Abstract
Introduction
Microtubule-associated doublecortin and CaM kinase-like-1 (DCLK1) is a novel candidate marker for intestinal stem cells. The aim of our study was to assess DCLK1 immunoreactivity in colorectal carcinogenesis and its correlation with prognosis.
Methods
DCLK1 immunostaining was performed in colorectal tissue from 71 patients, including 18 adenomatous polyps, 40 primary adenocarcinomas, and 14 metastatic lesions. Each case was evaluated by a combined scoring method based on the intensity of staining (score 0–3) and the percentage of tissue staining positive (score 0–3). Immunoexpression for DCLK1 was considered as positive when the combined score was 2–6 and negative with a score of 0–1.
Results
Overall, 14/18 (78%) of polyps, 30/40 (75%) of primary adenocarcinomas, and 7/14 (50%) of distant metastases were positive for DCLK1. In adenomatous polyps and primary cancer there was no association between DCLK1 staining score and tumor pathology. However, after curative colorectal cancer resection, patients whose tumor had a high (≥5) combined staining score had increased cancer-specific mortality compared to patients with low (0–4) staining score (hazard ratio 5.89; 95% confidence interval: 1.22–28.47; P = 0.027).
Conclusion
We found that DCLK1 is frequently expressed in colorectal neoplasia and may be associated with poor prognosis. Further studies are necessary to validate the use of DCLK1 as a prognostic marker.
Keywords: DCLK1, DCAMKL-1, gastrointestinal stem cell, cancer stem cell, adenomatous polyps, liver metastasis, immunohistochemistry
Introduction
Colonic carcinogenesis is known to occur through the accumulation of genetic mutations over a long time frame.1 Recently, lineage-tracing studies have shown that Apc deletion in long-lived stem cells expressing leucine-rich-repeat-containing G-protein-coupled receptor 5 (LGR5) gives rise to intestinal adenoma in mice, which lends support to the intestinal stem cell as the possible cell of origin for colorectal cancer.2 Several candidate markers of the intestinal stem cell population, such as LGR5, MSI1, and CD29, have been extensively studied.3 Recently, doublecortin and CaM kinase-like-1 (DCLK1, previously referred to as DCAMKL-1), a transmembrane microtubule-associated kinase found in post-mitotic neurons,4 has also been proposed as intestinal stem cell marker. In support of this, 1 DCLK1 was found to be abundant in mouse cDNA libraries from gastrointestinal progenitor cells.5 Moreover, DCLK1-positive cells were shown to retain bromodeoxyuridine and to form glandular epithelial structures when injected in nude mice.6 Although DCLK1 has been shown to be expressed in cancers, including pancreatic and esophageal,7–9 the data on DCLK1 immunoreactivity in human colorectal cancer are limited.7 Moreover, the expression of DCLK1 during progressive tumorigenesis has not been studied. The aim of this study was to further elucidate the expression of DCLK1 in colorectal carcinogenesis by evaluating DCLK1 immunoreactivity in colorectal adenomatous polyps, adenocarcinomas, and distant metastases. In addition, we sought to elucidate whether the expression of DCLK1 correlated with the degree of carcinogenesis and with prognosis.
Materials and methods
Patients
Pathology reports and the corresponding hematoxylin and eosin slides of patients treated at Tulane University Health Science Center between January 2000 and December 2010 were retrospectively reviewed. Patients with adenomatous polyps, primary colorectal adenocarcinomas, and colorectal metastases were identified. Representative tissue blocks were selected from 18 patients with benign colorectal polyps who underwent polypectomy and from 40 patients with primary colorectal adenocarcinomas who underwent surgical resection. We only included those colorectal cancer patients who had surgery before 2008 and excluded those who underwent neoadjuvant therapy. Colorectal distant metastases (13 liver and one lung) were selected among patients who underwent surgery for metastatic cancer. Demographics, tumor location, size, degree of dysplasia, American Joint Committee on Cancer (AJCC) stage, and degree of differentiation were extracted from the pathology reports. Lesions with the morphologic characteristics of adenocarcinomas but that had not invaded through the muscularis mucosae into the submucosa were classified as “intramucosal neoplasia”. For patients with primary colorectal cancer, updated follow-up information including disease status and cause of death was obtained from Tulane Cancer Center. The study was approved by the Tulane University Institutional Review Board.
