Abstract
Inflammation is a significant player in the progression of heart failure and has detrimental effects on cardiac function. Prostaglandin (PG)E2, a major proinflammatory prostanoid in the cardiovascular system, is a potent stimulus in inducing intracellular cAMP but minimally affects cardiac contractile function. Here, we show that the PGE2 stimulation attenuates the adrenergic-induced cardiac contractile response in animal hearts. Stimulation with PGE2 leads to stimulatory G protein (Gs)-dependent production of cAMP. However, the induced cAMP is spatially restricted because of its degradation by phosphodiesterase (PDE)4 and cannot access the intracellular sarcoplasmic reticulum (SR) for increasing calcium signaling and myocyte contraction. Moreover, pretreatment with PGE2 significantly inhibits PKA activities at the SR induced by a β-adrenergic agonist, isoproterenol, and subsequently blocks isoproterenol-induced PKA phosphorylation of phospholamban and contractile responses in myocytes. Further analysis reveals that the PGE2-induced cAMP/PKA is sufficient to phosphorylate and activate PDE4D isoforms, which, in turn, spatially inhibits the diffusion of adrenergic-induced cAMP from the plasma membrane to the SR. Inhibition of PDE4 rescues the adrenergic-induced increase in cAMP/PKA activities at the SR, PKA phosphorylation of phospholamban, and contractile responses in PGE2-pretreated myocytes. Thus, this offers an example that one Gs-coupled receptor is able to inhibit the intracellular signaling transduction initiated by another Gs-coupled receptor via controlling the diffusion of cAMP, presenting a paradigm for G protein-coupled receptor (GPCR) signal transduction. It also provides a mechanism for the integration of signaling initiated by different neurohormonal stimuli, as well as long-term effects of chronically circulating proinflammatory factors in myocardium.
Keywords: FRET, subcellular diffusion, cross-talk
Inflammation plays a significant role in the progression of heart failure (1, 2). The expression of prostaglandin (PG)E2, a major proinflammatory prostanoid in cardiovascular system, is elevated in the hearts of patients and animals with myocardial infarction and heart failure (3, 4). Clinically, heart failure is characterized with gradual development of cardiac hypertrophy and myocyte death attributable to elevated β-adrenergic stimulation and cardiac dysfunction (5, 6). However, cardiac function under E series prostaglandin (EP) receptor signaling is not clear, and the impact on cardiac β-adrenergic signaling and function during the development of heart diseases remains to be addressed.
In human and animal hearts, PGE2 and catecholamines activate divergent signaling cascades for distinct cellular and physiological function. EP4 is the most abundantly expressed EP subtype in the myocardium (7). Biochemically, activation of EP4 leads to an accumulation of cAMP through activation of stimulatory G (Gs) protein. However, the induced cAMP signal appears to be confined along the plasma membrane (PM) and has limited effect on myocyte contractile response (7). This confinement of cAMP is primarily regulated through cAMP hydrolysis by PDE4 isoforms (8, 9). Consequently, PGE2 stimulation fails to promote phosphorylation of proteins such as phospholamban (PLB), a critical player on the intracellular calcium storage compartment sarcoplasmic reticulum (SR) that releases calcium for contractile response. Catecholamines stimulate β adrenergic receptors (βARs) to induce Gs protein activation and cAMP production. In contrast to PGE2 stimulation, the adrenergic-induced cAMP signaling leads to robust PKA phosphorylation of substrates on the PM, the SR, and the myofibril, and promotes cardiomyocyte contractility (10, 11). Previous studies have also characterized the association of different PDE4D isoforms with βAR subtypes, which regulate subcellular distribution of the adrenergic signaling-induced cAMP activities (12, 13). Under agonist stimulation, these PDE4D isoforms dissociate from the activated βARs to allow cAMP diffusion from the PM to the intracellular compartments (12, 13). Interestingly, early studies have indicated that PGE2 treatment impairs the sympathetic regulation of cardiac contractile function in animal hearts (14). It is, thus, likely that the signaling induced by PGE2 and β-adrenergic stimuli are integrated in the myocardium. We hypothesize that PGE2 exerts inhibitory effects on βAR signaling cascades in the myocardium via a cross-talk by activating the βAR-associated PDE4D isoforms.
Here, we show that the PGE2-induced cAMP signal is confined within the PM domain and has minimal effects on PKA phosphorylation of PLB at the SR and contractile function in animal hearts. However, PGE2 stimulation inhibits intracellular βAR signaling transduction by blocking propagation of cAMP from the PM to the SR, which blocks PKA phosphorylation of PLB and inhibits calcium cycling regulation in myocytes. Further dissection reveals that PGE2 stimulation induces PKA phosphorylation of PDE4D isoforms, which prevents the βAR-induced cAMP diffusion to the SR. Consequently, the PGE2 stimulation blocks the βAR-induced contractile responses in cardiomyocytes and in animal hearts. The elegant cross-talk between two Gs-coupled receptors in shaping subcellular distribution of second messenger cAMP presents a paradigm on G protein-coupled receptor (GPCR) signaling transduction for integration of multiple extracellular stimuli.
