Abstract
Breast cancer is one of the most common cancers in humans. However, our understanding of the cellular and molecular mechanisms underlying tumorigenesis in breast tissues is limited. Here, we identified a molecular mechanism that controls the ability of breast cancer cells to form multicellular spheroids (mammospheres). We found that heregulin (HRG), a ligand for ErbB3, induced mammosphere formation of a breast cancer stem cell (BCSC)–enriched population as well as in breast cancer cell lines. HRG-induced mammosphere formation was reduced by treatment with inhibitors for phosphatidyl inositol 3-kinase (PI3K) or NF-κB and by expression of IκBα-Super Repressor (IκBαSR), a dominant-negative inhibitor for NF-κB. Moreover, the overexpression of IκBαSR in breast cancer cells inhibited tumorigenesis in NOD/SCID mice. Furthermore, we found that the expression of IL8, a regulator of self-renewal in BCSC-enriched populations, was induced by HRG through the activation of the PI3K/NF-κB pathway. These findings illustrate that HRG/ErbB3 signaling appears to maintain mammosphere formation through a PI3K/NF-κB pathway in human breast cancer.
Keywords: EGF, HER, tumor sphere, cancer stem cells, inflammation
Cancer stem cells (CSCs), which make up only a small proportion of heterogeneous tumor cells, may possess a greater ability to maintain tumorigenesis than other tumor cell types (1, 2). CSCs can self-renew and simultaneously produce differentiated daughter cells; thus they can strongly proliferate until they reach their final differentiated state. With improvements in the isolation of CSCs, there is now a growing body of evidence that, in some cases of hematologic and solid tumors, a cancer stem cell population can be enriched based on phenotype (3–10). In human breast cancers, breast cancer stem cells (BCSCs) are enriched in the CD44high/CD24low cell population, whereas the CD44low/CD24high cells represent a more differentiated phenotype with limited stem cell–like potential (3). Because BCSCs withstand anoikis in culture, they expand under anchorage-independent conditions, giving rise to clonal spheroids (mammospheres), which can be serially passaged in vitro (11, 12). These processes can in part recapitulate the breast tumorigenesis process (13–16). To develop more effective cancer therapies, it would be reasonable to target molecules that have a critical role in the maintenance of mammospheres. However, the molecular mechanism by which mammospheres are maintained is still largely obscure.
NF-κB is a transcription factor complex that is typically a heterodimer of p50, p52, p65 (RelA), RelB, and c-Rel. It is usually inactive and bound to IκB, an inhibitory protein, in the cytoplasm. The primary mechanism of regulation of NF-κB activity is through activation of the IKK complex, including heterodimers of IKKα and IKKβ, as a result of various signaling pathways. The serine–threonine kinase Akt is one of the activators of IKKβ (17), and the activated IKK complex phosphorylates the IκBα protein, resulting in its ubiquitination, proteasome-mediated degradation, and subsequent release of NF-κB for nuclear translocation. Released NF-κB translocates to the nucleus and binds to the κB sequence, where it promotes the transcription of various genes, including inflammatory chemokines. Recently, we found activation of inflammatory signaling pathways in association with an increase in NF-κB activity in BCSC-enriched populations (18, 19). However, the role of NF-κB and the molecular mechanisms by which NF-κB is activated during mammosphere formation remain unknown.
Heregulin (HRG, also called neuregulin) is a ligand for ErbB3, which is one of the four members of the EGF receptor ErbB family (20). Expression of HRG in the mammary gland induces adenocarcinomas in animal models (21) and favors metastatic spread of breast cancer cells (22). HRG is expressed in 30% of human breast cancer patients (23) and correlates with poor histological grade (24). Recently, it was reported that ErbB2 overexpression increases the stem/progenitor cell population of both normal and malignant mammary cells (25); however the role of HRG and ErbB3 in regulating the properties of BCSC-enriched populations remains largely unknown.
In the present study, we showed that HRG induced mammosphere formation of cancer cells from a BCSC-enriched population. Moreover, our findings suggest that the activity of phosphatidyl inositol 3-kinase (PI3K)/NF-κB is essential for the HRG-induced mammosphere formation.
Results
HRG Induces Mammosphere Formation of a BCSC-Enriched Population.
