Background: The molecular mechanisms regulating the invasive and metastatic phenotypes of ovarian cancer cells are poorly understood.
Results: BLT2 regulates invasion and metastasis of ovarian cancer cells through modulation of NOX4-ROS-STAT3-MMP2 axis.
Conclusion: BLT2 is overexpressed in ovarian cancer cells and is critical for the invasiveness and metastasis.
Significance: Our findings suggest BLT2 as a therapeutic target in ovarian cancer.
Keywords: Invasion, Leukotriene, Matrix Metalloproteinase (MMP), Metastasis, Ovarian Cancer, STAT3
Abstract
Ovarian cancer is the most lethal gynecologic malignancy in women. Despite the fact that the metastatic spread is associated with the majority of deaths from ovarian cancer, the molecular mechanisms regulating the invasive and metastatic phenotypes of ovarian cancer are poorly understood. In this study, we demonstrated that BLT2, a low affinity leukotriene B4 receptor, is highly expressed in OVCAR-3 and SKOV-3 human ovarian cancer cells, and that this receptor plays a key role in the invasiveness and metastasis of these cells through activation of STAT3 and consequent up-regulation of matrix metalloproteinase 2 (MMP2). In addition, our results suggest that activation of NAD(P)H oxidase-4 (NOX4) and subsequent reactive oxygen species (ROS) generation lie downstream of BLT2, mediating the stimulation of STAT3-MMP2 cascade in this process. For example, knockdown of BLT2 or NOX4 using each specific siRNA suppressed STAT3 stimulation and MMP2 expression. Similarly, inhibition of STAT3 suppressed the expression of MMP2, thus leading to attenuated invasiveness of these ovarian cancer cells. Finally, the metastasis of SKOV-3 cells in nude mice was markedly suppressed by pharmacological inhibition of BLT2. Together, our results implicate a BLT2-NOX4-ROS-STAT3-MMP2 cascade in the invasiveness and metastasis of ovarian cancer cells.
Introduction
Epithelial ovarian cancer is the second most common gynecologic malignancy in women, with the 5-year survival rate for all ovarian cancer patients having remained at only ∼40% for the past 30 years in the United States (1). Ovarian cancer is usually diagnosed at a late stage, at which time extensive local invasion and metastasis to the peritoneal cavity have already occurred (2). Currently available therapies, both surgical and cytotoxic, are not effective for the prevention or retardation of metastatic spread, with metastasis thus remaining a major clinical challenge in the treatment of ovarian cancer. A better understanding of the mechanisms that underlie invasion and metastasis in ovarian cancer is thus a key to the identification of new therapeutic targets for this malignancy.
An important first step in tumor metastasis is invasion of the cancer cells through the basement membrane, which requires degradation of extracellular matrix by matrix metalloproteinases (MMPs).2 MMPs constitute a family of secreted or membrane-bound enzymes that modulate the tissue microenvironment and regulate various cell behaviors related to cancer including the growth, apoptosis, migration, invasion, and metastasis of cancer cells, as well as angiogenesis (3). The expression of MMP2, also known as gelatinase A, was shown to be correlated with ovarian cancer progression, with increased levels of this enzyme in peritoneal metastases being found to be associated with an increased risk of death in women with ovarian carcinoma (4). MMP2 functions as an early response regulator in ovarian cancer cells, facilitating and accelerating initial metastasis. Inhibition of MMP2 proteolytic activity or expression was thus found to delay ovarian cancer metastasis in a xenograft mouse model (5). MMP2 expression is regulated by various transcription factors including PEA-3 and Foxp3 in ovarian cancer cells (6, 7). The transcription factor STAT3, which exists in a latent form in the cytosol, also regulates MMP2 expression through direct interaction with the MMP2 gene promoter (8), with inhibition of STAT3 also resulting in down-regulation of MMP2 expression (9). In addition, STAT3 was found to be overexpressed in ovarian cancer cell lines and ovarian cancer tissue, with constitutive activation of STAT3 signaling being implicated in invasion and metastasis in ovarian cancer (10, 11). Despite this association of STAT3 with ovarian cancer progression, however, the upstream signaling pathway responsible for activation of the STAT3-MMP2 axis in ovarian cancer cells has remained unknown.
Lipoxygenase pathway metabolites and their cognate receptors have also been implicated in ovarian cancer progression (12, 13). For example, expression of the leukotriene B4 (LTB4) receptors BLT1 and BLT2 was found to be increased in ovarian cancer tissue compared with normal ovarian tissue and to be preferentially associated with advanced stage disease (13). The mechanism by which BLT2 might contribute to ovarian cancer progression has remained unknown, however. We now show that increased BLT2 expression in OVCAR-3 and SKOV-3 human ovarian cancer cells results in activation of the STAT3-MMP2 axis and thereby promotes the invasiveness and metastasis of these cells. Furthermore, activation of NAD(P)H oxidase 4 (NOX4) and the consequent generation of reactive oxygen species (ROS) mediate activation of the STAT3-MMP2 axis by BLT2.
EXPERIMENTAL PROCEDURES
Cell Culture
The human ovarian cancer cell lines OVCAR-3 and SKOV-3 were obtained from the Korean Cell Line Bank (Seoul, Korea), and CAOV-3 human ovarian cancer cells were kindly provided by Hoi Young Lee (Konyang University, Daejeon, Korea). All of the cells were maintained under a humidified atmosphere of 5% CO2 at 37 °C in RPMI 1640 medium (Invitrogen) supplemented with 10% FBS (Hyclone, Logan, UT) and antibiotic-antimycotic solution (Invitrogen).
Reagents
Me2SO, diphenylene iodonium (DPI), and N-acetylcysteine were obtained from Sigma-Aldrich, the BLT2 antagonist LY255283 was from Cayman Chemical Company (Ann Arbor, MI), and the BLT1 antagonist U75302 was from BioMol (Plymouth Meeting, PA). AG490 was obtained from Calbiochem (La Jolla, CA), and the H2O2-sensitive fluorophore 2′,7′-dichlorofluorescein diacetate was from Molecular Probes (Eugene, OR). All other chemicals were from standard sources and were of molecular biology grade or higher.
Measurement of LTB4 and 12(S)-Hydroxyeicosatetraenoic Acid
CAOV-3, OVCAR-3, and SKOV-3 cells were seeded in 100-mm dishes and incubated in complete medium for 48 h, after which the culture supernatants were collected, freeze-dried overnight, and reconstituted with assay buffer supplied with ELISA kits for LTB4 or 12(S)-hydroxyeicosatetraenoic acid (12(S)-HETE) obtained from Assay Designs (Ann Arbor, MI). The concentrations of LTB4 and 12(S)-HETE were then measured with the kits.
