Four decades ago, it was demonstrated that the amino acid glutamate, when introduced directly into the central nervous system (CNS), could trigger convulsions (1, 2) by an excitatory (depolarizing) action on neural membrane (3). About the same time, others reported that s.c. glutamate can kill neurons in the retina (4) or brain (5, 6), and that the neuroexcitatory action of glutamate was responsible for the cell killing effect (7). From these early findings, glutamate gradually became recognized for its beneficial role as the predominant excitatory neurotransmitter in the mammalian CNS, and also for its detrimental potential as an “excitotoxic” molecule (8) that can destroy neurons throughout the entire CNS. A large family of glutamate transmitter receptors was then identified (9), which are classified as either ionotropic or metabotropic. The ionotropic subfamily is further divided into three subtypes, referred to as N-methyl-d-aspartate (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), and Kainate receptors. In recent decades, it has been shown that glutamate excitotoxicity is responsible for neuronal degeneration in anoxia (10) and in essentially all other acute brain injury conditions (e.g., stroke, hypoglycemia, epilepsy, and head trauma), and the excitotoxic action of glutamate has been shown in many cases to involve abnormal uptake or intracellular mobilization of Ca2+ (11). Substantial evidence has also been generated implicating abnormal glutamate signaling and/or excitotoxicity in the pathogenesis of numerous more chronic CNS diseases, such as Parkinson's and Alzheimer's disease (12), but also multiple sclerosis (13).
From the above discoveries, a burgeoning new field of research has emerged that is devoted to elucidating both the beneficial roles of glutamate in numerous important physiological functions (e.g., interneuronal signaling, sensory perception, memory, learning, and synaptic plasticity) and the undesirable roles of glutamate in the pathogenesis of CNS diseases. The undesirable effects of glutamate have been appropriately targeted by the pharmaceutical industry with glutamate receptor antagonists and/or partial agonists that have been shown in preclinical testing to have substantial therapeutic potential. Despite the promising preclinical results, large numbers of pharmacological compounds have now been clinically tested and, in the majority of cases, abandoned because in the clinical setting no benefit could be demonstrated. In many cases, compounds were abandoned because unacceptable neurotoxic side effects were encountered at doses required to achieve a therapeutic benefit.
Recently, increasing attention has been focused on the trophic functions of glutamate in the developing CNS. Glutamate has been implicated in neuronal proliferation and migration during early development (14), and is thought to critically regulate neuronal survival in the period of synaptogenesis, also known as the “brain growth spurt period.” The brain growth spurt occurs postnatally in rodents, but in humans extends from the sixth month of gestation to several years after birth (15). It was recently shown that, during the brain growth spurt, blockade of NMDA glutamate receptors by NMDA antagonist drugs, or by ethanol, triggers massive apoptotic neuronal death in the developing rodent brain (16, 17). Loss of neuronal mass and neurobehavioral disturbances associated with fetal alcohol syndrome, a well-described neurodevelopmental disorder in humans exposed to alcohol during gestation, can be attributed to this mechanism (17).
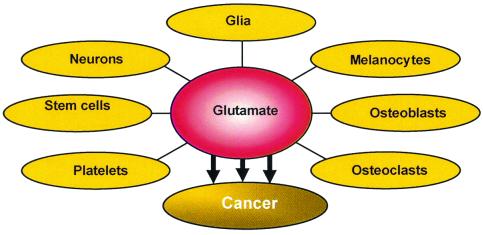
Although glutamate signaling has long been assumed to be restricted to the CNS, a growing body of recent evidence documents that non-neural cells throughout the body possess glutamate receptors, including bone osteoblasts and osteoclasts, megakaryocytes, keratinocytes, pancreatic islet cells, taste buds and cells in the lung, liver, heart, kidney, and adrenal (ref. 18 and Fig. 1). It remains to be determined what role glutamate plays in these various tissues, and whether glutamate-mediated signaling outside the CNS will ever constitute a valuable therapeutic target. This is certainly an intriguing question, in that huge numbers of glutamate antagonist drugs that were found unsuitable for treatment of neuropsychiatric disorders are stored on the shelves of pharmaceutical companies awaiting possible alternative indications.
Figure 1.
Glutamate signaling has long been assumed to be restricted to the CNS; however a growing body of recent evidence documents that non-neural cells throughout the body possess glutamate receptors, including bone osteoblasts and osteoclasta, megakaryocytes, keratinocytes, pancreatic islet cells, taste buds, and cells in the lung, liver, heart, kidney, and adrenal.