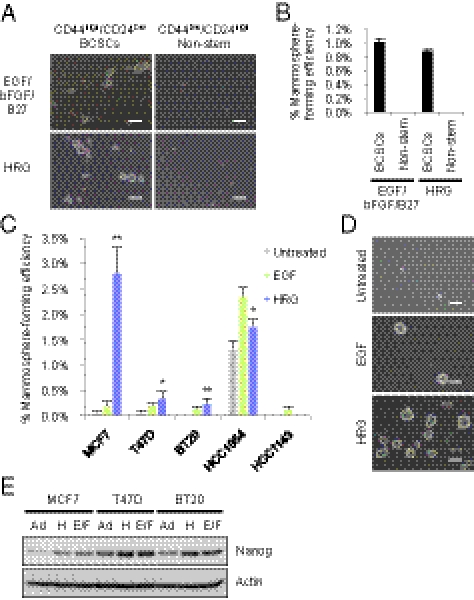
To test the mammosphere-forming ability of a BCSC-enriched population, we initially isolated CD44high/CD24low/lineage− BCSC-enriched population and CD44low/CD24high/lineage− nonstem cells from human breast cancer tissue. When these cells were cultured with conventional mammosphere culture media containing EGF, bFGF, and B27 supplement (13, 26), the CD44high/CD24low/lineage− BCSC-enriched population generated mammospheres, whereas the CD44low/CD24high/lineage− nonstem cells did not form mammospheres (Fig. 1 A and B). These observations suggest that cells with the capacity to form mammospheres are enriched in CD44high/CD24low/lineage− cells, confirming that mammospheres can be derived from BCSC-enriched populations as described previously (26, 27).
Fig. 1.
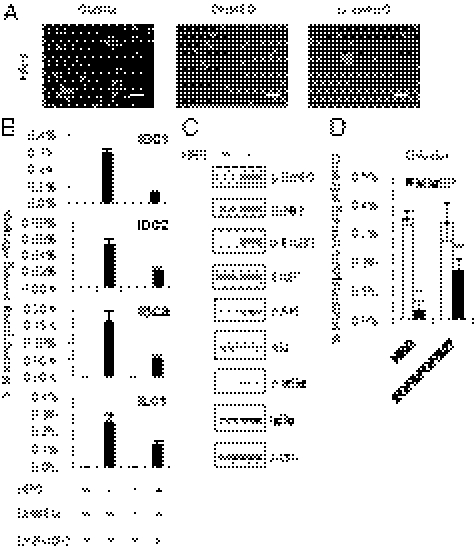
Effect of HRG on mammosphere formation of a BCSC-enriched population. (A) Representative images of primary cultures of mammospheres formed from the sorted CD44high/CD24low/lineage− BCSC-enriched population (Left) and the CD44low/CD24high/lineage− nonstem cell population (Right) obtained from a specimen of invasive ductal carcinoma (IDC1, Table S1). The cells from IDC1 were incubated with EGF/bFGF/B27 (Top) or with 20 ng/mL HRG (Bottom). Scale bar = 100 μm. (B) The spheres were counted and the percentage of mammosphere-forming cells were determined in each group (data are mean ± SD; n = 4). (C) Mammosphere assay in MCF7, T47D, BT20, HCC1954, and HCC1143 breast cancer cell lines treated with 20 ng/mL EGF or 20 ng/mL HRG (data are mean ± SD; n = 4, **P < 0.01, *P < 0.05, relative to the values in the respective untreated controls). (D) Images showing mammosphere formation in MCF7 cells treated as indicated in (C). Scale bar = 100 μm. (E) Nanog protein expression levels in the parental cells growing in 2D adherent (Ad) culture, sphere cells cultured with 20 ng/mL HRG (H) and sphere cells cultured with EGF/bFGF/B27 (E/F).
To examine the effects of HRG on the mammosphere formation, we cultured these cells with HRG in the absence of EGF, bFGF, or B27 supplement and then counted the number of mammospheres that formed. CD44high/CD24low/lineage− BCSC-enriched population cultured with HRG generated mammospheres at similar frequencies as those cultured with EGF/bFGF/B27, whereas CD44low/CD24high/lineage− nonstem cells did not generate mammospheres (Fig. 1 A and B). These findings suggest that HRG has the ability to induce mammosphere formation of BCSC-enriched population.
To further investigate the effect of HRG, we examined mammosphere formation in five breast cancer cell lines treated with HRG. Similar to the effects of HRG on primary human breast cancer cells, HRG increased mammosphere formation in four of the five breast cancer cell lines (Fig. 1 C and D) with an efficiency comparable to that of EGF. The HRG-induced mammospheres expressed the stem cell marker Nanog, comparable to mammospheres cultured with EGF/bFGF/B27 (Fig. 1E). Together, these results suggest that HRG plays an important role in enhancing the mammosphere formation of BCSC-enriched populations.
HRG Up-Regulates NF-κB Through PI3K/Akt Activation.
