Abstract
G protein-coupled receptors (GPCRs) have been shown to activate the mitogen-activated protein kinases, ERK1/2, through both G protein-dependent and -independent mechanisms. Here, we describe a G protein-independent mechanism that unravels an unanticipated role for β-arrestins. Stimulation of the V2 vasopressin receptor (V2R) in cultured cells or in vivo in rat kidney medullar collecting ducts led to the activation of ERK1/2 through the metalloproteinase-mediated shedding of a factor activating the insulin-like growth factor receptor (IGFR). This process was found to be both Src- and β-arrestin–dependent. Whereas Src was found to act upstream of the metalloproteinase activation and be required for the release of the IGFR-activating factor, β-arrestins were found to act downstream of the IGFR transactivation. Unexpectedly, the engagement of β-arrestins by the IGFR but not by the V2R was needed to promote the vasopressin-stimulated ERK1/2 activation, indicating that a pool of β-arrestins distinct from those β-arrestins recruited to the V2R acts downstream of the receptor tyrosine kinase to activate ERK1/2. Such a dual site of action for β-arrestins helps explain the pleiotropic actions of this scaffolding protein. Given the role that V2R-stimulated ERK1/2 plays in kidney cell proliferation, this transactivation mechanism may have important implications for renal pathophysiology. Still, the role of β-arrestins downstream of a transactivation event is not limited to the V2R, because we observed a similar involvement for an unrelated GPCR (the platelet-activating factor receptor), indicating that it may be a general mechanism shared among GPCRs.
Keywords: MAPK, paracrine factor, cell signalling, protein–protein interactions
The V2 vasopressin receptor (V2R), a member of the G protein-coupled receptor (GPCR) family, is predominantly expressed in the distal collecting tubules of the kidney, where it regulates water homeostasis. Binding of arginine vasopressin (AVP) to V2R leads to Gαs activation and subsequent cAMP accumulation that induces translocation of aquaporin-2 water channels (AQP2) to apical membranes, leading to increased water reabsorption. Although most of V2R biological actions have been attributed to this Gαs-adenylate cyclase pathway, V2R can signal to other cellular effectors such as ERK1/2 (1–3). GPCR activation of MAPK pathway has been shown to rely on numerous and sometimes overlapping mechanisms, such as stimulation of G proteins and subsequent generation of second messengers, recruitment of scaffold proteins like β-arrestins, and transactivation of receptor tyrosine kinases (RTKs) (4).
Multiple GPCRs have been shown to usurp RTK signaling machinery through transactivation, to convey stimulatory signals to the MAPK. One of the best described RTK transactivation mechanisms involves the GPCR-mediated activation of membrane-associated metalloproteinases, leading to the processing and shedding of tethered RTK precursor ligands, which in turn, can stimulate their cognate receptor in an autocrine–paracrine manner. This inside-out mode of transactivation is the prototypical mechanism of GPCR transactivation of the EGF receptor (EGFR) and has only been described for EGFR ligands thus far (5, 6). Although GPCR-mediated transactivation of several other RTKs [including the PDGF (7), FGF (8), VEGF (9), and tropomyosin-receptor kinase A (TrkA) (10) receptors] leading to MAPK activation has been documented, the specific mechanism responsible for the RTK engagement remains poorly characterized. Intracellular scaffolding that promotes the formation of protein complexes with nonreceptor tyrosine kinases, such as c-Src or Pyk2 (11–14), has also been implicated in MAPK activation downstream of GPCRs. For instance, β-arrestins, which are accessory proteins originally discovered for their role in GPCR desensitization, are also known to act as signal transducers by scaffolding members of the MAPK pathway (2, 15, 16) or recruiting and activating Src (17–19). However, the sequence of molecular events leading to the formation of these complexes and the role of scaffolding proteins such as β-arrestins in the inside-out mode of RTK transactivation remain to be established.
In the case of the V2R, we previously reported that AVP stimulates ERK1/2 independently of Gαs/i/q or Gβγ but involves c-Src and a metalloproteinase-dependent RTK transactivation event (3). As we were able to rule out EGFR as the transactivated RTK, our results suggest that another member of the RTK family can be activated through a metalloproteinase-dependent mechanism in response to V2R stimulation. However, the identity of the RTK involved remained elusive.
The V2R-mediated ERK1/2 activation was also found to require β-arrestins (3). Given that β-arrestins are classically regarded as GPCR regulators, it has been assumed that β-arrestins contribution to MAPK activation results from their recruitment to the stimulated GPCR. However, several studies suggested that RTKs can also recruit β-arrestins in response to their respective growth factors (20–22). In addition, β-arrestins were found to contribute to the activation of ERK1/2 and PI3K by the insulin-like growth factor receptor (IGFR) in response to its cognate ligand IGF1 (23, 24), suggesting that these scaffold proteins are not restricted to the regulation of GPCRs. These findings raise questions concerning the role and mechanism of β-arrestins in GPCR-mediated ERK1/2 activation, especially in a transactivation context, where the signal is conveyed through the sequential action of two membrane receptors that are both able to engage the scaffolding protein.
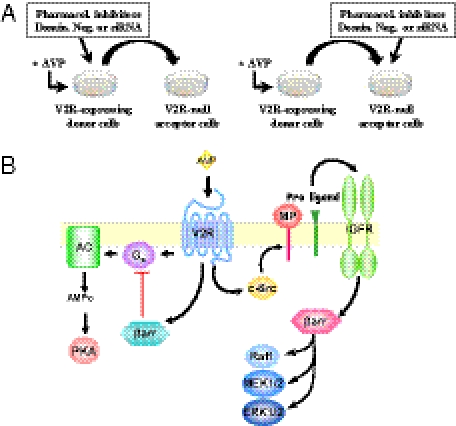
Here, we used a systematic approach to dissect the molecular sequence of events linking V2R to the activation of ERK1/2, with an emphasis on the specific role of β-arrestins. We identified IGFR as the transactivated RTK responsible for the V2R-mediated ERK1/2 activation in HEK293 cells. We also showed that this ERK1/2 activation relies on the metalloproteinase-dependent release of an extracellular ligand able to activate the IGFR in an autocrine–paracrine manner and confirmed the pertinence of this pathway in vivo in rat kidneys. Moreover, our results reveal an unanticipated mechanism, where the β-arrestin pool involved in the GPCR-mediated ERK1/2 activation is engaged by the transactivated RTK (IGFR) and not by the GPCR (V2R) itself. These findings clearly indicate the existence of different pools of β-arrestins that are involved in distinct cellular functions and provide a paradigm that may explain the apparently antagonistic roles attributed to β-arrestins. They also open perspectives for the development of pharmacological approaches aimed at selectively controlling the different pathways engaged by GPCRs.
Results
V2R-Mediated ERK1/2 Activation Involves the Metalloproteinase-Dependent Transactivation of the IGFR.
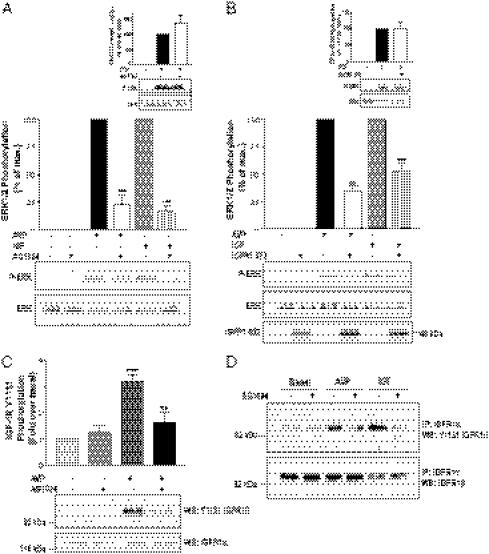
We previously showed that V2R-promoted ERK1/2 activation relied on the transactivation of an unknown RTK (3). To identify the RTK transactivated by V2R, we tested the ability of a panel of selective pharmacological RTK inhibitors to block AVP-promoted ERK1/2 activation in HEK293 cells stably expressing V2R. One of these compounds, the selective IGFR inhibitor AG1024, prevented ERK1/2 activation in response to both AVP and IGF1 (Fig. 1A). Although reportedly selective for IGFR at the concentration used, AG1024 shows some inhibitory action for the insulin receptor. Nevertheless, given the lack of detectable insulin-stimulated ERK1/2 activity in the HEK293 cell used, these data suggest a role for IGFR in the activation of the MAPK by GPCRs. In contrast, AG1024 did not alter EGF-induced ERK1/2 phosphorylation in the same conditions (Fig. 1A, Inset). Overexpression of the C-tail truncated dominant negative IGFR1-972 mutant (25) led to a strong inhibition of the AVP and IGF1-promoted MAPK activation (Fig. 1B), but it was without effect on EGF-induced ERK1/2 activation (Fig. 1B, Inset), supporting a role for IGFR in the studied signaling pathway. To confirm that IGFR is transactivated after V2R stimulation, we assessed the ability of vasopressin to directly promote IGFR activation by monitoring phosphorylation of a major autophosphorylation site of the activated endogenous IGFR (26, 27). As shown in Fig. 1C, AVP treatment led to a significant increase in IGFR phosphorylation at tyrosine 1131, a response prevented by AG1024. Ruling out any cross-reactivity of the phosphospecific antibody with other endogenously expressed RTKs, AVP- and IGF1-promoted IGF-1R phosphorylation at tyrosine 1131 was also detected after immunoprecipitation of endogenous IGF-1R (Fig. 1D).
Fig. 1.
