Background: The control of TGFβ signaling depends on many not well understood regulators.
Results: TGFβ transcriptionally induces SIK1, which cooperates with the ubiquitin ligase Smurf2 to negatively regulate the signaling output.
Conclusion: Transcriptional induction of SIK1 controls TGFβ signaling together with Smurf2 and Smad7.
Significance: The molecular interplay between SIK1 and Smurf2 provides new means for controlling TGFβ signaling.
Keywords: E3 Ubiquitin Ligase, Signal Transduction, SMAD Transcription Factor, Transcription Regulation, Transforming Growth Factor β (TGFβ)
Abstract
Transforming growth factor β (TGFβ) regulates many physiological processes and requires control mechanisms to safeguard proper and timely action. We have previously described how negative regulation of TGFβ signaling is controlled by the serine/threonine kinase salt-inducible kinase 1 (SIK1). SIK1 forms complexes with the TGFβ type I receptor and with the inhibitory Smad7 and down-regulates the type I receptor. We now demonstrate that TGFβ induces SIK1 levels via a direct transcriptional mechanism that implicates the Smad proteins, and we have mapped a putative enhancer element on the SIK1 gene. We provide evidence that the ubiquitin ligase Smurf2 forms complexes and functionally cooperates with SIK1. Both the kinase activity of SIK1 and the ubiquitin ligase activity of Smurf2 are important for proper type I receptor turnover. We also show that knockdown of endogenous SIK1 and Smurf2 enhances physiological signaling by TGFβ that leads to epithelial growth arrest. In conclusion, TGFβ induces expression of Smad7, Smurf2, and SIK1, the products of which physically and functionally interlink to control the activity of this pathway.
Introduction
TGFβ signaling initiates when the extracellular dimeric TGFβ ligand associates with serine/threonine kinase receptors type II (TβRII)5 and type I (TβRI), also known as activin receptor-like kinase 5 (ALK5) (1). TβRII trans-phosphorylates TβRI, which in turn phosphorylates receptor-regulated Smads (R-Smads, Smad2, and Smad3). R-Smad phosphorylation is necessary for their association with Smad4, accumulation in the nucleus, and cooperation with transcriptional complexes to regulate gene expression (2). TGFβ signaling is regulated by various mechanisms that operate outside the cell, at the cell membrane, in the cytoplasm, or in the nucleus (3). Intracellular regulation of the TGFβ signaling network relies to a large extent on the time point of signal transduction and on the cell compartment where various post-translational modifications of signaling proteins occur (4). In this manner, the multifaceted actions of TGFβ are regulated during embryonic development, adult organ homeostasis, and disease.
Among the negative regulators of TGFβ signaling are the inhibitory Smad7 and the E3 ubiquitin ligase Smurf2, both operating within a negative feedback mechanism and controlling the strength and duration of signal transduction (3, 4). The Smad7 and Smurf2 genes are immediate-early TGFβ-inducible genes (5, 6). Smad7 binds directly to ALK5, leading to competitive inhibition of Smad2 and Smad3 phosphorylation by the receptor (5, 7). Smad7 also binds directly to Smurf2 and its homolog Smurf1, thus leading to ALK5 ubiquitination and down-regulation (8, 9). In addition, Smurf1 and Smurf2 ubiquitinate and regulate the stability of Smad proteins (10), the mitotic checkpoint protein Mad2 that controls proper spindle assembly during cell division (11), and also of the serine/threonine kinase MEKK2, which is required for the differentiation of bone cells (12). Furthermore, Smurf1 and Smurf2 ubiquitinate the small GTPases RhoA and Rap1B, the actin-binding protein talin, and the planar cell polarity protein Prickle, thus regulating epithelial and neuronal cell polarity, contractility of the cytoskeleton, and amoeboid cell migration (13–17). Such molecular functions may explain the role of Smurf1 and Smurf2 in the process of breast cancer cell invasiveness and metastasis (18, 19).
We have previously identified a new gene target of TGFβ signaling, the salt-inducible kinase 1 (SIK1, hereby abbreviated as SIK), which encodes a serine/threonine kinase of the AMP-activated protein kinase (AMPK) family (20). SIK has a modular structure with an N-terminal kinase domain and a middle ubiquitin-associated domain, which is followed by a long C-terminal sequence (21). SIK expression is induced during cardiogenesis and skeletal muscle differentiation (22). SIK function is required for cardiomyocyte differentiation, where it affects the expression of the cell cycle inhibitor p57 (23), and for skeletal myogenesis, where SIK phosphorylates class II histone deacetylases (24). SIK is also induced in adrenal glands, leading to steroidogenesis (25) and regulation of sodium transport (26). Another important pathway under the control of SIK activity is the regulation of the cAMP-responsive element binding protein (CREB) (27). SIK directly phosphorylates and inactivates the transducer of regulated CREB activity (TORC), a critical transcriptional co-activator of CREB, and in this manner SIK represses CREB function. The same mechanism, when catalyzed by the SIK isoform SIK2 that phosphorylates TORC2, leads to recruitment of the COP1 signalosome regulator that mediates TORC2 ubiquitination and degradation (28). This specific mechanism appears to be defective in diabetes, resulting in TORC2 stabilization and enhancement of the gluconeogenic gene expression program. We have demonstrated that SIK also inhibits TGFβ signaling by inducing TβRI/ALK5 receptor down-regulation (29). Regulation of TGFβ signaling by SIK is compatible with independent reports on the Caenorhabditis elegans ortholog of SIK, KIN-29, that regulates chemosensory neuronal signaling and body size, processes dependent on TGFβ/Smad signaling (30, 31).
In this study we have explored the mechanisms by which TGFβ regulates SIK expression and achieves ALK5 down-regulation. Our findings clarify how the SIK gene is regulated by Smads and place SIK in close association and functional interaction with the ubiquitin ligase Smurf2.
EXPERIMENTAL PROCEDURES
Reagents
Human HaCaT keratinocytes, human embryonic kidney HEK-293T, green monkey kidney COS1, and mink lung epithelial Mv1Lu cells were cultured as described (29). Human immortalized breast epithelial cells MCF10A (MI) and corresponding Ras-transformed MCF10AneoT cells (MII), its derivative tumor-derived clone MCF10CA1h (MIII), and the metastatic clone MCF10CA1a.cl1 (MIV) were maintained in DMEM/F-12 supplemented with 5% fetal bovine serum, 20 ng/ml epidermal growth factor, 100 ng/ml cholera toxin, 0.5 μg/ml hydrocortisone, 10 μg/ml insulin, 100 units/ml penicillin, and 100 μg/ml streptomycin and were kindly provided by F. R. Miller (Fred Hutchinson Cancer Center, Seattle, WA) (32). The human metastatic breast cancer cell line MDA-MB-231 was maintained in DMEM supplemented with 10% bovine serum, 100 units/ml penicillin, and 100 μg/ml streptomycin. The mouse mammary epithelial cells NMuMG-Fucci that express two fluorescently labeled cell cycle markers (Cdt1, fused to the red fluorescent protein mKO2, and geminin, fused to the green fluorescent protein mAG) (33), were cultured in DMEM supplemented with 10% fetal bovine serum, 10 μg/ml insulin, 100 units/ml penicillin, and 100 μg/ml streptomycin and were kindly provided by S. Johansson (Uppsala University). All cell lines were grown in a humidified incubator at 37 °C and 5% CO2.
Adenoviruses expressing human SIK epitope tagged with FLAG at its N terminus and control virus expression bacterial lacZ protein were previously described by us and were amplified, titrated, and propagated as described before (29).
Recombinant human TGFβ1 was purchased from BIOSOURCE International Inc. (Camarillo, CA) or PeproTech EC Ltd. (London). The TGFβ type I receptor inhibitor SB505124 was purchased from Calbiochem/MERCK.
Anti-SIK antibody was made in-house and was described (29); anti-FLAG (M5) antibody was from Sigma; anti-GFP (A11122) and anti-GAPDH (AM4300, Ambion) were from Invitrogen; anti-Smurf2 (2078-1), anti-Smad2 (1736-1), and anti-Smad3 (1735–1) were from Epitomics, Inc. (Burlingame); anti-Smad4 (H-552) and anti-TβRI/ALK5 (V-22) were from Santa Cruz Biotechnology, Inc. (Santa Cruz); anti-Smad7 (IMG-531) was from Imgenex (San Diego, CA); anti-E-cadherin was from BD Transduction Laboratories; anti-HA (Y-11) and mouse monoclonal anti-myc (9E10) was made in-house or purchased from Santa Cruz Biotechnology; secondary antibodies coupled to horseradish peroxidase were from GE Healthcare; secondary antibodies coupled to fluorescein isothiocyanate and tetramethylrhodamine isothiocyanate were from DAKO (Glostrup, Denmark), Alexa fluor-546 was from Molecular Probes/Invitrogen, and AMCA7-(7-amino-4-methylcoumarin-3-acetic acid) was from Jackson ImmunoResearch (West Grove, PA).
