Background: LDLR is regulated by the E3 ubiquitin ligase Mylip/Idol.
Results: FGF21 down-regulates Mylip/Idol expression and up-regulates its inhibitor Cnpy2/Msap.
Conclusion: Increased LDLR by FGF21 results in increased lipoprotein uptake in human hepatocyte cells.
Significance: The FGF21-mediated effect on cholesterol uptake is additive to that of statin, which may be of clinical significance.
Keywords: Cholesterol, E3 Ubiquitin Ligase, Fibroblast Growth Factor (FGF), Lipoprotein Receptor, Low Density Lipoprotein (LDL), Canopy 2, FGF21, Mylip, Statin
Abstract
The LDLR is a critical factor in the regulation of blood cholesterol levels that are altered in different human diseases. The level of LDLR in the cell is regulated by both transcriptional and post-transcriptional events. The E3 ubiquitin ligase, myosin regulatory light chain-interacting protein (Mylip)/inducible degrader of the LDL-R (Idol) was shown to induce degradation of LDLR via protein ubiquitination. We have here studied novel factors and mechanisms that may regulate Mylip/Idol in human hepatocyte cells and in mouse macrophages. We observed that FGF21 that is present in serum in different conditions reduced Mylip/Idol at the RNA and protein level, and increased LDLR levels and stability in the cells. FGF21 also enhanced expression of Canopy2 (Cnpy2)/MIR-interacting Saposin-like protein (Msap) that is known to interact with Mylip/Idol. Overexpression of Cnpy2/Msap increased LDLRs, and knockdown experiments showed that Cnpy2/Msap is crucial for the FGF21 effect on LDLRs. Experiments using DiI-labeled LDL particles showed that FGF21 increased lipoprotein uptake and the effect of FGF21 was additive to that of statins. Our results are consistent with an important role of FGF21 and Cnpy2/Msap in the regulation of LDLRs in cultured cells, which warrants further studies using human samples.
Introduction
High blood low density lipoprotein (LDL)2 cholesterol is a risk for development of cardiovascular diseases and atherosclerosis (1, 2). Statins are used to lower cholesterol, and these drugs increase the amount of LDLRs at the cell surface (2, 3). The LDLR mediates the uptake of LDL particles into cells, mainly hepatocytes, and the level of this receptor is modulated by both transcriptional and post-transcriptional mechanisms (4–6). It was recently shown that LDLRs are regulated by protein ubiquitination via Mylip/Idol that leads to LDLR degradation (7). Mylip/Idol itself is induced by the sterol-responsive nuclear receptor, LXR that is activated by cellular oxysterols (7, 8). The down-regulation of LDLR by Mylip/Idol represents a novel pathway to influence LDLR levels that may be amenable for drug intervention in various diseases (7–9). Mylip/Idol was originally cloned in nervous tissue as a factor that inhibited neurite outgrowth, and it binds to myosin regulatory light chain (MRLC) and was therefore called MRLC-interacting protein, Mir (10, 11). Subsequently, Mylip/Idol was linked to signaling by platelet-derived growth factor in fibroblast (12), and to the regulation of LDLRs in macrophages/hepatocytes (7) and of VLDLR in neurons (13). These results showed that Mylip/Idol plays a role in cell signaling and influences lipoprotein receptors and cellular lipid metabolism.
In this study, we have searched for novel factors that modulate Mylip/Idol and thereby possibly increase LDLR levels in cells. We have previously, using the yeast two-hybrid system, discovered a protein named Msap (MIR-interacting Saposin-like Protein) that interacts with Mylip/Idol in cells (14). Msap is a type-2 transmembrane protein that has a saposin-like domain in its structure (14). Msap belongs to the larger Canopy gene family of saposin-like proteins (SAPLIPs) (15) and is therefore here named Cnpy2/Msap. Cnpy2/Msap was shown to counteract the activities of Mylip/Idol, as evident from an increase in neurite outgrowth and cell migration that are both reduced by Mylip/Idol (14, 16). We therefore hypothesized that Cnpy2/MSAP constitutes a natural inhibitor/blocker of Mylip/Idol activity in different systems (9). In this study, we examined the effects of Cnpy2/Msap on LDLR using mouse macrophage and human hepatocyte cell lines. We also studied fibroblast growth factor-21 (FGF21) that belongs to the FGF19 subfamily of fibroblast growth factors (FGFs) that has endocrine and metabolic functions in the body (17, 18). Particularly, FGF21 has been linked to lipid metabolism (19, 20). We, therefore, examined whether FGF21 may influence cellular lipid metabolism via LDLRs and whether the Mylip/Idol and Cnpy2/Msap pathways might be involved in these effects.
EXPERIMENTAL PROCEDURES
Reagents and Plasmids Constructs
Expression plasmids for Cnpy2/Msap (14, 16), Mylip/Idol (8, 13) have been previously reported. The promoter region of mouse Mylip/Idol was cloned into the luciferase reporter vector to study gene activity.
Cell Culture, Transfections, and LDL Uptake
Mouse macrophage, Raw 264,7 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (Invitrogen), and the human hepatocyte, Huh7 cell line in MEM with Glutamax (Invitrogen) at 37 °C and 5% CO2. Cells were transfected with 2–4 μg of expression plasmids or short hairpin (sh)-RNA constructs (SABiosciences, MD) using Transfectin or Fugene reagents. Cells were stimulated with different concentrations of FGF21 (R&D systems) alone or together with 3 μm simvastatin (Sigma) for various periods of times. The synthetic LXR ligand, GW3965 was used to induce Mylip/Idol, and actinomycin D (Sigma) to inhibit gene transcription, and Mg132 (Sigma) to block proteasomes.
To assay LDL uptake, 5 μg/ml DiL-LDL (Molecular Probes) was added to the medium, and cells incubated further for 6 h at 37 °C. Cells were lysed, and the amount of DiI-LDL taken up was measured by a fluorometer and the values corrected for the total amount of protein. Dynasore (Sigma) inhibiting endocytosis was used as a negative control for the assay and blocked LDL uptake.