Results
Prostaglandin Impairs β-Adrenergic Stimulation on Contractile Responses in Animal Hearts.
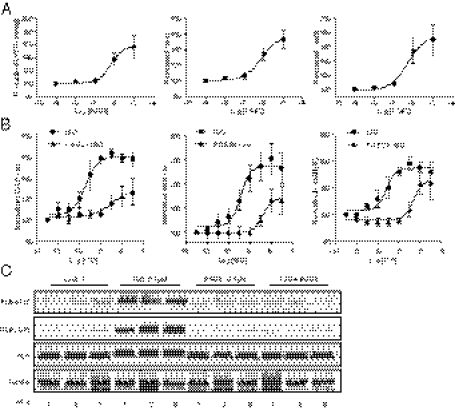
Proinflammatory factor PGE2 is a potent stimulus in inducing intracellular cAMP signaling but fails to promote cardiac contractile response (7). We envisioned that prostaglandin-induced PKA phosphorylation and activation of phosphodiesterase could impair signaling transduction induced by β-adrenergic stimulation in the myocardium and block adrenergic-induced contractile responses, offering a mechanism on how inflammation contributes to cardiac dysfunction. Using Langendorff perfusion of mouse hearts, we first tested different doses of PGE2 on cardiac contractility and found that PGE2 exerted a positive inotropic effect on the heart at minimal concentration of 1μM (Fig. 1A). In comparison, β-adrenergic stimulation with isoproterenol (ISO) induced a sensitive dose-dependent contractile response (Fig. 1B) with EC50 at 1.99 nM and maximal response at the concentrations >10 nM. ISO was able to increase peak left ventricular developed pressure (LVDP) from 71.4 ± 13.0 to 115.3 ± 12.0 mmHg (61.8%; EC50, −8.708), maximal dp/dt from 2,343.7 ± 159.7 to 5,984.4 ± 622.8 mmHg (150.6%; EC50, −8.675), and minimum –dp/dt from −1,787.8,± 325.2 to 3,492.2 ± 142.03 mmHg/s (95.4%; EC50, −8.729). In contrast, when the heart was pretreated with 0.1 μM PGE2, a concentration that was noneffective on cardiac function by itself, the ISO dose–response curve was shifted rightward, and the EC50 of ISO was changed to ∼199 nM, 100 times higher than the EC50 of ISO alone. Furthermore, the maximal responses of LVDP (from 63.8 ± 3.9 to 81.5 ± 10.9 mmHg; 27.8%; EC50, −6.891), dp/dt (from 2,229.5 ± 54.3 to 4,009.6 ± 504.3 mmHg/s; 77.1%; EC50, −6.78) and –dp/dt (from −1,840.8 ± 179.0 to −3,127.7 ± 750.9 mmHg; 70.0%; EC50, −6.577) were all substantially decreased. These data suggest that PGE2 pretreatment blunts β-adrenergic responsiveness of cardiomyocytes.
Fig. 1.
PGE2 inhibits adrenergic-induced phosphorylation of PLB and contractile response in animal hearts. Mouse hearts were cannulated for Langerdorff perfusion with prostaglandin or ISO or with prostaglandin for 5 min before addition of ISO at concentrations indicated. The LVDP, maximal dp/dt, and minimal −dp/dt were analyzed and normalized against basal levels. (A) Cardiac functional responses to different concentrations of PGE2. (B) Cardiac functional responses to different concentrations of ISO after PGE2 (0.1 μM) pretreatment. (C) Phosphorylation of PLB at the Ser16 site (PKA site) and Thr17 site (CaMKII site) after perfusion of the hearts with drugs indicated.
To validate the contractile response, we examined phosphorylation level of a PKA substrate PLB. PLB is located at the SR and plays a critical role in calcium cycling by regulating SERCA activity. Adrenergic stimulation, but not prostaglandin stimulation, significantly promotes both PKA and Ca2+/calmodulin-dependent protein kinase (CaMK)II phosphorylation of PLB in animal hearts. Pretreatment with 0.1 μM PGE2 abolished phosphorylation of PLB induced by ISO (Fig. 1C). These data suggest that although PGE2 stimulation fails to induce cAMP and PKA activities at the SR, it inhibits the adrenergic-induced PKA activities at the SR for cardiac contractile response in animal hearts.
Prostaglandin and Adrenergic Stimulation Induces Distinct Subcellular cAMP-PKA Activities in Cardiomyocytes.