Expression vectors pcDNA3-FLAG-hSIK, pCS2–6myc-hSIK, pEGFP-hSIK, and the SIK ATP-binding site mutant K56R were described before (29). Vectors pcDNA3-ALK5(CA)-HA (constitutively active T204D mutant receptor with C-terminal hemagglutinin tag), pRK1-myc-Smurf2, and its catalytically inactive point mutant pRK1-myc-Smurf2(CG), pcDNA3-FLAG-Smad3, pcDNA3-FLAG-Smad4, and pcDNA3-FLAG-Smad7 were described (34). All DNA constructs were sequence-verified.
Promoter Cloning and Luciferase Reporter Constructs
The human SIK promoter-enhancer sequences were amplified from genomic DNA isolated from human HaCaT keratinocytes using primers mapping upstream and downstream of the transcriptional start site (TSS) and upstream and downstream of the investigated putative enhancer element of the human gene. For the amplification of the promoter fragment the primers used were: forward (5′-GAGCTCATCCTCGTTTCTCCG-3′) and reverse (5′-GAGCTCGGGTGCCTACTGCT-3′). For the amplification of the enhancer fragment the primers used were forward (5′-GGATCCCATGAGGAGAGCAGGC-3′) and reverse (5′-GTCGACGAGGCTGCCTGGAGAC-3′). The amplified sequences were cloned into vector pGL4.12 (Promega Corp., Madison, WI) in two steps. (a) The PCR-amplified genomic DNA fragments were blunt end-ligated into the pGL4.12 vector after cutting with EcoRV. (b) The subcloned promoter and enhancer fragments were removed from the first recombinant plasmids with SacI (promoter) or SalI/BamHI (enhancer) and religated to pGL4.12, producing pGL4.12-hSIKP (carrying the human SIK promoter only) and pGL4.12-hSIKPE (carrying the SIK promoter and enhancer), the latter cloned downstream of the luciferase cDNA sequence, aiming at mimicking the endogenous SIK gene organization (Fig. 3E). The cloned promoter fragment corresponds to 1214 bp spanning −1151 to +63 bp relative to the TSS of the SIK gene. The cloned enhancer fragment corresponds to 451 bp spanning +14,423 to +14,874 bp relative to the TSS, the sequences located in the 3′ intergenic region downstream from the SIK gene (Fig. 3E). All SIK gene base pair coordinates are given based on the ENSEMBL GRCh37 version of the human genome. pEGFP-N3 (Takara Bio Europe/Clontech, France) was used for normalization of promoter assays.
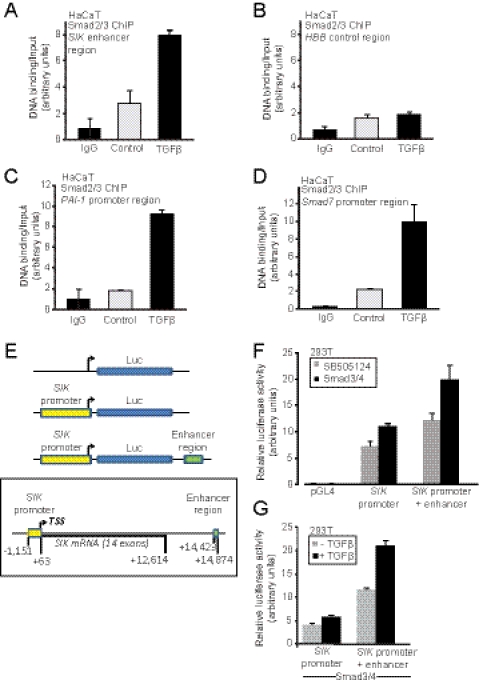
FIGURE 3.
Smads bind to and induce transcription from the SIK enhancer. ChIP assays are shown using an antibody against endogenous Smad2/Smad3 and amplification of genomic sequences corresponding to the SIK enhancer (A), the human HBB control region (B), the PAI-1 promoter (C), and the Smad7 promoter (D) in HaCaT cells stimulated with 5 ng/ml TGFβ for 1.5 h or not (Control). Control immunoprecipitations with mouse immunoglobulin (IgG) are also shown as reference. The amount of PCR-amplified DNA signal after ChIP is normalized against the equivalent PCR signal of the input chromatin before immunoprecipitation, and the relative ratios are shown in the diagrams as average values determined from triplicate determinations with their corresponding S.D. E, shown is cloning of the human SIK promoter and enhancer. A schematic diagram of the human SIK gene spanning the promoter sequences, the TSS, all exons and introns, and downstream intergenic sequence is shown. The genomic fragments that were cloned into the luciferase reporter are highlighted in colored boxes and are also shown relative to the luciferase cDNA in the corresponding constructs. F, the graph shows average luciferase activity determined from quadruplicate samples and the corresponding S.D. from transient transfection experiments in HEK-293T cells with each reporter construct transfected with empty expression vector pcDNA3 and treatment with TGFβ receptor inhibitor SB505124 or transfected with expression vectors for Smad3 and Smad4. G, an independent repeat of the experiment of panel F where Smad3/Smad4 was co-transfected under all conditions, and 5 ng/ml TGFβ (+TGFβ) or vehicle (control) was applied to the cells for 15 h.
Plasmid, Adenovirus, and siRNA Transient Transfections
COS1, Mv1Lu, and HEK-293T cells were transfected with plasmid DNA via calcium phosphate or via Lipofectamine 2000 as described (20). Transfection of siRNA oligonucleotides (25 nm) targeting human Smad2 (Dharmacon ON-TARGETplus SMARTpool L-003561-00), human Smad3 (Dharmacon ON-TARGETplus SMARTpool l-020067-00), human Smad4 (Dharmacon ON-TARGETplus SMARTpool L-003902-00), human SIK (Dharmacon ON-TARGETplus SMARTpool L-003959-00), human Smurf2 (Dharmacon ON-TARGETplus SMARTpool L-007194-00), or non-targeting control (Dharmacon ON-TARGETplus Non-targeting pool D-001810-10–20) was performed using siLentfect (Bio-Rad) transfection reagent. HaCaT cells were either transfected a single time with siRNAs for 36–48 h or transfected two times with a re-transfection after 18 h. Cells were cultured in DMEM containing 5% FBS before isolating the RNA. Adenoviral infections were performed as described (20) without any obvious signs of cytotoxicity, leading to a rate of 75–90% infected epithelial cells, as assessed by immunofluorescence microscopy. Infections with Ad-SIK usually followed on the next day after siRNA-mediated transfection and continued for 24 h before assays.
Real-time RT-PCR
HaCaT cells were treated and/or transfected as indicated in the figures before extraction of RNA using either RNeasy (Qiagen NORDIC, Sollentuna, Sweden) or the nucleic acid extractor NorDiag Arrow (CE, IVD) using the manufacturer's kit and protocol (NorDiag AB, Hägersten, Sweden). For the cycloheximide and actinomycin D experiments, the HaCaT cell culture was treated with 50 μm cycloheximide, 2 μg/ml actinomycin D (ActD), or the corresponding volume of vehicle (DMSO), which were added to the cells 1 h before the respective TGFβ stimulus per time point.
Measurements of mRNA expression were performed as described earlier (29). The primers used for PCR amplification were: human GAPDH, forward (5′-GGAGTCAACGGATTTGGTCGTA-3′) and reverse (5′-GGCAACAATATCCACTTTACCA-3′); human 5 S rRNA forward (5′-GGCCATACCACCCTGAACGC-3′) and reverse (5′-CAGCACCCGGTATTCCCAGG-3′); human Smad2 forward (5′-TGGCTGGCACCCTGCAACAG-3′) and reverse (5′-TGCCTTCGGTATTCTGCTCCCCA-3′); human Smad3 forward (5′-GCAATATTCCAGAGACCCCAC-3′) and reverse (5′-TAGGTTTGGAGAACCTGCGTC-3′); human Smad4 (forward, 5′-CATCCTGGACATTACTGGCCA-3′) and reverse, (5′-CCTACCTGAACGTCCATTTCAA-3′); human Smad7 (forward (5′-ACCCGATGGATTTTCTCAAACC-3′) and reverse (5′-GCCAGATAATTCGTTCCCCCT-3′); human SIK (forward 5′-CAACCTGGGCGACTACGATGAGCA-3′ and reverse (5′-GGGCGCACTGGGCATTCCGATACT-3′); human Smurf2 forward (5′-ACGCAACAAGGCCAGGTGTAT-3′) and reverse (5′-GGACCAAGCTCTTCACAATTGA-3′); human Gadd45B (forward 5′-GGGAAGGTTTTGGGCTCTCT-3′) and reverse (5′-CGGTCACCGTCCGCATCTT-3′); human (CDKN1A (p21) forward 5′-CTGCCCAAGCTCTACCTTCC-3′) and reverse (5′-CAGGTCCACATGGTCTTCCT-3′); human plasminogen activator inhibitor 1 (PAI1) forward (5′-GAGACAGGCAGCTCGGATTC-3′) and reverse (5′-GGCCTCCCAAAGTGCATTAC-3′); human fibronectin forward (5′-CATCGAGCGGATCTGGCCC-3′) and reverse (5′-GCAGCTGACTCCGTTGCCCA-3′).
