Background: Implication of Motin family members in canonical Wnt signaling is unknown.
Results: Amotl2 knockdown dorsalizes zebrafish embryos and Amotl2 can trap β-catenin in recycling endosomes.
Conclusion: Amotl2 regulates embryonic development by inhibiting canonical Wnt signaling.
Significance: The findings shed new light on developmental function of Motin family members and regulation of intracellular distribution of β-catenin.
Keywords: β-catenin, Embryo, Endosomes, Wnt Signaling, Zebra Fish, Angiomotin-like2
Abstract
The Motin family proteins can regulate cell polarity, cell mobility, and proliferation during embryonic development by controlling distinct signaling pathways. In this study, we demonstrate that amotl2 knockdown in zebrafish wild-type embryos results in embryonic dorsalization, and this effect can be antagonized by co-knockdown of the dorsal inducer β-catenin2. Overexpression of amotl2 in masterblind (mbl) homozygous embryos, in which canonical Wnt signaling is up-regulated due to an axin1 mutation, transforms eyeless phenotype into smaller eyes, whereas co-knockdown of amot, amotl1, and amotl2 leads to development of smaller eyes in mbl heterozygotes. In cultured mammalian cells, Motin family members all possess the ability to attenuate Wnt/β-catenin signaling. Focusing on Amotl2, we show that Amotl2 can associate with and trap β-catenin in the Rab11-positive recycling endosomes, and as a result, the amount of β-catenin in the cytosol and nucleus is reduced. Thus, our findings provide novel insights into functions of Motin family members and regulation of Wnt/β-catenin signaling.
Introduction
Canonical Wnt signaling, a pathway conserved in metazoan from hydra to human, is implicated in tumorigenesis, cell proliferation, stem cell renewal, and differentiation, cardiac hypertrophy, angiogenesis, and embryonic development (1–4). In the absence of Wnt ligands, cytoplasmic β-catenin is phosphorylated and degraded by the destruction complex consisting of APC, Axin, GSK3β, and CKI. After Wnt ligands bind to the receptors Frizzled and LRP5/6 on the membrane, β-catenin is stabilized by disassociating from the destruction complex and translocates into the nucleus where it binds to transcription factors TCF/LEF to initiate target gene transcription. Therefore, the regulation of β-catenin is the core event of Wnt/β-catenin signaling.
In zebrafish embryos, maternal and zygotic Wnt/β-catenin signaling functions differently (5). Maternal β-catenin is crucial for the formation of the dorsal organizer before the onset of gastrulation (6, 7). In contrast, zygotic Wnt/β-catenin signaling, which is activated in the ventral and lateral blastoderm during gastrulation, acts to prevent the expansion of the dorsal organizer after the onset of gastrulation (8, 9). Following neural induction in vertebrate embryos, Wnt/β-catenin signaling functions to posteriorize the neuroectoderm, and therefore inhibition of this pathway in the anterior neuroectoderm is required for forebrain formation and eyes induction (10, 11). For example, zebrafish masterblind (mbl) mutants, in which Wnt/β-catenin signaling is enhanced due to a point mutation in the GSK3β-binding domain of axin1 leading to weaker interaction between Axin1 and GSK3β, exhibit reduced or absent eyes and telencephalon (12, 13).
The Motin protein family in vertebrates consists of three members, angiomotin (Amot),2 Angiomotin-like 1 (Amotl1), and Angiomotin-like 2 (Amotl2), which share conserved coiled-coil domain and PDZ binding motif (14). These proteins have been demonstrated to play important roles in angiogenesis, cell mobility, cell polarity, and cell-cell junctions by regulating distinct signaling pathways (15–24). Interestingly, it appears that each Motin family member in human and mice has two isoforms, long and short isoform (14), which function differently in physiological processes (17, 25–28). However, it is unknown whether Motin members participate in regulation of canonical Wnt signaling pathway.
In this study, we demonstrate that knockdown of amotl2 in zebrafish embryos results in dorsalized phenotypes, and that amolt2 overexpression in mbl mutant embryos converts the eyeless phenotype into smaller eyes. In mammalian cells, transfection of Amotl2 or other Motin genes attenuates Wnt/β-catenin signaling whereas co-knockdown of three human Motin genes up-regulates Wnt/β-catenin signaling. Mechanistically, Amotl2 interacts with and traps β-catenin in Rab11-positive recycling endosomes, preventing its nuclear translocation and transcriptional activity. Thus, our study uncovers a novel function of Amotl2 in controlling Wnt/β-catenin signaling.
EXPERIMENTAL PROCEDURES
Zebrafish Strains
Wild-type and mbl mutant embryos of the Tuebingen strain were used. Embryos were incubated in Holtfreter's solution at 28.5 °C. Ethical approval was obtained from the Animal Care and Use Committee of Tsinghua University.
Plasmids
The plasmids Super TOPFlash, pXF2F-Flag and pLentiLox3.7 were gifts from Drs. Wei Wu, Xin-Hua Feng, and Qiang Wang, respectively. AMOT, AMOTL1, and AMOTL2 refer to human Motin family proteins. pXT7-Amotl2, pXT7-Amotl2m, pCMV5-HA-Amotl2, pCMV5-myc-Dvl2, β-cateninΔN,Wnt1, and LEF1 constructions were described previously (19, 20, 29). Amotl2 truncation mutants were amplified by polymerase chain reaction (PCR) and cloned into pCMV5-HA or pXT7. The mutants Amotl2ΔC1, ΔC2, ΔC3, ΔN2, ΔN3, ΔN4, and ΔM3 retained aa1–182, 1–268, 1–485, 183–721, 269–721, 486–721, and 1–268 joining to 486–721 of wild-type Amotl2, respectively. Unless otherwise stated, Amotl2 was referred to as zebrafish Amotl2. Human AMOT, AMOTL1, and AMOTL2 coding sequences were individually amplified from the cDNAs derived from HEK293T cells and cloned into pXF2F-Flag. The shorter form of human AMOTL2 was amplified using the forward primer (5′-ATATAGCATATGGAGGCCGTGCTGAG-3′) and the reverse primer (5′-ATATAGTCTAGATTAGATCAGTATCTCCACC-3′). The sequences of shRNA targeting human AMOT, AMOTL1, and AMOTL2 were previously described (21). The sequence of scramble shRNA was the same as Addgene plasmid 1864 (30). All the shRNA sequences were inserted into pLentiLox3.7. All of the constructs were verified by DNA sequencing.
Cell Culture and Cell Fractionation
HEK293T, HeLa, and L cell lines were grown in DMEM medium (Invitrogen) with 10% fetal bovine serum (HyClone) and 1% penicillin/streptomycin (Invitrogen). The L Wnt3a cell line was grown in the same way except addition of 0.4 mg/ml G-418 into the culture medium. The L conditional medium (L CM) and Wnt3a conditional medium (Wnt3a CM) were generated following the protocol of ATCC. For Wnt stimulation treatments, L CM or Wnt3a CM was mixed with normal culture medium at the volume ratio 1:1. Cell fractionation was done as described (31). The different fractions were collected separately for immunoblotting.