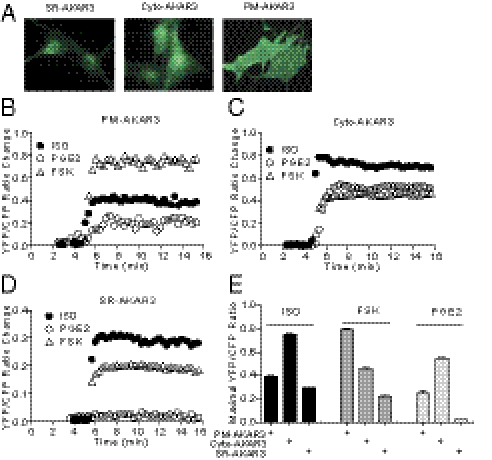
We sought to directly measure PKA activities in different intracellular compartments with biosensors under the signaling cross-talk between EP and βAR in cardiomyocytes. To achieve this, we used the recently developed PKA biosensors that are targeted to the PM, the cytoplasm, and the SR (PM-AKAR3, cyto-AKAR3, and SR-AKAR3, respectively) (Fig. 2A and Fig. S1) (15, 16). In neonatal cardiomyocytes, direct stimulation of adenylyl cyclases with forskolin (FSK) induced strong PKA activities at the PM, less robust PKA activities in the cytoplasm, and much lower PKA activities at the SR (Fig. 2 B–D). The pattern follows a diffusion of cAMP from the PM where cAMP is produced. In comparison, stimulation with ISO or PGE2 induced lower increases in PKA activities at the PM than FSK (Fig. 2 B–D). Interestingly, under ISO or PGE2 stimulation, the PKA activities in the cytoplasm were much more robust than those at the PM, respectively (Fig. 2 A–C). Furthermore, adrenergic stimulation induced significantly higher PKA activities at the SR than FSK stimulation, whereas PGE2 failed to generate significant increase (Fig. 2 D and E). The data indicate that the adrenergic-induced cAMP preferentially activates PKA at the intracellular compartments like the SR for myocyte contractile response, whereas PGE2 stimulation completely fails to do so. In addition, dopamine induced modest PKA activities at the SR, whereas adenosine failed to do so (Fig. S1 C and D). Together, different GPCRs have distinct effects in inducing local PKA activities at the SR in cardiomyocytes.
Fig. 2.
PGE2 and ISO induce distinct subcellular cAMP signaling in cardiomyocytes. Neonatal cardiomyocytes were infected with PKA biosensors targeted to different subcellular organelles. The expression of biosensors was detected by immunofluorescence staining (A). (B–D) Neonatal cardiomyocytes expressing different PKA biosensors were stimulated with PGE2 (1 μM), ISO (10 μM), or FSK (10 μM), and the changes in FRET ratio of the PKA biosensors were recorded. (E) The maximal increases in the FRET ratio of PKA biosensors were plotted.
Prostaglandin Stimulation Confines Adrenergic Signaling in Cardiomyocytes.
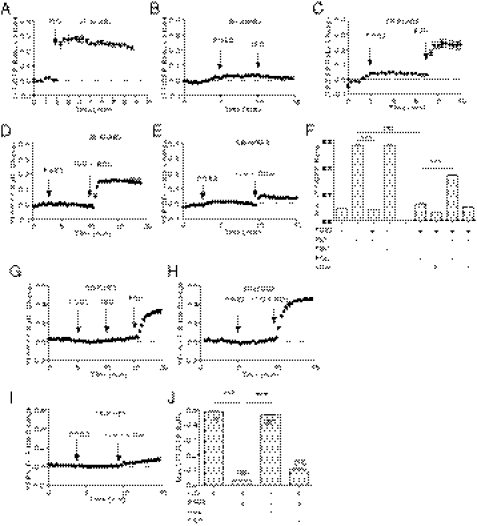
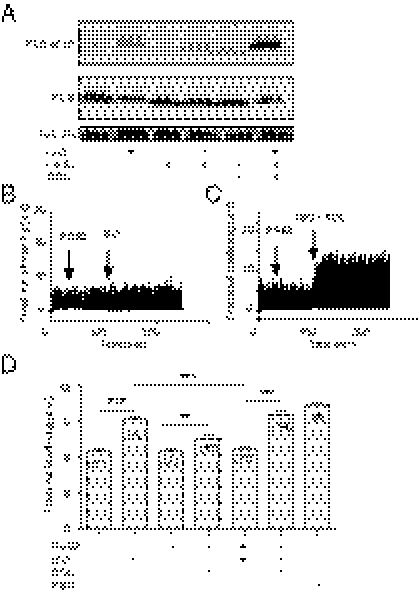
On GPCR stimulation, the small molecule cAMP is diffusible throughout the cell. However, the distribution of cAMP under specific GPCR stimuli is rather highly restricted because of hydrolysis of cAMP by a family of potent PDE enzymes. As a result, a specific set of PKA is activated for phosphorylation of substrates at the local vicinities. In rodent cardiomyocytes, PDE3 and PDE4 are two major PDE families expressed (17) to control cAMP distribution and diffusion in subcellular compartments. PDE3 and PDE4 can be selectively inhibited by cilostamide (CILO) and rolipram (ROL), respectively (Fig. S2A). PDE4, predominately located on the membrane (Fig. S2B), plays a major role in controlling cAMP activities induced by adrenergic stimulation in cardiomyocytes (12, 13). We hypothesized that the PGE2 inhibition on adrenergic signaling is mediated via activation of PDE4, which blocks cAMP diffusion and attenuates cardiac contractile response under adrenergic stimulation. Indeed, whereas PGE2 did not induce significant increase in PKA activities at the SR, pretreatment of myocytes with PGE2 blocked the ISO-induced PKA activities at the SR, but not the FSK-induced response (Fig. 3 A–C). Inhibition of PDE4 with ROL modestly enhanced the PGE2-induced PKA activities at the SR but significantly recovered the adrenergic-induced PKA activities at the SR in the presence of PGE2 pretreatment (Fig. 3 D and F). In contrast, inhibition of PDE3 with CILO did not significantly affect the PKA activities induced by PGE2, nor did it affect the PKA activities induced by ISO after PGE2 pretreatment (Fig. 3 E and F). As control, inhibition of PDE4 with ROL promoted PGE2-induced PKA activities at the SR and the PM, respectively (Fig. S3 A and B) but did not affect the activities in the cytoplasm (Fig. S3B).