Promoter Reporter Assays
The human SIK promoter-enhancer constructs were co-transfected with reporter plasmid pEGFP-N3 for normalization and Smad expression vectors as described in the figures in HEK-293T cells. The enhanced luciferase assay kit from BD Pharmingen was used. Normalized luciferase activity data are plotted in bar graphs representing the mean ± S.D. from triplicate samples.
Chromatin Immunoprecipitation (ChIP)
HaCaT cells were cultured in 10-cm plates to 80% confluence, and 1 plate was used per immunoprecipitation. Cells were fixed with 1% formaldehyde for 10 min at room temperature with swirling. Excess aldehyde was quenched with glycine, which was added to a final concentration of 0.125 m, and the incubation was continued for an additional 5 min. Cells were washed twice with ice-cold phosphate-buffered saline and harvested, and their pellets were resuspended in 1 ml of sodium dodecyl sulfate (SDS) lysis buffer (50 mm Tris-HCl (pH 8.1), 1% SDS, 10 mm EDTA, protease inhibitors (Complete EDTA-free protease inhibitors from Roche Diagnostics)). Samples were sonicated 3 times for 30 s each time (output H) at intervals of 30 s with a Diagenode Bioruptor sonicator.
Samples were then centrifuged at 14,000 rpm at 8 °C for 10 min. After removal of a control aliquot (whole-cell extract), supernatants were diluted 10-fold in ChIP dilution buffer (20 mm Tris-HCl, pH 8.0, 150 mm NaCl, 2 mm EDTA, 1% Triton X-100). Samples were incubated at 4 °C overnight in 2-methacryloyloxyethyl phosphorylcholine polymer-treated 15-ml polypropylene tubes (Assist) with anti-mouse IgG-Dynabeads that had been preincubated with 5 μg of antibodies in phosphate-buffered saline, 0.5% bovine serum albumin. The antibodies used were: mouse monoclonal anti-Smad2/Smad3 antibody (BD Transduction Laboratories), mouse monoclonal anti-Smad4 antibody (B8, Santa Cruz Biotechnology), or control, preimmune mouse immunoglobulin. The immunoprecipitated beads were then moved to 1.7-ml siliconized tubes (Corning Glass) and washed 5 times with ChIP wash buffer (50 mm HEPES-KOH (pH 7.0), 0.5 m LiCl, 1 mm EDTA, 0.7% deoxycholate, 1% Igepal CA630) and once with Tris-EDTA buffer (pH 8.0). Immunoprecipitated samples were eluted and reverse-cross-linked by incubation overnight at 65 °C in elution buffer (50 mm Tris-HCl (pH 8.0), 10 mm EDTA, 1% SDS). Genomic DNA was then extracted with a PCR purification kit (Qiagen).
The immunoprecipitated DNA was analyzed by quantitative PCR assay using specific primers for the human PAI-1 promoter region: forward (5′-GCAGGACATCCGGGAGAGA-3′) and reverse (5′-CCAATAGCCTTGGCCTGAGA-3′); for the human Smad7 promoter region, forward (5′-TGGGTTTCGCGGTGGCCATC-3′) and reverse (5′-CGCTCTCCTCCCCTTGCCCT-3′); for the human SIK enhancer region, forward (5′-CTGAGGTTGGCTGGGCATAAGTGTG-3′) and reverse (5′-TCAGACAGCCTCAAGCCACTAAGCC-3′); for the human β-globin (HBB) control region, forward (5′-AACGTGATCGCCTTTCTC-3′) and reverse (5′-GAAGCAGAACTCTGCACTTC-3′). The quantitative PCR protocol was 95 °C for 5 min followed by 39 cycles of 95 °C for 15 s, 60 °C for 1 min (or 65 °C for 1 min for the SIK sequences), and 95 °C for 15 s. Primer design and quantitative PCR conditions were according to the recently published ChIP analysis of Smad binding at a genome-wide level (35).
Immunoblotting and Immunoprecipitation Assays
SDS-PAGE, immunoblot, and co-immunoprecipitation analysis was as described (20, 34). Protein G-Sepharose was purchased from GE Healthcare and Dynabeads protein A from Invitrogen. For the endogenous co-immunoprecipitation experiment of Smad7, Smurf2, and ALK5 after anti-SIK immunoprecipitation, anti-FLAG (M5) IgG was used as the negative control (see Fig. 4C).
FIGURE 4.
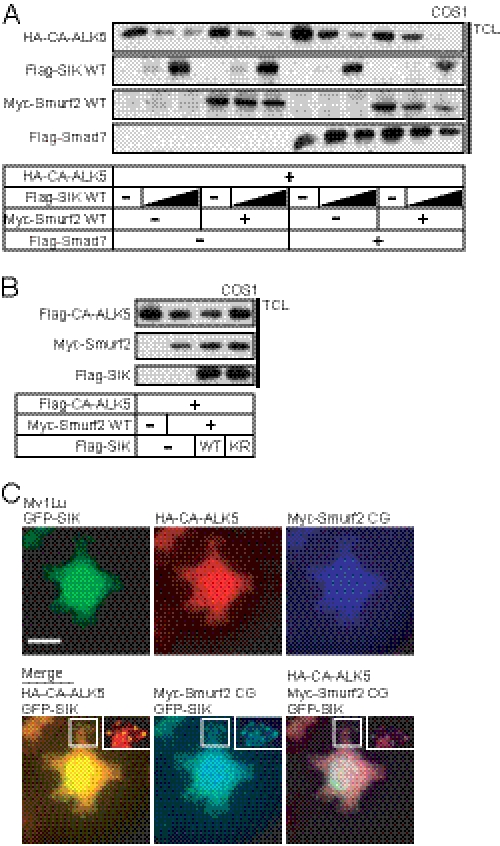
SIK forms protein complexes with Smad7 and Smurf2. A, TGFβ-induced Smurf2 mRNA expression is not dependent on de novo protein synthesis. Quantitative real-time RT-PCR analysis measuring Smurf2 mRNA levels with or without cycloheximide pretreatment for 1 h before stimulation with 5 ng/ml TGF-β for 1, 2, 4, 8, or 24 h is shown. The data are presented as in Fig. 1. B, co-precipitation of wild-type 6Myc-SIK and catalytically inactive Myc-Smurf2(CG) after immunoprecipitation of FLAG-Smad7 is shown. Total cell lysate (TCL) controls are shown. An asterisk indicates a nonspecific protein band. Note that the immunoprecipitation and total cell lysate proteins have been resolved on two different gels as illustrated by the size markers. C, shown is co-precipitation of endogenous Smad7, Smurf2, and ALK5 after immunoprecipitation (IP) of endogenous SIK in the absence (−) or presence (+) of TGFβ1 stimulation for 16 h. Immunoprecipitation with an unrelated immunoglobulin (IgG Ctrl) served as negative control. Total cell lysate controls are also shown, and GAPDH serves as the protein loading control. D, shown is co-precipitation of wild-type or kinase-dead (K56R) 6Myc-SIK and wild-type or catalytically inactive Myc-Smurf2(CG) after immunoprecipitation of wild-type FLAG-Smad7. Note that the two top immunoblots represent anti-Myc blots at two different exposure times. The top, long exposure shows the 6Myc-SIK, and the second, short exposure shows the Myc-Smurf2. Lane numbers are duplicated at the top and bottom of the immunoblots for convenience.