Raw 264,7 cells were also treated with native or acetylated LDL prior to stimulation with FGF21 to study foam cell formation. Native LDL was isolated from fresh human plasma by ultracentrifugation (21), and LDL was acetylated in the presence of acetic anhydride as described by Goldstein et al. (22). Cellular cholesterol ester deposition was imaged and quantified by Oil Red O staining. For colorimetric quantification, the cells were extensively washed with PBS, and the stain was solubilized in isopropanol. A490 was determined, and the results were normalized for total cell protein.
Immunoblotting and Immunoprecipitation
Cells were lysed, and immunoblots were made essentially as previously described (23, 24). Protein concentrations were determined using the Bradford assay and an equal amount of protein per sample was subjected to SDS-PAGE and blotted onto nitrocellulose filters (Amersham Biosciences, Helsinki, Finland). The filters were first incubated for 1 h in 50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 0.1% Tween 20, and 5% skimmed milk and then overnight at 4 °C with primary antibodies: Cnpy2/Msap (1:2000, Proteintech Europe), Mylip/Idol (1:1000, Abcam), anti-LDLR (1:2000; Cayman Chemical), rabbit anti-GFP (1:500, Chemicon), mouse anti-GFP (1:5000, Roche), and anti-β-actin (1:5000; Sigma). After washing, the filter was incubated with horseradish peroxidase-conjugated secondary antibodies (1:2500; Jackson ImmunoResearch, Espoo, Finland), followed by detection using enhanced chemiluminescence (Pierce, Helsinki, Finland). Quantification was performed using GelDoc (Bio-Rad, Espoo, Finland).
To study ubiquitination, N2a neuroblastoma cells were transfected with GFP-ubiquitin and Cnpy2/Msap-dsRed, and V5-Mylip/Idol plasmids for 24 h. Half of the cells received 5 μm MG321 (Sigma) to inhibit proteasomes. Lysates from precleared cell supernatants were incubated overnight at 4 °C on a rotary shaker using 2 μg/ml anti-LDLR or anti-V5 antibodies (Invitrogen). Immuncomplexes were bound to Sepharose-A for 2 h at 4 °C and recovered by centrifugation. Beads were washed three times, and the samples resuspended in SDS-PAGE buffer and subjected to immunoblotting using anti-LDLR, anti-Cnpy2/Msap, anti-Mylip/Idol, or anti-GFP antibodies. Blots were quantified by densitometry.
Mylip/Idol Promoter Assays
Cells in 6-well plates were transfected for 24 h with 0.5 μg of Mylip/Idol promoter luciferase reporter and using the Transfectin reagent followed by treatment with 50 ng/ml FGF21 and/or 1 μm GW3965. To control for transfection efficiency, 0.02 μg of Renilla luciferase pRL-TK was used. Cells were harvested after 48 h using Passive Lysis Buffer, and the Renilla and the firefly luciferase activities were measured using a luminometer (Promega, Biofellows, Helsinki, Finland) (25, 26). Results are shown as fold increase in firefly luciferase normalized to Renilla activity.
RNA Isolation and Quantitative PCR
Total RNA was extracted using the RNeasy tissue kit (Qiagen) followed by cDNA synthesis essentially as described (13, 25). DyNAmoTM HS SYBR® Green (Thermo Scientific) real-time quantitative (qt) PCR assays were performed on a LightCycler 480 (Roche) with 384-well block. Each 10-μl quantitative PCR reaction contained 1 μl of the cDNA product, 1 μl of 5 μm each of the forward and reverse primers. The reaction was ran for 15 min at 95 °C for initial activation of the enzyme, followed by 35 cycles of 10s at 95 °C for denaturation, 30s at 63 °C for annealing and extension. After completion of the reaction, the PCR products were subjected to a melting curve analysis spanning the temperature range from 65 °C to 95 °C with a ramping rate of 0.03 °C/s. The specificity of the amplification was further confirmed by electrophoresis on 2% agarose gels and stained with SYBR safe (Invitrogen). The results show the averages of four replicate experiments normalized to GAPDH. The following primer sequences were used for qtPCR: Cnpy2/Msap, forward (Fw), F 5′-GATCCTTCCGAATCAATCCA-3′ and Reverse (rev)5′-CTCTGAGCGGGCATAAGGTA-3′; Mylip/Idol, Fw, 5′-TGTGGAGCCTCATCTCA-TCTT-3′ and Rev, 5′-AGGGACTCTTTAA-TGTGCAAGAA-3′; LDLR, Fw, 5′-GCATC-AGCTTGGACAAGGTGT-3′ and Rev, 5′- GGGAACAGCCACCATTGTTG-3′; GAPDH: Fw- 5′-GGGTTCCTATAAATACGGACTGC-3′ and Rev, 5′-CCATTTTGTCTACGGGACGA-3′.
Stability of LDLR
Huh7 cells were stimulated with 50 ng/ml FGF21 to increase LDLR levels. 30 ng/ml actinomycinD was added to control and FGF21-treated cells to inhibit gene transcription (27). Cells were the incubated for various periods of time, and the amount of LDLR was determined by immunoblotting.
Quantification and Statistics
Statistical comparisons were performed using one-way Anova followed by a Bonferroni post-hoc test. The Student's t test was used in experiments with two groups with GraphPad Prism version 4.0 (GraphPad Software). Values are expressed as means ± S.E., and p ≤ 0.05 was considered significant.
