, 1357–1365 [DOI] [PubMed] [Google Scholar]
- 55. Li R., Gundersen G. G. (2008) Beyond polymer polarity. How the cytoskeleton builds a polarized cell. Nat. rev. Mol. Cell. Biol. 9, 860–873 [DOI] [PubMed] [Google Scholar]
- 56. Bielas S. L., Serneo F.F., Chechlacz M., Deerinck T. J., Perkins G. A., Allen P. B., Ellisman M. H., Gleeson J. G. (2007) Spinophilin facilitates dephosphorylation of doublecortin by PP1 to mediate microtubule bundling at the axonal wrist. Cell 129, 579–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tanaka T., Serneo F. F., Higgins C., Gambello M. J., Wynshaw-Boris A., Gleeson J. G. (2004) Lis1 and doublecortin function with dynein to mediate coupling of the nucleus to the centrosome in neuronal migration. J. Cell Biol. 165, 709–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.