Background: There are two isoforms of MeCP2: MeCP2_e1 and _e2. It is not known whether MeCP2_e2 has specific functions in vivo.
Results: Deletion of MeCP2_e2 results in no neurological phenotypes but confers a survival disadvantage to embryos and placenta defects.
Conclusion: MeCP2_e2 functions in placenta development and embryo survival.
Significance: MeCP2_e2 deletion results in a non-Rett syndrome phenotype but adversely affects embryo viability.
Keywords: Apoptosis, Embryo, Epigenetics, Gene Expression, Placenta, Mecp2_e2, Embryo Viability, Placenta
Abstract
Methyl CpG-binding protein 2 gene (MeCP2) mutations are implicated in Rett syndrome (RTT), one of the common causes of female mental retardation. Two MeCP2 isoforms have been reported: MeCP2_e2 (splicing of all four exons) and MeCP2_e1 (alternative splicing of exons 1, 3, and 4). Their relative expression levels vary among tissues, with MeCP2_e1 being more dominant in adult brain, whereas MeCP2_e2 is expressed more abundantly in placenta, liver, and skeletal muscle. In this study, we performed specific disruption of the MeCP2_e2-defining exon 2 using the Cre-loxP system and examined the consequences of selective loss of MeCP2_e2 function in vivo. We performed behavior evaluation, gene expression analysis, using RT-PCR and real-time quantitative PCR, and histological analysis. We demonstrate that selective deletion of MeCP2_e2 does not result in RTT-associated neurological phenotypes but confers a survival disadvantage to embryos carrying a MeCP2_e2 null allele of maternal origin. In addition, we reveal a specific requirement for MeCP2_e2 function in extraembryonic tissue, where selective loss of MeCP2_e2 results in placenta defects and up-regulation of peg-1, as determined by the parental origin of the mutant allele. Taken together, our findings suggest a novel role for MeCP2 in normal placenta development and illustrate how paternal X chromosome inactivation in extraembryonic tissues confers a survival disadvantage for carriers of a mutant maternal MeCP2_e2 allele. Moreover, our findings provide an explanation for the absence of reports on MeCP2_e2-specific exon 2 mutations in RTT. MeCP2_e2 mutations in humans may result in a phenotype that evades a diagnosis of RTT.
Introduction
Methyl CpG-binding protein 2 gene (MeCP2) mutations are implicated in Rett syndrome (RTT),5 one of the common causes of female mental retardation (1, 2). RTT patients exhibit apparently normal early psychomotor development and then gradually lose previously acquired psychomotor skills. Stereotypic hand movements and microcephaly are also clinical features of this disorder (3). MeCP2 binds to methylated CpG dinucleotides and functions as a transcriptional repressor through its interactions with the Sin3A/histone deacetylase complex and the SWI/SNF chromatin remodeling complex (4–8). To date, two MeCP2 isoforms have been characterized. The first reported MeCP2 isoform, referred to as MeCP2_e2 (translational start site in exon 2; also known as MeCP2A or MeCP2β), is generated by splicing of all four exons and has a translation start site in the middle of exon 2. The more recently discovered isoform, MeCP2_e1 (translational start site in exon 1; also known as MeCP2B or MeCP2α), results from alternative splicing of exons 1, 3, and 4 and has a translation start site in exon 1 (9, 10). Their relative expression levels vary among tissues, with MeCP2_e1 being more dominant in adult brain, whereas MeCP2_e2 is expressed more abundantly in placenta, liver, and skeletal muscle (10). The most common MeCP2 mutations in RTT occur in exons shared by both isoforms (11). However, no mutation in the MeCP2_e2-defining exon 2 has ever been reported in RTT. In this study, we performed specific disruption of the MeCP2_e2-defining exon 2 using the Cre-loxP system and examined the consequences of selective loss of MeCP2_e2 function in vivo.