RESULTS
Cnpy2/Msap Affects LDLR and Mylip/Idol Levels in Cells
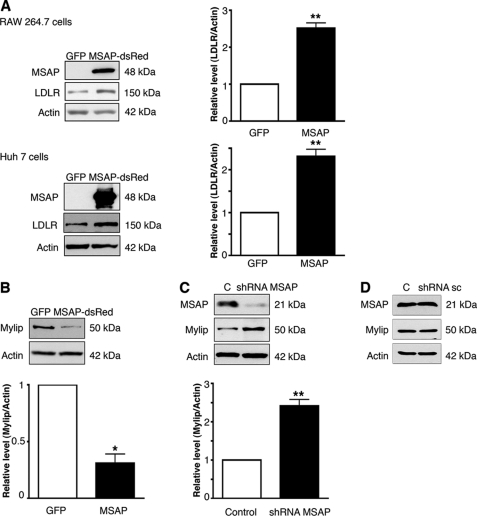
Cnpy2/Msap was previously shown to bind Mylip/Idol and to counteract the effect of this protein on neurite outgrowth (14). Data showed that overexpression of Cnpy2/Msap increased LDLR levels in the mouse macrophage Raw 264.7 cell line and in Huh7 human hepatocytes (Fig. 1A). Cnpy2/Msap decreased Mylip/Idol in these cells (Fig. 1B), while down-regulation of Cnpy2/Msap using shRNA constructs increased the levels of Mylip/Idol (Fig. 1C). In control experiments, employing scrambled shRNA, there was no decrease in Mylip/Idol (Fig. 1D). Experiments using GFP-Mylip and MSAP-dsRed constructs revealed a significant overlap of these two proteins showing that they partly reside in the similar compartment in the cell (supplemental Fig. S1A).
FIGURE 1.
Cnpy2/Msap increases LDLR levels and decreases Mylip/Idol levels in cells. Human hepatocytes (Huh7) and mouse macrophages (RAW) cell lines were transfected with 4 μg of EGFP or Cnpy2/Msap or shRNA-expressing plasmids and incubated for 24 h. Immunoblots were done as described under “Experimental Procedures” using antibodies against LDLR, Cnpy2/Msap, and Mylip/Idol. β-Actin was used as control. Values are means ± S.D., n = 3. **, p < 0.01 for Msap versus C. A, LDLR levels were increased by Cnpy2/Msap (MSAP) in both cell types as shown by immunoblots (left) and by using quantification (right). B and C, Mylip/Idol levels. Overexpression of Cnpy2/Msap decreased (B), whereas down-regulation of Cnpy2/Msap using shRNA (C) increased Mylip/Idol in Raw cells. Values are means ± S.D., n = 3. *, p < 0.05 for Msap versus GFP transfection. **, p < 0.01 for shRNA-Msap versus C. D, control experiment. Raw cells were transfected with 4 μg of scrambled shRNA (sc), and immunoblotting was done as above. No change in expression levels was observed.
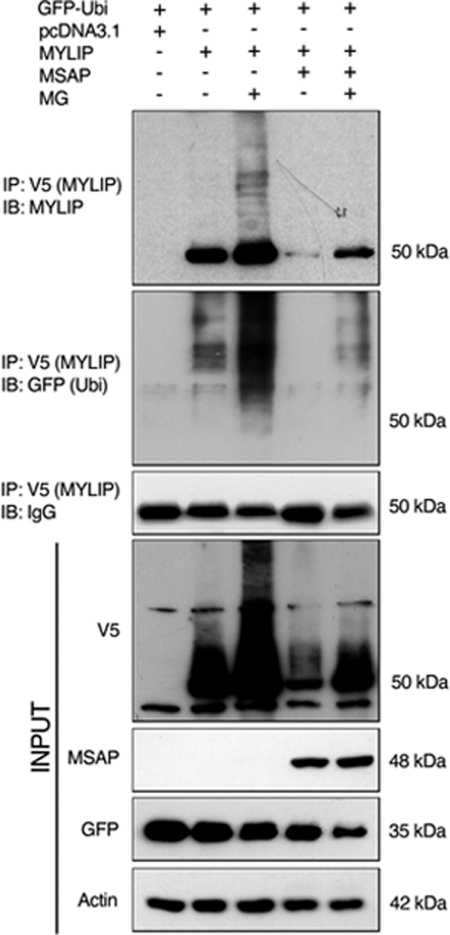
Mylip/Idol has previously been shown to be a rather unstable protein (7, 10–12), and we reasoned that Cnpy2/Msap might partly act by influencing the degradation of Mylip/Idol. We studied this using Mylip/Idol transfected cells in the absence or presence of Cnpy2/Msap. The ubiquitination of Mylip/Idol was increased by MG132, which inhibits proteasomes (Fig. 2B). Overexpression of Cnpy2/Msap strongly reduced Mylip/Idol levels and the ubiquitinated Mylip/Idol species were observed only after addition of MG321 to block further degradation (Fig. 2B).
FIGURE 2.
Cnpy2/Msap affects the ubiquitination of Mylip/Idol. N2A cells were transfected with 4 μg V5-Mylip/Idol for 24 h alone or together with 4 μg of Cnpy2/Msap. All cells also expressed GFP-ubiquitin. 5 μm MG132 was added for 4 h to inhibit proteasomes. Immunoprecipitation (IP) was done using anti-V5 antibodies as described under “Experimental Procedures” followed by immunoblotting (IB) using specific antibodies. Note a decrease of Mylip/Idol in Cnpy2/Msap-expressing cells. Ubiquitination of Mylip/Idol was increased by MG132 in control and in Cnpy2/Msap-expressing cells. A typical experiment is shown and was repeated three times.
