Background: Function of physiologically important α6β3*-nicotinic receptor (nAChR) is differentially impacted by β3 subunits.
Results: nAChR expressed in several novel ways indicates that β3 subunits mostly potentiate gain-of-function α6*-nAChR.
Conclusion: Extracellular domain loop E region in α6 subunits governs effect of β3 subunit on gain-of-function α6*-nAChR.
Significance: Novel α6β3*-nAChR reported could be used to assess and/or develop smoking cessation aids.
Keywords: Ion Channels, Ligand-binding Protein, Mutagenesis Site-specific, Nicotinic Acetylcholine Receptors, Receptor Structure-Function
Abstract
We previously have shown that β3 subunits either eliminate (e.g. for all-human (h) or all-mouse (m) α6β4β3-nAChR) or potentiate (e.g. for hybrid mα6hβ4hβ3- or mα6mβ4hβ3-nAChR containing subunits from different species) function of α6*-nAChR expressed in Xenopus oocytes, and that nAChR hα6 subunit residues Asn-143 and Met-145 in N-terminal domain loop E are important for dominant-negative effects of nAChR hβ3 subunits on hα6*-nAChR function. Here, we tested the hypothesis that these effects of β3 subunits would be preserved even if nAChR α6 subunits harbored gain-of-function, leucine- or valine-to-serine mutations at 9′ or 13′ positions (L9′S or V13′S) in their second transmembrane domains, yielding receptors with heightened functional activity and more amenable to assessment of effects of β3 subunit incorporation. However, coexpression with β3 subunits potentiates rather than suppresses function of all-human, all-mouse, or hybrid α6(L9′S or V13′S)β4*- or α6(N143D+M145V)L9′Sβ2*-nAChR. This contrasts with the lack of consistent function when α6(L9′S or V13′S) and β2 subunits are expressed alone or in the presence of wild-type β3 subunits. These results provide evidence that gain-of-function hα6hβ2*-nAChR (i.e. hα6(N143D+M145V)L9′Shβ2hβ3 nAChR) could be produced in vitro. These studies also indicate that nAChR β3 subunits can be assembly partners in functional α6*-nAChR and that 9′ or 13′ mutations in the nAChR α6 subunit second transmembrane domain can act as gain-of-function and/or reporter mutations. Moreover, our findings suggest that β3 subunit coexpression promotes function of α6*-nAChR.
Introduction
Nicotinic acetylcholine receptors (nAChR)3 are pentameric ligand-gated ion channels expressed throughout the nervous system. Those other than the muscle-type (embryonic α1β1γδ- or adult α1β1γϵ-) nAChR are thought to be composed of different permutations of eight α subunits (α2-α7, α9-α10) and three β subunits (β2-β4) in humans (1). Of specific interest to us in this study are α6β3*-nAChR (where * indicates the known or possible presence of nAChR subunits other than those specified) (2–4). α6β3*-nAChR have been implicated in dopaminergic neurotransmission, nicotine dependence, anxiety, and other important neurophysiological processes (5–13).
In vitro expression of functional, all-mouse (m) or all-human (h), wild-type α6β3*-nAChR has been difficult to achieve despite strong evidence for expression of α6β3*-nAChR in rodent brain (3, 4, 6, 7, 10, 12–15). Functional expression of α6*-nAChR only has been achieved in Xenopus oocytes when using specific forms of mutant or chimeric subunits or in hybrid α6*-nAChR composed of subunits from different species (16–20). For example, function is achieved when chimeric, hα6/hα3 subunits (composed of the N-terminal, first extracellular domain of the hα6 subunit fused to the first transmembrane domain through to the C terminus of the hα3 subunit) are coexpressed with hβ2 or hβ4 subunits alone or in the presence of hβ3 subunits (19). α6*-nAChR are functional when expressed as hybrids of mouse and human α6 and other subunits, and there is function of some complexes containing β3 subunits mutated at specific residues in their second transmembrane domains (leucine- or valine-to-serine mutations at 9′ or 13′ positions; L9′S or V13′S) to confer gain-of-function effects (4, 15, 21). Potentiation of function is sometimes seen when wild-type β3 subunits are incorporated into hybrid complexes, but this is in contrast to dominant-negative effects of coexpression with wild-type β3 subunits on function of α6β4*-nAChR when all subunits are from the same species (4, 21). There may be host cell specificity in some of these effects because nAChR hβ3 subunits promote expression and nicotine-induced up-regulation of h6*-nAChR in transfected cell lines (22).
We and others have taken advantage of gain-of-function mutations in the nAChR β3 subunit to produce functional nAChR, including those containing α6 subunits, in part to assess capabilities of subunits to coassemble, but also as a strategy to increase functional gain (signal:noise) to facilitate such assessments (4, 15, 21). For example, coexpression with β3V9′S subunits increases agonist sensitivity and efficacy for α6*-nAChR. We hypothesized that similar mutations in nAChR α6 subunits would increase agonist sensitivity and efficacy of α6(L9′S or V13′S)(β4 or β2)*-nAChR to provide enough functional gain to facilitate evaluation of effects of wild-type β3 subunits on complexes and even to ensure that we can detect incorporation of wild-type β3 subunits into α6(L9′S or V13′S)*-nAChR. We also hypothesized that wild-type β3 subunits would have the same effects, dominant-negative or potentiating, depending on the subunit combination investigated, on gain-of-function α6(L9′S or V13′S)*-nAChR as they did on wild-type α6*-nAChR. This would help us assess whether any reduction or abolishment of function is due to altered open channel probability (21) or due to reduced surface expression of nAChR because β3 subunit incorporation facilitates formation of dead end intermediates (23). Our results indicated that whenever nAChR β3 subunits are incorporated into (α6 or hα6(N143D+M145V))(L9′S or V13′S)*-nAChR, function is potentiated (i.e. there is higher agonist potency and larger magnitude responses) irrespective of whether there are dominant-negative or potentiating effects of β3 subunits on wild-type α6*-nAChR.
EXPERIMENTAL PROCEDURES
Chemicals
All chemicals for electrophysiology were obtained from Sigma. Fresh stock solutions of nicotine or mecamylamine were made daily in Ringer's solution and were diluted as needed.
Subcloning, Mutagenesis, and in Vitro Transcription of nAChR Subunits
Human or mouse nAChR α6, β2, β3, or β4 subunits were subcloned into the oocyte expression vector, pGEMHE, as earlier (4, 15). Fully synthetic, nAChR hβ2 subunit GenBank JN565027 with nucleotide sequence optimized for better heterologous expression (hβ2opt) was generated (GENEART, Burlingame, CA) and subcloned into the pCI vector (Promega, San Luis Obispo, CA) as earlier (4). Mutations in the nAChR subunits were introduced in the pGEMHE background using the QuikChange II site-directed mutagenesis kit (Stratagene, La Jolla, CA). Oligonucleotides used for a creation of the 9′ mutant in the hα6 subunit (L9′S; L280S) were 5′-cgctttgtatttcagtcctgtcttctctgactgtgtttttgc-3′ (forward) and 5′-gcaaaaacacagtcagagaagacaggactgaaatacaaagcg-3′ (reverse). Similarly, oligonucleotides used for creation of the 9′ mutant in the mα6 subunit (L9′S; L280S) were 5′-ctctttgcatctccgttctgagttctctcactgtctttttgc-3′ (forward) and 5′-gcaaaaagacagtgagagaactcagaacggagatgcaaagag-3′ (reverse). Also, a 13′ mutation in the mα6 subunit (V13′S; V284S) was created by using 5′-cgttctgctttctctcactagctttttgctggtgattacag-3′ (forward) and 5′-ctgtaatcaccagcaaaaagctagtgagagaaagcagaacg-3′ (reverse) oligonucleotides. Mutations in the N-terminal domain of the nAChR hα6 subunit (i.e. N143D+M145V) were introduced as earlier (4, 15). This hα6 subunit mutant (i.e. N143D+M145V) was further subjected to a 9′ mutation using the primers stated earlier. Identities of all wild-type or mutant subunits were confirmed by sequencing referenced to nucleotide/protein sequences available in GenBank.