Semiquantitative RT-PCR and Real Time PCR Analysis
Total RNA was extracted from cells with the use of Easy-Blue (Intron, Sungnam, Korea) and was subjected to RT by incubation at 37 °C for 50 min in a solution 20 μl containing 0.5 μg of oligo(dT)15 primer, 10 mm dithiothreitol, 0.5 mm deoxynucleoside triphosphates, and 200 units of Moloney murine leukemia virus reverse transcriptase (Beams Biotechnology, Kyunggi, Korea). The resulting cDNA was then subjected to semiquantitative RT-PCR analysis with the use of an RT-PCR PreMix kit (Intron) and with specific primers (forward and reverse, respectively) for human BLT1 (5′-TATGTCTGCGGAGTCAGCATGTACGC-3′ and 5′-CCTGTAGCCGACGCCCTATGTCCG-3′), human BLT2 (5′-AGCCTGGAGACTCTGACCGCTTTCG-3′ and 5′-GACGTAGCACCGGGTTGACGCTA-3′), human NOX4 (5′-CTCAGCGGAATCAATCAGCTGTG-3′ and 5′-AGAGGAACACGACAATCAGCCTTAG-3′), human NOX2 (5′-TGGAGTTGTCATCACGCTGTG-3′ and 5′-CTGCCCACGTACAATTCGTTC), human MMP2 (5′-GCTCAGATCCGTGGTGAGAT-3′ and 5′-GGTGCTGGCTGAGTAGATCC-3′), human MMP-9 (5′-CAACATCACCTATTGGATCC-3′ and 5′-TGGGTGTAGAGTCTCTCCCT-3′), and GAPDH (5′-CTGCACCACCAACTGCTTAGC-3′ and 5′-CTTCACCACCTTCTTGATGTC-3′; internal control). The PCR products were purified by 1.5% agarose gel electrophoresis and visualized by staining with ethidium bromide. For real time PCR analysis, cDNA derived from total RNA was subjected to PCR with the use of Moloney murine leukemia virus reverse transcriptase (Beams Biotechnology), and the data were analyzed with LightCycler software 3.3 (Roche Applied Science). Primers (forward and reverse, respectively) for real time PCR analysis were 5′-CCTGAAAAGGATGCAGAAGC-3′ and 5′-AAAAAGGGAGCAGTGAGCAA-3′ for human BLT1, 5′-CTTCTCATCGGGCATCACAG-3′ and 5′-ATCCTTCTGGGCCTACAGGT-3′ for human BLT2, and 5′-CTCAGCGGAATCAATCAGCTGTG-3′ and 5′-AGAGGAACACGACAATCAGCCTTAG-3′ for human NOX4. The amounts of BLT1 and BLT2 mRNAs were quantitative data normalized by GAPDH mRNA.
Flow Cytometric Analysis of BLT2 Expression
The cell surface expression of BLT2 in human ovarian cancer cell lines was evaluated by flow cytometry using a FACSCalibur instrument (BD Biosciences, San Jose, CA) analyzer. To evaluate the expression of cell surface BLT2, cells were fixed with 4% paraformaldehyde before incubation with rabbit polyclonal anti-BLT2 antibody (1:200 dilution; MBL Inc., Des Plaines, IL; catalogue number LS-A2099) for 2 h, followed by FITC-conjugated goat antibodies to anti-rabbit IgG (1:200 dilution; Sigma-Aldrich). Rabbit IgG was used as an isotype control. The data are expressed as the mean fluorescence intensities.
RNA Interference
BLT2-specific (5′-CCACGCAGUCAACCUUCUG-3′) (14), MMP2-specific (5′-AAACAGGUUGCAGCUCUCC-3′), and control (scrambled) siRNAs were obtained from Bioneer (Daejeon, Korea). The mammalian expression vector pSUPER (OligoEngine, Seattle, WA), pSUPER encoding NOX4 siRNA (pSUPER-siNOX4), or NOX2 siRNA (pSUPER-siNOX2) were kindly provided by Yoon-Soo Bae (Ewha Woman's University, Seoul, Korea); the NOX4 and NOX2 target sequences were described previously (15, 16). The siRNAs and siRNA vector were introduced into cells by transfection for the indicated times in Opti-MEM (Invitrogen) with the use of Oligofectamine and Lipofectamine reagents (Invitrogen), respectively.
Preparation of Cell Extracts and Immunoblot Analysis
The cells were washed with ice-cold PBS, scraped into a lysis buffer (20 mm Tris-HCl, pH 7.5, 150 mm NaCl, 0.5% Nonidet P-40, 5 mm EDTA, 1% Triton X-100, and protease inhibitors (100 mm phenylmethylsulfonyl fluoride, 1 mm sodium orthovanadate, 2 μg/ml leupeptin, and 2 μg/ml aprotinin)) at 4 °C, and heated at 95 °C for 5 min. The samples were then subjected to SDS-PAGE, and the separated proteins were transferred electrophoretically to a PVDF membrane for 90 min at 100 V. The membrane was exposed for 1 h to TBS containing 0.05% Tween 20 and 5% dried nonfat milk before incubation overnight at 4 °C with antibodies to STAT3, to phosphorylated STAT3 (Tyr705 or Ser727), to phosphorylated STAT1 (Tyr701), to STAT1, to 5-lipoxygenase (5-LO), to 12-lipoxygenase (12-LO), to FLAP (5-lipoxygenase-activating protein), or to α-tubulin (loading control), all of which were obtained from Cell Signaling Technology (Danvers, MA) and were used at a dilution of 1:2000 in TBS containing 0.05% Tween 20, with the exception of those to α-tubulin (1:4000 dilution). The membrane was then incubated for 2 h at room temperature with horseradish peroxidase-conjugated secondary antibodies before detection of immune complexes with the use of an enhanced chemiluminescence kit (Amersham Biosciences).
Luciferase Reporter Assay for MMP2 Promoter Activity
Cells in 6-well plates were transfected for 24 h with BLT2 siRNA or pSUPER-siNOX4, as well as with 1.5 μg of a luciferase reporter construct containing the human MMP2 promoter (kindly provided by Etty N. Benveniste, University of Alabama, Birmingham) (17) and 0.5 μg of the β-galactosidase expression plasmid pMDV-LacZ with the use of the Lipofectamine reagent (Invitrogen). The cells were then incubated in serum-free medium for 24 h before measurement of luciferase activity with the use of a Junior luminometer (Berthold, Bad Wilbad, Germany) as previously described (18). The activity of β-galactosidase was also measured and was used to normalize luciferase activity.
Assay of MMP2 Activity by Gelatin Zymography
The activity of MMP2 in culture supernatants was assessed by gelatin zymography. In brief, the cells were incubated in serum-free medium for 48 h, after which the conditioned medium was collected, mixed with Laemmli buffer without reducing agent, and subjected to SDS-PAGE on an 8% gel containing 0.2% gelatin. For detection of gelatinolytic activity, the gel was incubated for 24 h at 37 °C in a solution containing 50 mm Tris-HCl, pH 7.6, 150 mm NaCl, 5 mm CaCl2, and Brij-35 (200 μg/ml) and was then stained with Coomassie Brilliant Blue.
Matrigel Invasion Assay
Cell invasiveness was assayed in duplicate with the use of BioCoat Matrigel Invasion Chambers (BD Biosciences) as described previously (19). In brief, the cells (4 × 104) were seeded on the Matrigel inserts in RPMI 1640 supplemented with 0.5% FBS. Medium supplemented with 10% FBS was added to the lower chamber, and the cells were then incubated at 37 °C for 48 h. The cells on the filters were fixed in methanol and stained with hematoxylin/eosin, and the contents of the upper surface were then removed before the number of invading cells on the lower chamber surface was counted in 10 random high power (×20) fields per filter with a light microscope.
