Abstract
Opioid peptides are involved in various essential physiological processes, most notably nociception. Dipeptidyl peptidase III (DPP III) is one of the most important enkephalin-degrading enzymes associated with the mammalian pain modulatory system. Here we describe the X-ray structures of human DPP III and its complex with the opioid peptide tynorphin, which rationalize the enzyme's substrate specificity and reveal an exceptionally large domain motion upon ligand binding. Microcalorimetric analyses point at an entropy-dominated process, with the release of water molecules from the binding cleft (“entropy reservoir”) as the major thermodynamic driving force. Our results provide the basis for the design of specific inhibitors that enable the elucidation of the exact role of DPP III and the exploration of its potential as a target of pain intervention strategies.
Keywords: isothermal titration calorimetry, metallopeptidase, peptide binding, X-ray crystallography
The endogenous opioid system, composed of opioid peptides and their receptors, modulates a large number of physiological processes, such as endocrine and immune function, gastrointestinal motility, respiration, reward, stress, complex social behavior (e.g., sexual activity), vulnerability to drug addiction, and most notably the procession and transmission of pain stimuli (nociception) (1, 2). Two major types of endogenous opioid peptides are those containing enkephalin sequences at the N terminus (Tyr-Gly-Gly-Phe-Met/Leu) (3) and, more recently identified, endomorphins 1 and 2 (Tyr-Pro-Trp/Phe-Phe-NH2) (4, 5). Knowledge and control over synthesis and degradation pathways of this class of molecules is prerequisite for the development of new therapies that target pertinent physiological processes.
Dipeptidyl peptidase III (DPP III), also known as enkephalinase B, is an enkephalin-degrading enzyme that cleaves dipeptides sequentially from the N termini of substrates (6). All DPP IIIs described thus far contain the unique zinc-binding motif HEXXGH characteristic of metallopeptidase family M49 (7). Enzymes from several human and animal tissues, as well as from lower eukaryotes, were purified and biochemically characterized (8, 9). DPP III is largely found as a cytosolic protein, although membrane association in rat brain and Drosophila melanogaster has been described (10, 11). The 3D structure of the yeast ortholog has recently been determined, revealing a unique protein fold with two lobes forming a wide-open substrate-binding cleft (12). The lack of structural information on peptide complexes, however, left the question of substrate specificity largely unanswered.
DPP III purified from monkey brain microsomes is strongly inhibited by the neuropeptide spinorphin (Leu-Val-Val-Tyr-Pro-Trp-Thr), an endogenous factor isolated from bovine spinal cord that also inhibits other enkephalin-degrading enzymes, such as neutral endopeptidase (NEP, neprilysin), aminopeptidase, and angiotensin-converting enzyme (13). Because of a different mode of action compared with morphine, spinorphin is an analgesic, potentially useful for pain treatment in morphine-resistant cases (14). This opioid peptide was also shown to be a potent and selective antagonist of the receptor P2X3, which is involved in pain signaling in chronic inflammatory nociception and neuropathic pain due to nerve injury (15).
Tynorphin (Val-Val-Tyr-Pro-Trp), a synthetic, truncated form of spinorphin, is a highly specific inhibitor of DPP III and was shown to induce an even more potent antinociceptive effect (14, 16). An important role of DPP III in the mammalian pain modulatory system is supported by several recent findings: low levels of DPP III activity were detected in the cerebrospinal fluid of individuals suffering from acute pain (17); DPP III exhibits high in vitro affinity toward the important neuropeptides endomorphin-1 and endomorphin-2 (18); and neuroanatomical studies localized rat DPP III in the superficial laminae of the dorsal horn, where enkephalin-synthesizing neurons and high concentrations of endomorphin-2 are found (19, 20).
DPP III is also related to other physiologic processes. It is overexpressed in ovarian primary carcinomas, the aggressiveness of which is correlated with enhanced DPP III activity (21). In a recent genomic screen, it was also identified as a potential activator of the antioxidant response element by inducing nuclear translocation of NF-E2–related factor 2 (22). Recently, DPP III was found among the approximately 1,100 proteins that constitute the human central proteome, which is the set of proteins ubiquitously and abundantly expressed in all human cell lines (23).
Advances in pain therapy have been scarce in the past decades. Acute and chronic pain are disabling conditions affecting millions of people worldwide, with huge social costs associated (24). Novel pharmacological compounds such as enkephalin-degrading enzyme inhibitors were shown to possess analgesic properties while lacking the adverse effects of some current therapeutics agents, such as morphine and its surrogates (25). However, degradation of enkephalins is effected by the joint action of several enzymes [i.e., NEP, aminopeptidase N (APN), and DPP III] (26), and inhibiting only one of those enzymes yields just marginal effects. For instance, RB101, a dual inhibitor of APN and NEP, was able to increase enkephalin levels and to produce antinociceptive, antidepressant, and antianxiety effects in rodents without opioid-related side effects, but the potency of this compound was much lower compared with morphine (27).
