Abstract
Time series studies show that hot temperatures are associated with increased death rates in the short term. In light of evidence of adaptation to usual temperature but higher deaths at unusual temperatures, a long-term exposure relevant to mortality might be summertime temperature variability, which is expected to increase with climate change. We investigated whether the standard deviation (SD) of summer (June–August) temperatures was associated with survival in four cohorts of persons over age 65 y with predisposing diseases in 135 US cities. Using Medicare data (1985–2006), we constructed cohorts of persons hospitalized with chronic obstructive pulmonary disease, diabetes, congestive heart failure, and myocardial infarction. City-specific yearly summer temperature variance was linked to the individuals during follow-up in each city and was treated as a time-varying exposure. We applied a Cox proportional hazard model for each cohort within each city, adjusting for individual risk factors, wintertime temperature variance, yearly ozone levels, and long-term trends, to estimate the chronic effects on mortality of long-term exposure to summer temperature SD, and then pooled results across cities. Mortality hazard ratios ranged from 1.028 (95% confidence interval, 1.013– 1.042) per 1 °C increase in summer temperature SD for persons with congestive heart failure to 1.040 (95% confidence interval, 1.022–1.059) per 1 °C increase for those with diabetes. Associations were higher in elderly persons and lower in cities with a higher percentage of land with green surface. Our data suggest that long-term increases in temperature variability may increase the risk of mortality in different subgroups of susceptible older populations.
Keywords: health, climate variability, temperature related mortality, ozone related mortality
Records of daily weather conditions and air pollution concentrations measured at airports and other local stations, along with daily registries of health outcomes, such as mortality or hospitalizations, routinely compiled by health authorities, are sometimes merged to form multiyear time series datasets. These time series can be analyzed to yield information on how environmental conditions may contribute to increases in deaths and illness on a short-term time scale (days to weeks after the environmental exposure). In the last decade, numerous multicity time series analyses have demonstrated that cold and hot temperatures, as well as extremes of cold and hot temperature, are associated with increased death rates in the days after these weather conditions (1–10). These findings have important implications for understanding the health effects of climate change, given that climate change is increasing both the variability of temperatures and the frequency, duration, and intensity of heat waves (11–13).
As with the short-term associations between particulate air pollution and health, these findings, by their nature, are unable to address the question of the extent to which temperature exposure may decrease life expectancy. Studies of short-term mortality displacement demonstrate that some of the excess deaths associated with heat and heat waves are merely deaths brought forward by a few weeks (14–16), although heat may reduce life expectancy in a more substantial way, for example, among children in Delhi, India (14). In general, however, time series analyses are poorly designed for asking this question over longer time frames.
In the case of particulate air pollution, the question of whether exposure shortened lives has been answered affirmatively through cohort studies, which followed cohorts of individuals in locations with higher and lower particle concentrations for multiple years. Controlling for other determinants of reduced life expectancy, those living in more polluted areas died sooner than those living in cleaner communities (17–26). However, to date no such studies have been done for temperature, and addressing the question of life-shortening is more complicated for weather than for air pollution.
Unlike air pollution, which has a monotonic, linear dose–response relationship (with higher pollution associated with higher mortality), the associations observed with temperature are often nonlinear, especially in climates where physiologically stressful temperatures occur on either side (colder or hotter) of a zone of relatively comfortable temperatures (5, 9). In places like Delhi, India, where it does not get very cold, the relationship tends to be more linear.
Furthermore, there is strong evidence of adaptation to usual temperatures. Few people would expect to find higher mortality rates in the Mediterranean region than in Scandinavia, simply because of the higher mean temperature in the former. Indeed, time series studies in the United States have reported essentially no heat-related excess deaths in such cities as Houston, where summertime temperatures are regularly and persistently high (16); however, these studies have indicated that the variability of summertime temperature is a key factor explaining differences among cities in the effects of very hot days (16, 27).
Existing evidence suggests teperature variability may be an appropriate long-term indicator of weather conditions that may result in reduced life expectancy in an epidemiologic cohort. In addition to an increase in temperature, climate models predict an increase in summer temperature variability in mid-latitude Northern Hemisphere land areas, which may be a major public health concern (13).
The foregoing example raises another issue. Scandinavia differs from Greece in many other potential risk factors for longevity, including diet, stress patterns, smoking habits, and others. Although cohort studies studying longer-term environmental exposures and comparing communities in a cross-sectional manner attempt to control for these factors, residual confounding remains a concern. Causal modeling philosophy suggests that attempting to assign exposure as randomly as possible with respect to these factors can avoid such residual confounding.