Measurement of ROS
The cells plated in 60-mm dishes were subjected to measurement of intracellular H2O2. The actively growing cells were incubated for 10 min in the dark at 37 °C with 10 μm 2′,7′-dichlorofluorescein diacetate, after which dichlorofluorescein fluorescence was measured by flow cytometry with a FACSCalibur instrument (BD Biosciences).
In Vivo Metastasis Assay
The animals were treated according to guidelines approved by the institutional animal care and use committee of Korea University. Five-week-old female nude (BALB/c) mice (Charles River, Wilmington, MA) were injected intraperitoneally with SKOV-3 cells for examination of their peritoneal metastatic potential (20). In brief, the cells (5 × 106) were treated with 10 μm LY255283 or Me2SO vehicle for 3 h, isolated by exposure to 0.025% trypsin and 0.1% EDTA in Hanks' balanced salt solution, and suspended in Hanks' balanced salt solution for injection in a volume of 0.1 ml with a 30-gauge needle. The mice (n = 8/group) were injected intraperitoneal with LY255283 (2.5 mg/kg) or Me2SO twice a week for 21 days beginning 14 days after the injection of cells. The number and extent of overt metastases were then quantified 35 days after cell injection.
Statistical Analysis
The data are presented as the means ± S.D. and were analyzed by one-way analysis of variance or Student's t test for comparisons among multiple groups or between two groups, respectively. A p value of < 0.05 was considered statistically significant.
RESULTS
Up-regulation of BLT2 Expression in Aggressive Ovarian Cancer Cells
To elucidate the role of BLT2 in ovarian cancer progression, we first examined the expression of BLT1 and BLT2 in three ovarian cancer cell lines, including invasive CAOV-3 cells and the more invasive, aggressive OVCAR-3 and SKOV-3 cells. Semiquantitative RT-PCR and real time PCR analysis revealed that the abundance of BLT2 mRNA was markedly greater in OVCAR-3 and SKOV-3 cells than in CAOV-3 cells, whereas the amount of BLT1 mRNA was greater only in OVCAR-3 cells (Fig. 1A). Consistent with these results, the expression of BLT2 protein on the cell surface as determined by flow cytometry was significantly higher for OVCAR-3 and SKOV-3 cells than for CAOV-3 cells (Fig. 1B). We next determined the levels of the BLT2 ligands LTB4 and 12(S)-HETE produced by the ovarian cancer cells. The amount of LTB4 was substantially greater in the culture supernatants of OVCAR-3 and SKOV-3 cells than in those of CAOV-3 cells, whereas the amount of 12(S)-HETE was markedly increased in the culture supernatants of SKOV-3 cells and slightly increased in those of OVCAR-3 cells (Fig. 1C). Consistent with these results, immunoblot analysis revealed that the amounts of 5-LO and 12-LO, key enzymes in the synthesis of LTB4 and 12(S)-HETE, respectively, were greater in OVCAR-3 and SKOV-3 cells and SKOV-3 cells, respectively (Fig. 1D). Also, the amount of FLAP was slightly increased in SKOV-3 cells (Fig. 1D). As expected, a Matrigel invasion assay showed that the invasiveness of both OVCAR-3 and SKOV-3 cells was much higher than that of CAOV-3 cells (Fig. 1E). Together, these results suggested that the increased expression of BLT2 in OVCAR-3 and SKOV-3 cells might contribute to the highly invasive phenotype of these cells.
FIGURE 1.
Up-regulation of BLT2 expression in aggressive ovarian cancer cells. A, the abundances of BLT1 and BLT2 mRNAs in CAOV-3, OVCAR-3, and SKOV-3 ovarian cancer cells were determined by semiquantitative RT-PCR (left panel) and real time PCR analysis (middle and right panels). B, the expression of BLT2 on cell surface was determined by FACS analysis. MFI, mean fluorescence intensity. †, p < 0.01; ‡, p < 0.001. C, CAOV-3 (7 × 105), OVCAR-3 (7 × 105), and SKOV-3 (5 × 105) cells were cultured in 100-mm dishes for 48 h, after which the amounts of LTB4 and 12(S)-HETE in the culture supernatants were measured by ELISAs (*, p < 0.05). D, immunoblot analysis of 5-LO, 12-LO, and FLAP in ovarian cancer cell lines. E, cells (4 × 104) were seeded on Matrigel inserts of an invasion chamber in medium containing 0.5% FBS, and medium containing 10% FBS was placed in the lower compartment of the chamber. After incubation for 48 h, the number of cells that had invaded through the Matrigel matrix was determined. All of the quantitative data are the means ± S.D. from three independent experiments. †, p < 0.01.
BLT2 Expression Is Required for Invasiveness of Ovarian Cancer Cells
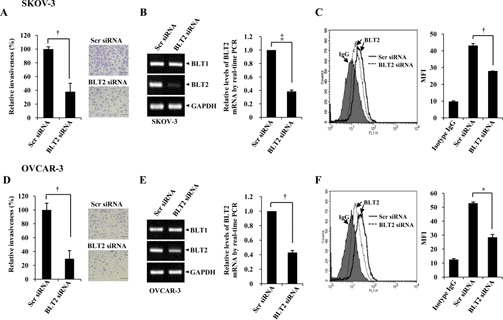
To investigate the potential role of BLT2 in the invasiveness of SKOV-3 and OVCAR-3 cells, we examined the effect of RNAi-mediated knockdown of BLT2 on the invasive phenotype of these cells. Depletion of BLT2 resulted in marked inhibition of the invasiveness of both SKOV-3 and OVCAR-3 cells (Fig. 2, A and D). Under these experimental conditions, the knockdown effects of BLT2 siRNA were clearly observed at the mRNA levels (Fig. 2, B and E) and the protein levels (Fig. 2, C and F). Similarly, treatment with the BLT2 antagonist LY255283, but not that with the BLT1 antagonist U75302, resulted in significant inhibition of the invasiveness of SKOV-3 and OVCAR-3 cells (supplemental Fig. S1, A and B). Also, knockdown of BLT2 did not affect the survival of these cells (supplemental Fig. S1C), suggesting that its effect on invasiveness is specific. Together, these results suggested that BLT2 is necessary for the highly invasive phenotype of SKOV-3 and OVCAR-3 cells.
FIGURE 2.
BLT2 is required for the invasiveness of ovarian cancer cells. SKOV-3 and OVCAR-3 cells were transfected with 50 nm BLT2 or control (scrambled, Scr) siRNAs for 24 h and then assayed for invasiveness (A and D). Stained cells that had invaded the lower chamber surface of the filter in a representative experiment were photographed with a CKX41 microscope equipped with a DP71 digital camera (Olympus). Scale bars, 100 μm. †, p < 0.01. The cells were transfected with 50 nm BLT2 or control (scrambled, Scr) siRNAs for 48 h and then either subjected to semiquantitative RT-PCR (BLT1 and BLT2) and real time PCR (BLT2) analysis (B and E) and FACS analysis for BLT2 protein levels (C and F). MFI, mean fluorescence intensity. *, p < 0.05; †, p < 0.01; ‡, p < 0.001. Quantitative data are the means ± S.D. from three independent experiments.