To date, DPP III is the only known enkephalin-degrading enzyme for which no structural data on substrate or inhibitor complexes are available. This lack of information has been a major obstacle for a detailed understanding of the enzyme's substrate specificity and for the development of specific inhibitors. Therefore, we determined the crystal structure of human DPP III and its complex with the opioid peptide tynorphin, revealing a large domain movement upon ligand binding, which possesses the signature of a distinctly entropy-driven process.
Results and Discussion
Initial attempts to crystallize the full-length human DPP III yielded crystals unsuitable for X-ray structure determination. To improve crystal quality a truncated form was designed (called here hDPP31–726), lacking 11 amino acid residues at the C terminus that were predicted to be unstructured (28). Monoclinic crystals (space group P21) obtained for this construct yielded diffraction data up to 1.9 Å resolution. The structure was solved by molecular replacement using the structure of yeast DPP III (12), resulting in one molecule in the asymmetric unit.
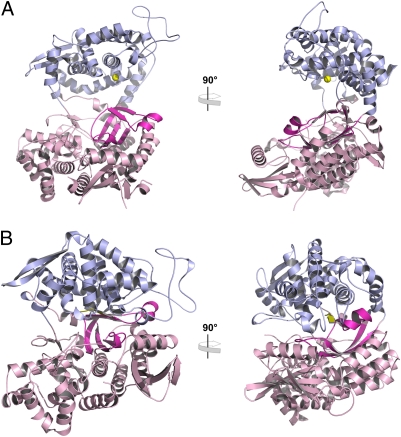
The overall fold of hDPP31–726 (Fig. 1A) is very similar to the yeast homolog (36% sequence identity). A wide cleft separates a mostly helical upper lobe containing the conserved zinc-binding motifs (450-HELLGH-455 and 507-EECRAE-512; zinc-coordinating residues in boldface in SI Appendix, Fig. S1) from a lower lobe of α/β-secondary structures containing a five-stranded β-barrel core (12). A superposition of the two structures yielded an rmsd of 1.4 Å for 523 superimposed Cα-atoms (SI Appendix, Fig. S2).
Fig. 1.
Overall structure of human DPP III. (A) Two perpendicular views of the overall structure of unbound hDPP31–726. The upper, zinc-binding lobe (residues 337–374 and 422–668) is shown in light blue, and the lower lobe (residues 1–336, 375–421, and 669–726) is shown in pink. The five-stranded β-core in the lower lobe (residues 307–335 and 376–409) is highlighted in magenta; the zinc ion is depicted as a yellow sphere. (B) Two perpendicular views of the overall structure of the complex with the opioid peptide tynorphin.
For cocrystallizing hDPP31–726 with the opioid peptide tynorphin, we exchanged Glu-451 for Ala, rendering the enzyme inactive (29) and preventing peptide cleavage. Two different, monoclinic crystal forms were obtained under the same conditions: space group P21 with two molecules per asymmetric unit and space group C2 with one molecule per asymmetric unit. These crystals diffracted to 2.4 and 3.0 Å resolution, respectively. Structure solution by molecular replacement failed when we used the complete structure of the unbound enzyme. However, when the two lobes were used separately as search templates, a solution was obtained (SI Appendix, Methods).
In both structures we observed well-defined electron density for tynorphin bound between the two lobes (SI Appendix, Fig. S3). The two independent molecules in the 2.4-Å structure are almost identical to each other (Cα-rmsd 0.03 Å) and—despite the different packing environments in the two crystal forms—they are also very similar to the 3.0-Å structure (Cα-rmsd of 0.5 Å). Because of the closely similar overall structures and conformations of the bound peptide (SI Appendix, Fig. S4), we are focusing our discussion on the higher-resolution structure. The most striking difference between unbound hDPP31–726 and its peptide complex is the closure of the binding cleft, which completely buries the bound ligand (Fig. 1). According to the program DynDom (30, 31), this conformational change can be described by a movement of two rigid bodies corresponding to the two lobes of the protein. This movement involves a rotation of approximately 60° of one domain relative to the other, with negligible translational contributions. A superposition on the corresponding upper and lower domains in the bound and unbound structures yielded Cα-rmsd values between 0.4 and 0.6 Å, indicating the rigidity of these domains. A difference distance matrix calculated from the Cα-coordinates of the bound and unbound structures revealed the same picture, with the upper and lower lobes moving as rigid bodies (SI Appendix, Fig. S5).
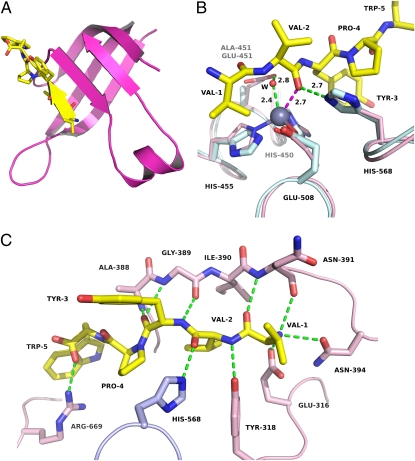
Tynorphin is bound in an extended conformation. The first three residues of the peptide form a β-strand, which binds to the five-stranded β-core of DPP III in an antiparallel fashion (Fig. 2A). Such a binding mode involving the extension of a β-sheet by the ligand is not uncommon in protein–peptide complexes (32).