Luciferase Reporter Assay, Immunoblotting, Co-immunoprecipitation, and Immunofluorescence
HEK293T cells lysates were used for luciferase reporter assay, immunoblotting and immunoprecipitation as described previously (20, 32, 33). Each luciferase reporter assay was repeated three times, and the average activity was calculated. Immunofluorescence was performed in HeLa cells as described (32). Primary antibodies were as fellows: anti-HA (F-7), anti-β-catenin (H102), anti-α-tubulin (H-300), and anti-actin (I-19) from Santa Cruz Biotechnology; anti-Flag (F1804) from Sigma; and anti-Lamin B (ab16048) from Abcam. Secondary antibodies conjugated with HRP or DyLight Fluorescent Dyes were purchased from Jackson ImmunoResearch Laboratories.
Quantitative Real-time PCR
Quantitative real-time PCR were performed as described previously (34). The primers for AMOT were: 5′-CTTGATGGCCAATAAGCGTTGCCT-3′ (forward) and 5′-GCAAGCCTGATCCAGCATTGGAAA-3′ (reverse); those for AMOT1 were: 5′- ACCCAGCAGAAACATGGAAATGGC-3′ (forward) and 5′-GGCATTCATGGCAAAGTGTCGGAT-3′ (reverse); those for AMOTL2 were: 5′-ACATGACCAAGTGGGA GCAGAAGT-3′ (forward) and 5′-ACCAGTGAGCAGACCCTCATTGAA-3′ (reverse). The primers for AXIN2 were described by others (35).
Morpholinos, mRNA Synthesis, and Microinjection
Antisense morpholinos targeting different zebrafish Motin members were: amotMO1 (5′-CCACTGACACAACTACCACCAAGTG-3′) (36), amotl1MO1 (5′-CCTCGATC TCCAACTGCAAATGTTC-3′) (37), and amotl2MO1 (5′-CTGATGATTC CTCTGCCGTTCTCAT-3′) (19). amotl2cMO1 (5′-TACTCTTGCCGTCTCCTTA GTAGTC-3′) was a control which was inverted from amotl2MO1 (19). The zebrafish β-catenin1 and β-catenin2 genes were depleted using morpholinos: β-cat1MO1 (5′-ATCAAGTCAGACTGGGTAGCCATGA-3′) (38) and β-cat2MO1 (5′-CCTTTAGCCTGAGCGACTTCCAAAC-3′) (6). All morpholinos were synthesized from GeneTools, LLC, and injected alone or in combination into the yolk of zebrafish embryos at the one-cell stage.
Capped mRNAs were synthesized using T7 mMessage mMachine Kit (Ambion) according to the manufacturer's instructions. The synthesized mRNAs were purified using RNeasy Mini Kit (Qiagen). amotl2m mRNA contained base substitutions in amotl2MO1 recognition sequences (20) and was only used for rescuing amotl2MO1-induced phenotypes. All of the mRNAs were injected into the yolk of zebrafish embryos at the one-cell stage.
Whole-mount in Situ Hybridization
Corresponding linearized plasmids were used for in vitro synthesizing antisense RNA in the presence of digoxingenin-UTP. Whole-mount in situ hybridization was performed following the standard procedure. Live embryos and stained embryos were mounted in 5% methylcellulose and glycerol, respectively, and photographed using a Leica MZ16 microscope with a Dage-MTI DC330 CCD camera. Immunohistochemical staining of β-catenin in embryos was carried out using anti-β-catenin antibody and DAB substrate.
Genotyping of mbl Embryos
Embryos produced by crosses of mbl heterozygotes were collected at 2 days postfertilization (dpf) after injection and genomic DNA was individually extracted. A 120-bp sequence at the mbl/axin1 locus was amplified by PCR using the forward primer (5′-GGATACCAAAGGATATTCACGTTG-3′) and the reverse primer (5′-ACCCTCTTGAGACGCTCCTCCAGC-3′) as described (13). The amplified products were subjected to BpmI restriction digestion and then separated on agarose gels. The digest derived from the wild-type allele consisted of 80-bp and 40-bp fragments, but digest derived from the mutant allele was 120-bp long.
Statistical Analysis
Statistical analyses were performed with Office Excel. The values were showed as means ± standard deviation for at least three independent experiments. Two-tailed t test was performed to show the significant differences.
RESULTS
amotl2 Knockdown Dorsalizes Embryos in Zebrafish
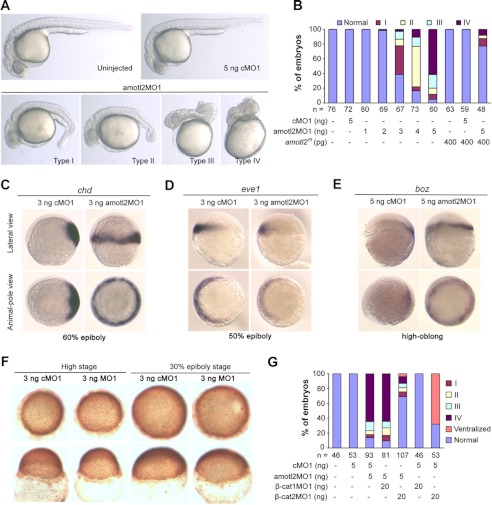
We previously reported that amotl2 knockdown in zebrafish embryos, by injecting the antisense morpholino amotl2MO1, results in defects in epiboly and convergent and extension movements (19). However, as observed at 24 hpf, we consistently noted that amotl2 morphants exhibited dorsalized phenotypes. Based on the degrees of dorsalization, the morphants could be categorized into four classes (Fig. 1A). Class I embryos had a smaller tail; class II showed a smaller poster trunk with a curly tail and a shortened yolk extension; class III embryos had visible head structures, but curly trunk and tail; and class IV embryos had undistinguishable tissue structures sitting on the yolk ball. The ratio of severely dorsalized morphants (classes III and IV) increased as higher doses of amotl2MO1 were used (Fig. 1B). Injection with 400 pg of amotl2m mRNA alone or its co-injection with 5 ng of the control morpholino cMO1, did not cause obvious morphological changes at 24 hpf. However, its co-injection with 5 ng amotl2MO1 led to a marked decrease of the ratio of dorsalized embryos compared with 5 ng amotl2MO1 injection alone (Fig. 1B). We then looked into the expression of dorsal and ventral markers in morphants during early development. The dorsal marker chordin (chd), which is expressed on the dorsal side in wild-type embryos, was expanded into the whole germ ring in 81.8% (n = 110) of embryos injected with 3 ng of amotl2MO1 at the 60% epiboly stage (Fig. 1C), whereas the ventral marker eve1 was markedly reduced in 90.2% (n = 41) of similarly injected embryos at the 50% epiboly stage (Fig. 1D). These observations support the idea that amotl2 knockdown results in embryonic dorsalization.