NOX4 Functions Downstream of BLT2 in Regulation of Ovarian Cancer Cell Invasiveness
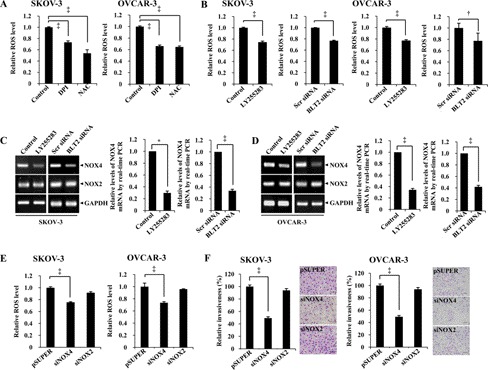
Increased levels of ROS have been shown to contribute to aspects of cancer progression, including cancer cell invasiveness and metastasis (21, 22). Indeed, we found that ROS levels were significantly higher in SKOV-3 and OVCAR-3 cells than in CAOV-3 cells (data not shown). These increased ROS levels in SKOV-3 and OVCAR-3 cells were significantly reduced by treatment with the ROS scavenger N-acetylcysteine or with DPI, an inhibitor of flavoenzymes such as NOX (Fig. 3A). The invasiveness of SKOV-3 and OVCAR-3 cells was also significantly inhibited by treatment with DPI or N-acetylcysteine (supplemental Fig. S2A), suggesting that NOX contributes to the generation of ROS and thereby promotes the invasiveness of these cells. We recently showed that NOX mediates BLT2-induced ROS generation and thereby promotes the tumorigenicity of various cancer cell types (19, 23–25). We therefore examined whether the increased ROS levels in SKOV-3 and OVCAR-3 cells might be due to NOX up-regulation. We found that NOX2 and NOX4 mRNAs are the dominant NOX mRNAs in SKOV-3 and OVCAR-3 cells (data not shown), and the amount of NOX4 mRNA, but not that of NOX2 mRNA (Fig. 3, C and D), and ROS levels (Fig. 3B) were significantly reduced as a result of LY255283 treatment or RNAi-mediated knockdown of BLT2 in these cells, suggesting that NOX4 likely functions downstream of BLT2 in ovarian cancer cells. Consistent with the suggested role for NOX4 in ROS generation, ROS levels were significantly reduced as a result of RNAi-mediated depletion of NOX4 mRNA, but not NOX2 mRNA, in SKOV-3 and OVCAR-3 cells (Fig. 3E and supplemental Fig. S2B). The invasiveness of SKOV-3 and OVCAR-3 cells was also significantly inhibited by depletion of NOX4, but not NOX2 (Fig. 3F). Collectively, these data thus implicated a BLT2-NOX4-ROS pathway in regulation of the invasiveness of SKOV-3 and OVCAR-3 ovarian cancer cells.
FIGURE 3.
NOX4 functions downstream of BLT2 in regulation of the invasiveness of ovarian cancer cells. A, SKOV-3 and OVCAR-3 cells were incubated for 30 min in the absence or presence of DPI (5 μm) or N-acetylcysteine (NAC; 10 mm) before exposure to 2′,7′-dichlorofluorescein diacetate (10 μm) for 10 min and measurement of intracellular H2O2 by flow cytometry. ‡, p < 0.001. B–D, cells were treated with LY255283 (10 μm) or transfected with 50 nm BLT2 or control (scrambled) siRNAs for 36 h and then either assayed for ROS levels (B) or subjected to semiquantitative RT-PCR or real time PCR analysis of NOX4 and NOX2 mRNA (C and D). *, p < 0.05; †, p < 0.01; ‡, p < 0.001. E, cells were transfected with 1 μg of pSUPER (control), pSUPER-siNOX4, or pSUPER-siNOX2 for 36 h and then assayed for ROS levels. ‡, p < 0.001. F, cells were transfected with pSUPER, pSUPER-siNOX4, or pSUPER-siNOX2 for 24 h and then assayed for invasiveness. Scale bars, 100 μm. All of the quantitative data are the means ± S.D. from three independent experiments. ‡, p < 0.001.
The BLT2-NOX4-ROS Pathway Up-regulates MMP2 in Ovarian Cancer Cells
MMP2 has been implicated in invasion and metastasis in ovarian cancer cells (4, 5). We therefore next examined whether MMP2 functions downstream of the BLT2-NOX4-ROS pathway in regulation of the invasiveness of ovarian cancer cells. Inhibition of BLT2 by LY255283 treatment or BLT2 depletion by RNAi knockdown resulted in down-regulation of the amount of MMP2 mRNA (as shown by RT-PCR; Fig. 4A), the activity of MMP2 (as shown by zymography; Fig. 4A), and the promoter activity of the MMP2 gene (Fig. 4B) in both SKOV-3 and OVCAR-3 cells. However, the amount of MMP9 mRNA was not affected by these BLT2 inhibitions (Fig. 4A), suggesting the specificity of MMP2 as a downstream component of BLT2. Depletion of ROS by knockdown of NOX4 had similar effects (Fig. 4, C and D). In addition, the invasiveness of SKOV-3 and OVCAR-3 cells was greatly inhibited by RNAi-mediated depletion of MMP2 (Fig. 4E). To confirm that BLT2 contributes to the up-regulation of MMP2, we transiently transfected CAOV-3 cells, in which endogenous BLT2 is expressed at a relatively low level (Fig. 1A), with an expression vector for BLT2. Such overexpression of BLT2 resulted in a marked increase in the abundance of MMP2 mRNA as well as in that of NOX4 mRNA, and also the invasiveness was increased in these cells (supplemental Fig. S3, A and B). Together, these results suggested that the BLT2-NOX4-ROS signaling pathway up-regulates MMP2 in SKOV-3 and OVCAR-3 cells and thereby promotes cell invasiveness.
FIGURE 4.
A BLT2-NOX4-ROS pathway up-regulates MMP2 in ovarian cancer cells. A and C, SKOV-3 and OVCAR-3 cells were treated with LY255283 (10 μm) or transfected for 48 h with 50 nm BLT2 or control (scrambled, Scr) siRNAs (A) or with 1 μg of pSUPER (control) or pSUPER-siNOX4 (C) and then either subjected to semiquantitative RT-PCR analysis of MMP2, MMP9, or NOX4 mRNAs or incubated in serum-free medium for 48 h, after which the culture supernatants were assayed for MMP2 activity by zymography. B and D, cells were transfected for 24 h as in A and C, respectively, but in the additional presence of an MMP2-luciferase reporter plasmid. The cells were then deprived of serum for 24 h before determination of normalized luciferase activity. *, p < 0.05; †, p < 0.01; ‡, p < 0.001). E, cells were transfected with 50 nm MMP2 or control (scrambled, Scr) siRNAs for 24 h and then assayed for invasiveness. All of the quantitative data are the means ± S.D. from three independent experiments. †, p < 0.01; ‡, p < 0.001.