We have adopted this philosophy when designing a cohort study to examine whether year-to-year variations in summertime temperature variability around its long-term trends within a city are associated with year-to-year variations in survival of city-specific cohorts. By restricting the analysis to within a city, we avoid all confounding by factors that can vary across a city or region. By looking only at year-to-year variations around the city-specific trend in exposure, we eliminate potential confounding by trends in other exposures, such as smoking, and focus on whether essentially random meteorological events are related to health. The year-to-year variability in exposure is more like a random fluctuation and is unlikely to be correlated with most other predictors of mortality, although ozone pollution and heat waves likely covary with these fluctuations. Because elderly persons and persons with chronic disease appear to be more susceptible to the acute effects of temperature, we focused on cohorts of these individuals.
Results
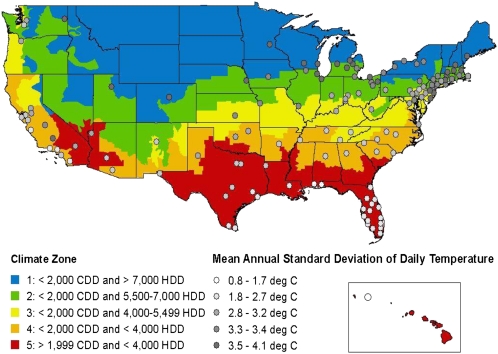
The cities used in the study, together with the city-specific distribution of the average and standard deviation (SD) of temperatures during summer (June–August), heat waves, and ozone are listed in Table S1. Ozone data were not available or were available for only a few years in nine cities, which were excluded from the analysis that included ozone in our models. Fig. 1 presents a map of the United States with the average SD of summer temperature of the 135 cities included in this study. The figure shows a higher summer temperature SD in the northern states compared with southern states.
Fig. 1.
Map of the 135 US cities included in the study and US Energy Information Administration climate zones. The size of the circle represents the SD of summer temperature in that city. CDD, cooling degree-days; HDD, heating degree-days.
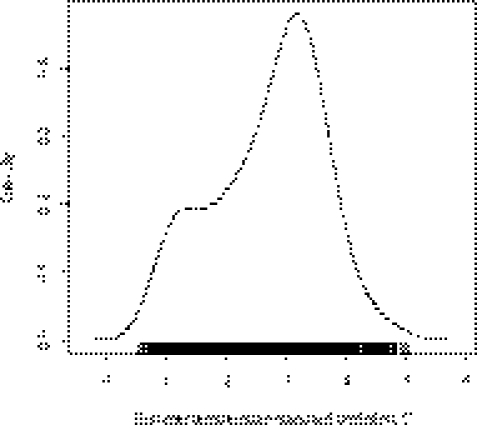
Fig. 2 presents the probability density estimates of the summer temperature SD across all of the cities; the distribution is slightly skewed to the left. The summer temperature SD across all cities varied between 0.5 °C and 5 °C. According to our definition of a heat wave, the annual mean number of days in a heat wave across all cities was 4 d (range, 0–36 d). The city-specific average number of heat waves is given in Table S1.
Fig. 2.
Density estimates of the summer temperature SD (°C).
Table 1 presents characteristics of the study population for all study cities together for each of the four cohorts. Overall, our cohorts comprised 3,749,096 persons with chronic obstructive pulmonary disease (COPD), 1,939,149 with congestive heart failure (CHF), 3,364,868 with diabetes, and 1,454,928 with myocardial infarction (MI). The median duration of follow-up is also shown in Table 1; in all cohorts, survival times ranged from 1 y to 21 y. Population characteristics varied among the four cohorts, although all cohorts had a predominance of females. In terms of racial distribution, blacks composed 17% of the diabetes cohort, but only 8% of the MI cohort. The CHF cohort was older than the others, with a mean age of 79 y, and the diabetes cohort was younger, with a mean age of 76 y. Data are expressed as hazard ratio (HR) for each 1 °C of temperature SD during summer months.
Table 1.
