Abstract
The Ca2+/Calmodulin-dependent phosphatase calcineurin is essential for exit from meiotic arrest at metaphases I and II in Drosophila and Xenopus oocytes. We previously found that Sarah, the Drosophila homolog of regulator of calcineurin, acts as a positive regulator of calcineurin and is required to complete anaphase I of female meiosis. Here, we undertook biochemical approaches, including MS and posttranslational modification analyses, to better understand the mechanism by which Sarah regulates calcineurin. A search for phosphorylated residues revealed that Sarah is highly phosphorylated at Ser100, Thr102, and Ser219 in both ovaries and activated eggs and that Ser215 is phosphorylated only in activated eggs. Functional analyses using mutant forms of Sarah showed that phosphorylation at Ser215, a consensus phosphorylation site for glycogen synthase kinase 3β (GSK-3β) and its priming kinase site Ser219, are essential for Sarah function. Furthermore, germ-line clones homozygous for a null allele of shaggy (Drosophila GSK-3β) both fail to complete meiosis and lack phosphorylation of Sarah at Ser215, suggesting that the phosphorylation of Sarah by Shaggy/GSK-3β is required to complete meiosis. Our findings suggest a mechanism in which Shaggy/GSK-3β activates calcineurin through Sarah phosphorylation on egg activation in Drosophila.
Posttranslational phosphorylation modification plays important roles in regulating the function of proteins in many aspects of cellular activities. Meiosis is one of the well-characterized processes for which progression is strictly regulated by protein phosphorylation/dephosphorylation in a spatiotemporal manner. Although calcineurin is a Ca2+/Calmodulin-dependent protein phosphatase that is required to complete meiosis during egg activation in Drosophila and Xenopus oocytes (1–3), little is known about the molecular mechanism by which calcineurin itself is activated and regulates downstream signaling pathways on egg activation.
Calcineurin is a highly conserved and ubiquitously distributed phosphatase that acts as a heterodimer composed of catalytic A (CnA) and regulatory B (CnB) subunits, and it is activated by Ca2+ and Calmodulin (4). In addition, recent studies have suggested new mechanisms of calcineurin regulation by intrinsic calcineurin binding proteins (5). The regulator of calcineurin (RCAN) family of proteins is conserved from fungi to humans, and it both stimulates and inhibits calcineurin by binding to CnA (6, 7). In yeast and mammalian culture cells, the action of RCANs as calcineurin activators is controlled by protein kinases. The most conserved region of RCANs, the serine-proline (SP) motif, contains two serine (Ser) residues that can be phosphorylated by glycogen synthase kinase 3β (GSK-3β) and its priming kinase MAPK (Fig. S1A) (8, 9). Several lines of evidence have shown that phosphorylation at these two Ser sites is required for the stimulatory function of RCANs (3, 8, 10). It has also been reported that phosphorylated RCANs exhibit decreased affinity for calcineurin and associate with other proteins such as 14-3-3 (11) and the E3 ubiquitin ligase SCFCdc4 (12), suggesting a relationship between RCAN phosphorylation and dissociation of RCAN protein from calcineurin complexes and/or degradation of RCAN. More recently, a study of mouse RCAN1 identified another pathway in which RCAN1 is phosphorylated by TGFβ-activated kinase 1 (TAK1) to induce calcineurin nuclear factor of activated T cells (NFAT) signaling pathway (13). However, whether RCANs are phosphorylated in vivo by a regulated process and how calcineurin is modulated through phosphorylation are still unclear.
We and others have previously shown that Sarah (Sra), the Drosophila homolog of RCAN (14, 15), and CanB2, which encodes CnB (3), are essential for completion of female meiosis. Genetic interactions between sra and calcineurin mutants suggested a role of Sra as the activator of calcineurin (3). We have also shown that Ser215 and Ser219 (consensus phosphorylation sites for GSK-3β and MAPK, respectively) are important for Sra function (3). Here, we extended our analysis to uncover protein interactions between Sra and calcineurin proteins and the phosphorylation profile of Sra. MS analysis identified calcineurin subunits, including two CnA subunits (Pp2B-14D and CanA-14F) and a CnB subunit (CanB2) as well as Calmodulin in coimmunoprecipitation experiments using Sra as a bait protein. Functional analysis of Pp2B-14D and CanA-14F suggests a redundant function of these genes in the completion of meiotic progression after metaphase I. Additionally, posttranslational modification (PTM) analysis revealed that phosphorylation at Ser215 is a regulated process that occurs during or after egg activation. A loss of function mutation in the shaggy (sgg) gene, which encodes Drosophila GSK-3β, caused similar meiotic defects in sra and calcineurin mutant eggs, supporting the hypothesis that Sgg/GSK-3β activates calcineurin during egg activation. Importantly, phosphorylation at Ser215 was absent in sgg mutant eggs. Our findings provide a model in which Sgg/GSK-3β regulates calcineurin activity through phosphorylation of Sra during egg activation.
Results
Sra Associates with Calcineurin Subunits in Vivo.
To investigate the interaction of calcineurin with Sra in vivo, we created an Sra overexpression construct tagged with a 3xFLAG sequence at the C-terminal end (Sra-FLAG). Sra-FLAG is functional, because its overexpression by a germ line-specific nos-GAL4 driver rescued both the female sterility and the meiotic phenotype seen in sraKO mutants; 95.6% of the laid eggs were able to complete meiosis (n = 45). Both Sra-FLAG and a nontagged Sra (for a negative control) were overexpressed by nos-GAL4 in an sraKO null mutant background to eliminate endogenous Sra.
The Drosophila development of the egg chamber in the ovary is subdivided into 14 stages. The release of meiotic arrest is one of the processes triggered by egg activation. In Drosophila, egg activation occurs when mature oocytes at stage 14 are ovulated through the oviduct, and it is independent of fertilization (16–18). To determine whether the interaction between Sra and calcineurin differs before and after egg activation, we examined both ovaries and activated eggs. Activated eggs were collected from females mated with spermless males (Methods). As a consequence, they undergo the normal egg activation processes but are not fertilized, and they do not enter mitosis. Protein extracts were immunoprecipitated with FLAG affinity beads and analyzed by Multidimensional Protein Identification Technology (MudPIT) (19) to search for Sra-interacting proteins. As shown in Table 1, a statistical analysis of four or five independent datasets using a Power Law Global Error Model (PLGEM) (20) revealed that two CnA subunits, Pp2B-14D and CanA-14F, and a CnB subunit, CanB2, were significantly more abundant in both ovary and activated egg samples containing Sra-FLAG than in the control samples containing nontagged Sra (P < 0.05). These findings are consistent with our previous expression analysis by RT-PCR that showed that these three genes are expressed in ovaries and early embryos (15). We also identified Calmodulin in the Sra immunoprecipitates at high abundance levels in both ovaries and activated eggs (Table 1). Because Calmodulin binds to the well-defined Calmodulin binding site of CnA and because Sra/RCAN does not possess this domain (4), it is likely that Calmodulin was identified through the association with Pp2B-14D and CanA-14F in our experiments. Furthermore, there were no significant differences in the abundance of calcineurin proteins and Calmodulin between two biologically different stages (see below), suggesting that Sra forms a stable complex with calcineurin regardless of whether egg activation occurs.