Immunohistochemistry
Immunohistochemical staining was carried out on 5-mm sections of formalin-fixed, paraffin-embedded samples. For all experiments we used a rabbit polyclonal anti-DCLK1 antibody (1:80; Abcam, Cambridge, MA). Heat-induced epitope retrieval was performed utilizing a pressurized food steamer in citrate buffer (pH 6.0) at 99°C. Primary antibody incubation was carried out overnight at 4°C. For all experiments we used the UltraVision LP Detection System kit (Thermo Fisher Scientific, Fremont, CA) following the instructions of the manufacturer. To exclude nonspecific staining, isotype (rabbit polyclonal IgG; Abcam) and negative controls were included for each experiment. A colorectal cancer with intense immunoreactivity for DCLK1 was used as positive control. Immunostaining was performed in duplicate.
Staining evaluation
DCLK1 staining intensity in tumor cells was evaluated in a blinded fashion by one pathologist (MG) to assign scores of average immunohistochemical signal intensity (ie, 0 = none, 1 = mild, 2 = moderate, and 3 = strong) as well as the percentage of tissue showing positive immunoreactivity (Table 1). Signal intensity and percentage of positive tissue were combined in a staining score similar to that described for the intestinal stem cell marker LGR5.10–12 Immunoexpression for DCLK1 was considered positive when the combined score was 2–6 and negative with a score of 0–1.
Table 1.
Scoring criteria
| Features | Score |
|---|---|
| Intensity of staining | |
| Nonreactive | 0 |
| Mild | 1 |
| Moderate | 2 |
| Strong | 3 |
| Percentage of positive cells | |
| <5% | 0 |
| 5%–30% | 1 |
| 31%–60% | 2 |
| 61%–100% | 3 |
Note: Total score: 0–1 = negative; 2–6 = positive.
Statistical analysis
The association between staining score, patient characteristics, and tumor features was tested using the Fisher’s exact test for qualitative variables and the Mann–Whitney test for quantitative ones. For the primary colorectal cancers, the cutoff point between staining scores with different prognostic values was calculated using a receiver-operating characteristic curve based on cancer-specific mortality (CSM). CSM curves were estimated by the Kaplan–Meier method and compared using the log-rank test; CSM was then analyzed by the Cox proportional hazards model, comparing the risk factors by the Wald test. No multivariate model was estimated, due to the low number of events. All reported P-values were obtained by the two-sided exact method, and significance was established at the conventional 5% level. Data were analyzed using SPSS software (version 19.0; SPSS, Chicago, IL).
Results
Adenomatous polyps
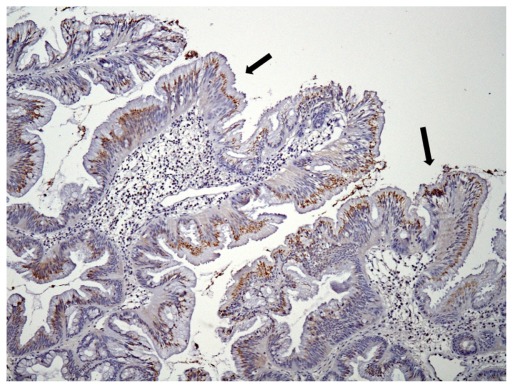
We identified 18 cases of adenomatous polyps. The mean age was 58 years (range 40–84 years). Nine of the polyps were located in the right colon, four in the left colon, and five in the rectum. Polyp architecture was tubular in three cases, tubulovillous in 13 cases, and villous in two cases (Table 2). The correlation of DCLK1 expression with various clinicopathologic features is detailed in Table 2. Of the 18 adenomas, 14 (78%) showed positive immunoreactivity for DCLK1 (Table 2). Staining was granular and localized to the apical (luminal) part of the cytoplasm (Figure 1). Staining was present focally or expressed over large areas of the adenoma. No significant associations were found between staining score and polyp location, size, morphology, architecture, degree of dysplasia, or the presence of carcinomas in situ. However, a staining score of ≥5 was found in 38% (5/13) of the adenomas with high-grade dysplasia compared to 0% (0/5) of the adenomas with low-grade dysplasia.
Table 2.
