Abstract
The ability to sense temperature is essential for organism survival and efficient metabolism. Body temperatures profoundly affect many physiological functions, including immunity. Transient receptor potential melastatin 2 (TRPM2) is a thermosensitive, Ca2+-permeable cation channel expressed in a wide range of immunocytes. TRPM2 is activated by adenosine diphosphate ribose and hydrogen peroxide (H2O2), although the activation mechanism by H2O2 is not well understood. Here we report a unique activation mechanism in which H2O2 lowers the temperature threshold for TRPM2 activation, termed “sensitization,” through Met oxidation and adenosine diphosphate ribose production. This sensitization is completely abolished by a single mutation at Met-214, indicating that the temperature threshold of TRPM2 activation is regulated by redox signals that enable channel activity at physiological body temperatures. Loss of TRPM2 attenuates zymosan-evoked macrophage functions, including cytokine release and fever-enhanced phagocytic activity. These findings suggest that redox signals sensitize TRPM2 downstream of NADPH oxidase activity and make TRPM2 active at physiological body temperature, leading to increased cytosolic Ca2+ concentrations. Our results suggest that TRPM2 sensitization plays important roles in macrophage functions.
Keywords: calcium, immune cells
The capacity to sense temperature is essential for organism survival and efficient metabolism, and body temperature has profound effects on many physiological functions, including immunity. Paradoxically, lowering body temperature with cyclooxygenase inhibitors worsens survival rates for bacterial infection (1), whereas fever elevates immune reactivity (2). Together, these effects suggest that elevated body temperature has beneficial effects for the immune system, although the molecular mechanisms underlying these effects remain largely unknown.
Transient receptor potential melastatin 2 (TRPM2) is a thermosensitive, Ca2+-permeable cation channel expressed by a wide range of immunocytes, including macrophages, whose function is gradually being clarified (3–9). We previously reported that heat stimulation activates TRPM2 in the presence of low concentrations of agonists, such as adenosine diphosphate ribose (ADPR) and related molecules (10). These agonists are believed to act on a unique C-terminal pyrophosphatase domain in TRPM2 (Nudix-like domain) (11–13). Temperature-dependent activation of TRPM2 plays significant roles in cellular functions, including insulin release from pancreatic β cells (10, 14). TRPM2 channels can be activated by hydrogen peroxide (H2O2) and are reported to be involved in cell death caused by oxidative stress via mechanisms that remain to be clarified (15, 16). ADPR released from intracellular organelles, such as the nucleus and mitochondria, may play a primary role in TRPM2 activation by H2O2 (17–19), although one report suggests involvement of an ADPR-independent activation mechanism (20).
H2O2, a reactive oxygen species (ROS) produced by NADPH oxidase (Nox), is crucial for microorganism removal, given that defects in H2O2 production lead to persistent infections (21). As the first line of defense against infections, the Toll-like receptors (TLRs) of phagocytes, including macrophages, recognize common microbial components, such as pathogen-associated molecular patterns. Then infective organisms are phagocytosed and cleared by systems in which Nox activity is engaged. Along with H2O2’s important role in microbicidal function inside the phagosomes, membrane-diffusible H2O2 also could play roles in cell signaling outside the phagosomes by acting on various proteins (22). ROS such as H2O2 are now considered to be signaling molecules, in parallel with reactive nitrogen species. These cellular “redox” signals play important roles in a wide range of physiological functions, including ion channel activity (23). We hypothesized that redox signals generated by microbicidal activity in macrophages could regulate the function of TRPM2, which is expressed in macrophages (8). To test the hypothesis, we investigated the regulation mechanisms of TRPM2.
Here we describe a unique mechanism for TRPM2 activation in which its temperature threshold is regulated dynamically by H2O2, termed “sensitization.” Sensitization of TRPM2 is caused by a reduction in its temperature threshold through oxidation of a single methionine at Met-214, and is partially attenuated by a poly(ADP ribose) polymerase (PARP) inhibitor. The loss of TRPM2 attenuates macrophage functions such as cytokine release at 37 °C and enhancement of phagocytic activity at febrile temperatures. We suggest that TRPM2 is sensitized by redox signals downstream of Nox activity, and contributes to macrophage functions.
Results
H2O2 Sensitizes TRPM2 to Heat.