Fig. 3.
PGE2 inhibits the adrenergic-induced cAMP and PKA activities at the SR. (A–E) Neonatal cardiomyocytes expressing the SR targeted PKA biosensor (SR-AKAR3) were stimulated with PGE2 (1 μM), ISO (10 μM), FSK (10 μM), ROL (0.1 μM), and CILO (1 μM) as indicated. The changes in FRET ratio of the PKA biosensors were recorded. (F) The maximal increases in the PKA FRET ratio were plotted. (G–I) Neonatal cardiomyocytes expressing the SR-targeted cAMP biosensor were stimulated with PGE2 (1 μM), ISO (10 μM), ROL (0.1 μM), and CILO (1 μM) as indicated. The changes in FRET ratio of the cAMP biosensors were recorded. (J) The maximal increases in the cAMP FRET ratio were plotted. ***P < 0.001 by one-way ANOVA.
We also measured cAMP activities directly using a cAMP biosensor targeted at the SR (SR-ICUE3). Pretreatment of myocytes with PGE2 completely blocked the adrenergic stimulation-induced cAMP accumulation at the SR but not the FSK-induced response (Fig. 3G). Moreover, inhibition of PDE4 with ROL, but not PDE3 with CILO, recovered the adrenergic-stimulated cAMP at the SR (Fig. 3 H–J). These data indicate that PGE2 activates PDE4 enzymes to prevent diffusion of the adrenergic stimulation-induced cAMP to the intracellular SR.
We then determined which βAR and EP subtypes are involved in the cross-talk. EP3 and EP4 are major subtypes expressed in rodent cardiomyocytes (7). Pretreatment with EP3 selective antagonist L-798106 or EP4 antagonist L-161982 did not affect the basal PKA activities at the SR in cardiomyocytes, nor did it affect the PGE2-induced PKA activities at the SR (Fig. S4A). However, EP4 antagonist L-161982 alone or together with EP3 antagonist L-798106 completely blocked the inhibitory effects of PGE2 on ISO-induced PKA activities at the SR (Fig. S4A). Meanwhile, in β2AR-KO myocytes, pretreatment with PGE2 did not affect ISO-induced PKA activities at the SR (Fig. S4B); in contrast, in β1AR-KO myocytes, pretreatment with PGE2 inhibited ISO-induced PKA activities, which was rescued by inhibition of PDE4 with ROL or by inhibition of PDE3 with CILO (Fig. S4B). Moreover, EP4 did not associate with either β1AR or β2AR when expressed in HEK293 cells, suggesting that the cross-talk occurs via downstream intracellular mechanisms (Fig. S5).
To explore whether the EP inhibition of adrenergic signaling is a general mechanism, we measured the cAMP activities in the nucleus in mouse embryonic fibroblasts under different stimulations. ISO and FSK induced robust cAMP activities in the nucleus in mouse embryonic fibroblasts (MEFs), whereas PGE2 failed to do so (Fig. S6 A–C). Pretreatment with PGE2 significantly reduced the ISO-induced cAMP signal in the nucleus (Fig. S6C). Inhibition of PDE4 with ROL, but not inhibition of PDE3 with CILO, abolished the inhibitory effect of PGE2 on the adrenergic-induced cAMP signal in the nucleus of MEFs (Fig. S6 D–F). Meanwhile, dopamine, urocortin, and relaxin all activate Gs-coupled receptors, but none of them induced cAMP activities in the nucleus in MEFs (Fig. S7 A–E). Pretreatment with dopamine almost completely inhibited the ISO-induced cAMP activities in the nucleus (Fig. S7 B and F). Urocortin partially inhibited the cAMP activities, whereas relaxin failed to do so (Fig. S7 D and E). In contrast, none of these agonists inhibited the cAMP activities in the nucleus induced by FSK (Fig. S7F). Together, these data indicate that the EP inhibition of adrenergic signaling is a general mechanism selectively shared by some Gs-coupled GPCRs.
Prostaglandin Activates βAR-Associated PDE4D to Restrict cAMP Signaling Under Adrenergic Stimulation.