Clinicopathologic and DCLK1 immunostaining characteristics in 18 adenomatous polyps
| Age at diagnosis (years) | Gender | Polyp location | Morphology | Architecture | Size (mm) | Grade of dysplasia | Intramucosal neoplasia | Intensity of staining | Area of staining (%) | Combined score |
|---|---|---|---|---|---|---|---|---|---|---|
| 51 | M | Right | Sessile | Tubulovillous | 20 | High | No | 2 | 10% | 3 |
| 57 | M | Left | Sessile | Tubulovillous | 18 | High | No | 3 | 100% | 6 |
| 56 | M | Right | Sessile | Tubulovillous | 20 | Low | No | 0 | 0% | 0 |
| 55 | M | Rectum | Pedunculated | Villous | 20 | Low | No | 2 | 60% | 4 |
| 40 | M | Left | Pedunculated | Tubular | 18 | High | Yes | 2 | 100% | 5 |
| 56 | M | Left | Pedunculated | Tubulovillous | 35 | High | No | 3 | 60% | 5 |
| 84 | M | Right | Pedunculated | Tubulovillous | N/A | Low | No | 2 | 50% | 4 |
| 42 | M | Rectum | Sessile | Tubulovillous | N/A | High | Yes | 0 | 0% | 0 |
| 57 | M | Right | Sessile | Tubulovillous | N/A | Low | No | 2 | 40% | 4 |
| 68 | M | Right | Sessile | Tubulovillous | 20 | High | No | 2 | 50% | 4 |
| 65 | F | Left | Pedunculated | Tubulovillous | 15 | Low | No | 2 | 10% | 3 |
| 66 | M | Rectum | Sessile | Villous | 50 | High | Yes | 2 | 50% | 4 |
| 66 | M | Rectum | Sessile | Tubular | 8 | High | No | 2 | 10% | 3 |
| 59 | F | Right | Sessile | Tubular | 50 | High | Yes | 0 | 0% | 0 |
| 64 | M | Right | Sessile | Tubulovillous | 50 | High | Yes | 1 | 25% | 2 |
| 50 | M | Right | Sessile | Tubulovillous | 15 | High | Yes | 2 | 80% | 5 |
| 64 | M | Rectum | Sessile | Tubulovillous | 25 | High | Yes | 3 | 70% | 5 |
| 50 | F | Right | Pedunculated | Tubulovillous | 50 | High | Yes | 0 | 0% | 0 |
Abbreviations: DCLK1, doublecortin and CaM kinase-like-1; N/A, not available; M, male; F, female.
Figure 1.
Moderate DCLK1 immunoreactivity in a tubulovillous adenoma with low-grade dysplasia (100×). Granular staining localized to the apical part of the cytoplasm (arrows).
Abbreviation: DCLK1, doublecortin and CaM kinase-like-1.
Adenocarcinomas
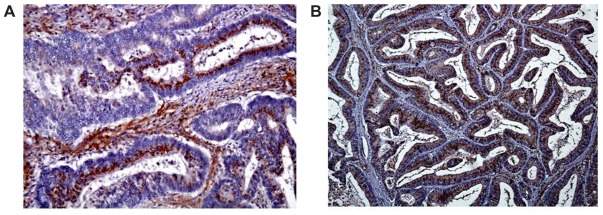
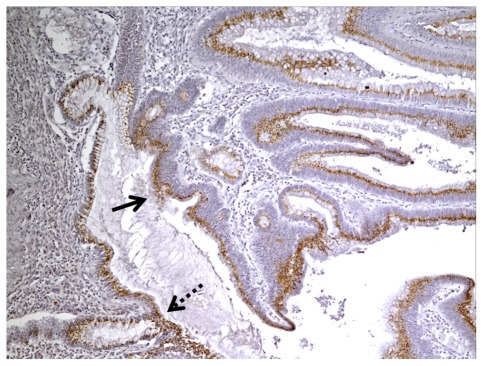
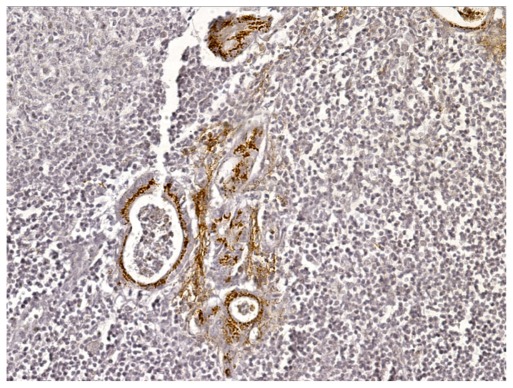
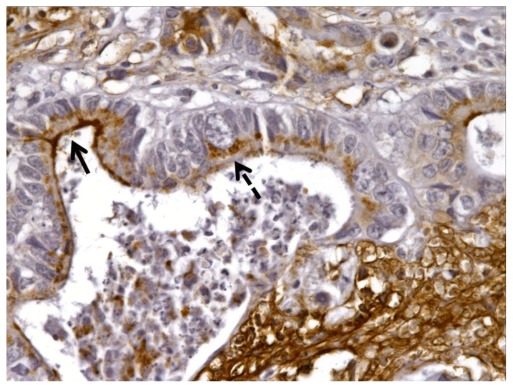
We identified 40 cases of colorectal adeoncarcinomas in the database of our institution for which paraffin blocks were available. The mean age of the patients was 66 years (range 30–98 years). Eighteen tumors were located in the right colon, 16 in the left colon, and six in the rectum. There were six AJCC stage I, eight stage II, 20 stage III, and six stage IV tumors (Table 3). The correlation of DCLK1 expression with various clinicopathologic features is detailed in Table 3. Immunostaining was positive in 30/40 (75%) of the adenocarcinomas (Table 3) with a staining pattern similar to that observed in the polyps, either focal (Figure 2A) or diffuse (Figure 2B). Focal immunoreactivity was also found in the