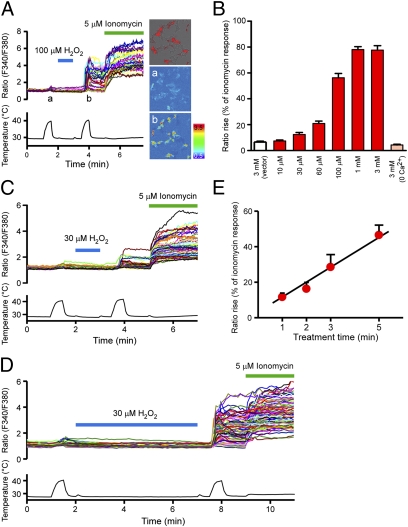
We first examined the effects of H2O2 on heat-evoked TRPM2 activities using a Ca2+-imaging method. Heat stimulation of up to ∼41 °C was applied before and after H2O2 treatment of mouse TRPM2-expressing HEK293 cells. Heat-evoked [Ca2+]i increases were dramatically enhanced by H2O2 treatment in a dose-dependent manner, whereas heat stimulation without H2O2 treatment caused only slight activation (Fig. 1 A and B). In addition to the concentration dependence, the duration of H2O2 treatment also affected the responses; increasing H2O2 (30 μM) treatment from 1 min to 5 min proportionally enhanced heat (∼41 °C)-evoked responses (Fig. 1 C–E). We observed no [Ca2+]i increases in DsRed-negative TRPM2-nonexpressing cells, and H2O2 treatment for 1 min at room temperature failed to increase [Ca2+]i, even in TRPM2-expressing cells (Fig. 1A). Furthermore, the heat-evoked [Ca2+]i increases were not observed in either vector-transfected cells or TRPM2-expressing cells in the absence of extracellular Ca2+ (Fig. 1B), suggesting that Ca2+ influx through TRPM2 caused the increase in heat-evoked [Ca2+]i. H2O2-dependent enhancement of heat-evoked TRPM2 responses was also observed in whole-cell patch-clamp recordings, confirming an event across the plasma membrane (Fig. S1). The heat-evoked TRPM2 currents gradually returned to basal levels after the temperature reduction, and the sustained currents were completely inhibited by the TRPM2 inhibitor 2-aminoethoxydiphenyl borate (2-APB; Fig. S1) (24), suggesting mediation of the sustained currents by TRPM2.
Fig. 1.
Heat-evoked responses of TRPM2 were elevated by H2O2 in a concentration- and time-dependent manner. (A) H2O2 (100 μM) enhanced heat-evoked increases in intracellular Ca2+ concentrations ([Ca2+]i) in DsRed(+) TRPM2-expressing cells (Left and Right Upper). Representative pseudocolor images of fluorescence intensity during heat stimulation before (a) and after (b) H2O2 treatment. (B) Each H2O2 concentration (10 μM, 30 μM, 60 μM, 100 μM, 1 mM, and 3 mM) was applied for 1 min as in A, and the heat-evoked [Ca2+]i increases after H2O2 treatment were normalized to the values in response to ionomycin for each experiment. Enhancement of the heat-evoked response was not observed in vector-transfected control cells (vector) or in TRPM2-expressing cells in the absence of extracellular Ca2+ (0 Ca2+), even at the highest H2O2 concentration (3 mM). Data are mean ± SEM (n = 5–13). (C and D) Representative traces of [Ca2+]i changes in TRPM2-expressing cells in response to heat before and after H2O2 (30 μM) treatment for 1 min (C) or 5 min (D) and later exposure to ionomycin (5 μM). (E) Heat-evoked responses of TRPM2 were elevated by prolonging H2O2 treatment. Data are mean ± SEM (n = 6 or 7). R2 = 0.97.
The observation that TRPM2 was significantly activated by heat stimulation after H2O2 treatment, whereas heat stimulation (∼41 °C) alone evoked only slight TRPM2 activation (Fig. 1A), might be explained if H2O2 reduces the temperature threshold for TRPM2 activation. Indeed, heat stimulation with higher temperatures induced potent [Ca2+]i increases even without H2O2 treatment (Fig. 2A, upper trace). When temperature thresholds were determined from temperatures causing [Ca2+]i increases in excess of those observed for DsRed-negative cells, the average threshold was 47.2 ± 0.2 °C (n = 5) (Fig. 2B). Treatment with H2O2 for 1 min significantly lowered this threshold [100 μM: 41.7 ± 0.1 °C (n = 5); 3 mM: 36.3 ± 0.4 °C (n = 8); P < 0.001 vs. H2O2 untreated] in a dose-dependent manner (Fig. 2 A and B). Similar to the time dependence of heat-evoked responses (Fig. 1 C–E), temperature threshold reductions also depended on the duration of H2O2 treatment (Fig. 2B). To more precisely determine the temperature thresholds, we used the heat-evoked currents observed in whole-cell patch-clamp recordings to generate Arrhenius plots, which displayed an explicit flex point during heating (Fig. 2C). The reductions in temperature thresholds were recapitulated in whole-cell patch-clamp recordings in which cells were exposed to H2O2 in the pipette solution [100 μM: 40.2 ± 1.3 °C (n = 11); 3 mM: 36.3 ± 0.6 °C (n = 10); P < 0.01] (Fig. 2 C and D). Of note, the sensitization of heat-evoked currents was more easily reproduced by lower concentrations when H2O2 was applied in the pipette solution rather than extracellularly (Fig. 2C and Fig. S1). In the whole-cell recordings, higher concentrations of H2O2 are needed when H2O2 is applied extracellularly, because H2O2 entering the cell can be diluted by the pipette solution. This suggests an intracellular site for H2O2 action. H2O2-mediated reduction in the temperature threshold for TRPM2 activation could explain the increased TRPM2 activity under physiological temperatures, as shown in Fig. S2A. Therefore, the effect of H2O2 on TRPM2 can be viewed as a “sensitization” to physiological body temperature.
Fig. 2.
