Background: Neuropeptide FF2 receptors interact with μ-opioid receptors and decrease their activity.
Results: The phosphorylation pattern of MOP receptor is similar after homologous (DAMGO) and heterologous (neuropeptide FF) stimulation.
Conclusion: GPCR kinase 2 (GRK2) contributes to the NPFF-induced phosphorylation and loss of function of MOP receptor.
Significance: GRK2-mediated transphosphorylation within receptor heteromers could play a role in heterologous desensitization of GPCRs.
Keywords: 7-Helix Receptor, Neuropeptide, Opiate Opioid, Phosphorylation, Signal Transduction
Abstract
Neuropeptide FF (NPFF) interacts with specific receptors to modulate opioid functions in the central nervous system. On dissociated neurons and neuroblastoma cells (SH-SY5Y) transfected with NPFF receptors, NPFF acts as a functional antagonist of μ-opioid (MOP) receptors by attenuating the opioid-induced inhibition of calcium conductance. In the SH-SY5Y model, MOP and NPFF2 receptors have been shown to heteromerize. To understand the molecular mechanism involved in the anti-opioid activity of NPFF, we have investigated the phosphorylation status of the MOP receptor using phospho-specific antibody and mass spectrometry. Similarly to direct opioid receptor stimulation, activation of the NPFF2 receptor by [d-Tyr-1-(NMe)Phe-3]NPFF (1DMe), an analog of NPFF, induced the phosphorylation of Ser-377 of the human MOP receptor. This heterologous phosphorylation was unaffected by inhibition of second messenger-dependent kinases and, contrarily to homologous phosphorylation, was prevented by inactivation of Gi/o proteins by pertussis toxin. Using siRNA knockdown we could demonstrate that 1DMe-induced Ser-377 cross-phosphorylation and MOP receptor loss of function were mediated by the G protein receptor kinase GRK2. In addition, mass spectrometric analysis revealed that the phosphorylation pattern of MOP receptors was qualitatively similar after treatment with the MOP agonist Tyr-d-Ala-Gly (NMe)-Phe-Gly-ol (DAMGO) or after treatment with the NPFF agonist 1DMe, but the level of multiple phosphorylation was more intense after DAMGO. Finally, NPFF2 receptor activation was sufficient to recruit β-arrestin2 to the MOP receptor but not to induce its internalization. These data show that NPFF-induced heterologous desensitization of MOP receptor signaling is mediated by GRK2 and could involve transphosphorylation within the heteromeric receptor complex.
Introduction
μ-Opioid (MOP)3 receptors are the main targets of analgesic drugs. However, the use of opiates gives rise to some undesirable effects, including tolerance, pain hypersensitivity, and addiction. Despite more than 40 years of research in the field, the mechanisms of action of these drugs are not completely understood. Tolerance to morphine has been explained by a variety of molecular mechanisms, including functional selective desensitization of receptor signaling and/or endocytosis, as well as cellular counter-adaptations such as hypertrophy of adenylyl cyclase signaling or shift from inhibitory to excitatory G protein coupling (1–5). But tolerance certainly results from complex integrated adaptive processes that contribute to counteract the opioid effects or to change the nociceptive threshold, leading in particular to the so-called paradoxical opioid-induced hyperalgesia (6). Among the peptidergic systems that modulate opiate action, the neuropeptide FF (NPFF) system has recently been shown to contribute to the tolerance to morphine analgesia (7, 8).
Neuropeptide FF (FLFQPQRF-amide, NPFF) belongs to a family of RF-amide peptides involved in the control of pain, cardiovascular functions, appetite, thirst, and body temperature (9). NPFF, together with other related neuropeptides, is produced from a precursor, pro-NPFFA, which is expressed in a few regions of the rodent central nervous system, namely the hypothalamus, the nucleus of the tractus solitarius in the brainstem, and the dorsal horn of the spinal cord (10). It is a high affinity agonist of two G protein-coupled receptors (GPCRs) called NPFF1 and NPFF2 (11). Injection of NPFF analogs in rodents has been shown to modulate both analgesic (12–14) and motivational properties of opioid drugs (15, 16). The NPFF system is therefore considered to be an opioid-modulating system involved in homeostasis that counteracts the action of opioids (17, 18) and thus an interesting therapeutic target for the management of opiate tolerance and dependence (7). In many cases, NPFF pretreatment inhibits opioid effects in rodents. For example, intracerebroventricular injection of NPFF analogs inhibits morphine-induced analgesia in rats (19) and mice (20) and morphine-induced neuronal activity in the mouse nucleus accumbens (21). Similarly to other opioid-modulating peptides (18), an important question is whether this inhibitory effect is indirect (mediated through neuronal circuitry) or direct (mediated by receptor cross-talk within the same neuron). The fact that 1DMe, an NPFF analog, reverses the inhibition of calcium conductance induced by the MOP agonist DAMGO in dissociated rat dorsal root ganglion neurons favors the second hypothesis (22).
To gain information on the molecular mechanisms responsible for the cellular anti-opioid activity of NPFF, we have developed a model by stably transfecting human NPFF2 receptors in the neuroblastoma-derived cell line SH-SY5Y (23). The neuroblastoma clone SH-SY5Y, derived from a human sympathetic ganglion tumor, endogenously expresses low levels of MOP receptors that are negatively coupled to adenylyl cyclase and voltage-gated N-type Ca2+ channels through heterotrimeric Gi/o proteins. Activation of NPFF2 receptors in the recombinant (SH2-D9) cell line inhibits the opioid modulation of Ca2+ channels, thus reproducing the cellular anti-opioid activity observed in isolated neurons (23). This anti-opioid effect is not prevented by inhibition of second messenger-dependent kinases PKA and PKC (23), suggesting that it does not involve typical pathways of heterologous desensitization (24).
GPCR function can also be modulated by heteromerization (25). Numerous studies have shown that the MOP receptor is subjected to this type of regulation by interacting with a large number of GPCRs (25, 26). In the case of MOP receptor dimerization with somatostatin sst2A (27), substance P NK1 (28), α2a-adrenergic (29), and nociceptin ORL1 (30) receptors, cross-desensitization occurs and could result from co-internalization of both partners upon activation of only one receptor or from allosteric changes in binding affinity (31). However, in the case of NPFF2 and MOP receptors previously shown to physically interact in SH-SY5Y cells (32), the stimulation of NPFF2 receptors does not induce the internalization of MOP receptors but, conversely, partly blocks its trafficking (32). Moreover, activation of NPFF2 receptors does not affect the ability of MOP agonists to bind to MOP receptors (23) and to stimulate GTPγS binding on membranes of SH-SY5Y cells (33) nor the distribution of MOP receptors in detergent-resistant membrane microdomains (34). In this study, we have pursued our investigations on the molecular mechanism of the anti-opioid activity of NPFF by testing the possibility that NPFF2 receptor-induced desensitization of the MOP receptor could result from an atypical mode of cross-phosphorylation.
Homologous desensitization results from the phosphorylation of activated GPCRs by a family of serine/threonine kinases called G protein-coupled receptor kinases (24). Previous studies have shown that Thr-370 and Ser-375 are the main targets of homologous regulation by G protein-coupled receptor kinases in the C-terminal tail of the rodent MOP receptor (35–38), although a potential role for Thr-180 in the second intracellular loop has also been suggested in Xenopus oocytes (39). Here, we have studied the phosphorylation status of the human MOP receptor after homologous stimulation by its agonist (DAMGO) or after heterologous regulation by NPFF2 receptors. In our SH-SY5Y model, we have used the only commercially available phospho-specific antibody to specifically monitor the phosphorylation of Ser-377 (corresponding to Ser-375 in the rodent sequence), and we have performed mass spectrometry to identify other phosphorylation sites in the MOP C-tail. Activation of the NPFF2 receptor by 1DMe induced the phosphorylation of MOP Ser-377. This heterologous phosphorylation was not blocked by inhibition of second messenger-dependent kinases (PKC and PKA) but, in contrast to homologous phosphorylation, was prevented by inactivation of Gi/o proteins by pertussis toxin (PTX). We also showed that Ser-377 cross-phosphorylation and MOP receptor cross-desensitization are mediated by GRK2. Moreover, NPFF2 receptor-mediated MOP receptor phosphorylation was accompanied by the recruitment of β-arrestin2 but not by receptor internalization. Finally, mass spectrometric analysis indicated that the phosphorylation pattern of immunopurified MOP receptor is similar after homologous and heterologous stimulation, but the phosphorylation level is more intense after DAMGO treatment.
EXPERIMENTAL PROCEDURES
Materials
Oligonucleotides were synthesized by Sigma Genosys. Restriction enzymes were purchased from New England Biolabs (Ozyme, France). Mouse monoclonal anti-GFP antibody was obtained from Invitrogen; rabbit polyclonal anti-GFP antibody and anti-GRK2 antibody were from Santa Cruz Biotechnology, and rabbit polyclonal anti-actin antibody was from Sigma. The Ser-375 phospho-specific antibody was from Cell Signaling Technology (Ozyme, France). Silencer Select validated siRNA (Ambion Silencer Negative control 1, GRK2 s1127 and GRK2 s1128) were purchased from Ambion (Applied Biosystems, France). 1DMe was synthesized with an automated peptide synthesizer (model 433A, Applied Biosystems). Tyr-d-Ala-Gly-(NMe)-Phe-Gly-ol (DAMGO) was purchased from Bachem (Switzerland); clonidine, H89, and chelerythrine were from Sigma, and PP2 and EGFR inhibitor were from Calbiochem. [3H]DAMGO (67 Ci/mmol) and [3H]adenine (26 Ci/mmol) were purchased from GE Healthcare. The NPFF2 receptor-selective radioligand [3H]EYF (Glu-Tyr-Trp-Ser-Leu-Ala-Ala-Pro-Gln-Arg-Phe-NH2) was tritiated by Tritec (Switzerland) as described (40).
Vector Construction
A truncated version of the enhanced YFP variant Venus encoding amino acids 155–238 of the protein was amplified by PCR using two overlapping forward primers introducing an NcoI restriction site and a DGGSGGGS 8-amino acid linker (5′-CATATACCATGGACGGCGGCAGCGGCGGCGGCAGCGACAAG-3′ and 5′-AGCGGCGGCGGCAGCGACAAGCAGAAGAACGGCATC-3′) and a reverse primer designed to introduce an XbaI restriction site (5′-AATCCTCTAGATCTTGTACAGCTCGTCCATGCCGAGAGT-3′). The PCR product was ligated in-frame at the 3′ end of the human MOP receptor cDNA (32) in pBluescript II SK− (Stratagene, Agilent Technologies) using NcoI and XbaI sites. The complete construct called MOP-VC was finally inserted into the EcoRV-XbaI sites of the mammalian expression vector pEFIB3 bearing the blasticidin selection marker. All constructs were verified by sequencing (Millegen, France). The cDNA for β-arrestin2 fused to the first 155 amino acids of Venus (β-arrestin2-VN) in a pcDNA3-zeo vector was kindly provided by Dr. N. Holliday (University of Nottingham, United Kingdom) (41).