tumor desmoplastic stroma. Histologically normal mucosa adjacent to tumors was evaluated in 37 cases of which 28 (76%) showed positive DCLK1 immunoreactivity (Figure 3). In three-paired nodal metastases the pattern of staining in metastatic tissue mirrored that of the primary tumor. An example of DCLK1 immunoreactivity in a metastatic lymph node is shown in Figure 4. No significant associations were found between staining score and tumor location, AJCC stage, or degree of differentiation.
Table 3.
Clinicopathologic survival and DCLK1 immunostaining characteristics in 40 colorectal adenocarcinomas
| Age at diagnosis | Gender | Primary tumor location | Pathology | AJCC stage | Degree of differentiation* | Survival (months) | Disease status | Intensity of staining | Area of staining (%) | Score |
|---|---|---|---|---|---|---|---|---|---|---|
| 53 | F | Right | Adenocarcinoma | IV | 3 | 117 | Alive without disease | 0 | 0 | 0 |
| 63 | M | Right | Adenocarcinoma | IV | 2 | 15 | Dead of disease | 3 | 20 | 4 |
| 68 | M | Right | Adenocarcinoma | III | 2 | 63 | Alive without disease | 0 | 0 | 0 |
| 74 | M | Right | Adenocarcinoma | III | 3 | 12 | Dead of disease | 3 | 80 | 6 |
| 61 | M | Left | Adenocarcinoma | III | 2 | 90 | Alive without disease | 2 | 50 | 4 |
| 72 | M | Rectum | Adenocarcinoma | III | 3 | 37 | Dead of disease | 2 | 75 | 5 |
| 43 | F | Left | Adenocarcinoma | IV | 3 | 0 | Postoperative death | 0 | 0 | 0 |
| 64 | M | Left | Adenocarcinoma | III | 2 | 72 | Alive without disease | 0 | 0 | 0 |
| 68 | M | Right | Mucinous adenocarcinoma | II | 2 | 48 | Alive without disease | 3 | 40 | 5 |
| 73 | F | Right | Adenocarcinoma | III | 3 | 110 | Alive without disease | 1 | 10 | 2 |
| 73 | F | Left | Adenocarcinoma | I | 2 | 72 | Alive without disease | 0 | 0 | 0 |
| 57 | M | Right | Adenocarcinoma | III | 2 | 94 | Dead without disease | 2 | 40 | 4 |
| 63 | M | Rectum | Adenocarcinoma | III | 3 | 15 | Dead of disease | 3 | 50 | 5 |
| 70 | F | Right | Mucinous adenocarcinoma | II | 2 | 38 | Alive without disease | 3 | 70 | 6 |
| 40 | F | Right | Adenocarcinoma | III | 2 | 16 | Dead of disease | 3 | 100 | 6 |
| 67 | M | Right | Adenocarcinoma | II | 2 | 46 | Alive without disease | 1 | 30 | 3 |
| 78 | M | Left | Mucinous adenocarcinoma | III | 1 | 70 | Dead of disease | 2 | 5 | 3 |
| 50 | M | Left | Adenocarcinoma | II | 2 | 48 | Alive without disease | 3 | 80 | 6 |
| 80 | M | Right | Adenocarcinoma | IV | 2 | 13 | Dead of disease | 3 | 50 | 5 |
| 63 | F | Left | Adenocarcinoma | III | 2 | 9 | Dead of disease | 3 | 80 | 6 |
| 76 | F | Right | Adenocarcinoma | III | 2 | 4 | Dead of disease | 3 | 80 | 6 |
| 60 | F | Left | Adenocarcinoma | II | 2 | 60 | Alive without disease | 1 | 50 | 3 |
| 50 | M | Rectum | Adenocarcinoma | I | 2 | 63 | Alive without disease | 0 | 0 | 0 |
| 36 | M | Rectum | Signet ring adenocarcinoma | III | 3 | 2 | Lost to follow-up | 0 | 0 | 0 |