H2O2 reduced the temperature threshold for TRPM2 activation. (A) Representative traces of temperature-response profiles in heat stimulation without H2O2 (Top) or after H2O2 treatment at 100 μM (Middle) or 3 mM (Bottom) for 1 min. (B) Averaged data for heat stimulation without and after H2O2 treatment at 100 μM or 3 mM for 1 min and at 60 μM for 1, 3, and 5 min. Mean ± SEM (n = 5–8). ###P < 0.001 vs. H2O2-untreated; ***P < 0.001 between indicated pairs (ANOVA). (C) A heat-evoked current after 1 min exposure to pipette solution containing 100 μM H2O2 (Top) obtained by whole-cell recording. Representative Arrhenius plot traces are shown for temperature vs. density of heat-evoked currents with 100 μM H2O2 (Middle, using upper trace) or 3 mM (Bottom) after 1 min exposure. 2-APB, a TRPM2 inhibitor. (D) Averaged data for whole-cell recordings of heat-evoked currents after H2O2 treatment at 100 μM or 3 mM for 1 min. Data are mean ± SEM (n = 10 or 11). *P < 0.05 (t test).
Molecular Mechanism of TRPM2 Sensitization to Heat.
Most previous studies have suggested that TRPM2 activation by H2O2 is caused by ADPR release from intracellular organelles (17–19). To test this possibility, we evaluated the effects of H2O2 in inside-out single-channel recordings in which intracellular components are absent. Consistent with the data from whole-cell recordings, heat-evoked currents in an inside-out configuration were dramatically enhanced by H2O2 treatment, whereas heat stimulation alone caused only slight activation (Fig. 3A). Single-channel openings of the heat-evoked current after H2O2 treatment were seen at temperatures as low as 37 °C, and the calculated conductance was 118.4 ± 10.1 pS (n = 7), higher than the reported 58 pS of the ADPR-evoked current of human TRPM2 at room temperature (12). In addition, the single-channel conductance increased concurrently with temperature (Fig. S3). Data from the single-channel recordings provide significant evidence that sensitization of TRPM2 could be caused independently of cytosolic ADPR, although ADPR production also could be involved in TRPM2 sensitization with intracellular components. In addition, TRPM2 sensitization in single-channel recordings was detected as long as 5 min after H2O2 removal (Fig. S4A), suggesting that H2O2 acts by oxidative modification of target amino acids.
Fig. 3.
H2O2 sensitizes TRPM2 to heat in a membrane-delimited manner. (A) A heat-evoked current in a TRPM2-expressing cell at −60 mV in an inside-out configuration. Magnified traces (a and b) correspond to the currents shown by the arrows in the left trace. (B and C) Heat-evoked responses of TRPM2 were sensitized by chloramine-T, a membrane-permeant oxidant that preferentially oxidizes Met residues, in both whole-cell (B) and inside-out single-channel (C) recordings, where the magnified traces (a and b) correspond to the currents shown by the arrows in the above trace. (D and E) 5,5′-Dithiobis-2-nitrobenzoic acid, a membrane-impermeant Cys-specific oxidant, did not sensitize the heat response of TRPM2 in either whole-cell (D) or inside-out single-channel (E) recordings.
The major candidate targets of ROS-mediated protein oxidation are cysteine (Cys) and Met residues (25). Thus, we evaluated the effects of various oxidants to identify the residues possibly involved in TRPM2 sensitization. Chloramine-T, a membrane-permeant oxidant that preferentially oxidizes Met residues, sensitized TRPM2 in both single-channel and whole-cell recordings (Fig. 3 B and C). In contrast, 5,5′-dithiobis-2-nitrobenzoic acid, a membrane-impermeant Cys-specific oxidant, did not induce sensitization of TRPM2 in either type of recording (Fig. 3 D and E). These data suggest that sensitization of TRPM2 can be mediated by direct oxidation of Met rather than by Cys. Unlike H2O2, S-nitroprusside, an NO donor, did not induce TRPM2 sensitization (Fig. S4B). In addition, even though the amino acid sequence of TRPM2 is very close to that of TRPM8, cold-evoked TRPM8 responses were not affected by H2O2 (Fig. S4C). These data suggest that H2O2-induced sensitization is unique to and characteristic of TRPM2.