EXPERIMENTAL PROCEDURES
Selective Targeting of MeCP2_e2
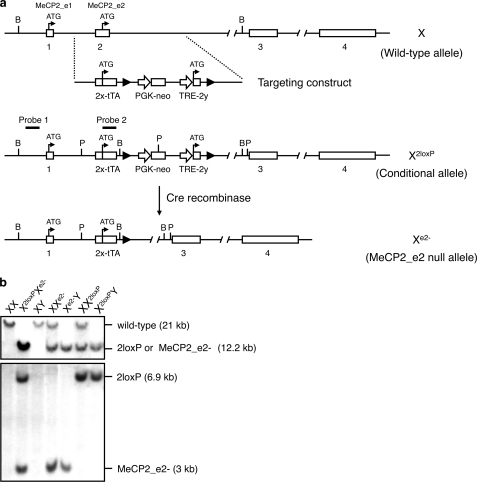
The MeCP2_e2 null allele was generated by Cre recombinase-mediated excision of exon 2 in MeCP2_e2 conditional mice (Fig. 1). MeCP2 sequences were either directly derived or amplified from genomic DNA obtained from CJ7 ES cells or a BAC clone carrying the MeCP2 locus. The 5′-end of the targeting vector consisted of a 1.2-kb region possessing homology to intron 1 and was generated by high fidelity PCR. The early part of exon 2 containing the untranslated region (referred to as exon 2x) was fused to the tetracycline transactivator (tTA) gene, having a stop codon and poly(A) sequence. The latter half of exon 2 (referred to as exon 2y) beginning from the ATG start site of MeCP2_e2 was placed under the control of the tetracycline-responsive promoter, TRE. A pair of loxP sites flanked this TRE-exon 2y sequence. A PGK-driven neomycin selection marker was positioned between the first loxP site and the TRE-2y region. The 3′ arm of the targeting vector consisted of a 5.9-kb EcoRI fragment derived from intron 2.
FIGURE 1.
Generation of MeCP2_e2-deficient mice. a, strategy for selective targeting of MeCP2_e2. Transcription start sites for MeCP2_e2 and MeCP2_e1 before and after exon 2 disruption are shown. loxP sites are denoted as filled triangles. Relative location of probes for Southern hybridization, and positions of restriction enzymes BamHI (B) and PvuII (P) are indicated. Crossing of MeCP2_e2 conditional mice with Nestin-Cre deleter mice results in the excision of the transcriptional start site of MeCP2_e2 and the creation of the MeCP2_e2 null allele, not only in neuronal cells but also in the germ line. Note that the transcriptional start of MeCP2_e1 remains intact after disruption of the MeCP2 locus. b, MeCP2_e2 wild-type and mutant alleles as differentiated by two sets of Southern hybridization. For the first screening (top), genomic DNA was digested with BamHI and probed to visualize the presence of the targeted MeCP2 locus containing the exon 2x-tTA sequence. In the second screening (bottom), PvuI-digested genomic DNA was probed to differentiate between the conditional (X2loxP) and null (Xe2−) alleles. Approximate band sizes are indicated in parentheses.
Generation of MeCP2_e2 Null Mice
A correctly targeted ES cell clone, confirmed by Southern blot analysis, was injected into 3.5-day postconception (dpc) C57BL/6J blastocysts. Approximately 10 ES cells were injected per blastocyst, and 20 blastocysts were transferred to each pseudopregnant recipient. The resulting chimeric offspring were intercrossed mice to generate F1 progeny. For deletion of MeCP2_e2, we crossed MeCP2_e2+/2loxP females with deleter mice carrying a Cre recombinase transgene under the control of the Nestin promoter. However, leaky expression from Nestin promoter-driven Cre recombinase induced a deletion in the germ line, resulting in progeny that carried the MeCP2_e2 null allele (Xe2−). This population was expanded and used in succeeding experiments. Genotypes of the resulting progeny were assessed by an initial PCR screen followed by two sets of Southern blotting. The MeCP2_e2 null allele was generated by Cre recombinase-mediated excision of exon 2 in MeCP2_e2 conditional mice (Fig. 1). A previously reported MeCP2 null mouse, B6.129P2(C)-Mecp2tm1.1Bird> (described as MeCP2−/y), generated by targeted disruption of exons 3 and 4 (12), was obtained from Jackson Laboratory (Bar Harbor, ME) and used as a control for some of the experiments. All animal studies were performed with the approval of the Animal Care Committee of the National Institute of Neuroscience, National Center of Neurology and Psychiatry, Japan.
RT-PCR and Real-time Quantitative PCR
We prepared 3–8 fresh frozen brains and placentas of various genotypes at 13.5 dpc and postnatal days 0 (P0) and 28 (P28). Total RNA was isolated from mouse tissue using the RNeasy minikit (Qiagen, Valencia, CA) following the manufacturer's recommendations. We carried out reverse transcription with the First-Strand cDNA synthesis kit (Amersham Biosciences) or TaqMan reverse transcription reagents (Applied Biosystems, Foster City, CA) using oligo(dT). Primer sequences and annealing conditions are as follows: for MECP2 exons 2 and 3, 5′-TTAGGGCTCAGGGAGGAAAA-3′ (forward) and 5′-CAAAATCATTAGGGTCCAAGG-3′ (reverse) with annealing temperature of 50 °C and expected PCR product size of 451 bp; for MECP2 exons 3 and 4, 5′-ATTATCCGTGACCGGGGA-3′ (forward) and 5′-TGATGCTGCTGCCTTTGGT-3′ (reverse) with annealing temperature of 55 °C and an expected PCR product size of 354 bp.