V2R-mediated ERK1/2 activation involves the transactivation of IGFR. (A) Serum-starved HEK293 cells stably expressing V2R were treated or not for 20 min at 37 °C with 5 μM IGFR inhibitor AG1024 before 1 μM AVP or 100 ng/mL IGF1 stimulation for 5 min. MAPK activity was detected by Western blot analysis using phosphospecific anti-ERK1/2 antibody (P-ERK). Expression levels of the MAPK were controlled using antibodies directed against the total kinase population (ERK). Data presented in the bar graph are expressed as a percent of P-ERK/ERK of the level observed in AVP-stimulated conditions. (Inset) EGF-induced ERK1/2 phosphorylation. (B) HEK293 cells transiently expressing myc-V2R were cotransfected or not with the IGFR dominant negative mutant IGFR1-972 and serum-starved before a 1 μM AVP or 100 ng/mL IGF1 stimulation of 5 min. Expression levels of IGFR1-972 were controlled by Western blot using anti-HA antibodies (Lower). (Inset) EGF-induced ERK1/2 phosphorylation. Data represent the mean ± SEM of at least three independent experiments. (C) Serum-starved HEK293 cells stably expressing V2R were pretreated or not for 20 min with 5 μM of the IGFR inhibitor AG1024 and stimulated or not for 5 min at 37 °C with either 1 μM AVP or 100 ng/mL IGF1. Tyrosine autophosphorylation of endogenous IGF1 receptor β-subunit was determined by immunoblotting (WB) whole-cell lysate with anti–IGF-1R pY1131 antibody, and total IGF-1R population was detected with an anti–IGF-1Rα antibody. (D) Endogenous IGFR was immunoprecipitated (IP) from samples derived from cells described in C using the anti–IGF-1Rα 1H7 antibody followed by Western blot (WB) detection of IGF-1R tyrosine autophosphorylation with anti–IGF-1Rβ pY1131 antibody. Total IGF-1R population was detected with an anti–IGF-1Rβ. Typical immunoblots representative of three independent experiments are shown. **P < 0.01; ***P < 0.001.
To further explore the mechanism linking V2R stimulation to IGFR transactivation, the potential role of metalloproteinases was investigated. As shown in Fig. 2A, whereas the selective inhibition of zinc-dependent metalloproteinases by marimastat treatment only promoted a modest inhibition (less than 10%) of ERK1/2 phosphorylation in response to IGF (Fig. 2A, Inset), it almost completely blocked (more than 75% inhibition) ERK1/2 activation induced by AVP, confirming the implication of this class of enzyme in V2R-mediated ERK1/2 signaling. To determine if, as shown for the transactivation of EGFR by some GPCRs (5, 28), the metalloproteinase could lead to the shedding of a ligand activating the IGFR from the extracellular milieu, we examined whether supernatant from AVP-stimulated, V2R-expressing cells could induce the activation of ERK1/2 in nontransfected (V2R null) HEK293 cells, which do not express endogenous V2R. As shown in Fig. 2B, the addition of the supernatant from AVP-treated, V2R-expressing cells to V2R-null cells increased ERK1/2 activity significantly above the level observed in cells treated with supernatant from nonstimulated V2R-expressing cells. This release of a transactivating factor in the extracellular medium was prevented by the metalloproteinase inhibitors marimastat (Fig. 2B) and 1,10-phenanthroline (Fig. S1), confirming the role of metalloproteinases in this process and ruling out the possibility of a direct effect of AVP present in the transferred supernatant on V2R-null cells. This finding is further supported by the observations that AVP stimulation of V2R-null cells failed to induce any detectable ERK1/2 phosphorylation (Fig. 2C) and that transfer of supernatant from AVP-stimulated V2R-null cells on other V2R-null cells did not promote any increase in ERK1/2 activity (Fig. S2). We next determined whether the factor released after AVP-induced metalloproteinase activity stimulated ERK1/2 through activation of the endogenously expressed IGFR. As seen in Fig. 2D, treatment of the recipient V2R-null cells with AG1024 completely abolished ERK1/2 activation promoted by the transferred supernatant of AVP-stimulated, V2R-expressing cells. Furthermore, transferred supernatant from AVP-stimulated V2R cells promoted IGFR phosphorylation at residue 1131 in V2R-null cells, a response prevented by preincubation of the recipient V2R-null cells with the selective IGFR inhibitor AG1024 (Fig. 2E). Interestingly, both IGFR and ERK phosphorylation occurred rapidly, being readily observed after 5 min stimulation. Taken together, these results reveal a distinct IGFR transactivation mechanism whereby V2R-mediated ERK1/2 activation relies on the metalloproteinase-dependent shedding of an autocrine–paracrine factor in the extracellular medium, which possesses IGFR stimulatory activity.
Fig. 2.
V2R-mediated ERK1/2 activation involves a metalloproteinase-dependent transactivation event. (A) Serum-starved HEK293 cells stably expressing V2R were treated or not for 30 min at 37 °C with 10 μM of the metalloproteinase inhibitor marimastat before 1 μM AVP stimulation for 5 min. ERK1/2 phosphorylation was detected and expressed as described in Fig. 1A. (Inset) IGF-induced ERK1/2 phosphorylation. (B) Serum-starved V2R-null cells were incubated for 5 min at 37 °C with the transferred supernatant from V2R-expressing cells previously incubated or not for 30 min with 10 μM marimastat and stimulated or not with AVP (1 μM for 5 min). ERK1/2 phosphorylation was detected as described in Fig. 1A, and data are expressed as fold increase of P-ERK/ERK ratio compared with basal conditions. (C) Serum-starved V2R-null cells were stimulated or not with 1 μM AVP or FBS for 5 min. (D) Serum-starved V2R-null cells previously incubated or not for 20 min with 5 μM AG1024 were incubated for 5 min at 37 °C with the transferred supernatant from V2R-expressing cells stimulated or not with AVP (1 μM for 5 min). ERK1/2 phosphorylation was detected as described in Fig. 1A, and data are expressed as fold increase of P-ERK/ERK ratio compared with basal conditions. (Inset) V2R-null HEK293 cells were stimulated or not with AVP (1 μM) or IGF (100 ng/mL) for 5 min. (E) Serum-starved V2R-null cells previously incubated or not for 20 min with 5 μM AG1024 were incubated for 5 min at 37 °C with the transferred supernatant from V2R-expressing cells stimulated or not with AVP (1 μM for 5 min). Tyrosine autophosphorylation of endogenous IGF1 receptor β-subunit was determined with anti–IGF-1R pY1131 antibody. Total IGF-1R population was detected in cell lysates with the anti–IGF-1Rα antibody. Data represent the mean ± SEM of at least four independent experiments. **P < 0.01; ***P < 0.001.
Metalloproteinase-Dependent Transactivation of IGFR Requires Src Activity.
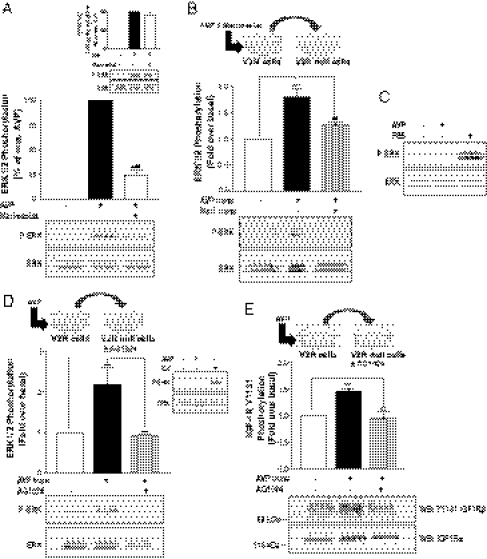
Given that Src has been shown to promote metalloproteinase-dependent MAPK activation by several GPCRs (29) but is also known to regulate ERK1/2 activity in response to many growth factor receptor ligands (30, 31), notably the IGFR (32, 33), we examined the role of Src in the signaling cascade uncovered in our study. To determine whether Src is involved in the transactivation of IGFR (acting upstream) or contributes to the activation of ERK1/2 downstream of IGFR, we took advantage of a phosphospecific antibody recognizing the activated form of Src phosphorylated at tyrosine 416. Fig. S3 shows that AVP can promote a transient time-dependent increase in Src phosphorylation at tyrosine 416 that peaked at 5 min. Consistent with our previous finding that V2R-promoted ERK1/2 activation is Gs-independent (3), down-regulating Gs with long-term cholera toxin treatment did not inhibit the AVP-stimulated Src phosphorylation (Fig. S4). More interestingly, whereas inhibition of IGFR by AG1024 completely abolished ERK1/2 activation in response to AVP (Fig. 3A, Inset), it did not alter the ability of AVP to promote Src phosphorylation at tyrosine 416 (Fig. 3A), suggesting that Src activation occurs upstream of IGFR transactivation. Similarly, V2R-promoted Src phosphorylation was not abolished by the metalloproteinase inhibitor phenanthroline (Fig. 3B). These results, although suggesting an early activation of Src by V2R upstream of the metalloproteinase-dependent IGFR transactivation, do not exclude an implication of Src tyrosine kinase activity downstream of IGFR transactivation. To establish whether Src activity was required downstream of IGFR transactivation in V2R-mediated ERK1/2 activation, we used the transferred supernatant assay in which we inhibited Src family kinases in either V2R-expressing donor cells (Fig. 3C) or V2R-null recipient cells (Fig. 3D) using the 4-Amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP2) inhibitor. Whereas the AVP-mediated increase in ERK1/2 activation in the receiving cells was completely abolished by PP2 treatment of the V2R-expressing donor cells (Fig. 3C), it was not affected after inhibition of Src in the V2R-null receiving cells (Fig. 3D). These results suggest that, after the transactivating ligand is released in the extracellular medium, Src activity is no longer required to orchestrate ERK activation downstream of the IGFR, and thus, Src acts only upstream of the metalloproteinase-dependent transactivation event.
Fig. 3.