All pGEMHE plasmids were linearized immediately downstream of the 3′-polyadenylation sequence. NheI was used to linearize nAChR hα6, hα6L9′S, hα6(N143D+M145V), hα6(N143D+M145V)L9′S, hβ3, hβ4, mα6, mα6L9′S, mα6L13′S, mβ2, mβ3, and mβ4 subunit-containing plasmids, and SbfI was used for linearizing the hβ2 subunit-containing plasmid. SwaI was used to linearize hβ2opt. Capped mRNA was transcribed from linearized plasmids in a reaction mixture (25 μl) containing 1× transcription buffer, 1.6 mm rNTPs (Promega, WI), 0.5 mm m7G(5′)ppp(5′)G RNA Cap Structure Analog (New England Biolabs), 1 μl of RNasin plus (New England Biolabs) and 1 μl T7 RNA polymerase (New England Biolabs) following standard protocols or using mMESSAGE mMACHINE® T7 kit (Ambion/Invitrogen) and following the manufacturer's instructions. The integrity and quality of the cRNA were checked by electrophoresis and UV spectroscopy.
Oocyte Preparation and cRNA Injection
Female Xenopus laevis (Xenopus I, Ann Arbor, MI) were anesthetized using 0.2% tricaine methanesulfonate (MS-222). The ovarian lobes were surgically removed from the frogs and placed in an incubation solution that consisted of (in mm) 82.5 NaCl, 2.5 KCl, 1 MgCl2, 1 CaCl2, 1 Na2HPO4, 0.6 theophylline, 2.5 sodium pyruvate, 5 HEPES supplemented with 50 mg/ml gentamycin, 50 units/ml penicillin, and 50 μg/ml streptomycin; pH 7.5. The frogs were allowed to recover from surgery before being returned to the incubation tank. Ovarian lobes were cut into small pieces and digested with 0.08 Wünsch units/ml Liberase blendzyme 3 (Roche Applied Science) with constant stirring at room temperature for 1.5–2 h. The dispersed oocytes were thoroughly rinsed with incubation solution. Stage VI oocytes were selected and incubated at 16 °C before injection. Micropipettes used for injection were pulled from borosilicate glass (Drummond Scientific, Broomall, PA) using a Sutter P87 horizontal puller, and the tips were broken with forceps to ∼40 μm in diameter. cRNA was drawn up into the micropipette and injected into oocytes using a Nanoject microinjection system (Drummond Scientific) at a total volume of ∼60 nl. To express nAChR in oocytes, about 4 ng of cRNA corresponding to each subunit was injected; i.e. at ratios of 1:1 or 1:1:1 for binary or trinary receptors, respectively, with the exception that for coexpression of hα6(N143D+M145V)+hβ2*-nAChR in the presence or absence nAChR hβ3 subunit, about 10 ng of cRNA corresponding to each subunit including nAChR hβ2opt subunit was injected.
Oocyte Electrophysiology
Two to seven days after injection, oocytes were placed in a small-volume chamber and continuously perfused with oocyte Ringer's solution, which consisted of (in mm) 92.5 NaCl, 2.5 KCl, 1 CaCl2, 1 MgCl2, and 5 HEPES; pH 7.5. The chamber was grounded through an agarose bridge. The oocytes were voltage-clamped at −70 mV (unless otherwise noted) to measure agonist-induced currents using an AxoClamp 900A and the pClamp 10.2 software (Axon Instruments, CA). The current signal was low pass-filtered at 10 Hz with the built-in low pass Bessel filter in the AxoClamp 900A and digitized at 20 Hz with an Axon Digidata1440A and the pClamp10.2 software. Electrodes contained 3 m KCl and had a resistance of 1–2 megaohms. Drugs (agonists and antagonists) were prepared daily in bath solution. Drug was applied using a Valvelink 8.2 perfusion system (Automate Scientific, Berkeley, CA). All electrophysiological measurements were conducted or checked in at least two batches of oocytes.
Experimental Controls
Injection of water or empty vector (used as two forms of negative controls) or of cRNA corresponding to one subunit alone or pairwise combinations of β3 subunits with either an α6 or a mutant α6 subunit or β2 or β4 subunits (8–20 ng total of cRNA) did not result in the expression of functional nAChR. Current responses to 100 μm nicotine were less than 5–10 nA (data not shown).
Data Analyses
Raw data were collected and processed in part using pClamp 10.2 (Molecular Devices, Sunnyvale, CA) and a spreadsheet (Excel; Microsoft, Bellevue, WA), using peak current amplitudes as measures of functional nAChR expression and results pooled across experiments (mean ± S.E. for data from at least three oocytes). In some cases, mean peak current amplitudes in response to a single concentration of an agonist were compared across different subunit combinations. However, assessment of true Imax values for different nAChR subunit combinations required assessment based on more complete concentration-response relationships, in which mean peak current amplitudes at specified ligand concentrations were fit to the Hill equation or its variants using Prism 4 (GraphPad Software, San Diego, CA). F-tests (p < 0.05 to define statistical significance) were carried out to compare the best fit values of log molar EC50 values across specific nAChR subunit combinations.
There are limitations in the ability to compare levels of functional nAChR expression, although we injected similar amounts of RNA for all constructs. This is because expression levels assessed as peak current amplitudes are affected by batch-to-batch variation in oocytes, time between cRNA injection and recording, and subunit combination-specific parameters, such as open probability (influenced by gating rate constants, rates, and extents of desensitization), single channel conductance, assembly efficiency, and efficiency of receptor trafficking to the cell surface (24). We made no attempt to measure or control for subunit combination-specific effects, but whenever preliminary studies revealed possible differences in peak current amplitudes, findings were further confirmed across different subunit combinations using the same batch of oocytes and the same time between cRNA injection and recording. Peak current amplitudes shown from representative traces in some figures presented below, pooled data from limited sets of studies, and mean peak current amplitudes across all studies for a given combination of subunits given in tables sometimes differ. However, when we make statements about results comparing ligand potencies and peak current amplitudes across subunit combinations, we do so for studies done under the same or very similar conditions, and the observations are clear, statistically significant, and in agreement whether for pooled data or for results from smaller sets of studies (one-way analyses of variance followed by Tukey's multiple comparison tests).