Met-Ala mutagenesis was performed to identify the Met residue(s) in TRPM2 involved in H2O2-induced sensitization. Mouse TRPM2 has 35 Met residues, 22 of which are conserved between humans and mice. Given that H2O2-induced sensitization was also observed in HEK293 cells expressing human TRPM2 in both whole-cell and single-channel recordings (Fig. S5 A and B), Ala substitutions were introduced at 21 of the conserved Met residues (the first Met was excluded; Fig. 4A), and the sensitization of each mutant was evaluated with a Ca2+-imaging method. In WT TRPM2, H2O2 (100 μM) treatment significantly reduced the temperature threshold for [Ca2+]i increases in a time-dependent manner (Fig. 4 B and D). Among the 21 mutants, only M214A completely lost H2O2-induced sensitization even after long exposure (3 min) (Fig. 4 C and D), and the corresponding human mutant M215A also showed similar properties (Fig. S5C). In addition, M214A did not exhibit H2O2-evoked [Ca2+]i increases at physiological temperature (Fig. S2B). We could not evaluate sensitization in two mutations (M815A and M1044A) because of their limited channel activity. ADPR release from organelles is thought to be involved in sensitization because it can be induced by H2O2 in [Ca2+]i imaging, and the threshold shift was partly attenuated by the PARP inhibitor PJ-34, which inhibits ADPR release from the nucleus (Fig. 4E, Left). Nevertheless, the significant threshold reductions seen in the presence of PJ-34 (Fig. 4E, Right) indicate that Met oxidation could be crucial for sensitization, consistent with the finding that oxidants, including H2O2 and chloramine-T, sensitized TRPM2 in a membrane-delimited manner. Unexpectedly, the density of ADPR (100 μM)-evoked currents was significantly reduced (5.5 ± 1.1 pA/pF) for M214A compared with WT (521.2 ± 153.1 pA/pF), although M214A demonstrated ADPR sensitivity to higher concentrations (500 μM) of ADPR (128.0 ± 89.7 pA/pF) (Fig. S6). This result suggests a possible interaction between M214 and the Nudix motif. To further confirm the importance of M214, we examined the TRPM2 splice variant with a C-terminal deletion (Δ1288–1321; ΔC) (18, 20). TRPM2ΔC still showed sensitization to H2O2 treatment while losing ADPR sensitivity, as reported previously (Fig. S7). This finding, along with rapid increases in H2O2-evoked [Ca2+]i at physiological temperature (Fig. S2A), support the possibility that the TRPM2 temperature threshold could be regulated in an ADPR-independent way.
Fig. 4.
Structural basis for TRPM2 sensitization by H2O2. (A) Putative membrane topology of TRPM2, with the Met residues conserved between mouse and human TRPM2 indicated. Mutations to Ala were introduced at the Met residues indicated by red circles. (B–D) Reduction in the temperature threshold for TRPM2 activation by H2O2 treatment (100 μM for 1 or 3 min) was completely abolished in the M214A mutant. Shown are temperature-response profiles of heat-evoked [Ca2+]i increases observed in DsRed(+), WT (B), or M214A-expressing cells (C) and nonexpressing DsRed(−) cells (D). Data are mean ± SEM (n = 4 or 5). P < 0.05, Student t test or ANOVA, unless noted otherwise, between without and after treatment with H2O2. NS, not significant (ANOVA). (E) Sensitization of WT TRPM2 in the presence (+) or absence (−) of PJ-34. In the PJ(+) groups, the potent PARP inhibitor PJ-34 (1 μM) was present during the entire experiment. Data are mean ± SEM (n = 4–8). *P < 0.05; **P < 0.01; ***P < 0.001 vs. 0 min (ANOVA).
TRPM2 Sensitization in Peritoneal Macrophages.
We performed additional studies to determine whether H2O2-induced sensitization could be recapitulated in native cells using peritoneal macrophages that endogenously produce ROS on phagocytosis. TRPM2 expression was detected by RT-PCR in freshly prepared WT macrophages but not in TRPM2-deficient cells, even though the two cell types had similar morphology (Fig. S8 A–C). Heat-evoked responses in WT macrophages were enhanced by H2O2 in Ca2+ imaging (Fig. 5A) and whole-cell patch-clamp methods (densities of heat-evoked current before and after H2O2 application were 4.1 ± 0.4 and 46.9 ± 22.2 pA/pF, respectively; n = 3) (Fig. 5C), similar to the response of HEK293 cells expressing TRPM2 (Fig. 1A and Fig. S1). Single-channel openings were detected in heat-evoked whole-cell currents. The sustained currents in WT macrophages were inhibited by 2-APB. Although 2-APB is not specific to TRPM2 and also affects store-operated Ca2+ entry (26), TRPM2 could mediate the heat-evoked responses (Fig. 5 A and C), given that TRPM2-deficient macrophages did not show such sensitization (Fig. 5 B and D). In addition, sensitization of heat-evoked responses in WT macrophages was not induced in the absence of extracellular Ca2+ (Fig. S8D). Together, these data indicate that the sensitization was inducible in murine macrophages by H2O2, and that these heat-evoked responses are attributable to endogenous TRPM2.
Fig. 5.
Sensitization is observed in WT macrophages, but not in TRPM2-deficient cells. (A and B) H2O2-induced sensitization of heat-evoked [Ca2+]i increases was observed in WT (A), but not in TRPM2-deficient (B) macrophages. (C and D) H2O2-induced sensitization of heat-evoked current was observed in WT (C), but not in TRPM2-deficient (D) macrophages. (C, Inset) A magnified trace corresponding to the red box shown in the upper trace.
TRPM2-Dependent Regulation of Macrophage Functions.