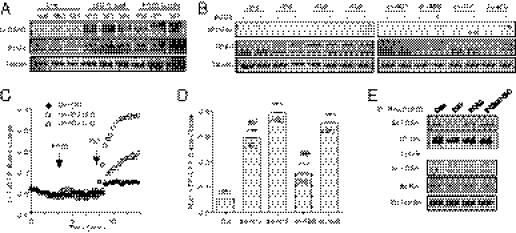
The members of PDE4 family can be activated via PKA phosphorylation (18). We examined the PKA phosphorylation of PDE4 isoforms in myocardium after Langendorff perfusion with drugs. Perfusion of mouse hearts with either PGE2 or ISO significantly increases in PKA phosphorylation of PDE4 isoforms in myocardium (Fig. 4A). Among the PDE4 family, we focused on PDE4D subfamily because its members are associated with adrenergic signaling in cardiomyocytes (12, 13). When wild-type PDE4D isoforms were overexpressed in H9C2 cardiac myoblasts, PGE2 failed to increase PKA phosphorylation of the isoforms (Fig. 4B), in agreement with the fact that overexpressed PDE4Ds suppress adrenergic-induced cAMP signal (19). However, PGE2 increased PKA phosphorylation of the dominant-negative forms of PDE4D5 and PDE4D9, and to a lesser extent, PDE4D8 and PDE4D3 (Fig. 4B). Accordingly, overexpression of dominant-negative PDE4D5 and PDE4D9 in neonatal cardiomyocytes fully rescued the adrenergic-induced cAMP signal at the SR after pretreatment with PGE2, and dominant-negative PDE4D3 and PDE4D8 showed partial rescue effects (Fig. 4 C and D). Stimulation with ISO promotes dissociation of PDE4D isoforms from the activated β1AR or β2AR, respectively (Fig. 4E and Fig. S8A) (12, 13). Whereas stimulation with PGE2 did not affect the βAR/PDE4D complexes, it completely blocked the dissociation of the βAR/PDE4D complexes under ISO stimulation (Fig. 4E and Fig. S8A). Interestingly, the inhibitory effect of PGE2 on the ISO-induced dissociation between β1AR/PDE4D8 complexes was not observed in β2AR-KO myocytes (Fig. S8B).
Fig. 4.
PGE2 induces PKA phosphorylation of phosphodiesterase 4D isoforms to attenuate cAMP signaling. (A) Animal hearts undergoing Langendorff perfusion with PGE2 (0.1 μM) or ISO (0.1 μM) were harvested. The tissues lysates were blotted with antibody against the PKA phosphorylated PDE4D. (B) H9C2 cardiac myoblasts overexpressing different wild-type or dominant-negative (dn) PDE4D isoforms were stimulated with PGE2 before being lysated. The cell lysates were blotted with antibody against the PKA phosphorylated PDE4D. (C) Neonatal cardiomyocytes expressing the SR-targeted cAMP biosensors together with different dominant-negative PDE4D isoforms were pretreated with PGE2 (1 μM) before stimulation with ISO (10 μM). The changes in the cAMP FRET ratio were recorded. (D) The maximal increases in cAMP FRET ratio were plotted. (E) Neonatal cardiomyocytes expressing hemagglutinin (HA)-β1AR and PDE4D8 were treated with PGE2 (0.1 μM) or ISO (0.1 μM) for 5 min, or ISO (0.1 μM) for 5 min after pretreatment with PGE2 (0.1 μM) for 5 min. Cell lysates were incubated with anti-HA beads to immunoprecipitate HA-β1AR. The pull-down receptor and associated PDE4D8 were blotted with antibodies indicated. ***P < 0.001 by one-way ANOVA in comparison with control; ##P < 0.01 by one-way ANOVA in comparison with PDE4D5 group.
In agreement with the PKA activity measurements, both adrenergic and prostaglandin stimulations promoted phosphorylation of the PKA biosensor at the PM, with a much smaller increase induced by PGE2 than ISO (Fig. S9A). In contrast, only adrenergic stimulation promoted phosphorylation of the PKA biosensor at the SR (Fig. S9B). As expected, PLB was also selectively phosphorylated by PKA under ISO but not PGE2 stimulation (Fig. S9C). Consequently, PGE2 stimulation failed to enhance calcium signaling and inotropic contractile shortening in adult myocytes (Fig. S9 D and E) and chronotropic contraction rate in neonatal myocytes (Fig. 9 F and G). Inhibition of PDE4 with ROL significantly enhanced PKA phosphorylation of PKA biosensor PM-AKAR3 at the PM and the PKA biosensor SR-AKAR3 and PLB at the SR (Fig. S9 A–C). Moreover, inhibition of PDE4 with ROL promoted increase in calcium signaling (Fig. S9D), inotropic contractile shortening in adult myocytes (Fig. S9E), and chronotropic contraction rate in neonatal myocytes (Fig. S9 F and G) under PGE2 stimulation.
Last, we examined whether inhibition of PDE4 is sufficient to abolish the inhibitory effects of PGE2 on ISO-induced PKA phosphorylation of PLB and contractile response in cardiomyocytes. Both adrenergic and prostaglandin stimulation promoted PKA phosphorylation of PDE4 isoforms (Fig. 4A), but only adrenergic stimulation is sufficient to promote increases in PKA phosphorylation of PLB and increase myocyte shortening (Fig. 5 A, B, and D). Pretreatment myocytes with PGE2 completely blocked adrenergic-induced increases in PKA phosphorylation of PLB and myocyte shortening (Fig. 5 A and B). Whereas addition of PDE4 inhibitor ROL promoted small increases in PGE2-induced PKA phosphorylation of PLB and myocyte shortening, it abolished the inhibitory effects of PGE2 on the ISO-induced increases in PKA phosphorylation of PLB (Fig. 5A) and myocyte shortening (Fig. 5 C and D).