RESULTS
Human nAChR α6L9′S Subunits Form Functional Receptors in Association with nAChR hβ4 and hβ3 Subunits with Increased Receptor Agonist Sensitivity and Efficacy
Earlier, we observed that oocytes coinjected with nAChR hα6 and hβ4 subunit cRNAs produce functional hα6hβ4-nAChR in only a few out of many injected oocytes and then only have minimal responses to nicotinic agonists (4). Although we could measure a peak current of 22 ± 3 nA for hα6hβ4-nAChR in response to 100 μm acetylcholine, we were unable to measure reliable and reproducible functional responses to nicotine. Also, oocytes injected with nAChR hα6, hβ4, and hβ3 subunit cRNAs do not produce reliable and reproducible functional hα6hβ4hβ3-nAChR, suggesting that the small amount of function seen for h6hβ4-nAChR is either reduced or completely eliminated, probably due to β3 subunits exerting a negative effect on function of hα6hβ4*-nAChR. We replicated those findings in the current work, and we also found that oocytes coexpressing nAChR hα6L9′S and hβ4 subunit cRNAs have marginally increased, but more reproducible, responses to nicotine (peak current of 32 ± 7 nA for hα6L9′Shβ4-nAChR in response to 100 μm nicotine; Fig. 1; Table 1). Thus, replacement of hα6L9′S for hα6 subunits does not have as great of a gain-of-function effect on α6β4*-nAChR as does introduction of hβ3V9′S subunits (4) into otherwise wild-type hα6hβ4*-nAChR.
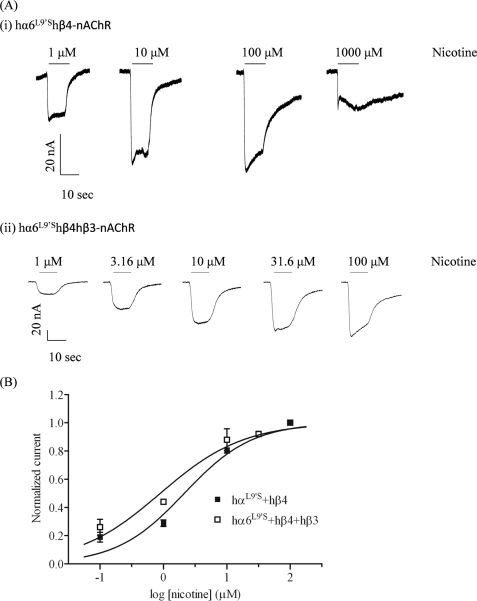
FIGURE 1.
Functional properties of hα6*-nAChR. A, representative traces are shown for inward currents in oocytes held at −70 mV, responding to application at the indicated concentrations of nicotine (shown with the duration of drug exposure as black bars above the traces), and expressing nAChR hα6L9′S and hβ4 subunits (i) or nAChR hα6L9′S, hβ4, and hβ3 subunits (ii). B, results for these and other studies averaged across experiments were used to produce concentration-response curves (ordinate, mean normalized current ± S.E.; abscissa, ligand concentration in log μm) for inward current responses to nicotine as indicated for oocytes expressing nAChR hα6L9′S and hβ4 subunits alone (■) or with hβ3 subunits (□), where current amplitudes are represented as a fraction of the peak inward current amplitude in response to the most efficacious concentration of nicotine. Much higher levels of evoked currents are evident for functional nAChR containing hα6L9′S, hβ4, and hβ3 subunits when compared with receptors lacking hβ3 subunits. See Table 1 for parameters.
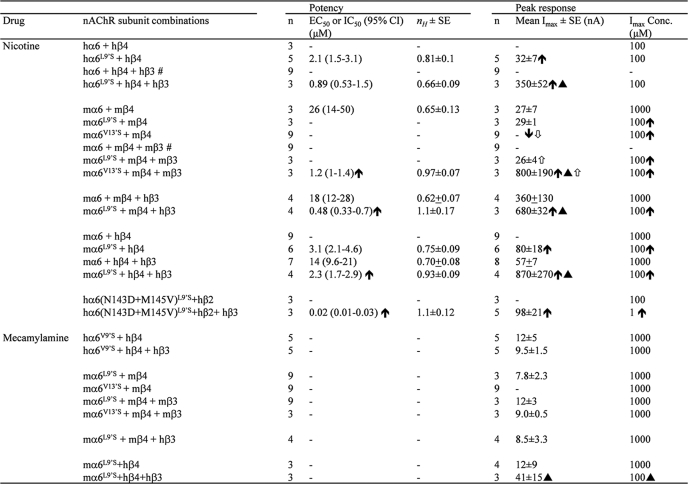
TABLE 1.
Parameters for agonist or antagonist action at nAChR containing gain-of-function α6 mutant subunits
Potencies (micromolar EC50 or IC50 values with 95% confidence intervals), Hill coefficients (nH ± S.E.), mean ± S.E. efficacies (two-electrode voltage-clamp peak responses, Imax, in nanoamperes), and concentrations where maximal peak current amplitudes (Imax concentration in micromolar) are achieved (μm) are provided for nicotine as an agonist or mecamylamine as an antagonist acting at nAChR composed of the indicated subunits derived from the specified species and from the indicated number of independent experiments (n) based on studies as shown the figures. Closed up arrows or closed down arrows indicate a significant (p < 0.05) increase or decrease, respectively, in potency or efficacy of the indicated agent at the indicated nAChR subtype relative to nAChR containing the wild type α6 subunit. Filled triangle indicates a significant increase in indicated agonist or antagonist potency or efficacy at the indicated nAChR containing α6L9′S or α6V13′S subunits relative to the same complex but lacking β3 subunits. Open up arrows or open down arrows indicate a significant increase or decrease, respectively, in potency or efficacy of the indicated agonist at the indicated nAChR containing α6V13′S subunits relative to nAChR containing α6L9′S subunits. Note that no or very rare and then small responses to nicotine were seen for the following subunit combinations (n = 6–9 each): hα6 or hα6L9′S plus hβ2 alone or with hβ3; mα6 or mα6L9′S or mα6V13′S plus mβ2 alone or with mβ3 or hβ3; and mα6 or mα6L9′S plus hβ2 alone or with hβ3. - indicates that absent or inconsistent functional responses in two-electrode voltage-clamp studies precluded determination of the parameter of interest; # indicates data from (4).
Consistent with our previous observations regarding introduction of gain-of-function β3 subunits into α6*-nAChR (4), oocytes coexpressing nAChR hα6L9′S and hβ4 subunits and exposed to the nAChR noncompetitive antagonist and open channel blocker, mecamylamine, respond with an apparent outward peak current of 12 ± 5 nA (Table 1). Because mecamylamine coexposure more than blocks inward currents produced by nicotinic agonists, also leading under those conditions to production of apparent outward current responses, and does so in a concentration-dependent manner, we again interpret these effects as showing the ability of mecamylamine to block spontaneous opening of α6L9′Shβ4-nAChR channels (Table 1). Given the magnitudes of peak current responses to nicotine alone and to mecamylamine alone, about 27% of hα6L9′Shβ4-nAChR appear to be spontaneously open at any given time (12/(12 + 32) = 0.27).
When nAChR hα6L9′S and hβ4 were coexpressed with hβ3 subunits instead of alone, oocyte responsiveness to nicotine (EC50 value of 0.9 μm) increases over 10-fold (to a peak current response of 350 ± 52 nA; Fig. 1, Table 1). This suggests that wild-type β3 subunits incorporate into hα6L9′Shβ4*-nAChR and strongly potentiate receptor function. However, this does not occur with a change in agonist potency upon hβ3 subunit incorporation into hα6L9′Shβ4*-nAChR because there is not a significant change in nicotine EC50 values (Table 1). Outward current production in the same oocytes (9.5 ± 1.5 nA) in response to 1000 μm mecamylamine indicates that there is spontaneous opening of hα6L9′Shβ4hβ3-nAChR, but levels of spontaneous opening are comparable with those for hα6L9′Shβ4-nAChR in the absence of hβ3 subunits, indicating that a smaller proportion of hα6L9′Shβ4hβ3-nAChR is spontaneously open at any time (9.5/(9.5 + 350) = 0.026; Table 1) than for hα6L9′Shβ4-nAChR. No function was observed in response to nicotine or mecamylamine in oocytes coexpressing nAChR hα6 or hα6L9′S subunits plus hβ2 subunits with or without hβ3 subunits.