Characteristics of the CHF, MI, diabetes, and COPD cohorts in 135 US cities
| CHF | MI | Diabetes | COPD | |
| Total cohort, % | 100 | 100 | 100 | 100 |
| Deaths, % | 60.9 | 41.9 | 43.0 | 49.8 |
| Sex, % | ||||
| Male | 41.2 | 50.9 | 41.6 | 46.4 |
| Female | 58.8 | 49.1 | 58.4 | 53.6 |
| Race, % | ||||
| White | 82.1 | 87.3 | 75.5 | 86.4 |
| Black | 13.1 | 7.9 | 17.1 | 9.1 |
| Other | 4.8 | 4.8 | 7.3 | 4.5 |
| Age, years, mean (95% CI) | 79.4 (66.9– 92.8) | 76.9 (66.8–90.2) | 76.2 (66.1–89.2) | 77.3 (66.6–90.5) |
| Days in coronary care, mean (95% CI) | 0.7 (0–4.2) | 1.4 (0–6.6) | 0.4 (0–2.6) | 0.5 (0–2.9) |
| Days in intensive care, mean (95% CI) | 0.87 (0–4.8) | 1.5 (0–6.7) | 0.6 (0–3.5) | 0.8 (0–4.7) |
| Median follow-up, years | 3.6 | 5.1 | 4.2 | 4.2 |
| Secondary or previous diagnoses, % | ||||
| COPD | 27.1 | 15.5 | 12.0 | |
| CHF | 35.2 | 16.1 | 21.6 | |
| Diabetes | 27.2 | 21.7 | 13.4 | |
| Hypertension | 38.5 | 36.3 | 41.4 | 28.7 |
| Previous admissions, % | ||||
| Atrial fibrillation | 15.0 | 5.5 | 4.3 | 6.0 |
| MI | 10.3 | 2.4 | 3.1 | |
Table 2 presents the increases in the risk of mortality in each of the four cohorts, associated with temperature SD in summer for four different models, pooled across all cities. In our preferred model, which included ozone levels, we found significant summer HRs for mortality, ranging from 1.028 [95% confidence interval (CI), 1.013–1.042] per 1 °C increase in summer temperature SD for persons with CHF to 1.040 (95% CI, 1.022–1.059) per 1 °C increase for persons with diabetes.
Table 2.
HR and 95% CI for a 1 °C increase in yearly summer temperature SD across 135 US cities in each of the four cohorts studied, 1985–2006
| HR | 95% CI | |
| COPD | 1.048 | 1.029–1.067 |
| Diabetes | 1.055 | 1.035–1.076 |
| MI | 1.050 | 1.030–1.069 |
| CHF | 1.038 | 1.024–1.052 |
| Adjusting for ozone | ||
| COPD | 1.037 | 1.019–1.055 |
| Diabetes | 1.040 | 1.022–1.059 |
| MI | 1.038 | 1.021–1.055 |
| CHF | 1.028 | 1.013–1.042 |
| Adjusting for heat waves | ||
| COPD | 1.069 | 1.052–1.087 |
| Diabetes | 1.076 | 1.058–1.095 |
| MI | 1.073 | 1.055–1.091 |
| CHF | 1.061 | 1.047–1.076 |
| Adjusting for ozone and heat waves | ||
| COPD | 1.064 | 1.044–1.083 |
| Diabetes | 1.052 | 1.036–1.069 |
| MI | 1.071 | 1.051–1.092 |
| CHF | 1.065 | 1.047–1.083 |
These results are similar (∼10% lower) to the results derived without adjusting for ozone levels presented in Table 2. We also found significant yearly ozone associations, with an HR of 1.040 (95% CI, 1.013–1.068) per 5-ppb increase in yearly ozone for MI, 1.019 (95% CI, 1.00–1.039) for CHF, 1.025 (95% CI, 1.002–1.049) for COPD, and 1.034 (95% CI, 1.010–1.060) for diabetes.
Table 2 also presents the results from two additional models, one adjusting only for heat waves and one adjusting for both heat waves and ozone. In these models, the effect estimates increased by ∼20% from the results without adjustment and by ∼30% from the results adjusting only for ozone.
Table 3 presents the results of the pooled analysis by region, as defined by the five climate regions. Our analysis included 12 cities in region 1 (the coldest region), 37 cities in region 2, 26 cities in region 3, 23 cities in region 4, and 28 cities in region 5 (the hottest region). In all of the cohorts, the summer temperature SD associations with mortality increased from lowest to highest as the regions went from coldest to hottest.
Table 3.
HR and 95% CI for a 1 °C increase in yearly summer temperature SD across 135 US cities in each of the four cohorts combined by climate region
| Summer (June–August) |
||
| HR | 95% CI | |
| CHF | ||
| 1 (coldest) | 0.997 | 0.957–1.039 |
| 2 | 1.013 | 0.994–1.031 |
| 3 | 1.020 | 0.995–1.046 |
| 4 | 1.042 | 1.018–1.067 |
| 5 (hottest) | 1.057 | 1.005–1.112 |
| MI | ||
| 1 (coldest) | 0.984 | 0.952–1.018 |
| 2 | 1.020 | 0.9997–1.045 |
| 3 | 1.030 | 1.001–1.060 |
| 4 | 1.051 | 1.018–1.085 |
| 5 (hottest) | 1.095 | 1.039–1.154 |
| Diabetes | ||
| 1 (coldest) | 0.998 | 0.949–1.049 |
| 2 | 1.020 | 0.994–1.045 |
| 3 | 1.022 | 0.991–1.054 |
| 4 | 1.052 | 1.024–1.080 |
| 5 (hottest) | 1.098 | 1.036–1.163 |
| COPD | ||
| 1 (coldest) | 1.015 | 0.962–1.071 |
| 2 | 1.019 | 0.995–1.044 |
| 3 | 1.021 | 0.994–1.048 |
| 4 | 1.047 | 1.015–1.080 |
| 5 (hottest) | 1.078 | 1.017–1.142 |
See Fig. 1 and text for a description of the climate regions.