Fig. 2.
Structure of human DPP III in complex with the opioid peptide tynorphin. (A) Close-up view of the five stranded β-core in the lower lobe (magenta), which is extended by the bound peptide (yellow). (B) Superposition of the zinc-binding residues in the structures of the unbound enzyme (light blue) and the peptide complex (pink). The peptide is shown in yellow, and the zinc ion is represented as a gray sphere. The supposed interaction of the P1 carbonyl group with the zinc ion is shown as a magenta dashed line. The interaction of a water molecule (W) with the zinc ion (see also SI Appendix, Fig. S1) as well as hydrogen bonds are depicted as green dashed lines. (C) Polar interactions of tynorphin with human DPP III. Residues from the lower lobe are shown in pink, His-568 (from the upper lobe) in light blue, and the peptide in yellow.
In both complex structures, electron density was missing for the zinc ion, which was very likely lost during crystallization. A superposition of the zinc-containing structure of unbound hDPP31–726 onto the structure of the peptide complex, however, reveals that the conformations of the zinc-coordinating residues are conserved in both structures (Fig. 2B). In this superposition, the carbonyl oxygen of the scissile peptide bond (P1, Val-2) is close to the zinc-bound water molecule (present in the structure of the unbound enzyme; SI Appendix, Fig. S1) and is at a distance of approximately 2.7 Å from the catalytic ion itself. Similarly, the side chain of Glu-451 (of the unbound protein) is appropriately positioned to activate this water molecule for the nucleophilic attack onto the amide bond. As in the proposed mechanisms of thermolysin (33) and neprilysin (34), the hydrogen bond between His-568 and the carbonyl group of Val-2 (Fig. 2B) likely provides additional stabilization of the tetrahedral intermediate (SI Appendix, Fig. S6). Both Glu-451 and His-568 are completely conserved among known DPP IIIs (9, 35).
The N terminus of the bound peptide is completely buried by the enzyme and anchored by polar interactions to the side chains of Glu-316 and Asn-394 and to the main-chain carbonyl group of Asn-391 (Fig. 2C). These tight interactions provide a rationale for the observation that peptides with modified N termini are not accepted as substrates by DPP III (36). Additional hydrogen bonds to the five-stranded β-core as well as to Tyr-318 suggest that the correct positioning of the P1 carbonyl group close to the catalytic zinc ion is the dominant basis of the dipeptidyl peptidase specificity of this enzyme. This is supported by the fact that all four of these catalytic residues are conserved among DPP IIIs (9, 35) and that a 125-fold decrease of kcat/Km was observed in the Y318F variant of human DPP III (37).
Substrate specificity of peptidases is likely governed by the shape and properties of the corresponding subsites in the binding site. In DPP IV and DPP VII, for example, subsite S1 is small and hydrophobic, restricting P1 to proline, alanine, or glycine (38). In contrast, all subsites in DPP III are deep and somewhat hydrophobic (SI Appendix, Fig. S7 and Table S2), in agreement with the relaxed specificity shown by this enzyme (18).
The C-terminal carboxylate of the bound peptide forms a salt bridge with Arg-669 (Fig. 2C). This residue is one of a series of conserved, positively charged amino acids (“Arg anchors”) in the binding cleft that have previously been predicted to bind the C termini of different-length peptides (12). Although the N terminus of the bound peptide is completely shielded from solvent, the cleft is more spacious on the opposite side, and a narrow tunnel connects the cleft with the exterior (SI Appendix, Fig. S7). This structural feature partly explains the observation that DPP III is able to accommodate peptides up to a length of 10 residues (39).
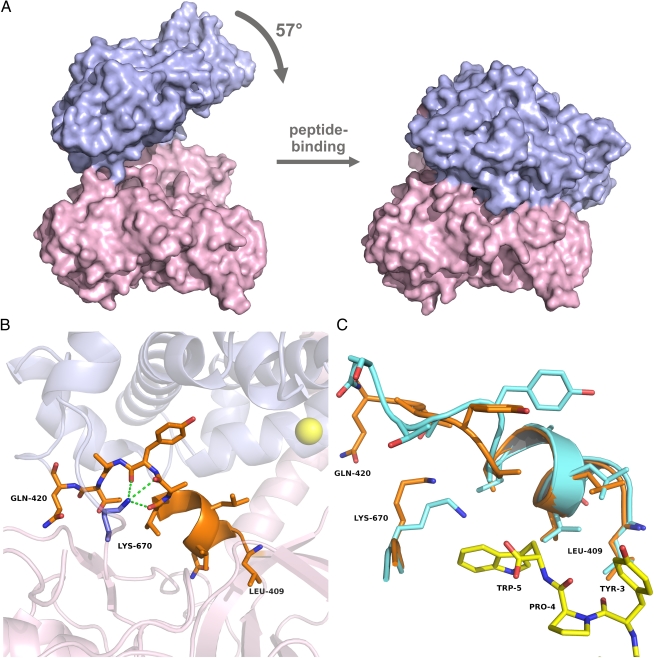
The analysis of the domain movement upon peptide binding using the program DynDom (30, 31) yielded a mainly rotational motion of approximately 60° and identified amino acids 409–420 as “mechanical hinge” residues (Fig. 3). The magnitude of this conformational change in enzymes is uncommon, probably because of the entropic costs involved (32).