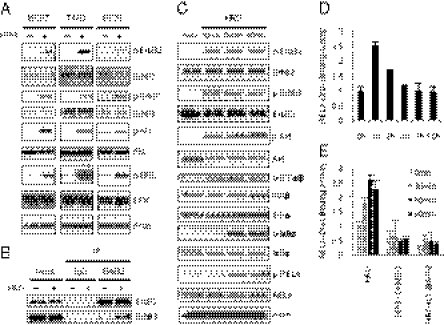
To examine whether HRG treatment activates the ErbB2/ErbB3 pathway, we investigated the effect of HRG on the phosphorylation levels of ErbB2, ErbB3, ERK, and Akt in three breast cancer cell lines. HRG markedly induced the phosphorylation of ErbB2, ErbB3, ERK, and Akt (Fig. 2A) in all three cell lines, suggesting that HRG strongly activates ErbB2 and ErbB3, which leads to the activation of ERK and the PI3K/Akt pathway. To confirm that HRG promotes the interaction between ErbB2 and ErbB3, we performed an immunoprecipitation analysis after treatment with HRG. The analysis revealed that treatment with HRG led to increased interactions between ErbB3 and ErbB2 (Fig. 2B).
Fig. 2.
HRG induces the ErbB/PI3K/Akt/NF-κB pathway. (A) MCF7, T47D, and BT20 cells were treated with 100 ng/mL HRG for 10 min, and protein expression levels were determined by immunoblotting. (B) MCF7 cells were treated with 100 ng/mL HRG for 10 min, and immunoprecipitation (IP) assays were performed with the indicated antibodies. (C) MCF7 cells were treated with 100 ng/mL HRG for 10, 30, and 60 min. Protein expression levels were determined by immunoblotting. (D) MCF7 cells were treated with 100 ng/mL HRG for 1, 2, 4, 8, and 16 h. The DNA binding activity of RELA was quantified by ELISA (data are mean ± SD; n = 3). (E) MCF7 cells were treated with 5 μg/mL DHMEQ (NF-κB inhibitor) or 10 μM LY294002 (PI3Kinase inhibitor) for 2 h, and then the cells were treated with 100 ng/mL HRG for 30, 60, and 90 min. The DNA-binding activity of RELA was quantified by ELISA (data are mean ± SD; n = 3).
Because NF-κB is a downstream target of Akt, we investigated whether the NF-κB signaling pathway was also altered by HRG treatment. IKKα/β are the upstream kinases involved in the phosphorylation of IκBα, which leads to the nuclear translocation of NF-κB. Treatment with HRG markedly induced the phosphorylation of Akt and IKKα/β within 10 min and the phosphorylation of IκBα and the NF-κB subunit RELA after 30 min (Fig. 2C). To examine the DNA-binding activity of RELA after HRG stimulation, we quantified the intensity of the RELA/DNA complex by ELISA at various time intervals. Treatment with HRG induced a marked increase in the binding activity of RELA after 1 h, and then this activation gradually decreased until 4 h (Fig. 2D). To test whether the activation of RELA by HRG was dependent on the PI3K/Akt pathway, we pretreated cells with LY294002, an inhibitor of PI3K before the addition of HRG. As anticipated, the HRG-induced activation of NF-κB was completely inhibited by LY294002 in a manner similar to the inhibition after treatment with DHMEQ, a specific inhibitor of NF-κB (28) (Fig. 2E and Fig. S1). These results showed that NF-κB was activated by HRG through the PI3K/Akt pathway. Because our previous observations suggested that the NF-κB pathway is enriched in BCSCs (19), we speculated that the HRG/PI3K/Akt/NF-κB axis may have a role in regulating mammosphere formation.
HRG/PI3K/NF-κB Axis Controls Mammosphere Formation.
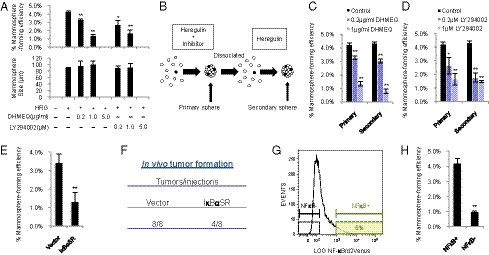
To elucidate whether NF-κB or PI3K influences HRG-induced mammosphere formation, we treated MCF7 cells with HRG, together with DHMEQ or LY294002. Treatment with DHMEQ or LY294002 decreased the frequency of mammosphere formation in a dose-dependent manner (Fig. 3A); however, the sizes of the mammospheres were not significantly changed, suggesting that the activities of NF-κB or PI3K affect mammosphere initiation but do not primarily influence cell proliferation during mammosphere growth. To test secondary mammosphere formation, primary mammospheres generated in the presence of DHMEQ or LY294002 were dissociated into single cells and incubated with HRG in the absence of the inhibitors (Fig. 3B). We found that the cells derived from primary mammospheres formed in the presence of DHMEQ or LY294002 did not form secondary mammospheres as efficiently as cells from untreated mammospheres (Fig. 3 C and D). These findings suggest that the activities of NF-κB and PI3K are required to maintain mammosphere cells with the ability to initiate HRG-induced mammosphere formation. In agreement with these findings, we found that lapatinib, an inhibitor of EGF receptor and ErbB2 tyrosine kinases, decreased NF-κB activity and mammosphere formation (Fig. S2 and SI Results). To determine whether DHMEQ or LY294002 induces apoptosis, we stained mammosphere cells with propidium iodide (PI) following inhibitor treatments and then analyzed the cell-cycle status by flow cytometry (Fig. S3). There was no apparent sub-G1 cell population, indicating that the inhibitors did not induce apoptosis at the effective concentrations for mammosphere formation.