Mouse nAChR α6V13′S Subunits Form Functional Receptors in Association with nAChR mβ4 and mβ3 Subunits with Increased Receptor Agonist Sensitivity and Efficacy
We have shown earlier that oocytes coinjected with mα6 and mβ4 nAChR subunit cRNAs form functional nAChR, but with minimal responses to nicotinic agonists, and function is further reduced in the presence of nAChR mβ3 subunits, indicating that nAChR mβ3 subunits exert dominant-negative effects on the function of mα6mβ4*-nAChR (4). Here, we observed that oocytes coexpressing either wild-type mα6 or mutant mα6L9′S along with mβ4 subunits give comparably modest peak current responses to 100 μm nicotine (Imax = 27 ± 7 or 29 ± 1 nA, respectively; Table 1). Thus, although oocytes expressing mα6L9′S and mβ4 subunits give outward current responses to mecamylamine, consistent with spontaneous channel opening, the 9′ mutation in the nAChR mα6 subunit does not significantly increase the magnitude of functional responsiveness (Table 1). Similarly, there is no increase in functional responsiveness to nicotine for oocytes coexpressing nAChR mα6L9′S, mβ4, and mβ3 subunits (peak current = 26 ± 4 nA), although mecamylamine-induced outward current indicated that there is spontaneous opening of mα6L9′Smβ4mβ3-nAChR (Table 1).
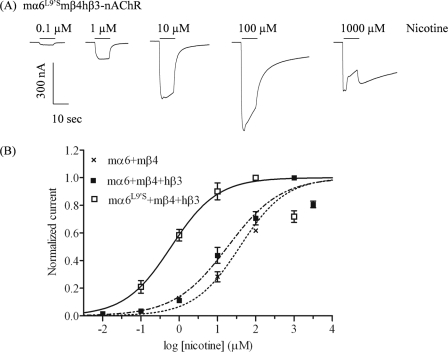
By contrast, unexpectedly, and interestingly, oocytes expressing nAChR mα6V13′S, mβ4, and mβ3 subunits exhibit a >25-fold increase in peak current responses to nicotine (800 ± 190 nA) relative to responses of mα6L9′Smβ4- or mα6L9′Smβ4mβ3-nAChR. Also, we were able to define an increase in nicotine potency when acting at mα6V13′Smβ4mβ3-nAChR (EC50 = 1.2 μm) relative to nicotine potency at mα6mβ4-nAChR (EC50 value of 26 μm; Table 1, Fig. 2). In addition, there also is spontaneous opening of mα6V13′Smβ4mβ3-nAChR, although responses to nicotine or mecamylamine are absent for mα6V13′Smβ4-nAChR (Table 1). No function was observed in response to nicotine or mecamylamine in oocytes coexpressing nAChR mα6 or mα6L9′S or mα6V13′S subunits plus mβ2 subunits with or without mβ3 subunits.
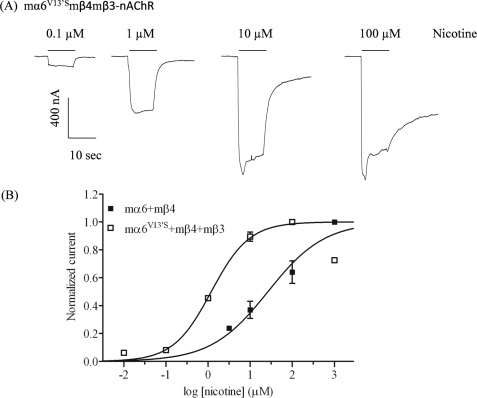
FIGURE 2.
Functional properties of mα6*-nAChR. A, representative traces are shown for inward currents in oocytes held at −70 mV, responding to application at the indicated concentrations of nicotine (shown with the duration of drug exposure as black bars above the traces), and expressing nAChR mα6V13′S, mβ4, and mβ3 subunits. B, results for these and other studies averaged across experiments were used to produce concentration-response curves (ordinate, mean normalized current ± S.E.; abscissa, ligand concentration in log μm) for inward current responses to nicotine as indicated for oocytes expressing nAChR mα6 and mβ4 subunits (■) or mα6V13′S and mβ4 and mβ3 subunits (□), where current amplitudes are represented as a fraction of the peak inward current amplitude in response to the most efficacious concentration of nicotine. Much higher levels of evoked currents are evident for functional nAChR containing mα6V13′S, mβ4, and mβ3 subunits when compared with receptors lacking mα6V13′S subunits. See Table 1 for parameters.
Mouse nAChR α6L9′S Subunits Form Functional Receptors in Association with nAChR hβ4 and hβ3 Subunits with Increased Receptor Agonist Sensitivity and Efficacy
We reported earlier that oocytes coinjected with nAChR mα6, hβ4, and hβ3 subunit cRNAs tend to form functional mα6hβ4hβ3-nAChR, whereas oocytes coexpressing nAChR mα6 and hβ4 subunit cRNAs do not respond to nicotinic agonists (4). Here, we show that oocytes coexpressing nAChR mα6L9′S and hβ4 subunits yield peak function of 80 ± 18 nA in response to 100 μm nicotine (Fig. 3, Table 1) and outward current responses (12 ± 9 nA) to mecamylamine, consistent with spontaneous channel opening of mα6L9′Shβ4-nAChR (Table 1). Moreover, oocytes coexpressing nAChR mα6L9′S, hβ4, and hβ3 subunits respond to nicotine with an EC50 value of 2.3 μm and give an even larger peak current (870 ± 270 nA; Fig. 3; Table 1). Also, oocytes coexpressing mα6L9′S, hβ4, and hβ3 subunits give relatively large, outward current responses (peak current of 41 ± 15 nA) to mecamylamine, again an indication that functional and spontaneously opening mα6L9′Shβ4hβ3-nAChR are formed (Table 1). The kinetics of traces generated in response to application of nicotine differs between mα6L9′Shβ4- and mα6L9′Shβ4hβ3-nAChR. mα6L9′Shβ4hβ3-nAChR in response to activation by 1000 μm nicotine exhibit a tail current that is significantly reduced or absent in mα6L9′Shβ4-nAChR when activated by the same concentration of nicotine. Precisely, in the presence of hβ3 subunits, there is a pronounced functional block of the receptor at higher concentration of nicotine. Removal of the functional block imposed by nicotine (probably acting as an open channel blocker), as a result of switching out to buffer application, leads to activation of the receptor by the residual nicotine that results in formation of a tail current. This is not unusual given that incorporation of accessory subunits into nAChR differentially affects various functional characteristics of the receptor (25).
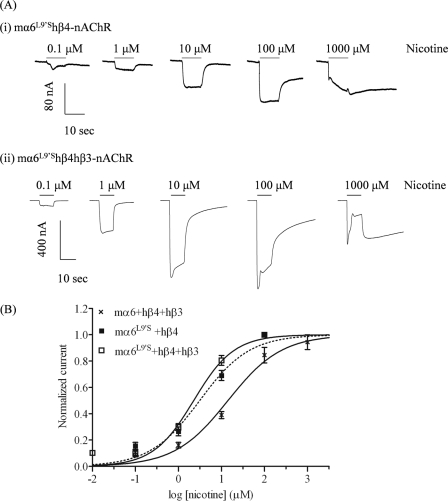
FIGURE 3.