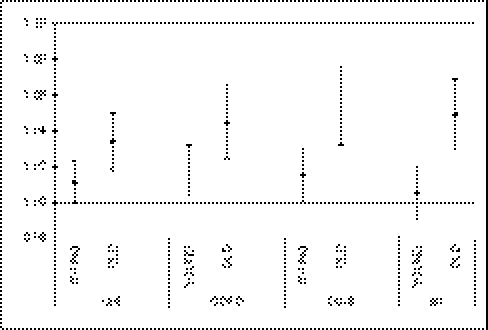
Our analysis including interaction terms between the individual characteristics and summer temperature SD in the model revealed no difference in associations by sex or by race. However, we found significantly higher associations in the subjects aged >75 y compared with those aged 65–74 y (Fig. 3).
Fig. 3.
Combined results across the 135 cities of the modification of the effect by age in the four cohorts. YOUNG, subjects aged ≤74 y and summer temperature SD; OLD, subjects aged >74 y and summer temperature SD.
We analyzed geographic modifiers at the zip code level by including interaction terms between the modifiers and summer temperature SD in the model, and found no significant interactions. However, all of the cohorts showed significant main effects, with decreased survival time with an increasing proportion of the population below the poverty level, an increasing proportion of blacks, or increasing population density and increased survival time with increasing proportion of the population over 25 y of age who completed college and increasing proportion of green surface (Table S2). For example, for each 15% increase in green surface within a zip code, the HR for the MI cohort was 0.98 (95% CI, 0.97–1.00) and the HR for the COPD cohort was 0.98 (95% CI, 0.97–0.99); for each 9% increase in the proportion of college-educated adults in a zip code, the HR for the MI cohort was 0.97 (95% CI, 0.95–0.98); and for each 4% increase in the proportion of the population below the poverty level, the HR for the MI cohort was 1.03 (95% CI, 1.02–1.04). These and other results are presented in Table S2.
In the second stage of our analysis, we examined the city-specific characteristics as potential geographic-level modifiers of the temperature SD–mortality association at the city level in a meta-regression. We found a significant effect of the proportion of green surface in all of the cohorts, with lower associations in cities with a higher percentage of land in green surface. We also found significant modification by the proportion of nonwhite residents, with a stronger association in cities with higher percentage of nonwhite residents (Table 4). Applying a multiple meta-regression with those city-specific characteristics that were not highly correlated revealed significant modifications of the temperature SD–mortality association by the proportion of green surface and the proportion of nonwhite residents in a city in all four cohorts.
Table 4.
Modification of the variance of summer temperature association by proportion of green surface and proportion of nonwhite residents across 135 US cities
| HR at the 25th percentile |
HR at the 75th percentile |
||||||
| Cohort | P value for modifier | 25% | HR | 95% CI | 75% | HR | 95% CI |
| Proportion green space modifier | |||||||
| CHF | 0.01 | 0.8 | 1.04 | 1.02–1.05 | 0.9 | 1.01 | 0.99–1.03 |
| COPD | 0.02 | 1.04 | 1.03–1.06 | 1.02 | 1.00–1.04 | ||
| Diabetes | 0.03 | 1.05 | 1.03–1.07 | 1.02 | 1.00–1.05 | ||
| MI | 0.03 | 1.05 | 1.03–1.06 | 1.02 | 1.00–1.04 | ||
| Proportion nonwhite modifier | |||||||
| CHF | 0.00 | 0.16 | 1.01 | 0.99–1.03 | 0.33 | 1.04 | 1.03–1.06 |
| COPD | 0.00 | 1.02 | 0.99–1.04 | 1.05 | 1.03–1.07 | ||
| Diabetes | 0.00 | 1.02 | 1.00–1.04 | 1.06 | 1.04–1.08 | ||
| MI | 0.01 | 1.02 | 1.00–1.04 | 1.05 | 1.03–1.07 | ||
Results are expressed as HR and 95% CI for a 1 °C increase in the SD of summer temperature estimated at the 25th percentile and the 75th percentile of the effect modifier.