V2R-mediated ERK1/2 activation involves an Src-dependent metalloproteinase-mediated transactivation event. (A and B) Serum-starved HEK293 cells stably expressing V2R were pretreated or not with either (A) the IGFR inhibitor AG1024 (5 μM for 20 min) or (B) the metalloproteinase inhibitor 1,10-phenanthroline (500 μM for 30 min) at 37 °C before AVP stimulation (1 μM for 5 min); c-Src phosphorylation at Y416 was detected and quantified using phosphospecific anti-Src (P-Src) and anti-Src GD11 (Src) antibodies. (Insets) AVP-induced ERK1/2 phosphorylation. (C) Serum-starved V2R-null cells were incubated for 5 min at 37 °C with the transferred supernatant from V2R-expressing cells previously incubated or not with PP2 (10 μM for 1 h) and stimulated or not with AVP (1 μM for 5 min). (Inset) V2R-null cells were stimulated or not with AVP or IGF. (D) Serum-starved V2R-null cells pretreated or not with PP2 (10 μM for 1 h) were incubated for 5 min at 37 °C with the transferred supernatant from V2R-expressing cells previously stimulated or not with AVP (1 μM for 5 min). ERK1/2 phosphorylation was detected as described in Fig. 1A, and data are expressed as fold increase of P-ERK/ERK ratio compared with basal conditions. Data represent the mean ± SEM of three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001.
β-Arrestins Are Required Downstream of IGFR Transactivation for V2R-Mediated ERK1/2 Stimulation.
β-Arrestins have been shown to play a role in ERK1/2 activation in response to the stimulation of many GPCRs (34), including V2R (3). To explore the role of β-arrestins in the IGFR transactivation mechanism, we used a C-tail truncated form of β-arrestins-1 (βarr Δ318–419), which has been shown to act as a dominant negative (3, 35). Coexpression of this β-arrestin dominant negative mutant with V2R significantly blunted ERK1/2 activation in response to AVP stimulation (Fig. 4A). Surprisingly, however, it did not inhibit ERK1/2 phosphorylation in the transferred supernatant assay in which ERK1/2 activation was monitored in V2R-null cells receiving the supernatant from the donor cells coexpressing V2R and βarr Δ318–419 (Fig. 4B). Those results, although confirming the involvement of β-arrestins in V2R-mediated ERK1/2 activation, rule out a proximal role of these scaffold proteins in the V2R-stimulated, metalloproteinase-dependent proligand shedding. siRNA silencing of both β-arrestin isoforms (Fig. 4C, Lower) not only confirmed that β-arrestins are not required for the V2R-mediated, metalloproteinase-dependent shedding of the IGFR transactivating ligand but also revealed an unexpected role of β-arrestins downstream of IGFR transactivation. Indeed, β-arrestin knockdown in the donor cells did not inhibit the activation of ERK1/2 in the receiving cells (Fig. 4C), whereas β-arrestin depletion in the V2R-null receiving cells resulted in a drastic inhibition of ERK1/2 activation promoted by supernatant transfer (Fig. 4D). Consistent with the idea that β-arrestins act downstream of IGFR transactivation, whereas Src is involved upstream (Fig. 3), β-arrestin knockdown did not block the AVP-promoted increase in Src phosphorylation (Fig. 4E), indicating that β-arrestins are not involved in V2R-stimulated Src activation.
Fig. 4.
β-Arrestins are required downstream of the Src- and metalloproteinase-dependent release of IGFR transactivation factor. (A) HEK293 cells transiently expressing myc-V2R were cotransfected or not with the β-arrestin dominant negative mutant (βarrΔ319–418) and serum-starved before AVP stimulation. Expression levels of βarrΔ319–418 were controlled using the anti–β-arrestin H9 antibody (Lower). ERK1/2 phosphorylation was detected and expressed as described in Fig. 1A. (B) Serum-starved V2R-null cells were incubated for 5 min at 37 °C with the transferred supernatant from HEK293 cells transiently expressing myc-V2R, cotransfected or not with βarrΔ319–418 and previously stimulated or not with AVP (1 μM for 5 min). (Inset) V2R-null cells were stimulated or not with AVP or IGF. (C) Serum-starved V2R-null cells were incubated for 5 min at 37 °C with the transferred supernatant from V2R-expressing cells previously transfected with siRNAs targeting both β-arrestin isoforms or nonspecific siRNA (−) and stimulated or not with AVP (1 μM for 5 min). β-Arrestin knockdown was assessed with the anti–β-arrestin-2 (H9) antibody (Lower). (D) Serum-starved V2R-null cells transfected with siRNAs targeting both β-arrestin isoforms or with nonspecific siRNA (−) were incubated for 5 min at 37 °C with the transferred supernatant from V2R-expressing cells previously stimulated or not with AVP (1 μM for 5 min). ERK1/2 phosphorylation was detected as described in Fig. 1A, and data are expressed as fold increase of P-ERK/ERK ratio compared with basal conditions. (E) HEK293 cells stably expressing V2R were transfected or not with siRNAs targeting both β-arrestin isoforms and serum-starved before AVP stimulation (1 μM for 5 min), and c-Src phosphorylation at Y416 was detected and quantified using phosphospecific anti-Src (P-Src) and anti-Src GD11 (Src) antibodies. β-Arrestin knockdown was assessed with the anti–β-arrestin-1/2 (D24H9) antibody. Data represent the mean ± SEM of at least three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001.
β-Arrestin Action Downstream of GPCR-Promoted Transactivation Is Not Limited to V2R.
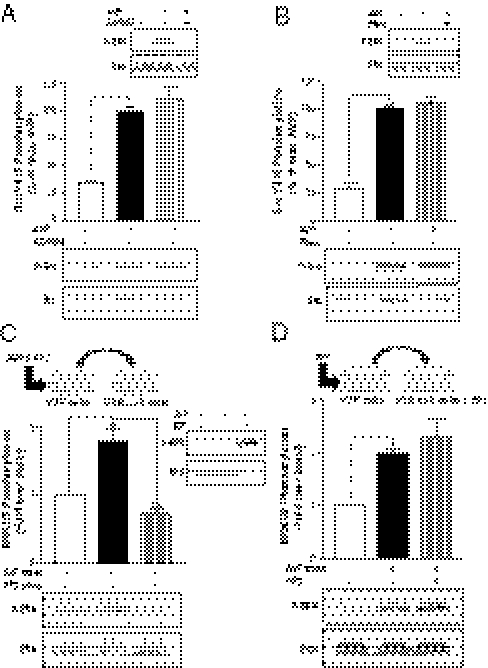
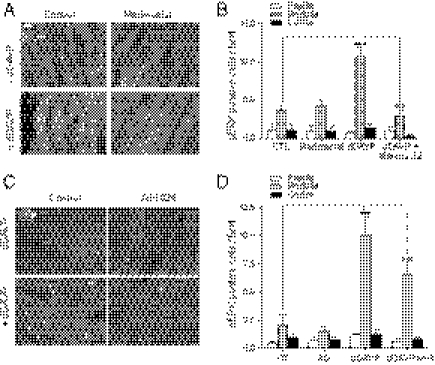
To assess whether a β-arrestin action downstream of a transactivation event could also be involved in the ERK1/2 activation by another GPCR, a supernatant transfer assay was performed using donor cells expressing the platelet-activating factor receptor (PAFR). As shown in Fig. 5 A and B, transferring the supernatant from PAF-stimulated cells to HEK293 cells lacking PAFR (PAFR-null cells) resulted in a robust activation of ERK1/2 in the receiving cells. The lack of PAFR in the receiving cells was confirmed by the absence of PAF-promoted ERK1/2 activation in these cells (Fig. 5C). These data indicate that, as was the case for V2R, PAFR activation led to the release of a transactivating factor in the extracellular medium. This effect required receptor activation, because the transfer of supernatant from nonstimulated PAFR-expressing cells did not promote significant activation. As observed for the V2R, down-regulating β-arrestins through siRNA silencing in the PAFR-null receiving cell (Fig. 5B) but not the PAFR-expressing donor cells (Fig. 5A) largely inhibited ERK1/2 activation, indicating that β-arrestins act downstream of the transactivation event. These data indicate that the unraveled mechanism is not restricted to the V2R.
Fig. 5.
β-Arrestins are required downstream of an ectodomain shedding event for PAF-mediated ERK1/2 activation. (A) Serum-starved PAFR-null cells were incubated for 5 min at 37 °C with the transferred supernatant from PAFR-expressing cells previously transfected with siRNAs targeting both β-arrestin isoforms or nonspecific siRNA (−) and stimulated or not with PAF (200 nM for 5 min). (B) Serum-starved PAFR-null cells transfected with siRNAs targeting both β-arrestin isoforms or nonspecific siRNA (−) were incubated for 5 min at 37 °C with the transferred supernatant from PAFR-expressing cells previously stimulated or not with PAF (200 nM for 5 min). β-arrestin knockdown (βarr) was assessed with the anti–β-arrestin-1/2 (D24H9) antibody (A Lower and B Lower). Data represent the mean ± SEM of five independent experiments. (C) PAFR-null cells were stimulated or not with 200 nM PAF or 100 ng/mL IGF for 5 min. ERK1/2 phosphorylation was detected as described in Fig. 1A, and data are expressed as fold increase of P-ERK/ERK ratio compared with basal conditions. *P < 0.05; **P < 0.01.
V2R Activation Can Promote β-Arrestin-1 Association to the Transactivated IGFR.