Fig. 5.
Inhibition of PDE4 abolishes the inhibitory effect of PGE2 on the adrenergic signaling for myocyte contractile response. Adult cardiomyocyte were stimulated with different PGE2 (1 μM), ISO (10 μM), FSK (10 μM), or ROL (1 μM) as indicated. The phosphorylation of PDE4D and PLB at the PKA site were blotted with anti-phospho PLB antibody (A), the increases in iontropic contractile response were recorded (B and C), and the maximal increases were plotted (D). **P < 0.05 and ***P < 0.001 by one-way ANOVA.
Discussion
GPCRs are the biggest family of receptors that respond to a variety of stimuli, ranging from neurotransmitters and hormones to light and mechanical pressure. Α large group of GPCRs is able to couple Gs protein, which leads to activation of adenylyl cyclases to produce cAMP, a critical second messenger that elicits a wide range of cellular functions via specific activation of locally tethered subcellular PKA. Here, we present a mechanism by which one Gs-coupled receptor (EP) inhibits the signaling transduction of another Gs-coupled receptor (βAR) via activation of intracellular cAMP degradation enzyme PDE4Ds. The EP-induced PDE4D activation limits the diffusion of cAMP induced by βAR stimulation in cardiomyocytes and MEFs, preventing PKA activation and phosphorylation of PLB and increase in calcium cycling for contractile responses in animal hearts. This elaborate spatial regulation of intracellular second messenger by two Gs-coupled receptors presents a paradigm in GPCR signaling.
EPs and βARs represent two distinct families of GPCRs that are coexpressed in many cells such as cardiomyocytes, neurons, and astrocytes. Despite the activation of both receptors transduces extracellular stimuli to the cAMP and PKA signaling pathway, these stimuli can lead to divergent and sometimes even opposing cellular processes in cells. As proinflammatory factors, the prostaglandin-induced cAMP and PKA activities play important roles in diversified inflammatory responses in fibroblasts in the hearts (20) and in microglia cells in the brain (21) but have limited roles in promoting muscle contraction (7) and possibly neuron excitation (21). In contrast, the βAR-induced cAMP has a broad reach into different intracellular compartments. Consequently, activation of PKA leads to a wide range of responses, including muscle contraction and relaxation and neuron excitation. Traditionally, it is thought that these two signaling machineries are physically segregated via enrichment in different lipid raft microdomains on the PM (22), through which the cAMP is confined at distinct subcellular locations by PDE4 enzymes (8, 9). Thus, the stimulated signaling exerts different cellular responses via targeting selective sets of substrates.
However, it is also well known that EPs and βARs act reciprocally to blunt the function of one another in vivo. In the myocardium, early studies show that PGEs have a negative effect on inotropy under sympathetic regulation (14). In microglia cells, EP signaling promotes activation of glia cells, whereas βAR activation inhibits the inflammatory response. These studies suggest that these two signaling systems converge and interact intracellularly. Here, we show that PGE2 stimulation of EP4 induces cAMP, which activates PKA to phosphorylate PDE4 isoforms (Fig. 4). This phosphorylation enhances the activities of PDE4 isoforms (18) that not only play a role in attenuating the EP signaling (9) but also blocks the diffusion of cAMP induced by βAR to the intracellular SR and nucleus. The observation is also consistent with the functional association between these PDE4 isoforms and βAR subtypes (12, 13); however, PGE2 stimulation also blocks the dissociation of PDE4 isoforms from both βAR subtypes (Fig. 4E and Fig. S8A). As a result, PGE2 treatment attenuates the adrenergic-induced contractile response in myocytes, as well as in animal hearts. The elegant and elaborate cross-talk between these two Gs-coupled receptors in shaping subcellular distribution of second messenger, which is shared by some but not all Gs-coupled receptors. This study provides a mechanism for understanding coordination and integration of extracellular stimuli for diversified cellular functions under stress. It also offers an aspect in understanding long-term effect of chronically circulating proinflammatory factors in myocardium.
Materials and Methods
Adenovirus Construction.
Adenoviruses expressing individual wild-type and dominant-negative forms of PDE4D-mcherry that lack catalytic activity because of point mutation (e.g., D556A in PDE4D5 isoform) were described previously (13). The regular cytoplasmic, PM- and SR-specific PKA activity biosensors cyto-AKAR3, PM-AKAR3, and SR-AKAR3 were published previously (16, 23). The SR-specific cAMP biosensor SR-ICUE3 was generated from ICUE3 with the same strategy used for SR-AKAR3 (16), and the nuclear-specific cAMP biosensor NLS-ICUE3 was generated with the same strategy used for NLS-AKAR3 (16, 23). These reporters were subcloned into the shuttle vector of the AdEasy system for virus production according to the instructions of the manufacturer (MP Biomedical).