Table 1.
Identification of Sra-interacting proteins by MudPIT MS analysis
| PLGEM |
dNSAF average |
dNSAF ratio Sra-FLAG: control |
||||||||||
| Ovary Sra-FLAG/control |
Activated egg Sra-FLAG/control |
Ovary† Sra-FLAG |
Activated egg Sra-FLAG |
Control‡ |
||||||||
| Protein name* | STN | P value | STN | P value | dNSAF average | Detected of four | dNSAF average | Detected of five | dNSAF average | Detected of four | Ovary | Activated egg |
| Sra | 1.74 | 1.09E-05 | 1.69 | 1.39E-05 | 5.37E-02 | 4 | 4.52E-02 | 5 | 0 | 0 | Undefined | Undefined |
| CanB2 | 1.31 | 5.80E-04 | 1.42 | 2.42E-04 | 1.05E-02 | 3 | 1.37E-02 | 5 | 0 | 0 | Undefined | Undefined |
| Pp2B-14D | 1.18 | 1.41E-03 | 1.18 | 1.29E-03 | 5.60E-03 | 3 | 5.26E-03 | 4 | 3.90E-05 | 1 | 143.5 | 134.8 |
| CanA-14F | 1.25 | 8.87E-04 | 0.91 | 6.70E-03 | 5.93E-03 | 4 | 1.15E-03 | 3 | 0 | 0 | Undefined | Undefined |
| Calmodulin | 1.47 | 1.78E-04 | 1.32 | 5.24E-04 | 2.92E-02 | 3 | 1.53E-02 | 4 | 5.05E-04 | 2 | 57.9 | 30.4 |
Protein extracts from ovaries and activated eggs expressing WT nontagged Sra (control) or FLAG-tagged Sra (Sra-FLAG) were immunoprecipitated by FLAG affinity beads and analyzed by MS (SI Methods). The data were statistically analyzed by PLGEM (20). dNSAF, distributed normalized spectral abundance factor; PLGEM, Power Law Global Error Model; STN, signal to noise ratio.
*Other calcineurin subunit proteins CanA1 and CanB and Sgg were not identified as interactors of Sra at statistically significant levels of P < 0.05. CG32667 was identified as a protein with abundance that was statistically significant in the Sra-immunoprecipitates from both ovaries and activated eggs. However, the dNSAF values for this protein were substantially lower than the values of the proteins listed, and attempts to confirm the interaction of hemagglutinin (HA)-tagged CG32667 with Sra/calcineurin complexes by immunoprecipitation were unsuccessful.
†Because the ovary samples were collected from whole ovaries rather than being isolated from developed oocytes, our data should include a small fraction of proteins from germ-line cells at earlier stages.
‡Combined data from two independent control (nontagged Sra) datasets from ovary and activated egg samples were analyzed.
Pp2B-14D and CanA-14F Double KO Mutants Fail to Complete Female Meiosis.
We previously showed that loss of CanB2 in germ-line cells results in a meiotic arrest phenotype at anaphase I as seen in sraKO mutants, indicating a role of calcineurin in meiotic progression after metaphase I arrest (3). To examine whether mutations in the catalytic subunit CnA also affect meiotic progression, we tested null mutants of CanA-14F and Pp2B-14D. Females homozygous for either CanA-14F or Pp2B-14D KO allele are viable and fertile (21). These two genes are located next to each other on the X chromosome and are thought to be generated by local gene duplication (22), suggesting that they might have redundant functions. Therefore, we created a KO allele that ablates both genes (CnAdKO) by homologous recombination (Methods). Because CnAdKO homozygotes were larval lethal, we performed germ-line clone analysis to determine whether female meiosis is affected in this mutant. Mature oocytes generated by CnAdKO germ-line clones had a normal metaphase I chromosome configuration (Fig. 1 A and B). However, the mothers bearing CnAdKO germ-line clones were sterile, and the eggs exhibited a meiotic arrested phenotype at anaphase I (Fig. 1C and Table 2). These meiotic defects were rescued in 97.3% of the eggs by overexpression of WT Pp2B-14D (Pp2B-14DWT) in the female germ line. In contrast, expression of a catalytically inactive mutant form of Pp2B-14D, which has an amino acid substitution at His217 to glutamine (Pp2B-14DH217Q) (23), could not rescue the meiotic phenotype in CnAdKO (Table 2), showing the requirement of phosphatase activity for the function of CnA. Thus, CanA-14F and Pp2B-14D are indeed functionally redundant, and calcineurin activity is essential for meiotic progression from metaphase I arrest.
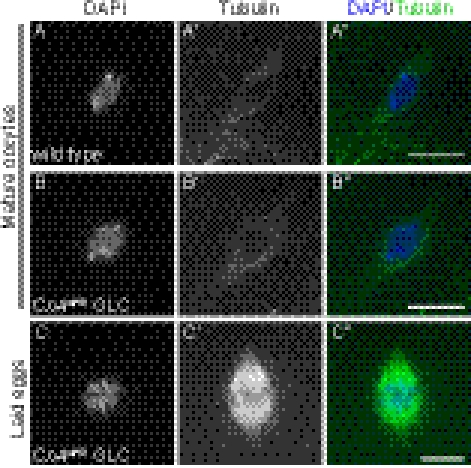
Fig. 1.
Pp2B-14D and CanA-14F double KO (CnAdKO) mutants fail to complete meiosis. (A and B) Metaphase I arrest is normal in mature oocytes from CnAdKO germ-line clones (GLCs). (C) Laid eggs from CnAdKO GLCs exhibit an arrested anaphase I chromosome configuration. Chromosomes (A–C) and spindles (A′–C′) are stained with DAPI and anti–α-tubulin antibody, respectively, and the merged images are shown in A″–C″. (Scale bars: 5 μm.)
Table 2.
CnA subunits are required in the germ line for completion of meiosis
| Maternal genotype | Arrested anaphase I (%) | Meiosis completed* (%) | Other phenotypes (%) | N† |
| CnA+ GLC | 0 | 100 | 0 | 78 |
| CnAdKO GLC | 100 | 0 | 0 | 29 |
| CnAdKO GLC + nos > Pp2B-14DWT | 2.6 | 94.8 | 2.6‡ | 38 |
| CnAdKO GLC + nos > Pp2B-14DH217Q | 97.3 | 0 | 2.7§ | 37 |
GLC, germ-line clone.