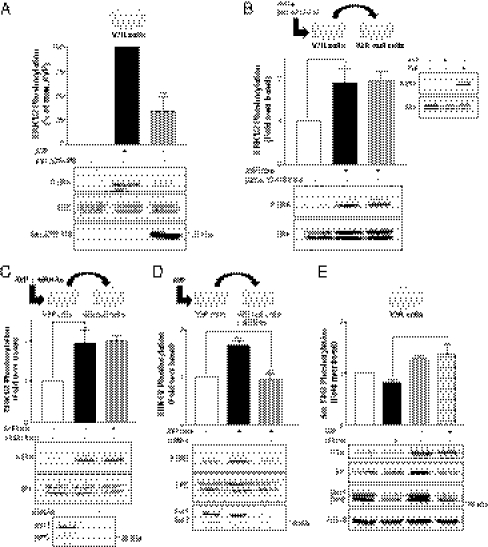
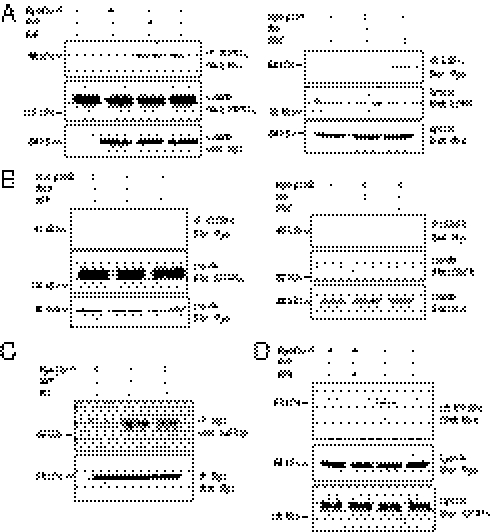
Given that V2R-null HEK293 cells do not express any endogenous V2R (Fig. 2C and Fig. S2), the role of β-arrestins downstream of IGFR transactivation in these cells after supernatant transfer implicates the existence of a distinct stimulatory signal triggering β-arrestin engagement. The recently appreciated ability of some RTK to recruit β-arrestins in response to their cognate ligands (21) makes IGFR an attractive candidate as the membrane receptor initiating β-arrestin translocation and activation. Indeed, IGF1 has been shown to promote β-arrestin-1 translocation to the IGFR (20, 23, 36). We thus hypothesized that IGFR could possibly conserve this signaling capacity in the context of a transactivation event when stimulated by a GPCR ligand. Supporting this hypothesis, coimmunoprecipitation experiments revealed that AVP as well as IGF1 can promote β-arrestin-1 association to the endogenously expressed IGFR (Fig. 6 A, Left and C). To rule out a possible nonspecific translocation of β-arrestins to membrane receptors after vasopressin stimulation of the V2R, we also performed coimmunoprecipitation experiments using the endogenously expressed EGFR. As seen in Fig. 6A, Right, whereas EGF can induce β-arrestin-1 translocation to the EGFR, AVP cannot, indicating that the AVP-promoted β-arrestin-1/IGFR association is specific for the transactivated RTK. Interestingly, no interaction of β-arrestin-2 with either IGFR or EGFR after growth factor or AVP stimulation could be detected (Fig. 6B). To confirm the molecular sequence of events leading to IGFR-promoted β-arrestin-1 recruitment, the association of β-arrestin-1 to IGFR was assessed in the presence of the Src family kinase inhibitor PP2. The Src inhibitor blocked the AVP-promoted β-arrestin-1 association to IGFR (Fig. 6D). This finding most likely results from an action of Src upstream of the transactivating factor release, because in the supernatant transfer assay, PP2 inhibited the supernatant-promoted ERK1/2 activation when administered in the donor (V2R) but not the receiving (V2R-null) cells (Fig. 3 C and D). Altogether, these results show that, in addition to promoting β-arrestin recruitment to the V2R itself, AVP also triggers β-arrestin-1 recruitment to the IGFR after its transactivation through an Src- and metalloproteinase-dependant ligand shedding. From these results, we cannot completely exclude that the coimmunoprecipitation of β-arrestin with IGFR could result from the formation of a ternary complex between β-arrestin, V2R, and IGFR in the V2R-expressing cells. However, it is clear that the involvement of β-arrestins downstream of IGFR in the AVP-triggered ERK1/2 activation does not require V2R in a complex with IGFR, because ERK1/2 activation is observed in the receiving cells lacking V2R (Fig. 4D). Combined with the fact that down-regulating β-arrestins in the donor cells did not affect the activation of ERK1/2 after supernatant transfer (Fig. 4 B and C), these results indicate that β-arrestin recruitment to V2R is not required for the AVP-promoted ERK1/2 activation.
Fig. 6.
V2R-promoted association of β-arrestins to the transactivated IGFR. (A and B) HEK293 cells stably expressing V2R were transiently transfected or not with either myc-β-arrestin-1 (A) or myc-β-arrestin-2 (B) and serum-starved before a 5-min AVP (1 μM), IGF (100 ng/mL), or EGF (10 ng/mL) stimulation at 37 °C. Cells were then subjected to immunoprecipitation (IP) with an antibody specific to the α-subunit of the IGF-1R (Left) or an antibody specific to the N terminus of EGFR (Right). Coimmunoprecipitation of β-arrestins was assessed by blotting with an anti-myc antibody. Endogenous IGFR and EGFR expression levels were detected in cell lysates with the anti–IGF-1Rα and the anti-EGFR antibodies, respectively. Expression levels of the transfected constructs were detected in cell lysates using an anti-myc antibody. (C) myc-β-arrestin-1 was immunoprecipitated using an anti-myc antibody, and the presence of IGFR and β-arrestin-1 were probed using anti-IGFRβ and anti-myc antibodies, respectively. (D) HEK293 cells stably expressing V2R were transiently transfected with myc-β-arrestin-1, serum-starved, and pretreated or not with PP2 (10 μM for 1 h) before AVP (1 μM for 5 min) stimulation at 37 °C. Endogenous IGF-1R was then immunoprecipitated, and β-arrestin-1 association was detected with anti-Myc antibody. Typical immunoblots representative of at least three independent experiments are shown.
β-Arrestin Recruitment to V2R Is Not Essential for AVP-Stimulated ERK1/2.
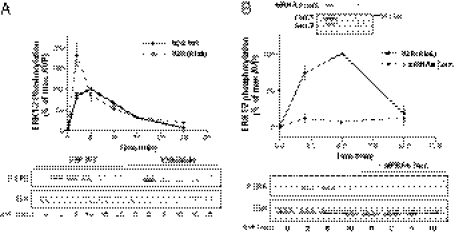
To confirm that the recruitment of β-arrestins to IGFR and not V2R is responsible for the V2R-mediated ERK1/2 activation, we took advantage of a mutant form of V2R [V2R(Ala6)] in which phosphate acceptor residues that stabilize the receptor's interaction with β-arrestins were replaced by alanines (37), thus decreasing V2R affinity for β-arrestin-2 (38) and -1 (39). Mutation of Ser-362, Ser-363, Ser-364, Thr-369, Ser-370, and Ser-371 to alanines in V2R C-tail [V2R(Ala6)] did not affect the ability of the receptor to stimulate ERK1/2 activation in response to AVP (Fig. 7A). In fact, the response promoted by the V2R(Ala6) was of larger amplitude than the response promoted by the WT-V2R for identical receptor expression levels. This finding suggests that the recruitment of β-arrestins to the V2R itself leads to an attenuation of the AVP-stimulated ERK1/2 activity, consistent with its role in desensitization. Still, the V2R(Ala6)-promoted ERK1/2 activation remained β-arrestin–dependent, because the β-arrestin dominant negative mutant βarrΔ318–419 (Fig. S5) and siRNA-targeting β-arrestin-1/2 (Fig. 7B) significantly inhibited AVP-stimulated ERK1/2 activation. These results, although confirming the crucial role of β-arrestins in V2R-mediated ERK1/2 stimulation, indicate that the β-arrestin pool involved in the MAPK activation is distinct from the pool recruited directly to the V2R, and they suggest that the IGF-1R-recruited β-arrestin pool underlies the engagement of the ERK1/2 pathway. Taken together, these data allow us to propose a model where V2R-promoted activation of ERK1/2 relies on β-arrestin engagement downstream of the Src- and metalloproteinase-dependant IGFR transactivation.
Fig. 7.
The phosphorylation-deficient V2R(Ala6) conserves its ability to stimulate ERK1/2 activity in a β-arrestin–dependent manner. (A) Serum-starved HEK293 cells stably expressing either WT-V2R or V2R(Ala6) were stimulated at 37 °C with 1 μM AVP for the indicated times. (B) HEK293 cells stably expressing V2R(Ala6) were transfected with siRNAs targeting both β-arrestin isoforms (dotted line) or not (solid line) and serum-starved before 1 μM AVP stimulation at 37 °C for the indicated times. ERK1/2 phosphorylation was detected and expressed as described in Fig. 1A. (Inset) β-arrestin knockdown was assessed with the anti–β-arrestin-2 (H9) antibody.
V2R-Mediated ERK1/2 Activity Is Metalloproteinase- and IGFR-Dependent in Vivo.