Functional properties of hybrid mα6hβ4*-nAChR. A, representative traces are shown for inward currents in oocytes held at −70 mV, responding to application at the indicated concentrations of nicotine (shown with the duration of drug exposure as black bars above the traces), and expressing nAChR mα6L9′S and hβ4 subunits (i) or nAChR mα6L9′S, hβ4, and hβ3 subunits (ii). B, results for these and other studies averaged across experiments were used to produce concentration-response curves (ordinate, mean normalized current ± S.E.; abscissa, ligand concentration in log μm) for inward current responses to nicotine as indicated for oocytes expressing nAChR mα6L9′S and hβ4 subunits alone (■) or with hβ3 subunits (□) or expressing mα6 and hβ4 and hβ3 subunits (×; data from Ref. 4), where current amplitudes are represented as a fraction of the peak inward current amplitude in response to the most efficacious concentration of nicotine. Much higher levels of evoked currents are evident for functional nAChR containing mα6L9′S, hβ4, and hβ3 subunits when compared with receptors lacking hβ3 subunits. See Table 1 for parameters.
Minimal and inconsistent nAChR function was observed when mα6L9′S subunits were coexpressed with hβ2 and hβ3 subunits. This is in contrast to our earlier observation that nAChR mα6 subunits along with hβ2 subunits and gain-of-function hβ3 subunits (hβ3V9′S; hβ3V273S) form functional mα6hβ2hβ3V9′S-nAChR (4). This suggests that the gain-of-function mutation in the mα6L9′S subunit is inadequate to overcome what seems to be a strong, dominant-negative effect of nAChR β3 subunits in the presence of β2 subunits.
Mouse nAChR α6L9′S Subunits Form Functional Receptors in Association with nAChR mβ4 and hβ3 Subunits with Increased Receptor Agonist Sensitivity and Efficacy
Oocytes coinjected with nAChR mα6, mβ4, and hβ3 subunit. cRNAs give >10-fold larger and ∼2-fold more sensitive responses to nicotinic agonists than do oocytes coinjected mα6 and mβ4 subunit cRNAs and in stark contrast to the elimination of functional responses in oocytes coexpressing nAChR mα6, mβ4, and mβ3 subunits (Table 1). Interestingly, here we found that oocytes coexpressing nAChR mα6L9′S, mβ4, and hβ3 subunits responded to nicotine with an EC50 value of 0.48 μm and with large peak currents 680 ± 32 nA; Fig. 4; Table 1) and gave outward current responses (8.5 ± 3.3 nA) when exposed to 1000 μm mecamylamine (Table 1). No function was observed when mα6L9′S subunits were coexpressed with mβ2 and hβ3 subunits.
FIGURE 4.
Functional properties of gain-of-function hybrid mα6hβ3*-nAChR. A, representative traces are shown for inward currents in oocytes held at −70 mV, responding to application at the indicated concentrations of nicotine (shown with the duration of drug exposure as black bars above the traces), and expressing nAChR mα6L9′S, mβ4 and hβ3 subunits. B, results for these and other studies averaged across experiments were used to produce concentration-response curves (ordinate, mean normalized current ± S.E.; abscissa, ligand concentration in log μm) for inward current responses to nicotine as indicated for oocytes expressing nAChR mα6 and mβ4 subunits alone (32 ×) or with hβ3 subunits (■) or expressing mα6L9′S and mβ4 and hβ3 subunits (□), where current amplitudes are represented as a fraction of the peak inward current amplitude in response to the most efficacious concentration of nicotine. Much higher levels of evoked currents are evident for functional nAChR containing mα6L9′S, hβ4, and hβ3 subunits when compared with receptors lacking hβ3 subunits. See Table 1 for parameters.
Human nAChR α6(N143D+M145V)L9′S Subunits Form Functional Receptors in Association with nAChR hβ2 and hβ3 Subunits with Increased Receptor Agonist Sensitivity and Efficacy
Earlier (4), we had shown that mutations in the N-terminal domain of the nAChR hα6 subunit enable nAChR hβ3V9′S subunits to exert a gain-of-function effect at hα6(N143D+M145V)hβ2*-nAChR (i.e. hα6(N143D+M145V)hβ2hβ3V9′S-nAChR are functional). This finding led us to explore effects of incorporation of hβ3 subunits into hα6(N143D+M145V)L9′Shβ2*-nAChR.
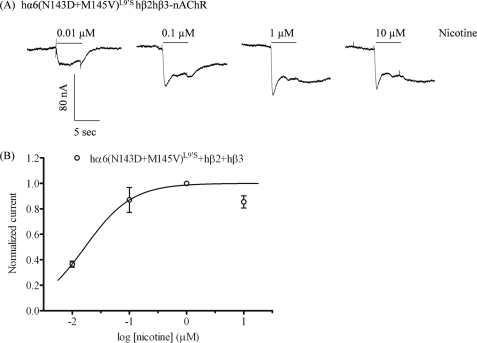
Oocytes injected with nAChR hα6(N143D+M145V)L9′S and hβ2 subunit cRNAs did not yield functional nicotinic responses (Table 1). However, oocytes injected with hβ3 subunit cRNAs along with nAChR hα6(N143D+M145V)L9′S and hβ2 subunit cRNAs yielded functional responses. Oocytes coexpressing nAChR hα6(N143D+M145V)L9′S, hβ2, and hβ3 subunits responded to nicotine with an EC50 value of 0.02 μm and with a maximal peak current of 98 ± 21 nA (Fig. 5; Table 1). We also found that oocytes coexpressing nAChR hα6(N143D+M145V)L9′S, hβ2, and hβ3 gave outward current responses when exposed to 1000 μm mecamylamine (data not shown).
FIGURE 5.
Functional properties of gain-of-function hα6(N143D+M145V)L9′Shβ3*-nAChR. A, representative traces are shown for inward currents in oocytes held at −80 mV, responding to application at the indicated concentrations of nicotine (shown with the duration of drug exposure as black bars above the traces), and expressing nAChR hα6(N143D+M145V)L9′S, hβ2, and hβ3 subunits. B, results for these and other studies averaged across experiments were used to produce concentration-response curves (ordinate, mean normalized current ± S.E.; abscissa, ligand concentration in log μm) for inward current responses to nicotine for oocytes expressing nAChR hα6(N143D+M145V)L9′S, hβ2, and hβ3 subunits (○), where current amplitudes are represented as a fraction of the peak inward current amplitude in response to the most efficacious concentration of nicotine. Much higher levels of evoked currents are evident for functional nAChR containing hα6(N143D+M145V)L9′S, hβ2, and hβ3 subunits when compared with receptors lacking hβ3 subunits. See Table 1 for parameters.
DISCUSSION
Recent studies have investigated how nAChR β3 subunits might incorporate as accessory partners into nAChR subtypes, specifically into α6*-nAChR (4). To further understand how β3 subunits might incorporate into α6*-nAChR, we exploited the gain-of-function/reporter mutant strategy (4, 15, 26) to reveal whether β3 subunits integrate into α6*-nAChR complexes that are on the cell surface and functional. This approach allows us to focus on cell surface, functional receptors without complications due to ambiguities of protein chemical or immunochemical studies confounded by the prevalent expression of intracellular and perhaps partially assembled receptor complexes and the unreliable quality and/or availability of most anti-nAChR antibodies for use in immunoprecipitation and/or immunoblot studies (15). In addition, we based the current studies on our findings (4) that (i) incorporation of nAChR β3 subunits into α6*-nAChR, mouse or human, has a dominant-negative effect; (ii) incorporation of nAChR hβ3 subunits into mα6hβ4*- or mα6mβ4*- nAChR leads to formation of functional nAChR; and (iii) mutations in the E1 N-terminal domain of the nAChR hα6 subunit are essential for successful assembly and formation of functional hα6(N143D+M145V)hβ2hβ3V9′S-nAChR.