FGF21 Increases Cnpy2/Msap and LDLR Levels in Cells
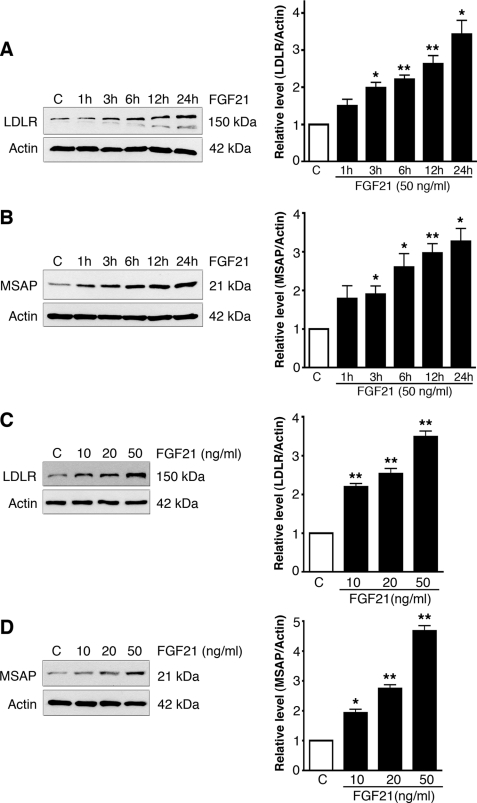
FGF21 is a circulating endocrine signal known to have metabolic effects on glucose and lipid metabolism (18–20, 26). We therefore studied whether the effects of FGF21 on lipids are linked to LDLR and its regulation. Addition of FGF21 significantly increased LDLR and Cnpy2/Msap in cultured human hepatocyte and mouse macrophage cells (Fig. 3). The effect of FGF21 on LDLRs was time and dose-dependent with an about 3.5-fold increase at 24 h of incubation (Fig. 3A), and using 50 ng/ml FGF21 (Fig. 3C). A similar time course and dose-dependence was also observed with regard to the increase in Cnpy2/Msap by FGF21 (Fig. 3, B and D).
FIGURE 3.
FGF21 increases LDLR and Cnpy2/Msap in cells. Cells were treated as below. Left, immunoblots; Right, quantification. Values are means ± S.D., n = 3. *, p < 0.05 and **, p < 0.01 for FGF21 versus C. A and B, raw cells were treated with 50 ng/ml FGF21 for different times and LDLR (A) and Cnpy/Msap (B) levels were determined by immunoblots. β-Actin was used as control. LDLR levels. Left, immunoblots; Right, quantification. C and D, Huh7 cells were treated for 24 h using different FGF21 concentrations followed by immunblotting using LDLR (C) and Cnpy2/Msap (D) antibodies.
Quantitative real-time PCR analyses revealed that FGF21 increased LDLR and Cnpy2/Msap mRNA levels in these cells showing an effect of FGF21 on gene expression (supplemental Fig. S1). This was also supported by data obtained using actinomycin D to block RNA synthesis that inhibited the increase in gene expression upon FGF21 stimulation.
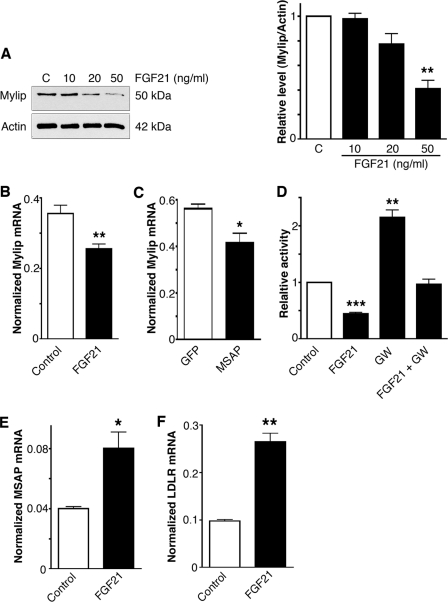
FGF21 Decreases Mylip/Idol in Cells and Affects the Half-life of LDLR
As shown in Fig. 1, there is a reciprocal regulation of Cnpy2/Msap and Mylip/Idol in cells. In line with this, we observed that FGF21 decreased Mylip/Idol levels particularly at 50 ng/ml FGF21 (Fig. 4A). Qt-PCR analyses showed that FGF21 decreased the mRNA levels of Mylip/Idol in these cells (Fig. 4B). This was confirmed in experiments employing the Mylip/Idol promoter luciferase reporter construct that showed a significant reduction in gene activity by FGF21 (Fig. 4D). Stimulation of Liver X Receptor (LXR) using the synthetic ligand, GW3695 was recently shown to increase gene expression of Mylip/Idol (7, 8). We observed that the GW3695-mediated increase in Mylip/Idol was counteracted by FGF21 (Fig. 4D), suggest that there is interplay between the LXR and FGF21 signaling systems in the regulation of Mylip/Idol in cells.
FIGURE 4.
FGF21 decreases Mylip/Idol and increases Cnpy2/Msap at the RNA level. A, Huh7 cells were treated for 24 h with different concentrations of FGF21 and Mylip/Idol was determined. Left, immunoblots; Right, quantification. Values are means ± S.D., n = 3. **, p < 0.01 for treated versus C. B, quantitative PCR. Mylip/Idol-mRNA levels decreased in Raw cells after treatment with 50 ng/ml FGF21. Values are means ± S.D., n = 4. **, p < 0.01 for treated versus C. C, overexepression of Cnpy2/Msap for 24 h decreased Mylip/Idol-mRNA levels. Values are means ±S.D., n = 3. *, p < 0.05 for treated versus C. D, raw cells were transfected with the Mylip/Idol-promoter luciferase construct and further treated for 24 h with 50 ng/ml FGF21 alone or together with 1 μm GW3695 (GW). Luciferease activity was measured as described and corrected for that of Renilla. Mylip/Idol promoter activity was decreased by FGF21 and was increased by GW. Values are means ± S.D., n = 3. **, p < 0.01 and ***, p < 0.01 for treated versus C. E and F, quantitative PCR. Raw cells were stimulated with 50 ng/ml FGF21and the mRNA levels of Cnpy2/Msap and LDLR determined. Values are means ± S.D., n = 3. *, p < 0.05 and **, p < 0.01 for treated versus C.
Apart from its effects on Mylip/Idol transcription, FGF21 increased LDLR and Cnpy2/Msap mRNA levels in these cells (Fig. 4, E and F), while Cnpy2/Msap overexpression decreased Mylip/Idol expression (Fig. 4C). Collectively, thus data shows that FGF21 has a dual gene effect in these cells decreasing Mylip/Idol and increasing Cnpy2/Msap that leads to increased LDLR levels.