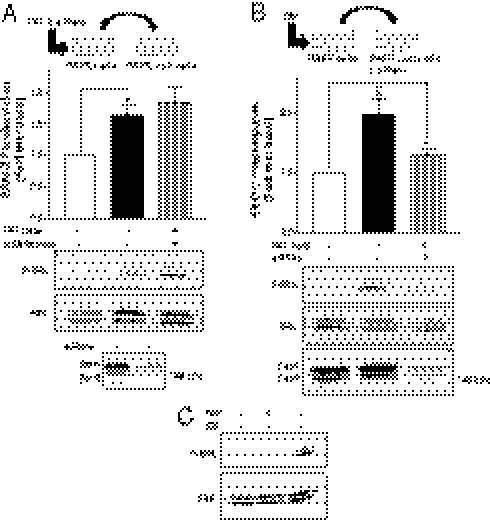
To determine if the signaling cascade uncovered in HEK293 cells could also be relevant in native tissue, we investigated whether administration of desmopressin (dDAVP) to rats could lead to the metalloproteinase- and IGFR-dependent activation of ERK1/2 in the kidney. As shown in Fig. S6A, a 3-d infusion with the V2R-selective agonist dDAVP in rats led to a strong stimulation of ERK1/2 phosphorylation, which was revealed by the increased number of immunofluorescent-positive cells detected by a selective antibody directed against phosphorylated ERK1/2. The elevation of ERK phosphorylation was observed specifically in the collecting ducts of the renal medulla, which is known to express AQP2 and V2R (40). In contrast, no change in phospho-ERK1/2 levels was observed after dDAVP treatment in the papilla or cortex. The dDAVP-promoted increase in ERK1/2 phosphorylation in the rat medulla was dose-dependent and saturable (Fig. S6B). To determine whether the metalloproteinase-dependent transactivation of IGFR described above is involved in the V2R-stimulated ERK1/2 activation in the kidney, rats infused for 3 d with dDAVP were concomitantly treated or not with the metalloproteinase inhibitor, marimastat, or the IGFR inhibitor AG1024. As shown in Fig. 8A, marimastat treatment blocked the dDAVP-promoted increase in phospho-ERK1/2–positive cells in the rat medulla region. Quantitative analysis shows that, whereas administration of marimastat did not alter basal ERK1/2 activity in any of the considered kidney regions, it completely blocked the significant dDAVP-promoted ERK1/2 activation in the kidney medulla (Fig. 8B). Similarly, inhibiting IGFR with AG1024 significantly blocked the dDAVP-stimulated ERK1/2 phosphorylation in the kidney medulla (Fig. 8 C and D), confirming that the transactivation of IGFR resulting from metalloproteinases activity significantly contributes to the V2R-stimulated MAPK activation in vivo.
Fig. 8.
The AVP-stimulated ERK1/2 activity in the rat kidney is metalloproteinase- and IGFR-dependent. Phospho-ERK1/2 activity was assessed in kidney slices from rat treated or not (control) for 3 d with dDAVP (0.13 μg/rat per day) in the absence or presence of (A) marimastat (3.6 mg/rat per day) or (C) AG1024 (0.30 mg/rat per day) using an antiphospho-ERK1/2 antibody. pERK activity was detected in the rat kidney medulla by epifluorescence microscopy (objective = 40×). (B and D) The histograms depict the number of pERK1/2-positive cells detected per confocal microscope field (20× objective) in 50-μm-thick slices of rat papilla (white), medulla (gray), or cortex (black) after the indicated treatment. Three to four kidney sections were evaluated for each animal, and three animals were tested for each condition. The data shown represent the mean ± SEM of three independent experiments. ***P < 0.001.
Discussion
Our results lead us to propose a unique model for the activation of ERK1/2 by a GPCR. This model involves the transactivation of an RTK, the IGFR, which had not been shown to be involved in GPCR-mediated MAPK activation. The mechanism of IGFR activation unraveled here is also distinctive, involving metalloproteinase-induced liberation of an autocrine–paracrine-activating ligand. Mechanistically, this signaling pathway involves β-arrestins in ways that had not been anticipated. Indeed, in contrast to the general view linking the recruitment of β-arrestins to the GPCR for the transactivation of an RTK, our data show that β-arrestin involvement occurs downstream of IGFR transactivation after their recruitment to the RTK. These changes in paradigm open avenues for the development of pharmacological strategies aimed at selectively regulating the activation of MAPK by GPCRs.
Activation of MAPK by several GPCRs has been shown to result from the transactivation of many distinct RTKs, including EGF (41), FGF (8), PDGF (7), VEGF (9), and TrkA (10) receptors. Although IGFR has also been shown to be transactivated by the thrombin (42), μ-opioid (43), γ-aminobutyric acid type B (GABAB) (44), and angiotensin II type-1 (AT1) (45) receptors, leading to the activation of the PI3K/AKT pathway for the latter two receptors, our study distinctively links IGFR transactivation to MAPK activation. Not only was the contribution of IGFR transactivation on the ERK1/2 stimulation confirmed by pharmacological inhibition and dominant negative approaches, but the activating autophosphorylation of the RTK was directly shown after V2R stimulation.
The V2R-stimulated IGFR transactivation and ERK1/2 activation were found to result from a metalloproteinase-dependent liberation of an IGFR-activating ligand. Although the identity of the ligand remains to be established, it was clearly released in the medium of AVP-stimulated, V2R-expressing cells, because supernatant transfer led to IGFR autophosphorylation and IGFR-dependent ERK1/2 activation in HEK293 cells devoid of endogenous V2R. To date, GPCR-induced ligand shedding from proligands has only been documented for EGFR (6, 29, 46). Our results, although extending this mode of transactivation to another RTK, raise the question of the existence of similar proligands for other RTK that have yet to be discovered. Interestingly, protease-activated PDGF receptor ligands have been identified (47, 48), which suggests that this receptor also has the potential to be transactivated by an inside-out model. No such membrane-tethered proligand has been identified yet for IGFR either experimentally or through database analysis. Alternatively, IGFR transactivation by V2R could rely on the metalloproteinase-mediated cleavage of IGF binding proteins, which are a family of secreted proteins specifically binding and sequestering IGFR ligands with affinities equal to or greater than those affinities of the IGFR. Zinc-dependent metalloproteinase-mediated proteolysis of the IGF binding proteins has previously been shown to regulate the release of biologically active IGF (49–55).
Several metalloproteinases have been shown to promote ERK1/2 activation in response to GPCR stimulation (56–58), but none has been linked to V2R stimulation of MAPK. Interestingly, however, AVP has been shown to induce translocation and activation of the zinc-dependent metalloproteinase Phospholipase A2-activating protein (P-LAP) through V2R in cells derived from rat renal tubules (59). However, this enzyme is most likely not the one involved in our system, because DNA CHIP expression array experiments did not reveal a significant expression of P-LAP in the HEK293 cells used in the present study. Taking advantage of either dominant negative mutants or selective pharmacological metalloproteinase inhibitors currently available, we were also able to rule out the implication of a disintegrin and metalloproteinase (ADAM)17, matrix metalloproteinase (MMP)9, MMP2, and MMP13 in the V2R stimulation of ERK1/2, but we could not identify the metalloproteinase involved.
Our observation that Src activity is required for the metalloproteinase-dependent release of a transactivating ligand in the extracellular medium after V2R stimulation confirms Src family kinases as the GPCR effectors contributing to the regulation of metalloproteinase activity. Interestingly, Src has been shown to interact with members of the ADAM family of zinc-dependent metalloproteinase through its SH3 domain and phosphorylate several metalloproteinases on their C terminus (60–62). In the case of EGFR transactivation by the α2AAR, Src was required for both the GPCR-promoted shedding of EGF from its proligand heparin-binding EGF-like growth factor (HB-EGF) and the activation of ERK downstream of EGFR transactivation (63). For the V2R-mediated activation of ERK1/2, no implication of Src downstream of the IGFR transactivation was detected, because the inhibition of Src in V2R-null receiving cells did not alter the ability of the transferred supernatant of AVP-stimulated, V2R-expressing cells to promote ERK1/2 activation. Also consistent with the notion that Src acted exclusively upstream of the metalloproteinase-dependent ligand shedding to promote IGFR transactivation is our observation that Src activation in response to IGF1 could not be observed in our cells.
β-Arrestin engagement by activated GPCRs has been shown to promote Src recruitment and activation, an event required for downstream signal transduction (17, 18). This scaffolding mechanism is, however, unlikely to account for the V2R-promoted Src activation detected in our study given that the recruitment of β-arrestins to the V2R is not needed for AVP-stimulated ERK1/2 activation. Indeed, β-arrestin knockdown in V2R-expressing cells did not abolish the AVP-stimulated release of the IGFR transactivating ligand. We uncovered a role for β-arrestins downstream of IGFR transactivation, and in contrast to the current view, we showed that the β-arrestin pool involved in ERK1/2 stimulation is engaged by the transactivated RTK rather than V2R. This unique model of β-arrestin–dependent ERK1/2 activation is consistent with recent findings highlighting the importance of β-arrestins in RTK regulation and signaling (21). Several RTKs have indeed been shown to recruit β-arrestins in response to their cognate ligands, including the IGFR after IGF1 stimulation (20, 36, 64). β-Arrestins were also found to be required for IGF1 stimulation of the ERK1/2 and PI3K pathways (23, 24) as well as IGFR endocytosis (36). In Caenorhabditis elegans, direct engagement of arrestin by the IGFR homolog, Daf2, has been shown to play an important role in longevity, thus supporting a role for this pathway in physiologically relevant phenomenon (65). β-arrestins therefore seem to be true RTK signaling effectors. Adding to this pleiotropic nature of β-arrestins, our results reveal another level of complexity in the regulation of these scaffold proteins. Not only can β-arrestins be recruited to the transactivated IGFR in response to V2R stimulation, but they do so in response to the metalloproteinase-mediated release of an IGFR ligand in the extracellular medium. Indeed, siRNA-mediated silencing of β-arrestins in naïve HEK293 cells, devoid of V2R, abolished the ability of the transferred IGFR transactivating ligand to stimulate ERK1/2, indicating that V2R, as a signaling platform, is not required to stimulate the β-arrestin pool involved in ERK1/2 activation. This notion is substantiated by our observation that V2R(Ala6), in which a cluster of serine and threonine phosphorylation sites were mutated to alanines, conserved its ability to fully activate the ERK1/2 pathway in a β-arrestin–dependent manner, despite a reduced ability of this mutant receptor to recruit this scaffolding protein. This paradox of preserved β-arrestin–dependent GPCR-stimulated MAPK activity, despite an altered ability of the receptor to recruit β-arrestins, has been described previously. Indeed, mutation of five serines/threonines to alanine residues in the FSH receptor C tail resulted in a reduced ability of the receptor to recruit β-arrestins. However, similar to what was observed with V2R(Ala6), these mutations were found to potentiate the activation of ERK1/2, which was still β-arrestin–dependent (66). Similarly, the siRNA silencing of G protein–coupled receptor kinase (GRK)-2 and -3 has previously been shown to impair agonist-promoted phosphorylation of both V2R and AT1R and subsequent β-arrestin association to the receptors, but paradoxically, it was found to potentiate the β-arrestin–dependent ERK1/2 activation (67, 68). The V2R-mediated ERK1/2 activation mechanism described in this study allows us to propose an explanation for this apparent paradox. Indeed, the transactivated RTK and not the GPCR triggers the recruitment of the β-arrestin pool involved in ERK1/2 stimulation. The GPCR-recruited β-arrestin pool would rather promote receptor desensitization and endocytosis, thus accounting for the ERK1/2 pathway potentiation observed when using the β-arrestin recruitment-defective V2R(Ala6). The preceding discussion suggests that a role for β-arrestins downstream of a transactivation event, independent from its recruitment to a GPCR, may not be restricted to V2R. Consistent with this notion, we found that β-arrestins also act downstream of a transactivating event for the PAFR.