*Embryos with a signal of the rosette structure (Methods) or undergoing synchronous mitotic nuclear division were categorized.
†Number of eggs examined for each genotype.
‡One egg appeared to be in the normal process of meiotic completion, because it contained a DAPI-stained nucleus undergoing pronuclear fusion and three polar bodies in interphase.
§One egg showed a meiotic arrested chromosome configuration at anaphase II.
Sra Is Phosphorylated in Vivo.
The stimulatory function of RCANs in calcineurin signaling is regulated by their phosphorylation (8, 10, 13). Our previous study showed that two serine residues, Ser215 and Ser219, within the SP motif of Sra are necessary for Sra function. To examine if these residues and other Ser/Thr/Tyr residues in Sra are indeed phosphorylated in vivo, we carried out PTM analysis (SI Methods). As shown in Table 3, three residues (Ser100, Thr102, and Ser219) were phosphorylated in 50% or more spectra in the ovary. Thr196 and Thr246 were also phosphorylated, but the modified proportion was low (less than 1%). Interestingly, Sra seems to have a different phosphorylation profile in activated eggs than ovaries. Phosphorylation levels at Ser100, Thr102, and Ser219 were lower in activated eggs than those levels observed in ovaries (21%, 52%, and 46% reduction, respectively). Moreover, three residues (Ser67, Ser72, and Ser215) were phosphorylated only in activated eggs, although phosphorylation levels at these sites were relatively low (2–8%). Ser219 is thought to be the priming kinase site for Ser215 phosphorylation based on previous phosphorylation analyses in other organisms and the high homology of the SP motif among RCANs (8, 9), and Ser215 phosphorylation indeed occurs after eggs are activated; therefore, it is likely that Ser215 is phosphorylated by a regulated process that is induced during or after egg activation.
Table 3.
Identification of phosphorylation sites of Sra by PTM analysis
| Ovary |
Activated egg |
|||||||
| No. of spectra |
No. of spectra |
|||||||
| Residue position | Modified | Total | Modified (%) | Modified | Total | Modified (%) | NetPhos score* | Rescue by A substitution (%)† |
| Ser67 | 0 | 32 | 0 | 7 | 130 | 5.38 | 0.37 | 87.9 |
| Ser72 | 0 | 59 | 0 | 4 | 191 | 2.09 | 0.97 | 97.6 |
| Ser100 | 96 | 117 | 82.05 | 42 | 65 | 64.62 | 0.99 | 100 |
| Thr102 | 64 | 117 | 54.70 | 17 | 65 | 26.15 | 0.48 | 100 |
| Thr196 | 2 | 361 | 0.55 | 0 | 133 | 0 | 0.17 | 97.1 |
| Ser215 | 0 | 148 | 0 | 6 | 75 | 8.00 | 0.90 | 0 |
| Ser219 | 99 | 148 | 66.89 | 27 | 75 | 36.00 | 0.94 | 26.2 |
| Thr246 | 1 | 116 | 0.86 | 1 | 76 | 1.32 | 0.86 | 93.2 |
A total of three or four independent datasets from three different enzymatic digestions of ovary and activated egg samples were analyzed as described in Methods. The total numbers of distributed spectra and sequence coverage for Sra were 1,297 spectra and 93.5% in ovaries and 1,134 spectra and 93.8% in activated eggs, respectively.
*The NetPhos (version 2.0) score indicates the probability of phosphorylation at each Ser/Thr residue, with increasing probability as the value tends to 1.0 (36).
†Sramut-FLAG constructs were expressed by nos-GAL4 in an sraKO background. Percentage of eggs that completed meiosis is represented; 23–44 eggs were examined for each genotype.
Phosphorylation at Ser100, Thr102, Ser215, and Ser219 was also detected by Western blot analyses. We used the Mn2+ phosphate binding tag (Phos-tag) system applied to the 1D SDS gel electrophoresis (24) to separate phosphorylated species of Sra-FLAG. As shown in Fig. 2, multiple bands with different mobilities were detected (Fig. 2). By testing mutant forms of Sra-FLAG (Sramut-FLAG), we were able to map the positions of each phosphorylated species of Sra-FLAG. The middle two bands indicate phosphorylation at least at Ser219, because they are absent when this site is mutated to alanine (Ala) (Fig. S1 B and C). The slower of these middle bands seems to represent additional phosphorylation at Ser100 and Thr102, and the fastest doublet signal at the bottom of the blot likely indicates phosphorylated forms at Ser100 and/or Thr102, because these signals were absent when either or both of these residues was mutated to Ala (Fig. S1C). More importantly, Ser215 phosphorylation was detected as the slowest migrating signal, which was confirmed by observations that it was detected only in activated eggs and was absent in eggs expressing SraS215A–FLAG (Fig. 2). Overall, it is clear that Sra is phosphorylated in vivo.
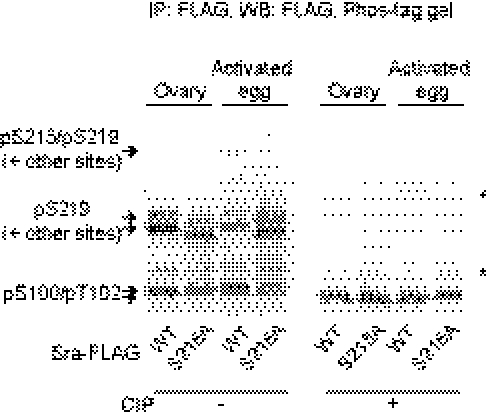
Fig. 2.
Sra is phosphorylated at multiple sites in vivo. Separation of affinity-purified Sra-FLAG proteins on the Phos-tag gel followed by Western blot analysis. WT Sra-FLAG and SraS215A-FLAG (S215A) were overexpressed by nos-GAL4, and protein lysates from ovaries and activated eggs were immunoprecipitated with FLAG affinity beads. The bands, which include Ser219 phosphorylation, migrate slightly faster when Ser215 is substituted to Ala for unknown reasons (Fig. S1B). Asterisks indicate nonspecific signals. The upper nonspecific signals appear in all samples treated with CIP (Fig. S4 B and D). Because the lower nonspecific signals are detected in samples both with and without CIP, they seem to be caused by cross-activity of the anti-FLAG antibody with other unknown proteins present in immunoprecipitated samples.
Phosphorylation of Ser215 and Ser219 Is Required for Sra Function.
To understand the significance of phosphorylation at the identified sites, we first determined whether these eight Ser/Thr residues are conserved in human RCANs. Comparison of amino acid sequences between Sra and three human RCANs (hRCAN1–3) shows that only Ser215 and Ser219 in Sra and their corresponding residues in hRCAN1–3 are identical among all four proteins (Fig. S1A). A threonine at the corresponding position of Thr196 of Sra is present in hRCAN2, but phosphorylation at Thr196 was observed at only 0.86% in ovaries (Table 3). The other five Ser/Thr residues in Sra are not conserved within hRCAN1–3.
