Few recent scientific advances have captured the imagination of biologists and the general public like the prospect of animal cloning (1, 2). The procedure is elegantly simple. A nucleus from a mature cell is transferred into the cytoplasm of an enucleated egg and becomes “reprogrammed” to re-execute embryogenesis. That cloning has been successful at all seems biologically remarkable and has forced biologists to assess what cell differentiation is all about. However, although possible, the process has many complications (3–5). Fetal and placental weight are often dramatically increased. Animals also frequently suffer from congenital anomalies and die within hours of birth. Embryonic and fetal losses are also extremely high, such that far less than 1% of manipulated embryos give rise to live-born animals. These grim facts, collectively termed here cloned offspring syndrome, have raised considerable concern about the cloning process. The reasons for these complications have remained a mystery. However, work from the Jaenisch lab, culminating in a paper by Eggan et al. in this issue (6), has systematically addressed the problem and revealed surprising conclusions.
Mechanisms related to fetoplacental growth should be the focus of research aimed at understanding nuclear reprogramming.
Three potential causes of cloned offspring syndrome have been considered. As somatic cells are mostly used for cloning, it is argued that they have lost their developmental potential and can only rarely be reprogrammed (3, 7, 8). Surprisingly, this occurrence turns out not to be true. This was tested by using donor nuclei from embryonic stem (ES) cells. Murine ES cells, when injected into early embryos, contribute to all fetal structures, implying that they are totipotent. However, cloned embryos produced by using ES cell nuclei suffer a similar ill fate, in that only a tiny fraction of embryos survive to term and survive only rarely to adulthood (9, 10). Another popular idea is that culture of embryos prior to their transfer to the uterus introduces developmental errors (11). Studies with cattle and sheep embryos imply that indeed some fetal overgrowth results from procedures that involve embryo culture (11). However, the extent of this problem has not been directly compared with the effects of nuclear transfer. Notably, congenital anomalies and perinatal death are not associated with the culture of embryos. The final hypothesis is that the nuclear transfer process itself somehow results in complications of development. One of the problems in discriminating between these hypotheses is that all of the features of cloned offspring syndrome have been lumped together, an assumption that now appears to be incorrect.
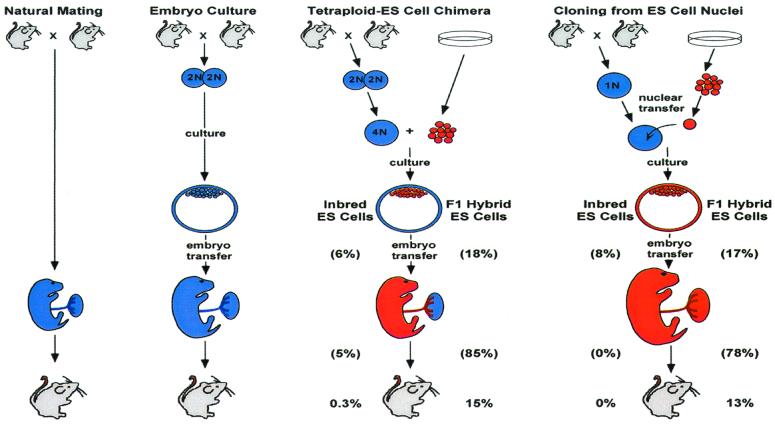
In earlier work with cloned mice made by transfer of ES cell nuclei, the Jaenisch lab made a fortuitous observation (9). Most ES cell lines are derived from inbred strains of mice. Although cloned mice derived from their 129/Sv ES cell line invariably died perinatally, cloned animals derived from a (129/Sv × C57BL/6) F1 hybrid ES cell line survived to adulthood (9). In the present paper, they extended the observations to several other donor ES cell lines and found that embryonic development to term and postnatal survival were higher when F1 hybrid ES cells were used as the source of donor nuclei. Impressively, cloned animals survived to adulthood only when they were derived from F1 hybrid cells (Fig. 1) (6).
Figure 1.