Immunofluorescence and Confocal Microscopy
Approximately 70% confluent-transfected Mv1Lu monolayers were analyzed by immunofluorescence 24 h post-transfection as described (29). Nuclei were counterstained with 4′,6′-diamidino-2-phenylindole (DAPI) or propidium iodide. A Zeiss Axiovert 200M confocal microscope equipped with LSM 510 laser was used with the Zeiss 63×/0.75 objective lens and photographing at ambient temperature in the presence of immersion oil. For the endogenous immunofluorescence experiments, MDA-MB-231 cells were cultured on standard 8-well glass plates before fixation, and photomicrographs were obtained by a Zeiss Axioplan 2 microscope with a Hammamatsu C4742–95 digital camera using the Zeiss Plan-neofluar 100×/01.4 Iris objective lens. For fluorescence microscopy of live NMuMG-Fucci cells growing on a culture dish, a Zeiss Axiovert 40 CFL with an AxioCam MRc digital camera was used using the Zeiss Plan-neofluar 10×/0.3 objective lens. Primary images were acquired with the camera's Volocity (MDA-MB-231 assays), QED Camera Plug-in v.1.1.6 (QED Imaging Inc.) (Mv1Lu assays) and AxioVision v4.8.2.0 (NMuMG-Fucci) software. Image memory content was reduced, and brightness-contrast was adjusted using Adobe Photoshop CS3 Extended.
Live Cell Cycle Analysis Assay
NMuMG-Fucci cells were transiently transfected with siRNAs twice followed by a transient adenoviral infection with control Ad-LacZ or Ad-SIK viruses as described above. Forty-eight hours after the first siRNA transfection and 24 h after the adenoviral infection and second siRNA transfection, cells were stimulated with vehicle or TGFβ1 for 56 h before fluorescence microscopy and image acquisition as explained above. The red and green fluorescent cells were counted using the ImageJ software (rsbweb.nih.gov) and are expressed as percent of red or green cells relative to the total number of cells counted. For each independent condition, two photomicrographs were captured by the microscope's camera, and two separate fields of 500 cells were counted. The numbers of cells were averaged among the two fields and the two independent photomicrographs to calculate the percentage of red or green cells.
RESULTS
Comparative Analysis of SIK and Smad7 mRNA Induction by TGFβ
We have previously shown that SIK down-regulates the TβRI/ALK5 in cooperation with Smad7 (29). Interestingly, both SIK and Smad7 are up-regulated relatively early after TGFβ stimulation, and their mRNAs showed a roughly 7-fold peak induction after 1 h of TGFβ stimulation in HaCaT cells (Fig. 1, A and B). The transcriptional induction of SIK mRNA has previously been seen in mouse mammary epithelial NMuMG cells (36), human breast cancer MDA-MB-468 cells (20), and in independent studies of transcriptomic responses to TGFβ (37, 38). We confirmed such previous reports beyond the HaCaT cell system by measuring induction of SIK and Smad7 mRNAs by TGFβ after 1 h of stimulation of human breast epithelial cells MCF10A (MI), their Ras-transformed derivatives (MII), and tumorigenic clones derived from the latter (MIII and MIV) and in the human metastatic breast cancer cell line MDA-MB-231 (supplemental Fig. 1, A and B).
FIGURE 1.
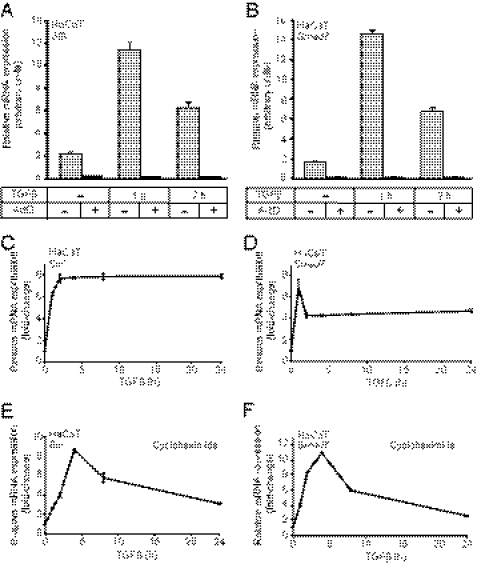
SIK and Smad7 mRNA induction by TGFβ is direct and independent of de novo protein synthesis. A and B, shown is quantitative real-time RT-PCR analysis measuring SIK (A) and Smad7 (B) mRNA levels with or without ActD pretreatment for 1 h before stimulation with 5 ng/ml TGFβ for 1 or 2 h. The data are plotted as the average mRNA levels with S.D. determined from triplicate measurements. C–F, shown is quantitative real-time RT-PCR analysis measuring SIK (C and E) and Smad7 (D and F) mRNA levels with (E and F) or without (C and D) cycloheximide pretreatment for 1 h before stimulation with 5 ng/ml TGFβ for 1, 2, 4, 8, or 24 h. The data are plotted as the average -fold induction of TGFβ-stimulated mRNA levels relative to unstimulated levels (0 h set to 1) with S.D. determined from triplicate measurements. In all RT-PCR assays, the specific mRNA level is normalized to the corresponding mRNA level of the housekeeping gene GAPDH, analyzed in the same mRNA preparation.
The increase on SIK and Smad7 mRNA levels depended on RNA polymerase II activity as ActD reduced the levels of each mRNA to background, and TGFβ was unable to exert any effect in the presence of ActD (Fig. 1, A and B). As control, ActD was shown to minimally or not at all affect expression of the 5 S rRNA that is transcribed by RNA polymerase III (supplemental Fig. 1C). TGFβ signaling did not appreciably alter 5 S rRNA levels as expected. These data confirm that TGFβ does not act by stabilizing the mRNA of SIK or Smad7.
Next, we compared the regulation of SIK and Smad7 mRNA levels over longer times of TGFβ stimulation (0–24 h) and examined whether both genes are direct targets of TGFβ/Smad signaling (Fig. 1, C–F). Both SIK and Smad7 mRNAs rapidly reached peak levels after 1–2 h stimulation, and their expression remained elevated above basal levels throughout the 24 h period (Fig. 1, C and D).
Treating HaCaT cells with cycloheximide before stimulation to block de novo protein synthesis did not inhibit SIK and Smad7 mRNA induction by TGFβ (Fig. 1, E and F). This indicates that both SIK and Smad7 are direct target genes of TGFβ signaling. Interestingly, however, the long term pattern of mRNA expression changed with the addition of cycloheximide. Both SIK and Smad7 mRNAs accumulated slower, reaching their peak levels at 4 h (Fig. 1, E and F). Also notable is that when de novo protein synthesis was blocked, neither of the two genes could uphold its plateau levels, as the peak of expression was followed by a slow but steady decline toward basal levels during the 24-h time course.
In summary, the rapid mRNA accumulation of SIK and Smad7 by TGFβ is independent of de novo protein synthesis (Fig. 1). This strongly suggests that both genes are direct targets of the TGFβ signaling pathway, and their regulation can be verified in all mouse and human cell models examined so far.
Smad-dependent Transcriptional Regulation of SIK in Response to TGFβ
The direct effect of TGFβ on SIK gene expression (Fig. 1) suggested that Smad signaling might be responsible for this regulation. Knockdown of each Smad protein of the TGFβ pathway in HaCaT cells showed that Smad2 and Smad3 as well as Smad4 contribute to the up-regulation of SIK and Smad7 mRNAs (Fig. 2, A and B). We confirmed the efficiency of Smad knockdown by measuring their respective mRNA level (Fig. 2, C–E) and the corresponding protein level (Fig. 2, F and G). It is worth noting that the Smad3 siRNA pool used was less efficient compared with the Smad2 and Smad4 siRNA pools. Despite this, the knockdown of Smad3 had a strong impact on SIK and Smad7 gene expression (Fig. 2, A and B). Thus, quantitatively Smad3 and Smad4 have a larger impact on the induction of SIK and Smad7 mRNA than Smad2. The contribution of each Smad to the induction of SIK and Smad7 mRNA seems to be similar between these two genes, suggesting that the same organization of Smad complexes might regulate their enhancers/promoters.
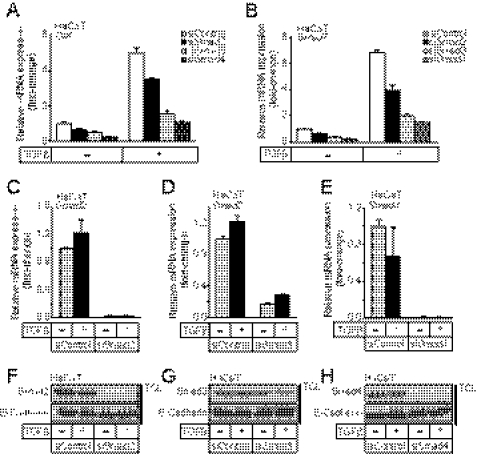
FIGURE 2.