Cell Culture and Transfection
Human neuroblastoma SH-SY5Y cells were grown in Dulbecco's modified Eagle's medium (4.5 g/liter glucose, GlutaMAXI) containing 10% fetal calf serum (FCS) and 50 μg/ml gentamicin (Invitrogen), in a 37 °C humidified atmosphere containing 5% CO2. 400 μg/ml G418 (Invitrogen) was added to the culture medium for the SH2-D9 clone stably transfected with the human NPFF2 receptor (23). 2 μg/ml blasticidin (Cayla, France) was also added for the (SH2-D9)MOP-YFP (clone B7) cell line that expresses nontagged NPFF2 receptors and YFP-tagged MOP receptors (32). The MOP-VC vector was used to transfect the SH2-D9 clone previously characterized (23) to give the (SH2-D9)MOP-VC cell line that was maintained in 400 μg/ml G418 and 2 μg/ml blasticidin. All transfections were performed according to the manufacturer's instruction using FuGENE 6 (Roche Diagnostics) for stable or transient transfection of cDNAs and Lipofectamine RNAiMAX (Invitrogen) for transfection of siRNAs. Cells were used undifferentiated.
Binding and Biological Assays
Membrane preparation, binding of [3H]EYF (NPFF2 receptors) or [3H]DAMGO (MOP receptors), and cAMP measurements were performed as described previously (23). The MOP-VC cell line established in this study expressed 1.3 pmol of receptor/mg of membrane proteins. The receptor was fully functional in cAMP assays. Intracellular calcium concentration ([Ca2+]i) was monitored in living, perfused SH2-D9 cells by quantitative photometry using the fluorescent Ca2+ indicator Fluo-4 (23).
Western Blotting
Cells seeded in 6- or 24-well plates (1 million and 300,000, respectively) were incubated in culture medium containing 0.5% FCS for 2 h. Ligands were then applied in HEPES-buffered medium (150 mm NaCl, 2.5 mm KCl, 2 mm CaCl2, 1 mm MgCl2, 10 mm glucose, 0.1% BSA, 10 mm HEPES; pH adjusted to 7.3) at room temperature. Cells were rinsed with ice-cold PBS and scraped directly in Laemmli sample buffer containing 5% β-mercaptoethanol on ice. Samples were homogenized using a 1-ml syringe, boiled for 5 min, and subjected to 10% SDS-PAGE. Proteins in the gel were transferred to a polyvinylidene difluoride membrane for immunoblotting under standard conditions in Tris-buffered saline (20 mm Tris, pH 7.6, 137 mm NaCl) containing 0.1% Tween and 1% bovine serum albumin. Immunoreactivity was revealed with peroxidase-conjugated goat anti-rabbit IgG (Jackson ImmunoResearch) using the ECL Plus Western blotting detection kit (GE Healthcare). X-ray films were scanned using a GS-800 calibrated densitometer (Bio-Rad). When required, blots were quantified using Quantity One software (Bio-Rad) relative to actin used as internal standard.
Immunofluorescence
Cells were seeded on glass coverslips in 24-well plates. The following day, cells were incubated for 2 h in DMEM containing 0.5% FCS, rinsed twice with KRH buffer (124 mm NaCl, 5 mm KCl, 1.25 mm MgSO4, 1.5 mm CaCl2, 1.25 mm KH2PO4, 25 mm HEPES, 8 mm glucose, 0.5 mg/ml bovine serum albumin, pH 7.4), and then incubated for 15 or 30 min at room temperature with 0.5 ml of buffer (control) or 0.5 ml of buffer plus 1 μm 1DMe or DAMGO. Cells were rinsed three times (with PBS and were fixed with 3.8% formaldehyde (Sigma, France) in PBS containing protease and phosphatase inhibitors (Complete EDTA-free and PhosStop, respectively, Roche Diagnostics) for 20 min at room temperature. After three washes with 0.5 ml of PBS, cells were incubated in PBS containing 10% FCS, 2% BSA, and protease and phosphatase inhibitors for 1 h at room temperature. Coverslips were then incubated with anti-phospho-Ser-377 primary antibody (1:100) in PBS, 2% FCS inhibitor mixtures for 1 h. Cells were rinsed three times for 10 min in PBS, 2% SVF and incubated overnight at 4 °C with Alexa Fluor-633 goat anti-rabbit IgG diluted 1:400 in the same solution for 30 min at room temperature. After three rinses, cells were mounted in Vectashield medium (Vector Laboratories, AbCys, France) and observed on a Zeiss 710 NLO inverted microscope with a ×40 objective (1.3 numerical aperture, oil). Cells were excited at 488 nm (band pass 493–598 nm for emission) and 633 nm (band pass 652–720 nm for emission) under sequential mode. Images were processed with Image J (National Institutes of Health, Bethesda).
β-Arrestin2 Recruitment
Bimolecular fluorescence complementation (BiFC) was detected in (SH2-D9)MOP-VC cells 48 h after transient transfection with β-arrestin2-VN in Lab-Tek II chambered coverglasses (Nunc, Thermo Fisher Scientific, France) using FuGENE 6 transfection reagent. Cells were rinsed with KRH buffer, treated with agonists, and observed live at room temperature under an inverted confocal Zeiss 510 microscope (×63 water objective, 488 nm excitation wavelength, LP505). Images were acquired using the AMS software (Zeiss, France).
Receptor Purification
MOP-YFP receptors were isolated by immunoprecipitation using anti-GFP monoclonal antibodies. Each sample was prepared from two confluent 140-mm culture dishes. Cells were incubated in culture medium containing 0.5% FCS for 2 h. Ligands were then applied in KRH buffer for 20 min at room temperature, and cells were scraped directly on ice. After a short centrifugation (1000 × g, 5 min, 4 °C), cells were incubated for 1 h at 4 °C with gentle agitation in 1 ml of freshly prepared solubilization buffer consisting of 50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 5 mm EDTA, 0.5% Nonidet P-40, and protease and phosphatase inhibitor mixtures (Complete EDTA-free and PhosStop respectively, Roche Diagnostics). The homogenate was then centrifuged at 20,000 × g for 2 min at 4 °C. The supernatant was precleared by incubation with 50 μl of EZView red protein A affinity gel (Sigma) for 3 h at 4 °C and centrifugation at 12,000 × g for 30 s. Immunoprecipitation was then performed with 4 μg of mouse monoclonal anti-GFP antibody for 1 h at 4 °C, followed by overnight incubation at 4 °C with protein A-agarose beads. The washing procedure was exactly as described by the manufacturer (Invitrogen). Beads were resuspended in 2× Laemmli sample buffer containing 30 mm DTT, boiled for 5 min at 100 °C, and alkylated in 90 mm iodoacetamide for 30 min in the dark. Proteins were separated by SDS-PAGE on 10% polyacrylamide gels. Gels were stained with colloidal Coomassie Blue (17% (w/v) ammonium sulfate, 34% (v/v) methanol, 3% (v/v) orthophosphoric acid, and 0.1% (w/v) Brilliant Blue G-250) for 24 h.
In-gel Tryptic Digestion and NanoLC-MS/MS Analysis
The Coomassie Blue-stained band corresponding to MOP-YFP receptor in each condition was excised and subjected to in-gel tryptic digestion using modified porcine trypsin (Promega, France) at 20 ng/μl as described previously (42). The dried peptide extracts obtained were dissolved in 14 μl of 0.05% trifluoroacetic acid in 2% acetonitrile and analyzed by on-line nanoLC using an Ultimate 3000 System (Dionex, Amsterdam, The Netherlands) coupled to an ETD-enabled LTQ Orbitrap Velos mass spectrometer (Thermo Fisher Scientific, Bremen, Germany). 5 μl of each peptide extract were loaded on a 300-μm inner diameter × 5-mm PepMap C18 precolumn (LC Packings, Dionex) at 20 μl/min in 2% acetonitrile, 0.05% trifluoroacetic acid. After desalting for 5 min, peptides were separated on line on a 75-μm inner diameter × 15-cm C18 column (packed in-house with Reprosil C18-AQ Pur 3-μm resin, Dr. Maisch; Proxeon Biosystems, Odense, Denmark). The flow rate was set at 300 nl/min. Peptides were eluted using a 0–50% linear gradient of solvent B in 60 min (solvent A was 0.2% formic acid in 5% acetonitrile, and solvent B was 0.2% formic acid in 80% acetonitrile). Two MS/MS fragmentation techniques were used for both LTQ Orbitrap experiments as follows: first, collision-induced dissociation (CID) was first performed for exhaustive MOP receptor identification, and second, ETD was next carried out for unambiguous MOP receptor phosphopeptide site determination. Both instrument methods for the LTQ Orbitrap were operated in data-dependent scan with the Xcalibur software (version 2.0 SR2, Thermo Fisher Scientific), using a 60-s dynamic exclusion window to prevent repetitive selection of the same peptide. The survey scan MS was performed in the Orbitrap on the 300–2000 m/z mass range with the resolution set to a value of 60,000 at m/z 400. Then the 20 most intense ions per survey scan were selected for subsequent CID or ETD fragmentation using optimal settings for each activation technique, and the resulting fragments were analyzed in the linear trap (LTQ). The normalized collision energy was set to 35% for both CID and ETD. Supplemental activation was enabled for ETD. To increase the ETD experiment selectivity and sensitivity, a parent mass list was used, including the m/z of all charge states of phosphorylated peptides previously identified in the CID experiment. For internal calibration, the 445.120025 ion was used as lock mass with target lock mass abundance of 0%.
Database Search and Determination of MOP Receptor Phosphopeptide Abundance Ratio
Peak lists extraction from Xcalibur raw files were automatically performed using Proteome Discoverer software (version 1.2, Thermo Fisher Scientific). For both fragmentation techniques, the following parameters were set for creation of the peak lists, parent ions in the mass range 300–5000 and no grouping of MS/MS scans. The nonfragmented filter was used to simplify ETD spectra with the following settings: the precursor peak was removed within a 4-Da window, charged reduced precursors were removed within a 2-Da window, and neutral losses from charged reduced precursors were removed within a 2-Da window (the maximum neutral loss mass was set to 120 Da). Peak lists were searched against SwissProt and TrEMBL human databases implemented with the YFP-tagged MOP receptor sequence and using Mascot software (version 2.3.01, Matrix Science, London, UK). Cysteine carbamidomethylation was set as a fixed modification and methionine oxidation, protein N-terminal acetylation and serine/threonine/tyrosine phosphorylations as variable modifications. Up to two missed trypsin cleavages were allowed. Mass tolerances in MS and MS/MS were set to 10 ppm and 0.6 Da, respectively. Phosphorylated site identification was confirmed by manual interpretation of corresponding MS/MS data. The following relative abundance ratios 1DMe/CTRL, DAMGO/CTRL, and DAMGO/1DMe were calculated by computing the peak area ratio of all MOP receptor peptides containing phosphorylation site(s) for pairwise comparison. Peak areas were automatically measured from extracted ion chromatograms of each peptide (sum of all observed charge states) in the nano-LC-MS raw file using the label-free module of the in-house developed MFPaQ version 4.0.0 software (43). To calibrate the amounts of MOP receptor in the three treatment conditions, a normalization of the calculated ratios was performed. The normalization was based on the sum of the extracted ion chromatogram area of identified MOP receptor reference peptides (unmodified and unequivocally cleaved peptides). The reference peptides were as follows: ALDER (620.3282 Da), FICT TGK (825.4055 Da), YIAVCHPVK (1085.5692 Da) FSVSGEGEGDATYGK (1502.6526 Da), GEELFTGVVPILVELDGDVNGHK (2436.2537 Da), and HNIEDGSVQL ADHYQQNTPIGDGPVLLPDNHYLSYQSK (2436.2537 Da).