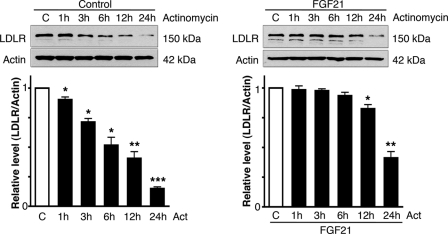
To study whether the decrease in Mylip/Idol induced by FGF21 influences the stability of LDLR, we pretreated cells for 20 h with 50 ng/ml FGF21 followed by the addition of actinomycin D to block gene transcription. LDLR levels decreased in control cells with a half-life about 6 h (Fig. 5). However, this half-life was increased by FGF21 and LDLR levels remained stable for up to 12 h in FGF21-treated cells (Fig. 5). Increased half-life and a decrease in receptor decay indicate enhanced stability of LDLR in these cells, in keeping with lower Mylip/Idol expression after FGF21 treatments.
FIGURE 5.
FGF21 increases the stability of LDLRs in cells. Raw cells were left untreated (controls) or treated with 50 ng/ml FGF21 for 20h. 30 ng/ml actinomycin-D was then added to block gene transcription, and cells were incubated further for various periods of time. LDLR levels were determined. Note that the control immunoblot shown was exposed longer time to reveal the 24 h-LDLR value. Values are means ± S.D., n = 3. *, p < 005 and **, p < 0.01 for treated versus C. Left, control cells. Calculated half-life of LDLR was about 6 h. Right, in FGF21-treated cells, LDLR was stable for more than 6 h, and the half-life was about 15 h.
The Effects of FGF21 Is Dependent on Cnpy2/Msap
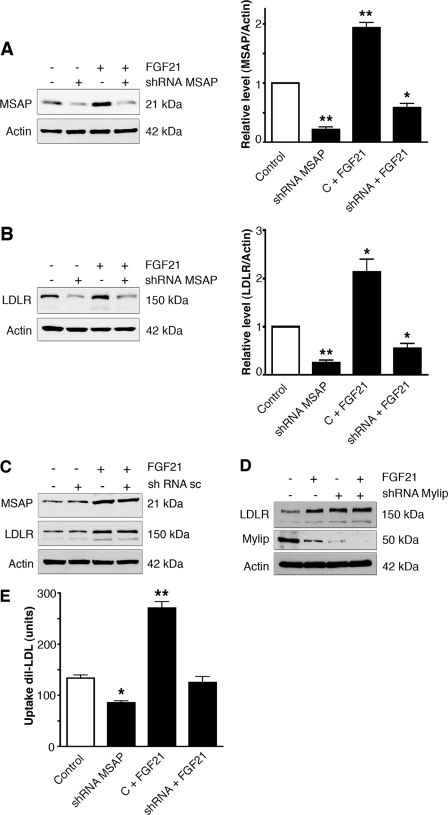
To study more directly the role of Cnpy2/Msap in the FGF21-mediated increase of LDLR, we employed shRNA constructs that decreased the level of this protein about 80% in mouse Raw 264.7 (Fig. 6A). The knockdown of Cnpy2/Msap reduced the levels of LDLR in control cells (Figs. 6B and 1B) and counteracted the increase brought about by FGF21 (Fig. 6B). In control experiments, employing scrambled shRNAs, there was no decrease in Cnpy2/Msap nor in LDLR (Fig. 6C). This shows that Cnpy2/Msap is crucially involved in the FGF21-mediated increase in LDLR.
FIGURE 6.
Down-regulation of Cnpy2/Msap inhibits the effect of FGF21 on LDLR and lipid uptake. Raw cells were transfected with 4 μg shRNA against Cnpy2/Msap for 24 h and half of the cells were further stimulated for 20 h with 50 ng/ml FGF21 followed by immunoblotting. Values are means ± S.D., n = 3. *, p < 0.05 and **, p < 0.01 for treated versus control. A, Cnpy2/Msap. Left, immunoblots. Right, quantification. Note down-regulation of Cnpy2/Msap by shRNA. The increase in Cnpy2/Msap by FGF21 was blocked by the shRNA. B, LDLR. Left, immunoblots; Right, quantification. Down-regulation of Cnpy2/Msap by shRNA decreased LDLR levels and reduced the increase in LDLR by FGF21. C, control experiment. Cells were transfected with 4 μg of scrambled shRNA (sc), and immunoblotting was done as above. FGF21 increased LDLR levels in sc-treated cells. D, Mylip/Idol down-regulation. Cells were transfected with 4 μg of shRNA against Mylip/Idol. Note a decrease in Mylip/Idol and an increase in LDLR by the shRNA. Treatment with 50 ng/ml FGF21 for 24 h further reduced Mylip/Idol levels. E, lipoprotein uptake. Cnpy2/Msap was down-regulated using shRNA for 24 h, and cells were incubated with 50 ng/ml FGF21 for an additional 20 h. 5 μg/ml DiI-labeled LDL was then added for 6 h, and the uptake was determined as described under “Experimental Procedures.” Values are means ± S.D., n = 3. **, p < 0.01 and ***, p < 0.001 and for treated versus C.
It was also found important to study whether the regulation of LDLR by FGF21 requires Mylip/Idol using shRNA. This treatment itself significantly down-regulated Mylip/Idol and up-regulated LDLR levels (Fig. 6D). FGF21 acted in the same direction and further decreased the level of Mylip/Idol reduced by the shRNA (Fig. 6D).
We then studied whether Cnpy2/Msap levels influence the capacity of the cells to take up lipoprotein particles. Data showed that down-regulation of Cnpy2/Msap by shRNA decreased the uptake of DiI-labeled LDL into macrophage cells both in control and in cells treated with FGF21 (Fig. 6E).