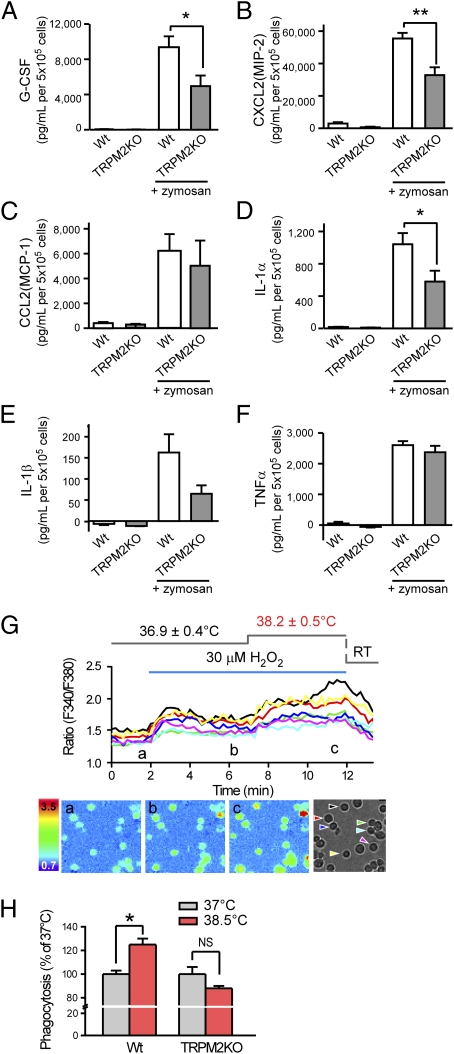
On infection, macrophage activation induces the release of cytokines for the recruitment and activation of immune cells. To examine the involvement of TRPM2, we compared the release of cytokines in WT and TRPM2-deficient macrophages using ELISA of culture media. In these assays, macrophages were stimulated for 24 h at 37 °C with the TLR2 agonist zymosan (50 μg/mL), which induces Nox activation and ROS production (27). ROS generation downstream of TLR2 activation could cause Ca2+ influx through TRPM2 sensitization, which in turn would enhance macrophage functions. As such, we focused on the cytokines regulated by NF-ĸB, whose activity is regulated by cytosolic Ca2+ levels (28). Macrophage stimulation with zymosan elicited the release of granulocyte colony stimulating factor (G-CSF), TNFα, IL-1α, IL-1β, macrophage inflammatory protein-2 (CXCL2), and monocyte chemotactic protein-1 (CCL2) (Fig. 6 A–F). Among them, release of G-CSF, CXCL2, and IL-1α were significantly reduced in TRPM2-deficient macrophages compared with WT cells. IL-1β release tended to be lower in TRPM2-deficient macrophages without statistical significance (P = 0.10; values were 163 ± 43 and 65 ± 20 pg/mL) (Fig. 6E). These data suggest that TRPM2-mediated pathways, including ROS-induced sensitization, contribute to the increased release of G-CSF, CXCL2, IL-1α, and possibly IL-1β. Considering TRPM2 sensitization, temperature elevation should affect H2O2-evoked [Ca2+]i increases in macrophages. Indeed, temperature elevations as small as 1.3 °C enhanced H2O2 (30 μM)-evoked [Ca2+]i increases (Fig. 6G). These data indicate that sensitized-TRPM2 channel function can be further enhanced by temperature elevation, suggesting that redox signals and fever can act cooperatively on macrophage functions via TRPM2. To examine the effects of small temperature increases on other macrophage functions, we examined phagocytic activity at normal (37 °C) and febrile (38.5 °C) temperatures (2). Phagocytosis was significantly increased at 38.5 °C compared with 37 °C in WT macrophages, whereas TRPM2-deficient macrophages showed no such temperature-dependent effect (Fig. 6H). These data suggest that TRPM2 could mediate the enhanced phagocytic activity at elevated temperatures.
Fig. 6.
Zymosan-induced cytokine release and phagocytic activity in WT and TRPM2-deficient macrophages. (A–F) Amount of released cytokines from unstimulated and zymosan (50 μg/mL)-stimulated macrophages from WT and TRPM2-deficient mice. Data are mean ± SEM (n = 4–5). *P < 0.05; **P < 0.01 (ANOVA). (G) A small temperature elevation caused further enhancement of H2O2 (30 μM)-induced [Ca2+]i increases in WT macrophages. Mean values for the normal and elevated temperatures are reported as mean ± SD. The lower panels show pseudocolor images of fluorescence intensity corresponding to the time points in the upper trace (a–c) and a phase-contrast image. Colored wedges in the phase-contrast image indicate the cells corresponding to each colored ratio trace. (H) Enhancement of phagocytic activity by elevated temperature (38.5 °C) was abolished in TRPM2-deficient macrophages. The ratio of cells that phagocytized zymosan particle(s) was normalized to the average values at 37 °C in each genotype. Data are mean ± SEM (n = 3). *P < 0.05; NSP > 0.05 for 37 °C vs. 38.5 °C (Student t test).
Discussion
In the present study, we have identified a unique mechanism for “sensitization” of TRPM2. In the absence of H2O2 treatment, the temperature threshold for TRPM2 activation remained at supraphysiological temperature levels, whereas H2O2 treatment lowered the threshold to physiological temperatures. Our present results differ somewhat from those of our previous study, which showed that TRPM2 was activated by heat alone at around body temperature and maximal single-channel openings occurred at ∼36 °C (10). However, in that study, to obtain sufficient current size, HEK293 cells were cultured for longer periods (more than 36 h) after transfection, compare with only 20–36 h in the present study. These different culture conditions might have affected the sensitivity to heat stimulation.