TGFβ-induced SIK and Smad7 mRNA expression is dependent on Smad signaling. A and B, quantitative real-time RT-PCR analysis measured the effects of Smad2, Smad3, and Smad4 siRNA knock-down on SIK (A) and Smad7 (B) mRNA levels with (+) or without (−) stimulation with 5 ng/ml TGFβ for 2 h. C–E, shown is confirmation of the knock-down levels of Smad2 (C), Smad3 (D), and Smad4 (E) mRNA analyzed in panels A and B by real-time RT-PCR. The data are plotted as the average -fold induction of TGFβ-stimulated mRNA levels (+TGFβ) relative to unstimulated levels (control set to 1), with S.E. determined from triplicate measurements. F–H, shown is confirmation of the knockdown levels of Smad2 (F), Smad3 (G), and Smad4 (H) protein analyzed in panels A and B by immunoblotting of total cell lysates (TCL) from parallel HaCaT cultures using Smad-specific antibodies and E-cadherin antibody to verify equal protein loading.
We then verified the presence of Smad complexes on a SIK enhancer region residing downstream of the 3′ end of the SIK gene (Fig. 3). This region was previously identified in a genome-wide screen for TGFβ-induced Smad2/3 binding using ChIP-chip analysis in HaCaT cells (35). Immunoprecipitation of Smad2/Smad3 revealed recruitment of these Smads to the SIK enhancer under control conditions, and this recruitment was enhanced ∼2.7-fold after TGFβ stimulation (Fig. 3A). This recruitment was specific to the SIK enhancer as shown by a lack of Smad2/3 recruitment to the control HBB gene sequence (Fig. 3B). Smad2/3 binding to the SIK enhancer exhibited similar pattern to those of the well characterized promoters of the PAI-1 and Smad7 genes (Fig. 3, C and D). A similar experiment with a Smad4-specific antibody confirmed the above result (supplemental Fig. 2). A clear TGFβ-dependent enrichment of SIK enhancer chromatin was measured in the Smad4 immunocomplexes (supplemental Fig. 2A), similar to the PAI-1 promoter enrichment (supplemental Fig. 2C) and unlike the negative control HBB sequence (supplemental Fig. 2B).
To confirm the endogenous ChIP data on the role of the SIK enhancer, we cloned the human SIK promoter and enhancer into a luciferase reporter construct (Fig. 3E) and performed promoter activation experiments in transfected HEK-293T cells. As predicted from the Smad-binding ChIP assays, the upstream promoter could be activated weakly by TGFβ and/or co-transfection of Smad3 and Smad4 (Fig. 3, F and G). However, a luciferase construct that carried both the SIK promoter and the enhancer cloned downstream from the luciferase cDNA showed higher basal activity (Fig. 3F) and responded better to TGFβ and/or Smad3/Smad4 co-transfection (Fig. 3, F and G).
In conclusion, this analysis demonstrates that TGFβ signaling sends the Smad complex to the 3′ intergenic enhancer of the SIK gene. This mechanism of gene regulation can only be partially recapitulated in vitro after cloning the enhancer element downstream of the basic promoter unit of this gene, which implies that additional regulatory sequences mediating TGFβ responses may exist on the SIK gene.
Smurf2 Forms Complexes with SIK and Smad7
We have previously investigated the cooperation between SIK and Smad7 in down-regulating the TβRI/ALK5 receptor (29); however, the mechanism whereby SIK works together with Smad7 has not been clear. A possible link could be the E3 ubiquitin ligase Smurf2, which is known to be induced upon TGFβ stimulation, interacts with ALK5 and Smad7, and targets ALK5 receptors for ubiquitination (8, 9). We verified that Smurf2 mRNA is rapidly but weakly up-regulated by TGFβ signaling in HaCaT cells even in the presence of cycloheximide (Fig. 4A). Furthermore, the weak but reproducible induction of Smurf2 mRNA by TGFβ could also be verified in three (MI, MII, MIV) of the five breast cancer cell lines tested (supplemental Fig. 3A).
Interaction between Smad7 and SIK has been shown previously (29). Here we further investigated a possible complex formation between Smad7, Smurf2, and SIK. First, we immunoprecipitated FLAG-Smad7 and confirmed that it could bind both 6Myc-SIK and catalytically inactive Myc-Smurf2 (C716G), which was used to avoid strong degradation of the proteins in the complex (Fig. 4B). However, immunoblotting with the Myc antibody suggested a weaker binding of SIK to Smad7 compared with binding of Smad7 to Smurf2. Next, we also tested whether Smurf2 could interact with both SIK and Smad7. Indeed, co-immunoprecipitation experiments with catalytically inactive Smurf2 (C716G), 6Myc-SIK, and FLAG-Smad7 showed that they interacted also when Smurf2 was immunoprecipitated (supplemental Fig. 3B). Using our homemade anti-SIK antibody and HaCaT cell extracts, we immunoprecipitated endogenous SIK from cells stimulated with vehicle or TGFβ (Fig. 4C). In the absence of TGFβ stimulation we observed a complex with endogenous Smad7 and ALK5. After TGFβ stimulation, the complex between SIK, Smad7, and ALK5 was again visible, and it now had also incorporated Smurf2. This experiment also demonstrated the induction of endogenous protein levels of SIK, Smad7, and Smurf2 by TGFβ and the concomitant down-regulation of the ALK5 receptor (Fig. 4C).
We then examined the role of the kinase activity of SIK on formation of protein complexes between Smad7, Smurf2, and SIK (Fig. 4D). Smad7 and SIK interacted irrespective of the kinase activity of SIK (Fig. 4D, lanes 4 and 7). The protein complex between SIK and Smad7 was weakly enhanced by the presence of wild-type Smurf2 (Fig. 4D, lane 5); however, the addition of catalytically inactive Smurf2(C716G) dramatically enhanced the complex between the three proteins (Fig. 4D, lane 6) at equal expression levels of wild-type and mutant Smurf2 (Fig. 4D). Surprisingly, when the same co-immunoprecipitation experiment was repeated with the SIK(K56R) mutant instead of wild-type kinase, the ability of Smurf2(C716G) to promote an enhanced SIK/Smad7/Smurf2 complex was reduced (Fig. 4D, lanes 8 and 9). This suggests that the catalytic activity of SIK has an impact on formation of the complex between these three proteins. Lack of strongly enhanced protein complex accumulation by the wild-type Smurf2 is most likely due to the rapid dissociation or degradation caused by the recruitment of active Smurf2 into this complex, making it difficult to visualize the dynamics of this protein complex. Overall, these biochemical experiments suggested that SIK, Smad7, and Smurf2 are induced by TGFβ signaling and can engage with each other in mutual complexes.
SIK Cooperates with Smurf2 and Smad7 to Down-regulate TβRI/ALK5 Receptor
The interaction data suggested that the two enzymes, SIK and Smurf2, cooperate or depend on each other during TGFβ receptor down-regulation. We tested this possibility by co-expressing wild-type SIK and Smurf2 (Fig. 5A). Increasing levels of SIK led to down-regulation of the constitutively active (CA) ALK5 receptor and Smurf2 combined with wild-type SIK had the same effect. Combining Smad7 with SIK had a similar effect on receptor down-regulation, but when we co-expressed SIK, Smurf2, and Smad7, we then observed essentially complete loss of the receptor (Fig. 5A). In a similar experiment where the level of Smurf2 was increased, we observed the same cooperation between SIK and Smurf2 on ALK5 receptor down-regulation (supplemental Fig. 4A). SIK also led to a significant down-regulation of Smurf2, and the presence of Smad7 enhanced this effect.
FIGURE 5.
SIK cooperates with Smurf2 in down-regulation of the ALK5 receptor. A, shown is the immunoblot analysis of CA-ALK5 protein stability in COS-1 cells co-expressing the indicated combinations of wild-type Smurf2 and Smad7 constructs in the absence or presence of two doses of wild-type SIK (triangles). TCL, total cell lysates. B, shown is immunoblot analysis of CA-ALK5 protein levels in COS-1 cells co-transfected with the indicated combinations of wild-type Smurf2 and wild-type or kinase inactive (KR) mutant SIK. C, shown is triple immunofluorescence analysis of wild-type GFP-SIK, CA-ALK5, and catalytically inactive Smurf2(CG) in transfected Mv1Lu cells. Insets show higher magnifications of peripheral punctate structures just below the plasma membrane. The bar represents 10 μm.
We examined the role of the kinase activity of SIK for TβRI/ALK5 receptor down-regulation and its cooperation with Smurf2 (Fig. 5B). Catalytically inactive SIK(K56R) blocked the effects of Smurf2 on receptor down-regulation (Fig. 5B). This implies that Smurf2 down-regulates the TGFβ receptor more effectively when it cooperates with a catalytically active SIK.