Fig. 3.
Domain movement upon tynorphin binding to human DPP III. (A) Surface representation of unbound hDPP31–726 (Left) and of the peptide complex (Right). The coloring scheme is identical to that in Fig. 1. (B) Close-up view of the hinge region (residues 409–420) shown in orange. Lys-670 is shown in blue, together with its polar interactions with the U-shaped part of the hinge region. (C) Superposition of the hinge regions and Lys-670 in the unbound structure (orange) and in the peptide complex (cyan).
Residues in the hinge region are evolutionarily conserved among DPP IIIs, particularly at the beginning of the stretch, where they form secondary structures (9, 35). The C-terminal part of this hinge region adopts a distinct U-shaped conformation that is stabilized by hydrogen bonds to Lys-670 (Fig. 3B). With tynorphin binding, this lysine residue moves away to interact with the C-terminal tryptophan, leading to a conformational change in the hinge region (Fig. 3C). Interestingly, Lys-670 is also highly conserved. In the yeast enzyme this position is occupied by an arginine that forms similar interactions with the corresponding part of the hinge region (SI Appendix, Fig. S8).
The secondary structure at the beginning of the hinge is largely preserved upon ligand binding and conformational change (Fig. 3). The majority of the hinge residues have hydrophobic side chains, and therefore primarily weak interactions have to be rearranged upon domain motion, mainly as a result of side-chain rotations. In addition, the temperature factors of these residues are not significantly different from the average B-factors in the rest of the protein. These observations suggest that the hinge motion is a low-energy deformation similar to the situation first observed in T4 lysozyme (40).
In the complex structure, tynorphin forms polar interactions almost exclusively with residues of the lower lobe (Fig. 2C). The only exception is the hydrogen bond between His-568 and the carbonyl group of residue P1, which is important for catalysis (SI Appendix, Fig. S6). In addition, the larger part of the surface of the bound peptide is buried through interactions with the lower lobe. On the basis of this pattern of interactions, we suggest an order of peptide binding to human DPP III: the peptide binds first to the lower lobe (especially by interactions with five-stranded β-core; Fig. 2); the C terminus of the bound peptide induces conformational changes in the hinge region (Fig. 3C), possibly triggering the observed domain motion; and the latter brings the catalytic zinc ion (over a distance of approximately 16 Å) in close juxtaposition to the carbonyl group of the scissile amide bond. A possible role of substrate binding in triggering the conformational change is corroborated by the observation that a minimum number of four residues are required for a peptide being efficiently cleaved by DPP III (36, 41, 42). Furthermore, the close similarity of the unbound, open structures of human and yeast DPP III, despite their moderate sequence identity (SI Appendix, Fig. S2), indicates that this open structure represents a defined, even fixed conformation of the protein rather than a random snapshot from a continuum of flexible, open conformations.
Because the bound peptide is completely buried between the lobes and only narrow channels connect the binding site with the bulk solvent (SI Appendix, Fig. S7), product release quite likely occurs after reopening of the cleft.
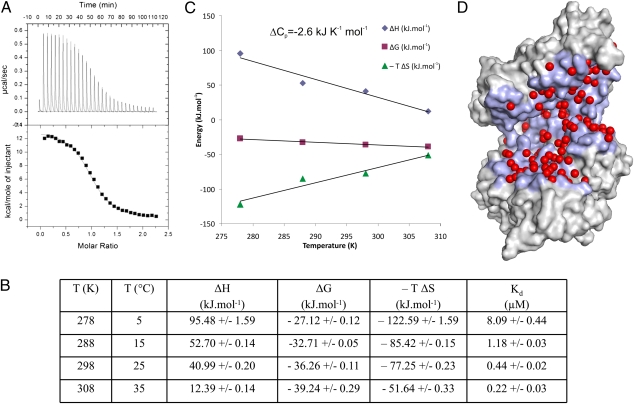
To further characterize tynorphin binding, we performed a series of isothermal titration calorimetry experiments at four different temperatures ranging from 5 to 35 °C. In this temperature range peptide binding was strongly endothermic (Fig. 4A), although exothermic binding was expected on the basis of the supposition that peptide interactions with proteins are mainly governed by optimization of hydrogen bonds (32). To have a spontaneous process, the free energy (ΔG), at constant pressure, must be negative, and hence the entropy term has to outweigh the enthalpy term (i.e., TΔS > ΔH) (43) in an endothermic process. As shown in Fig. 4, the positive ΔH in the entire temperature range studied is counteracted by a large gain in entropy, resulting in an exergonic binding process. The enthalpy term is less unfavorable at higher temperatures and overcompensates the smaller contribution by the entropy term, resulting in almost 40-fold tighter binding at 35 °C compared with 5 °C. Overall, the temperature dependence of the enthalpy (heat capacity change, ΔCp) has a negative slope, indicating that the binding process is dominated by the burial of hydrophobic surfaces. An analysis of the buried surface area upon peptide binding [using the program NACCESS (44)] indeed yielded a large loss of solvent-accessible area of approximately 3,500 Å2, with a ratio between hydrophobic and hydrophilic areas of 60:40. The calculated ΔCp using empirical area coefficients (45) is −2.5 kJ K−1 mol−1, in close agreement with the experimentally determined value of −2.6 kJ K−1 mol−1 (Fig. 4C).