Data Analysis
Nonlinear regression and statistical analysis of the data were performed using Prism 4.01 (GraphPad Software Inc.) as described in the figure legends.
RESULTS
NPFF2 Receptor Activation Induces Heterologous Phosphorylation of Ser-377 of the Human MOP Receptor
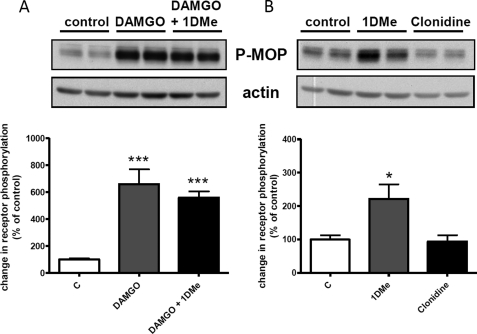
The ability of the anti-Ser-377 phosphospecific antibody to detect MOP receptor phosphorylation was first tested after a 30-min treatment with 1 μm of the MOP agonist DAMGO. As the antibody was not sensitive enough to detect the endogenous MOP receptor, which is expressed at low levels in SH-SY5Y cells (23), phosphorylation experiments were performed in the SH2D9 MOP-YFP cell line that stably expresses the human NPFF2 receptor and the human MOP receptor fused to the yellow fluorescent protein (YFP) at its C terminus (32). Western blots (Fig. 1A) show that the phospho-Ser-377 antibody detects a band (110–120 kDa), previously shown to correspond to the glycosylated MOP-YFP receptor (32), which is about 6-fold more intense after DAMGO treatment. Interestingly, treatment with 1 μm 1DMe alone for 30 min also induced a significant 2-fold increase in MOP receptor Ser-377 phosphorylation (Fig. 1B). This effect seems specific for the NPFF2 receptor because activation of another Gi/o-coupled receptor endogenously expressed in SH-SY5Y cells, the α2-adrenergic receptor, with 10 μm clonidine for 30 min did not modify Ser-377 phosphorylation (Fig. 1B). Co-treatment with 1 μm MOP and NPFF2 agonists did not induce a further increase in phosphorylation (Fig. 1A), indicating that both agonists act on the same population of receptors. An anti-GFP antibody was used to verify that the total amount of MOP-YFP receptor was not changed after a 30-min treatment with DAMGO or 1DMe (supplemental Fig. 1A), indicating that the changes detected with the phospho-specific antibody were indeed due to increased phosphorylation. When testing various doses of 1DMe, we observed that Ser-377 phosphorylation was already detectable at 10 nm (supplemental Fig. 1B). Importantly, 1DMe was unable to induce MOP receptor phosphorylation in cells that did not express NPFF2 receptors (supplemental Fig. 1C).
FIGURE 1.
Homologous (A) and heterologous (B) phosphorylation of MOP receptor Ser-377. Representative Western blots show the levels of Ser-377 phosphorylation induced by treatment with 1 μm DAMGO, 1 μm DAMGO + 1 μm 1DMe, 1 μm 1DMe, or 10 μm clonidine for 30 min in (SH2-D9)MOP-YFP cells. Samples were immunoblotted with anti-Ser(P)-377 antibody (P-MOP) followed by anti-actin antibody (actin) for normalization. Upper panels show representative results from at least three independent experiments. The P-MOP band migrated between the 95- and 130-kDa molecular mass markers. The actin band was detected at the level of the 43-kDa molecular mass marker. Lower panels show means ± S.E. of Ser(P)-377 quantification normalized to actin from at least three independent experiments performed in duplicate. c indicates control cells. *, p < 0.05; ***, p < 0.001; one-way ANOVA followed by Bonferroni post-tests.
Ser-377 Heterologous Phosphorylation Time Course Correlates with Kinetics of Desensitization of Opioid Response
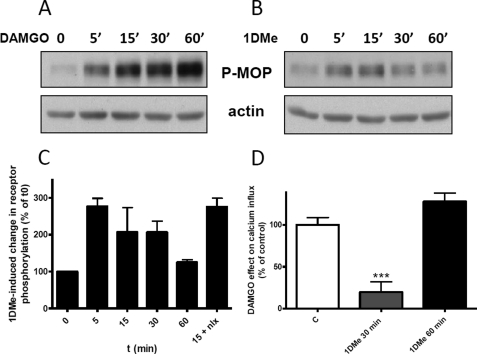
The time course of MOP Ser-377 phosphorylation was also assessed, and interestingly, although DAMGO induced a continuous increase in MOP receptor phosphorylation over 60 min (Fig. 2A), 1DMe-induced heterologous phosphorylation was transient, with Ser-377 phosphorylation returning to base-line level after 60 min of treatment (Fig. 2, B and C). In addition, Ser-377 heterologous phosphorylation was not blocked by treatment with 10 μm of the opioid antagonist naloxone, indicating that it did not involve any direct activation of the MOP receptor (Fig. 2C). Interestingly, the time course of cross-phosphorylation could be correlated to the kinetics of the effect of 1DMe on MOP receptor activity. We have previously reported that, in SH-SY5Y cells, increasing extracellular KCl concentration induced a transient rise in intracellular calcium (resulting from the opening of N-type voltage-gated calcium channels) that was sensitive to opioids (23). As shown in Fig. 2D, stimulation of endogenous MOP receptors by 0.1 μm DAMGO in SH2-D9 cells reduced by 46.5% the calcium influx. This effect of DAMGO was inhibited by 80% in cells that had been pretreated with 1 μm 1DMe for 30 min, revealing the loss of opioid receptor function at a time point when it is phosphorylated by the activation of NPFF receptors. However, when 1DMe pretreatment time was extended to 60 min, the effect of DAMGO returned to control level (Fig. 2D), indicating that MOP receptors recover their activity (resensitization) when Ser-377 is no longer phosphorylated (Fig. 2, B and C). The correlation of kinetic data thus supports a role of the transient MOP receptor Ser-377 phosphorylation in the opioid receptor desensitization and resensitization during 1DMe treatment.
FIGURE 2.
Correlation between the kinetics of heterologous phosphorylation of MOP receptor Ser-377 and desensitization of MOP receptor signaling. Representative Western blots showing the levels of Ser-377 phosphorylation induced by treatment with 1 μm DAMGO (A) or 1 μm 1DMe (B) for the indicated time at room temperature. Samples were immunoblotted with anti-Ser(P)-377 antibody followed by anti-actin antibody for normalization. Upper panels show representative results from at least three independent experiments. C shows the quantification of Ser-377 phosphorylation induced by 1DMe, normalized to actin. Data are expressed as means ± S.E. of three independent experiments performed with 1DMe. The 15 + nlx bar corresponds to a 15-min treatment with 1DMe together with 10 μm naloxone (nlx). D, inhibition of potassium-evoked calcium influx by 30 s of treatment with 0.1 μm DAMGO in control cells (c) or in cells pretreated with 1 μm 1DMe for 30 or 60 min. Results are expressed as % of the DAMGO effect (46.5% inhibition) in control cells (c). Intracellular calcium was measured using Fluo-4. ***, p < 0.001; one-way ANOVA followed by Bonferroni post tests. Number of cells: 48 (C), 47 (1DMe 30 min), and 25 (1DMe 60 min).
GRK2 Contributes to Ser-377 Heterologous Phosphorylation and Inhibition of MOP Receptor Signaling
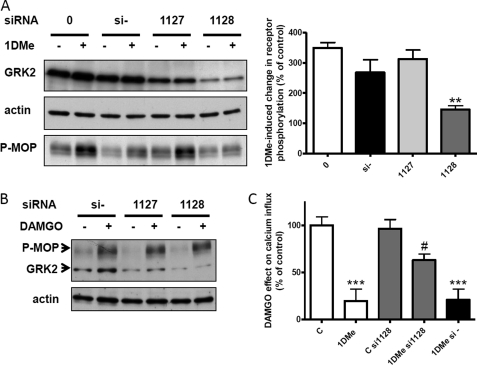
Because Ser-375 of the rodent MOP receptor (equivalent to Ser-377 in the human receptor) has been shown to be a GRK2 target (35–37), we tested the possible involvement of this kinase in Ser-377 heterologous phosphorylation by 1DMe. (SH2-D9)MOP-YFP cells were transfected with two validated siRNAs (1127 and 1128) directed against human GRK2. Levels of GRK2 were determined by Western blot 48 h after transfection. Fig. 3A shows that GRK2 was strongly down-regulated after transfection of siRNA 1128 compared with untransfected cells or cells transfected with a negative control siRNA. siRNA 1127 induced a much lower down-regulation of GRK2. When GRK2 was knocked down by siRNA 1128, 1DMe lost its ability to induce the heterologous phosphorylation of Ser-377 (Fig. 3A). This result indicates that in SH-SY5Y cells, GRK2 is the only kinase responsible for Ser-377 cross-phosphorylation induced by the activation of NPFF2 receptor. Binding of GRK2 to activated NPFF2 receptors was confirmed by co-immunoprecipitation experiments (supplemental Fig. 2). In contrast, the homologous phosphorylation induced by DAMGO was not significantly affected by GRK2 knockdown (Fig. 3B). This suggests that, in the case of homologous phosphorylation of Ser-377, either GRK2 is not involved or other G protein-coupled receptor kinases present in the cell can compensate for the lack of GRK2, or finally, the low level of GRK2 still present after siRNA transfection is sufficient for DAMGO-induced phosphorylation. Having found a way to specifically block 1DMe-induced Ser-377 phosphorylation, we could directly test the role of this phosphorylation in the anti-opioid effect of 1DMe on MOP receptor function. We compared the ability of a 30-min pretreatment with 1 μm 1DMe to affect the inhibition of calcium influx by 0.1 μm DAMGO in SH2-D9 control cells and in cells transfected with control (si-) or GRK2 (si1128) siRNAs (Fig. 3C). GRK2 knockdown did not affect DAMGO response, although it significantly reduced by 43% the effect of 1DMe on DAMGO responses. 1DMe inhibited 80.2% of the effect of DAMGO in control cells versus 34.4% in siRNA 1128-treated cells. Moreover, 1DMe effect was not affected in cells transfected with control siRNAs. Therefore our data demonstrate that GRK2 is a major contributor to the heterologous phosphorylation and the loss of function of the MOP receptor induced by the NPFF2 receptor in SH-SY5Y cells, suggesting that GRK2-mediated transphosphorylation played a role in MOP receptor desensitization.