Although five of eight residues identified by PTM search undergo low levels of phosphorylation, it is possible that the phosphorylated state is transient and rapidly converted to a dephosphorylated state. Thus, we mutated all of the eight Ser/Thr residues individually to Ala and tested if expression of mutant form of Sra-FLAG transgenes in the germ line could rescue the meiotic phenotype seen in sraKO mutants. To avoid the possibility that expressing these constructs at different levels caused by positional effects of the insertion sites affects our quantitative phenotypic analysis, we created UASp-sramut-FLAG transgenic lines using the ΦC31-mediated site-specific integration system (SI Methods) (25, 26). As mentioned above, the meiotic phenotype in sraKO eggs was rescued by germ line-specific expression of SraWT-FLAG. To our surprise, all mutant constructs (except the mutations at Ser215 and Ser219) were also able to rescue the meiotic phenotypes of sraKO eggs (88–100%) (Table 3). These results suggest that phosphorylation at any of these six residues is not required for Sra function in completion of female meiosis. We also tested a mutant at Ser199 (Fig. S1A) that might correspond to a TAK1 phosphorylation site, Ser136 of mouse RCAN1 (13), although phosphorylation at this residue was not identified in either ovaries or activated eggs by PTM analysis. Expression of SraS199A rescued the meiotic defect in sraKO mutants (86.2%, n = 65), suggesting that TAK1-mediated phosphorylation of Sra is not involved in calcineurin activation in Drosophila oocytes.
SraS215A failed to restore the female fertility of sraKO homozygotes, and all laid eggs from nos > sraS215A in sraKO females exhibited an arrested phenotype at anaphase I or II, which was seen in sraKO eggs. SraS219A expression resulted in partial rescue (26.2%) and induced multiple meiotic spindles in 73.8% of eggs (Table 3). These results are consistent with our previous observations using randomly inserted P-element lines (3). We hypothesized that if phosphorylation at Ser215 and Ser219 is important for Sra function, the mutants in which Ser residues are converted to phosphomimetic residues, glutamic acid (Glu, E) or aspartic acid (Asp, D), may behave differently from Ala mutants. Therefore, we generated transgenic lines expressing SraS215E, SraS215D, SraS219E, and SraS219D mutant variants and tested them for their ability to rescue the meiotic phenotype of sraKO. The expression of SraS219E or SraS219D increased levels of the rescue from the observed 26.2% with SraS219A to 71.9% (n = 57) and 60.0% (n = 40), respectively, suggesting that these forms do act as phosphomimetic proteins and that phosphorylation of Ser219 is important for Sra function. Intriguingly, SraS215E and SraS215D were not able to rescue the meiotic phenotypes of sraKO (0%, n = 44 and 36, respectively). Although it is possible that the substitutions of Ser to Glu/Asp at Ser215 might not fully mimic phosphorylations or might adversely affect conformation of Sra to be functional, we favor the possibility that phosphorylation at Ser215 has to occur at egg activation for Sra protein to function properly. These observations support the idea that phosphorylation at Ser219 and Ser215 of Sra plays a critical role in calcineurin activation.
Our previous studies that used P-element transgenic lines with random insertion sites showed that expression of SraS215A in the presence of endogenous sra dominantly prevents meiotic progression, resulting in low levels of egg hatchability, and that the lethality is enhanced by reducing the dose of endogenous sra gene in sraKO/+ heterozygotes (3). In other words, SraS215A acts as an antimorphic protein. We, thus, examined whether phosphomimetic mutants of Sra cause similar antimorphic effects. Expression of SraS215A in a WT background containing two copies of the endogenous sra gene did not induce significant levels of embryonic lethality (Fig. S2A), suggesting that the expression level of the transgenes integrated into the attP40 site is lower than the expression level of the transgenic lines used in our previous study (3). Regardless of this weaker phenotypic effect in the presence of two copies of endogenous sra, reducing the dose of sra by one-half resulted in 8% egg hatchability (Fig. S2A), and meiotic defects similar to those defects seen in sra and calcineurin mutants were observed (Fig. S2B). In contrast, we did not observe such strong antimorphic effects in sraKO/+ heterozygotes when Ser215 was mutated to Glu or Asp (Fig. S2A). As we previously observed (3), mutations at Ser219 caused relatively lower egg hatchability in sraKO/+ heterozygotes than in the WT background, suggesting subtle antimorphic effects. However, there was no significant difference in hatch rates between Ala, Glu, and Asp substitutions of Ser219 (Fig. S2A). Furthermore, the mutant forms with the substitutions at both Ser215 and Ser219 acted similarly to those forms with a single substitution at Ser215 (Fig. S2A). These results show that substituting Ser215 to an unphosphorylatable Ala residue causes the strongest antimorphic effect, supporting the critical role of phosphorylation of Ser215 in regulation of Sra function.
Sgg/GSK-3β Is Essential for Completion of Female Meiosis.
Our findings that the GSK-3β consensus site Ser215 of Sra is phosphorylated during or after egg activation and that the phosphorylation is essential for Sra function suggested a possible role of GSK-3β in female meiosis. Western blot analysis using the anti–GSK-3 antibody revealed that Sgg, the Drosophila homolog of GSK-3β, is expressed in both WT ovaries and activated eggs (Fig. 3A). We next addressed if Sgg is required for meiotic progression in Drosophila females and analyzed sgg mutant phenotypes. sggb12 and sggD127 have been widely used as genetically null alleles of the sgg gene, whereas sggD127 is known to be the only protein null allele (27). In addition, we tested a molecularly mapped deficiency strain Df(1)ED6584 (sggDf) that uncovers a genomic region 3A9-3B1 and deletes three genes (sgg, HLH3B, and CG2652). Because these three alleles do not produce viable homozygotes in adults, we performed germ-line clone analysis. Expression levels of Sgg protein were strongly reduced in activated eggs from germ-line clones of all three sgg alleles compared with WT (Fig. 3A). An early study in which mitotic recombination was induced by X-ray and embryonic phenotypes were determined by histological sections stained with methylene blue reported that embryos from sgg germ-line clones exhibited disordered cellularization which was rescued by zygotic expression from the paternal sgg+ chromosome (28), suggesting that completion of female meiosis is not affected in sgg mutants.
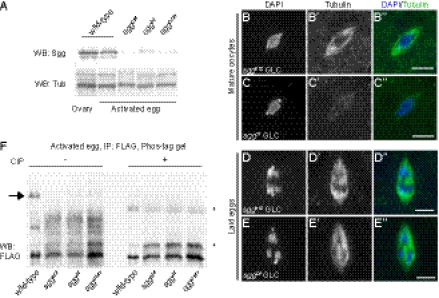
Fig. 3.