Fig. 4.
Microcalorimetric analysis. (A) ITC measurement at 15 °C. Upper: Time-dependent deflection of heat for each injection. Lower: Peak integral as a function of molar ratio of hDPP31–726 to tynorphin. The continuous curve represents the best fit using a one-site binding model. (B) Table with thermodynamic data derived from the ITC measurements at different temperatures. (C) Temperature dependence of ΔG, ΔH, and -TΔS. (D) Surface representation of unbound hDPP31–726, indicating water molecules bound in the cleft and the part of the surface buried upon complex formation (light blue).
Thus, binding of tynorphin to human DPP III clearly is an entropy-driven process, with the major contribution very likely being the release of ordered water molecules from the binding cleft of the unbound enzyme (Fig. 4). A layer of water molecules (≈7 Å deep) consisting of different shells with varying rigidity is thought to be present on the surface of protein molecules, and the shells are in dynamic equilibrium with each other and the bulk solvent (46, 47). In an analysis of crystallization processes, the entropy contribution of the release of structured water molecules has been estimated to be in the range of 100 to 700 J mol−1 K−1, corresponding to approximately 5–30 water molecules displaced upon the incorporation of a protein molecule into a crystal (48). In the ligand-free structure of DPP III approximately 60 water molecules are positioned in the binding cleft (Fig. 4D), and we assume that the displacement of all (or most of) these waters is sufficient to explain the favorable entropy contribution to ligand binding (from 170 to 450 J mol−1 K−1 in the temperature range of our measurements). Therefore, we consider the water molecules bound in the large cleft between the two lobes as an “entropy reservoir” used to drive the binding process. The endothermic binding behavior of endomorphin-1, Leu-enkephalin, and IVYPW (SI Appendix, Fig. S9 and Table S3) indicates that the same concept very likely applies more generally to the binding of other opioid peptides to DPP III.
DPP III is one of very few peptidases known to display an entropy-driven binding mode (49) and/or a large domain movement upon substrate binding (50). The latter was suggested to regulate the enzyme in a mechanical rather than chemical fashion, with the rate-determining step being the closure of the active site (50). It is also conceivable that the closure of the binding cleft contributes to the promiscuous substrate specificity of DPP III. The domain movement may be seen as a way to accommodate diverse substrates through “conformational plasticity” (51) (i.e., by closing differently around different peptides). Along the same lines, the release of a large number of water molecules from the binding cleft (Fig. 4D) and the concomitant entropy gain facilitate the binding of different peptides, almost irrespective of the exact (enthalpic) interaction between enzyme and substrate.
The insights gained by our structure determination and the thermodynamic characterization of the binding process also provide a starting point for the rational design of molecular tools specific for DPP III and pave the way for new efforts to exploit this enzyme as drug target for pain intervention strategies. DPP III inhibitors might offer alternatives to conventional treatments in the nociceptive field, especially the use of opioid receptor agonists—such as morphine and its derivatives—that possess severe side effects. The data also allow the design of inhibitors that do not target the zinc ion but rather interfere with the domain motion. This would decrease cross-reactivity with other metallopeptidases and thus unwanted side effects.
Methods
A truncated variant of human dipeptidyl peptidase III consisting of residues 1–726 (hDPP31–726) was expressed in SF9 cells, purified to homogeneity by column chromatography, and crystallized. For cocrystallization with the opioid peptide tynorphin an inactive variant (E451A) of the enzyme expressed in Escherichia coli was used. Diffraction data to 1.9 Å (unbound enzyme) as well as to 2.4 Å and 3.0 Å (complexes with tynorphin) were collected using synchrotron radiation, and the structures were solved by molecular replacement using the structure of the yeast homolog as template (12).