FIGURE 1.
amotl2 knockdown results in embryonic dorsalization. Wild-type embryos at the one-cell stage were injected with indicated morpholinos and mRNA and observed at later stages. A, morphology of representative live embryos at 24 hpf. The dorsalized phenotypes of types I-V were shown. All embryos were viewed laterally. B, percentage of embryos in different categories after amotl2 knockdown. n, the number of analyzed embryos. Higher doses of amotl2MO1 caused more severe dorsalization that was rescued by co-injection of amotl2m mRNA. C–E, expansion of the dorsal markers chd (C) and the Wnt target boz (E) and reduction of the ventral makrer eve1 (D) in amotl2 morphants, detected by in situ hybridization. amotl2 morphants developed slower and so were fixed when they matched the indicated stages of control embryos. F, immunohistochemical staining of β-catenin in embryos using anti-β-catenin antibody. Upper panel, animal-pole views; lower panel, dorsal view with animal-pole to the top. G, percentage of embryos in different categories after amotl2 and β-catenin1/β-catenin2 co-knockdown. Co-injection of β-cat2MO but not β-cat1MO lessened amotl2MO1-induced dorsalization.
amotl2MO1-induced Dorsalization Is Associated with Up-regulation of Wnt/β-catenin Signaling
It has been found that β-catenin2-mediated maternal canonical Wnt signaling is essential for dorsal development of zebrafish embryos while maternal β-catenin1 has no effect (6). We asked whether embryonic dorsalization caused by amotl2 knockdown was related to up-regulation of Wnt/β-catenin signaling. To address this issue, we examined the expression of bozozok (boz), a direct target gene of Wnt/β-catenin signals (39), in amotl2 morphants. In cMO1-injected embryos at the high-oblong stages, boz expression was restricted to the dorsal blastodermal margin (Fig. 1E). In contrast, boz expression could be detected in the whole germ ring in 87.5% (n = 40) of amotl2 morphants (Fig. 1E). Immunostaining experiments revealed that more blastomeres of amotl2 morphants had more nuclear β-catenin compared with those in control embryos (Fig. 1F). In addition, only a small proportion of embryos co-injected with 5 ng of amotl2MO1 and 20 ng of β-catenin2MO1 exhibited dorsalized phenotypes compared with those co-injected with 5 ng of amotl2MO1 and 20 ng of β-catenin1MO1 (Fig. 1G), which was indicative of an antagonistic effect. These results together suggest that amotl2 knockdown up-regulates Wnt/β-catenin signaling and as a result causes embryonic dorsalization.
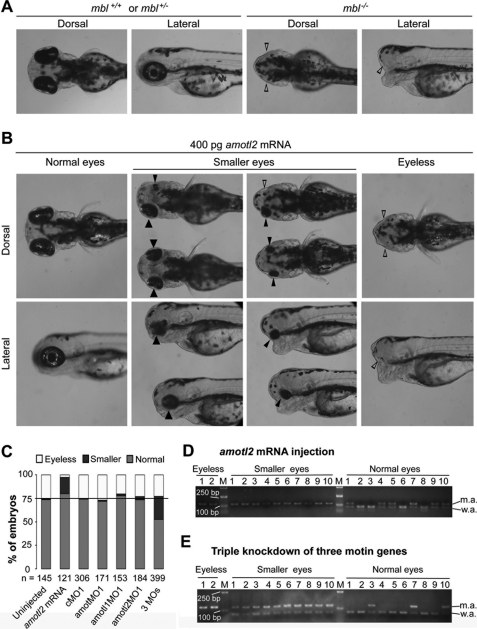
amotl2 Overexpression Rescues the Eyeless Phenotype of Masterblind Mutants
To further confirm the involvement of amotl2 in Wnt/β-catenin signaling, we performed amolt2 overexpression or knockdown in mbl mutants, which carry a mutation in the axin1 locus resulting in up-regulated Wnt/β-catenin signaling (13). Among 145 embryos observed at 3 dpf, which were produced by crosses between mbl heterozygotes (Tubingen background), 37 (25.5%) did not have eyes (eyeless phenotype), 1 (0.7%) had smaller eyes, and 107 had eyes of normal size (Fig. 2, A and C), suggesting a Mendelian segregation of the eyeless phenotype. When amotl2 mRNA was injected into one-cell stage embryos of mbl intercrosses at a dose of 400 pg, only 2.5% (n = 121) of embryos at 3 dpf exhibited the eyeless phenotype and 17.4% had a pair of smaller eyes or one smaller eye with the other disappeared (Fig. 2, B and C), suggesting that the eyes of a large proportion of mbl mutants were partially recovered by amotl2 overexpression. Genotyping of a batch of injected embryos disclosed that all of 10 analyzed embryos with smaller eyes were mbl−/− mutants, two tested eyeless embryos were also homozygous mutants, while 10 embryos with normal eyes had either mbl+/+ (4/10) or mbl+/− (6/10) genotypes (Fig. 2D). Thus, we conclude that amotl2 overexpression can rescue the eyeless phenotype of mbl−/− mutants.
FIGURE 2.
amotl2 overexpression or knockdown in mbl embryos affects eye phenotypes. All of used embryos were derived from crosses of mbl hetergygotes. A and B, different types of eyes of uninjected (A) or amotl2 mRNA-injected (B) live embryos at 3 dpf. The heads were shown. Large solid, small solid, and small empty arrowheads indicated normal eyes, small eyes, and absence of eyes, respectively. C, ratios of embryos with different eye phenotypes at 3 dpf. Injection doses: cMO1, amotMO1, and amotl1MO1, 10 ng; amotl2MO1, 2 ng amotl2MO + 8 ng cMO1; 3MOs, 4 ng amotMO1 + 4 ng amotl1MO1 + 2 ng amotl2MO1; amotl2 mRNA, 400 pg. D and E, genotyping of individual embryos with different eye types after injection with 400 pg amotl2 mRNA (D) or 3MOs (E). BpmI digests of amplified fragments from individual embryos were separated on agarose gels. M, molecular weight markers; w.a., wild-type allele; m.a., mutant allele.
Next, we asked whether amotl2 knockdown could enhance the abnormal eye phenotype of mbl mutants. To avoid early dorsoventral patterning defects caused by a high dose (5 ng) of amotl2MO1, we reduced the injection dose of amotl2MO1 to 2 ng, which did not produce obvious morphological defects when applied to wild-type embryos (20). When embryos produced from mbl intercrosses were injected with amotl2MO1 at this dose, the ratio of embryos with eyeless phenotype was not biased. Individual knockdown of amot and amotl1, two other genes of Motin family in the zebrafish genome, did not prejudice the ratio of eyeless embryos among those of mbl intercrosses. However, when amotl2, amotl1 and amot in embryos of mbl intercrosses were simultaneously knocked down, 24.8% (n = 399) had smaller eyes at 3dpf in addition to 22.6% showing the eyeless phenotype (Fig. 2C). Genotyping of triple knockdown embryos indicated that all of 10 analyzed embryos with smaller eyes were heterozygous for mbl (mbl+/−), two eyeless embryos were mutants, while embryos with normal eyes had heterogeneous genotypes (mbl+/+ or mbl+/−) (Fig. 2E). These data support the idea that simultaneous depletion of Motin family members can suppress eye development of mbl heterozygotes.