Perhaps a cure for some forms of cancer is hiding within such drug libraries. In this issue of PNAS, Rzeski, Turski, and Ikonomidou (19) report that glutamate antagonists at NMDA and AMPA glutamate receptor/ion channel complexes limit growth of human cancers. The authors were led to this discovery by logical deduction. Glutamate has trophic functions in the developing CNS; it regulates proliferation, migration, and survival of neuronal progenitors. Cancer cells have properties in common with neuronal progenitor cells, and cells throughout the body have glutamate receptors, so why not test whether interference with glutamate receptor function might influence growth of cancer cells?
Rzeski et al. demonstrate that the NMDA antagonist dizocilpine and the AMPA antagonist GYKI52466 limit proliferation of cancer cells in a wide variety of human non-neuronal cancers, including colon, breast, lung, and thyroid carcinoma, and also in glial and neuronal tumors, such as astrocytoma, neuroblastoma, and medulloblastoma/rhabdomyosarcoma. This antiproliferative effect is attributable to both decreased cell division and increased cell death, and can be reproduced by several other NMDA and AMPA receptor antagonists, supporting involvement of NMDA and AMPA receptors. In addition, the antiproliferative effect of glutamate antagonists is calcium dependent, which is consistent with knowledge that glutamate receptor/ion channel complexes are permeable to calcium.
Why not test whether interference with glutamate receptor function might influence growth of cancer cells?
It is potentially of considerable interest that glutamate antagonists, in addition to their antiproliferative action, produce motility-related morphological changes and interfere with migration of tumor cells. Inhibition of tumor cell migration, which is considered an indicator of reduced metastatic potential, can be achieved at much lower concentrations of glutamate antagonists than the antiproliferative effect. Limiting tumor metastasis is a high priority in cancer therapy, because metastatic disease is more important than local tumor growth as a determinant of mortality in most peripheral cancers. The opposite is the case in treatment of CNS tumors, where antiproliferative action is of crucial importance to preserve neuronal tissue and function.
Also important is the finding by Rzeski et al. of a synergistic action between glutamate antagonists and common cytostatic agents used in cancer therapy (19). This finding implies that, by combining glutamate antagonists with existing chemotherapeutic regimens, one might achieve superior cytostatic effects compared with either therapy alone.
Much work remains to be done to elucidate the mechanisms involved in the cytostatic effects of glutamate antagonists. Calcium appears to play a critical role, in that the antiproliferative effect was markedly diminished when calcium was removed from the extracellular medium. As the authors point out, calcium stimulates tumor growth (20, 21), regulates protein trafficking through the nuclear membrane (22), and plays important roles in axonal extension and pathfinding, and in cell division, migration, and survival (23–25). It has been shown that glutamate receptor ion channels on embryonic neurons are permeable to calcium (26–28). The authors note that tumor cells have a relatively low resting membrane potential, and advance the interesting hypothesis that this low potential promotes a high rate of calcium entry through glutamate receptor-gated ion channels that, in turn, would stimulate proliferation and migratory activity of tumor cells. This hypothesis, if confirmed, would provide a plausible explanation for inhibition by glutamate receptor antagonists of tumor cell proliferation and motility.
This study provides important new challenges for cancer researchers and the pharmaceutical industry. It will be necessary to determine whether glutamate antagonists exert similar cytostatic effects in vivo, and to clarify the molecular pathways used by glutamate antagonists to inhibit tumor cell proliferation and migration. In addition, it will be important to characterize the electrophysiological and binding properties and the subunit composition of glutamate receptors on tumor cells. When such information is available, hopefully it will be possible to add to the cancer chemotherapy armamentarium a new class of drugs that can contribute significantly to the therapeutic management of several different types of cancer. It is interesting that glutamate antagonists were more effective in suppressing proliferation of tumor cells derived from peripheral (non-CNS) tissues than those of CNS (either neuronal or glial) origin. This effect is potentially important, in that there are many glutamate receptor antagonists already available that do not readily penetrate blood brain barriers, and such agents can be used in relatively high concentrations to treat peripheral cancers without inducing adverse neurological side effects.
Footnotes
See companion article on page 6372.