In several studies, physical association between a GPCR, β-arrestins, and an RTK in a multiprotein complex has been invoked as a mechanism explaining the RTK- and β-arrestin–dependent activation of ERK1/2 by a GPCR (14, 69, 70). Although such protein–protein interactions may contribute to the response, our finding that the IGFR transactivating ligand released after V2R stimulation can activate ERK in cells devoid of V2R in a β-arrestin–dependent manner suggests that a complex between the V2R and IGFR is not an essential part of the activation mechanism.
Our data also confirm that the activation of ERK1/2 after V2R stimulation is physiologically relevant, because it can be observed in rat kidney medulla after in vivo stimulation of the receptor (40). As it was found in the HEK293 cells, the mechanism leading to the MAPK activation in the kidney cells relied on the activation of a metalloproteinase and IGFR. Indeed, the metalloproteinase and IGFR inhibitors significantly blocked the dDAVP-stimulated ERK1/2 phosphorylation in the medulla kidney cells (known to express the V2R) without affecting the basal ERK1/2 activity in adjacent regions of the kidney. Given the potential role of ERK1/2 in controlling phosphorylation of AQP2, cell growth (40), and apoptosis (71), the metalloproteinase- and IGFR-dependent activation of ERK1/2 by V2R could play functional roles in water homeostasis and oncogenic or procystic processes. Interestingly, metalloproteinase inhibitors have been shown to present anticancer properties in many tissues, and marimastat, in particular, was developed for several cancer indications (72). Although these metalloproteinases were selected largely for their action on the cellular matrix that could prevent invasion, their role in controlling MAPK activation by GPCRs could also be part of their action mechanism. IGFR for its part has been shown to increase kidney cell growth (73, 74) and has been identified as a potential target for the treatment of some renal cancers (75, 76). Future work will be required to establish the identity of the metalloproteinase and the processed IGFR ligand involved in the pathway in order to design the experiments to directly test the importance of the V2R-stimulated ERK1/2 pathway in normal and pathological conditions.
Materials and Methods
Western Blotting.
Cells were grown in six-well plates and rendered quiescent by serum starvation for 16 h before incubation in the presence or absence of the specified inhibitors for the indicated time followed by the different stimulations: 5 min with 1 μM AVP, 200 nM PAF, 100 ng/mL IGF1, or 10 ng/mL EGF. When assessing ectodomain shedding transactivation, cells were incubated with 1 μM AVP; the supernatant was taken after 5 min stimulation and transferred on V2R-null HEK293 cells that were then incubated for 5 min before being harvested, lysed, and prepared for Western blot analysis. ERK1/2 or ERK2-GFP phosphorylation was detected by protein immunoblotting using mouse monoclonal anti–P-ERK (E-4) and anti-mouse HRP-conjugated antibodies for chemiluminescence detection. After quantification of the bands by densitometry, nitrocellulose membranes were stripped of Igs and reprobed using rabbit polyclonal anti-ERK (K-23) to assess total ERK expression. ERK phosphorylation was normalized according to the loading of proteins by expressing the data as a percentage of P-ERK over total ERK (or P-ERK2-GFP over total ERK2-GFP) of the level observed in the agonist-stimulated condition. When performing the ectodomain shedding transactivation assay, because a basal activity was detectable, ERK activity was expressed as the fold increase of P-ERK/total ERK over basal P-ERK/total ERK. c-Src and IGFR activation and phosphorylation were detected and quantified similarly using either the rabbit polyclonal anti–P-Src antibody that recognizes c-Src phosphorylated at tyrosine 416 and the mouse monoclonal anti-Src (GD11) antibody or the phosphospecific IGF-1R antibody directed against phosphorylated tyrosine 1131 and the mouse anti–IGF-1Rα 1H7 antibody. Detection of myc- and HA-tagged constructs was performed using mouse monoclonal anti-myc 9E10 and anti-HA 12CA5 antibodies, respectively, followed by an anti-mouse HRP-conjugated IgG. Detection of β-arrestin-1 and -2 was achieved using either the mouse monoclonal anti–β-arrestin-2 (H9) antibody that recognizes both β-arrestin isoforms followed by anti-mouse HRP-conjugated IgG or the rabbit monoclonal anti–β-arrestin-1/2 (D24H9) antibody followed by anti-rabbit HRP-conjugated IgG.
Immunohistochemistry.
Fixed kidney tissue was cut with a vibratome into 50-μm-thick sections. The vibratome sections were rinsed in PBS and incubated for 48 h at 4 °C with an anti-pERK1/2 rabbit polyclonal IgG antibody (Biosource) at a working dilution of 1:500 in PBS:BSA 1 mg/mL. After rinsing in PBS, sections were incubated for 4 h at 4 °C with a donkey anti-rabbit IgG secondary antibody conjugated to the Cy3 fluorophore from Jackson ImmunoResearch Laboratories used at 1:1,000. Primary and secondary antibodies were diluted in PBS containing 1% normal goat or donkey serum and 0.1% Triton X-100.
Additional information concerning materials, expression vectors, cell culture and transfections, immunoprecipitation, animals, animal treatment, imaging and quantification of animal tissue sections, and data analysis is presented in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Monique Lagacé for critical reading of the manuscript; and Rafik Marir, Evelyne Gallibert, and Dominique Haddou for technical assistance with experiments. Confocal microscopy has been realized using the Montpellier Bio Imaging Facility, with specific help from Julien Cau and Nicole Lautredou. G.O-L. was supported by a doctoral studentship from the Canadian Institute of Health Research. J.Z. holds a studentship from the Fonds de Recherche en Santé du Québec. M.C. and G.G. were supported by grants from Centre National de la Recherche Scientifique. M.B. holds a Canada Research Chair in Signal Transduction and Molecular Pharmacology. This work was supported by grants from the Kidney Foundation of Canada and Canadian Institutes for Health Research (to M.B.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
See Author Summary on page 6374 (volume 109, number 17).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1112422109/-/DCSupplemental.