| 30 | M | Rectum | Signet ring adenocarcinoma | III | 3 | 11 | Dead of disease | 0 | 0 | 0 |
| 77 | F | Right | Adenocarcinoma | III | 2 | 72 | Alive without disease | 0 | 0 | 0 |
| 53 | F | Left | Adenocarcinoma | IV | 2 | 6 | Dead of disease | 3 | 100 | 6 |
| 81 | M | Left | Adenocarcinoma | III | 3 | 2 | Lost to follow-up | 3 | 90 | 6 |
| 63 | M | Right | Adenocarcinoma | II | 2 | 43 | Alive without disease | 3 | 10 | 4 |
| 98 | F | Left | Signet ring adenocarcinoma | II | 3 | 0 | Postoperative death | 1 | 50 | 3 |
| 74 | M | Rectum | Adenocarcinoma | I | 2 | 52 | Alive without disease | 2 | 50 | 4 |
| 78 | F | Right | Adenocarcinoma | I | 2 | 55 | Alive without disease | 3 | 60 | 5 |
| 92 | F | Left | Adenocarcinoma | II | 2 | 58 | Dead without disease | 2 | 80 | 6 |
| 66 | F | Right | Adenocarcinoma | III | 2 | 46 | Alive without disease | 3 | 90 | 6 |
| 90 | F | Right | Mucinous adenocarcinoma | III | 3 | 0 | Postoperative death | 3 | 90 | 6 |
| 52 | M | Left | Adenocarcinoma | I | 2 | 46 | Alive without disease | 1 | 5 | 2 |
| 75 | M | Left | Adenocarcinoma | IV | 3 | 26 | Dead of disease | 2 | 20 | 4 |
| 81 | M | Left | Mucinous adenocarcinoma | III | 3 | 25 | Dead of disease | 3 | 100 | 6 |
| 34 | F | Right | Adenocarcinoma | I | 2 | 78 | Alive without disease | 0 | 0 | 0 |
| 81 | M | Left | Adenocarcinoma | III | 3 | 25 | Dead of disease | 1 | 10 | 2 |
Note:
1 = well differentiated; 2 = moderately differentiated; 3 = poorly differentiated.
Abbreviations: DCLK1, doublecortin and CaM kinase-like-1; AJCC, American Joint Committee on Cancer; M, male; F, female.
Figure 2.
(A) Focal DCLK1 immunoreactivity in a moderately differentiated adenocarcinoma (200×). (B) Diffuse DCLK1 immunoreactivity in a moderately differentiated adenocarcinoma (100×).
Abbreviation: DCLK1, doublecortin and CaM kinase-like-1.
Figure 3.
DCLK1 immunoreactivity in the normal colonic mucosa (dashed arrow) adjacent to the tumor (solid arrow) (100×).
Abbreviation: DCLK1, doublecortin and CaM kinase-like-1.
Figure 4.
DCLK1 immunoreactivity in a metastatic lymph node (200×).
Abbreviation: DCLK1, doublecortin and CaM kinase-like-1.
Distant metastasis
Of the 14 distant (13 liver and one lung) colorectal metastases, seven (50%) had immunoreactivity for DCLK1. Similar to that observed in the primary tumors, the staining was predominately cytoplasmic. In addition, three tumors showed areas with strong apical membrane localization. An example of a liver metastasis with both cytoplasmic and membrane staining is shown in Figure 5. Focal immunoreactivity was found in the desmoplastic stroma. For one liver metastasis, the primary tumor was available. Interestingly, there was DCLK1 immunoreactivity in both the primary and metastatic tissue.
Figure 5.
DCLK1 immunoreactivity in a liver metastasis showing both cytoplasmic (dashed arrow) and apical membrane (solid arrow) localization (400×).
Abbreviation: DCLK1, doublecortin and CaM kinase-like-1.