FIGURE 3.
Effect of GRK2 knockdown on MOP receptor phosphorylation and desensitization. A, heterologous phosphorylation. Left panel, representative Western blots showing the levels of GRK2, actin, and MOP receptor Ser-377 phosphorylation (P-MOP) in cells transfected with transfection agent alone (0), negative control siRNA (si−), or GRK2 siRNAs (1127 and 1128) and treated or not with 1 μm 1DMe for 30 min. Right panel, histogram showing the quantification of Ser-377 phosphorylation induced by 1 μm 1DMe in cells transfected with transfection agent alone (0), negative control siRNA (si−), or GRK2 siRNAs (1127 and 1128). Data are expressed as percent of stimulation over nonstimulated cells (means ± S.E. of three independent experiments). **, p < 0.01; one-way ANOVA followed by Bonferroni post-tests. B, homologous phosphorylation. Representative Western blot showing the levels of GRK2, actin, and MOP receptor Ser-377 phosphorylation (P-MOP) in cells transfected with negative control siRNA (si−) or GRK2 siRNAs (1127 and 1128) and treated or not with 1 μm DAMGO for 30 min. C, heterologous desensitization. Inhibition of potassium-evoked calcium influx by 30 s of treatment with 0.1 μm DAMGO and effect of pretreatment with 1 μm 1DMe for 30 min in control cells and cells transfected with control (si−) or GRK2 (si1128) siRNAs. Results are expressed as percent of the DAMGO effect in control cells (c). Intracellular calcium was measured using Fluo-4. ***, p < 0.001 versus control (c); #, p < 0.05 versus 1DMe; one-way ANOVA followed by Bonferroni post-tests. Number of cells: 48 (c), 47 (1DMe), 60 (C si1128), 58 (1DMe si1128), and 41 (1DMe si−).
Ser-377 Heterologous Phosphorylation Depends on Inhibitory Heterotrimeric G Proteins but Not on Second Messenger-activated Protein Kinases
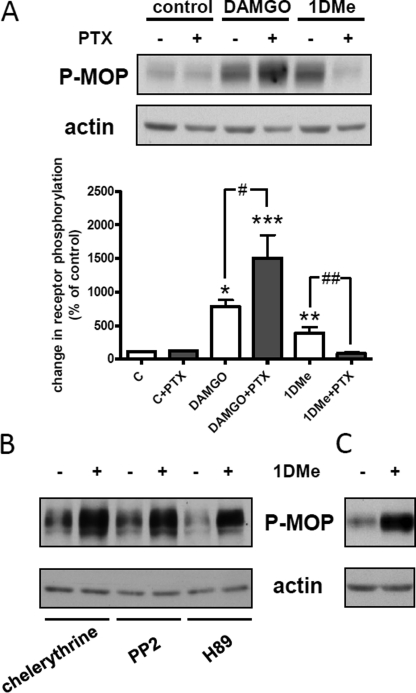
We next addressed the mechanism by which NPFF2 receptor activation could recruit GRK2 to the MOP receptor. We first inhibited Gi/o heterotrimeric G protein with PTX (100 ng/ml for 18 h). The PTX treatment was efficient because it fully prevented the inhibition of adenylyl cyclase by both DAMGO and 1DMe in (SH2-D9)MOP-YFP cells (supplemental Fig. 3). Uncoupling receptors from inhibitory G proteins had opposite effects on homologous and heterologous phosphorylation of Ser-377. It potentiated DAMGO-induced phosphorylation but completely blocked the effect of 1DMe (Fig. 4A). Therefore, G protein activation is not necessary for homologous phosphorylation of the MOP receptor on Ser-377, although it is mandatory for MOP receptor heterologous phosphorylation.
FIGURE 4.
Effect of pertussis toxin and protein kinase inhibitors on MOP receptor Ser-377 phosphorylation. A, representative Western blot showing the levels of Ser-377 phosphorylation induced by treatment with 1 μm DAMGO or 1 μm 1DMe for 30 min in control cells or cells pretreated with 100 ng/ml of PTX for 18 h. Samples were immunoblotted with anti-Ser(P)-377 antibody followed by anti-actin antibody for normalization. Histogram shows the quantification of Ser-377 phosphorylation, and data are expressed as means ± S.E. of three independent experiments. c indicates control cells. *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus untreated cell; #, p < 0.05; ##, p < 0.01; one-way ANOVA followed by Bonferroni post-tests. B, Western blot showing the levels of Ser-377 phosphorylation induced by treatment with 1 μm 1DMe for 30 min in the presence of 10 μm of protein kinase inhibitors (representative of three independent experiments). C, Western blot showing the levels of Ser-377 phosphorylation induced by treatment with 1 μm 1DMe for 30 min in the presence of 10 μm of EGF receptor inhibitor (representative of three independent experiments).
Recruitment of GRK2 to the MOP receptor after NPFF2 receptor activation could result from an indirect activation of GRK2 via its phosphorylation by second messenger activated kinase. GRK2 activity can be enhanced by protein kinase A (PKA), protein kinase C (PKC), or c-Src (44). 1DMe was still able to induce a strong increase in Ser-377 phosphorylation in the presence of 10 μm H89 (PKA inhibitor), chelerythrine (PKC inhibitor), or PP2 (Src inhibitor). Activation of EGFR is also able to phosphorylate and activate GRK2, resulting in cross-phosphorylation of opioid receptors (45). Because GPCRs can transactivate EGFR (46) and SH-SY5Y cells express EGFR (47), we explored the possibility that Ser-377 phosphorylation could be linked to EGFR signaling. Similarly to what we observed with second messenger activated kinases, 1DMe-induced increase in Ser-377 phosphorylation was unaffected by the presence of 10 μm of the EGFR inhibitor cyclopropanecarboxylic acid-(3-(6-(3-trifluoromethyl-phenylamino)-pyrimidin-4-ylamino)-phenyl)-amide (Fig. 4C). Taken together, these results show that MOP receptor phosphorylation by GRK2 after NPFF2 receptor activation necessitates G protein coupling but is independent from GRK2 regulation downstream of G protein signaling.
NPFF2 Receptor Activation Induces β-Arrestin2-VN Recruitment to MOP Receptors but Not Their Internalization
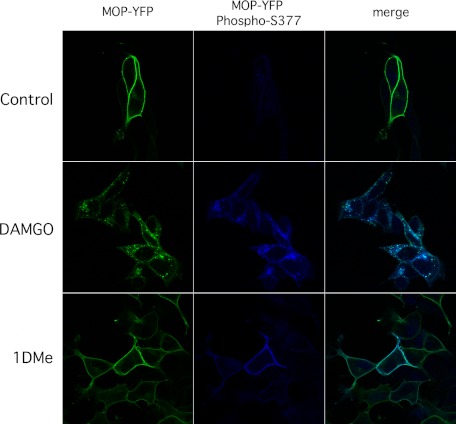
Ser-375 phosphorylation of rodent MOP receptors has been implicated in receptor internalization in addition to desensitization (36, 37). However, morphine has been shown to induce the phosphorylation of MOP receptor Ser-375 at the plasma membrane without triggering endocytosis of the receptor (37). We thus tested the consequences of DAMGO or 1DMe treatment on trafficking of total (MOP-YFP) and Ser-377 phosphorylated MOP receptors. (SH2-D9)MOP-YFP cells were incubated at room temperature with 1 μm 1DMe or DAMGO for 15 or 30 min, respectively. Incubation with 1DMe was shorter to match the peak of Ser-377 phosphorylation. When total MOP receptors were visualized using the fluorescence of the YFP tag, a strong internalization was observed with DAMGO, whereas receptors had remained at the plasma membrane after 1DMe treatment (Fig. 5). The same pattern was observed when Ser-377 phosphorylated MOP receptors were specifically immunolabeled by the phospho-specific MOP antibody. Incubation with 1DMe for 30 min did not produce any sign of MOP receptor internalization, although it is able to induce NPFF2 receptor endocytosis (see Ref. 32 and data not shown). This indicates that the Ser-377 phosphorylation of MOP receptor induced by the NPFF agonist is not sufficient to trigger MOP receptor internalization.
FIGURE 5.
Differential localization of Ser-377-phosphorylated MOP receptors after DAMGO or 1DMe treatment in (SH2-D9)MOP-YFP cells. Cells were treated with buffer alone, 1 μm DAMGO, or 1DMe for 30 or 15 min at room temperature, respectively, before formaldehyde fixation. Total MOP receptors were visualized using the fluorescence of the YFP tag. MOP receptors phosphorylated on Ser-377 were visualized by immunofluorescence using the phospho-specific MOP antibody. Confocal images were taken with a Zeiss 710 NLO inverted microscope with ×40 objective as described under “Experimental Procedures.”
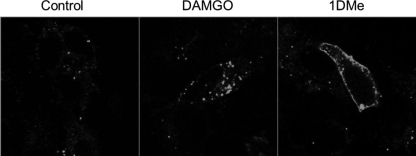
β-Arrestin2 has been implicated in MOP receptor desensitization, endocytosis, and recycling (3). We used BiFC to test whether NPFF2 receptor activation could recruit β-arrestin2 to MOP receptors (48). SH2D9 cells stably expressing MOP receptors fused to the C-terminal part of the fluorescent protein Venus (MOP-VC) were transiently transfected with β-arrestin2 fused to the N-terminal part of the Venus protein (β-arrestin2-VN). 48 h later, living cells were incubated with 1 μm DAMGO or 1DMe at room temperature and observed under an inverted confocal microscope for 30–60 min to allow for the refolding of the fluorescent protein. Compared with control, cells incubated with DAMGO exhibited highly fluorescent intracellular punctae (Fig. 6), indicating that activated MOP receptor could recruit β-arrestin2-VN, enabling fluorescence complementation and internalization of the complex. Cells treated with 1DMe showed a different profile. Fluorescence complementation also occurred, demonstrating β-arrestin2-VN recruitment to MOP receptors, but in this case the fluorescence remained at the plasma membrane (Fig. 6). Thus, 1DMe-induced recruitment of β-arrestin2 to MOP receptor is not sufficient to trigger its intracellular trafficking. A ligand-dependent selective association of β-arrestin1 and β-arrestin2 to the MOP receptor has been recently described (49). Using BiFC, we confirmed that DAMGO induced β-arrestin1 association to the MOP receptor and endocytosis of the complex in our model, but because constitutive association was observed at the membrane of control cells, it was not possible to conclude for NPFF2 receptor activation (data not shown).