Langendorff Perfusion Heart Preparation.
Animal experiments were performed following the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and all procedures were approved by the Institutional Animal Care and Use Committee at the University of Illinois at Urbana and Temple University. The isolated heart perfusion technique was described previously (24). See SI Materials and Methods for more details.
Cardiomyocyte Culture and Adenoviral Infection.
Ventricular neonatal and adult cardiomyocytes were isolated from wild-type FVB animals and cultured as described previously (25). For expressing the PKA or cAMP activity biosensors, neonatal myocytes were infected with adenoviruses at the multiplicity of infection (MOI) of 100 in serum-free Dulbecco modified Eagle/F12 media. After 24 h, myocytes were fixed with 4% paraformaldehyde and blocking with 5% goat serum. The fluorescent images were captured by Leica SP2 Vis Laser confocal microscope. Other experiments were carried out after expression for 24h.
Fluorescent Resonance Energy Transfer Measurement.
Myocytes expressing PKA or cAMP biosensors were rinsed and maintained in PBS for fluorescent resonance energy transfer (FRET) recording as described previously (25). Cells were imaged on a Zeiss Axiovert 200M microscope with a 40×/1.3NA oil-immersion objective lens and cooled CCD camera. Dual emission ratio imaging was acquired with a 420DF20 excitation filter, a 450DRLP dichroic mirror, and two emission filters (475DF40 for cyan and 535DF25 for yellow). The acquisition was set with 200-ms exposure in both channels and 20-s elapses. Images in both channels were subjected to background subtraction, and ratios of yellow-to-cyan color were calculated at different time points.
Neonatal Myocyte Contraction Rate Assay and Adult Myocyte-Shortening Assay.
Measurement of spontaneous contraction rate and adult myocyte shortening was carried out as described previously (25). Responses in myocyte beating rate and contractile shortening after drug treatments were analyzed by Metamorph software. Myocytes were stimulated with FSK (Sigma), ISO (Sigma), or PGE2 (Sigma) at indicated doses and times. Cells were also treated with ROL (Calbiochem) or CILO (Calbiochem) at indicated doses and times.
Western Blotting.
Drug-treated myocytes were lysed in lysis buffer containing 25 mM Hepes (pH 7.5), 2.5 mM EDTA, 50 mM NaCl, 30 mM sodium pyrophosphate, 10% (vol/vol) glycerol, and 1% (vol/vol) Triton X-100 and protease inhibitor mixture tablets (Pierce) after washing twice with ice-cold PBS. Cell lysates were separated by SDS/PAGE for Western blotting with antibodies against phosphorylated serine 16 and threonine 17 of PLB and PLB (Badrilla), PDE4 and phosphorylated PDE4D S190 (Fabgennix), γ-tubulin, GFP, and phosphorylated PKA substrate (SCBT). Primary antibodies were visualized with IRDye 680CW goat-anti mouse or with IRDye 800CW goat-anti rabbit secondary antibodies using an Odyssey scanner (Li-cor biosciences). Quantification of phosphoproteins was performed using densitometry software Quantity One. Arbitrary phosphorylation units were calculated, and results were plotted against controls.
Statistical Analysis.
One-way ANOVA and Student t test were performed using Prism (GraphPad Software, CA).
Supplementary Material
Acknowledgments
This study was supported by National Institutes of Health Grants HL082646 (to Y.K.X.) and HL088243 and American Heart Association Scientist Development Grant 0730347N (to X.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1117862109/-/DCSupplemental.
References
- 1.Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res. 2004;94:1543–1553. doi: 10.1161/01.RES.0000130526.20854.fa. [DOI] [PubMed] [Google Scholar]
- 2.Mann DL. Inflammatory mediators and the failing heart: Past, present, and the foreseeable future. Circ Res. 2002;91:988–998. doi: 10.1161/01.res.0000043825.01705.1b. [DOI] [PubMed] [Google Scholar]
- 3.Yuhki K, et al. Roles of prostanoids in the pathogenesis of cardiovascular diseases: Novel insights from knockout mouse studies. Pharmacol Ther. 2011;129:195–205. doi: 10.1016/j.pharmthera.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Rabinowitz B, Schollmayer E, Weiss M. Prostaglandin E1 in heart disease: Review and perspective. Am J Ther. 1997;4:353–358. doi: 10.1097/00045391-199711000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Lohse MJ, Engelhardt S, Eschenhagen T. What is the role of beta-adrenergic signaling in heart failure? Circ Res. 2003;93:896–906. doi: 10.1161/01.RES.0000102042.83024.CA. [DOI] [PubMed] [Google Scholar]
- 6.Lefkowitz RJ, Rockman HA, Koch WJ. Catecholamines, cardiac beta-adrenergic receptors, and heart failure. Circulation. 2000;101:1634–1637. doi: 10.1161/01.cir.101.14.1634. [DOI] [PubMed] [Google Scholar]