References
- 1.Charest PG, Bouvier M. Palmitoylation of the V2 vasopressin receptor carboxyl tail enhances beta-arrestin recruitment leading to efficient receptor endocytosis and ERK1/2 activation. J Biol Chem. 2003;278:41541–41551. doi: 10.1074/jbc.M306589200. [DOI] [PubMed] [Google Scholar]
- 2.Tohgo A, et al. The stability of the G protein-coupled receptor-beta-arrestin interaction determines the mechanism and functional consequence of ERK activation. J Biol Chem. 2003;278:6258–6267. doi: 10.1074/jbc.M212231200. [DOI] [PubMed] [Google Scholar]
- 3.Charest PG, Oligny-Longpré G, Bonin H, Azzi M, Bouvier M. The V2 vasopressin receptor stimulates ERK1/2 activity independently of heterotrimeric G protein signalling. Cell Signal. 2007;19:32–41. doi: 10.1016/j.cellsig.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 4.Werry TD, Sexton PM, Christopoulos A. “Ins and outs” of seven-transmembrane receptor signalling to ERK. Trends Endocrinol Metab. 2005;16:26–33. doi: 10.1016/j.tem.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Wetzker R, Böhmer FD. Transactivation joins multiple tracks to the ERK/MAPK cascade. Nat Rev Mol Cell Biol. 2003;4:651–657. doi: 10.1038/nrm1173. [DOI] [PubMed] [Google Scholar]
- 6.Prenzel N, et al. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;402:884–888. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- 7.Oak JN, Lavine N, Van Tol HH. Dopamine D(4) and D(2L) receptor stimulation of the mitogen-activated protein kinase pathway is dependent on trans-activation of the platelet-derived growth factor receptor. Mol Pharmacol. 2001;60:92–103. doi: 10.1124/mol.60.1.92. [DOI] [PubMed] [Google Scholar]
- 8.Tsuchioka M, Takebayashi M, Hisaoka K, Maeda N, Nakata Y. Serotonin (5-HT) induces glial cell line-derived neurotrophic factor (GDNF) mRNA expression via the transactivation of fibroblast growth factor receptor 2 (FGFR2) in rat C6 glioma cells. J Neurochem. 2008;106:244–257. doi: 10.1111/j.1471-4159.2008.05357.x. [DOI] [PubMed] [Google Scholar]
- 9.Korzh A, Keren O, Gafni M, Bar-Josef H, Sarne Y. Modulation of extracellular signal-regulated kinase (ERK) by opioid and cannabinoid receptors that are expressed in the same cell. Brain Res. 2008;1189:23–32. doi: 10.1016/j.brainres.2007.10.070. [DOI] [PubMed] [Google Scholar]
- 10.El Zein N, Badran BM, Sariban E. The neuropeptide pituitary adenylate cyclase activating protein stimulates human monocytes by transactivation of the Trk/NGF pathway. Cell Signal. 2007;19:152–162. doi: 10.1016/j.cellsig.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 11.Keely SJ, Calandrella SO, Barrett KE. Carbachol-stimulated transactivation of epidermal growth factor receptor and mitogen-activated protein kinase in T(84) cells is mediated by intracellular ca(2+), PYK-2, and p60(src) J Biol Chem. 2000;275:12619–12625. doi: 10.1074/jbc.275.17.12619. [DOI] [PubMed] [Google Scholar]
- 12.Biscardi JS, et al. c-Src-mediated phosphorylation of the epidermal growth factor receptor on Tyr845 and Tyr1101 is associated with modulation of receptor function. J Biol Chem. 1999;274:8335–8343. doi: 10.1074/jbc.274.12.8335. [DOI] [PubMed] [Google Scholar]
- 13.Drube S, Stirnweiss J, Valkova C, Liebmann C. Ligand-independent and EGF receptor-supported transactivation: Lessons from beta2-adrenergic receptor signalling. Cell Signal. 2006;18:1633–1646. doi: 10.1016/j.cellsig.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Maudsley S, et al. The beta(2)-adrenergic receptor mediates extracellular signal-regulated kinase activation via assembly of a multi-receptor complex with the epidermal growth factor receptor. J Biol Chem. 2000;275:9572–9580. doi: 10.1074/jbc.275.13.9572. [DOI] [PubMed] [Google Scholar]
- 15.DeFea KA, et al. Beta-arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J Cell Biol. 2000;148:1267–1281. doi: 10.1083/jcb.148.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luttrell LM, et al. Activation and targeting of extracellular signal-regulated kinases by beta-arrestin scaffolds. Proc Natl Acad Sci USA. 2001;98:2449–2454. doi: 10.1073/pnas.041604898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buchanan FG, et al. Role of beta-arrestin 1 in the metastatic progression of colorectal cancer. Proc Natl Acad Sci USA. 2006;103:1492–1497. doi: 10.1073/pnas.0510562103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luttrell LM, et al. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283:655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- 19.DeFea KA, et al. The proliferative and antiapoptotic effects of substance P are facilitated by formation of a beta-arrestin-dependent scaffolding complex. Proc Natl Acad Sci USA. 2000;97:11086–11091. doi: 10.1073/pnas.190276697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dalle S, Ricketts W, Imamura T, Vollenweider P, Olefsky JM. Insulin and insulin-like growth factor I receptors utilize different G protein signaling components. J Biol Chem. 2001;276:15688–15695. doi: 10.1074/jbc.M010884200. [DOI] [PubMed] [Google Scholar]
- 21.Hupfeld CJ, Olefsky JM. Regulation of receptor tyrosine kinase signaling by GRKs and beta-arrestins. Annu Rev Physiol. 2007;69:561–577. doi: 10.1146/annurev.physiol.69.022405.154626. [DOI] [PubMed] [Google Scholar]
- 22.Rakhit S, Pyne S, Pyne NJ. Nerve growth factor stimulation of p42/p44 mitogen-activated protein kinase in PC12 cells: Role of G(i/o), G protein-coupled receptor kinase 2, beta-arrestin I, and endocytic processing. Mol Pharmacol. 2001;60:63–70. doi: 10.1124/mol.60.1.63. [DOI] [PubMed] [Google Scholar]
- 23.Girnita L, et al. Beta-arrestin and Mdm2 mediate IGF-1 receptor-stimulated ERK activation and cell cycle progression. J Biol Chem. 2007;282:11329–11338. doi: 10.1074/jbc.M611526200. [DOI] [PubMed] [Google Scholar]
- 24.Povsic TJ, Kohout TA, Lefkowitz RJ. Beta-arrestin1 mediates insulin-like growth factor 1 (IGF-1) activation of phosphatidylinositol 3-kinase (PI3K) and anti-apoptosis. J Biol Chem. 2003;278:51334–51339. doi: 10.1074/jbc.M309968200. [DOI] [PubMed] [Google Scholar]
- 25.Sachdev D, Hartell JS, Lee AV, Zhang X, Yee D. A dominant negative type I insulin-like growth factor receptor inhibits metastasis of human cancer cells. J Biol Chem. 2004;279:5017–5024. doi: 10.1074/jbc.M305403200. [DOI] [PubMed] [Google Scholar]
- 26.Peterson JE, et al. Src phosphorylates the insulin-like growth factor type I receptor on the autophosphorylation sites. Requirement for transformation by src. J Biol Chem. 1996;271:31562–31571. doi: 10.1074/jbc.271.49.31562. [DOI] [PubMed] [Google Scholar]
- 27.Hernández-Sánchez C, Blakesley V, Kalebic T, Helman L, LeRoith D. The role of the tyrosine kinase domain of the insulin-like growth factor-I receptor in intracellular signaling, cellular proliferation, and tumorigenesis. J Biol Chem. 1995;270:29176–29181. doi: 10.1074/jbc.270.49.29176. [DOI] [PubMed] [Google Scholar]
- 28.Schäfer B, Gschwind A, Ullrich A. Multiple G-protein-coupled receptor signals converge on the epidermal growth factor receptor to promote migration and invasion. Oncogene. 2004;23:991–999. doi: 10.1038/sj.onc.1207278. [DOI] [PubMed] [Google Scholar]
- 29.Rozengurt E. Mitogenic signaling pathways induced by G protein-coupled receptors. J Cell Physiol. 2007;213:589–602. doi: 10.1002/jcp.21246. [DOI] [PubMed] [Google Scholar]
- 30.Biscardi JS, Ishizawar RC, Silva CM, Parsons SJ. Tyrosine kinase signalling in breast cancer: Epidermal growth factor receptor and c-Src interactions in breast cancer. Breast Cancer Res. 2000;2:203–210. doi: 10.1186/bcr55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim LC, Song L, Haura EB. Src kinases as therapeutic targets for cancer. Nat Rev Clin Oncol. 2009;6:587–595. doi: 10.1038/nrclinonc.2009.129. [DOI] [PubMed] [Google Scholar]
- 32.Boney CM, Sekimoto H, Gruppuso PA, Frackelton AR., Jr Src family tyrosine kinases participate in insulin-like growth factor I mitogenic signaling in 3T3-L1 cells. Cell Growth Differ. 2001;12:379–386. [PubMed] [Google Scholar]
- 33.Lieskovska J, Ling Y, Badley-Clarke J, Clemmons DR. The role of Src kinase in insulin-like growth factor-dependent mitogenic signaling in vascular smooth muscle cells. J Biol Chem. 2006;281:25041–25053. doi: 10.1074/jbc.M602866200. [DOI] [PubMed] [Google Scholar]
- 34.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 35.Galandrin S, et al. Conformational rearrangements and signaling cascades involved in ligand-biased mitogen-activated protein kinase signaling through the beta1-adrenergic receptor. Mol Pharmacol. 2008;74:162–172. doi: 10.1124/mol.107.043893. [DOI] [PubMed] [Google Scholar]
- 36.Lin FT, Daaka Y, Lefkowitz RJ. Beta-arrestins regulate mitogenic signaling and clathrin-mediated endocytosis of the insulin-like growth factor I receptor. J Biol Chem. 1998;273:31640–31643. doi: 10.1074/jbc.273.48.31640. [DOI] [PubMed] [Google Scholar]
- 37.Oakley RH, Laporte SA, Holt JA, Barak LS, Caron MG. Association of beta-arrestin with G protein-coupled receptors during clathrin-mediated endocytosis dictates the profile of receptor resensitization. J Biol Chem. 1999;274:32248–32257. doi: 10.1074/jbc.274.45.32248. [DOI] [PubMed] [Google Scholar]
- 38.Barak LS, Oakley RH, Laporte SA, Caron MG. Constitutive arrestin-mediated desensitization of a human vasopressin receptor mutant associated with nephrogenic diabetes insipidus. Proc Natl Acad Sci USA. 2001;98:93–98. doi: 10.1073/pnas.011303698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oakley RH, Laporte SA, Holt JA, Barak LS, Caron MG. Molecular determinants underlying the formation of stable intracellular G protein-coupled receptor-beta-arrestin complexes after receptor endocytosis*. J Biol Chem. 2001;276:19452–19460. doi: 10.1074/jbc.M101450200. [DOI] [PubMed] [Google Scholar]