FIGURE 6.
β-Arrestin2 bimolecular fluorescence complementation assay. (SH2-D9)MOP-VC cells were transiently transfected with β-arrestin2-VN. 48 h post-transfection, living cells were incubated at room temperature in KRH buffer alone (Control) or containing 1 μm DAMGO or 1DMe and observed for 30 to 60 min under a Zeiss LSM 510 inverted confocal microscope with ×63 objective at λexc 488 nm. Representative images of the 30-min time point are shown.
Mass Spectrometric Analysis Reveals a Similar Pattern for Homologous and Heterologous Phosphorylation of MOP Receptor
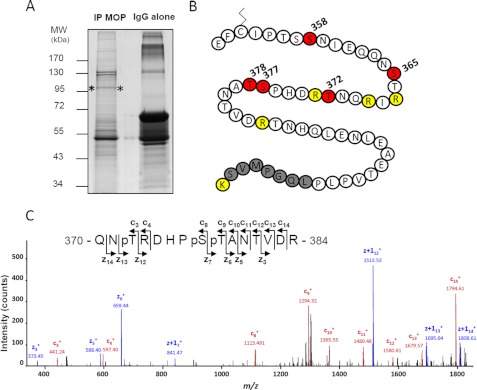
The difference in MOP receptor trafficking after DAMGO and 1DMe could be due to quantitative or qualitative differences in the receptor phosphorylation pattern, similarly to what has been suggested for morphine and DAMGO differential effects (38, 50). MOP receptor phosphorylation was thus analyzed by on-line nanoLC-MS/MS LTQ Orbitrap. Receptors were purified from (SH2-D9)MOP-YFP cells treated or not with 1 μm, 1DMe or DAMGO for 20 min by immunoprecipitation using the YFP tag followed by SDS-PAGE (Fig. 7A). After trypsin digestion, and nanoLC-MS/MS CID and ETD experiments, Mascot database searches considering putative phosphorylation modification identified the MOP receptor fused to YFP sequence with 39.9–41.5% coverage, depending on the sample. No phosphorylation was detected in the parts of the intracellular loops from which tryptic peptides were present, so we focused our analysis on the C-tail. Full coverage was obtained for the 351–408 sequence that starts between the two putatively palmitoylated cysteines (Fig. 7B). The phosphorylated or unphosphorylated peptides identified within this region are presented in Table 1, and the detailed MS/MS spectra of phosphorylated peptides are displayed in supplemental Fig. 4. The effects of agonists were evaluated by calculating the relative abundance ratios for each phosphorylated peptide (see “Experimental Procedures”).
FIGURE 7.
NanoLC-MS/MS analysis of MOP receptor phosphorylation sites. A, immunoprecipitated (IP) MOP receptors visualized after colloidal Coomassie staining (between asterisks). IgG alone correspond to the antibody used for immunoprecipitation, without cellular extract. MW, molecular weight. B, amino acid sequence of the part of the C-tail of MOP that was covered by our study. The putatively palmitoylated cysteine is shown. Phosphorylated residues are shown in red. Trypsin cleavage sites are in yellow. The gray sequence corresponds to the beginning of the YFP fusion. C, ETD MS/MS spectrum of the triply phosphorylated peptide, 370QNpTRDHPpSpTANTVDR384 (triply charged precursor ion, MH3+, at m/z 651.2417), displays series of c- and z-ions indicating that Thr-372, Ser-377, and Thr-378 are phosphorylated. Sequence and phosphorylated positions are indicated.
TABLE 1.
List of C-tail MOP receptor phosphorylated and unphosphorylated peptides identified by nanoLC-MS/MS CID and/or ETD analysis from cells treated for 20 min at room temperature with KRH buffer alone (CTRL), 1 μm DAMGO, or 1 μm 1DMe
The abbreviations used are as follows: Theo. Mass, theoretical mass; MC, missed cleavage.
| Theo. Mass | MC | Position | Sequence | Phospho-residue | Present in sample |
Agonist effect | DAMGO/1DMe | ||
|---|---|---|---|---|---|---|---|---|---|
| CTRL | DAMGO | 1DMe | |||||||
| 2009.9113 | 0 | 351–367 | EFCIPTSSNIEQQNSTR | √ | √ | √ | |||
| 2089.8776 | 0 | 351–367 | EFCIPTSSNIEQQNSTR | Ser-365 | √ | √a | √a | No increase | 1.1 |
| 2089.8776 | 0 | 351–367 | EFCIPTSSNIEQQNSTR | Ser-358 | √ | ||||
| 1211.5531 | 0 | 374–384 | DHPSTANTVDR | √ | √ | √ | |||
| 1291.5194 | 0 | 374–384 | DHPSTANTVDR | Ser-377 or Thr-378 | √ | √ | √ | Increase | 1.9 |
| 1371.4857 | 0 | 374–384 | DHPSTANTVDR | Ser-377 + Thr-378 | √ | √ | Increase | 6.6 | |
| 1710.8034 | 1 | 370–384 | QNTRDHPSTANTVDR | √ | √ | √ | |||
| 1790.7697 | 1 | 370–384 | QNTRDHPSTANTVDR | Thr-372 | √ | √ | √ | Decrease | 0.4 |
| 1870.7360 | 1 | 370–384 | QNTRDHPSTANTVDR | Thr-372 + Ser-377 or Thr-378 | √ | √ | √ | Increase | 2.6 |
| 1950.7023 | 1 | 370–384 | QNTRDHPSTANTVDR | Thr-372 + Ser-377 + Thr-378 | √ | √ | Increase | 20.1 | |
| 2644.3530 | 0 | 385–408 | TNHQLENLEAETVPLPLQGPMVSK | √ | √ | √ | |||
a No distinction between Ser-365 and Thr-366 was obtained for the phosphorylation site. All MS/MS spectra of phosphorylated peptides were manually interpreted and are displayed in supplemental Fig. 4. For each peptide, the normalized DAMGO/1DMe relative abundance ratios were calculated from peak area automatically extracted using the label-free module of the in-house developed MFPaQ version 4.0.0 software as described under “Experimental Procedures.”
The first peptide, EFCIPTSSNIEQQNSTR, existed as three forms as follows: unphosphorylated, phosphorylated on Ser-365, and phosphorylated on Ser-358. Phosphorylation of Ser-365 (corresponding to Ser-363 in the rodent sequence) was unambiguously identified in control condition (ETD MS/MS spectrum presented in supplemental Fig. 4), whereas the only obtained CID MS/MS spectra did not allow us to discriminate between Ser-365 and Thr-366 for DAMGO and 1DMe treatments. However, the agonists had minimal effect on the relative amount of this phosphopeptide suggesting it is constitutively phosphorylated on Ser-365. The Ser-358 phosphorylated EFCIPTSSNIEQQNSTR peptide was present, albeit in very low abundance, only in the DAMGO sample.
The second peptide, DHPSTANTVDR, containing Ser-377, existed as four forms as follows: unphosphorylated, bearing a single phosphorylation either on Ser-377 or Thr-378, and with a dual phosphorylation on these two residues (Table 1). 1DMe and DAMGO induced an increase in single phosphorylation, in agreement with immunoblotting data focusing on Ser-377. Interestingly, the peptide with dual phosphorylation was only present in agonist-treated samples. It is worth noting that the DAMGO effect was always more intense than the 1DMe effect, especially when considering dual versus single phosphorylation (DAMGO/1DMe abundance ratio = 6.6- and 1.9-fold, respectively).
A third peptide, QNTRDHPSTANTVDR, containing the second peptide sequence extended at its N terminus resulted from a trypsin missed cleavage. Four different forms of this peptide were identified as follows: unphosphorylated, phosphorylated on Thr-372 (corresponding to Thr-370 in the rodent sequence), bearing a dual phosphorylation on Thr-372 and Ser-377 or Thr-378, and a triple phosphorylation on Thr-372, Ser-377, and Thr-378 (Table 1). The nonphosphorylated form was present in very low relative abundance, probably because the missed cleavage was favored only in the presence of a phosphate on Thr-372, adjacent to the digestion site. Similarly to the shorter DHPSTANTVDR peptide, DAMGO and 1DMe treatment favored the appearance of multiphosphorylated species (causing a parallel decrease in monophosphorylated peptides), the triphosphate peptide being present only after agonist treatments (Fig. 7C). Again, although both ligands induced the same qualitative pattern, DAMGO was much more efficacious in inducing multiple phosphorylation than 1DMe, up to 20-fold for triple modification.
A two-residue extended IRQNTRDHPSTANVDR peptide containing two missed cleavages was also detected, but its detailed analysis was not presented for the sake of clarity because it behaved exactly as the QNTRDHPSTANTVDR peptide described above. Finally, the peptide TNHQLENLEAETVPLPLQGPMVSK, corresponding to the last 16 amino acids of the C-tail followed by the first 8 residues of the fused YFP, was present in all samples but exclusively in an unphosphorylated form.
Unexpectedly, our proteomics analysis thus revealed that stimulation of the NPFF2 receptor induces the same pattern of phosphorylation of MOP receptor as the one induced by direct MOP receptor activation. However, the dual phosphorylation on Ser-377 and Thr-378 shows a strong “selectivity” for DAMGO versus 1DMe with a DAMGO/1DMe abundance ratio of 6.6, and even 20.1, when associated with Thr-372 phosphorylation.
DISCUSSION
All GPCRs possess several phosphorylation sites (serine, threonine, and tyrosine) in their intracellular domain (51–53). Differential phosphorylation, tissue-, cell-, or agonist-dependent, has recently been postulated to create a “phosphorylation bar code” that offers a way to regulate signaling and trafficking of GPCRs (52). Such a mechanism could explain, for example, the differences in MOP receptor desensitization and trafficking induced by morphine versus DAMGO (5, 38, 49, 50, 54) or could determine the signaling pathway (PKCϵ versus β-arrestin2) by which MOP agonists activate ERK (55). Cross- or transphosphorylation between receptors offers an additional level of complexity to the regulation of GPCR activity and trafficking (56) that could be involved in the functional inhibition of MOP receptors by NPFF receptors. Indeed, activation of NPFF receptors negatively modulates MOP receptor pharmacological effects in vivo, as well as MOP receptor signaling in cellular and neuronal models (17). Because MOP and NPFF2 receptors have been shown to heteromerize in recombinant SH-SY5Y cells (32), we have investigated here the phosphorylation status of MOP receptor in relation to the anti-opioid effect produced by NPFF2 receptor activation in the SH-SY5Y model.