Smurf2 resides in the nucleus and moves together with Smad7 to the cytoplasm in response to TGFβ to reach the ALK5 receptor (9). SIK shuttles between the cytoplasm and the nucleus, and its localization can be regulated by steroids or by the 14-3-3 adaptor protein (25, 39). Using immunofluorescence experiments, we investigated localization of SIK, Smurf2, and ALK5. In transfected, TGFβ-sensitive Mv1Lu cells, SIK showed both nuclear and cytoplasmic distribution, as expected but additionally localized close to the plasma membrane in pronounced punctated clusters (Fig. 5C). Co-localization of SIK, CA-ALK5, and Smurf2 was observed in these peripheral clusters (Fig. 5C, insets), with no obvious co-localization in the more diffuse pattern scattered in the rest of the cell body. Thus, SIK, Smurf2, and ALK5 may be able to form complexes in cytoplasmic regions proximal to the plasma membrane. We also attempted to perform the co-localization experiments at the endogenous level. We were hampered from succeeding in this aim as all of our antibodies that gave positive and specific results with endogenous proteins are raised in rabbits and thus prohibit us from performing double or triple immunofluorescence experiments. Despite this, single antibody experiments verified TGFβ-induced expression and distribution of endogenous SIK, Smad7, and Smurf2 in the nucleus and cytoplasm of HaCaT (not shown) and MDA-MB-231 cells (supplemental Fig. 4B). In conclusion, the evidence so far supports the existence of protein complexes between SIK, Smad7, and Smurf2 that could initiate the process of receptor turnover.
Functional Cooperation of SIK and Smurf2 in Regulation of Endogenous TGFβ Signaling
To further investigate the effects of endogenous SIK and Smurf2 on TGFβ signaling, we performed siRNA knockdowns and subsequently analyzed established cellular responses to TGFβ. The efficiency of silencing of SIK, Smurf2, or both was significant at both mRNA and protein levels (supplemental Fig. 5). We first analyzed the mRNA levels of well established target genes of TGFβ, such as p21, Smad7, Gadd45β, fibronectin, and PAI-1 (Fig. 6A, and supplemental Fig. 6). When SIK, Smurf2, or both were silenced, all these target genes showed enhanced magnitude of response to TGFβ (Fig. 6A, supplemental Fig. 6). We did not observe any gene-specific differences in terms of the effect of silencing of SIK or Smurf2 on this set of genes, which is compatible with a role of SIK and Smurf2 at the receptor level. Overall, the observed enhancement in gene responses was similar after SIK or Smurf2 silencing (Fig. 6A, supplemental Fig. 6). Interestingly, during simultaneous silencing of both SIK and Smurf2, we observed similar gene responses as after single silencing (Fig. 6A, supplemental Fig. 6). The latter result is compatible with the model that SIK and Smurf2 participate in the same linear pathway or possibly act as part of one and the same functional protein complex as suggested by the biochemical evidence.
FIGURE 6.
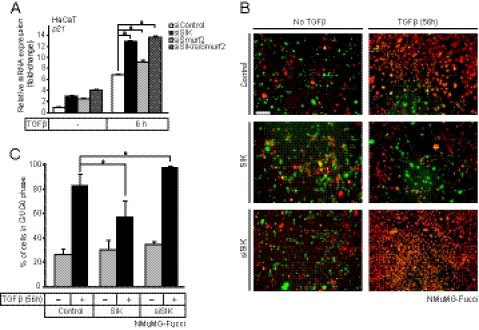
Endogenous SIK regulates epithelial growth arrest by TGFβ signaling. A, quantitative real-time RT-PCR analysis measuring the effects of SIK, Smurf2, and combined SIK/Smurf2 siRNA knockdown on the cell cycle inhibitor gene p21 mRNA levels in HaCaT cells with or without (−) stimulation with 5 ng/ml TGF-β1 for 6 h. QRT-PCR analysis was performed and are presented as in Fig. 1. Asterisks indicate significant differences determined by Student's t test with significance at p < 0.01. B, shown is live fluorescence microscopy of mammary epithelial NMuMG-Fucci cells transiently transfected with control scrambled siRNA followed by infection with control Ad-LacZ virus (control, upper panels), transiently transfected with specific siRNA targeting SIK (siSIK, bottom panels) or transiently infected with an adenoviral vector expressing SIK (middle panels). The cells were treated with vehicle (no TGFβ) or 5 ng/ml TGFβ1 for 56 h before photography. A bar indicates 20 μm. C, shown is quantitative analysis of the cell cycle from experiments such as that shown in panel B. The number of red cells (G1/G0 phase) were counted in duplicate photos from two independent experiments from each experimental condition and are plotted as % relative to the total number of cells. Statistical significance between conditions is shown with a asterisk that indicates p < 0.05.
One of the hallmarks of biological TGFβ responses in epithelial cells is the cell cycle arrest at the early G1 phase mediated by transcriptional induction of cell cycle inhibitors such as p21 (40). We, therefore, examined the impact of SIK on TGFβ-mediated epithelial cell growth arrest using the well established model of mouse mammary epithelial NMuMG cells. We employed a stable clone of NMuMG that expresses two fluorescent proteins providing the cell with a fluorescent ubiquitination-based cell cycle indication (Fucci) (33). In this system the green fluorescent protein mAG fused to geminin marks cells in S/M/G2 phases, whereas the red fluorescent protein mKO2 fused to Cdt1 marks cells in G1/G0 phases. The cells were either transiently transfected with siRNA against endogenous SIK or with an adenoviral vector expressing SIK (Fig. 6B). TGFβ clearly induced cell cycle arrest in control cells as it shifted the percentage of cells in G1/G0 from 25 to 80% (Fig. 6C). SIK overexpression via the adenoviral vector had minor effects on the basal level of cycling cells but had a strong negative effect on the response to TGFβ, reducing the cell cycle arrested cells from 80 to 58%. Conversely, silencing the endogenous SIK led again to minimal basal effects, but essentially 98% of the cells in multiple cultures became arrested in G1/G0 (Fig. 6, B and C). The above data collectively demonstrate that SIK mediates a significant negative regulatory effect that appears to be specific to TGFβ, and for SIK to elicit this function, complementation with additional inhibitor proteins of the TGFβ pathway is required.
DISCUSSION
SIK has been shown to be a negative regulator of TGFβ receptor signaling (29). Here we provide insights into the mechanisms of (i) TGFβ-induced SIK gene expression and (ii) TGFβ type I receptor down-regulation by SIK (Fig. 7). We describe that (a) the SIK gene is a direct target of TGFβ/Smad signaling, and its protein product participates in protein complexes to down-regulate ALK5, (b) the kinase activity of SIK and the ubiquitin ligase activity of Smurf2 affect the dynamics of protein complexes with Smad7, and both are required for optimal ALK5 down-regulation, and (c) the regulation of receptor levels has an immediate impact on physiological signaling by TGFβ, and this includes several genes and the cytostatic response.
FIGURE 7.
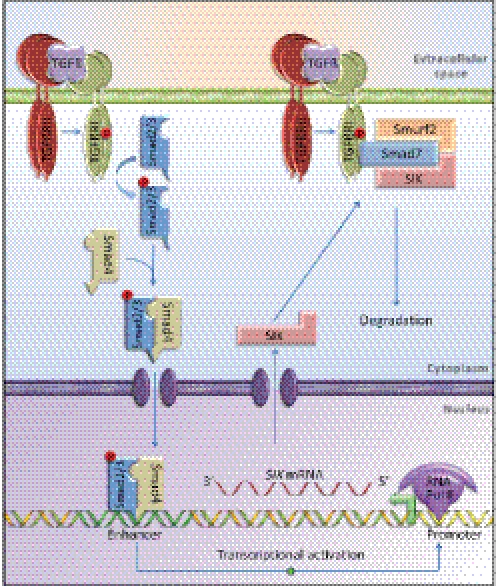
The role of SIK during TGFβ signaling. Shown is a graphic presentation of the TGFβ receptor-Smad pathway leading to transcriptional induction of the SIK gene in the nucleus and formation of a protein complex involving SIK, Smad7, Smurf2, and TβRI/ALK5. The hetero-tetrameric TβRII/TβRI receptor complex is shown bound to extracellular TGFβ. A circled P indicates established phosphorylation events. The individual Smad2 and Smad3 proteins are shown as a single Smad2/3 molecule for simplicity. On the DNA double helix, the promoter and enhancer sequences are indicated with distinct coloration, the promoter is shown occupied by the RNA polymerase II (Pol II), and the TSS of the gene is illustrated as a green arrow pointing in the direction of transcription.