- 40.Alonso G, et al. Sustained elevated levels of circulating vasopressin selectively stimulate the proliferation of kidney tubular cells via the activation of V2 receptors. Endocrinology. 2009;150:239–250. doi: 10.1210/en.2008-0068. [DOI] [PubMed] [Google Scholar]
- 41.Daub H, Weiss FU, Wallasch C, Ullrich A. Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors. Nature. 1996;379:557–560. doi: 10.1038/379557a0. [DOI] [PubMed] [Google Scholar]
- 42.Rao GN, Delafontaine P, Runge MS. Thrombin stimulates phosphorylation of insulin-like growth factor-1 receptor, insulin receptor substrate-1, and phospholipase C-gamma 1 in rat aortic smooth muscle cells. J Biol Chem. 1995;270:27871–27875. doi: 10.1074/jbc.270.46.27871. [DOI] [PubMed] [Google Scholar]
- 43.Spartà A, Baiula M, Campbell G, Spampinato S. beta-Arrestin 2-mediated heterologous desensitization of IGF-IR by prolonged exposure of SH-SY5Y neuroblastoma cells to a mu opioid agonist. FEBS Lett. 2010;584:3580–3586. doi: 10.1016/j.febslet.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 44.Tu H, et al. GABAB receptor activation protects neurons from apoptosis via IGF-1 receptor transactivation. J Neurosci. 2010;30:749–759. doi: 10.1523/JNEUROSCI.2343-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zahradka P, Litchie B, Storie B, Helwer G. Transactivation of the insulin-like growth factor-I receptor by angiotensin II mediates downstream signaling from the angiotensin II type 1 receptor to phosphatidylinositol 3-kinase. Endocrinology. 2004;145:2978–2987. doi: 10.1210/en.2004-0029. [DOI] [PubMed] [Google Scholar]
- 46.Gschwind A, Hart S, Fischer OM, Ullrich A. TACE cleavage of proamphiregulin regulates GPCR-induced proliferation and motility of cancer cells. EMBO J. 2003;22:2411–2421. doi: 10.1093/emboj/cdg231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li X, et al. PDGF-C is a new protease-activated ligand for the PDGF alpha-receptor. Nat Cell Biol. 2000;2:302–309. doi: 10.1038/35010579. [DOI] [PubMed] [Google Scholar]
- 48.Bergsten E, et al. PDGF-D is a specific, protease-activated ligand for the PDGF beta-receptor. Nat Cell Biol. 2001;3:512–516. doi: 10.1038/35074588. [DOI] [PubMed] [Google Scholar]
- 49.Bunn RC, Fowlkes JL. Insulin-like growth factor binding protein proteolysis. Trends Endocrinol Metab. 2003;14:176–181. doi: 10.1016/s1043-2760(03)00049-3. [DOI] [PubMed] [Google Scholar]
- 50.Byun D, et al. Pregnancy-associated plasma protein-A accounts for the insulin-like growth factor (IGF)-binding protein-4 (IGFBP-4) proteolytic activity in human pregnancy serum and enhances the mitogenic activity of IGF by degrading IGFBP-4 in vitro. J Clin Endocrinol Metab. 2001;86:847–854. doi: 10.1210/jcem.86.2.7223. [DOI] [PubMed] [Google Scholar]
- 51.Lawrence JB, et al. The insulin-like growth factor (IGF)-dependent IGF binding protein-4 protease secreted by human fibroblasts is pregnancy-associated plasma protein-A. Proc Natl Acad Sci USA. 1999;96:3149–3153. doi: 10.1073/pnas.96.6.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boldt HB, Conover CA. Pregnancy-associated plasma protein-A (PAPP-A): A local regulator of IGF bioavailability through cleavage of IGFBPs. Growth Horm IGF Res. 2007;17:10–18. doi: 10.1016/j.ghir.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 53.Yan X, Baxter RC, Firth SM. Involvement of pregnancy-associated plasma protein-A2 in insulin-like growth factor (IGF) binding protein-5 proteolysis during pregnancy: A potential mechanism for increasing IGF bioavailability. J Clin Endocrinol Metab. 2010;95:1412–1420. doi: 10.1210/jc.2009-2277. [DOI] [PubMed] [Google Scholar]
- 54.Hemers E, et al. Insulin-like growth factor binding protein-5 is a target of matrix metalloproteinase-7: Implications for epithelial-mesenchymal signaling. Cancer Res. 2005;65:7363–7369. doi: 10.1158/0008-5472.CAN-05-0157. [DOI] [PubMed] [Google Scholar]
- 55.Imai Y, et al. Protease-resistant form of insulin-like growth factor-binding protein 5 is an inhibitor of insulin-like growth factor-I actions on porcine smooth muscle cells in culture. J Clin Invest. 1997;100:2596–2605. doi: 10.1172/JCI119803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schäfer B, Marg B, Gschwind A, Ullrich A. Distinct ADAM metalloproteinases regulate G protein-coupled receptor-induced cell proliferation and survival. J Biol Chem. 2004;279:47929–47938. doi: 10.1074/jbc.M400129200. [DOI] [PubMed] [Google Scholar]
- 57.Fischer OM, Hart S, Gschwind A, Ullrich A. EGFR signal transactivation in cancer cells. Biochem Soc Trans. 2003;31:1203–1208. doi: 10.1042/bst0311203. [DOI] [PubMed] [Google Scholar]
- 58.Ohtsu H, Dempsey PJ, Eguchi S. ADAMs as mediators of EGF receptor transactivation by G protein-coupled receptors. Am J Physiol Cell Physiol. 2006;291:C1–C10. doi: 10.1152/ajpcell.00620.2005. [DOI] [PubMed] [Google Scholar]
- 59.Masuda S, et al. Involvement of the V2 receptor in vasopressin-stimulated translocation of placental leucine aminopeptidase/oxytocinase in renal cells. Eur J Biochem. 2003;270:1988–1994. doi: 10.1046/j.1432-1033.2003.03570.x. [DOI] [PubMed] [Google Scholar]
- 60.Nyalendo C, et al. Src-dependent phosphorylation of membrane type I matrix metalloproteinase on cytoplasmic tyrosine 573: Role in endothelial and tumor cell migration. J Biol Chem. 2007;282:15690–15699. doi: 10.1074/jbc.M608045200. [DOI] [PubMed] [Google Scholar]
- 61.Suzuki A, et al. Meltrin alpha cytoplasmic domain interacts with SH3 domains of Src and Grb2 and is phosphorylated by v-Src. Oncogene. 2000;19:5842–5850. doi: 10.1038/sj.onc.1203986. [DOI] [PubMed] [Google Scholar]
- 62.Poghosyan Z, et al. Phosphorylation-dependent interactions between ADAM15 cytoplasmic domain and Src family protein-tyrosine kinases. J Biol Chem. 2002;277:4999–5007. doi: 10.1074/jbc.M107430200. [DOI] [PubMed] [Google Scholar]
- 63.Pierce KL, et al. Epidermal growth factor (EGF) receptor-dependent ERK activation by G protein-coupled receptors: A co-culture system for identifying intermediates upstream and downstream of heparin-binding EGF shedding. J Biol Chem. 2001;276:23155–23160. doi: 10.1074/jbc.M101303200. [DOI] [PubMed] [Google Scholar]
- 64.Girnita L, et al. beta-Arrestin is crucial for ubiquitination and down-regulation of the insulin-like growth factor-1 receptor by acting as adaptor for the MDM2 E3 ligase. J Biol Chem. 2005;280:24412–24419. doi: 10.1074/jbc.M501129200. [DOI] [PubMed] [Google Scholar]
- 65.Palmitessa A, Benovic JL. Arrestin and the multi-PDZ domain-containing protein MPZ-1 interact with phosphatase and tensin homolog (PTEN) and regulate Caenorhabditis elegans longevity. J Biol Chem. 2010;285:15187–15200. doi: 10.1074/jbc.M110.104612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kara E, et al. A phosphorylation cluster of five serine and threonine residues in the C-terminus of the follicle-stimulating hormone receptor is important for desensitization but not for beta-arrestin-mediated ERK activation. Mol Endocrinol. 2006;20:3014–3026. doi: 10.1210/me.2006-0098. [DOI] [PubMed] [Google Scholar]
- 67.Ren XR, et al. Different G protein-coupled receptor kinases govern G protein and beta-arrestin-mediated signaling of V2 vasopressin receptor. Proc Natl Acad Sci USA. 2005;102:1448–1453. doi: 10.1073/pnas.0409534102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim J, et al. Functional antagonism of different G protein-coupled receptor kinases for beta-arrestin-mediated angiotensin II receptor signaling. Proc Natl Acad Sci USA. 2005;102:1442–1447. doi: 10.1073/pnas.0409532102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alderton F, et al. Tethering of the platelet-derived growth factor beta receptor to G-protein-coupled receptors. A novel platform for integrative signaling by these receptor classes in mammalian cells. J Biol Chem. 2001;276:28578–28585. doi: 10.1074/jbc.M102771200. [DOI] [PubMed] [Google Scholar]
- 70.Tilley DG, Kim IM, Patel PA, Violin JD, Rockman HA. beta-Arrestin mediates beta1-adrenergic receptor-epidermal growth factor receptor interaction and downstream signaling. J Biol Chem. 2009;284:20375–20386. doi: 10.1074/jbc.M109.005793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cagnol S, Chambard JC. ERK and cell death: Mechanisms of ERK-induced cell death—apoptosis, autophagy and senescence. FEBS J. 2010;277:2–21. doi: 10.1111/j.1742-4658.2009.07366.x. [DOI] [PubMed] [Google Scholar]
- 72.Hidalgo M, Eckhardt SG. Development of matrix metalloproteinase inhibitors in cancer therapy. J Natl Cancer Inst. 2001;93:178–193. doi: 10.1093/jnci/93.3.178. [DOI] [PubMed] [Google Scholar]
- 73.Rosendahl A, Forsberg G. Influence of IGF-IR stimulation or blockade on proliferation of human renal cell carcinoma cell lines. Int J Oncol. 2004;25:1327–1336. [PubMed] [Google Scholar]
- 74.Cheung CW, Vesey DA, Nicol DL, Johnson DW. The roles of IGF-I and IGFBP-3 in the regulation of proximal tubule, and renal cell carcinoma cell proliferation. Kidney Int. 2004;65:1272–1279. doi: 10.1111/j.1523-1755.2004.00535.x. [DOI] [PubMed] [Google Scholar]
- 75.Yuen JS, et al. Validation of the type 1 insulin-like growth factor receptor as a therapeutic target in renal cancer. Mol Cancer Ther. 2009;8:1448–1459. doi: 10.1158/1535-7163.MCT-09-0101. [DOI] [PubMed] [Google Scholar]
- 76.Major JM, Pollak MN, Snyder K, Virtamo J, Albanes D. Insulin-like growth factors and risk of kidney cancer in men. Br J Cancer. 2010;103:132–135. doi: 10.1038/sj.bjc.6605722. [DOI] [PMC free article] [PubMed] [Google Scholar]