FGF21 Acts in a Parallel to Statin and Increases Uptake of LDL into Cells
Statins elevate LDLR in cells by enhancing gene expression subsequent to a reduction in cellular cholesterol levels (3, 6). We were interested to examine how the effect of statin relates to that observed with FGF21. Statin and FGF21 both significantly increased the levels of LDLR in human Huh7 cells (Fig. 7A). FGF21 further increased the LDLR levels in cells that were treated with statins showing an additive effect of these two compounds (Fig. 7B).
FIGURE 7.
FGF21 and statin increase LDLR levels and lipid uptake in an additive manner. A, raw cells (upper panel) and Huh7 cells (lower panel) were treated for 20 h with 50 ng/ml FGF21 alone or together with 3 μm simvastatin. LDLR levels were determined. B, quantification shown for Huh7 cells. Values are means ± S.D., n = 3. **, p < 0.01 and ***, p < 0.001 for treated versus C. C, Huh7 cells were incubated for 20 h with 50 ng/ml FGF21 alone or together with 3 μm simvastatin 5 μg/ml DiI-labeled LDL was added for 6 h, and the uptake was determined. Values are means ± S.D., n = 3. **, p < 0.01 and ***, p < 0.001 for treated versus C. NC, is a negative control in the presence of Dynasore to inhibit endocytosis.
As shown using DiI-labeled LDL, treatment with FGF21 for 20 h increased the capacity of these cells to take up lipoprotein particles (Fig. 7C). Co-treatment of the cells with FGF21 and statins further enhanced LDL uptake (Fig. 7C), in accordance with the data on the LDLR levels. These results show that the increase in LDLRs by FGF21 is reflected in an increased cholesterol uptake into cells. In view of this, we also studied whether FGF21 may lead to foam cell formation that in the long-run might be harmful. However, data showed that FGF21 does not appreciably increase LDLR levels in mouse macrophage cells loaded with acetylated cholesterol (supplemental Fig. S1, B and C), nor does FGF21 increase the cellular lipid content in already cholesterol loaded macrophages (supplemental Fig. S1D). These data indicate that FGF does not lead to a cholesterol deposit as seen in foam cell macrophages in different cardiovascular diseases.
DISCUSSION
FGF21 together with the related molecules FGF19 and FGF23 constitute a subfamily of FGFs having endocrine functions in the body (17, 18). FGF21 is present in human serum, and the levels are linked to metabolic diseases, such as type-2 diabetes and nonalcoholic fatty liver that are characterized by insulin resistance (28, 29). FGF21, like FGF19, protects animals from diet-induced obesity and when overexpressed in transgenic mice (18, 30). FGF21 also plays a role in lipid metabolism, and is increased by starvation (19, 20, 28–32). Levels of FGF21 in serum closely associate with liver fat content (32), but the precise mechanisms by which FGF21 influences lipid metabolism in man is not fully understood. Recently, it was also shown that FGF21 could constitute a biomarker for human mitochondrial disorders (33).
We show here that FGF21 rapidly elevated LDLRs in human hepatocyte and in mouse macrophage cell lines. The effect of FGF21 depended on the presence of Cnpy2/Msap and involved a down-regulation in Mylip/Idol levels. The increase in LDLR by FGF21 was reflected by an enhanced lipoprotein uptake into cells, which may be of physiological significance in clinical states with elevated FGF21 in the blood. It is not excluded that FGF21 or related factors might play a role also in the regulation of LDLRs under steady state conditions as this factor seems to be present normally in blood.
Statins are drugs in the clinic to lower blood LDL-cholesterol and to prevent against atherosclerosis and other cardiovascular diseases (1–3). These drugs act by inhibiting cholesterol synthesis in the cells, which leads to an increased LDLR gene expression. This is reflected in higher LDLR abundance on the cell surface with a beneficial effect on blood LDL-cholesterol. Like statins, treatment of cells with FGF21 increased LDLRs and LDL uptake. Important enough, this effect was additive to that of simvastatin, suggesting that it may be possible to enhance the effect of statins on LDLRs with FGF21, which could be beneficial in cases where statins show adverse effects or cannot be used in higher doses.
Cnpy2/Msap belongs to the Cnpy family of proteins having a saposin domain like that in the SAPLIPs with the ability to associate with cellular membranes (15). Four Cnpy genes exist in the human genome and these proteins have a four amino acid sequence at their C-terminal end that resembles the classical KDEL motif for ER retention (15). However, the precise functions of the Cnpy family proteins in the cell are not fully understood. We have previously shown that Cnpy2/Msap interacts with Mylip/Idol, counteracting the effects of Mylip/Idol on neurite outgrowth and on glioma cell migration (14, 16). As shown here, Cnpy2/Msap had an opposite effect to that of Mylip/Idol in LDLR regulation. The precise domains in Cnpy2/Msap binding to Mylip/Idol (14) are currently not known. As shown in immunoprecipitation experiments, Cnpy2/Msap influenced the ubiquitination and degradation of Mylip/Idol, acting possibly via an autoubiquitination mechanism that needs to be studied further. Apart from protein binding, we observed that Cnpy2/Msap overexpression decreased Mylip/Idol mRNA levels. The mechanisms behind this remains to be studied, but it is interesting to note that Cnpy2/Msap has been associated with the endoplasmic reticulum and could thus affect signals activating different genes including Mylip/Idol. As shown here the stimulation with FGF21 increased Cnpy2/Msap and decreased Mylip/Idol, which both led to an increase in the levels of LDLR in the cell. Experiments using RNA silencing showed that Cnpy2/Msap expression is crucial for the effect of FGF21 to down-regulate FGF2 lending credence to the view that Cnpy2/Msap plays an important role in LDLR and Mylip/Idol regulation.