SIK and Smad7 exhibit both direct (cycloheximide-insensitive/actinomycin D-sensitive) early transcriptional peaks and prolonged profiles of sustained expression that are indirect (cycloheximide-sensitive/actinomycin D-sensitive) (Fig. 1). It is possible that newly synthesized Smads are required for the expression of SIK and Smad7 over long periods of time. More likely, sustained SIK and Smad7 induction by TGFβ requires the synthesis of additional transcriptional cofactors or regulators of mRNA processing, as has been previously established for other genes responding to TGFβ signaling (40). At this stage we have not yet identified putative regulatory proteins whose synthesis is required for the sustained SIK or Smad7 expression.
The regulation of SIK gene expression by TGFβ was previously found to be dependent on Smad4, based on genome-wide studies in the Smad4-deficient breast cancer cell line MDA-MB-468 (20). Here we established that all three Smads of the TGFβ pathway, Smad2, Smad3, and Smad4, contribute to the accumulation of SIK and Smad7 mRNA in response to TGFβ (Fig. 2). This is an important finding, as previous genome-wide attempts to define the contribution of each one of the TGFβ pathway Smads to target gene expression have not delivered clear conclusions due to differences in the technical platforms used or differences in the cell models used (35, 41–43). Finally, ChIP assays at the endogenous level and cloned promoter assays in transfected cells established that at least one Smad-sensitive genomic region resides in the 3′ direction of the SIK gene (Fig. 3). This enhancer element seems to account for a significant part of the SIK gene response to TGFβ; however, the experimental evidence suggests the existence of additional TGFβ-responding regulatory sequences on this gene.
The mechanism by which SIK acts on TβRI/ALK5 clearly involves the adaptor protein Smad7 (29) and the E3 ubiquitin ligase Smurf2 (Figs. 4 and 5). Because Smurf2 ubiquitination activity has been linked to the regulation of Smad protein stability and function (4), it is possible that SIK might also regulate Smad protein function and turnover in addition to the regulation of the TGFβ type I receptor.
An important event during the cooperative action of these proteins toward TβRI/ALK5 seems to be phosphorylation by SIK, as its kinase activity is critical for receptor down-regulation. At this stage we do not know the substrate(s) of the SIK kinase in the TβRI-Smad7-Smurf2 complex. One possibility is that formation and function of this protein complex requires phosphorylation by SIK. Alternatively, SIK-mediated phosphorylation might promote receptor trafficking to lysosomes/proteasomes for degradation. The latter model is compatible with our unpublished evidence, which does not support a role of SIK in inducing ALK5 ubiquitination. Rather, SIK recognizes ubiquitinated Smad7 (or other proteins) via its ubiquitin-associated domain and localizes in proteasome-rich locations (29). Future work in the direction of understanding the role of SIK-mediated phosphorylation during TGFβ receptor internalization and degradation is warranted.
Altering the levels of SIK in epithelial cells clearly showed an impact on cell cycle regulation by TGFβ (Fig. 6). However, we failed to observe the effects on the cell cycle in the absence of TGFβ signaling (Fig. 6C), suggesting that SIK may not play an important functional role in regulating the cell cycle but, rather, acts as a regulator of other pathways, such as TGFβ, that feed into the control of cell division.
In summary, SIK provides molecular means for multifunctional regulation of the TGFβ receptor-Smad7 complex (Fig. 7). More work into the mechanistic details of SIK-mediated TGFβ receptor regulation could uncover novel targets for therapeutic intervention against the TGFβ pathway.
Supplementary Material
Acknowledgments
We thank D. Koinuma (Tokyo University) for assistance with Smad2/3 ChIP analysis and mapping of the SIK enhancer, S. Johansson (Uppsala University, Sweden) and T. Imamura (Ehime University, Japan) for the NMuMG-Fucci cells and introduction to this technology, and F. R. Miller (Fred Hutchinson Cancer Center, Seattle) for the MCF10A series of cell lines. We also thank members of our Institute for assistance and suggestions during the course of this work.
The work was supported by the Ludwig Institute for Cancer Research, the Swedish Cancer Society (project 4855-B03-01XAC), the Natural Sciences Foundation of Sweden (project K2007-66X-14936-04-03 and K2010-67X-14936-07-03), and the Strategic Japanese-Swedish Cooperative Program “Multidisciplinary BIO” (Project 26575-1) supported by the Swedish Agency for Innovation Systems and the Swedish Foundation for Strategic Research.

This article contains supplemental Figs. 1–6.
- TβRI and TβRII
- TGFβ type I and II, respectively
- ALK
- activin receptor-like kinase
- CA
- constitutively active
- SIK
- salt-inducible kinase
- CREB
- cAMP-responsive element-binding protein
- TORC
- transducer of regulated CREB activity
- TSS
- transcriptional start site
- ActD
- actinomycin D
- HBB
- human β-globin
- PAI
- plasminogen activator inhibitor 1.
REFERENCES
- 1. Moustakas A., Heldin C. H. (2009) The regulation of TGFβ signal transduction. Development 136, 3699–3714 [DOI] [PubMed] [Google Scholar]
- 2. Feng X. H., Derynck R. (2005) Specificity and versatility in TGF-β signaling through Smads. Annu. Rev. Cell Dev. Biol. 21, 659–693 [DOI] [PubMed] [Google Scholar]
- 3. Itoh S., ten Dijke P. (2007) Negative regulation of TGF-β receptor/Smad signal transduction. Curr. Opin. Cell Biol. 19, 176–184 [DOI] [PubMed] [Google Scholar]
- 4. Lönn P., Morén A., Raja E., Dahl M., Moustakas A. (2009) Regulating the stability of TGFβ receptors and Smads. Cell Res. 19, 21–35 [DOI] [PubMed] [Google Scholar]
- 5. Nakao A., Afrakhte M., Morén A., Nakayama T., Christian J. L., Heuchel R., Itoh S., Kawabata M., Heldin N. E., Heldin C. H., ten Dijke P. (1997) Identification of Smad7, a TGFβ-inducible antagonist of TGFβ signaling. Nature 389, 631–635 [DOI] [PubMed] [Google Scholar]
- 6. Ohashi N., Yamamoto T., Uchida C., Togawa A., Fukasawa H., Fujigaki Y., Suzuki S., Kitagawa K., Hattori T., Oda T., Hayashi H., Hishida A., Kitagawa M. (2005) Transcriptional induction of Smurf2 ubiquitin ligase by TGF-β. FEBS Lett. 579, 2557–2563 [DOI] [PubMed] [Google Scholar]
- 7. Hayashi H., Abdollah S., Qiu Y., Cai J., Xu Y. Y., Grinnell B. W., Richardson M. A., Topper J. N., Gimbrone M. A., Jr., Wrana J. L., Falb D. (1997) The MAD-related protein Smad7 associates with the TGFβ receptor and functions as an antagonist of TGFβ signaling. Cell 89, 1165–1173 [DOI] [PubMed] [Google Scholar]
- 8. Ebisawa T., Fukuchi M., Murakami G., Chiba T., Tanaka K., Imamura T., Miyazono K. (2001) Smurf1 interacts with transforming growth factor-β type I receptor through Smad7 and induces receptor degradation. J. Biol. Chem. 276, 12477–12480 [DOI] [PubMed] [Google Scholar]
- 9. Kavsak P., Rasmussen R. K., Causing C. G., Bonni S., Zhu H., Thomsen G. H., Wrana J. L. (2000) Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGF β receptor for degradation. Mol. Cell 6, 1365–1375 [DOI] [PubMed] [Google Scholar]
- 10. Inoue Y., Imamura T. (2008) Regulation of TGF-β family signaling by E3 ubiquitin ligases. Cancer Sci. 99, 2107–2112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Osmundson E. C., Ray D., Moore F. E., Gao Q., Thomsen G. H., Kiyokawa H. (2008) The HECT E3 ligase Smurf2 is required for Mad2-dependent spindle assembly checkpoint. J. Cell Biol. 183, 267–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yamashita M., Ying S. X., Zhang G. M., Li C., Cheng S. Y., Deng C. X., Zhang Y. E. (2005) Ubiquitin ligase Smurf1 controls osteoblast activity and bone homeostasis by targeting MEKK2 for degradation. Cell 121, 101–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang C., Rajfur Z., Yousefi N., Chen Z., Jacobson K., Ginsberg M. H. (2009) Talin phosphorylation by Cdk5 regulates Smurf1-mediated talin head ubiquitylation and cell migration. Nat. Cell Biol. 11, 624–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Narimatsu M., Bose R., Pye M., Zhang L., Miller B., Ching P., Sakuma R., Luga V., Roncari L., Attisano L., Wrana J. L. (2009) Regulation of planar cell polarity by Smurf ubiquitin ligases. Cell 137, 295–307 [DOI] [PubMed] [Google Scholar]