Isothermal titration calorimetry (ITC) data were carried out at various temperatures in 25 mM Tris·HCl (pH 7.5) and 200 mM NaCl. Binding of the peptides (tynorphin, endomorphin-1, Leu-enkephalin, and IVYPW) to hDPP31–726 E451A was analyzed with a VP-ITC microcalorimeter (MicroCal) equilibrated at the respective temperature. A 200-μM peptide solution in the syringe was titrated into a 40-μM solution of the enzyme in the measuring cell. The corresponding heats of binding were determined by integration of the observed peaks after correction for the heat of dilution of the peptide and plotted against the ratio of peptide vs. protein concentration in the cell. Nonlinear least-squares fitting to these binding isotherms was used to obtain association constants (Ka), heats of binding (ΔH), and stoichiometries. Kd and Gibbs free energy (ΔG) were calculated.
Supplementary Material
Acknowledgments
We thank Lissete Crombet for cloning services; Alma Seitova for generating recombinant baculovirus; Denise Cavalcante Hissa and Bernhard Lehofer for assistance in protein purification; Christian C. Gruber for assistance in generating the distance difference matrix; and the beamline staff at the Advanced Photon Source, the Deutsches Elektronensynchrotron/European Molecular Biology Laboratory, and the European Synchrotron Radiation Facility for support during diffraction data collection. Funding was provided by Project W901 (Doktoratskolleg “Molecular Enzymology”) from the Austrian Science Funds; the Austrian Agency for International Cooperation in Research and Education; Project 098-1191344-2938 from the Croatian Ministry of Science, Education and Sport; the Canadian Institutes for Health Research; the Canadian Foundation for Innovation; Genome Canada through the Ontario Genomics Institute; GlaxoSmithKline; Karolinska Institutet; the Knut and Alice Wallenberg Foundation; the Ontario Innovation Trust; the Ontario Ministry for Research and Innovation; Merck & Co., Inc.; the Novartis Research Foundation; the Swedish Agency for Innovation Systems; the Swedish Foundation for Strategic Research; and the Wellcome Trust.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. W.W.B. is a guest editor invited by the Editorial Board.
Data deposition: Crystallography, atomic coordinates, and structure factors reported in this paper have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 3FVY, 3T6B, and 3T6J).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1118005109/-/DCSupplemental.
References
- 1.Fichna J, Janecka A, Costentin J, Do Rego JC. The endomorphin system and its evolving neurophysiological role. Pharmacol Rev. 2007;59:88–123. doi: 10.1124/pr.59.1.3. [DOI] [PubMed] [Google Scholar]
- 2.Bodnar RJ. Endogenous opiates and behavior: 2009. Peptides. 2010;31:2325–2359. doi: 10.1016/j.peptides.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 3.Hughes J, et al. Identification of two related pentapeptides from the brain with potent opiate agonist activity. Nature. 1975;258:577–580. doi: 10.1038/258577a0. [DOI] [PubMed] [Google Scholar]
- 4.Zadina JE, Hackler L, Ge LJ, Kastin AJ. A potent and selective endogenous agonist for the mu-opiate receptor. Nature. 1997;386:499–502. doi: 10.1038/386499a0. [DOI] [PubMed] [Google Scholar]
- 5.Zadina JE. Isolation and distribution of endomorphins in the central nervous system. Jpn J Pharmacol. 2002;89:203–208. doi: 10.1254/jjp.89.203. [DOI] [PubMed] [Google Scholar]
- 6.Prajapati SC, Chauhan SS. Dipeptidyl peptidase III: A multifaceted oligopeptide N-end cutter. FEBS J. 2011;278:3256–3276. doi: 10.1111/j.1742-4658.2011.08275.x. [DOI] [PubMed] [Google Scholar]
- 7.Rawlings ND, Morton FR, Kok CY, Kong J, Barrett AJ. MEROPS: The peptidase database. Nucleic Acids Res. 2008;36(Database issue):D320–D325. doi: 10.1093/nar/gkm954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J-M, Barrett AJ. Dipeptidyl-peptidase III. In: Barrett AJ, Rawlings ND, Woessner JF, editors. Handbook of Proteolytic Enzymes. Vol 1. Amsterdam: Elsevier Academic Press; 2004. pp. 809–812. [Google Scholar]
- 9.Abramić M, et al. Highly reactive cysteine residues are part of the substrate binding site of mammalian dipeptidyl peptidases III. Int J Biochem Cell Biol. 2004;36:434–446. doi: 10.1016/s1357-2725(03)00267-x. [DOI] [PubMed] [Google Scholar]