STAT3 Functions Downstream of BLT2-NOX4-ROS Signaling to Mediate MMP2 Up-regulation
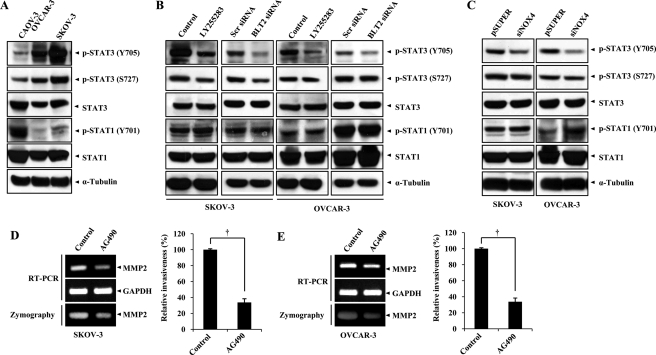
STAT3 plays an important tumor-promoting role in a variety of cancer cells including ovarian cancer cells (26, 27). STAT3 is regulated by cellular redox status and controls the transcription of various genes related to cell invasiveness including MMP2 (28–30). We therefore investigated whether BLT2 signaling might influence the activity of STAT3. Immunoblot analysis revealed that the amounts of STAT3 phosphorylated at Tyr705 or Ser727, which correspond to the activated forms of the protein, were markedly increased in SKOV-3 and OVCAR-3 cells compared with CAOV-3 cells (Fig. 5A). BLT2 inhibition by LY255283 treatment or depletion of BLT2 by RNAi knockdown clearly diminished the amount of Tyr705-phosphorylated STAT3 but did not affect those of Ser727-phosphorylated STAT3 or Tyr701-phosphorylated STAT1 in SKOV-3 and OVCAR-3 cells (Fig. 5B), implicating BLT2 in the selective regulation of STAT3 phosphorylation at Tyr705. Moreover, transient transfection of CAOV-3 cells with an expression plasmid for BLT2 resulted in a marked increase in the amount of Tyr705-phosphorylated STAT3 without an effect on that of the Ser727-phosphorylated form (supplemental Fig. S3C). These data thus indicated that STAT3 (Tyr705) is a target of BLT2 signaling. To investigate whether ROS generated by NOX4 mediate the effect of BLT2 signaling on STAT3 phosphorylation, we examined the effect of NOX4 knockdown. The amount of STAT3 phosphorylation at Tyr705 in SKOV-3 or OVCAR-3 cells was selectively diminished by NOX4 knockdown, whereas the amounts of phosphorylation of STAT3 at Ser727 or STAT1 at Tyr701 were not affected by NOX4 knockdown (Fig. 5C). Finally, we examined whether STAT3 inhibition affects the invasiveness of SKOV-3 and OVCAR-3 cells. The invasiveness of both cell lines was reduced after treatment with AG490, an inhibitor of JAK2 (Janus kinase 2), an upstream kinase of STAT3 (Fig. 5, D and E, right panels). In addition, AG490 induced marked down-regulation of the amounts of MMP2 mRNA and activity in both cell lines (Fig. 5, D and E, left panels). Collectively, these results suggested that STAT3 functions downstream of the BLT2-NOX4-ROS pathway and mediates up-regulation of MMP2 in ovarian cancer cells.
FIGURE 5.
STAT3 functions downstream of BLT2-NOX4-ROS signaling to mediate up-regulation of MMP2. A, immunoblot analysis of Tyr705-phosphorylated (Y705), Ser727-phosphorylated (S727) forms of STAT3 (p-STAT3) or Tyr701-phosphorylated (Y701) forms of STAT1 (p-STAT1) in CAOV-3, OVCAR-3, and SKOV-3 cells. B, cells were treated with LY255283 (10 μm) for 30 min or transfected with 50 nm BLT2 or control (scrambled, Scr) siRNAs for 48 h and then subjected to immunoblot analysis as in A. C, cells were transfected with pSUPER (control) or pSUPER-siNOX4 for 48 h and then subjected to immunoblot analysis as in A. All of the immunoblot data are representative of three independent experiments. D and E, SKOV-3 (D) and OVCAR-3 (E) cells were incubated in the absence or presence of AG490 (50 μm) for 24 h and were then either subjected to semiquantitative RT-PCR analysis of MMP2 mRNA, incubated for 48 h in serum-free medium in the continued absence or presence of AG490 for assay of MMP2 activity in culture supernatants by zymography, or assayed for invasiveness in the continued absence or presence of AG490, as indicated. The quantitative data are the means ± S.D. from three independent experiments. †, p < 0.01.
In Vivo Metastatic Potential of SKOV-3 Cells Is Suppressed by BLT2 Inhibition
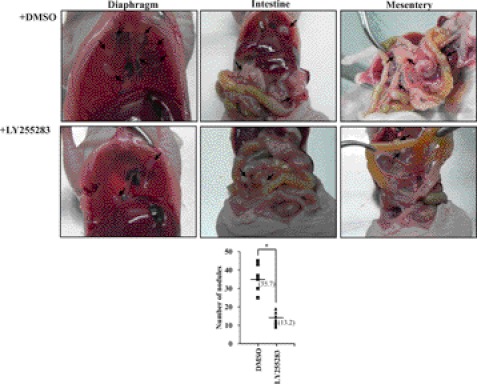
To examine the possible role of BLT2 in metastasis of ovarian cancer in vivo, we determined the effect of BLT2 inhibition on this process. Intraperitoneal injection of ovarian cancer cells into immune-compromised mice has been studied as a model for peritoneal metastasis of human ovarian cancer (20, 31). We therefore injected SKOV-3 cells into athymic mice, which were then treated twice a week with LY255283 (2.5 mg/kg) or Me2SO vehicle beginning 2 weeks later, with the treatment regimen being based on our previous results on the dose-response relation for the efficacy of this drug in vivo (19). At 35 days after cell injection, the mice were killed and examined for intraperitoneal dissemination. Whereas control mice manifested several disseminated and enlarged metastatic nodules throughout the peritoneal cavity, including the diaphragm, intestine, and mesentery, the number of metastatic nodules was significantly reduced (by ∼60%) in mice treated with LY255283 (Fig. 6). These results thus suggested that BLT2 signaling contributes to the metastasis of SKOV-3 ovarian cancer cells.
FIGURE 6.
Suppression of the in vivo metastatic potential of SKOV-3 cells by BLT2 inhibition. Female nude mice (n = 8/group) were intraperitoneally injected with SKOV-3 cells (5 × 106) that had been treated with 10 μm LY255283 or Me2SO vehicle (DMSO) for 3 h. After 14 days, the mice were injected intraperitoneally with LY255283 (2.5 mg/kg) or Me2SO vehicle, respectively, twice a week for 21 days. The development of metastatic nodules (arrows) in the peritoneal cavity was then examined, and their number was determined. The number of nodules in each mouse is shown in the bottom panel, with the median values indicated by the horizontal lines. *, p < 0.05.
DISCUSSION
Ovarian cancer is the second most common gynecologic malignancy but the most common cause of gynecologic cancer-related death (1). Approximately 70% of women with ovarian cancer are diagnosed at an advanced stage with metastases present outside of the ovaries. Despite advances in cytotoxic therapy, only 30% of patients with advanced stage ovarian cancer survive 5 years after initial diagnosis (32). There is thus an urgent need for more effective therapeutic strategies and a better understanding of the molecular mechanisms underlying ovarian cancer invasion and metastasis. Our present results now suggest that a BLT2-NOX4-ROS-STAT3-MMP2 cascade plays a central role in the invasiveness and metastasis of ovarian cancer cells.