Finally, to test whether the association of summer temperature SD with survival was linear, we fit a piecewise linear model estimating the effect of summer temperature SD for levels below and above the median temperature SD (2.9 °C) across all of the cities. We found a slightly different association for summer temperature SDs at the higher end of the range compared with those at the lower end of the range; for example, in the COPD cohort, we found an HR of 1.050 (95% CI, 1.025–1.076) for a 1 °C increase in summer temperature SD for the linear piece less than the median SD (2.9 °C) and an HR of 1.040 (95% CI, 1.022–1.059) for the linear piece greater than the median SD. None of the differences were statistically significant, however. Moreover, a sensitivity analysis for violation of proportionality by including interactions between time and the covariates in the model produced similar estimates for temperature SD.
Discussion
Higher temperature SD in the warm season was significantly associated with shorter survival time in a large multicity study of older subjects discharged alive following an admission for MI, COPD, CHF, or diabetes. Associations were stronger in the subjects age ≥75 y. For all of the cohorts, the temperature SD had a weaker association with survival time in the cities with a higher proportion of green surface and a stronger association in cities with a higher proportion of nonwhite residents. The associations varied by geographical regions, with the stronger associations with summertime temperature variability in the warmer regions.
Our results are not confounded by ozone level; adjusting for yearly averages of ozone reduced the effect estimates by ∼10%. Our recent study (23) applying the same survival analysis approach found that long-term ozone exposure alone is associated with increased risk of death in Medicare subjects with the same specific chronic conditions as in the present study. Our current results support this association with ozone. In addition, our effect sizes increased with control for number of heat waves in each summer, indicating that our results are not driven by heat waves.
A major advance in the present study is the ability to examine effect modification by covariates measured at the zip code level rather than at the more commonly studied city level. Notwithstanding, we found no difference in the association of temperature variability with survival by percentage of the population living in poverty, percentage with a college degree, percentage of nonwhite residents, or proportion of green space at the zip code level. In addition, at the individual level, associations did not differ by race. This is in contrast to the results of time series studies of the acute effects of heat, which have reported stronger associations in blacks and persons with less than a college degree (28, 29). Also in contrast to results from time series studies, air-conditioning prevalence at the city level did not modify the association between temperature exposure and mortality (1). A limitation of the present study was our inability to control for particles with an aerodynamic diameter of 2.5 μm or less (PM2.5), because it was not available in these cities until 1999.
To the best of our knowledge, this is the first study to examine the longer-term effects of temperature variability on survival in susceptible older people. Evidence from the physiology literature suggests that older people and those with chronic health conditions have a harder time thermoregulating and acclimating to heat (30), suggesting that they also may be less resilient to significant swings in temperature. Moreover, controlling for the number of heat waves in each summer season does not diminish the estimated effect of temperature variability, indicating that we are not simply looking at the long-term effects of heat waves and that temperature variability that does not reach the threshold for a heat wave still affects life expectancy. This suggests that adaptation and intervention strategies solely targeted to heat waves may miss an important opportunity to improve public health. Taken together, our present findings and previous evidence suggest that summer temperature variability could plausibly impair the health and shorten the life expectancy of older adults, particularly those with chronic medical conditions.
Given our city-specific analysis, the associations revealed by our analysis are not related to differences between cities in exposure. Rather, they reflect the overall impact of year-to-year changes in mortality risk with year-to-year changes in exposure. Moreover, our study design avoids confounding by cross-sectional factors that vary by city. In addition, a sensitivity analysis for lack of proportionality in the covariates controlled demonstrated no impact on the estimated effects of temperature variability.
A 1 °C increase in temperature SD is a plausible increase in some regions (31). Based on our findings, this increase in temperature SD would increase all-cause mortality in our MI cohort by 5%, for example. Based on an average of 270,000 deaths per year across all four cohorts, a 5% increase in mortality would correspond to ∼14,000 additional deaths per year due to an increase in temperature variability in the United States. Our findings suggest that long-term increases in temperature SD may increase the risk of mortality in different subgroups of susceptible older populations, although further investigation of appropriate adaptation measures is needed.
Materials and Methods
Study Population.
The US Medicare program covers hospitalization for all residents aged 65 and older. Using data for the years 1985–2006, we constructed four cohorts of persons with potentially predisposing conditions. These were defined as persons discharged alive after emergency admission for four specific conditions that we hypothesized might put subjects at greater risk, defining cases as primary discharge diagnoses of COPD [International Classification of Disease ninth revision (ICD-9) codes 490–496, except 493], diabetes (ICD-9 code 250), CHF (ICD-9 code 428), and MI (ICD-9 code 410).