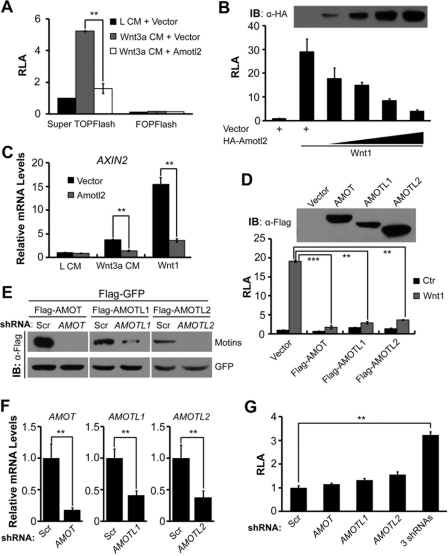
Amotl2 Inhibits Wnt/β-Catenin Signaling in Mammalian Cells
The above data obtained in zebrafish embryos suggest that Amotl2 inhibits Wnt/β-catenin signaling. To elucidate the underlying mechanism, we switched to mammalian cell system for easy manipulations. The expression of the Wnt responsive reporter Super TOPFlash in HEK293T cells was activated in the Wnt3a conditional medium and co-transfection of zebrafish Amotl2 inhibited the activated reporter expression (Fig. 3A). Amotl2 also attenuated Wnt1-activated TOPFlash reporter expression in a dose dependent manner (Fig. 3B). The Wnt-stimulated expression of endogenous AXIN2, a direct target gene of Wnt/β-catenin signaling, was also inhibited by overexpression of zebrafish Amotl2 (Fig. 3C). Like zebrafish Amotl2, overexpression of human AMOT, AMOTL1, or AMOTL2 in HEK293T cells all repressed Wnt1-activated TOPFlash reporter expression (Fig. 3D). These results indicate that all members of Motin family are able to interfere in Wnt signaling.
FIGURE 3.
Amotl2 and other two Motin members inhibit Wnt/β-catenin signaling in mammalian cells. All assays were performed in HEK293T cells. A, overexpression of zebrafish Amotl2 attenuated Super TOPFlash reporter expression in Wnt3a conditional medium. RLA, relative luciferase activity. B, zebrafish Amotl2 inhibited Wnt1-stimulated Super TOPflash expression. Wnt1 plasmid was co-transfected with different amounts of HA-Amotl2 plasmid. HA-Amotl2 level was detected by Western blotting and shown in the top panel. C, transfection of zebrafish Amotl2 reduced AXIN2 transcription. AXIN2 mRNA level was quantified by real-time PCR 48 h after transfection and normalized to GAPDH mRNA level. D, human Motin proteins inhibited Super TOPflash expression. Expression levels of Flag-tagged human AMOT, AMOTL1, and AMOTL2 were detected by Western blotting and shown in the top panel. E and F, knockdown efficiency of human Motin family members as examined by Western blotting (E) and real-time PCR (F). Flag-GFP and scramble shRNA (scr) were served as transfection and specificity control, respectively. G, simultaneous knockdown of three Motin family members up-regulated Wnt1-stimulated Super TOPflash expression. The luciferase activity was normalized to that using scramble shRNA. Statistical significance levels: **, p < 0.01; ***, p < 0.001.
To test whether endogenous Motin proteins act to suppress Wnt signaling, we used shRNA to knock down AMOT, AMOTL1 and AMOTL2 in HEK293T cells (Fig. 3, E and F). Individual knockdown of these genes failed to significantly activate TOPFlash expression in HEK293T cells (Fig. 3G). However, co-knockdown of the three genes resulted in a significant increase of the reporter expression. These results suggest that members of Motin family are functionally redundant and are required for repression of Wnt signaling.
Amotl2 Blocks β-catenin Nuclear Accumulation by Detaining It in Cytoplasmic Punctate Structures
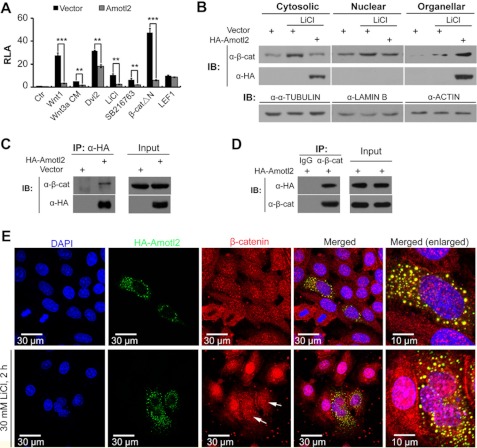
Wnt/β-catenin signaling consists of multiple steps and involves many proteins (40). We set to determine at which step Amotl2 could inhibit Wnt/β-catenin signaling. The reporter assays in HEK293T cells showed that co-transfection of Amotl2 was able to suppress the TOPFlash reporter expression activated by Wnt3a conditional medium, transfection of plasmids Wnt1, Dvl2, or β-cateninΔN (constitutively active form due to loss of GSK3β-binding domain), or treatment with the GSK3β inhibitors LiCl or SB216763 while it failed to block LEF1-promoted reporter expression (Fig. 4A). These results indicate that Amotl2 most likely plays a role in regulating β-catenin function.
FIGURE 4.
Amotl2 interacts with β-catenin and arrests it in cytoplasmic punctate structures. A, transfection of zebrafish Amotl2 inhibited TOPFlash reporter expression activated by different ways in HEK293T cells. The reporter was activated by co-transfection of Wnt1, Dvl2, β-cateninΔN, or LEF1 or by addition of Wnt3a CM, LiCl or SB216763. **, p < 0.01; ***, p < 0.001. B, transfection of Amotl2 reduced cytosolic and nuclear β-catenin but enriched it in organellar fraction in HEK293T cells. LiCl was added to a final concentration of 30 mm 44 h after transfection. 4 h later, cells were collected and subjected to cell fractionation assay. α-TUBULIN, LAMIN B, and ACTIN were markers for cytosolic, nuclear and organellar fractions, respectively. C and D, zebrafish Amotl2 expressed in HEK293T cells associated with endogenous β-catenin. IP: immunoprecipitation; IB, immunoblot. E, Amotl2 co-localized with β-catenin in cytoplasmic punctuate structures and resulted in a reduction of nuclear β-catenin in HeLa cells. 22 h after transfection of HA-Amotl2, LiCl was added to a final concentration of 30 mm and cells were subjected to immunofluorescence assay 2 h later using anti-HA and anti-β-catenin antibodies. Arrows indicated cells expressing Amotl2 with reduced nuclear β-catenin.
Then we tested whether intracellular locations of endogenous β-catenin could be affected by transfection of Amotl2. Western blot analysis revealed that overexpressed HA-Amotl2 protein in HEK293T cells was detected in cytosolic and organellar fractions but not in nuclear fraction (Fig. 4B). LiCl treatment led to an increase of β-catenin protein in cytosolic, nuclear and organellar fractions (Fig. 4B). When LiCl was applied, overexpression of Amotl2 caused a decrease of β-catenin in cytosolic and nuclear fractions but an increase in the organellar fraction (Fig. 4B). These results imply that Amotl2 overexpression may reduce cytosolic and nuclear β-catenin by recruiting it in some cytoplasmic organelles.