For quantitative analysis, we carried out PCR amplifications using Universal PCR Master Mix (Applied Biosystems) according to the manufacturer's recommendations in a real-time ABI PRISM 7700 platform (Applied Biosystems). Relative transcript ratios were normalized to GAPDH RNA. Primers and probes for mouse MeCP2 (common sequence of MeCP2_e2 and MeCP2_e1), MeCP2_e2, MAP2, IGFBP3, and BDNF are available from Applied Biosystems. The probes 5′-CGCCGAGCGGAGGAG-3′ and 5′-CCTGGTCTTCTGACTTTTCTTCCA were designed to amplify a portion of the MeCP2_e1 transcript, and a probe of CCTCCTCGCCTCCTCC-3′ was used. Sequence Detection System 1.7 software (Applied Biosystems) was used for analysis.
Immunohistochemical Analysis and TUNEL Assay
Tissues were fixed in 4% paraformaldehyde and then embedded in paraffin. Three-micrometer sections were prepared and stained with cresyl violet to visualize neurons. Purified MeCP2 antibody (provided by Dr. S. Kudo, Hokkaido Institute of Public Health, Sapporo, Japan), cleaved caspase-3 antibody (Chemicon International Inc., Temecula, CA), Peg-1 antibody (Atlas Antibodies AB, Stockholm, Sweden), and CRCX4 antibody (Abnova, Taipei, Taiwan) were used for immunohistological experiments. TUNEL assays were performed using terminal deoxynucleotidyltransferase (Roche Applied Science) following the manufacturer's recommendations.
Behavior Analysis
We performed tail suspension, footprinting, and open field analysis, using 4- or 5-week-old wild-type, MeCP2_e2−, MeCP2_e22loxP, and MeCP2−/y males.
Statistical Analysis
Statistical analysis was performed using the χ2 test. Animal crossings were performed to evaluate the effect of parent-specific transmission of the MeCP2_e2 null allele using appropriate sample sizes. Statistical significance of the expression levels was evaluated using Student's t test with a significance level of p < 0.05.
RESULTS AND DISCUSSION
MeCP2_e2-null Mouse Generation
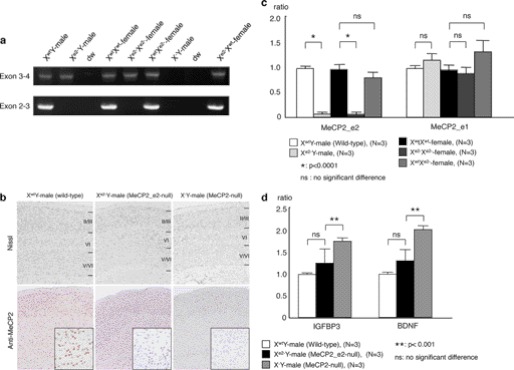
We generated the MeCP2_e2 mutant allele (Xe2−) by crossing mice carrying a tetracycline-inducible MeCP2_e2 conditional allele (X2loxP) with deleter mice carrying a Nestin-driven Cre recombinase transgene (Fig. 1). We observed germ line transmission of the MeCP2_e2 null allele in some of the F3 generation (Fig. 1), probably resulting from leaky expression of Nestin-driven Cre recombinase in non-brain tissue. This subpopulation was expanded, and the F10 to F12 generations were used for the experiments in this study. We confirmed loss of MeCP2_e2 expression, whereas MeCP2_e1 transcription remained intact in these animals (Fig. 2, a and c). Brain histological analysis showed no difference between MeCP2_e2 null mouse and wild-type mice (Fig. 2b).
FIGURE 2.