For each subject in these four cohorts, we recorded the date of death or whether still living at the end of 2006, as well as information on age, sex, race, number of coronary and medical intensive care days, and medical conditions that might affect the risk of survival. Age was updated each year for the next year's follow-up period. We defined medical conditions as previous admissions with a diagnosis of atrial fibrillation (ICD-9 code 427.3) or MI, and secondary (on the index admission) or previous diagnosis of COPD, diabetes, CHF, and essential hypertension (ICD-9 code 401) for cohorts in which that was not the primary definition (e.g., COPD in the MI cohort).
Subjects alive on January 1 of the year following the index admission were entered into the cohort, and follow-up periods were calendar years. We excluded subjects whose death or subsequent admission occurred within the first 3 mo of their index admission, as well as those who were admitted in 2006.
Environmental Data.
We choose 135 US cities that represented diverse geographic and climatic features, with good representation from most regions of the United States. Cities were defined according to the county or counties within a corresponding metropolitan statistical area (http://www.census.gov/population/www/metroareas/metrodef.html). These 201 counties were selected based on the size of the metropolitan statistical area and the number of admissions for cardiovascular disease in 2004–2006. We obtained daily mean temperature data from the National Oceanic and Atmospheric Administration (http://www.ncdc.noaa.gov/oa/ncdc.html); a single weather station was selected for each city based on the proximity of the station to the city's population center and the availability of data for 1985–2006. We then created, for each year, a variable for the SD of mean daily summertime (June–August) temperature in each city. We also created a variable representing heat waves, defined as the count of days in each year when the 2-d average temperature exceeded the 99th percentile of daily mean temperature for 1985–2006.
We obtained ozone (daily 8-h mean) data from the US Environmental Protection Agency's Air Quality System Technology Transfer Network. Daily 8-h mean ozone data were available for 126 of the 135 cities that we examined. For each follow-up period, we created yearly averages of the 8-h mean daily ozone concentrations.
We examined whether the risk differed by prevailing climate by dividing the US into five climate regions based on the US Department of Energy, Energy Information Administration's Commercial Buildings Energy Consumption Survey climate zones (available at http://www.eia.doe.gov/emeu/cbecs/climate_zones.html). These five climate zones are based on the 30-y average of cooling degree-days (i.e., the sum of daily mean temperatures above 18.3 °C) and heating degree-days (i.e., the sum of daily mean temperatures below 18.3 °C) for the period 1971–2000.
Other Geographic Data.
We considered several factors as potential geographical-level modifiers of the response to temperature SD. Using the 2000 US Census Planners Package Plus data product (GeoLytics), we obtained data on population density, proportion of the population below the poverty level, proportion of the population over 25 y of age (25+) who completed college, proportion of the population 25+ who did not complete high school, proportion of Hispanic population, and proportion of black population. From the 2001 National Land Use Cover dataset, we calculated the proportion of land with green surface and proportion of land with a water body. Finally, we calculated the percentage of households in each city with central air-conditioning based on the Census Bureau's American Housing Survey. More details on these geographic data are available elsewhere (32). The census and land use data were merged with individuals based on their postal code of residence, whereas air-conditioning data were available only for the county or metropolitan area. Because these variables vary substantially between cities as well as within a city, city average values were computed and used as effect modifiers in the second-stage analysis defined below.
Statistical Methods.
To avoid cross-sectional confounding, we fit separate survival analyses in each city. The exposures were summertime temperature SDs in each year, adjusting for wintertime (December–February) temperature SDs, both of which were entered into the regression models simultaneously and treated as time-varying covariates. To do this, we used the counting process extension of the proportional hazards model pioneered by Andersen and Gill (33). In this formulation, one observation is created for each person for each year of mortality follow-up. We analyzed the data using Proc PHREG in SAS version 9.1.1 (SAS Institute). To control for tied observations, we used the appropriate likelihood function as given by Kalbfleisch and Prentice (34).
City-specific cohorts were created for each of the four health conditions that we identified, using the aforementioned inclusion criteria. Separate survival analyses, with failure defined as death, were conducted for each city and each cohort. For each subject, follow-up was each 1-y period (January–December) until the year of death or until December 2006 (censoring). This method has been described previously (23, 25).
The focus of our analysis was on whether year-to-year variations in summer temperature SD within each city were associated with year-to-year variations in survival. To avoid confounding by long-term time trends, we entered a linear term for year of follow-up. Thus, we examined whether year-to-year variations in survival around its long-term trend were associated with year-to-year variations in summer temperature SD around its long-term trend. Our model also included indicator variables for season of index admission, defined as cold (December–February), hot (June–August), or transitional. Another possible confounder is ozone, a secondary pollutant whose levels increase during warmer months; thus, we included the yearly (January–December) averages of the daily 8-h mean ozone concentrations in the models.