- 10.Čičin-Sain L, Šimaga S, Froebe A, Abramić M. Central aminopeptidase and serotonin system activities: Possible relationship. Neuropeptides. 2008;42:435–440. doi: 10.1016/j.npep.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Mazzocco C, Fukasawa KM, Auguste P, Puiroux J. Characterization of a functionally expressed dipeptidyl aminopeptidase III from Drosophila melanogaster. Eur J Biochem. 2003;270:3074–3082. doi: 10.1046/j.1432-1033.2003.03689.x. [DOI] [PubMed] [Google Scholar]
- 12.Baral PK, et al. The first structure of dipeptidyl-peptidase III provides insight into the catalytic mechanism and mode of substrate binding. J Biol Chem. 2008;283:22316–22324. doi: 10.1074/jbc.M803522200. [DOI] [PubMed] [Google Scholar]
- 13.Nishimura K, Hazato T. Isolation and identification of an endogenous inhibitor of enkephalin-degrading enzymes from bovine spinal cord. Biochem Biophys Res Commun. 1993;194:713–719. doi: 10.1006/bbrc.1993.1880. [DOI] [PubMed] [Google Scholar]
- 14.Ueda H, Matsunaga S, Inoue M, Yamamoto Y, Hazato T. Complete inhibition of purinoceptor agonist-induced nociception by spinorphin, but not by morphine. Peptides. 2000;21:1215–1221. doi: 10.1016/s0196-9781(00)00262-x. [DOI] [PubMed] [Google Scholar]
- 15.Jung KY, et al. Structure-activity relationship studies of spinorphin as a potent and selective human P2X(3) receptor antagonist. J Med Chem. 2007;50:4543–4547. doi: 10.1021/jm070114m. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto Y, Hashimoto J, Shimamura M, Yamaguchi T, Hazato T. Characterization of tynorphin, a potent endogenous inhibitor of dipeptidyl peptidaseIII. Peptides. 2000;21:503–508. doi: 10.1016/s0196-9781(00)00174-1. [DOI] [PubMed] [Google Scholar]
- 17.Sato H, Kimura K, Yamamoto Y, Hazato T. [Activity of DPP III in human cerebrospinal fluid derived from patients with pain] Masui. 2003;52:257–263. [PubMed] [Google Scholar]
- 18.Baršun M, Jajčanin N, Vukelić B, Špoljarić J, Abramić M. Human dipeptidyl peptidase III acts as a post-proline-cleaving enzyme on endomorphins. Biol Chem. 2007;388:343–348. doi: 10.1515/BC.2007.039. [DOI] [PubMed] [Google Scholar]
- 19.Chiba T, et al. Inhibition of recombinant dipeptidyl peptidase III by synthetic hemorphin-like peptides. Peptides. 2003;24:773–778. doi: 10.1016/s0196-9781(03)00119-0. [DOI] [PubMed] [Google Scholar]
- 20.Martin-Schild S, Gerall AA, Kastin AJ, Zadina JE. Differential distribution of endomorphin 1- and endomorphin 2-like immunoreactivities in the CNS of the rodent. J Comp Neurol. 1999;405:450–471. [PubMed] [Google Scholar]
- 21.Šimaga Š, Babić D, Osmak M, Šprem M, Abramić M. Tumor cytosol dipeptidyl peptidase III activity is increased with histological aggressiveness of ovarian primary carcinomas. Gynecol Oncol. 2003;91:194–200. doi: 10.1016/s0090-8258(03)00462-1. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, et al. A genomic screen for activators of the antioxidant response element. Proc Natl Acad Sci USA. 2007;104:5205–5210. doi: 10.1073/pnas.0700898104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burkhard TR, et al. Initial characterization of the human central proteome. BMC Bioinformatics. 2011;5:17. doi: 10.1186/1752-0509-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melzack R. The future of pain. Nat Rev Drug Discov. 2008;7:629. doi: 10.1038/nrd2640. [DOI] [PubMed] [Google Scholar]
- 25.Noble F, Roques BP. Protection of endogenous enkephalin catabolism as natural approach to novel analgesic and antidepressant drugs. Expert Opin Ther Targets. 2007;11:145–159. doi: 10.1517/14728222.11.2.145. [DOI] [PubMed] [Google Scholar]
- 26.Thanawala V, Kadam VJ, Ghosh R. Enkephalinase inhibitors: Potential agents for the management of pain. Curr Drug Targets. 2008;9:887–894. doi: 10.2174/138945008785909356. [DOI] [PubMed] [Google Scholar]
- 27.Jutkiewicz EM. RB101-mediated protection of endogenous opioids: Potential therapeutic utility? CNS Drug Rev. 2007;13:192–205. doi: 10.1111/j.1527-3458.2007.00011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones DT. Protein secondary structure prediction based on position-specific scoring matrices. J Mol Biol. 1999;292:195–202. doi: 10.1006/jmbi.1999.3091. [DOI] [PubMed] [Google Scholar]
- 29.Fukasawa K, Fukasawa KM, Iwamoto H, Hirose J, Harada M. The HELLGH motif of rat liver dipeptidyl peptidase III is involved in zinc coordination and the catalytic activity of the enzyme. Biochemistry. 1999;38:8299–8303. doi: 10.1021/bi9904959. [DOI] [PubMed] [Google Scholar]