Derivation and developmental potential of ES cell clones produced either by injection of ES cells into tetraploid blastocysts to produce chimeras or by transfer of ES cell nuclei into enucleated oocytes in mice. With both procedures, the manipulated embryos are cultured, and those that develop to the blastocyst stage are transferred to recipient females. The cumulative fraction of transferred blastocysts that result in adult mice is shown, based on the data from Eggan et al. (6). The fraction that develops from blastocyst to term gestation and from term gestation to adulthood is indicated in brackets. Tissues derived from the host embryo and ES cells are shown by blue and red, respectively. Note that in tetraploid chimeras, the placenta is mostly derived from the tetraploid cells, although the mesenchymal and vascular components are derived from the ES cells.
Embryos completely derived from 129/Sv ES cells, by making chimeras between the ES cells and host embryos, also develop to term at a low rate and typically die soon after birth (12). This finding raised the possibility that the poor developmental potential of cloned embryos derived from inbred strains was unrelated to the nuclear transfer process. To test this idea, embryos were generated by making ES cell-tetraploid chimeras (6). This approach takes advantage of the fact that blastomeres made tetraploid (4N) by electrofusion of a two-cell embryo replicate as tetraploid cells, which, although forming trophoblast and endoderm of the placenta and extraembryonic membranes, fail to form fetal structures. ES cells, by contrast, cannot form trophoblast and extraembryonic endoderm but do form fetal tissues. Chimeras made from ES cells and tetraploid embryos therefore form normal concepti because of complementary cell contributions (Fig. 1) (13). Strikingly, the fate of ES cell-derived fetuses produced by tetraploid complementation is similar to that of cloned embryos in that they survive to adulthood if they were derived from F1 hybrid ES cell lines (Fig. 1) (6).
The results from the ES cell-tetraploid chimeras indicate that the poor postnatal survival of cloned mouse embryos is not because of the nuclear transfer process itself. But what about other aspects of cloned offspring syndrome? With respect to embryonic survival, F1 hybrid-derived embryos develop to term at a significantly higher rate, whether they are produced as ES cell-tetraploid chimeras or via nuclear transfer. Notably, the rates of development from blastocyst to term were not different between the two methods (Fig. 1) (6). In contrast, one difference between embryos produced by cloning and tetraploid chimeras is that fetal, and particularly placental, size is significantly larger in the cloned animals. This is true for both inbred and F1 hybrid-derived animals. Although the cloned fetuses and placentas are extremely large, it is notable that animals produced as ES cell-tetraploid chimeras are intermediate in size (6). One interpretation of these data would be that a normal placenta cannot “rescue” the growth phenotype. It is important to remember, though, that ES cells do contribute to mesenchymal and vascular elements of the placenta (13–15). Therefore, without knowing the cellular composition of the large placentas, this conclusion is premature. Notably, embryos that are simply cultured to the blastocyst stage without any other manipulation before embryo transfer also develop into larger fetuses and placentas (6). These results imply that fetoplacental overgrowth in cloned offspring is related in part to culture effects, supporting reports from other species (11), but that the magnitude of overgrowth is much greater than can be attributed to culture alone.
Overall, the results from Eggan et al. (6) are important because they indicate that different aspects of cloned offspring syndrome are attributable to distinct problems. Specifically, poor embryonic and postnatal survival is not specifically associated with cloning. The underlying mechanistic defects can now better be addressed by focusing on the effects of culture conditions, embryo transfer, etc. That the problem can be dramatically improved by using F1 hybrid rather than inbred donor cells is a major advance, but whether the effect is specific to ES cells must be evaluated. One discouraging clue is that cloned embryos produced from somatic cells produced from F1 hybrid somatic cells were not reported to have a different fate than clones based on inbred strains (16). Fetoplacental overgrowth, by contrast, does appear to be a specific complication of cloned animals. Therefore, mechanisms related to fetoplacental growth should be the focus of research aimed at understanding nuclear reprogramming.
Does this new information give insights into the problems of cloning in domestic animals? With respect to embryonic and postnatal survival, it is unclear whether the beneficial effect of using F1 hybrid cells is applicable to domestic species. First, a requirement to outbreed would defeat the purpose of cloning in cases where the object is to propagate a valuable animal's genotype. Second, although it is difficult to compare the extent of inbreeding in mouse strains with breeds of cattle or sheep, it is likely that there is less inbreeding within a breed of livestock than in a strain of mice. It is noteworthy that the rates of embryonic/fetal and postnatal survival are extremely variable in farm animals. Most attempts to clone domestic animals have used cells derived from purebred animals. Whether the variability is because of differences in inbreeding deserves evaluation.