Sgg is required for completion of meiosis and phosphorylation of Sra at Ser215. (A) Sgg proteins are expressed in ovaries and activated eggs in WT. Protein lysates from w1118 (WT) and three alleles of sgg mutants were analyzed by Western blot with anti–GSK-3 and anti–α-tubulin (for loading control) antibodies. The faint signals of Sgg in sgg mutant eggs could be caused by contamination of a small proportion of survived ovoD1-18-bearing eggs that have one copy of the sgg+ gene (Methods) or nonspecific signals. (B and C) Metaphase I arrest is normal in mature oocytes from sggb12 (B) and sggDf (C) GLCs. (D and E) sgg mutants fail to complete meiosis. Laid eggs from sggb12 (D) and sggDf (E) GLCs show a meiotic arrested anaphase I chromosome configuration. Chromosomes (B–E) and spindles (B′–E′) are stained with DAPI and anti–α-tubulin antibody, respectively, and the merged images are shown in B″–E″. (Scale bars: 5 μm.) (F) Sra phosphorylation at Ser215 is absent in sgg eggs. Lysates from the WT and sgg mutants, all of which overexpress Sra-FLAG, were immunoprecipitated with FLAG affinity beads and analyzed by Phos-tag/Western blot. The slowest band (arrow) is absent in sgg eggs, suggesting that Sgg phosphorylates Sra at Ser215. The amount of WT samples loaded for both CIP-treated and nontreated is one-fifth of sgg samples, because Sra-FLAG proteins are more abundant in the input and immunoprecipitation samples in the WT (Fig. S4). Asterisks indicate nonspecific signals.
Our analysis using a flippase-dominant female sterile technique (29) revealed that sgg mutants show meiotic defects; 29.2% of sggD127 eggs were able to complete meiosis, and the remaining 70.8% exhibited abnormal meiotic defects (Table 4 and Fig. S3). However, most of the laid eggs from mothers bearing sggb12 and sggDf germ-line clones failed to complete meiosis properly, whereas metaphase I arrest seemed normal in the mature oocytes (Fig. 3 B–E and Table 4). Indeed, the majority of sgg mutant eggs (86.4% in sggb12 and 91.9% in sggDf) showed an anaphase I-arrested chromosome configuration identical to the one observed in sra and calcineurin mutant eggs (Fig. 3 D and E and Table 4). Overexpression of the WT sgg+ transgene rescued the defects seen in sgg eggs from all three alleles (Table 4). These results contradict the general understanding that sggD127 is the null allele of the sgg and show that sggD127 is, in fact, a hypomorphic allele at least with respect to the female germ line. It is possible that sggD127 produces a small amount of functional Sgg proteins that are sufficient to allow meiotic progression beyond anaphase I. Additionally, because the sgg transgene rescued the meiotic defect in sggDf eggs, we conclude that HLH3B and CG2652 are not essential for completion of female meiosis. Most importantly, the fact that loss of sgg function results in the same meiotic phenotype as sra and calcineurin mutants strongly suggests a role for sgg in calcineurin regulation.
Table 4.
sgg is essential for completion of female meiosis
| Maternal genotype | Arrested anaphase I (%) | Meiosis completed* (%) | Other phenotypes (%) | N† |
| sgg+ GLC | 0 | 100 | 0 | 78 |
| sggb12 GLC | 86.4 | 9.1 | 4.5‡ | 22 |
| sggDf GLC | 91.9 | 0 | 8.1¶ | 37 |
| sggD127 GLC | 0 | 29.2 | 70.8§ | 24 |
| sggb12 GLC + nos > sggWT | 0 | 93.8 | 6.2|| | 48 |
| sggDf GLC + nos > sggWT | 0 | 100 | 0 | 63 |
| sggD127 GLC + nos > sggWT | 0 | 100 | 0 | 49 |
GLC, germ-line clone.
*Embryos with a signal of the rosette structure (unfertilized) or undergoing synchronous mitotic nuclear division were categorized.
†Number of eggs examined for each genotype.
‡One egg contained an anaphase I spindle and a signal of the rosette structure.
¶Two eggs showed an arrested chromosome configuration at meiotic anaphase II, and one egg contained a monopolar meiotic spindle.
§Fig. S3.
||Two eggs contained three meiotic spindles or three signals of the rosette-like structure.
Sgg/GSK-3β Is Required for Phosphorylation of Ser215 in Sra.
We next examined whether Sgg is required for phosphorylation of Ser215 in Sra. We took advantage of the Phos-tag gel and analyzed the phosphorylation pattern of Sra in sgg mutants. The phosphorylation profile in the ovary did not seem to differ between control and sgg mutants, although mutations in sgg likely affect the stability of Sra protein (Fig. S4 A–C). However, it is clear that the slowest migrating band that represents Ser215 phosphorylation is absent in activated eggs from sgg germ-line clones (Fig. 3F and Fig. S4D). Thus, although we did not determine that Ser215 is the direct phosphorylation target site of Sgg, Sgg is required for Ser215 phosphorylation.
Sra Is Associated with Calcineurin After Egg Activation.
One possible explanation for the dominant negative effect of SraS215A is that phosphorylation of Sra at Ser215 releases its physical interaction from calcineurin complexes to allow calcineurin activation. To test this hypothesis, we examined whether the protein interaction between Sra and calcineurin changes before and after egg activation. Reciprocal immunoprecipitation experiments using protein lysates from both ovaries and activated eggs expressing Sra-FLAG and CnA-myc revealed that Sra does not dissociate from CnA after egg activation. Also, the levels of interaction between Sra and CnA were unaltered when Ser215 was mutated to Ala (Fig. S5A). In agreement with these results, analysis of MudPIT data showed no significant difference in abundance of any calcineurin subunits and Calmodulin that were coimmunoprecipitated with SraWT-FLAG or SraS215A-FLAG (Fig. S5B). These observations show that phosphorylation of Sra does not dramatically change the physical association between Sra and calcineurin complexes on calcineurin activation, but rather, we propose that Sra phosphorylation functions to modify the conformation and activity of calcineurin (Discussion).
Discussion
The calcineurin signaling pathway is involved in a number of biological responses and implicated in pathogenesis of diseases such as schizophrenia, Down syndrome, and Alzheimer's disease (4, 30–32). However, the mechanism by which calcineurin activity is modulated by endogenous regulators in vivo is poorly understood. In this study, we presented evidence strongly supporting a model in which the temporally controlled phosphorylation of Sra/RCAN by Sgg/GSK-3β plays an essential role in calcineurin activation and completion of female meiosis in Drosophila.
Calcineurin Activity Is Essential for Completion of Meiotic Exit from Metaphase I.