Co-immunoprecipitation experiments disclosed that co-expressed HA-Amotl2 could interact with endogenous β-catenin (Fig. 4, C and D). We performed immunofluorescence assay to detect the locations of Amotl2 and β-catenin in HeLa cells. In cells without LiCl treatment, HA-Amotl2 and endogenous β-catenin were co-localized in cytoplasmic punctate structures (Fig. 4E, upper panel). LiCl treatment led to nuclear accumulation of β-catenin, but this effect was blocked by overexpression of HA-Amotl2 (Fig. 4E, lower panel). We hypothesize that Amotl2 prevents translocation of β-catenin into nuclei by retaining it in cytoplasmic punctate structures.
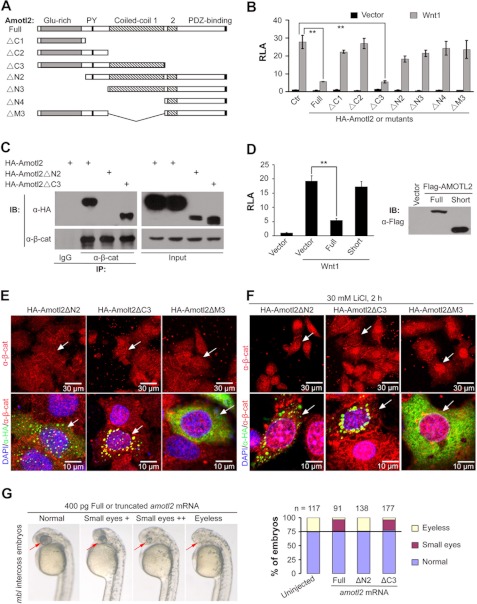
The N-terminal Glutamine-rich and the Middle Coiled-coil Domain 1 Are Essential For Amotl2 to Inhibit Wnt/β-catenin Signaling
To identify domains of Amotl2 for inhibiting Wnt/β-catenin signaling, we generated various truncation mutant forms of zebrafish Amotl2 (Fig. 5A). Reporter assays in HEK293T cells showed that transfection of Amotl2ΔC3, which retained the N-terminal glutamine-rich and the first coiled-coil domain, attenuated Wnt1-stimulated TOPFlash reporter expression, and that overexpression of the mutant forms without either of these domains failed to efficiently block the reporter expression (Fig. 5B). Further co-immunoprecipitation analysis showed that Amotl2ΔN2, a mutant lacking the N-terminal glutamine-rich domain, could not interact with β-catenin, but Amotl2ΔC3 lacking the C-terminal PDZ-binding motif still associated with β-catenin (Fig. 5C). These data suggest that the N-terminal glutamine-rich and the middle coiled-coil domain 1 mediate the inhibitory effect of Amotl2 on Wnt/β-catenin signaling while the C-terminal PDZ-binding domain is not required for this function. Interestingly, a human cDNA deposited in the GenBankTM (accession number AAH11454) encodes a 466-aa peptide, a short isoform lacking 1- 313 aa of the full (long) AMOTL2 (780 aa) and resembling our Amotl2ΔN3 mutant. Reporter assays showed that human full-length AMOTL2 but not short AMOTL2 was able to inhibit Wnt1-stimulated TOPFlash reporter expression (Fig. 5D). The physiological importance of the short AMOTL2 needs to be investigated in the future.
FIGURE 5.
The N-terminal and coiled-coil domain of Amotl2 are essential for its inhibition of Wnt/β-catenin signaling. A, sketch map of truncation mutants of zebrafish Amotl2. Conserved domains were indicated: glutamine-rich domain, PPXY motif (PY), coiled-coil domains 1 and 2, and PDZ-binding motif. B, effects of various constructs on TOPFlash reporter expression in HEK293T cells. **, p < 0.01; ***, p < 0.001. C, Amotl2ΔC3 retained but Amotl2ΔN2 lost the ability to bind to β-catenin in HEK293T cells. D, full-length isoform but not short isoform of huma AMOTL2 attenuated TOPFlash reporter expression in HEK293T cells. The expression levels of transfected AMOTL2 were examined by Western blotting and shown on the right side. E and F, co-localization of representative Amotl2 mutants with endogenous β-catenin in HeLa cells without (E) or with LiCl treatment (F). Arrows indicated transfected cells. G, rescuing effects of overexpression of representative amotl2 mutant mRNAs on the eyeless phenotype of zebrafish mbl emryos. Embryos produced by mbl heterozygote intercrosses were injected at the one-cell stage and observed at 30 hpf for eye morphology. The statistical data were shown in a bar graph on the right.
We next examined, by immunofluorescence staining, the subcellular localization of representative Amotl2 mutants in HeLa cells. As shown in Fig. 5E, Amotl2ΔC3 was distributed in a way similar to full-length Amotl2 (Fig. 4E) and was co-localized with β-catenin in cytoplasmic punctate structures; however, Amotl2ΔN2 was rarely co-localized with β-catenin though it appeared to be in some punctuate structures; Amotl2ΔM3 was evenly distributed in the cytosol. Following LiCl treatment, Amotl2ΔC3, but not Amotl2ΔN2 or Amotl2ΔM3, could inhibit nuclear accumulation of β-catenin (Fig. 5F). These results support the idea that Amotl2 requires the N-terminal glutamine-rich domain and the middle coiled-coil domain for capturing β-catenin in specialized cytosolic vesicles.
To investigate the requirement of the N-terminal glutamine-rich and the middle coiled-coil domain 1 for Amotl2 functioning in vivo, we injected one-cell stage embryos of mbl intercrosses with mRNA of the representative amotl2 mutant amotl2ΔN2 or amotl2ΔC3. Like full length amotl2 mRNA, amotl2ΔC3 injection markedly reduced the ratio of eyeless embryos at 30 hpf while amotl2ΔN2 had no effect (Fig. 5G). Therefore, the N-terminal glutamine-rich and the middle coiled-coil domains of Amotl2 is essential for Amotl2 function in zebrafish embryos.
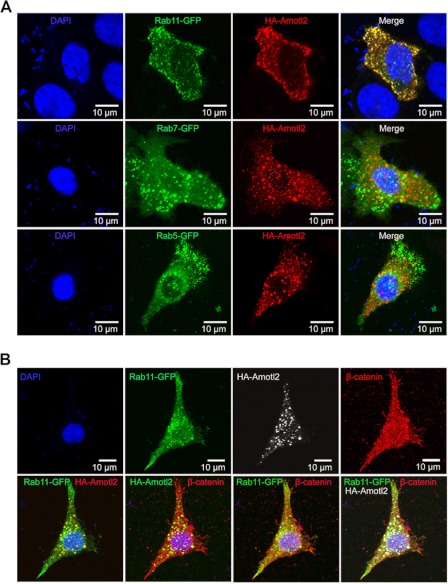
Amotl2 Is Localized in Recycling Endosomes
A previous report demonstrates that AMOT is localized in endocytic recycling compartments through the coiled-coil domain (41). We asked whether the Amotl2-containing cytosolic punctuate structures are also recycling compartments. Co-immunostaining analyses showed that Amotl2 was co-localized with the recycling endosomal marker Rab11, but not with the early endosomal marker Rab5 or the late endosomal marker Rab7 (Fig. 6A). Furthermore, HA-Amotl2, Rab11-GFP, and endogenous β-catenin could be co-detected in some vesicles (Fig. 6B). Therefore, we conclude that Amotl2 binds to and arrest β-catenin in recycling endosomes.