Survival analysis in primary colorectal cancer
At a median follow-up of 52 months (range 2–110) of 40 patients with primary colorectal cancer, 19 were alive without evidence of recurrence, 16 died of colorectal cancer, three died of other causes (including two perioperative deaths) and two were lost to follow-up (Table 3). Using cancer-specific mortality as the endpoint, receiver-operating characteristic curve calculations showed the most accurate cutoff point for the staining score was ≥5, with an accuracy of 68.9%, a sensitivity of 64.3%, and a specificity of 69.2%. Using two different staining score cutoff points (either ≥2 or ≥5), patients and tumor characteristics were tested in univariate binary logistic regression models as independent predictors of staining score. No associations were found between score and AJCC stage or degree of differentiation using either the cutoff point of ≥2 or ≥5.
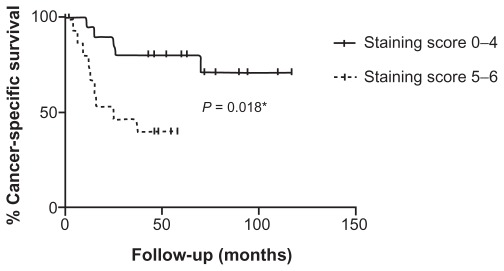
CSM was severely increased in patients with a high (≥5) staining score compared to patients with low (0–4) staining score (hazard ratio 4.16; 95% confidence interval [CI]: 1.28–13.57; P = 0.018). The Kaplan–Meier cancer-specific survival curve for patients with high (≥5) and low (0–4) staining score is shown in Figure 6. This strong risk factor persisted even after eliminating patients diagnosed at stage IV (hazard ratio 5.89; 95% CI: 1.22–28.47; P = 0.027).
Figure 6.
Kaplan–Meier cancer-specific survival curve for patients with high (≥5) and low (0–4) DCLK1 staining score.
Note: *Log-rank test.
Abbreviation: DCLK1, doublecortin and CaM kinase-like-1.
Discussion
The relationship of DCLK1-expressing cells to crucial events in tumor progression is poorly understood. Using standard immunostaining, we noted DCLK1 immunoreactivity in 78% of colorectal adenomas, and in 75% of primary colorectal cancers. This finding suggests that the upregulation of DCLK1 might be an early event in colorectal tumorigenesis. We also noted that nearly 40% of adenomatous polyps with high-grade dysplasia expressed intense DCLK1 immunoreactivity. This finding is similar to the results of other investigators who found an increased expression of the putative intestinal stem cell marker LGR5 in high-grade precancerous colorectal lesions.12 These findings are also consistent with the animal data showing that Apc gene deletion in LGR5-positive cells gives rise to intestinal adenomas,2 and provide indirect evidence of the involvement of adult intestinal stem cells in colorectal carcinogenesis. However, the role of DCLK1 as an intestinal stem cell marker is still a matter of debate, since recent studies in a mouse model suggest that DCLK1-expressing cells represent postmitotic differentiated tuft cells and enteroendocrine cells.13
While these results confirm the increased immunohistochemical expression of DCLK1 in primary colorectal cancer, we also noted DCLK1 immunoreactivity in the histologically normal mucosa immediately adjacent to the adenocarcinomas in 76% of cases. This is in contrast to the findings of Sureban and colleagues, who did not identify any immunoreactivity in paired normal mucosa from tissue microarrays.7,14 However, studies of tissue microarrays do not examine the tumor–mucosal junction, which in colorectal cancer, is known to be hyperplastic and to contain more immature and undifferentiated cells.15–19 These findings may therefore reflect a process of clonal activation starting in intestinal stem cells and the phenomenon of field cancerization.20
The presence of DCLK1-expressing cells in colorectal metastases has not been described. In our study, we found high DCLK1 expression not only in the primary tumors but also in the distant metastases, which is consistent with the results of other investigators who found a similar expression of the putative intestinal stem cell marker LGR5 in primary colorectal carcinomas and liver metastases.21 In distant metastases, we noted that DCLK1 immunoreactivity was positive in 50% of our specimens, with a staining pattern similar to that observed in the primary tumors. These results raise the question of whether DCLK1 in metastases might have their origin in primary tumors. We believe that DCLK1-expressing tumor cells become detached from the primary tumor site and migrate to the metastatic site via vascular and/or perineural spaces. However, our results are difficult to interpret, because paired tissue from the primary tumors was available in only a minority of samples.