We showed by MS analysis that Sra forms a complex with three calcineurin subunit proteins (Pp2B-14D, CanA-14F, and CanB2) in ovaries and activated eggs. Functional analysis of CnA genes revealed that the catalytic activity of calcineurin is essential for meiotic progression after the release from metaphase I arrest. Because a single mutant of either Pp2B-14D or CanA-14F does not show meiotic defects and expression of Pp2B-14D is alone sufficient for the rescue of meiotic arrest phenotypes of CnAdKO, these two CnA genes function redundantly in the female germ line.
In our previous model (3), we proposed that calcineurin is activated on egg activation, although no evidence for Ca2+ increase in oocytes on egg activation has been reported in Drosophila so far. However, surprisingly, we found that Calmodulin is associated with calcineurin complexes both before and after egg activation. It has been thought that the activation of Calmodulin by Ca2+ binding abrogates the autoinhibitory function of CnA, allowing the association of the substrates with the catalytic domain and full calcineurin activation (5). The identification of Calmodulin in Sra-containing calcineurin complexes raises another possibility that calcineurin may be already active in ovaries. Because it has been shown by in vitro experiments that the SP motif of RCANs can be dephosphorylated by calcineurin (8, 9), Sra phosphorylation at Ser215 might be blocked by calcineurin in ovaries. Perhaps, calcineurin signaling might be regulated by a more complicated interplay among Sra/RCAN, calcineurin, Sgg/GSK-3β, and other proteins (see below).
Mechanism for Calcineurin Regulation by Sra Phosphorylation.
We performed comprehensive and quantitative identification of in vivo phosphorylation sites of Sra; we found that three residues (Ser100, Thr102, and Ser219) were highly phosphorylated in both ovaries and activated eggs and that Ser215 is phosphorylated only in activated eggs. Although the phosphorylation of Ser100 and Thr102 is not required for Sra function in completion of female meiosis, phosphorylation at Ser215 and Ser219 is essential. One possible explanation for the low levels of phosphorylation at Ser215 (8%) in activated eggs is that its phosphorylation state is transient. Alternatively, Ser215 phosphorylation in a small fraction of overexpressed Sra might be enough to fulfill the role as the calcineurin regulator.
How does Sra phosphorylation regulate calcineurin? Based on our findings, we suggest the following model for calcineurin activation by Sra. Before eggs are activated, the association between calcineurin and Sra is sufficiently tight that other substrate proteins are not accessible to calcineurin, which might explain the inhibitory function of Sra on enzymatic activity of calcineurin. This inhibition model is supported by the fact that SraS215A acts as a strong antimorphic allele of Sra, but SraS215E and SraS215D do not. Our biochemical analyses suggest that Sra is associated with the calcineurin complex even after egg activation, and thus, a simple model that Sra phosphorylation allows its dissociation from calcineurin complexes is unlikely. Instead, Ser215 phosphorylation might cause a conformational change of calcineurin complexes by which Sra exposes the catalytic domain of calcineurin to other specific substrates. The timing of Ser215 phosphorylation needs to be accurate, because the phosphomimetic variants were not able to rescue the meiotic phenotypes of sra mutants. This model for stimulation of calcineurin by Sra phosphorylation is in excellent agreement with the hypothesis from studies in yeast (10).
How Is Sgg/GSK-3β Activated During Egg Activation?
GSK-3, which includes GSK-3α and GSK-3β, is a highly conserved Ser/Thr kinase and mediates a wide range of signal transduction cascades in response to many cellular stimuli, including Wnt, insulin, and growth factors (33). We here presented direct evidence that Sgg/GSK-3β is essential for meiotic progression after metaphase I arrest. Also, the meiotic phenotypes of sgg mutant eggs were identical to sra and calcineurin mutants, and Sra phosphorylation at Ser215 was absent in sgg eggs. However, Ser219, the consensus site for priming phosphorylation recognized by GSK-3β, was already phosphorylated in the ovary. Our findings suggest that Sgg/GSK-3β functions as the upstream trigger of calcineurin activation through Sra phosphorylation.
In many vertebrate oocytes, a transient increase of Ca2+ activates the Ca2+/Calmodulin-dependent Ser/Thr kinase CaMKII at fertilization and releases metaphase II arrest. In Xenopus oocytes, calcineurin is also activated by Ca2+ increase and plays important roles in meiotic exit and entry into mitosis (1, 2), whereas calcineurin seems not to be essential for metaphase II exit in mice (34). In Drosophila, however, metaphase I arrest is released at the time of ovulation and independently of fertilization (16, 17). Two possible triggers, hydration and mechanical stimuli, are thought to induce egg activation when mature oocytes pass through the oviduct (18). Sgg/GSK-3β may be the downstream effector activated by these stimuli.
Conclusions
Our proteomic approaches combined with genetic analyses provide important insights into the molecular mechanism of calcineurin regulation in the female germ line. MS analysis was not able to identify Sgg and other proteins that were evidently enriched in Sra/calcineurin (Table 1), suggesting that the interaction between kinase/phosphatase and their substrates is transient, which is known for many other enzymes and their substrates. Additional biochemical analyses and the identification of other components, such as calcineurin substrate(s) and the kinase that phosphorylates Ser219 of Sra, will likely reveal the regulatory network of calcineurin signaling and its function in completion of Drosophila female meiosis.
Methods
Fly Stocks.
w1118 was used as the WT strain. sraKO, UASp-sra, and nos-GAL4:VP16 have been described previously (15). Two alleles of sgg mutants, sggb12, which has an inversion breakpoint in the middle of the sgg gene, and sggD127, which was generated by ethyl methanesulfonate (EMS)-induced mutagenesis (but the lesion has not been identified), were obtained from Pat Simpson (University of Cambridge, Cambridge, UK) (27). Df(1)ED6584, UAS-sgg, P{FRT}19A, and P{FRT}19A P{hsp70-FLP}1 ovoD1-18/C(1)DX/Y stocks were obtained from the Bloomington Drosophila Stock Center. Methods for generating transgenic constructs are described in SI Methods.
Generation of the CnAdKO Mutant.
The CnAdKO mutant was generated by sequentially performing ends-out gene targeting of Pp2B-14D and CanA-14F as previously described (21). A miniwhite gene flanked by loxP sequences in the Pp2B-14DKO (w+) line was removed by crossing with nos-Cre flies, and the Pp2B-14DKO (w) stock was established. The gene targeting for CanA-14F was then carried out against Pp2B-14DKO (w) chromosomes.
Germ-Line Clone Analysis.
Germ-line clones of CnAdKO and sgg mutants were produced by the flippase-dominant female sterile technique (29). The mutation (mut) was recombined on the P{FRT}19A chromosome, and P{FRT}19A mut/FM7 B females were crossed to P{FRT}19A P{hsp70-FLP}1 ovoD1-18/Y males. Third instar larvae were heat-treated two times for 2 h at 37 °C, and the eclosed B+ females were then crossed to WT males. We noticed during egg collection that there were a few laid eggs that did not fully develop. They appeared to be ovoD1-18-bearing escapers, because such eggs were observed from the females that were not heat-treated as well, and they were morphologically distinguishable from sgg mutant eggs in immunostaining experiments.