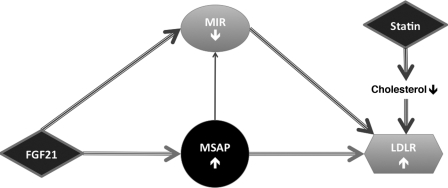
Fig. 8 summarizes the results obtained, and illustrates how FGF21 increases LDLR levels in cells by acting via the Cnpy2/Msap and Mylip/Idol pathways. Cnpy2/Msap is crucial for the effects of FGF21 as shown by RNA silencing, and Cnpy2/Msap further reduces the Mylip/Idol. The decreased level of Mylip/Idol leads to a stabilization of LDLR levels in the cells.
FIGURE 8.
Summary of the effects of FGF21 and Cnpy2/Msap on LDLR. Schematic drawing of the roles of FGF21 and Cnpy2/Msap in the regulation of LDLRs. FGF21 increases Cnpy2/Msap and LDLR levels, and decreases Mylip/Idol. Cnpy2/Msap interacts with Mylip/Idol and decreases its levels. Reduced Mylip/Idol levels stabilize LDLR and increases LDL uptake into cells in an additive manner to that of statins.
Taken together, we show here that Cnpy2/Msap is an important regulator of LDLR levels in human hepatocytes and in mouse macrophage cells. Cnpy2/Msap acts in conjunction with Mylip/Idol, inhibiting its effects on LDLR degradation. Cnpy2/Msap is crucial for the action of FGF21 in regulation of LDLR and cellular lipoprotein metabolism and is thus a novel factor to consider in lipid disorders. In future experiments, it will be interesting to study in more detail the mechanisms by which FGF21 influences Cnpy2/Msap and whether this protein is affected in human lipid disorders.
Supplementary Material
Acknowledgments
We thank Kristiina Söderholm and Eeva Lehto for skillful technical assistance, Dr. Matti Jauhiainen and Dr. Hanna Heikkilä for native and acetylated LDL, respectively, You Zhou for help with the Oil Red O imaging and quantification, and Terhi Vihervaara for cells.
This work was supported by Finska Läkaresällskapet, Sigrid Juselius Foundation, Liv och Hälsa, Magnus Ehrnrooth, Minerva Foundation, and the Academy of Finland.

This article contains supplemental Fig. S1.
- LDL
- low density lipoprotein
- Cnpy
- Canopy gene family
- Cnpy2
- Canopy-2 protein
- FGF
- fibroblast growth factor
- FGF21
- fibroblast growth factor-21
- Mylip
- myosin regulatory light chain-interacting protein
- Msap
- MIR-interacting Saposin-like protein
- Idol
- inducible degrader of the LDLR
- LDLR
- low density lipoprotein receptor
- LXR
- liver X receptors.
REFERENCES
- 1. Nabel E. G. (2003) Cardiovascular disease. N. Engl. J. Med. 349, 60–72 [DOI] [PubMed] [Google Scholar]
- 2. Ridker P. M., Danielson E., Fonseca F. A., Genest J., Gotto A. M., Jr., Kastelein J. J., Koenig W., Libby P., Lorenzatti A. J., MacFadyen J. G., Nordestgaard B. G., Shepherd J., Willerson J. T., Glynn R. J. and JUPITER Study Group (2008) Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 359, 2195–2207 [DOI] [PubMed] [Google Scholar]
- 3. Knopp R. H. (1999) Drug treatment of lipid disorders. N. Engl. J. Med. 341, 498–511 [DOI] [PubMed] [Google Scholar]
- 4. Brown M. S., Goldstein J. L. (1979) Receptor-mediated endocytosis: insights from the lipoprotein receptor system. (1979) Proc. Natl. Acad. Sci. U.S.A. 76, 3330–3337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goldstein J. L., DeBose-Boyd R. A., Brown M. S. (2006) Protein sensors for membrane sterols. Cell 124, 35–46 [DOI] [PubMed] [Google Scholar]
- 6. Goldstein J. L., Brown M. S. (2009) The LDL receptor. Arterioscler. Thromb. Vasc. Biol. 29, 431–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zelcer N., Hong C., Boyadjian R., Tontonoz P. (2009) LXR regulates cholesterol uptake through Idol-dependent ubiquitination of the LDL receptor. Science 325, 100–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sorrentino V., Scheer L., Santos A., Reits E., Bleijlevens B., Zelcer N. (2011) Distinct functional domains contribute to degradation of the low density lipoprotein receptor (LDLR) by the E3 ubiquitin ligase inducible Degrader of the LDLR (IDOL). J. Biol. Chem. 286, 30190–30199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lindholm D., Bornhauser B. C., Korhonen L. (2009) Mylip makes an Idol turn into regulation of LDL receptor. Cell Mol. Life Sci. 66, 3399–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Olsson P. A., Korhonen L., Mercer E. A., Lindholm D. (1999) MIR is a novel ERM-like protein that interacts with myosin regulatory light chain and inhibits neurite outgrowth. J. Biol. Chem. 274, 36288–36292 [DOI] [PubMed] [Google Scholar]
- 11. Bornhauser B. C., Johansson C., Lindholm D. (2003) Functional activities and cellular localization of the ezrin, radixin, moesin (ERM) and RING zinc finger domains in MIR. FEBS Lett. 553, 195–199 [DOI] [PubMed] [Google Scholar]
- 12. Nagano K., Bornhauser B. C., Warnasuriya G., Entwistle A., Cramer R., Lindholm D., Naaby-Hansen S. (2006) PDGF regulates the actin cytoskeleton through hnRNP-K-mediated activation of the ubiquitin E3-ligase MIR. EMBO J. 25, 1871–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hong C., Duit S., Jalonen P., Out R., Scheer L., Sorrentino V., Boyadjian R., Rodenburg K. W., Foley E., Korhonen L., Lindholm D., Nimpf J., van Berkel T. J., Tontonoz P., Zelcer N. (2010) The E3 ubiquitin ligase IDOL induces the degradation of the low density lipoprotein receptor family members VLDLR and ApoER2. J. Biol. Chem. 285, 19720–19726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bornhauser B. C., Olsson P. A., Lindholm D. (2003) MSAP is a novel MIR-interacting protein that enhances neurite outgrowth and increases myosin regulatory light chain. J. Biol. Chem. 278, 35412–35420 [DOI] [PubMed] [Google Scholar]