We controlled for individual risk factors, including age, sex, race, number of days of coronary and medical intensive care, previous diagnoses for atrial fibrillation and MI, and secondary or previous diagnoses for COPD, diabetes, CHF, and hypertension. To allow for possible nonproportionality of the survival rates, age (by 5-y categories), sex, and race (white, black, and other) were treated as stratification variables. We further tested for nonproportionality by adding interaction terms between the other covariates and time to the model to examine the effects on our temperature SD associations.
To examine whether the summertime temperature SD is merely representative of the presence of heat waves in that year, we reran our models including a variable for counts of heat waves in each year. We also examined age (defined as ≤74 y and >74 y), sex, and race (white, black, and other) as modifiers of the temperature effects by including interaction terms between the individual characteristic and summer temperature SD in the model. Similarly, we examined interactions by the zip code level covariates defined above. To test the linearity of summer temperature SD, we fit a piecewise linear model with a separate slope above and below the median summer temperature SD across all of the cities.
In the second stage of the analysis, we combined the results of these city-specific analyses (for each chronic condition) in a random-effects meta-analysis (35). To examine city-specific characteristics as potential geographic-level modifiers, we regressed the city-specific coefficients obtained in the first stage of the analysis for each cohort against each city-level modifier in a meta-regression and expressed the results as HR for a 1 °C increase in the summer temperature SD at the 25th percentile and the 75th percentile of the potential effect modifier. The results are expressed as HR for 1 °C increments of summertime temperature SD during 1985–2006 over all 135 communities. This HR can be interpreted as the relative risk of dying earlier for people living in a community with a 1 °C higher summertime temperature SD.
Supplementary Material
Acknowledgments
This work was supported by US Environmental Protection Agency (USEPA) Grants RD 83479801, RD-83241601, and R83275201 and National Institute of Environmental Health Sciences Grant R21 ES020695.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/10.1073/pnas.1113070109/-/DCSupplemental.
References
- 1.Anderson BG, Bell ML. Weather-related mortality: How heat, cold, and heat waves affect mortality in the United States. Epidemiology. 2009;20:205–213. doi: 10.1097/EDE.0b013e318190ee08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson GB, Bell ML. Heat waves in the United States: Mortality risk during heat waves and effect modification by heat wave characteristics in 43 US communities. Environ Health Perspect. 2011;119:210–218. doi: 10.1289/ehp.1002313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basu R. High ambient temperature and mortality: A review of epidemiologic studies from 2001 to 2008. Environ Health. 2009;8:40. doi: 10.1186/1476-069X-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conlon KC, Rajkovich NB, White-Newsome JL, Larsen L, O'Neill MS. Preventing cold-related morbidity and mortality in a changing climate. Maturitas. 2011;69:197–202. doi: 10.1016/j.maturitas.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curriero FC, et al. Temperature and mortality in 11 cities of the eastern United States. Am J Epidemiol. 2002;155:80–87. doi: 10.1093/aje/155.1.80. [DOI] [PubMed] [Google Scholar]
- 6.D'Ippoliti D, et al. The impact of heat waves on mortality in 9 European cities: Results from the EuroHEAT project. Environ Health. 2010;9:37. doi: 10.1186/1476-069X-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gosling S, Lowe J, McGregor G, Pelling M, Malamud B. Associations between elevated atmospheric temperature and human mortality: A critical review of the literature. Clim Change. 2009;92:299–341. [Google Scholar]
- 8.Hajat S, Kosatky T. Heat-related mortality: A review and exploration of heterogeneity. J Epidemiol Community Health. 2010;64:753–760. doi: 10.1136/jech.2009.087999. [DOI] [PubMed] [Google Scholar]
- 9.McMichael AJ, et al. International study of temperature, heat and urban mortality: The ISOTHURM project. Int J Epidemiol. 2008;37:1121–1131. doi: 10.1093/ije/dyn086. [DOI] [PubMed] [Google Scholar]
- 10.Analitis A, et al. Effects of cold weather on mortality: Results from 15 European cities within the PHEWE project. Am J Epidemiol. 2008;168:1397–1408. doi: 10.1093/aje/kwn266. [DOI] [PubMed] [Google Scholar]
- 11.Diffenbaugh NS, Ashfaq M. Intensification of hot extremes in the United States. Geophys Res Lett. 2010;37:L15701. [Google Scholar]
- 12.Meehl GA, Tebaldi C. More intense, more frequent, and longer-lasting heat waves in the 21st century. Science. 2004;305:994–997. doi: 10.1126/science.1098704. [DOI] [PubMed] [Google Scholar]