FIGURE 6.
Co-localization of Amotl2, Rab11, and β-catenin. A, HA-Amotl2 was co-localized with Rab11-GFP but not Rab5-GFP and Rab7-GFP. HA-Amotl2 was co-transfected with Rab5-GFP, Rab7-GFP, or Rab11-GFP into HeLa cells, respectively. 24 h after transfection, cells were subjected to immunostaining using anti-HA antibody and DAPI and observed by confocal microscopy. B, HA-Amotl2 was co-localized with Rab11-GFP and endogenous β-catenin. HA-Amotl2 and Rab11-GFP were co-transfected into HeLa cells. Cells were immunostained 24 h after transfection using anti-HA and anti-β-catenin antibodies as well as DAPI. A single cell was shown for individual markers (upper panel) and for merged multiple markers (lower panel).
DISCUSSION
Although Motin family members have been shown to control cell migration, proliferation, and differentiation by regulating several signaling pathways, it is unclear whether they could play a role in embryonic patterning and participate in canonical Wnt signaling. In this study, we demonstrate that amotl2 is essential for repressing dorsal development and normal development of the forebrain/eye development in zebrafish embryos. Mechanistically, Amolt2 can associate with and trap β-catenin in Rab11-positive recycling endosomes and thus prevent translocation of β-catenin into nuclei.
In zebrafish, amot, amotl1, and amotl2 are all expressed during early development. We found that amotl2 knockdown alone caused embryonic dorsalization whereas knockdown of amot or amotl1 knockdown (even at a dose of 10 ng) did not affect early development. It is likely that, among the Motin family members, amotl2 is a major player in germ layer formation and immediate patterning process. Injection of 2 ng of amotl2MO1 into one-cell embryos derived from mbl heterozygote mating did not change the ratio of eyeless embryos at later stages. Similarly, injection of 4 ng (or 10 ng) of amotMO or amotl1MO did not affect eye development. However, co-injection of three MOs converted normal eyes of mbl heterozygotes into smaller eyes (Fig. 2, C and D). Since repression of Wnt/β-catenin signaling is crucial for the forebrain and eye development after the onset of gastrulation (10, 11), we believe that all of the Motin family members are required for controlling this signaling pathway during the anteroposterior patterning of the neuroectoderm. Much more work are needed in the future to address the specific and cooperative functions of Motin family members in various developmental processes.
We showed that zebrafish Amotl2, human AMOT, AMOTL1, or AMOTL2 overexpressed in HEK293T cells could inhibit Wnt/β-catenin reporter expression (Fig. 3, A and D). Co-knockdown but not individual knockdown of human Motin genes by shRNA enhanced Wnt/β-catenin signaling (Fig. 3G). Therefore, all of three Motin members function to repress Wnt/β-catenin signaling. Focusing on zebrafish Amotl2, we demonstrated that Amotl2 trapped β-catenin in Rab11-posititve recycling endosomes, which resulted in a reduction of cytosolic and nuclear β-catenin. It appears that Amotl2 is involved in intracellular trafficking of β-catenin. It has been well known that β-catenin not only transduces Wnt signals but participates in cadherins-mediated cell adhesions (42). Redistribution of intracellular β-catenin may require active endocytosis and exocytosis (43, 44). However, we did not observe an obvious increase of β-catenin attached to the plasma membrane in Amotl2-overexpressing cells by immunofluorescence analysis. Overexpression of Amotl2 in HEK293T cells did not increase the amount of β-catenin bound to cadherins as examined by co-immunoprecipitation (data not shown). These observations exclude the possibility that Amotl2 promotes cell adhesion, which is consistent with our previous finding that Amotl2 overexpression accelerates cell migration (19, 20). Therefore, Amotl2 may just act to recruit free cytosolic β-catenin to recycling endosomes, which may be released into the cytosol upon unknown stimulation.
We have previously shown that Amotl2 can up-regulate FGF/MAPK signaling and phosphorylation of tyrosine 103 is necessary for this function (20). MAPK signaling has been found to enhance Wnt/β-catenin signaling by phosphorylating and inactivating GSK3β (45). Logically, one would not believe that Motin proteins suppress Wnt/β-catenin signal by activating MAPK pathway. Nevertheless, we found that Amotl2(Y103F) mutant, which loses the ability to up-regulate FGF/MAPK signaling (20), still suppressed Wnt1- or LiCl-stimulated Super TOPFlash reporter expression (data not shown). This result excludes the possibility that Amotl2 suppresses Wnt/β-catenin signaling through activation of the MAPK cascade.
Motin proteins have been reported to regulate Hippo signaling pathway by activating LATS and trapping YAP and TAZ in the cytoplasm (21, 23, 31, 46). On the other hand, Hippo signaling has been found to suppress Wnt/β-catenin signal transduction via distinct mechanisms (47–49). Logically, one would not believe that Motin proteins suppress Wnt/β-catenin signal by inhibition of Hippo pathway. We found that the zebrafish Amotl2 mutant Amotl2(Y208A), which would not associate with YAP and TAZ (21, 31), retained the ability to suppress Wnt1- or β-catenin-stimulated TOPFlash reporter expression (data not shown). We hypothesize that Amotl2 traps β-catenin and YAP/TAZ using different motifs. It remains unclear whether β-catenin and YAP/TAZ are co-trapped by Motin proteins in the same vesicles.
In summary, we uncover a novel function of Motin family members in down-regulating Wnt/β-catenin signaling. This function plays a role in dorsoventral patterning and neuroectodermal anteroposterior differentiation in zebrafish embryos. Thus, Motin proteins act as scaffolds to regulate various signaling pathways and developmental processes.
Acknowledgments
We thank Drs. Bernard Thisse and Christine Thisse for assistance and discussion. We are grateful to Drs. Wei Wu, Xinhua Feng, and Qiang Wang for plasmids. We also thank Yeguang Chen and Wei Wu for helpful discussion.
This work was supported by grants from the Major Science Programs of China (2011CB943800) and from National Natural Science Foundation of China (30921004/C061003).
- Amot
- Angiomotin
- masterblind
- mbl
- MO
- morpholino
- dpf
- days postfertilization
- hpf
- hours postfertilization
- Wnt3a CM
- Wnt3a conditional medium.