The principal findings of this study, whenever functional expression levels are adequate to allow comparisons, and with exceptions that could be informative as discussed below, are: (i) that introduction of 9′ or 13′ mutations into the second transmembrane domain of mα6 or hα6 subunits typically has a gain-of-function effect, leading to production of (α6 or α6(N143D+M145V))(L9′S or V13′S)(β2 or β4)*-nAChR that have 6–34-fold higher sensitivity to nicotine and much higher levels of function than do nAChR containing the same subunit combinations but with wild-type α6 subunits; (ii) that incorporation of β3 subunits into (α6 or α6(N143D+M145V))(L9′S or V13′S)(β2 or β4)*-nAChR typically increases levels of receptor function with or without concomitant increase in agonist potency; and (iii) that gain-of-function mutations in α6 or α6(N143D+M145V) subunits still do not allow for formation of functional α6(L9′S or V13′S)β2-nAChR complexes, thus continuing to confound assessments of roles played by β3 subunits in modulation of α6β2*-nAChR.
The amount of functional expression for hα6L9′Shβ4-, mα6L9′Smβ4-, or mα6L9′Shβ4-nAChR is modest in absolute terms (27–80-nA peak current). However, with the exception of the insignificant difference in the magnitude of function seen for all-mouse mα6L9′Smβ4- and mα6mβ4-nAChR, the increase in function upon expression with the α6 subunit 9′ mutants is remarkable because of the lack of reliable function for wild-type, all human hα6hβ4-, or hybrid mα6hβ4-nAChR. The little-if-any function for all-wild-type α6β4-nAChR complicates quantitative assessment of effects of α6 subunit gain-of-function mutations on agonist potency, although qualitatively, nicotine EC50 values are over 10 μm for α6β4-nAChR and never higher than 3.1 μm for α6(L9′S or V13′S)β4-nAChR. However, gain-of-function effects manifest as increases in agonist potency and in peak current magnitudes are very clear based on comparisons of hα6hβ4hβ3- with hα6L9′Shβ4hβ3-nAChR and comparisons of mα6hβ4hβ3- with mα6L9′Shβ4hβ3-nAChR. A difference in agonist potency is also clear for comparison of mα6mβ4hβ3- with mα6L9′Smβ4hβ3-nAChR, although there is only a 2-fold difference in peak current response across these receptors, partly due to the relatively high absolute levels of function for the hybrid mα6mβ4hβ3-nAChR. Once again, however, all-mouse α6β4β3-nAChR are outliers because there is only modest function for mα6L9′Smβ4mβ3-nAChR, although there is no reliable function for the all-wild-type analog, mα6mβ4mβ3-nAChR.
Nevertheless, and very interestingly, for all-mouse α6*-receptors, although there is not reproducible function for mα6V13′Smβ4-nAChR, there are increases both in agonist potency and in response magnitude for mα6V13′Smβ4mβ3-nAChR when compared with those parameters for any form of mα6mβ4-nAChR or for mα6mβ4mβ3- or mα6L9′Smβ4mβ3-nAChR. Our initial studies of mouse α6*-nAChR were prompted because of the reported difficulties in heterologous expression of all-human α6*-nAChR and because so many data on naturally expressed α6*-nAChR function came from studies using rodents, but we have found all-mouse α6*-nAChR no easier to express than human α6*-nAChR. Expression of hybrid nAChR made up of subunits from different species has been more productive, suggesting that subtle differences for a given subunit across species in amino acid sequences in N-terminal, extracellular domains, but also in cytoplasmic and perhaps transmembrane domains, and at what must be at subunit interfaces not heretofore recognized as being functionally important, can strongly influence whether functional α6*-nAChR can be produced (4). The fact that mα6 L9′S and V13′S mutations differing in position by just one turn in the second transmembrane domain α-helix can have such a large difference in their impact on mα6mβ4*-nAChR function indicates unexpectedly important roles for this channel-lining region in α6*-nAChR function. More work is warranted to more thoroughly characterize the bases for these influences.
Our findings demonstrate that α6 subunit L9′S or V13′S modifications can function as reporter and/or gain-of-function mutations, leading to production of receptors with heightened sensitivity to agonists, thus confirming the presence of α6 subunits in functional receptor complexes, as expected. These studies also further affirm and recapture the strategy applied to exploit gain-of-function α6 subunit mutations expressed in vivo to enhance sensitivity to agonists and thus to help reveal roles played by α6*-nAChR in dopaminergic pathways relevant to movement disorders and nicotine dependence (13).
This study was also initiated largely to assess whether effects previously described of nAChR β3 subunit incorporation into α6*-nAChR would be preserved when receptor functional levels at baseline were intentionally elevated by using reporter mutation/gain-of-function α6 subunits as coexpression partners. By contrast to earlier work by others (21), in which β3 subunits were coexpressed in excess over other subunits, we chose to introduce equal amounts of subunit cRNAs into oocytes for the current work, anticipating that approximately equal amounts of subunit proteins would be made and that this more closely approximates conditions in vivo. We confirmed our previous observations (4, 15) that hβ3 subunit incorporation into hα6hβ4*-nAChR has an uncertain effect on functional expression, that mβ3 subunit incorporation into mα6mβ4*-nAChR has a dominant-negative effect on receptor function, that hβ3 subunit incorporation into hybrid mα6hβ4*-nAChR potentiates function, but that there is even larger potentiation of function when hβ3 subunits are incorporated into hybrid mα6mβ4*-nAChR. However, with the exception of the lack of an obvious effect of mβ3 subunit incorporation on low functioning mα6L9′Smβ4*-nAChR, wild-type β3 subunit incorporation into any of the tested α6(L9′S or V13′S)β4-nAChR potentiated levels of function by >11-fold, notably including effects of hβ3 subunits on low functioning mα6L9′Smβ4*-nAChR and effects of mβ3 subunits on mα6V13′Smβ4*-nAChR. These findings indicate that β3 subunits do not always have dominant-negative effects on α6*-nAChR function as suggested earlier (21) and do not always promote formation of dead end, α6β4*-nAChR intermediates as suggested previously (23). Instead, based on our results shown here, we can hypothesize that β3 subunits seem to promote assembly, cell surface expression, and/or functional responsiveness of α6β4*-nAChR, at least when there is enough function for α6β4(non-β3)-nAChR to allow assessment of effects of β3 subunit incorporation. Our findings using the oocyte expression system are in line with observations made regarding β3 subunit effects on α6*-nAChR functional expression in cell lines (22), suggesting that successful, functional α6β4*-nAChR expression in oocytes does not require coexpression with chaperones missing from oocytes but present in neurons or selected cell lines. Notably, although peak current potentiation upon substitution of gain-of-function α6 subunits (or β3 subunits; see Refs. 4 and 15) occurs along with an increase in agonist potency, wild-type β3 incorporation into complexes increases peak current responses without affecting agonist potency.