Fig. 3.
Role of the HRG/PI3K/NF-κB axis on mammosphere formation. (A) MCF7 cells were incubated with 20 ng/mL HRG and/or the indicated concentrations of DHMEQ and LY294002. The number of formed mammospheres was counted, and the percentage of mammosphere-forming cells was determined [data are mean ± SD; n = 3, **P < 0.01, *P < 0.05, relative to the values in the HRG(+)]. (B) Experimental strategies for evaluating the effect of initial treatment with inhibitors on secondary mammosphere formation. (C and D) Effects of DHMEQ and LY294002 on primary and secondary mammosphere formation. MCF7 cells were incubated with 20 ng/mL HRG and/or the indicated concentrations of DHMEQ and LY294002. The formed primary mammospheres were dissociated into single cells and grown as secondary mammospheres without treatment with inhibitors. The mammospheres were counted, and the percentage of mammosphere-forming cells was determined (data are mean ± SD; n = 3, **P < 0.01, *P < 0.05, relative to the values in the respective controls). (E) MCF7 cells expressing the indicated lentiviral vectors were incubated with 20 ng/mL HRG, and the percentage of mammosphere-forming cells was determined (data are mean ± SD; n = 4, **P < 0.01). (F) NOD/SCID mice were injected in the mammary fat pad with 1 × 105 of vector- or IκBαSR-transduced MCF7 cells. Tumor formation was indicated by tumors/injections at 6 wk after injection. (G) NF-κB reporter activity of mammospheres derived from MCF7 cells. (H) NF-κB+ cells and NF-κB− cells (Fig. 3G) were sorted by FACS and cultured with 20 ng/mL HRG. The percentage of mammosphere-forming cells was determined (data are mean ± SD; n = 4, **P < 0.01).
NF-κB Is Required to Maintain Mammosphere-Forming Ability and Tumorigenic Potential of MCF7 Breast Cancer Cells.
To further validate these findings, we overexpressed mutant IκBα (IκBαSR), a dominant-negative inhibitor of NF-κB, in MCF7 cells with a lentiviral vector. Overexpression of mutant IκBα resulted in a decrease in the number of HRG-induced mammospheres compared with the vector-transduced cells (Fig. 3E). We also attempted to determine whether NF-κB regulates the mammosphere-forming ability in culture containing EGF/bFGF/B27, and found that NF-κB activity was required for mammosphere formation in such a culture condition. These results suggest that NF-κB is a mediator of mammosphere-forming capacity in both HRG- and EGF/bFGF/B27-containing media (Figs. S4 and S5 and SI Results).
To test whether the down-regulation of NF-κB signaling alters the tumor-initiating ability in vivo, we injected 1 × 105 MCF7 cells constitutively expressing mutant IκBα into the mammary fat pads of NOD/SCID mice. There were no significant morphological differences between these cells and control cells in culture (Fig. S6, Upper). All eight mice injected with control cells developed tumors within 6 wk, whereas tumor formation was inefficient in the mice injected with cells expressing mutant IκBα (four of eight mice) (Fig. 3F). Histological analysis showed that tumors derived from the vector-transduced cells or IκBαSR-transduced cells had a similar morphology (Fig. S6, Lower). Therefore, it is unlikely that the reduced incidence of tumor formation by expression of IκBαSR is due to cell differentiation; rather, it appears that NF-κB activity is required for tumor initiation of MCF7 cells in vivo.
By extension, we speculated that an NF-κB-negative subpopulation could not generate mammospheres. To isolate living cells based on NF-κB activity, we used an NF-κB reporter lentiviral vector expressing d2Venus (a yellow fluorescent protein) driven by four copies of the NF-κB response element located upstream of the minimal TATA promoter. We isolated MCF7 cells expressing d2Venus at high (NFkB+) or low (NFkB−) levels (Fig. 3G); the activity of NF-κB is thought to be higher in the former cells than in the latter cells. As expected, the NF-κB–negative subpopulation showed significantly decreased mammosphere formation capacity compared with the NF-κB–positive subpopulation (Fig. 3H). Taken together, these in vitro and in vivo results showed that NF-κB is required for mammosphere formation and tumor initiation of MCF7 cells.