REFERENCES
- 1. Clevers H. (2006) Wnt/β-catenin signaling in development and disease. Cell 127, 469–480 [DOI] [PubMed] [Google Scholar]
- 2. Huelsken J., Birchmeier W. (2001) New aspects of Wnt signaling pathways in higher vertebrates. Curr. Opin. Genet. Dev. 11, 547–553 [DOI] [PubMed] [Google Scholar]
- 3. Logan C. Y., Nusse R. (2004) The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 20, 781–810 [DOI] [PubMed] [Google Scholar]
- 4. Nusse R., Fuerer C., Ching W., Harnish K., Logan C., Zeng A., ten Berge D., Kalani Y. (2008) Wnt signaling and stem cell control. Cold Spring Harb. Symp. Quant. Biol. 73, 59–66 [DOI] [PubMed] [Google Scholar]
- 5. Langdon Y. G., Mullins M. C. (2011) Maternal and zygotic control of zebrafish dorsoventral axial patterning. Annu. Rev. Genet. 45, 357–377 [DOI] [PubMed] [Google Scholar]
- 6. Bellipanni G., Varga M., Maegawa S., Imai Y., Kelly C., Myers A. P., Chu F., Talbot W. S., Weinberg E. S. (2006) Essential and opposing roles of zebrafish β-catenins in the formation of dorsal axial structures and neurectoderm. Development 133, 1299–1309 [DOI] [PubMed] [Google Scholar]
- 7. Xiong B., Rui Y., Zhang M., Shi K., Jia S., Tian T., Yin K., Huang H., Lin S., Zhao X., Chen Y., Chen Y. G., Lin S. C., Meng A. (2006) Tob1 controls dorsal development of zebrafish embryos by antagonizing maternal β-catenin transcriptional activity. Dev. Cell 11, 225–238 [DOI] [PubMed] [Google Scholar]
- 8. Lekven A. C., Thorpe C. J., Waxman J. S., Moon R. T. (2001) Zebrafish wnt8 encodes two wnt8 proteins on a bicistronic transcript and is required for mesoderm and neurectoderm patterning. Dev. Cell 1, 103–114 [DOI] [PubMed] [Google Scholar]
- 9. Ramel M. C., Lekven A. C. (2004) Repression of the vertebrate organizer by Wnt8 is mediated by Vent and Vox. Development 131, 3991–4000 [DOI] [PubMed] [Google Scholar]
- 10. Stern C. D., Charité J., Deschamps J., Duboule D., Durston A. J., Kmita M., Nicolas J. F., Palmeirim I., Smith J. C., Wolpert L. (2006) Head-tail patterning of the vertebrate embryo: one, two or many unresolved problems? Int. J. Dev. Biol. 50, 3–15 [DOI] [PubMed] [Google Scholar]
- 11. Wilson S. W., Houart C. (2004) Early steps in the development of the forebrain. Dev. Cell 6, 167–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heisenberg C. P., Brand M., Jiang Y. J., Warga R. M., Beuchle D., van Eeden F. J., Furutani-Seiki M., Granato M., Haffter P., Hammerschmidt M., Kane D. A., Kelsh R. N., Mullins M. C., Odenthal J., Nusslein-Volhard C. (1996) Genes involved in forebrain development in the zebrafish, Danio rerio. Development 123, 191–203 [DOI] [PubMed] [Google Scholar]
- 13. Heisenberg C. P., Houart C., Take-Uchi M., Rauch G. J., Young N., Coutinho P., Masai I., Caneparo L., Concha M. L., Geisler R., Dale T. C., Wilson S. W., Stemple D. L. (2001) A mutation in the Gsk3-binding domain of zebrafish Masterblind/Axin1 leads to a fate transformation of telencephalon and eyes to diencephalon. Genes Dev. 15, 1427–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bratt A., Wilson W. J., Troyanovsky B., Aase K., Kessler R., Van Meir E. G., Holmgren L. (2002) Angiomotin belongs to a novel protein family with conserved coiled-coil and PDZ binding domains. Gene 298, 69–77 [DOI] [PubMed] [Google Scholar]
- 15. Troyanovsky B., Levchenko T., Månsson G., Matvijenko O., Holmgren L. (2001) Angiomotin: an angiostatin-binding protein that regulates endothelial cell migration and tube formation. J. Cell Biol. 152, 1247–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Levchenko T., Aase K., Troyanovsky B., Bratt A., Holmgren L. (2003) Loss of responsiveness to chemotactic factors by deletion of the C-terminal protein interaction site of angiomotin. J. Cell Sci. 116, 3803–3810 [DOI] [PubMed] [Google Scholar]
- 17. Bratt A., Birot O., Sinha I., Veitonmäki N., Aase K., Ernkvist M., Holmgren L. (2005) Angiomotin regulates endothelial cell-cell junctions and cell motility. J. Biol. Chem. 280, 34859–34869 [DOI] [PubMed] [Google Scholar]
- 18. Wells C. D., Fawcett J. P., Traweger A., Yamanaka Y., Goudreault M., Elder K., Kulkarni S., Gish G., Virag C., Lim C., Colwill K., Starostine A., Metalnikov P., Pawson T. (2006) A Rich1/Amot complex regulates the Cdc42 GTPase and apical-polarity proteins in epithelial cells. Cell 125, 535–548 [DOI] [PubMed] [Google Scholar]
- 19. Huang H., Lu F. I., Jia S., Meng S., Cao Y., Wang Y., Ma W., Yin K., Wen Z., Peng J., Thisse C., Thisse B., Meng A. (2007) Amotl2 is essential for cell movements in zebrafish embryo and regulates c-Src translocation. Development 134, 979–988 [DOI] [PubMed] [Google Scholar]
- 20. Wang Y., Li Z., Xu P., Huang L., Tong J., Huang H., Meng A. (2011) Angiomotin-like2 gene (amotl2) is required for migration and proliferation of endothelial cells during angiogenesis. J. Biol. Chem. 286, 41095–41104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang W., Huang J., Chen J. (2011) Angiomotin-like proteins associate with and negatively regulate YAP1. J. Biol. Chem. 286, 4364–4370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yi C., Troutman S., Fera D., Stemmer-Rachamimov A., Avila J. L., Christian N., Persson N. L., Shimono A., Speicher D. W., Marmorstein R., Holmgren L., Kissil J. L. (2011) A tight junction-associated Merlin-angiomotin complex mediates Merlin's regulation of mitogenic signaling and tumor suppressive functions. Cancer Cell 19, 527–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Paramasivam M., Sarkeshik A., Yates J. R., 3rd, Fernandes M. J., McCollum D. (2011) Angiomotin family proteins are novel activators of the LATS2 kinase tumor suppressor. Mol. Biol. Cell 22, 3725–3733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ranahan W. P., Han Z., Smith-Kinnaman W., Nabinger S. C., Heller B., Herbert B. S., Chan R., Wells C. D. (2011) The adaptor protein AMOT promotes the proliferation of mammary epithelial cells via the prolonged activation of the extracellular signal-regulated kinases. Cancer Res. 71, 2203–2211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ernkvist M., Aase K., Ukomadu C., Wohlschlegel J., Blackman R., Veitonmäki N., Bratt A., Dutta A., Holmgren L. (2006) p130-angiomotin associates to actin and controls endothelial cell shape. FEBS J 273, 2000–2011 [DOI] [PubMed] [Google Scholar]