References
- 1.Hayashi T. Jpn J Pharmacol. 1952;3:45–64. [Google Scholar]
- 2.Hayashi T. Nature (London) 1958;182:1076–1077. doi: 10.1038/1821076a0. [DOI] [PubMed] [Google Scholar]
- 3.Curtis D R, Watkins J C. J Physiol (London) 1963;166:1–14. doi: 10.1113/jphysiol.1963.sp007087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lucas D R, Newhouse J P. Arch Ophthalmol. 1957;58:193–201. doi: 10.1001/archopht.1957.00940010205006. [DOI] [PubMed] [Google Scholar]
- 5.Olney J W. Science. 1969;164:719–721. doi: 10.1126/science.164.3880.719. [DOI] [PubMed] [Google Scholar]
- 6.Olney J W, Sharpe L G. Science. 1969;166:386–388. doi: 10.1126/science.166.3903.386. [DOI] [PubMed] [Google Scholar]
- 7.Olney J W, Ho O L, Rhee V. Exp Brain Res. 1971;14:61–76. doi: 10.1007/BF00234911. [DOI] [PubMed] [Google Scholar]
- 8.Olney J W. In: Heritable Disorders of Amino Acid Metabolism. Nyhan W N, editor. New York: Wiley; 1974. pp. 501–512. [Google Scholar]
- 9.Watkins J C, Evans R H. Annu Rev Pharmacol Toxicol. 1981;21:165–204. doi: 10.1146/annurev.pa.21.040181.001121. [DOI] [PubMed] [Google Scholar]
- 10.Rothman S M. Science. 1983;220:536–537. doi: 10.1126/science.6836300. [DOI] [PubMed] [Google Scholar]
- 11.Zipfel G L, Lee J M, Choi D W. N Engl J Med. 1999;341:543–544. doi: 10.1056/NEJM199911113412011. [DOI] [PubMed] [Google Scholar]
- 12.Price D L. Nature (London) 1999;399,Suppl.:A3–A5. doi: 10.1038/399a003. [DOI] [PubMed] [Google Scholar]
- 13.Smith T, Groom A, Zhu B, Turski L. Nat Med. 2000;6:62–66. doi: 10.1038/71548. [DOI] [PubMed] [Google Scholar]
- 14.Komuro H, Rakic P. Science. 1993;260:95–97. doi: 10.1126/science.8096653. [DOI] [PubMed] [Google Scholar]
- 15.Dobbing J, Sands J. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- 16.Ikonomidou C, Bosch F, Miksa M, Bittigau P, Voeckler J, Dikranian K, Tenkova T, Stefovska V, Turski L, Olney J W. Science. 1999;283:355–358. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- 17.Ikonomidou C, Bittigau P, Ishimaru M J, Wozniak D F, Koch C, Genz K, Price M T, Stefovska V, Hoerster F, Tenkova T, Dikranian K, Olney J W. Science. 2000;287:1056–1060. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- 18.Skerry T M, Genever P G. Trends Pharmacol Sci. 2001;22:174–181. doi: 10.1016/s0165-6147(00)01642-4. [DOI] [PubMed] [Google Scholar]
- 19.Rzeski W, Turski L, Ikonomidou C. Proc Natl Acad Sci USA. 2001;98:6372–6377. doi: 10.1073/pnas.091113598. . (First Published May 1, 2001; 10.1073/pnas.091113598) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meloni F, Brochieri A, Ballabio P C, Tua A, Grignani G, Grassi G G. Monaldi Arch Chest Dis. 1998;53:405–409. [PubMed] [Google Scholar]
- 21.Celli A, Treves C, Nassi P, Stio M. Neurochem Res. 1999;24:691–698. doi: 10.1023/a:1021060610958. [DOI] [PubMed] [Google Scholar]
- 22.Stehno-Bittel L, Perez-Terzic C, Clapham D E. Science. 1995;270:1835–1838. doi: 10.1126/science.270.5243.1835. [DOI] [PubMed] [Google Scholar]
- 23.Clapham D E. Cell. 1995;80:259–268. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- 24.Lawson M A, Maxfield F R. Nature (London) 1995;377:75–79. doi: 10.1038/377075a0. [DOI] [PubMed] [Google Scholar]
- 25.Gomez T M, Spitzer N C. Nature (London) 1999;397:350–355. doi: 10.1038/16927. [DOI] [PubMed] [Google Scholar]
- 26.Gallo V, Pende M, Cherer S, Molne M, Wright P. Neurochem Res. 1995;20:549–560. doi: 10.1007/BF01694536. [DOI] [PubMed] [Google Scholar]
- 27.Bardoul M, Levallois C, Konig N. J Chem Neuroanat. 1998;14:79–85. doi: 10.1016/s0891-0618(97)10016-3. [DOI] [PubMed] [Google Scholar]
- 28.Iwata M, Komori S, Unno T, Minamoto N, Ohashi H. Br J Pharmacol. 1999;126:1691–1698. doi: 10.1038/sj.bjp.0702473. [DOI] [PMC free article] [PubMed] [Google Scholar]