Although TRPM2 is activated by ROS and is involved in cell death after oxidative stress (15, 16), the activation mechanisms involved are unclear (17–20). The primary activator of TRPM2 is thought to be ADPR, with most previous studies suggesting that the release of ADPR from the nucleus and mitochondria plays a primary role in TRPM2 activation by H2O2 (17–19), although one study has reported that H2O2 acts on TRPM2 directly (20). We show here that H2O2 can sensitize TRPM2 in the absence of these organelles and activate it at physiological temperatures in a membrane-delimited manner by reducing the temperature threshold for activation. We also found that PARP inhibition attenuates H2O2-evoked reductions in the temperature threshold. Nevertheless, we believe that ADPR participation is modest, given that single-channel activation is induced at ∼37 °C after H2O2 treatment (Fig. 3A), and that the PARP inhibitor used might have an additional effect in this system. These results suggest that H2O2 causes TRPM2 sensitization through two different mechanisms in parallel, which might explain the different proposals for the action of H2O2 on TRPM2 (17–20). Along with PARP-dependent ADPR release from the nucleus, ADPR release from mitochondria is also reportedly involved in TRPM2 activation by H2O2. Although our results using intact cells cannot rule out mitochondrial involvement, the fact that H2O2-evoked activation of TRPM2 at physiological temperature is more rapid (Fig. S2) than TRPM2 currents mediated by ADPR released from mitochondria (18) make its participation less likely. Interestingly, TRPM2 sensitization was completely abolished by a single mutation at Met-214 (M214A) (Fig. 4) and a corresponding mutation in human TRPM2 (M215A) (Fig. S5C), strongly supporting the importance of this Met residue in TRPM2 sensitization. Previous studies on a TRPM2 splice variant with diminished ADPR-evoked activation also support our idea that ADPR-independent sensitization of TRPM2 occurs through Met oxidation. Unexpectedly, the M214 mutant affected TRPM2 activation by ADPR, even though the site is apart from the C-terminal Nudix-like domain, suggesting that an interaction between the TRPM2 N- and C-terminal regions regulates TRPM2 activity.
Although Met oxidation in the regulation of TRPM6 has been reported previously (29), our study demonstrates that Met oxidation is involved in regulation of the temperature threshold for activation of thermosensitive TRP channels (thermo-TRPs). Among the known thermo-TRPs, TRPV1 was found to exhibit a reduction in temperature threshold through serine phosphorylation by protein kinases A and C on proinflammatory mediator production (30, 31). Thus, the temperature thresholds for activating thermo-TRPs might be regulated by various mechanisms depending on the channel and its cellular environment.
Sensitization of TRPM2 was found to be involved in macrophage functions. Pathogen-associated molecular patterns of invading microorganisms can activate the TLR pathway, leading to production of ROS for microbicidal activity. Among the produced ROS, H2O2 has weaker reactivity and higher membrane permeability, allowing it to diffuse over long distances (32), thus making H2O2 a suitable signaling molecule (33). H2O2 concentrations are reported to reach mM levels within phagosomes (34) and 10–100 μM in inflammatory environments (35), which would be sufficient to sensitize TRPM2. We first hypothesized that TRPM2 activity could enhance cytokine release, given that intracellular Ca2+ activates NF-kB (28), which in turn regulates the expression of various cytokines (36). However, we found that the loss of TRPM2 affected the release of cytokine subsets. One possible explanation for this finding is that direct regulation of transcription factors by redox signals (37) leads to complex changes in cytokine release. Other mechanisms lying downstream of TRPM2 activity could be involved as well, given that cytokine release is not caused simply by effects on transcription regulation. For example, the calcium-dependent proteases calpains are involved in the maturation and release of IL-1α (38) as well as in phagocytosis (39). A recent in vivo study reported significantly enhanced susceptibility to Listeria monocytogenes in TRPM2-deficient mice (40), which can be partly explained by the impaired macrophage functions observed in the present study.
Of note, TRPM2 is expressed by lymphocytes, neutrophils, and monocytes/macrophages (3–8), whose activities have a strong relationship with body temperature (2, 41). This suggests that TRPM2 might have a broader role in the temperature sensitivity of the immune system. Fever or hyperthermia is a widely conserved phenomenon involved in host defenses against infections in both endotherms and ectotherms (42, 43) and is considered to enhance immune reactivity (2). Thus, fever is considered a beneficial response in host defenses, but the underlying mechanism remains unclear. Given that TRPM2 is conserved among a wide range of species (44) and is thought to be widely expressed in immunocytes, ROS-sensitized TRPM2 can act as a thermosensor to regulate immune reactivities at body temperatures ranging from nonfebrile to febrile. Redox signals are also known to affect Ca2+ release from Ca2+ stores (37), suggesting that ROS can regulate Ca2+ signals in various ways. TRPM2 could play a part in this regulatory system, as suggested by a recent report demonstrating negative regulation of ROS by TRPM2 (9). Our findings suggest that the study of TRPM2 sensitization might identify unique approaches for determining the physiological function of TRPM2 that focus on body temperature and redox signals.