Our results show that, similarly to the MOP agonist DAMGO, the NPFF2 agonist 1DMe induced the phosphorylation of the C-terminal tail of the human MOP receptor, in particular at a residue (Ser-377) known to play a key role in desensitization and internalization of the opioid receptor (35–37). This heterologous phosphorylation was specific to NPFF receptor activation because it did not occur when another Gi/o-coupled receptor, the α2-adrenergic receptor, was activated. NPFF2-induced MOP receptor phosphorylation on Ser-377 was GRK2-dependent and was associated with a reduction of opioid response on calcium signaling. However, contrary to opioid stimulation that induced recruitment of β-arrestin2-VN and internalization of MOP receptor, stimulation of the NPFF receptor promoted the association of β-arrestin2-VN with MOP receptor but not the internalization of the opioid receptor that remained associated with arrestin and phosphorylated on Ser-377 at the plasma membrane. This suggests that the recruitment of β-arrestin2 is not sufficient for receptor internalization and that NPFF-induced phosphorylation of MOP receptor Ser-377 contributes to the loss of opioid receptor function rather than to the receptor internalization. Finally, mass spectrometric analysis of MOP receptor phosphorylation revealed that 1DMe induced the same pattern of phosphorylation as DAMGO, but with a lower phosphorylation rate, indicating that differences between NPFF and opioid agonist effects do not result from a specific pattern of phosphorylation but rather from a quantitative phosphorylation threshold, or other events not dependent on phosphorylation.
The heterologous phosphorylation of MOP receptors following the activation of other GPCRs (CCR5 chemokine (57), somatostatin sst2A (27), substance P NK1 (28), and nociceptin (58)) has been described previously but without identification of the phosphorylated residues nor characterization of the kinases involved (except for nociceptin receptor, see below). Our study is the first one that identifies sites of MOP receptor heterologous phosphorylation after activation of another GPCR. It also shows that the NPFF2-induced phosphorylation of MOP receptor on Ser-377 and inactivation of the MOP receptor are mediated by GRK2, a protein kinase described to mediate GPCR homologous desensitization, although it is able to contribute also to heterologous phosphorylation by indirect regulation via second messenger-activated kinases (24, 44). Such indirect regulation has been described in the case of MOP and nociceptin receptors in BE(2)-C neuroblastoma cells, where activation of nociceptin receptors potentiates DAMGO-induced phosphorylation of the MOP receptor by GRK2, by a mechanism that necessitates PKC activation and the presence of the MOP agonist (58). In our case, the fact that inhibitors of several protein kinases (PKC, PKA, Src, or EGF receptor tyrosine kinase), all known to stimulate GRK2 activity (44, 45), did not inhibit the 1DMe-induced phosphorylation of the MOP receptor indicates that GRK2 is directly involved in this heterologous regulation. Transphosphorylation within GPCR oligomers has been previously described for rhodopsin (59) and chemokine CCR5 receptor in homo-association or hetero-association with C5a anaphylatoxin receptor (60) and could be a general regulatory mechanism (56). In the case of chemokine receptors, one part of the transphosphorylation was attributed to direct G protein-coupled receptor kinase activation, as observed in our study.
Because MOP and NPFF2 receptors interact physically in an NPFF-regulated way (32), the heterologous phosphorylation described here could result from the recruitment of GRK2 by activated NPFF2 receptors within a MOP/NPFF heteromer by two possible mechanisms: (i) GRK2 could be recruited to the activated NPFF2 receptor (supplemental Fig. 2) and, because of the close proximity of MOP receptor within the complex, could transphosphorylate, with a low efficiency, the C-terminal tail of the opioid receptor; (ii) the dimerization induced by NPFF receptor stimulation could allosterically modify the conformation of MOP receptor toward an activated-like state able to recruit GRK2. In both cases, interaction with a Gi/o protein is a prerequisite because PTX prevents the MOP Ser-377 phosphorylation induced by 1DMe. The G protein could be necessary for the formation and/or the stability of the heteromer as reported for some receptors (61–63). Moreover, although it was originally thought that translocation of GRK2 to the plasma membrane was primarily mediated by interaction with βγ subunits released by activated receptors (44), it has been recently reported that GRK2 could also interact with Gαi (64) and could be already present in preassembled receptor-G protein complexes (65). In our case, we have previously observed that activation of NPFF receptors increased the association of Gβγ and some Gα subtypes to the MOP receptor (33), and we show here that activated NPFF2 receptors recruit GRK2. However, the precise stoichiometry and dynamic organization of MOP-NPFF2-G protein-GRK2 complexes, as well as the putative contribution of phosphorylation-independent properties of GRK2 (64–67) to the regulation of MOP signaling, remain to be determined.
We used mass spectrometry to analyze whether DAMGO or 1DMe treatment induced a different pattern of MOP receptor phosphorylation that could explain the differences in MOP receptor trafficking. A recent study used similar techniques to compare the effect of DAMGO and morphine on the phosphorylation pattern of the MOP C-tail (50). As an unexpected result, the phosphorylation pattern of MOP receptor is the same whether it is stimulated by the opioid agonists DAMGO or morphine (50) or by the NPFF agonist 1DMe (this study). However, the phosphorylation level was lower with morphine and 1DMe, suggesting that, in the case of MOP receptor, the specificity of ligand-induced signaling is not encoded by a specific pattern of phosphorylation but rather depends on quantitative variations in phosphorylation, as proposed by Lau et al. (50).
Our study confirms the data from Lau et al. (50), with more precision on the identification of two residues. Like them, we found no phosphorylated residue in the last 16 residues of the MOP C-tail, thus confirming that Thr-396 (Thr-394 in rodent) is not phosphorylated under basal conditions and after DAMGO (or 1DMe) treatment, contrary to what was suggested by indirect studies (68). In agreement with previous studies (38, 50), we found that Ser-365 (Ser-363 in rodent), the main site of MOP receptor phosphorylation by PKC when the kinase is directly activated by phorbol ester (69), was phosphorylated under basal conditions and not affected by DAMGO treatment nor by 1DMe treatment. This is in accordance with the fact that the effects of 1DMe are not sensitive to PKC inhibitors (this study and see Ref. 23). Lau et al. (50) found one phosphorylated residue in the TSST (354–357) rodent sequence that was subjected to agonist regulation (50). We identified Ser-358 in the equivalent TSS (356–358) human sequence, as a phosphorylated residue, only after DAMGO treatment. But, as this phosphopeptide was present at the detection limit, it was not possible to say if this phosphorylation was specific to DAMGO stimulation. In any case, there is some discrepancy in the literature about the TSST motif because MOP receptor phosphorylation, internalization, and β-arrestin2 association are not impaired when this motif is mutated by alanine substitution (36, 50). Finally, similarly to what was shown in HEK cells (50) but with faster kinetics (20 min instead of 3 h), we also found that DAMGO treatment induced multiple phosphorylation on Thr-372 + Ser-377 + Thr-378. Thr-372 (Thr-370 in rodents) and Ser-377 (Ser-375 in rodents) were known from the literature (36, 38, 50) but Thr-378 is identified for the first time by a direct method. This triple phosphorylation was also present, however, in much lower relative abundance compared with DAMGO in morphine- (50) and 1DMe (this study)-treated samples (15- and 20-fold, respectively). Taken together, all these data show that the same phosphorylated residues are identified in both homologous and heterologous conditions. This is consistent with the fact that both treatments are able to induce the recruitment of β-arrestin2 to the MOP receptor. However, dual phosphorylation on Ser-377 and Thr-378 and triple phosphorylation on Thr-372, Ser-377, and Thr-378 show a strong selectivity (up to 20-fold) for DAMGO versus 1DMe that could explain the difference in receptor trafficking.
Of particular interest is the finding that the MOP receptor appears to be similarly regulated by the NPFF agonist 1DMe (this study) and the partial opioid agonist morphine (37, 50). This could have some mechanistic importance because NPFF receptors have been involved in the tolerance to morphine analgesia (7, 8). Both ligands are able to increase the phosphorylation of the same residues in the C-terminal tail of the MOP receptor but to a lower (up to 15–20-fold) extent compared with DAMGO (Ref. 50 and this study). They recruit β-arrestin2 (Ref. 49 and this study), but, in contrast to DAMGO, they promote no or low internalization of MOP receptor, leaving the receptor under a phosphorylated state at the plasma membrane (Ref. 37 and this study). Alanine substitution of Ser-375 in the rodent MOP receptor shows that Ser-375 phosphorylation is responsible for morphine-induced desensitization of MOP receptor signaling (adenylyl cyclase inhibition and ERK activation) (37). Here, we show that depletion of GRK2 abolishes both 1DMe-induced phosphorylation of Ser-377 and inhibition of MOP receptor function. These data therefore support the hypothesis that an initial step of Ser-377 (Ser-375) phosphorylation leads to acute MOP receptor desensitization not only in the case of homologous stimulation but also in the case of heterologous stimulation, but a higher level of phosphorylation implicating multiple residues is necessary for internalization. However, in contrast to morphine-induced modification of Ser-375 and desensitization, which were shown to be persistent (37), the Ser-377 phosphorylation and MOP receptor desensitization induced by 1DMe were transient (return to basal after 1 h, Fig. 2, B and D), confirming the recent observation that MOP receptor endocytosis and recycling are not necessary for dephosphorylation and resensitization (3, 38). This also suggests that, while sharing a common initial step, 1DMe effect on opioid signaling is of short duration, whereas morphine can induce prolonged alterations in receptor signaling and trafficking likely to contribute to tolerance (3).
Because NPFF2 and MOP receptors are able to heteromerize, one could have thought that NPFF2 receptor internalization induced by 1DMe could result in the co-internalization of the associated MOP receptor, as suggested for other receptor pairs (27, 28). The absence of co-internalization suggests that the interaction between the two receptors is transient or, in contrast, that the dimer is resistant to internalization, as recently observed for angiotensin AT1-AT2 receptor complexes, specifically visualized by BiFC (70). β-Arrestin is a major partner for desensitization and internalization (3, 44). Its interaction with GPCRs is regulated by receptor phosphorylation, but it also highly depends on receptor-active conformation (71). In the case of MOP receptor regulation, a recent study has shown that for many opioid agonists, including morphine and DAMGO, there is a good correlation between the rate of phosphorylation of Ser-375 and the ability to recruit β-arrestin2 and to induce internalization (72). Accordingly, MOP receptor heterologously phosphorylated by activation of NPFF2 receptor can recruit β-arrestin2, but because its phosphorylation level is too low, and probably also because it does not adopt a proper agonist-active conformation, the receptor-β-arrestin complex could either be not stable enough or in an inappropriate conformation to recruit the endocytic machinery. However, β-arrestin2 can also play a role in desensitization independently from internalization (5, 71) and could therefore contribute to NPFF2-induced MOP receptor loss of function by disrupting Gi protein activation or by means of its scaffolding properties.