Consistent with our observations, recent studies have suggested that BLT2 expression is increased in ovarian cancer (13, 33, 34). Thus, although the role of BLT2 in ovarian cancer progression has not previously been elucidated, an increased level of BLT2 expression was thus found to be associated with an advanced stage of the disease (13). Moreover, we previously showed that BLT2 expression was increased in various cancer specimens including those of ovarian cancer tissue by in situ hybridization (33–35). We have now shown that a BLT2 signaling pathway up-regulates the invasiveness and metastasis of ovarian cancer cells. We found that NOX4 functions downstream of BLT2 in this pathway, that NOX4 expression was increased in SKOV-3 and OVCAR-3 ovarian cancer cells relative to that in CAOV-3 cells (data not shown), and that inhibition of NOX4-dependent.
ROS generation attenuated invasiveness in these cells (Fig. 3). Consistent with our findings, ovarian cancer cells have thus previously been found to have increased levels of NOX4 expression, and the increased generation of ROS by NOX4 plays an important role in tumor angiogenesis (36). Similarly, we recently showed that a BLT2-NOX1/4-ROS pathway contributes to the invasiveness and metastasis of bladder cancer cells (19). In addition, NOX4-generated ROS are required for the proliferation and survival of melanoma and glioma cells (37, 38) and contribute to cell survival by regulating the AKT-ASK1 signaling pathway in pancreatic cancer cells (39). These various observations thus support the notion that maintenance of a high level of intracellular ROS by NOX family proteins is important for the aggressive phenotype of cancer cells, including ovarian cancer cells.
Our results also show that MMP2 is up-regulated by the BLT2-NOX4-ROS pathway in SKOV-3 and OVCAR-3 cells and contributes to cell invasiveness (Fig. 4). MMPs represent the predominant family of proteinases associated with tumorigenesis, being implicated in the degradation of basement membrane and extracellular matrix (40). In particular, MMP2 overexpression in peritoneal metastases was found to be associated with poor prognosis in women with ovarian cancer, and MMP2 promotes ovarian cancer cell invasion and metastasis (4, 5). Knockdown of MMP2 expression in vivo also reduced both the number of metastases and tumor weight in a xenograft mouse model of ovarian cancer (5). We recently showed that MMP9 is up-regulated by a BLT2-ROS-NF-κB signaling pathway in bladder cancer cells and mediates the invasiveness and metastasis of bladder cancer cell lines (19). We did not detect up-regulation of MMP9 by BLT2-ROS signaling in ovarian cancer cells, and we found that inhibition of the transcription factor NF-κB did not interfere with the up-regulation of MMP2 induced by BLT2-ROS signaling in these cells (data not shown), suggesting that the mediators of BLT2 signaling to MMPs may be dependent on cell type.
Our results also implicate STAT3 as a key downstream component of the BLT2-ROS pathway that up-regulates MMP2 in SKOV-3 and OVCAR-3 ovarian cancer cells (Fig. 5). STAT3 has previously been shown to be of potential prognostic relevance in ovarian cancer (11, 28, 29, 41). In particular, activation of STAT3 and its translocation to the nucleus are frequent events in ovarian carcinoma (41), and the levels of phosphorylated STAT3 were found to correlate with disease stage, differentiation of carcinoma, and the presence of lymph node metastasis (11). STAT3 is activated by numerous cytokines, growth factors, and oncogenic proteins, including Src and Ras (42), and aberrant activity of STAT3 contributes to invasive growth in cancer cells (43, 44). Also, consistent with our present observations, the activation of STAT3 has previously been shown to be inhibited by antioxidants or inhibitors of NOX enzymes, suggesting that STAT3 activation is dependent on ROS (45, 46). Furthermore, activated STAT3 was shown to regulate cancer invasion through control of MMP gene transcription (42). In particular, increased STAT3 activity was shown to contribute to ovarian cancer invasion through modulation of MMP2 activity (47). The STAT3-MMP2 axis may thus play a key role in ovarian cancer progression, and our results now suggest that BLT2-NOX4-ROS signaling regulates the STAT3-MMP2 pathway and thereby promotes the invasiveness and metastasis of aggressive ovarian cancer cells. Consistent with this notion, we found that the level of phospho-STAT3 was reduced by RNAi-mediated knockdown of BLT2 or NOX4 in SKOV-3 and OVCAR-3 cells and that treatment with AG490, an inhibitor of JAK2, an upstream kinase of STAT3, attenuated both the expression of MMP2 and cell invasiveness (Fig. 5). From these results, we predict that the generation of ROS through BLT2-NOX4 likely results in JAK activation and consequent activation of STAT3. Recently, it was reported that NOX4-derived ROS mediated inhibition of protein-tyrosine phosphatases, resulting in sustained activation of JAK2-STAT1/3 (48). Similarly, treatment with DPI, an inhibitor of flavoprotein-dependent oxidases such as NOX, was previously shown to suppress activation of JAK2-STAT3 (28), and inhibition of the NOX subunit p47phox was found to abolish angiotensin II-induced JAK-STAT3 activation (49), implicating ROS in JAK-STAT3 activation. Thus, one interesting possibility is that BLT2-NOX4-derived ROS sustain the phosphorylated (activated) state of JAK2-STAT3 by inactivating protein-tyrosine phosphatases. Further studies are needed to investigate the exact mechanism among BLT2-NOX4-ROS and STAT3 signaling pathway and their relevance for ovarian cancer progression.
In summary, we have shown that a BLT2-NOX4-ROS-STAT3 cascade mediates up-regulation of MMP2 in SKOV-3 and OVCAR-3 cells and thereby contributes to the invasiveness and metastasis of these aggressive ovarian cancer cells. The elucidation of this mechanism provides important insight into ovarian cancer progression as well as a basis for the development of new therapeutic agents for this malignance.
Supplementary Material
This work was supported by General Researcher Support Project Grant 2011-0004241 and the Bio & Medical Technology Development Program of the National Research Foundation funded by Korean government Ministry of Education and Science Technology Grant 2011-0027753. This work was also supported by Grant A101032 from the Korea Healthcare Technology R & D Project, Ministry of Health & Welfare.

This article contains supplemental text, references, and Figs. S1–S3.
- MMP
- matrix metalloproteinase
- BLT
- leukotriene B4 receptor
- LO
- lipoxygenase
- LT
- leukotriene
- HETE
- hydroxyeicosatetraenoic acid
- ROS
- reactive oxygen species
- NOX
- NAD(P)H oxidase
- DPI
- diphenylene iodonium
- JAK
- Janus kinase.