HRG Elicits PI3K/NF-κB–Dependent Up-Regulation of IL8 mRNA Expression.
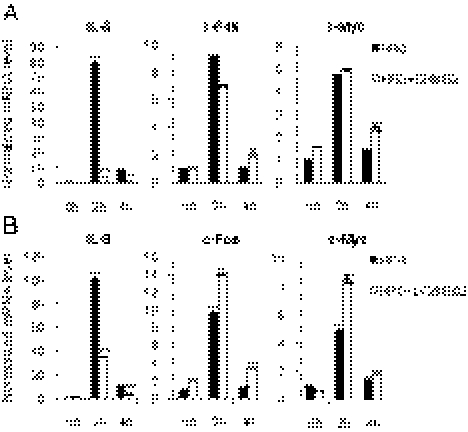
IL8 signaling has been shown to play a role in BCSC self-renewal (29, 30). Because the expression of IL8 is regulated by NF-κB activity (31), we investigated whether HRG induces the expression of IL8. We also examined the expression of representative immediate early genes, c-Fos and c-Myc. Treatment with HRG resulted in a dramatic increase of IL8 expression (up to a 100-fold increase) after 2 h (Fig. 4 A and B). The expression levels of c-Fos and c-Myc were also increased (10-fold and fivefold, respectively) (Fig. 4 A and B). The levels of these mRNAs were decreased rapidly after 4 h. To determine whether the activity of NF-κB or PI3K is involved in the induction of IL8 expression by HRG, cells were stimulated with HRG in the presence of DHMEQ or LY294002. We found that the levels of IL8 induction by HRG were decreased by treatment with inhibitors, although the induction levels of c-Fos or c-Myc were not significantly changed (Fig. 4 A and B). These results suggest that the expression of IL8 is induced by the HRG/PI3K/NF-κB axis.
Fig. 4.
IL8 is a transcriptional target of NF-κB. (A and B) MCF7 cells were treated with 5 μg/mL DHMEQ or 5 μM LY294002 for 2 hours, and then the cells were treated with 100 ng/mL HRG for 2 h and 4 h. Expression levels of IL8, c-Fos, and c-Myc were examined by quantitative RT-PCR (data are mean ± SD; n = 3, **P < 0.01, relative to the values in the respective controls).
HRG/PI3K/NF-κB Axis Controls Mammosphere Formation of Primary Tumor Cells Derived from Breast Cancer Patients.
We extended our analyses to primary tumor cells isolated directly from human breast cancer tissues (Table S1). To assess the effect of HRG, PI3K, and NF-κB on mammosphere formation, primary tumor cells were treated with HRG, together with DHMEQ or LY294002. Treatment with HRG induced mammosphere formation in all tumor samples, and the effect of HRG was blocked when DHMEQ or LY294002 was added with HRG (Fig. 5A and B). We confirmed that both ErbB2 and ErbB3 were expressed in these cells and that the phosphorylation of Akt and IκBα was induced in response to HRG (Fig. 5C). When we treated primary tumor cells with HRG, together with LY294002, the phosphorylation levels of Akt and IκBα were decreased, suggesting that NF-κB was activated by HRG through the PI3K/Akt pathway in primary tumor cells. (Fig. S1). Furthermore, overexpression of mutant IκBα in primary tumors cells with a lentiviral vector led to a decreased frequency of mammosphere formation compared with the control vector–transduced cells (Fig. 5D). Similar results were obtained in mammospheres cultured with EGF/bFGF/B27 (Fig. 5D and Fig. S7 A and B). These results suggest that the mammosphere formation of primary tumor cells derived from breast cancer patients is regulated by the HRG/PI3K/NF-κB pathway, which is consistent with the results obtained from the analysis performed with breast cancer cell lines.
Fig. 5.
The HRG/PI3K/NF-κB axis controls mammosphere formation of primary tumor cells derived from breast cancer patients. (A) Representative images of primary cultures of mammospheres incubated with 20 ng/mL HRG, 5 μg/mL DHMEQ and 5 μM LY294002. Scale bar = 100 μm. (B) Primary breast cancer cells obtained from specimens of invasive ductal carcinoma (IDC1, 2, and 3) or invasive lobular carcinoma (ILC1) were treated as indicated in A, and the percentage of mammosphere-forming cells was determined (data are mean ± SD; n = 4). (C) Cells from IDC1 were incubated with 100 ng/mL HRG for 10 min, and protein expression levels were determined by immunoblotting. (D) Cells from IDC1 expressing the indicated lentiviral vectors were incubated with 20 ng/mL HRG or with EGF/bFGF/B27, and the percentage of mammosphere-forming cells was determined (data are mean ± SD; n = 4, **P < 0.01, relative to the values in the respective controls).