- 26. Ernkvist M., Birot O., Sinha I., Veitonmaki N., Nyström S., Aase K., Holmgren L. (2008) Differential roles of p80- and p130-angiomotin in the switch between migration and stabilization of endothelial cells. Biochim. Biophys. Acta 1783, 429–437 [DOI] [PubMed] [Google Scholar]
- 27. Gagné V., Moreau J., Plourde M., Lapointe M., Lord M., Gagnon E., Fernandes M. J. (2009) Human angiomotin-like 1 associates with an angiomotin protein complex through its coiled-coil domain and induces the remodeling of the actin cytoskeleton. Cell Motil. Cytoskeleton 66, 754–768 [DOI] [PubMed] [Google Scholar]
- 28. Roudier E., Chapados N., Decary S., Gineste C., Le Bel C., Lavoie J. M., Bergeron R., Birot O. (2009) Angiomotin p80/p130 ratio: a new indicator of exercise-induced angiogenic activity in skeletal muscles from obese and non-obese rats? J. Physiol. 587, 4105–4119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gao X., Wen J., Zhang L., Li X., Ning Y., Meng A., Chen Y. G. (2008) Dapper1 is a nucleocytoplasmic shuttling protein that negatively modulates Wnt signaling in the nucleus. J. Biol. Chem. 283, 35679–35688 [DOI] [PubMed] [Google Scholar]
- 30. Sarbassov D. D., Guertin D. A., Ali S. M., Sabatini D. M. (2005) Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307, 1098–1101 [DOI] [PubMed] [Google Scholar]
- 31. Zhao B., Li L., Lu Q., Wang L. H., Liu C. Y., Lei Q., Guan K. L. (2011) Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Genes Dev. 25, 51–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang L., Zhou H., Su Y., Sun Z., Zhang H., Zhang L., Zhang Y., Ning Y., Chen Y. G., Meng A. (2004) Zebrafish Dpr2 inhibits mesoderm induction by promoting degradation of nodal receptors. Science 306, 114–117 [DOI] [PubMed] [Google Scholar]
- 33. Su Y., Zhang L., Gao X., Meng F., Wen J., Zhou H., Meng A., Chen Y. G. (2007) The evolutionally conserved activity of Dapper2 in antagonizing TGF-β signaling. FASEB J. 21, 682–690 [DOI] [PubMed] [Google Scholar]
- 34. Zhang Y., Li X., Qi J., Wang J., Liu X., Zhang H., Lin S. C., Meng A. (2009) Rock2 controls TGFβ signaling and inhibits mesoderm induction in zebrafish embryos. J. Cell Sci. 122, 2197–2207 [DOI] [PubMed] [Google Scholar]
- 35. Labbé E., Lock L., Letamendia A., Gorska A. E., Gryfe R., Gallinger S., Moses H. L., Attisano L. (2007) Transcriptional cooperation between the transforming growth factor-β and Wnt pathways in mammary and intestinal tumorigenesis. Cancer Res. 67, 75–84 [DOI] [PubMed] [Google Scholar]
- 36. Aase K., Ernkvist M., Ebarasi L., Jakobsson L., Majumdar A., Yi C., Birot O., Ming Y., Kvanta A., Edholm D., Aspenström P., Kissil J., Claesson-Welsh L., Shimono A., Holmgren L. (2007) Angiomotin regulates endothelial cell migration during embryonic angiogenesis. Genes Dev. 21, 2055–2068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zheng Y., Vertuani S., Nyström S., Audebert S., Meijer I., Tegnebratt T., Borg J. P., Uhlén P., Majumdar A., Holmgren L. (2009) Angiomotin-like protein 1 controls endothelial polarity and junction stability during sprouting angiogenesis. Circ. Res. 105, 260–270 [DOI] [PubMed] [Google Scholar]
- 38. Rottbauer W., Saurin A. J., Lickert H., Shen X., Burns C. G., Wo Z. G., Kemler R., Kingston R., Wu C., Fishman M. (2002) Reptin and pontin antagonistically regulate heart growth in zebrafish embryos. Cell 111, 661–672 [DOI] [PubMed] [Google Scholar]
- 39. Leung T., Söll I., Arnold S. J., Kemler R., Driever W. (2003) Direct binding of Lef1 to sites in the boz promoter may mediate pre-midblastula-transition activation of boz expression. Dev. Dyn. 228, 424–432 [DOI] [PubMed] [Google Scholar]
- 40. MacDonald B. T., Tamai K., He X. (2009) Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev. Cell 17, 9–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Heller B., Adu-Gyamfi E., Smith-Kinnaman W., Babbey C., Vora M., Xue Y., Bittman R., Stahelin R. V., Wells C. D. (2010) Amot recognizes a juxtanuclear endocytic recycling compartment via a novel lipid binding domain. J. Biol. Chem. 285, 12308–12320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Brembeck F. H., Rosário M., Birchmeier W. (2006) Balancing cell adhesion and Wnt signaling, the key role of β-catenin. Curr. Opin. Genet. Dev. 16, 51–59 [DOI] [PubMed] [Google Scholar]
- 43. Pellón-Cárdenas O., Schweitzer J., D'Souza-Schorey C. (2011) Endocytic trafficking and Wnt/β-catenin signaling. Curr. Drug Targets 12, 1216–1222 [DOI] [PubMed] [Google Scholar]
- 44. Baum B., Georgiou M. (2011) Dynamics of adherens junctions in epithelial establishment, maintenance, and remodeling. J. Cell Biol. 192, 907–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ding Q., Xia W., Liu J. C., Yang J. Y., Lee D. F., Xia J., Bartholomeusz G., Li Y., Pan Y., Li Z., Bargou R. C., Qin J., Lai C. C., Tsai F. J., Tsai C. H., Hung M. C. (2005) Erk associates with and primes GSK-3β for its inactivation resulting in upregulation of β-catenin. Mol. Cell 19, 159–170 [DOI] [PubMed] [Google Scholar]
- 46. Chan S. W., Lim C. J., Chong Y. F., Pobbati A. V., Huang C., Hong W. (2011) Hippo pathway-independent restriction of TAZ and YAP by angiomotin. J. Biol. Chem. 286, 7018–7026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Varelas X., Miller B. W., Sopko R., Song S., Gregorieff A., Fellouse F. A., Sakuma R., Pawson T., Hunziker W., McNeill H., Wrana J. L., Attisano L. (2010) The Hippo pathway regulates Wnt/β-catenin signaling. Dev. Cell 18, 579–591 [DOI] [PubMed] [Google Scholar]
- 48. Heallen T., Zhang M., Wang J., Bonilla-Claudio M., Klysik E., Johnson R. L., Martin J. F. (2011) Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science 332, 458–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Imajo M., Miyatake K., Iimura A., Miyamoto A., Nishida E. (2012) EMBO J. 31, 1109–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]