- 13.Intergovernmental Panel on Climate Change . Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge Univ Press; 2007. p. 996. [Google Scholar]
- 14.Hajat S, Armstrong BG, Gouveia N, Wilkinson P. Mortality displacement of heat-related deaths: A comparison of Delhi, São Paulo, and London. Epidemiology. 2005;16:613–620. doi: 10.1097/01.ede.0000164559.41092.2a. [DOI] [PubMed] [Google Scholar]
- 15.Braga AL, Zanobetti A, Schwartz J. The effect of weather on respiratory and cardiovascular deaths in 12 U.S. cities. Environ Health Perspect. 2002;110:859–863. doi: 10.1289/ehp.02110859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braga AL, Zanobetti A, Schwartz J. The time course of weather-related deaths. Epidemiology. 2001;12:662–667. doi: 10.1097/00001648-200111000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Pope CA, 3rd, et al. Particulate air pollution as a predictor of mortality in a prospective study of U.S. adults. Am J Respir Crit Care Med. 1995;151:669–674. doi: 10.1164/ajrccm/151.3_Pt_1.669. [DOI] [PubMed] [Google Scholar]
- 18.Dockery DW, et al. An association between air pollution and mortality in six U.S. cities. N Engl J Med. 1993;329:1753–1759. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- 19.Pope CA, III, et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287:1132–1141. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krewski D, et al. Extended Follow-Up and Spatial Analysis of the American Cancer Society Study Linking Particulate Air Pollution and Mortality. Boston: Health Effects Institute; 2009. [PubMed] [Google Scholar]
- 21.Pope CA., III Mortality effects of longer-term exposures to fine particulate air pollution: Review of recent epidemiological evidence. Inhal Toxicol. 2007;19(Suppl 1):33–38. doi: 10.1080/08958370701492961. [DOI] [PubMed] [Google Scholar]
- 22.Laden F, Schwartz J, Speizer FE, Dockery DW. Reduction in fine particulate air pollution and mortality: Extended follow-up of the Harvard Six Cities study. Am J Respir Crit Care Med. 2006;173:667–672. doi: 10.1164/rccm.200503-443OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zanobetti A, Schwartz J. Ozone and survival in four cohorts with potentially predisposing diseases. Am J Respir Crit Care Med. 2011;184:836–841. doi: 10.1164/rccm.201102-0227OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zanobetti A, Schwartz J. Particulate air pollution, progression, and survival after myocardial infarction. Environ Health Perspect. 2007;115:769–775. doi: 10.1289/ehp.9201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zanobetti A, Bind MA, Schwartz J. Particulate air pollution and survival in a COPD cohort. Environ Health. 2008;7:48. doi: 10.1186/1476-069X-7-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoek G, Brunekreef B, Goldbohm S, Fischer P, van den Brandt PA. Association between mortality and indicators of traffic-related air pollution in the Netherlands: A cohort study. Lancet. 2002;360:1203–1209. doi: 10.1016/S0140-6736(02)11280-3. [DOI] [PubMed] [Google Scholar]
- 27.Medina-Ramón M, Schwartz J. Temperature, temperature extremes, and mortality: A study of acclimatization and effect modification in 50 United States cities. Occup Environ Med. 2007;64:827–833. doi: 10.1136/oem.2007.033175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Medina-Ramón M, Zanobetti A, Cavanagh DP, Schwartz J. Extreme temperatures and mortality: Assessing effect modification by personal characteristics and specific cause of death in a multi-city case-only analysis. Environ Health Perspect. 2006;114:1331–1336. doi: 10.1289/ehp.9074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Neill MS, Zanobetti A, Schwartz J. Modifiers of the temperature and mortality association in seven US cities. Am J Epidemiol. 2003;157:1074–1082. doi: 10.1093/aje/kwg096. [DOI] [PubMed] [Google Scholar]
- 30.Kenny GP, Yardley J, Brown C, Sigal RJ, Jay O. Heat stress in older individuals and patients with common chronic diseases. CMAJ. 2010;182:1053–1060. doi: 10.1503/cmaj.081050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schär C, et al. The role of increasing temperature variability in European summer heatwaves. Nature. 2004;427:332–336. doi: 10.1038/nature02300. [DOI] [PubMed] [Google Scholar]
- 32.Reid CE, et al. Mapping community determinants of heat vulnerability. Environ Health Perspect. 2009;117:1730–1736. doi: 10.1289/ehp.0900683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andersen P, Gill R. Cox's regression model counting process: A large-sample study. Ann Stat. 1982;10:1100–1120. [Google Scholar]
- 34.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York: Wiley-Interscience; 1980. [Google Scholar]
- 35.Berkey CS, Hoaglin DC, Antczak-Bouckoms A, Mosteller F, Colditz GA. Meta-analysis of multiple outcomes by regression with random effects. Stat Med. 1998;17:2537–2550. doi: 10.1002/(sici)1097-0258(19981130)17:22<2537::aid-sim953>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.