REFERENCES
- 1. Jemal A., Siegel R., Xu J., Ward E. (2010) Cancer statistics, 2010. CA Cancer J. Clin. 60, 277–300 [DOI] [PubMed] [Google Scholar]
- 2. Holschneider C. H., Berek J. S. (2000) Ovarian cancer. Epidemiology, biology, and prognostic factors. Semin. Surg. Oncol. 19, 3–10 [DOI] [PubMed] [Google Scholar]
- 3. Egeblad M., Werb Z. (2002) New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer 2, 161–174 [DOI] [PubMed] [Google Scholar]
- 4. Périgny M., Bairati I., Harvey I., Beauchemin M., Harel F., Plante M., Têtu B. (2008) Role of immunohistochemical overexpression of matrix metalloproteinases MMP-2 and MMP-11 in the prognosis of death by ovarian cancer. Am. J. Clin. Pathol. 129, 226–231 [DOI] [PubMed] [Google Scholar]
- 5. Kenny H. A., Lengyel E. (2009) MMP-2 functions as an early response protein in ovarian cancer metastasis. Cell Cycle 8, 683–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Davidson B., Goldberg I., Gotlieb W. H., Kopolovic J., Ben-Baruch G., Reich R. (2003) PEA3 is the second Ets family transcription factor involved in tumor progression in ovarian carcinoma. Clin. Cancer Res. 9, 1412–1419 [PubMed] [Google Scholar]
- 7. Zhang H. Y., Sun H. (2010) Up-regulation of Foxp3 inhibits cell proliferation, migration and invasion in epithelial ovarian cancer. Cancer Lett. 287, 91–97 [DOI] [PubMed] [Google Scholar]
- 8. Xie T. X., Wei D., Liu M., Gao A. C., Ali-Osman F., Sawaya R., Huang S. (2004) Stat3 activation regulates the expression of matrix metalloproteinase-2 and tumor invasion and metastasis. Oncogene 23, 3550–3560 [DOI] [PubMed] [Google Scholar]
- 9. Huang C., Cao J., Huang K. J., Zhang F., Jiang T., Zhu L., Qiu Z. J. (2006) Inhibition of STAT3 activity with AG490 decreases the invasion of human pancreatic cancer cells in vitro. Cancer Sci. 97, 1417–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang M., Page C., Reynolds R. K., Lin J. (2000) Constitutive activation of stat 3 oncogene product in human ovarian carcinoma cells. Gynecol. Oncol. 79, 67–73 [DOI] [PubMed] [Google Scholar]
- 11. Min H., Wei-hong Z. (2009) Constitutive activation of signal transducer and activator of transcription 3 in epithelial ovarian carcinoma. J. Obstet. Gynaecol. Res. 35, 918–925 [DOI] [PubMed] [Google Scholar]
- 12. Guo A. M., Liu X., Al-Wahab Z., Maddippati K. R., Ali-Fehmi R., Scicli A. G., Munkarah A. R. (2011) Role of 12-lipoxygenase in regulation of ovarian cancer cell proliferation and survival. Cancer Chemother. Pharmacol. 68, 1273–1283 [DOI] [PubMed] [Google Scholar]
- 13. Rocconi R. P., Kirby T. O., Seitz R. S., Beck R., Straughn J. M., Jr., Alvarez R. D., Huh W. K. (2008) Lipoxygenase pathway receptor expression in ovarian cancer. Reprod. Sci. 15, 321–326 [DOI] [PubMed] [Google Scholar]
- 14. Hennig R., Osman T., Esposito I., Giese N., Rao S. M., Ding X. Z., Tong W. G., Büchler M. W., Yokomizo T., Friess H., Adrian T. E. (2008) BLT2 is expressed in PanINs, IPMNs, pancreatic cancer and stimulates tumour cell proliferation. Br. J. Cancer 99, 1064–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Park H. S., Lee S. H., Park D., Lee J. S., Ryu S. H., Lee W. J., Rhee S. G., Bae Y. S. (2004) Sequential activation of phosphatidylinositol 3-kinase, β Pix, Rac1, and Nox1 in growth factor-induced production of H2O2. Mol. Cell. Biol. 24, 4384–4394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bae Y. S., Lee J. H., Choi S. H., Kim S., Almazan F., Witztum J. L., Miller Y. I. (2009) Macrophages generate reactive oxygen species in response to minimally oxidized low-density lipoprotein. Toll-like receptor 4- and spleen tyrosine kinase-dependent activation of NADPH oxidase 2. Circ. Res. 104, 210–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Qin H., Sun Y., Benveniste E. N. (1999) The transcription factors Sp1, Sp3, and AP-2 are required for constitutive matrix metalloproteinase-2 gene expression in astroglioma cells. J. Biol. Chem. 274, 29130–29137 [DOI] [PubMed] [Google Scholar]
- 18. Woo C. H., Lim J. H., Kim J. H. (2005) VCAM-1 upregulation via PKCδ-p38 kinase-linked cascade mediates the TNF-α-induced leukocyte adhesion and emigration in the lung airway epithelium. Am. J. Physiol. Lung Cell Mol. Physiol. 288, L307–L316 [DOI] [PubMed] [Google Scholar]
- 19. Kim E. Y., Seo J. M., Kim C., Lee J. E., Lee K. M., Kim J. H. (2010) BLT2 promotes the invasion and metastasis of aggressive bladder cancer cells through a reactive oxygen species-linked pathway. Free Radic. Biol. Med. 49, 1072–1081 [DOI] [PubMed] [Google Scholar]
- 20. Ponnusamy M. P., Lakshmanan I., Jain M., Das S., Chakraborty S., Dey P., Batra S. K. (2010) MUC4 mucin-induced epithelial to mesenchymal transition. A novel mechanism for metastasis of human ovarian cancer cells. Oncogene 29, 5741–5754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liou G. Y., Storz P. (2010) Reactive oxygen species in cancer. Free Radic. Res. 44, 479–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wu W. S. (2006) The signaling mechanism of ROS in tumor progression. Cancer Metastasis Rev. 25, 695–705 [DOI] [PubMed] [Google Scholar]
- 23. Choi J. A., Lee J. W., Kim H., Kim E. Y., Seo J. M., Ko J., Kim J. H. (2010) Pro-survival of estrogen receptor-negative breast cancer cells is regulated by a BLT2-reactive oxygen species-linked signaling pathway. Carcinogenesis 31, 543–551 [DOI] [PubMed] [Google Scholar]
- 24. Ryu H. C., Kim C., Kim J. Y., Chung J. H., Kim J. H. (2010) UVB radiation induces apoptosis in keratinocytes by activating a pathway linked to “BLT2-reactive oxygen species.” J. Invest. Dermatol. 130, 1095–1106 [DOI] [PubMed] [Google Scholar]
- 25. Seo J. M., Cho K. J., Kim E. Y., Choi M. H., Chung B. C., Kim J. H. (2011) Up-regulation of BLT2 is critical for the survival of bladder cancer cells. Exp. Mol. Med. 43, 129–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Badgwell D. B., Lu Z., Le K., Gao F., Yang M., Suh G. K., Bao J. J., Das P., Andreeff M., Chen W., Yu Y., Ahmed A. A., S.-L., Liao W., Bast R. C., Jr. (2012) The tumor-suppressor gene ARHI (DIRAS3) suppresses ovarian cancer cell migration through inhibition of the Stat3 and FAK/Rho signaling pathways. Oncogene 31, 68–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Saydmohammed M., Joseph D., Syed V. (2010) Curcumin suppresses constitutive activation of STAT-3 by up-regulating protein inhibitor of activated STAT-3 (PIAS-3) in ovarian and endometrial cancer cells. J. Cell. Biochem. 110, 447–456 [DOI] [PubMed] [Google Scholar]