In almost every case, α6(L9′S or V13′S)*-nAChR spend a finite amount of time in a spontaneously open channel state, as judged by the ability of mecamylamine to block those open channels, giving the appearance of production of outward currents. This is a common feature for nAChR containing subunits with second transmembrane domain mutations that give gain-of-function effects (27, 28). Interestingly, the absolute magnitudes of responses to mecamylamine generally are quite similar across all the α6*-nAChR studied (7.8–12 nA), even when magnitudes of agonist-induced inward currents varied much more widely (26–800 nA). The only exceptions are for mα6V13′Smβ4-nAChR, which curiously have no reproducible responses to nicotine or to mecamylamine, despite there being strong responses upon incorporation of mβ3 subunits to form mα6V13′Smβ4mβ3-nAChR, and for mα6L9′Shβ4hβ3-nAChR, which have slightly larger responses to mecamylamine (41 nA) but also have the largest responses to nicotine (870 nA).
Although the current findings support a role for β3 subunits in potentiating function of α6β4*-nAChR with at least a modicum of baseline functional activity, we were confounded in our studies of α6β2*-nAChR by a general lack of function. This made it impossible to assess effects of β3 subunit incorporation on α6β2*-nAChR, but the results indicate that any gain-of-function earned by incorporation of α6(L9′S or V13′S) subunits into complexes is inadequate to reveal effects of β3 subunits, perhaps due to the surprising incompatibilities (illuminated in Refs. 4 and 15) that often occur in attempts to use α6, β2, and β3 subunits to form functional receptors. In order for us to show that in fact a variant of gain-of-function hα6 subunit can partner with hβ2 and hβ3 subunit to form functional nAChR, we took advantage of our site-directed mutagenesis work (4, 15), which has implicated α6 residues 143 and 145 in the ability of β3 subunits to affect α6β2*-nAChR function. The hα6(N143D+M145V) mutations change the indicated residues to those that are in the mα6 subunit and permit mutated hα6 subunits to show function when coexpressed with hβ2 and hβ3 subunits when wild-type hα6 subunits do not. Human nAChR α6 subunit residues 143 and 145 are in the E1 domain, in loop E, on the (−) or complementary face of the subunit. This suggests that interactions between the α6 subunit (−) face with the (+) face from either β2 subunits or β3 subunits are important for functional α6*-nAChR expression. In order for us to prove that the nAChR β3 subunit does affect the function of α6β2*-nAChR, a 9′ mutation was introduced into the hα6(N143D+M145V) subunit. Although coexpression of hα6(N143D+M145V)L9′S and hβ2 subunits did not yield receptors with reliable function, upon inclusion of the hβ3 subunit, function was evident in all oocytes coexpressing the three subunits together. These hα6(N143D+M145V)L9′Shβ2*-nAChR mimic the gain-of-function, high-affinity mα6*-nAChR artificially expressed in mouse midbrain dopamine neurons (13).
We conclude, based on the current and previous findings, that gain-of-function/reporter mutations introduced into α6 subunits in α6(β2 or β4)β3-nAChR are effective in potentiating receptor function. This potentiation yields receptors with higher agonist potency and larger magnitude responses to agonists, and also a finite likelihood of existing in a spontaneously open channel state. We also conclude from the present studies that wild-type β3 subunit incorporation into functionally competent (α6 or α6(N143D+M145V)) (L9′S or V13′S)(β4 or β2)*-nAChR has a potentiating effect irrespective of whether there are dominant-negative, null, or potentiating effects of β3 subunits on wild-type α6(β2 or β4)*-nAChR. In fact, reliable expression of functional gain-of-function α6*-nAChR is achieved only in the presence of nAChR β3 subunits. These results suggest that wild-type β3 subunit coexpression is at least permissive for cell surface expression of α6β4*-nAChR and very likely promotes function of these receptors. The strategies and results demonstrated here to increase function of α6*-nAChR to levels compatible with drug screening could facilitate the development of new drugs selective for α6*-nAChR. This is of increasing importance given the potentially important roles for α6*-nAChR in movement and movement disorders, mood disorders, and drug reinforcement (5, 13, 29–31).
Acknowledgments
We thank Dr. Jerry A. Stitzel (Department of Integrative Physiology, University of Colorado, Boulder, CO) for providing mouse nAChR subunits. We also thank Drs. Yongchang Chang and Paul Whiteaker of the Barrow Neurological Institute for comments about the project and manuscript and for technical advice and Minoti Bhakta for assistance.
This work was supported, in whole or in part, by National Institutes of Health Grant DA015389 (to R. J. L.), by Philip Morris USA Inc. and Philip Morris International through their External Research Program, and by endowment and capitalization funds from the Men's and Women's Boards of the Barrow Neurological Foundation. Portions of this work have been presented in abstract form (Dash, B., Chang, Y., and Lukas, R. J. (2008) Molecular and pharmacological characterization of recombinant α6β4*-nAChR. Soc. Neurosci. Abst. 34, 233.13).
- ACh
- acetylcholine
- nAChR
- nicotinic acetylcholine receptor(s)
- Imax
- peak current response
- m
- mouse
- h
- human.
REFERENCES
- 1. Lukas R. J., Changeux J. P., Le Novère N., Albuquerque E. X., Balfour D. J., Berg D. K., Bertrand D., Chiappinelli V. A., Clarke P. B., Collins A. C., Dani J. A., Grady S. R., Kellar K. J., Lindstrom J. M., Marks M. J., Quik M., Taylor P. W., Wonnacott S. (1999) International Union of Pharmacology. XX. Current status of the nomenclature for nicotinic acetylcholine receptors and their subunits. Pharmacol. Rev. 51, 397–401 [PubMed] [Google Scholar]
- 2. Capelli A. M., Castelletti L., Chen Y. H., Van der Keyl H., Pucci L., Oliosi B., Salvagno C., Bertani B., Gotti C., Powell A., Mugnaini M. (2011) Stable expression and functional characterization of a human nicotinic acetylcholine receptor with α6β2 properties: discovery of selective antagonists. Br. J. Pharmacol. 163, 313–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yang K., Buhlman L., Khan G. M., Nichols R. A., Jin G., McIntosh J. M., Whiteaker P., Lukas R. J., Wu J. (2011) Functional nicotinic acetylcholine receptors containing α6 subunits are on GABAergic neuronal boutons adherent to ventral tegmental area dopamine neurons. J. Neurosci. 31, 2537–2548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dash B., Bhakta M., Chang Y., Lukas R. J. (2011) Identification of N-terminal extracellular domain determinants in nicotinic acetylcholine receptor (nAChR) α6 subunits that influence effects of wild-type or mutant β3 subunits on function of α6β2*- or α6β4*-nAChR. J. Biol. Chem. 286, 37976–37989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cui C., Booker T. K., Allen R. S., Grady S. R., Whiteaker P., Marks M. J., Salminen O., Tritto T., Butt C. M., Allen W. R., Stitzel J. A., McIntosh J. M., Boulter J., Collins A. C., Heinemann S. F. (2003) The β3 nicotinic receptor subunit: a component of α-conotoxin MII-binding nicotinic acetylcholine receptors that modulate dopamine release and related behaviors. J. Neurosci. 23, 11045–11053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Exley R., Clements M. A., Hartung H., McIntosh J. M., Cragg S. J. (2008) α6-Containing nicotinic acetylcholine receptors dominate the nicotine control of dopamine neurotransmission in nucleus accumbens. Neuropsychopharmacology 33, 2158–2166 [DOI] [PubMed] [Google Scholar]