- 15. Bruhn H. (2005) A short guided tour through functional and structural features of saposin-like proteins. Biochem. J. 389, 249–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bornhauser B. C., Lindholm D. (2005) MSAP enhances migration of C6 glioma cells through phosphorylation of the myosin regulatory light chain. Cell. Mol. Life Sci. 62, 1260–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nishimura T., Nakatake Y., Konishi M., Itoh N. (2000) Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim. Biophys. Acta 1492, 203–206 [DOI] [PubMed] [Google Scholar]
- 18. Kharitonenkov A., Shiyanova T. L., Koester A., Ford A. M., Micanovic R., Galbreath E. J., Sandusky G. E., Hammond L. J., Moyers J. S., Owens R. A., Gromada J., Brozinick J. T., Hawkins E. D., Wroblewski V. J., Li D. S., Mehrbod F., Jaskunas S. R., Shanafelt A. B. (2005) FGF-21 as a novel metabolic regulator. J. Clin. Invest. 115, 1627–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Badman M. K., Pissios P., Kennedy A. R., Koukos G., Flier J. S., Maratos-Flier E. (2007) Hepatic fibroblast growth factor 21 is regulated by PPARα and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 5, 426–437 [DOI] [PubMed] [Google Scholar]
- 20. Inagaki T., Dutchak P., Zhao G., Ding X., Gautron L., Parameswara V., Li Y., Goetz R., Mohammadi M., Esser V., Elmquist J. K., Gerard R. D., Burgess S. C., Hammer R. E., Mangelsdorf D. J., Kliewer S. A. (2007) Endocrine regulation of the fasting response by PPARα-mediated induction of fibroblast growth factor 21. Cell Metab. 5, 415–425 [DOI] [PubMed] [Google Scholar]
- 21. Havel R. J., Eder H. A., Bragdon J. H. (1955) The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Invest. 34, 1345–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goldstein J. L., Ho Y. K., Basu S. K., Brown M. S. (1979) Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc. Natl. Acad. Sci. U.S.A. 76, 333–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Korhonen L., Belluardo N., Lindholm D. (2001) Regulation of X-chromosome-linked inhibitor of apoptosis protein in kainic acid-induced neuronal death in the rat hippocampus. Mol. Cell Neurosci. 17, 364–372 [DOI] [PubMed] [Google Scholar]
- 24. Sokka A. L., Putkonen N., Mudo G., Pryazhnikov E., Reijonen S., Khiroug L., Belluardo N., Lindholm D., Korhonen L. (2007) Endoplasmic reticulum stress inhibition protects against excitotoxic neuronal injury in the rat brain. J. Neurosci. 27, 901–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mudò G., Mäkelä J., Liberto V. D., Tselykh T. V., Olivieri M., Piepponen P., Eriksson O., Mälkiä A., Bonomo A., Kairisalo M., Aguirre J. A., Korhonen L., Belluardo N., Lindholm D. (2012) Transgenic expression and activation of PGC-1α protect dopaminergic neurons in the MPTP mouse model of Parkinson's disease. Cell Mol. Life Sci. 69, 1153–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kairisalo M., Korhonen L., Sepp M., Pruunsild P., Kukkonen J. P., Kivinen J., Timmusk T., Blomgren K., Lindholm D. (2009) NF-κB-dependent regulation of brain-derived neurotrophic factor in hippocampal neurons by X-linked inhibitor of apoptosis protein. Eur. J. Neurosci. 30, 958–966 [DOI] [PubMed] [Google Scholar]
- 27. Lindholm D., Heumann R., Hengerer B., Thoenen H. (1988) Interleukin 1 increases stability and transcription of mRNA encoding nerve growth factor in cultured rat fibroblasts. J. Biol. Chem. 263, 16348–16351 [PubMed] [Google Scholar]
- 28. Rydén M. (2009) Fibroblast growth factor 21: an overview from a clinical perspective. Cell Mol. Life Sci. 66, 2067–2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang X., Yeung D. C., Karpisek M., Stejskal D., Zhou Z. G., Liu F., Wong R. L., Chow W. S., Tso A. W., Lam K. S., Xu A. (2008) Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes 57, 1246–1253 [DOI] [PubMed] [Google Scholar]
- 30. Xu J., Lloyd D. J., Hale C., Stanislaus S., Chen M., Sivits G., Vonderfecht S., Hecht R., Li Y. S., Lindberg R. A., Chen J. L., Jung D. Y., Zhang Z., Ko H. J., Kim J. K., Véniant M. M. (2009) Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes 58, 250–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Murata Y., Konishi M., Itoh N. (2011) FGF21 as an endocrine regulator in lipid metabolism: from molecular evolution to physiology and pathophysiology. J. Nutr. Metab. 2011, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yan H., Xia M., Chang X., Xu Q., Bian H., Zeng M., Rao S., Yao X., Tu Y., Jia W., Gao X. (2011) Circulating fibroblast growth factor 21 levels are closely associated with hepatic fat content: a cross-sectional study. PLoS One. 6, e24895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Suomalainen A., Elo J. M., Pietiläinen K. H., Hakonen A. H., Sevastianova K., Korpela M., Isohanni P., Marjavaara S. K., Tyni T., Kiuru-Enari S., Pihko H., Darin N., Õunap K., Kluijtmans L. A., Paetau A., Buzkova J., Bindoff L. A., Annunen-Rasila J., Uusimaa J., Rissanen A., Yki-Järvinen H., Hirano M., Tulinius M., Smeitink J., Tyynismaa H. (2011) FGF-21 as a biomarker for muscle-manifesting mitochondrial respiratory chain deficiencies: a diagnostic study. Lancet Neurol. 10, 806–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.