Immunostaining.
Immunostaining of mature oocytes and eggs was performed as previously described (3). Images were collected under a DeltaVision deconvolution microscope system (Applied Precision) equipped with an Olympus 1670 inverted microscope and high-resolution CCD camera. Images were acquired with the PlanApo N 60×/1.42 oil objective lens with 1.5× auxiliary magnification at 0.2-μm intervals along the z axis that was deconvolved using the softWoRx v.3.5.1 software and analyzed using the softWoRx Explorer v.1.3.0 (Applied Precision). Eggs that contained only a rosette structure were viewed like those eggs that had completed meiosis. These eggs were probably either the eggs that failed to be fertilized or the eggs that were laid from unmated females.
Hatchability Test.
Eggs from females 2–4 d after mating with WT males were collected on grape juice-agar media, and the hatchability of embryos was determined after 24 h or later; 200–1,000 eggs were scored for each genotype. Hatch rates were analyzed by t test, and 95% confidence intervals are shown in the graphs.
Immunoprecipitation.
Ovaries from females mated with WT males were dissected in Robb's buffer (55 mM NaOAc, 8 mM KOAc, 20 mM sucrose, 2 mM glucose, 0.44 mM MgCl2, 0.01 mM CaCl2, 20 mM Hepes, pH. 7.4) containing 1% BSA. Activated eggs from the cross of virgin test females with sterile males (sons of tudor that were made from the cross of tud1 homozygous females and WT males) (35) were collected at every 2 h, dechorionated in 50% bleach, and washed with embryo wash buffer (140 mM NaCl, 0.03% Triton-X100); 30–50 ovaries and 20–30 μL packed activated eggs were used for each immunoprecipitation to analyze phosphorylation of Sra-FLAG and the interaction between Sra-FLAG and CnA-Myc, and 120–200 ovaries and >100 μL packed activated eggs were used for each immunoprecipitation followed by MudPIT. The samples were homogenized with a lysis buffer (50 mM Tris⋅HCl, 150 mM NaCl, 0.5% Nonidet-P40, 0.5% Triton X-100, 1 mM PMSF, protease inhibitor mixtures, pH 8.0). Lysates were cleared by centrifugation two times at 20,817 × g for 15 min at 4 °C. Protein concentrations were measured using a Quick Start Bradford Protein Assay (Bio-Rad Laboratories) to normalize amounts of total proteins for immunoprecipitation. Lysates were added to EZview Red ANTI-FLAG M2 Affinity Gel (Sigma-Aldrich) and mixed for >2 h at 4 °C. The beads were washed with 50 mM Tris⋅HCl (pH 8.0), 150 mM NaCl, and 0.1% Triton X-100 five times for 5 min at 4 °C. Proteins were eluted with 150 ng/μL 3xFLAG peptide (Sigma) in tris-buffered saline (TBS) by incubating for 30 min at 4 °C. Immunoprecipitation using anti-Myc antibody (A-14; Santa Cruz Biotechnology) was performed as previously reported (3). Methods for subsequent proteomic analyses are described in SI Methods.
Western Blot.
Calf intestinal alkaline phosphatase (CIP) treatment was performed in 1× NEBuffer 3 with the addition of 10 units CIP (NEB) for 30 min at 37 °C. Protein samples were run on 10% SDS-acrylamide gels. For phosphorylation analyses, we added Phos-tag (Wako) and MnCl2 to a final concentration of 100 μM for each in the separation gels and followed the manufacturer's procedures. The primary antibodies used were mouse anti-FLAG (M2, 1:1,000; Sigma-Aldrich), rabbit anti-Myc (9E10, 1:200; Santa Cruz Biotechnology), mouse anti–α-tubulin (DM1A, 1:1,000; Sigma-Aldrich), and anti–GSK-3 (4G-1E, 1:500; Millipore). Immunoactivity was detected using alkaline phosphatase-conjugated anti-mouse IgG secondary antibody (Jackson ImmunoResearch) and the nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphatase (Invitrogen) reagents.
Supplementary Material
Acknowledgments
We thank members of the R.S.H. laboratory for helpful discussions and critical reading of the manuscript. We also thank Dr. Christian Lehner, Dr. Satoru Kobayashi, Dr. Pat Simpson, and the Bloomington Drosophila Stock Center for providing us with reagents. S.T. was supported by a fellowship from the Japan Society for the Promotion of Science (JSPS) as a JSPS Postdoctoral Fellow for Research Abroad, and R.S.H. is an American Cancer Society Research Professor.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1120367109/-/DCSupplemental.
References
- 1.Mochida S, Hunt T. Calcineurin is required to release Xenopus egg extracts from meiotic M phase. Nature. 2007;449:336–340. doi: 10.1038/nature06121. [DOI] [PubMed] [Google Scholar]
- 2.Nishiyama T, Yoshizaki N, Kishimoto T, Ohsumi K. Transient activation of calcineurin is essential to initiate embryonic development in Xenopus laevis. Nature. 2007;449:341–345. doi: 10.1038/nature06136. [DOI] [PubMed] [Google Scholar]
- 3.Takeo S, Hawley RS, Aigaki T. Calcineurin and its regulation by Sra/RCAN is required for completion of meiosis in Drosophila. Dev Biol. 2010;344:957–967. doi: 10.1016/j.ydbio.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Rusnak F, Mertz P. Calcineurin: Form and function. Physiol Rev. 2000;80:1483–1521. doi: 10.1152/physrev.2000.80.4.1483. [DOI] [PubMed] [Google Scholar]
- 5.Li H, Rao A, Hogan PG. Interaction of calcineurin with substrates and targeting proteins. Trends Cell Biol. 2011;21:91–103. doi: 10.1016/j.tcb.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies KJA, et al. Renaming the DSCR1/Adapt78 gene family as RCAN: Regulators of calcineurin. FASEB J. 2007;21:3023–3028. doi: 10.1096/fj.06-7246com. [DOI] [PubMed] [Google Scholar]
- 7.Rothermel BA, Vega RB, Williams RS. The role of modulatory calcineurin-interacting proteins in calcineurin signaling. Trends Cardiovasc Med. 2003;13:15–21. doi: 10.1016/s1050-1738(02)00188-3. [DOI] [PubMed] [Google Scholar]
- 8.Hilioti Z, et al. GSK-3 kinases enhance calcineurin signaling by phosphorylation of RCNs. Genes Dev. 2004;18:35–47. doi: 10.1101/gad.1159204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vega RB, Yang J, Rothermel BA, Bassel-Duby R, Williams RS. Multiple domains of MCIP1 contribute to inhibition of calcineurin activity. J Biol Chem. 2002;277:30401–30407. doi: 10.1074/jbc.M200123200. [DOI] [PubMed] [Google Scholar]