- 7. Meyer E. L., Yoshikami D., McIntosh J. M. (2008) The neuronal nicotinic acetylcholine receptors α4* and α6* differentially modulate dopamine release in mouse striatal slices. J. Neurochem. 105, 1761–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pons S., Fattore L., Cossu G., Tolu S., Porcu E., McIntosh J. M., Changeux J. P., Maskos U., Fratta W. (2008) Crucial role of α4 and α6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration. J. Neurosci. 28, 12318–12327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jackson K. J., Martin B. R., Changeux J. P., Damaj M. I. (2008) Differential role of nicotinic acetylcholine receptor subunits in physical and affective nicotine withdrawal signs. J. Pharmacol. Exp. Ther. 325, 302–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gotti C., Guiducci S., Tedesco V., Corbioli S., Zanetti L., Moretti M., Zanardi A., Rimondini R., Mugnaini M., Clementi F., Chiamulera C., Zoli M. (2010) Nicotinic acetylcholine receptors in the mesolimbic pathway: primary role of ventral tegmental area α6β2* receptors in mediating systemic nicotine effects on dopamine release, locomotion, and reinforcement. J. Neurosci. 30, 5311–5325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Booker T. K., Butt C. M., Wehner J. M., Heinemann S. F., Collins A. C. (2007) Decreased anxiety-like behavior in β3 nicotinic receptor subunit knockout mice. Pharmacol. Biochem. Behav. 87, 146–157 [DOI] [PubMed] [Google Scholar]
- 12. Drenan R. M., Grady S. R., Steele A. D., McKinney S., Patzlaff N. E., McIntosh J. M., Marks M. J., Miwa J. M., Lester H. A. (2010) Cholinergic modulation of locomotion and striatal dopamine release is mediated by α6α4* nicotinic acetylcholine receptors. J. Neurosci. 30, 9877–9889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Drenan R. M., Grady S. R., Whiteaker P., McClure-Begley T., McKinney S., Miwa J. M., Bupp S., Heintz N., McIntosh J. M., Bencherif M., Marks M. J., Lester H. A. (2008) In vivo activation of midbrain dopamine neurons via sensitized, high affinity α6 nicotinic acetylcholine receptors. Neuron 60, 123–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gotti C., Moretti M., Clementi F., Riganti L., McIntosh J. M., Collins A. C., Marks M. J., Whiteaker P. (2005) Expression of nigrostriatal α6-containing nicotinic acetylcholine receptors is selectively reduced, but not eliminated, by β3 subunit gene deletion. Mol. Pharmacol. 67, 2007–2015 [DOI] [PubMed] [Google Scholar]
- 15. Dash B., Chang Y., Lukas R. J. (2011) Reporter mutation studies show that nicotinic acetylcholine receptor (nAChR) α5 subunits and/or variants modulate function of α6*-nAChR. J. Biol. Chem. 286, 37905–37918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fucile S., Matter J. M., Erkman L., Ragozzino D., Barabino B., Grassi F., Alemà S., Ballivet M., Eusebi F. (1998) The neuronal α6 subunit forms functional heteromeric acetylcholine receptors in human transfected cells. Eur. J. Neurosci. 10, 172–178 [DOI] [PubMed] [Google Scholar]
- 17. Evans N. M., Bose S., Benedetti G., Zwart R., Pearson K. H., McPhie G. I., Craig P. J., Benton J. P., Volsen S. G., Sher E., Broad L. M. (2003) Expression and functional characterization of a human chimeric nicotinic receptor with α6β4 properties. Eur. J. Pharmacol. 466, 31–39 [DOI] [PubMed] [Google Scholar]
- 18. Gerzanich V., Kuryatov A., Anand R., Lindstrom J. (1997) “Orphan” α6 nicotinic AChR subunit can form a functional heteromeric acetylcholine receptor. Mol. Pharmacol. 51, 320–327 [PubMed] [Google Scholar]
- 19. Kuryatov A., Olale F., Cooper J., Choi C., Lindstrom J. (2000) Human α6 AChR subtypes: subunit composition, assembly, and pharmacological responses. Neuropharmacology 39, 2570–2590 [DOI] [PubMed] [Google Scholar]
- 20. Kuryatov A., Lindstrom J. (2011) Expression of functional human α6β2β3* acetylcholine receptors in Xenopus laevis oocytes achieved through subunit chimeras and concatamers. Mol. Pharmacol. 79, 126–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Broadbent S., Groot-Kormelink P. J., Krashia P. A., Harkness P. C., Millar N. S., Beato M., Sivilotti L. G. (2006) Incorporation of the β3 subunit has a dominant-negative effect on the function of recombinant central-type neuronal nicotinic receptors. Mol. Pharmacol. 70, 1350–1357 [DOI] [PubMed] [Google Scholar]
- 22. Tumkosit P., Kuryatov A., Luo J., Lindstrom J. (2006) β3 subunits promote expression and nicotine-induced up-regulation of human nicotinic α6* nicotinic acetylcholine receptors expressed in transfected cell lines. Mol. Pharmacol. 70, 1358–1368 [DOI] [PubMed] [Google Scholar]
- 23. Kuryatov A., Onksen J., Lindstrom J. (2008) Roles of accessory subunits in α4β2* nicotinic receptors. Mol. Pharmacol. 74, 132–143 [DOI] [PubMed] [Google Scholar]
- 24. Groot-Kormelink P. J., Boorman J. P., Sivilotti L. G. (2001) Formation of functional α3β4α5 human neuronal nicotinic receptors in Xenopus oocytes: a reporter mutation approach. Br. J. Pharmacol. 134, 789–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li P., McCollum M., Bracamontes J., Steinbach J. H., Akk G. (2011) Functional characterization of the α5(Asn-398) variant associated with risk for nicotine dependence in the α3β4α5 nicotinic receptor. Mol. Pharmacol. 80, 818–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Labarca C., Nowak M. W., Zhang H., Tang L., Deshpande P., Lester H. A. (1995) Channel gating governed symmetrically by conserved leucine residues in the M2 domain of nicotinic receptors. Nature 376, 514–516 [DOI] [PubMed] [Google Scholar]
- 27. Miko A., Werby E., Sun H., Healey J., Zhang L. (2004) A TM2 residue in the β1 subunit determines spontaneous opening of homomeric and heteromeric γ-aminobutyric acid-gated ion channels. J. Biol. Chem. 279, 22833–22840 [DOI] [PubMed] [Google Scholar]
- 28. Chang Y., Weiss D. S. (1998) Substitutions of the highly conserved M2 leucine create spontaneously opening rho1 γ-aminobutyric acid receptors. Mol. Pharmacol. 53, 511–523 [DOI] [PubMed] [Google Scholar]
- 29. Wang N., Orr-Urtreger A., Chapman J., Rabinowitz R., Nachman R., Korczyn A. D. (2002) Autonomic function in mice lacking α5 neuronal nicotinic acetylcholine receptor subunit. J. Physiol. 542, 347–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Salas R., Orr-Urtreger A., Broide R. S., Beaudet A., Paylor R., De Biasi M. (2003) The nicotinic acetylcholine receptor subunit α5 mediates short-term effects of nicotine in vivo. Mol. Pharmacol. 63, 1059–1066 [DOI] [PubMed] [Google Scholar]
- 31. Wang N., Orr-Urtreger A., Chapman J., Rabinowitz R., Korczyn A. D. (2004) Nicotinic acetylcholine receptor α5 subunits modulate oxotremorine-induced salivation and tremor. J. Neurol. Sci. 222, 87–91 [DOI] [PubMed] [Google Scholar]