- 30.Hayward S, Berendsen HJC. Systematic analysis of domain motions in proteins from conformational change: New results on citrate synthase and T4 lysozyme. Proteins. 1998;30:144–154. [PubMed] [Google Scholar]
- 31.Hayward S, Kitao A, Berendsen HJC. Model-free methods of analyzing domain motions in proteins from simulation: A comparison of normal mode analysis and molecular dynamics simulation of lysozyme. Proteins. 1997;27:425–437. doi: 10.1002/(sici)1097-0134(199703)27:3<425::aid-prot10>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 32.London N, Movshovitz-Attias D, Schueler-Furman O. The structural basis of peptide-protein binding strategies. Structure. 2010;18:188–199. doi: 10.1016/j.str.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 33.Matthews BW. Thermolysin. In: Messerschmidt A, Cygler M, Bode W, editors. Handbook of Metalloproteins. Vol 3. Chichester, UK: John Wiley & Sons; 2004. pp. 85–94. [Google Scholar]
- 34.Dale GE, Oefner C. Neprilysin. In: Messerschmidt A, Cygler M, Bode W, editors. Handbook of Metalloproteins. Vol 3. Chichester, UK: John Wiley & Sons; 2004. pp. 104–115. [Google Scholar]
- 35.Abramić M, Špoljarić J, Šimaga Š. Prokaryotic homologs help to define consensus sequences in peptidase family M49. Period Biol. 2004;106:161–168. [Google Scholar]
- 36.Ellis S, Nuenke JM. Dipeptidyl arylamidase III of the pituitary. Purification and characterization. J Biol Chem. 1967;242:4623–4629. [PubMed] [Google Scholar]
- 37.Salopek-Sondi B, et al. Functional tyrosine residue in the active center of human dipeptidyl peptidase III. Biol Chem. 2008;389:163–167. doi: 10.1515/BC.2008.021. [DOI] [PubMed] [Google Scholar]
- 38.Soisson SM, et al. Structural definition and substrate specificity of the S28 protease family: The crystal structure of human prolylcarboxypeptidase. BMC Struct Biol. 2010;10:16. doi: 10.1186/1472-6807-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abramić M, Zubanović M, Vitale L. Dipeptidyl peptidase III from human erythrocytes. Biol Chem Hoppe Seyler. 1988;369:29–38. doi: 10.1515/bchm3.1988.369.1.29. [DOI] [PubMed] [Google Scholar]
- 40.Faber HR, Matthews BW. A mutant T4 lysozyme displays five different crystal conformations. Nature. 1990;348:263–266. doi: 10.1038/348263a0. [DOI] [PubMed] [Google Scholar]
- 41.Abramić M, et al. Human and rat dipeptidyl peptidase III: Biochemical and mass spectrometric arguments for similarities and differences. Biol Chem. 2000;381:1233–1243. doi: 10.1515/BC.2000.151. [DOI] [PubMed] [Google Scholar]
- 42.Dhanda S, Singh H, Singh J, Singh TP. Isolation, purification and characterization of a DPP-III homologue from goat brain. Protein Expr Purif. 2007;52:297–305. doi: 10.1016/j.pep.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 43.Vekilov PG, et al. Intermolecular interactions, nucleation, and thermodynamics of crystallization of hemoglobin C. Biophys J. 2002;83:1147–1156. doi: 10.1016/S0006-3495(02)75238-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hubbart SJ, Thornton JM. NACCESS 2.1.1 (computer program) (Department of Biochemistry and Molecular Biology, University College, London) 1996 [Google Scholar]
- 45.Murphy KP, Freire E. Thermodynamics of structural stability and cooperative folding behavior in proteins. Adv Protein Chem. 1992;43:313–361. doi: 10.1016/s0065-3233(08)60556-2. [DOI] [PubMed] [Google Scholar]
- 46.Bhattacharyya SM, Wang ZG, Zewail AH. Dynamics of water near a protein surface. J Phys Chem B. 2003;107:13218–13228. [Google Scholar]
- 47.Pal SK, Zewail AH. Dynamics of water in biological recognition. Chem Rev. 2004;104:2099–2123. doi: 10.1021/cr020689l. [DOI] [PubMed] [Google Scholar]
- 48.Derewenda ZS, Vekilov PG. Entropy and surface engineering in protein crystallization. Acta Crystallogr D Biol Crystallogr. 2006;62:116–124. doi: 10.1107/S0907444905035237. [DOI] [PubMed] [Google Scholar]
- 49.Téllez-Sanz R, García-Fuentes L, Barón C. Calorimetric analysis of lisinopril binding to angiotensin I-converting enzyme. FEBS Lett. 1998;423:75–80. doi: 10.1016/s0014-5793(98)00069-6. [DOI] [PubMed] [Google Scholar]
- 50.Li M, Chen C, Davies DR, Chiu TK. Induced-fit mechanism for prolyl endopeptidase. J Biol Chem. 2010;285:21487–21495. doi: 10.1074/jbc.M109.092692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nobeli I, Favia AD, Thornton JM. Protein promiscuity and its implications for biotechnology. Nat Biotechnol. 2009;27:157–167. doi: 10.1038/nbt1519. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.