Materials and Methods
HEK293T cells transfected with cDNAs or peritoneal macrophages prepared from female C57BL/6NCr and TRPM2-deficient mice (7) were used for Ca2+ imaging with fura-2 and patch-clamp recordings to study TRPM2-mediated channel properties. Zymosan-evoked cytokine release and phagocytic activity were compared in WT and TRPM2-deficient macrophages. Data are presented as mean ± SEM or mean ± SD. Statistical analysis was performed using the Student t test or ANOVA, followed by the Bonferroni-type multiple t test. P values < 0.05 were considered significant. Synthetic oligonucleotide primers constructing specific mutations and splice variant are shown in Table S1. A detailed description of the experimental procedures is provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Drs. Shin-ichiro Saitoh (Tokyo University), Masatsugu Ohora (Tokyo Medical and Dental University), Yoshihiro Kubo, and Masaki Fukata (National Institute for Physiological Sciences) for their helpful advice. This work was supported by grants from the Japanese Ministry of Education, Culture, Sports, Science and Technology (to M.T.) and the Mitsubishi Foundation (to M.T.); and by a postdoctoral fellowship from the Japan Society for Promotion of Science postdoctoral fellowship (to M.K.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1114193109/-/DCSupplemental.
References
- 1.Kluger MJ, Kozak W, Conn CA, Leon LR, Soszynski D. Role of fever in disease. Ann N Y Acad Sci. 1998;856:224–233. doi: 10.1111/j.1749-6632.1998.tb08329.x. [DOI] [PubMed] [Google Scholar]
- 2.Hasday JD, Fairchild KD, Shanholtz C. The role of fever in the infected host. Microbes Infect. 2000;2:1891–1904. doi: 10.1016/s1286-4579(00)01337-x. [DOI] [PubMed] [Google Scholar]
- 3.Heiner I, Eisfeld J, Lückhoff A. Role and regulation of TRP channels in neutrophil granulocytes. Cell Calcium. 2003;33:533–540. doi: 10.1016/s0143-4160(03)00058-7. [DOI] [PubMed] [Google Scholar]
- 4.Inada H, Iida T, Tominaga M. Different expression patterns of TRP genes in murine B and T lymphocytes. Biochem Biophys Res Commun. 2006;350:762–767. doi: 10.1016/j.bbrc.2006.09.111. [DOI] [PubMed] [Google Scholar]
- 5.Carter RN, et al. Molecular and electrophysiological characterization of transient receptor potential ion channels in the primary murine megakaryocyte. J Physiol. 2006;576:151–162. doi: 10.1113/jphysiol.2006.113886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lange I, Penner R, Fleig A, Beck A. Synergistic regulation of endogenous TRPM2 channels by adenine dinucleotides in primary human neutrophils. Cell Calcium. 2008;44:604–615. doi: 10.1016/j.ceca.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamamoto S, et al. TRPM2-mediated Ca2+ influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat Med. 2008;14:738–747. doi: 10.1038/nm1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sumoza-Toledo A, Penner R. TRPM2: A multifunctional ion channel for calcium signalling. J Physiol. 2011;589:1515–1525. doi: 10.1113/jphysiol.2010.201855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di A, et al. The redox-sensitive cation channel TRPM2 modulates phagocyte ROS production and inflammation. Nat Immunol. 2012;13:29–34. doi: 10.1038/ni.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Togashi K, et al. TRPM2 activation by cyclic ADP-ribose at body temperature is involved in insulin secretion. EMBO J. 2006;25:1804–1815. doi: 10.1038/sj.emboj.7601083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perraud AL, et al. ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature. 2001;411:595–599. doi: 10.1038/35079100. [DOI] [PubMed] [Google Scholar]
- 12.Sano Y, et al. Immunocyte Ca2+ influx system mediated by LTRPC2. Science. 2001;293:1327–1330. doi: 10.1126/science.1062473. [DOI] [PubMed] [Google Scholar]
- 13.Fleig A, Penner R. The TRPM ion channel subfamily: Molecular, biophysical and functional features. Trends Pharmacol Sci. 2004;25:633–639. doi: 10.1016/j.tips.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Uchida K, et al. Lack of TRPM2 impaired insulin secretion and glucose metabolisms in mice. Diabetes. 2011;60:119–126. doi: 10.2337/db10-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hara Y, et al. LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol Cell. 2002;9:163–173. doi: 10.1016/s1097-2765(01)00438-5. [DOI] [PubMed] [Google Scholar]
- 16.Kolisek M, Beck A, Fleig A, Penner R. Cyclic ADP-ribose and hydrogen peroxide synergize with ADP-ribose in the activation of TRPM2 channels. Mol Cell. 2005;18:61–69. doi: 10.1016/j.molcel.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 17.Fonfria E, et al. TRPM2 channel opening in response to oxidative stress is dependent on activation of poly(ADP-ribose) polymerase. Br J Pharmacol. 2004;143:186–192. doi: 10.1038/sj.bjp.0705914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perraud AL, et al. Accumulation of free ADP-ribose from mitochondria mediates oxidative stress-induced gating of TRPM2 cation channels. J Biol Chem. 2005;280:6138–6148. doi: 10.1074/jbc.M411446200. [DOI] [PubMed] [Google Scholar]