- 28. Ju K. D., Lim J. W., Kim K. H., Kim H. (2011) Potential role of NADPH oxidase-mediated activation of Jak2/Stat3 and mitogen-activated protein kinases and expression of TGF-β1 in the pathophysiology of acute pancreatitis. Inflamm. Res. 60, 791–800 [DOI] [PubMed] [Google Scholar]
- 29. Li L., Cheung S. H., Evans E. L., Shaw P. E. (2010) Modulation of gene expression and tumor cell growth by redox modification of STAT3. Cancer Res. 70, 8222–8232 [DOI] [PubMed] [Google Scholar]
- 30. Thornber K., Colomba A., Ceccato L., Delsol G., Payrastre B., Gaits-Iacovoni F. (2009) Reactive oxygen species and lipoxygenases regulate the oncogenicity of NPM-ALK-positive anaplastic large cell lymphomas. Oncogene 28, 2690–2696 [DOI] [PubMed] [Google Scholar]
- 31. Shaw T. J., Senterman M. K., Dawson K., Crane C. A., Vanderhyden B. C. (2004) Characterization of intraperitoneal, orthotopic, and metastatic xenograft models of human ovarian cancer. Mol. Ther. 10, 1032–1042 [DOI] [PubMed] [Google Scholar]
- 32. Naora H., Montell D. J. (2005) Ovarian cancer metastasis. Integrating insights from disparate model organisms. Nat. Rev. Cancer 5, 355–366 [DOI] [PubMed] [Google Scholar]
- 33. Kim E. Y., Seo J. M., Cho K. J., Kim J. H. (2010) Ras-induced invasion and metastasis are regulated by a leukotriene B4 receptor BLT2-linked pathway. Oncogene 29, 1167–1178 [DOI] [PubMed] [Google Scholar]
- 34. Yoo M. H., Song H., Woo C. H., Kim H., Kim J. H. (2004) Role of the BLT2, a leukotriene B4 receptor, in Ras transformation. Oncogene 23, 9259–9268 [DOI] [PubMed] [Google Scholar]
- 35. Ihara A., Wada K., Yoneda M., Fujisawa N., Takahashi H., Nakajima A. (2007) Blockade of leukotriene B4 signaling pathway induces apoptosis and suppresses cell proliferation in colon cancer. J. Pharmacol. Sci. 103, 24–32 [DOI] [PubMed] [Google Scholar]
- 36. Xia C., Meng Q., Liu L. Z., Rojanasakul Y., Wang X. R., Jiang B. H. (2007) Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer Res. 67, 10823–10830 [DOI] [PubMed] [Google Scholar]
- 37. Yamaura M., Mitsushita J., Furuta S., Kiniwa Y., Ashida A., Goto Y., Shang W. H., Kubodera M., Kato M., Takata M., Saida T., Kamata T. (2009) NADPH oxidase 4 contributes to transformation phenotype of melanoma cells by regulating G2-M cell cycle progression. Cancer Res. 69, 2647–2654 [DOI] [PubMed] [Google Scholar]
- 38. Shono T., Yokoyama N., Uesaka T., Kuroda J., Takeya R., Yamasaki T., Amano T., Mizoguchi M., Suzuki S. O., Niiro H., Miyamoto K., Akashi K., Iwaki T., Sumimoto H., Sasaki T. (2008) Enhanced expression of NADPH oxidase Nox4 in human gliomas and its roles in cell proliferation and survival. Int. J. Cancer 123, 787–792 [DOI] [PubMed] [Google Scholar]
- 39. Mochizuki T., Furuta S., Mitsushita J., Shang W. H., Ito M., Yokoo Y., Yamaura M., Ishizone S., Nakayama J., Konagai A., Hirose K., Kiyosawa K., Kamata T. (2006) Inhibition of NADPH oxidase 4 activates apoptosis via the AKT/apoptosis signal-regulating kinase 1 pathway in pancreatic cancer PANC-1 cells. Oncogene 25, 3699–3707 [DOI] [PubMed] [Google Scholar]
- 40. Kessenbrock K., Plaks V., Werb Z. (2010) Matrix metalloproteinases. Regulators of the tumor microenvironment. Cell 141, 52–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rosen D. G., Mercado-Uribe I., Yang G., Bast R. C., Jr., Amin H. M., Lai R., Liu J. (2006) The role of constitutively active signal transducer and activator of transcription 3 in ovarian tumorigenesis and prognosis. Cancer 107, 2730–2740 [DOI] [PubMed] [Google Scholar]
- 42. Huang S. (2007) Regulation of metastases by signal transducer and activator of transcription 3 signaling pathway. Clinical implications. Clin. Cancer Res. 13, 1362–1366 [DOI] [PubMed] [Google Scholar]
- 43. Masuda M., Suzui M., Yasumatu R., Nakashima T., Kuratomi Y., Azuma K., Tomita K., Komiyama S., Weinstein I. B. (2002) Constitutive activation of signal transducers and activators of transcription 3 correlates with cyclin D1 overexpression and may provide a novel prognostic marker in head and neck squamous cell carcinoma. Cancer Res. 62, 3351–3355 [PubMed] [Google Scholar]
- 44. Kusaba T., Nakayama T., Yamazumi K., Yakata Y., Yoshizaki A., Inoue K., Nagayasu T., Sekine I. (2006) Activation of STAT3 is a marker of poor prognosis in human colorectal cancer. Oncol. Rep. 15, 1445–1451 [PubMed] [Google Scholar]
- 45. Simon A. R., Rai U., Fanburg B. L., Cochran B. H. (1998) Activation of the JAK-STAT pathway by reactive oxygen species. Am. J. Physiol. 275, C1640–C1652 [DOI] [PubMed] [Google Scholar]
- 46. Manea A., Tanase L. I., Raicu M., Simionescu M. (2010) Jak/STAT signaling pathway regulates nox1 and nox4-based NADPH oxidase in human aortic smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 30, 105–112 [DOI] [PubMed] [Google Scholar]
- 47. Landen C. N., Jr., Lin Y. G., Armaiz Pena G. N., Das P. D., Arevalo J. M., Kamat A. A., Han L. Y., Jennings N. B., Spannuth W. A., Thaker P. H., Lutgendorf S. K., Savary C. A., Sanguino A. M., Lopez-Berestein G., Cole S. W., Sood A. K. (2007) Neuroendocrine modulation of signal transducer and activator of transcription-3 in ovarian cancer. Cancer Res. 67, 10389–10396 [DOI] [PubMed] [Google Scholar]
- 48. Lee J. K., Edderkaoui M., Truong P., Ohno I., Jang K. T., Berti A., Pandol S. J., Gukovskaya A. S. (2007) NADPH oxidase promotes pancreatic cancer cell survival via inhibiting JAK2 dephosphorylation by tyrosine phosphatases. Gastroenterology 133, 1637–1648 [DOI] [PubMed] [Google Scholar]
- 49. Schieffer B., Luchtefeld M., Braun S., Hilfiker A., Hilfiker-Kleiner D., Drexler H. (2000) Role of NAD(P)H oxidase in angiotensin II-induced JAK/STAT signaling and cytokine induction. Circ. Res. 87, 1195–1201 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.