While there was no association of DCLK1 expression with the stage of disease or the degree of tumor differentiation, we found that a high level of DCLK1 immunoreactivity correlated with a worse cancer specific survival and, therefore, may reflect a more aggressive tumor phenotype. The reason why colorectal cancers expressing high levels of DCLK1 behave more aggressively may be related to the cancer stem cell theory. This theory postulates that only a subset of cancer cells with stem-like features are capable of reproducing the tumor and metastasizing and that, in colorectal cancer, these cells express intestinal stem cell markers.3,22 Although an association between tumor expression and a worse colorectal cancer prognosis has been observed with other candidate intestinal stem cell markers including LGR5 and Musashi-1,23,24 the small number of subjects in our cohort limits the power of the study and gives rise to wide confidence intervals. Another limit of our study is that the follow-up was not standardized. Therefore our results must be viewed with caution and must be confirmed in a larger patient population. Moreover, our results are only based on immunohistochemical analysis which does not quantify protein expression. Future studies should include quantitative evaluation of DCLK1 by reverse transcriptase polymerase chain reaction or fluorescence-activated cell sorting in both normal and neoplastic colorectal tissue.
Although several putative colorectal cancer stem cells markers have been identified, it is not clear how these markers can be used clinically. Interestingly, Sureban and colleagues showed that after small-interfering RNA blockage of DCLK1, colon cancer cells had reduced in vivo tumorigenic potential. This functional role was mediated by a decrease of the MIRLET7 A primary transcript and an increase of Myc expression, both related to loss of epithelial differentiation.7 In a recent study, administration of a nanoparticle-based DCLK1 small interfering RNA into a colorectal tumor xenograft inhibited tumor growth and downregulated Myc and Notch1.14 These studies suggest that DCLK1 may be a marker of colorectal stem cells with a functional role and thus may be an important therapeutic target.
In conclusion, these results demonstrate that DCLK1 is commonly expressed in colorectal adenomas and carcinomas and that a high DCLK1 staining score may have prognostic value. Our results suggest an active involvement of DCLK1 in colorectal carcinogenesis. However, further studies are necessary to validate the use of DCLK1 as a colorectal cancer stem cell marker and as a possible therapeutic target.
Acknowledgments
This work was supported by a South Central Veterans Affairs Health Care Network Research Pilot Study Award.
Footnotes
Disclosure
The authors declare no conflicts of interest.
References
- 1.Shackleton M, Quintana E, Fearon ER, Morrison SJ. Heterogeneity in cancer: cancer stem cells versus clonal evolution. Cell. 2009;138(5):822–829. doi: 10.1016/j.cell.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 2.Barker N, Ridgway RA, van Es JH, et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457(7229):608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- 3.Todaro M, Francipane MG, Medema JP, Stassi G. Colon cancer stem cells: promise of targeted therapy. Gastroenterology. 2010;138(6):2151–2162. doi: 10.1053/j.gastro.2009.12.063. [DOI] [PubMed] [Google Scholar]
- 4.Lin PT, Gleeson JG, Corbo JC, Flanagan L, Walsh CA. DCAMKL1 encodes a protein kinase with homology to doublecortin that regulates microtubule polymerization. J Neurosci. 2000;20(24):9152–9161. doi: 10.1523/JNEUROSCI.20-24-09152.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giannakis M, Stappenbeck TS, Mills JC, et al. Molecular properties of adult mouse gastric and intestinal epithelial progenitors in their niches. J Biol Chem. 2006;281(16):11292–11300. doi: 10.1074/jbc.M512118200. [DOI] [PubMed] [Google Scholar]