- 10.Mehta S, Li H, Hogan PG, Cunningham KW. Domain architecture of the regulators of calcineurin (RCANs) and identification of a divergent RCAN in yeast. Mol Cell Biol. 2009;29:2777–2793. doi: 10.1128/MCB.01197-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abbasi S, et al. Protein kinase-mediated regulation of calcineurin through the phosphorylation of modulatory calcineurin-interacting protein 1. J Biol Chem. 2006;281:7717–7726. doi: 10.1074/jbc.M510775200. [DOI] [PubMed] [Google Scholar]
- 12.Kishi T, Ikeda A, Nagao R, Koyama N. The SCFCdc4 ubiquitin ligase regulates calcineurin signaling through degradation of phosphorylated Rcn1, an inhibitor of calcineurin. Proc Natl Acad Sci USA. 2007;104:17418–17423. doi: 10.1073/pnas.0704951104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Q, Busby JC, Molkentin JD. Interaction between TAK1-TAB1-TAB2 and RCAN1-calcineurin defines a signalling nodal control point. Nat Cell Biol. 2009;11:154–161. doi: 10.1038/ncb1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horner VL, et al. The Drosophila calcipressin sarah is required for several aspects of egg activation. Curr Biol. 2006;16:1441–1446. doi: 10.1016/j.cub.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 15.Takeo S, Tsuda M, Akahori S, Matsuo T, Aigaki T. The calcineurin regulator sra plays an essential role in female meiosis in Drosophila. Curr Biol. 2006;16:1435–1440. doi: 10.1016/j.cub.2006.05.058. [DOI] [PubMed] [Google Scholar]
- 16.Doane WW. Completion of meiosis in uninseminated eggs of Drosophila melanogaster. Science. 1960;132:677–678. doi: 10.1126/science.132.3428.677. [DOI] [PubMed] [Google Scholar]
- 17.Heifetz Y, Yu J, Wolfner MF. Ovulation triggers activation of Drosophila oocytes. Dev Biol. 2001;234:416–424. doi: 10.1006/dbio.2001.0246. [DOI] [PubMed] [Google Scholar]
- 18.Horner VL, Wolfner MF. Transitioning from egg to embryo: Triggers and mechanisms of egg activation. Dev Dyn. 2008;237:527–544. doi: 10.1002/dvdy.21454. [DOI] [PubMed] [Google Scholar]
- 19.Washburn MP, Wolters D, Yates JR., 3rd Large-scale analysis of the yeast proteome by multidimensional protein identification technology. Nat Biotechnol. 2001;19:242–247. doi: 10.1038/85686. [DOI] [PubMed] [Google Scholar]
- 20.Pavelka N, et al. Statistical similarities between transcriptomics and quantitative shotgun proteomics data. Mol Cell Proteomics. 2008;7:631–644. doi: 10.1074/mcp.M700240-MCP200. [DOI] [PubMed] [Google Scholar]
- 21.Nakai Y, et al. Calcineurin and its regulator sra/DSCR1 are essential for sleep in Drosophila. J Neurosci. 2011;31:12759–12766. doi: 10.1523/JNEUROSCI.1337-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miskei M, Ádám C, Kovács L, Karányi Z, Dombrádi V. Molecular evolution of phosphoprotein phosphatases in Drosophila. PLoS One. 2011;6:e22218. doi: 10.1371/journal.pone.0022218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shibasaki F, Price ER, Milan D, McKeon F. Role of kinases and the phosphatase calcineurin in the nuclear shuttling of transcription factor NF-AT4. Nature. 1996;382:370–373. doi: 10.1038/382370a0. [DOI] [PubMed] [Google Scholar]
- 24.Kinoshita E, Kinoshita-Kikuta E, Takiyama K, Koike T. Phosphate-binding tag, a new tool to visualize phosphorylated proteins. Mol Cell Proteomics. 2006;5:749–757. doi: 10.1074/mcp.T500024-MCP200. [DOI] [PubMed] [Google Scholar]
- 25.Bischof J, Maeda RK, Hediger M, Karch F, Basler K. An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc Natl Acad Sci USA. 2007;104:3312–3317. doi: 10.1073/pnas.0611511104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Markstein M, Pitsouli C, Villalta C, Celniker SE, Perrimon N. Exploiting position effects and the gypsy retrovirus insulator to engineer precisely expressed transgenes. Nat Genet. 2008;40:476–483. doi: 10.1038/ng.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruel L, Pantesco V, Lutz Y, Simpson P, Bourouis M. Functional significance of a family of protein kinases encoded at the shaggy locus in Drosophila. EMBO J. 1993;12:1657–1669. doi: 10.1002/j.1460-2075.1993.tb05811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bourouis M, Heitzler P, el Messal M, Simpson P. Mutant Drosophila embryos in which all cells adopt a neural fate. Nature. 1989;341:442–444. doi: 10.1038/341442a0. [DOI] [PubMed] [Google Scholar]
- 29.Chou TB, Perrimon N. The autosomal FLP-DFS technique for generating germline mosaics in Drosophila melanogaster. Genetics. 1996;144:1673–1679. doi: 10.1093/genetics/144.4.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fuentes JJ, et al. DSCR1, overexpressed in Down syndrome, is an inhibitor of calcineurin-mediated signaling pathways. Hum Mol Genet. 2000;9:1681–1690. doi: 10.1093/hmg/9.11.1681. [DOI] [PubMed] [Google Scholar]
- 31.Ladner CJ, Czech J, Maurice J, Lorens SA, Lee JM. Reduction of calcineurin enzymatic activity in Alzheimer's disease: Correlation with neuropathologic changes. J Neuropathol Exp Neurol. 1996;55:924–931. doi: 10.1097/00005072-199608000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Miyakawa T, et al. Conditional calcineurin knockout mice exhibit multiple abnormal behaviors related to schizophrenia. Proc Natl Acad Sci USA. 2003;100:8987–8992. doi: 10.1073/pnas.1432926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hur EM, Zhou FQ. GSK3 signalling in neural development. Nat Rev Neurosci. 2010;11:539–551. doi: 10.1038/nrn2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suzuki T, et al. Mouse Emi2 as a distinctive regulatory hub in second meiotic metaphase. Development. 2010;137:3281–3291. doi: 10.1242/dev.052480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boswell RE, Mahowald AP. tudor, a gene required for assembly of the germ plasm in Drosophila melanogaster. Cell. 1985;43:97–104. doi: 10.1016/0092-8674(85)90015-7. [DOI] [PubMed] [Google Scholar]
- 36.Blom N, Gammeltoft S, Brunak S. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J Mol Biol. 1999;294(5):1351–62. doi: 10.1006/jmbi.1999.3310. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.