- 19.Buelow B, Song Y, Scharenberg AM. The poly(ADP-ribose) polymerase PARP-1 is required for oxidative stress-induced TRPM2 activation in lymphocytes. J Biol Chem. 2008;283:24571–24583. doi: 10.1074/jbc.M802673200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wehage E, et al. Activation of the cation channel long transient receptor potential channel 2 (LTRPC2) by hydrogen peroxide: A splice variant reveals a mode of activation independent of ADP-ribose. J Biol Chem. 2002;277:23150–23156. doi: 10.1074/jbc.M112096200. [DOI] [PubMed] [Google Scholar]
- 21.Roos D, et al. Mutations in the X-linked and autosomal recessive forms of chronic granulomatous disease. Blood. 1996;87:1663–1681. [PubMed] [Google Scholar]
- 22.Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 23.Brown DI, Griendling KK. Nox proteins in signal transduction. Free Radic Biol Med. 2009;47:1239–1253. doi: 10.1016/j.freeradbiomed.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Togashi K, Inada H, Tominaga M. Inhibition of the transient receptor potential cation channel TRPM2 by 2-aminoethoxydiphenyl borate (2-APB) Br J Pharmacol. 2008;153:1324–1330. doi: 10.1038/sj.bjp.0707675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spickett CM, Pitt AR, Morrice N, Kolch W. Proteomic analysis of phosphorylation, oxidation and nitrosylation in signal transduction. Biochim Biophys Acta. 2006;1764:1823–1841. doi: 10.1016/j.bbapap.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 26.DeHaven WI, Smyth JT, Boyles RR, Bird GS, Putney JW., Jr Complex actions of 2-aminoethyldiphenyl borate on store-operated calcium entry. J Biol Chem. 2008;283:19265–19273. doi: 10.1074/jbc.M801535200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J Exp Med. 2003;197:1107–1117. doi: 10.1084/jem.20021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386:855–858. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- 29.Cao G, et al. Methionine sulfoxide reductase B1 (MsrB1) recovers TRPM6 channel activity during oxidative stress. J Biol Chem. 2010;285:26081–26087. doi: 10.1074/jbc.M110.103655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tominaga M, Wada M, Masu M. Potentiation of capsaicin receptor activity by metabotropic ATP receptors as a possible mechanism for ATP-evoked pain and hyperalgesia. Proc Natl Acad Sci USA. 2001;98:6951–6956. doi: 10.1073/pnas.111025298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sugiura T, Tominaga M, Katsuya H, Mizumura K. Bradykinin lowers the threshold temperature for heat activation of vanilloid receptor 1. J Neurophysiol. 2002;88:544–548. doi: 10.1152/jn.2002.88.1.544. [DOI] [PubMed] [Google Scholar]
- 32.Winterbourn CC. Reconciling the chemistry and biology of reactive oxygen species. Nat Chem Biol. 2008;4:278–286. doi: 10.1038/nchembio.85. [DOI] [PubMed] [Google Scholar]
- 33.Forman HJ, Maiorino M, Ursini F. Signaling functions of reactive oxygen species. Biochemistry. 2010;49:835–842. doi: 10.1021/bi9020378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haas A, Goebel W. Microbial strategies to prevent oxygen-dependent killing by phagocytes. Free Radic Res Commun. 1992;16:137–157. doi: 10.3109/10715769209049167. [DOI] [PubMed] [Google Scholar]
- 35.Nathan CF, Root RK. Hydrogen peroxide release from mouse peritoneal macrophages: Dependence on sequential activation and triggering. J Exp Med. 1977;146:1648–1662. doi: 10.1084/jem.146.6.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kamata H, Hirata H. Redox regulation of cellular signalling. Cell Signal. 1999;11:1–14. doi: 10.1016/s0898-6568(98)00037-0. [DOI] [PubMed] [Google Scholar]
- 38.Carruth LM, Demczuk S, Mizel SB. Involvement of a calpain-like protease in the processing of the murine interleukin 1α precursor. J Biol Chem. 1991;266:12162–12167. [PubMed] [Google Scholar]
- 39.Dewitt S, Hallett MB. Cytosolic free Ca(2+) changes and calpain activation are required for β integrin–accelerated phagocytosis by human neutrophils. J Cell Biol. 2002;159:181–189. doi: 10.1083/jcb.200206089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knowles H, et al. Transient Receptor Potential Melastatin 2 (TRPM2) ion channel is required for innate immunity against Listeria monocytogenes. Proc Natl Acad Sci USA. 2011;108:11578–11583. doi: 10.1073/pnas.1010678108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blatteis CM. Fever: Is it beneficial? Yale J Biol Med. 1986;59:107–116. [PMC free article] [PubMed] [Google Scholar]
- 42.Dinarello CA. Infection, fever, and exogenous and endogenous pyrogens: Some concepts have changed. J Endotoxin Res. 2004;10:201–222. doi: 10.1179/096805104225006129. [DOI] [PubMed] [Google Scholar]
- 43.Vaughn LK, Bernheim HA, Kluger MJ. Fever in the lizard Dipsosaurus dorsalis. Nature. 1974;252:473–474. doi: 10.1038/252473a0. [DOI] [PubMed] [Google Scholar]
- 44.Saito S, Shingai R. Evolution of thermoTRP ion channel homologs in vertebrates. Physiol Genomics. 2006;27:219–230. doi: 10.1152/physiolgenomics.00322.2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.