In conclusion, our data show that NPFF2 receptor activation is able to induce the heterologous phosphorylation of MOP receptor on three residues (Thr-372, Ser-377, and Thr-378). In the case of Ser-377, the phosphorylation is mediated by GRK2, is transient, and is associated with the NPFF-induced decrease in MOP receptor ability to inhibit N-type voltage-gated calcium channels. Phosphorylated Ser-377 is thus the first post-translational modification characterized at the molecular level related to the anti-opioid activity of NPFF agonists. Moreover, our data support the concept that GRK2 plays a role in some type of heterologous desensitization, in addition to its granted contribution to homologous desensitization. The rapid and reversible heterologous regulation described here could be a way for NPFF agonists to switch off MOP receptor signaling in neurons that co-express the two types of receptors. The detailed molecular organization of these events, in particular the possible role of direct protein/protein interactions, remains to be characterized. Considering that opioid tolerance is linked in part to MOP phosphorylation/desensitization, arrestin association, and endocytosis (3), the possibility that the mechanism of heterologous phosphorylation presented here contributes to the regulation of the pharmacological effects of opioids by NPFF analogs in vivo deserves further investigation.
Supplementary Material
Acknowledgments
We thank Dr. Laurence Salomé (Institut de Pharmacologie et Biologie Structurale, Toulouse) for critical reading of the manuscript, Dr. Nicholas Holliday (University of Nottingham, United Kingdom) for providing us with the β-arrestin constructs, and Karima Chaoui (Institut de Pharmacologie et Biologie Structurale, Toulouse) for technical assistance with the MS experiments. This work used the facilities of the TRI RIO Imaging platform at Institut de Pharmacologie et Biologie Structurale (Genotoul, Toulouse, France).
This work was supported by French Agence Nationale de la Recherche Grant ANR-09-PIRI-0008-01, piribio09_445026.

This article contains supplemental “Experimental Procedures” and Figs. 1–4.
- MOP
- μ-opioid
- BiFC
- bimolecular fluorescence complementation
- CID
- collision-induced dissociation
- EGFR
- epidermal growth factor receptor
- ETD
- electron transfer dissociation
- GPCR
- G protein-coupled receptor
- LTQ
- linear trap quadrupole
- NPFF
- neuropeptide FF
- PTX
- pertussis toxin
- 1DMe
- [d-Tyr-1-(NMe)Phe-3]NPFF
- DAMGO
- Tyr-d-Ala-Gly-(NMe)-Phe-Gly-ol
- ANOVA
- analysis of variance
- GTPγS
- guanosine 5′-3-O-(thio)triphosphate.
REFERENCES
- 1. Bailey C. P., Connor M. (2005) Opioids. Cellular mechanisms of tolerance and physical dependence. Curr. Opin. Pharmacol. 5, 60–68 [DOI] [PubMed] [Google Scholar]
- 2. Christie M. J. (2008) Cellular neuroadaptations to chronic opioids. Tolerance, withdrawal, and addiction. Br. J. Pharmacol. 154, 384–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dang V. C., Christie M. J. (2012) Mechanisms of rapid opioid receptor desensitization, resensitization, and tolerance in brain neurons. Br. J. Pharmacol. 165, 1704–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Martini L., Whistler J. L. (2007) The role of μ-opioid receptor desensitization and endocytosis in morphine tolerance and dependence. Curr. Opin. Neurobiol. 17, 556–564 [DOI] [PubMed] [Google Scholar]
- 5. Raehal K. M., Schmid C. L., Groer C. E., Bohn L. M. (2011) Functional selectivity at the μ-opioid receptor. Implications for understanding opioid analgesia and tolerance. Pharmacol. Rev. 63, 1001–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ossipov M. H., Lai J., King T., Vanderah T. W., Porreca F. (2005) Underlying mechanisms of pronociceptive consequences of prolonged morphine exposure. Biopolymers 80, 319–324 [DOI] [PubMed] [Google Scholar]
- 7. Elhabazi K., Trigo J. M., Mollereau C., Moulédous L., Zajac J. M., Bihel F., Schmitt M., Bourguignon J. J., Meziane H., Petit-demoulière B., Bockel F., Maldonado R., Simonin F. (2012) Involvement of neuropeptide FF receptors in neuroadaptive responses to acute and chronic opiate treatments. Br. J. Pharmacol. 165, 424–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Simonin F., Schmitt M., Laulin J. P., Laboureyras E., Jhamandas J. H., MacTavish D., Matifas A., Mollereau C., Laurent P., Parmentier M., Kieffer B. L., Bourguignon J. J., Simonnet G. (2006) RF9, a potent and selective neuropeptide FF receptor antagonist, prevents opioid-induced tolerance associated with hyperalgesia. Proc. Natl. Acad. Sci. U.S.A. 103, 466–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Panula P., Aarnisalo A. A., Wasowicz K. (1996) Neuropeptide FF, a mammalian neuropeptide with multiple functions. Prog. Neurobiol. 48, 461–487 [DOI] [PubMed] [Google Scholar]
- 10. Vilim F. S., Aarnisalo A. A., Nieminen M. L., Lintunen M., Karlstedt K., Kontinen V. K., Kalso E., States B., Panula P., Ziff E. (1999) Gene for pain modulatory neuropeptide NPFF. Induction in spinal cord by noxious stimuli. Mol. Pharmacol. 55, 804–811 [PubMed] [Google Scholar]
- 11. Zajac J. M. (2001) Neuropeptide FF. New molecular insights. Trends Pharmacol. Sci. 22, 63. [DOI] [PubMed] [Google Scholar]
- 12. Yang H. Y., Tao T., Iadarola M. J. (2008) Modulatory role of neuropeptide FF system in nociception and opiate analgesia. Neuropeptides 42, 1–18 [DOI] [PubMed] [Google Scholar]
- 13. Dupouy V., Zajac J. M. (1995) Effects of neuropeptide FF analogs on morphine analgesia in the nucleus raphe dorsalis. Regul. Pept. 59, 349–356 [DOI] [PubMed] [Google Scholar]
- 14. Dupouy V., Zajac J. M. (1997) Neuropeptide FF receptors control morphine-induced analgesia in the parafascicular nucleus and the dorsal raphe nucleus. Eur. J. Pharmacol. 330, 129–137 [DOI] [PubMed] [Google Scholar]
- 15. Kotlinska J., Pachuta A., Dylag T., Silberring J. (2007) Neuropeptide FF (NPFF) reduces the expression of morphine- but not of ethanol-induced conditioned place preference in rats. Peptides 28, 2235–2242 [DOI] [PubMed] [Google Scholar]
- 16. Marchand S., Betourne A., Marty V., Daumas S., Halley H., Lassalle J. M., Zajac J. M., Frances B. (2006) A neuropeptide FF agonist blocks the acquisition of conditioned place preference to morphine in C57Bl/6J mice. Peptides 27, 964–972 [DOI] [PubMed] [Google Scholar]
- 17. Moulédous L., Mollereau C., Zajac J. M. (2010) Opioid-modulating properties of the neuropeptide FF system. Biofactors 36, 423–429 [DOI] [PubMed] [Google Scholar]
- 18. Mollereau C., Roumy M., Zajac J. M. (2005) Opioid-modulating peptides. Mechanisms of action. Curr. Top. Med. Chem. 5, 341–355 [DOI] [PubMed] [Google Scholar]
- 19. Yang H. Y., Fratta W., Majane E. A., Costa E. (1985) Isolation, sequencing, synthesis, and pharmacological characterization of two brain neuropeptides that modulate the action of morphine. Proc. Natl. Acad. Sci. U.S.A. 82, 7757–7761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gicquel S., Mazarguil H., Allard M., Simonnet G., Zajac J. M. (1992) Analogs of F8Famide resistant to degradation, with high affinity and in vivo effects. Eur. J. Pharmacol. 222, 61–67 [DOI] [PubMed] [Google Scholar]
- 21. Moulédous L., Frances B., Zajac J. M. (2010) Modulation of basal and morphine-induced neuronal activity by a NPFF(2) selective agonist measured by c-Fos mapping of the mouse brain. Synapse 64, 672–68120336629 [Google Scholar]
- 22. Rebeyrolles S., Zajac J. M., Roumy M. (1996) Neuropeptide FF reverses the effect of μ-opioid on Ca2+ channels in rat spinal ganglion neurones. Neuroreport 7, 2979–2981 [DOI] [PubMed] [Google Scholar]
- 23. Mollereau C., Mazarguil H., Zajac J. M., Roumy M. (2005) Neuropeptide FF (NPFF) analogs functionally antagonize opioid activities in NPFF2 receptor-transfected SH-SY5Y neuroblastoma cells. Mol. Pharmacol. 67, 965–975 [DOI] [PubMed] [Google Scholar]
- 24. Chuang T. T., Iacovelli L., Sallese M., De Blasi A. (1996) G protein-coupled receptors. Heterologous regulation of homologous desensitization and its implications. Trends Pharmacol. Sci. 17, 416–421 [DOI] [PubMed] [Google Scholar]
- 25. Rozenfeld R., Devi L. A. (2010) Receptor heteromerization and drug discovery. Trends Pharmacol. Sci. 31, 124–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van Rijn R. M., Whistler J. L., Waldhoer M. (2010) Opioid-receptor-heteromer-specific trafficking and pharmacology. Curr. Opin. Pharmacol. 10, 73–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pfeiffer M., Koch T., Schröder H., Laugsch M., Höllt V., Schulz S. (2002) Heterodimerization of somatostatin and opioid receptors cross-modulates phosphorylation, internalization, and desensitization. J. Biol. Chem. 277, 19762–19772 [DOI] [PubMed] [Google Scholar]
- 28. Pfeiffer M., Kirscht S., Stumm R., Koch T., Wu D., Laugsch M., Schröder H., Höllt V., Schulz S. (2003) Heterodimerization of substance P and μ-opioid receptors regulates receptor trafficking and resensitization. J. Biol. Chem. 278, 51630–51637 [DOI] [PubMed] [Google Scholar]
- 29. Tan M., Walwyn W. M., Evans C. J., Xie C. W. (2009) p38 MAPK and β-arrestin2 mediate functional interactions between endogenous micro-opioid and α2A-adrenergic receptors in neurons. J. Biol. Chem. 284, 6270–6281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Evans R. M., You H., Hameed S., Altier C., Mezghrani A., Bourinet E., Zamponi G. W. (2010) Heterodimerization of ORL1 and opioid receptors and its consequences for N-type calcium channel regulation. J. Biol. Chem. 285, 1032–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vilardaga J. P., Nikolaev V. O., Lorenz K., Ferrandon S., Zhuang Z., Lohse M. J. (2008) Conformational cross-talk between α2A-adrenergic and μ-opioid receptors controls cell signaling. Nat. Chem. Biol. 4, 126–131 [DOI] [PubMed] [Google Scholar]
- 32. Roumy M., Lorenzo C., Mazères S., Bouchet S., Zajac J. M., Mollereau C. (2007) Physical association between neuropeptide FF and micro-opioid receptors as a possible molecular basis for anti-opioid activity. J. Biol. Chem. 282, 8332–8342 [DOI] [PubMed] [Google Scholar]
- 33. Kersanté F., Moulédous L., Zajac J. M., Mollereau C. (2010) Modulation by neuropeptide FF of the interaction of μ-opioid (MOP) receptor with G proteins. Neurochem. Int. 56, 768–773 [DOI] [PubMed] [Google Scholar]