- 7.Di Benedetto G, et al. Protein kinase A type I and type II define distinct intracellular signaling compartments. Circ Res. 2008;103:836–844. doi: 10.1161/CIRCRESAHA.108.174813. [DOI] [PubMed] [Google Scholar]
- 8.Terrin A, et al. PGE(1) stimulation of HEK293 cells generates multiple contiguous domains with different [cAMP]: Role of compartmentalized phosphodiesterases. J Cell Biol. 2006;175:441–451. doi: 10.1083/jcb.200605050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christian F, et al. Small molecule AKAP-protein kinase A (PKA) interaction disruptors that activate PKA interfere with compartmentalized cAMP signaling in cardiac myocytes. J Biol Chem. 2011;286:9079–9096. doi: 10.1074/jbc.M110.160614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiang Y, Kobilka BK. Myocyte adrenoceptor signaling pathways. Science. 2003;300:1530–1532. doi: 10.1126/science.1079206. [DOI] [PubMed] [Google Scholar]
- 11.Xiao RP, et al. Subtype-specific alpha1- and beta-adrenoceptor signaling in the heart. Trends Pharmacol Sci. 2006;27:330–337. doi: 10.1016/j.tips.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Richter W, et al. Signaling from beta1- and beta2-adrenergic receptors is defined by differential interactions with PDE4. EMBO J. 2008;27:384–393. doi: 10.1038/sj.emboj.7601968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Arcangelis V, Liu R, Soto D, Xiang Y. Differential association of phosphodiesterase 4D isoforms with beta2-adrenoceptor in cardiac myocytes. J Biol Chem. 2009;284:33824–33832. doi: 10.1074/jbc.M109.020388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Endoh M. Effects of prostaglandin E1 on the positive inotropic actions of noradrenaline, nerve stimulation and calcium in the isolated blood-perfused papillary muscle of the dog. Eur J Pharmacol. 1976;39:259–265. doi: 10.1016/0014-2999(76)90134-5. [DOI] [PubMed] [Google Scholar]
- 15.Allen MD, et al. Reading dynamic kinase activity in living cells for high-throughput screening. ACS Chem Biol. 2006;1:371–376. doi: 10.1021/cb600202f. [DOI] [PubMed] [Google Scholar]
- 16.Liu S, Zhang J, Xiang YK. FRET-based direct detection of dynamic protein kinase A activity on the sarcoplasmic reticulum in cardiomyocytes. Biochem Biophys Res Commun. 2011;404:581–586. doi: 10.1016/j.bbrc.2010.11.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richter W, Jin SL, Conti M. Splice variants of the cyclic nucleotide phosphodiesterase PDE4D are differentially expressed and regulated in rat tissue. Biochem J. 2005;388:803–811. doi: 10.1042/BJ20050030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baillie G, MacKenzie SJ, Houslay MD. Phorbol 12-myristate 13-acetate triggers the protein kinase A-mediated phosphorylation and activation of the PDE4D5 cAMP phosphodiesterase in human aortic smooth muscle cells through a route involving extracellular signal regulated kinase (ERK) Mol Pharmacol. 2001;60:1100–1111. doi: 10.1124/mol.60.5.1100. [DOI] [PubMed] [Google Scholar]
- 19.De Arcangelis V, Liu S, Zhang D, Soto D, Xiang YK. Equilibrium between adenylyl cyclase and phosphodiesterase patterns adrenergic agonist dose-dependent spatiotemporal cAMP/protein kinase A activities in cardiomyocytes. Mol Pharmacol. 2010;78:340–349. doi: 10.1124/mol.110.064444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brilla CG, Zhou G, Rupp H, Maisch B, Weber KT. Role of angiotensin II and prostaglandin E2 in regulating cardiac fibroblast collagen turnover. Am J Cardiol. 1995;76:8D–13D. doi: 10.1016/s0002-9149(99)80485-8. [DOI] [PubMed] [Google Scholar]
- 21.Furuyashiki T, Narumiya S. Stress responses: The contribution of prostaglandin E(2) and its receptors. Nat Rev Endocrinol. 2011;7:163–175. doi: 10.1038/nrendo.2010.194. [DOI] [PubMed] [Google Scholar]
- 22.Agarwal SR, et al. Effects of cholesterol depletion on compartmentalized cAMP responses in adult cardiac myocytes. J Mol Cell Cardiol. 2011;50:500–509. doi: 10.1016/j.yjmcc.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allen MD, Zhang J. Subcellular dynamics of protein kinase A activity visualized by FRET-based reporters. Biochem Biophys Res Commun. 2006;348:716–721. doi: 10.1016/j.bbrc.2006.07.136. [DOI] [PubMed] [Google Scholar]
- 24.MacDonnell SM, et al. Adrenergic regulation of cardiac contractility does not involve phosphorylation of the cardiac ryanodine receptor at serine 2808. Circ Res. 2008;102:e65–e72. doi: 10.1161/CIRCRESAHA.108.174722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soto D, De Arcangelis V, Zhang J, Xiang Y. Dynamic protein kinase a activities induced by beta-adrenoceptors dictate signaling propagation for substrate phosphorylation and myocyte contraction. Circ Res. 2009;104:770–779. doi: 10.1161/CIRCRESAHA.108.187880. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.