The most immediate impact of the work from Eggan et al. (6) is the practical implication for production of mutant mice. Currently, ES cells carrying a mutated gene or transgene are used to make chimeras with normal diploid embryos. The resulting animals are only partially derived from the ES cells and therefore must be bred to obtain heterozygous progeny. Producing homozygotes requires further breeding. This approach could be bypassed completely by using F1 hybrid ES cells and tetraploid chimeras. Making tetraploid chimeras is a simple technique, particularly when chimeras are made by aggregation rather than by injection (13). Theoretically, it then becomes possible to do in a few weeks what otherwise would takes months in animal breeding. This is an exciting prospect.
Footnotes
See companion article on page 6209.
References
- 1.Wilmut I. Sci Am. 1998;279:58–63. doi: 10.1038/scientificamerican1298-58. [DOI] [PubMed] [Google Scholar]
- 2.Lupton M L. Med Law. 1999;18:107–123. [PubMed] [Google Scholar]
- 3.Westhusin M E, Long C R, Shin T, Hill J R, Looney C R, Pryor J H, Piedrahita J A. Theriogenology. 2001;55:35–49. doi: 10.1016/s0093-691x(00)00444-1. [DOI] [PubMed] [Google Scholar]
- 4.Young L E, Sinclair K D, Wilmut I. Rev Reprod. 1998;3:155–163. doi: 10.1530/ror.0.0030155. [DOI] [PubMed] [Google Scholar]
- 5.Hill J R, Burghardt R C, Jones K, Long C R, Looney C R, Shin T, Spencer T E, Thompson J A, Winger Q A, Westhusin M E. Biol Reprod. 2000;63:1787–1794. doi: 10.1095/biolreprod63.6.1787. [DOI] [PubMed] [Google Scholar]
- 6.Eggan K, Aksutsu H, Loring J, Jackson-Grusby L, Klemm M, Rideout W M, 3rd, Yanagimachi R, Jaenisch R. Proc Natl Acad Sci USA. 2001;98:6209–6214. doi: 10.1073/pnas.101118898. . (First Published May 1, 2001, 10.1073/pnas.101118898). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell K H S. In: Cloned Animal and Placentation. Roberts R M, Yanagimachi R, Kariya T, Hasizume K, editors. Tokyo: Yokendo; 2000. pp. 36–43. [Google Scholar]
- 8.Wilmut I, Young L, Campbell K H. Reprod Fertil Dev. 1998;10:639–643. doi: 10.1071/rd98047. [DOI] [PubMed] [Google Scholar]
- 9.Rideout W M, 3rd, Wakayama T, Wutz A, Eggan K, Jackson-Grusby L, Dausman J, Yanagimachi R, Jaenisch R. Nat Genet. 2000;24:109–110. doi: 10.1038/72753. [DOI] [PubMed] [Google Scholar]
- 10.Wakayama T, Rodriguez I, Perry A C, Yanagimachi R, Mombaerts P. Proc Natl Acad Sci USA. 1999;96:14984–14989. doi: 10.1073/pnas.96.26.14984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sinclair K D, Young L E, Wilmut I, McEvoy T G. Hum Reprod. 2000;15 Suppl. 5:68–86. doi: 10.1093/humrep/15.suppl_5.68. [DOI] [PubMed] [Google Scholar]
- 12.Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder J C. Proc Natl Acad Sci USA. 1993;90:8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagy A, Rossant J. In: Gene Targeting: A Practical Approach. Joyner A, editor. Oxford, U.K.: Oxford Univ. Press; 1993. pp. 147–179. [Google Scholar]
- 14.Nagy A, Gocza E, Diaz E M, Prideaux V R, Ivanyi E, Markkula M, Rossant J. Development (Cambridge, UK) 1990;110:815–821. doi: 10.1242/dev.110.3.815. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka M, Gertsenstein M, Rossant J, Nagy A. Dev Biol. 1997;190:55–65. doi: 10.1006/dbio.1997.8685. [DOI] [PubMed] [Google Scholar]
- 16.Wakayama T, Yanagimachi R. Mol Reprod Dev. 2001;58:376–383. doi: 10.1002/1098-2795(20010401)58:4<376::AID-MRD4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]