Discussion
Accumulating evidence indicates that BCSCs are responsible for the initiation, propagation, recurrence, and radioresistance of breast cancers (1, 15, 32); hence, BCSCs are considered to be critical therapeutic targets (30, 33, 34). Recent studies have indicated that BCSC-enriched populations give rise to mammospheres in anchorage-independent conditions (11, 12). An understanding of the molecular mechanisms involved in the regulation of mammosphere formation is important for the design of efficient therapeutic strategies and improvements in conventional anticancer treatments. Recently, several inflammatory chemokines have been found to play a role in regulating the mammosphere-forming ability of breast cancer cells (18). However, the molecular pathways linking inflammation to mammosphere-forming ability are still largely unknown. In the present study, we describe one such molecular pathway that involves HRG, PI3K/Akt, NF-κB, and IL8.
HRG is widely expressed in numerous tissues, including breast, brain, heart, skeletal muscle, liver, and lung, and it is implicated in the regulation of a variety of biological processes, including cell proliferation, apoptosis and differentiation (35). We have identified yet another role of HRG in human breast cancer: induction of the mammosphere-forming capacity of BCSC-enriched populations. We demonstrated that the effects of HRG on mammosphere formation are mediated through a PI3K/NF-κB pathway in breast cancer cell lines and primary tumor cells derived from surgically resected breast cancer tissues. The first step of HRG stimulation is activation of PI3K, followed by the phosphorylation of Akt, which occurs within 10 min after treatment with HRG. Activated Akt phosphorylates IKKα/β and then leads to phosphorylation of IκBα, resulting in NF-κB activation. Once the signal has been activated, production of IL8 is induced at high levels. IL8 increases the self-renewal capacity of BCSC-enriched populations through nuclear translocation of β-catenin (36), indicating that the HRG/NF-κB pathway-mediated initiation of mammosphere formation is regulated by this pathway, at least in part. Together, these observations suggest a tight link between the HRG-induced mammosphere formation and the NF-κB–dependent inflammatory signaling pathway.
NF-κB is a central regulator of inflammatory gene expression (37). The findings presented here describe an important role of NF-κB in regulating mammosphere formation. NF-κB was activated by HRG stimulation as well as by culture with EGF/bFGF/B27, and it regulated the mammosphere formation under each culture condition. Down-regulation of NF-κB led to a decreased frequency of tumor initiation of MCF7 cells and mammosphere formation, and the cells that had low NF-κB activity showed a decreased frequency of mammosphere formation. These observations suggest that NF-κB activity is required to maintain the mammosphere-forming ability of breast cancer cells.
ErbB3 is the only ErbB family member that directly binds to PI3K (38). As ErbB3 has six direct binding sites for p85, a subunit of PI3K, the signaling output of ErbB3 is dominated by activation of the PI3K cascade, leading to activation of Akt. Although ErbB3 lacks intrinsic kinase activity, it is well known that ErbB family members homodimerize or heterodimerize to activate signaling pathways. Among the various combinations of family members, the ErbB2/ErbB3 heterodimer is considered the most potent ErbB pair with respect to the strength of interaction, ligand-induced tyrosine phosphorylation, and downstream signaling (39). Because there is no ligand for ErbB2, HRG is among the most efficient ligands to activate ErbB2/ErbB3 heterodimers. This could be the reason why HRG stimulates strong activation of the PI3K/NF-κB pathway for the mammosphere formation of breast cancer cells.
Trastuzumab (Herceptin, Genentech), a humanized monoclonal antibody directed at the ErbB2 ectodomain, is effective in the treatment of some human breast cancers that overexpress ErbB2 (40). In this study, we showed that the HRG signaling pathway plays important roles for mammosphere formation in primary tumor cells derived from human breast cancer tissues in which ErbB2 was expressed at moderate levels (Table S1) as well as in breast cancer cell lines in which ErbB2 was expressed at various levels. This raises the intriguing possibility that trastuzumab effectively targets BCSCs in the breast cancer tissues in which HRG is overexpressed even if ErbB2 is expressed at moderate levels. Indeed, it was recently reported that trastuzumab sensitizes HRG-overexpressing breast cancer cells to chemotherapy (23).
In conclusion, our results suggest that HRG/ErbB/PI3K/NF-κB signaling regulates the mammosphere formation of human breast cancer cells. Hence, in the future it will be important to develop compounds or antibodies targeted at the signaling molecules involved in this pathway to improve the prognosis of breast cancer patients.
Materials and Methods
Cell Lines and Primary Cell Culture.