- 34. Moulédous L., Merker S., Neasta J., Roux B., Zajac J. M., Mollereau C. (2008) Neuropeptide FF-sensitive confinement of μ-opioid receptor does not involve lipid rafts in SH-SY5Y cells. Biochem. Biophys. Res. Commun. 373, 80–84 [DOI] [PubMed] [Google Scholar]
- 35. Chu J., Zheng H., Loh H. H., Law P. Y. (2008) Morphine-induced μ-opioid receptor rapid desensitization is independent of receptor phosphorylation and β-arrestins. Cell. Signal. 20, 1616–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. El Kouhen R., Burd A. L., Erickson-Herbrandson L. J., Chang C. Y., Law P. Y., Loh H. H. (2001) Phosphorylation of Ser-363, Thr-370, and Ser-375 residues within the carboxyl tail differentially regulates μ-opioid receptor internalization. J. Biol. Chem. 276, 12774–12780 [DOI] [PubMed] [Google Scholar]
- 37. Schulz S., Mayer D., Pfeiffer M., Stumm R., Koch T., Höllt V. (2004) Morphine induces terminal micro-opioid receptor desensitization by sustained phosphorylation of serine 375. EMBO J. 23, 3282–3289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Doll C., Konietzko J., Pöll F., Koch T., Höllt V., Schulz S. (2011) Agonist-selective patterns of μ-opioid receptor phosphorylation revealed by phosphosite-specific antibodies. Br. J. Pharmacol. 164, 298–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Celver J. P., Lowe J., Kovoor A., Gurevich V. V., Chavkin C. (2001) Threonine 180 is required for G protein-coupled receptor kinase 3- and β-arrestin 2-mediated desensitization of the μ-opioid receptor in Xenopus oocytes. J. Biol. Chem. 276, 4894–4900 [DOI] [PubMed] [Google Scholar]
- 40. Talmont F., Garcia L. P., Mazarguil H., Zajac J. M., Mollereau C. (2009) Characterization of two novel tritiated radioligands for labeling neuropeptide FF (NPFF(1) and NPFF(2)) receptors. Neurochem. Int. 55, 815–819 [DOI] [PubMed] [Google Scholar]
- 41. Kilpatrick L. E., Briddon S. J., Hill S. J., Holliday N. D. (2010) Quantitative analysis of neuropeptide Y receptor association with β-arrestin2 measured by bimolecular fluorescence complementation. Br. J. Pharmacol. 160, 892–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shevchenko A., Wilm M., Vorm O., Mann M. (1996) Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 68, 850–858 [DOI] [PubMed] [Google Scholar]
- 43. Mouton-Barbosa E., Roux-Dalvai F., Bouyssié D., Berger F., Schmidt E., Righetti P. G., Guerrier L., Boschetti E., Burlet-Schiltz O., Monsarrat B., Gonzalez de Peredo A. (2010) In-depth exploration of cerebrospinal fluid by combining peptide ligand library treatment and label-free protein quantification. Mol. Cell. Proteomics 9, 1006–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kohout T. A., Lefkowitz R. J. (2003) Regulation of G protein-coupled receptor kinases and arrestins during receptor desensitization. Mol. Pharmacol. 63, 9–18 [DOI] [PubMed] [Google Scholar]
- 45. Chen Y., Long H., Wu Z., Jiang X., Ma L. (2008) EGF transregulates opioid receptors through EGFR-mediated GRK2 phosphorylation and activation. Mol. Biol. Cell 19, 2973–2983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Prenzel N., Zwick E., Daub H., Leserer M., Abraham R., Wallasch C., Ullrich A. (1999) EGF receptor transactivation by G protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature 402, 884–888 [DOI] [PubMed] [Google Scholar]
- 47. Offermanns S., Bombien E., Schultz G. (1993) Stimulation of tyrosine phosphorylation and mitogen-activated protein (MAP) kinase activity in human SH-SY5Y neuroblastoma cells by carbachol. Biochem. J. 294, 545–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rose R. H., Briddon S. J., Holliday N. D. (2010) Bimolecular fluorescence complementation. Lighting up seven transmembrane domain receptor signaling networks. Br. J. Pharmacol. 159, 738–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Groer C. E., Schmid C. L., Jaeger A. M., Bohn L. M. (2011) Agonist-directed interactions with specific β-arrestins determine μ-opioid receptor trafficking, ubiquitination, and dephosphorylation. J. Biol. Chem. 286, 31731–31741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lau E. K., Trester-Zedlitz M., Trinidad J. C., Kotowski S. J., Krutchinsky A. N., Burlingame A. L., von Zastrow M. (2011) Quantitative encoding of the effect of a partial agonist on individual opioid receptors by multisite phosphorylation and threshold detection. Sci. Signal. 4, ra52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Busillo J. M., Armando S., Sengupta R., Meucci O., Bouvier M., Benovic J. L. (2010) Site-specific phosphorylation of CXCR4 is dynamically regulated by multiple kinases and results in differential modulation of CXCR4 signaling. J. Biol. Chem. 285, 7805–7817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Butcher A. J., Prihandoko R., Kong K. C., McWilliams P., Edwards J. M., Bottrill A., Mistry S., Tobin A. B. (2011) Differential G protein-coupled receptor phosphorylation provides evidence for a signaling bar code. J. Biol. Chem. 286, 11506–11518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tobin A. B., Butcher A. J., Kong K. C. (2008) Location, location, location. Site-specific GPCR phosphorylation offers a mechanism for cell-type-specific signaling. Trends Pharmacol. Sci. 29, 413–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kelly E., Bailey C. P., Henderson G. (2008) Agonist-selective mechanisms of GPCR desensitization. Br. J. Pharmacol. 153, S379–S388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zheng H., Chu J., Zhang Y., Loh H. H., Law P. Y. (2011) Modulating micro-opioid receptor phosphorylation switches agonist-dependent signaling as reflected in PKCϵ activation and dendritic spine stability. J. Biol. Chem. 286, 12724–12733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Maurice P., Kamal M., Jockers R. (2011) Asymmetry of GPCR oligomers supports their functional relevance. Trends Pharmacol. Sci. 32, 514–520 [DOI] [PubMed] [Google Scholar]
- 57. Chen C., Li J., Bot G., Szabo I., Rogers T. J., Liu-Chen L. Y. (2004) Heterodimerization and cross-desensitization between the μ-opioid receptor and the chemokine CCR5 receptor. Eur. J. Pharmacol. 483, 175–186 [DOI] [PubMed] [Google Scholar]
- 58. Ozsoy H. Z., Thakker D. R., Standifer K. M. (2005) Orphanin FQ/nociceptin potentiates [d-Ala2,N-Me-Phe4,Gly5-ol]enkephalin-induced μ-opioid receptor phosphorylation. Mol. Pharmacol. 68, 447–456 [DOI] [PubMed] [Google Scholar]
- 59. Shi G. W., Chen J., Concepcion F., Motamedchaboki K., Marjoram P., Langen R. (2005) Light causes phosphorylation of nonactivated visual pigments in intact mouse rod photoreceptor cells. J. Biol. Chem. 280, 41184–41191 [DOI] [PubMed] [Google Scholar]
- 60. Hüttenrauch F., Pollok-Kopp B., Oppermann M. (2005) G protein-coupled receptor kinases promote phosphorylation and β-arrestin-mediated internalization of CCR5 homo- and hetero-oligomers. J. Biol. Chem. 280, 37503–37515 [DOI] [PubMed] [Google Scholar]
- 61. Estes A. M., McAllen K., Parker M. S., Sah R., Sweatman T., Park E. A., Balasubramaniam A., Sallee F. R., Walker M. W., Parker S. L. (2011) Maintenance of Y receptor dimers in epithelial cells depends on interaction with G protein heterotrimers. Amino Acids 40, 371–380 [DOI] [PubMed] [Google Scholar]
- 62. Law P. Y., Erickson-Herbrandson L. J., Zha Q. Q., Solberg J., Chu J., Sarre A., Loh H. H. (2005) Heterodimerization of μ- and δ-opioid receptors occurs at the cell surface only and requires receptor-G protein interactions. J. Biol. Chem. 280, 11152–11164 [DOI] [PubMed] [Google Scholar]
- 63. Parker S. L., Parker M. S., Sallee F. R., Balasubramaniam A. (2007) Oligomerization of neuropeptide Y (NPY) Y2 receptors in CHO cells depends on functional pertussis toxin-sensitive G proteins. Regul. Pept. 144, 72–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Breton B., Lagacé M., Bouvier M. (2010) Combining resonance energy transfer methods reveals a complex between the α2A-adrenergic receptor, Gαi1β1γ2, and GRK2. FASEB J. 24, 4733–4743 [DOI] [PubMed] [Google Scholar]
- 65. Namkung Y., Dipace C., Urizar E., Javitch J. A., Sibley D. R. (2009) G protein-coupled receptor kinase-2 constitutively regulates D2 dopamine receptor expression and signaling independently of receptor phosphorylation. J. Biol. Chem. 284, 34103–34115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Fernandez N., Gottardo F. L., Alonso M. N., Monczor F., Shayo C., Davio C. (2011) Roles of phosphorylation-dependent and -independent mechanisms in the regulation of histamine H2 receptor by G protein-coupled receptor kinase 2. J. Biol. Chem. 286, 28697–28706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Pao C. S., Benovic J. L. (2002) Phosphorylation-independent desensitization of G protein-coupled receptors? Sci. STKE 2002, pe42. [DOI] [PubMed] [Google Scholar]
- 68. Pak Y., O'Dowd B. F., George S. R. (1997) Agonist-induced desensitization of the μ-opioid receptor is determined by threonine 394 preceded by acidic amino acids in the C-terminal tail. J. Biol. Chem. 272, 24961–24965 [DOI] [PubMed] [Google Scholar]
- 69. Feng B., Li Z., Wang J. B. (2011) Protein kinase C-mediated phosphorylation of the μ-opioid receptor and its effects on receptor signaling. Mol. Pharmacol. 79, 768–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Porrello E. R., Pfleger K. D., Seeber R. M., Qian H., Oro C., Abogadie F., Delbridge L. M., Thomas W. G. (2011) Heteromerization of angiotensin receptors changes trafficking and arrestin recruitment profiles. Cell. Signal. 23, 1767–1776 [DOI] [PubMed] [Google Scholar]
- 71. Gurevich V. V., Gurevich E. V. (2006) The structural basis of arrestin-mediated regulation of G protein-coupled receptors. Pharmacol. Ther. 110, 465–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. McPherson J., Rivero G., Baptist M., Llorente J., Al-Sabah S., Krasel C., Dewey W. L., Bailey C. P., Rosethorne E. M., Charlton S. J., Henderson G., Kelly E. (2010) μ-Opioid receptors. Correlation of agonist efficacy for signaling with ability to activate internalization. Mol. Pharmacol. 78, 756–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.