- 15. Sahai E., Garcia-Medina R., Pouysségur J., Vial E. (2007) Smurf1 regulates tumor cell plasticity and motility through degradation of RhoA leading to localized inhibition of contractility. J. Cell Biol. 176, 35–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schwamborn J. C., Müller M., Becker A. H., Püschel A. W. (2007) Ubiquitination of the GTPase Rap1B by the ubiquitin ligase Smurf2 is required for the establishment of neuronal polarity. EMBO J. 26, 1410–1422 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17. Wang H. R., Zhang Y., Ozdamar B., Ogunjimi A. A., Alexandrova E., Thomsen G. H., Wrana J. L. (2003) Regulation of cell polarity and protrusion formation by targeting RhoA for degradation. Science 302, 1775–1779 [DOI] [PubMed] [Google Scholar]
- 18. Fukunaga E., Inoue Y., Komiya S., Horiguchi K., Goto K., Saitoh M., Miyazawa K., Koinuma D., Hanyu A., Imamura T. (2008) Smurf2 induces ubiquitin-dependent degradation of Smurf1 to prevent migration of breast cancer cells. J. Biol. Chem. 283, 35660–35667 [DOI] [PubMed] [Google Scholar]
- 19. Jin C., Yang Y. A., Anver M. R., Morris N., Wang X., Zhang Y. E. (2009) Smad ubiquitination regulatory factor 2 promotes metastasis of breast cancer cells by enhancing migration and invasiveness. Cancer Res. 69, 735–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kowanetz M., Valcourt U., Bergström R., Heldin C. H., Moustakas A. (2004) Id2 and Id3 define the potency of cell proliferation and differentiation responses to transforming growth factor β and bone morphogenetic protein. Mol. Cell. Biol. 24, 4241–4254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jaleel M., Villa F., Deak M., Toth R., Prescott A. R., Van Aalten D. M., Alessi D. R. (2006) The ubiquitin-associated domain of AMPK-related kinases regulates conformation and LKB1-mediated phosphorylation and activation. Biochem. J. 394, 545–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ruiz J. C., Conlon F. L., Robertson E. J. (1994) Identification of novel protein kinases expressed in the myocardium of the developing mouse heart. Mech. Dev. 48, 153–164 [DOI] [PubMed] [Google Scholar]
- 23. Romito A., Lonardo E., Roma G., Minchiotti G., Ballabio A., Cobellis G. (2010) Lack of sik1 in mouse embryonic stem cells impairs cardiomyogenesis by down-regulating the cyclin-dependent kinase inhibitor p57kip2. PLoS One 5, e9029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Berdeaux R., Goebel N., Banaszynski L., Takemori H., Wandless T., Shelton G. D., Montminy M. (2007) SIK1 is a class II HDAC kinase that promotes survival of skeletal myocytes. Nat. Med. 13, 597–603 [DOI] [PubMed] [Google Scholar]
- 25. Okamoto M., Takemori H., Katoh Y. (2004) Salt-inducible kinase in steroidogenesis and adipogenesis. Trends Endocrinol. Metab. 15, 21–26 [DOI] [PubMed] [Google Scholar]
- 26. Sjöström M., Stenström K., Eneling K., Zwiller J., Katz A. I., Takemori H., Bertorello A. M. (2007) SIK1 is part of a cell sodium-sensing network that regulates active sodium transport through a calcium-dependent process. Proc. Natl. Acad. Sci. U.S.A. 104, 16922–16927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Takemori H., Kajimura J., Okamoto M. (2007) TORC-SIK cascade regulates CREB activity through the basic leucine zipper domain. FEBS J. 274, 3202–3209 [DOI] [PubMed] [Google Scholar]
- 28. Dentin R., Liu Y., Koo S. H., Hedrick S., Vargas T., Heredia J., Yates J., 3rd, Montminy M. (2007) Insulin modulates gluconeogenesis by inhibition of the coactivator TORC2. Nature 449, 366–369 [DOI] [PubMed] [Google Scholar]
- 29. Kowanetz M., Lönn P., Vanlandewijck M., Kowanetz K., Heldin C. H., Moustakas A. (2008) TGFβ induces SIK to negatively regulate type I receptor kinase signaling. J. Cell Biol. 182, 655–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lanjuin A., Sengupta P. (2002) Regulation of chemosensory receptor expression and sensory signaling by the KIN-29 Ser/Thr kinase. Neuron 33, 369–381 [DOI] [PubMed] [Google Scholar]
- 31. Maduzia L. L., Roberts A. F., Wang H., Lin X., Chin L. J., Zimmerman C. M., Cohen S., Feng X. H., Padgett R. W. (2005) C. elegans serine-threonine kinase KIN-29 modulates TGFβ signaling and regulates body size formation. BMC Dev. Biol. 5, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Santner S. J., Dawson P. J., Tait L., Soule H. D., Eliason J., Mohamed A. N., Wolman S. R., Heppner G. H., Miller F. R. (2001) Malignant MCF10CA1 cell lines derived from premalignant human breast epithelial MCF10AT cells. Breast Cancer Res. Treat. 65, 101–110 [DOI] [PubMed] [Google Scholar]
- 33. Sakaue-Sawano A., Kurokawa H., Morimura T., Hanyu A., Hama H., Osawa H., Kashiwagi S., Fukami K., Miyata T., Miyoshi H., Imamura T., Ogawa M., Masai H., Miyawaki A. (2008) Visualizing spatiotemporal dynamics of multicellular cell cycle progression. Cell 132, 487–498 [DOI] [PubMed] [Google Scholar]
- 34. Morén A., Imamura T., Miyazono K., Heldin C. H., Moustakas A. (2005) Degradation of the tumor suppressor Smad4 by WW and HECT domain ubiquitin ligases. J. Biol. Chem. 280, 22115–22123 [DOI] [PubMed] [Google Scholar]
- 35. Koinuma D., Tsutsumi S., Kamimura N., Taniguchi H., Miyazawa K., Sunamura M., Imamura T., Miyazono K., Aburatani H. (2009) Chromatin immunoprecipitation on microarray analysis of Smad2/3 binding sites reveals roles of ETS1 and TFAP2A in transforming growth factor β signaling. Mol. Cell. Biol. 29, 172–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Valcourt U., Kowanetz M., Niimi H., Heldin C. H., Moustakas A. (2005) TGF-β and the Smad signaling pathway support transcriptomic reprogramming during epithelial-mesenchymal cell transition. Mol. Biol. Cell 16, 1987–2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen C. R., Kang Y., Massagué J. (2001) Defective repression of c-myc in breast cancer cells. A loss at the core of the transforming growth factor β growth arrest program. Proc. Natl. Acad. Sci. U.S.A. 98, 992–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kang Y., Chen C. R., Massagué J. (2003) A self-enabling TGFβ response coupled to stress signaling. Smad engages stress response factor ATF3 for Id1 repression in epithelial cells. Mol. Cell 11, 915–926 [DOI] [PubMed] [Google Scholar]
- 39. Al-Hakim A. K., Göransson O., Deak M., Toth R., Campbell D. G., Morrice N. A., Prescott A. R., Alessi D. R. (2005) 14-3-3 cooperates with LKB1 to regulate the activity and localization of QSK and SIK. J. Cell Sci. 118, 5661–5673 [DOI] [PubMed] [Google Scholar]
- 40. Massagué J., Gomis R. R. (2006) The logic of TGFβ signaling. FEBS Lett. 580, 2811–2820 [DOI] [PubMed] [Google Scholar]
- 41. Deckers M., van Dinther M., Buijs J., Que I., Löwik C., van der Pluijm G., ten Dijke P. (2006) The tumor suppressor Smad4 is required for transforming growth factor β-induced epithelial to mesenchymal transition and bone metastasis of breast cancer cells. Cancer Res. 66, 2202–2209 [DOI] [PubMed] [Google Scholar]
- 42. Levy L., Hill C. S. (2005) Smad4 dependency defines two classes of transforming growth factor β (TGF-β) target genes and distinguishes TGF-β-induced epithelial-mesenchymal transition from its antiproliferative and migratory responses. Mol. Cell. Biol. 25, 8108–8125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yang Y. C., Piek E., Zavadil J., Liang D., Xie D., Heyer J., Pavlidis P., Kucherlapati R., Roberts A. B., Böttinger E. P. (2003) Hierarchical model of gene regulation by transforming growth factor β. Proc. Natl. Acad. Sci. U.S.A. 100, 10269–10274 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.