Breast cancer cell lines MCF7, T47D, BT20 HCC1954, and HCC1143 were purchased from the American Type Culture Collection (ATCC). Cells were cultured in RPMI1640 with 10% (vol/vol) FBS. Primary cultures of tumor cells and mammospheres were generated as described previously (12–14). Briefly, tumor samples were processed within 1 h after surgical resection. Minced pieces of human breast tumor samples were digested with 2 mg/mL collagenase A (Roche), 1 mM CaCl2, and DNaseI (Roche) in RPMI1640 with 10% FBS. Tumors were digested for 1.5–2.5 h at 37 °C with shaking and pipetting every 30 min for mechanical dissociation. Once tumors were digested, the resulting single-cell suspension was filtered through a 100-μm and 40-μm cell strainer (BD Falcon) and washed with PBS. After isolation of lineage-negative (Lin−) breast cancer cells, cells were cultured in human mammary epithelial cell growth medium (HuMEC, GIBCO) or in mammosphere culture medium, which consisted of serum-free Dulbecco's modified Eagle's Medium:Nutrient Mixture F-12 (DMEM/F-12) medium (GIBCO) supplemented with 20 ng/mL EGF (Millipore), 20 ng/mL bFGF (PeproTech), B27 (GIBCO), and heparin (Stem Cell Technologies). B27 supplement has been shown to support the growth of mammospheres from human breast tissue (41). Alternatively, mammospheres were grown in DMEM/F12 medium supplemented with 20 ng/mL HRG-β1 (R&D).
Human breast carcinoma specimens were obtained from the University of Tokyo Hospital and Showa General Hospital. This study was approved by the institutional review boards of the Institute of Medical Science, University of Tokyo, and Showa General Hospital.
Cell Isolation.
To isolate Lin− breast cancer cells, cells obtained from breast tumor specimens were incubated with a mixture of biotin-conjugated antibodies against Lin+ cells. The mixture of antibodies included a MACS lineage depletion kit for hematopoietic and erythrocyte precursor cells (CD2, CD3, CD11b, CD14, CD15, CD16, CD19, CD56, CD123, and CD235a, Miltenyi Biotec), CD31 (for endothelial cells, eBioscience) and CD140b (for stromal cells, Biolegend) antibody. After incubation, cells were separated using the MACS magnetic cell separation system according to the manufacturer's instructions (Miltenyi Biotec). To isolate putative stem cells, Lin− breast cancer cells were then sorted after staining with CD24-FITC or CD44-PE antibody (BD Pharmingen) using a FACSAria Cell Sorter (BD Bioscience). Dead cells were excluded by propidium iodide (PI, Sigma) staining. Data were analyzed by FlowJo software.
Mammosphere Assay.
Cells were plated as single cells in ultralow attachment plates at a low density (5,000 cells/mL) and were grown in mammosphere culture medium with or without NF-κB inhibitor DHMEQ (28), PI3K inhibitor LY294002 (Cell Signaling), lapatinib (Selleck Chemicals), gefitinib (AstraZeneca), dasatinib (Selleck Chemicals), or sunitinib (Sigma-Aldrich) at the indicated concentrations for 4–7 d. To test whether the low-density conditions, such as 5,000 cells/mL, represented clonal expansion rather than aggregation, as previously reported (11), we performed mammosphere assays using primary tumor cells from patients under the condition of 50 cells/well in 96-well plates (SI Materials and Methods). We found a comparable frequency of mammosphere formation irrespective of cell-plating density, strongly suggesting that mammospheres are not formed by simple cell aggregation but by clonal expansion of single cells. To culture secondary mammospheres, primary mammospheres were collected by gentle centrifugation (400 × g), and cells were dissociated enzymatically and mechanically into a single-cell suspension. The single-cell suspension was replated as described above.
Statistical Analysis.
All data were presented as mean ± SD. The unpaired Student t test was used for the statistical analysis. P values less than 0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
We thank H. Nakauchi and Y. Ishii for their help with flow cytometry, N. Yamaguchi for preparing the IκBαSR construct, N. Haraguchi for advising on the design of the in vivo experiments, T. Haraguchi for advising on the generation of the lentiviruses, and Y. Murakami, H. Kawahara and M. Kuroda for preparing the H&E sections. This work was supported by a grant from the Ministry of Health, Labor and Welfare of Japan for the Third Term Comprehensive 10-Year Strategy for Cancer Control; by a Grant-in-Aid from the Ministry of Education, Science, Sports and Culture of Japan for Scientific Research on Innovative Areas; and by grants from the Cell Science Research Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1113271109/-/DCSupplemental.