- 6.May R, Sureban SM, Hoang N, et al. Doublecortin and CaM kinase-like-1 and leucine-rich-repeat-containing G-protein-coupled receptor mark quiescent and cycling intestinal stem cells, respectively. Stem Cells. 2009;27(10):2571–2579. doi: 10.1002/stem.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sureban SM, May R, Ramalingam S, et al. Selective blockade of DCAMKL-1 results in tumor growth arrest by a Let-7a MicroRNA-dependent mechanism. Gastroenterology. 2009;137(2):649–659. e641–e642. doi: 10.1053/j.gastro.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vega KJ, May R, Sureban SM, et al. Identification of the putative intestinal stem cell marker DCAMKL-1 in Barrett’s esophagus and esophageal adenocarcinoma. J Gastroenterol Hepatol. 2011 doi: 10.1111/j.1440-1746.2011.06928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sureban SM, May R, Lightfoot SA, et al. DCAMKL-1 regulates epithelial-mesenchymal transition in human pancreatic cells through a miR-200a-dependent mechanism. Cancer Res. 2011;71(6):2328–2338. doi: 10.1158/0008-5472.CAN-10-2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Becker L, Huang Q, Mashimo H. Lgr5, an intestinal stem cell marker, is abnormally expressed in Barrett’s esophagus and esophageal adenocarcinoma. Dis Esophagus. 2010;23(2):168–174. doi: 10.1111/j.1442-2050.2009.00979.x. [DOI] [PubMed] [Google Scholar]
- 11.Fan XS, Wu HY, Yu HP, Zhou Q, Zhang YF, Huang Q. Expression of Lgr5 in human colorectal carcinogenesis and its potential correlation with beta-catenin. Int J Colorectal Dis. 2010;25(5):583–590. doi: 10.1007/s00384-010-0903-z. [DOI] [PubMed] [Google Scholar]
- 12.Takeda K, Kinoshita I, Shimizu Y, Matsuno Y, Shichinohe T, Dosaka-Akita H. Expression of LGR5, an intestinal stem cell marker, during each stage of colorectal tumorigenesis. Anticancer Res. 2011;31(1):263–270. [PubMed] [Google Scholar]
- 13.Bjerknes M, Khandanpour C, Möröy T, et al. Origin of the brush cell lineage in the mouse intestinal epithelium. Dev Biol. 2012;362(2):194–218. doi: 10.1016/j.ydbio.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sureban SM, May R, Mondalek FG, et al. Nanoparticle-based delivery of siDCAMKL-1 increases microRNA-144 and inhibits colorectal cancer tumor growth via a Notch-1 dependent mechanism. J Nanobiotechnology. 2011;9(1):40. doi: 10.1186/1477-3155-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong WM, Mandir N, Goodlad RA, et al. Histogenesis of human colorectal adenomas and hyperplastic polyps: the role of cell proliferation and crypt fission. Gut. 2002;50(2):212–217. doi: 10.1136/gut.50.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shamsuddin AK, Weiss L, Phelps PC, Trump BF. Colon epithelium. IV. Human colon carcinogenesis. Changes in human colon mucosa adjacent to and remote from carcinomas of the colon. J Natl Cancer Inst. 1981;66(2):413–419. [PubMed] [Google Scholar]
- 17.Dawson PA, Filipe MI. An ultrastructural and histochemical study of the mucous membrane adjacent to and remote from carcinoma of the colon. Cancer. 1976;37(5):2388–2398. doi: 10.1002/1097-0142(197605)37:5<2388::aid-cncr2820370531>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 18.Riddell RH, Levin B. Ultrastructure of the “transitional” mucosa adjacent to large bowel carcinoma. Cancer. 1977;40(Suppl 5):2509–2522. doi: 10.1002/1097-0142(197711)40:5+<2509::aid-cncr2820400918>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 19.Kuniyasu H, Yasui W, Shinohara H, et al. Induction of angiogenesis by hyperplastic colonic mucosa adjacent to colon cancer. Am J Pathol. 2000;157(5):1523–1535. doi: 10.1016/S0002-9440(10)64790-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Humphries A, Wright NA. Colonic crypt organization and tumorigenesis. Nat Rev Cancer. 2008;8(6):415–424. doi: 10.1038/nrc2392. [DOI] [PubMed] [Google Scholar]
- 21.Kleist B, Xu L, Li G, Kersten C. Expression of the adult intestinal stem cell marker Lgr5 in the metastatic cascade of colorectal cancer. Int J Clin Exp Pathol. 2011;4(4):327–335. [PMC free article] [PubMed] [Google Scholar]
- 22.Merlos-Suárez A, Barriga FM, Jung P, et al. The intestinal stem cell signature identifies colorectal cancer stem cells and predicts disease relapse. Cell Stem Cell. 2011;8(5):511–524. doi: 10.1016/j.stem.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 23.Li D, Peng X, Yan D, et al. Msi-1 is a predictor of survival and a novel therapeutic target in colon cancer. Ann Surg Oncol. 2011;18(7):2074–2083. doi: 10.1245/s10434-011-1567-9. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi H, Ishii H, Nishida N, et al. Significance of Lgr5(+ve) cancer stem cells in the colon and rectum. Ann Surg Oncol. 2011;18(4):1166–1174. doi: 10.1245/s10434-010-1373-9. [DOI] [PubMed] [Google Scholar]