References
- 1.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 2.Gotoh N. Potential signaling pathways activated in cancer stem cells in breast cancer. In: Shostak S, editor. Cancer Stem Cells Theories and Practice. Vienna, Austria: InTech; 2011. p. 6. [Google Scholar]
- 3.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boiko AD, et al. Human melanoma-initiating cells express neural crest nerve growth factor receptor CD271. Nature. 2010;466:133–137. doi: 10.1038/nature09161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 6.Chan KS, et al. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc Natl Acad Sci USA. 2009;106:14016–14021. doi: 10.1073/pnas.0906549106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalerba P, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci USA. 2007;104:10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 9.Prince ME, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci USA. 2007;104:973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh SK, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 11.Cordenonsi M, et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell. 2011;147:759–772. doi: 10.1016/j.cell.2011.09.048. [DOI] [PubMed] [Google Scholar]
- 12.Pece S, et al. Biological and molecular heterogeneity of breast cancers correlates with their cancer stem cell content. Cell. 2010;140:62–73. doi: 10.1016/j.cell.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Dontu G, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ponti D, et al. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65:5506–5511. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- 15.Yu F, et al. let-7 Regulates self renewal and tumorigenicity of breast cancer cells. Cell. 2007;131:1109–1123. doi: 10.1016/j.cell.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 16.Scheel C, et al. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell. 2011;145:926–940. doi: 10.1016/j.cell.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsing CH, et al. Anesthetic propofol reduces endotoxic inflammation by inhibiting reactive oxygen species-regulated Akt/IKKβ/NF-κB signaling. PLoS ONE. 2011;6:e17598. doi: 10.1371/journal.pone.0017598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hinohara K, Gotoh N. Inflammatory signaling pathways in self-renewing breast cancer stem cells. Curr Opin Pharmacol. 2010;10:650–654. doi: 10.1016/j.coph.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Murohashi M, et al. Gene set enrichment analysis provides insight into novel signalling pathways in breast cancer stem cells. Br J Cancer. 2010;102:206–212. doi: 10.1038/sj.bjc.6605468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayes NV, Gullick WJ. The neuregulin family of genes and their multiple splice variants in breast cancer. J Mammary Gland Biol Neoplasia. 2008;13:205–214. doi: 10.1007/s10911-008-9078-4. [DOI] [PubMed] [Google Scholar]
- 21.Krane IM, Leder P. NDF/heregulin induces persistence of terminal end buds and adenocarcinomas in the mammary glands of transgenic mice. Oncogene. 1996;12:1781–1788. [PubMed] [Google Scholar]
- 22.Atlas E, et al. Heregulin is sufficient for the promotion of tumorigenicity and metastasis of breast cancer cells in vivo. Mol Cancer Res. 2003;1:165–175. [PubMed] [Google Scholar]
- 23.Menendez JA, Mehmi I, Lupu R. Trastuzumab in combination with heregulin-activated Her-2 (erbB-2) triggers a receptor-enhanced chemosensitivity effect in the absence of Her-2 overexpression. J Clin Oncol. 2006;24:3735–3746. doi: 10.1200/JCO.2005.04.3489. [DOI] [PubMed] [Google Scholar]
- 24.Dunn M, et al. Co-expression of neuregulins 1, 2, 3 and 4 in human breast cancer. J Pathol. 2004;203:672–680. doi: 10.1002/path.1561. [DOI] [PubMed] [Google Scholar]
- 25.Korkaya H, Paulson A, Iovino F, Wicha MS. HER2 regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion. Oncogene. 2008;27:6120–6130. doi: 10.1038/onc.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ginestier C, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mani SA, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishimura D, et al. DHMEQ, a novel NF-kappaB inhibitor, induces apoptosis and cell-cycle arrest in human hepatoma cells. Int J Oncol. 2006;29:713–719. [PubMed] [Google Scholar]
- 29.Charafe-Jauffret E, et al. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res. 2009;69:1302–1313. doi: 10.1158/0008-5472.CAN-08-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ginestier C, et al. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J Clin Invest. 2010;120:485–497. doi: 10.1172/JCI39397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 32.Diehn M, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780–783. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta PB, et al. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645–659. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morrison BJ, Schmidt CW, Lakhani SR, Reynolds BA, Lopez JA. Breast cancer stem cells: Implications for therapy of breast cancer. Breast Cancer Res. 2008;10:210. doi: 10.1186/bcr2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stove C, Bracke M. Roles for neuregulins in human cancer. Clin Exp Metastasis. 2004;21:665–684. doi: 10.1007/s10585-004-6917-6. [DOI] [PubMed] [Google Scholar]
- 36.Liu S, Wicha MS. Targeting breast cancer stem cells. J Clin Oncol. 2010;28:4006–4012. doi: 10.1200/JCO.2009.27.5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karin M, Greten FR. NF-kappaB: Linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 38.Hynes NE, Lane HA. ERBB receptors and cancer: The complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 39.Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: Receptor heterodimerization in development and cancer. EMBO J. 2000;19:3159–3167. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vogel CL, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 41.Lim E, et al. kConFab Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat Med. 2009;15:907–913. doi: 10.1038/nm.2000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.