Absence of RTT-associated phenotypes in MeCP2_e2-deficient mice. a, reverse transcription PCR showing the selective loss of MeCP2_e2 transcripts in brains of MeCP2_e2 null males and females at P28. b, sections of P28 mouse brain were stained with cresyl violet to visualize neurons. Immunochemical staining was performed using anti-MeCP2 antibody. The MeCP2-deficient mouse, a previously reported MeCP2_e2 and MeCP2_e1 knockout (12), shows thinning of the cerebral cortex and no MeCP2-immunopositive cells. MeCP2_e2 null mouse exhibits MeCP2-immunopositive cells in the cerebral cortex. c, real-time PCR analysis of MeCP2_e2 and MeCP2_e1 of P28 brains. The MeCP2_e2-deficient mouse shows MeCP2_e1 expression but not MeCP2_e2, as indicated by the presence of exons 3 and 4 and the absence exons 2 and 3. d, quantitation of BDNF and IGFBP3 transcripts in P0 MeCP2_e2-deficient mice by real-time PCR. An XwtY male mouse was used as a reference. Statistical analysis was performed using Student's t test at p < 0.0001 (*) and p < 0.001 (**). Error bars, S.D.
Phenotypes and Expression Analyses of MeCP2_e2-null Mice
At birth, mice carrying MeCP2_e2 mutant alleles were indistinguishable from wild-type littermates. They developed into fertile adults and did not display any neurological deficits observed in murine models for RTT (12, 13), indicating that MeCP2_e1 is sufficient to carry on the functions of MeCP2 in the brain. Moreover, mice carrying MeCP2_e2 mutant alleles lived as long as their wild-type siblings, over 2 years (data not shown). Immunohistochemical staining of brain tissue from Xe2−Y and XwtXe2− animals at 28 days of age revealed normal morphology of neuronal layers in contrast to the denser packaging of neurons in a previously reported RTT model wherein both MeCP2 isoforms have been knocked out (Fig. 2b) (14, 15). Taken together, these results demonstrate that loss of MeCP2_e1 function is not sufficient to cause RTT-associated neurological phenotypes.
To examine the implications of MeCP2_e2 deficiency on MeCP2 transcriptional silencing activity, we checked mRNA levels of two MeCP2-regulated genes, insulin like growth factor binding protein 3 (IGFBP3) (16, 17) and brain-derived nerve growth factor (BDNF) (18). The mRNA levels of these genes in brains of Xe2−Y mice did not significantly differ from those of age-matched wild-type males (Fig. 2d). In contrast, IGFBP3 and BDNF transcript levels increased by 1.6- and 2-fold, respectively, in the X−Y total MeCP2 knockout. These findings indicate that MeCP2_e2 is not essential for mediating transcriptional silencing of MeCP2 target genes in the brain.
Parent-specific Effects of MeCP2_e2 Null Allele Birth Rates
We next examined whether MeCP2_e2 deficiency mediated any other non-neuronal phenotype. Interestingly, we observed reduced births of progeny that carried MeCP2_e2 null allele of maternal origin. Specifically, we found a 76% reduction in Xe2−Y males and a 44% reduction in Xe2−Xwt females born to XwtXe2− female and wild-type male pairings (Table 1). Similarly, in Xe2−Xwt and Xe2−Y pairings, Xe2−Y and Xe2−Xe2− births were reduced by 50 and 60%, respectively (Table 2). In contrast, birth rates of XwtXe2− females (having a paternal Xe2−) did not deviate from the expected values (Tables 2 and 3). We exclude the possibility that these were nonspecific effects resulting from toxicity of the tTA in the targeting vector because no such decreases in births were observed in an unrelated transgenic mouse model carrying the same vector backbone.6 Taken together, these results point to an association between reduced embryo viability and a maternally transmitted MeCP2_e2 null allele.
TABLE 1.
Offspring distribution at 4 weeks of age; crossing of XwtXe2− females and XwtY males (maternal transmission of MeCP2_e2 null allele)
χ sum = 107.04, p < 0.0001. % Change = (% observed value − % expected value)/% expected value) × 100.
| XwtXwt | Xe2−Xwt | XwtY | Xe2−Y | Total | |
|---|---|---|---|---|---|
| Observed | 52 | 27 | 101 | 12 | 192 |
| (27%) | (14%) | (53%) | (6%) | ||
| Estimated | 48 | 48 | 48 | 48 | 192 |
| (25%) | (25%) | (25%) | (25%) | ||
| % Change | 8% | −44% | −112% | −76% |
TABLE 2.
Offspring distribution at 4 weeks of age; crossing of XwtXe2− females and Xe2−Y males (biparental transmission of MeCP2_e2 null allele)
χ sum = 16.20, p < 0.002. % Change = (% observed value − % expected value)/% expected value) × 100.
| XwtXe2− | Xe2−Xe2− | XwtY | Xe2−Y | Total | |
|---|---|---|---|---|---|
| Observed | 11 | 4 | 20 | 5 | 40 |
| (28%) | (10%) | (50%) | (12%) | ||
| Estimated | 10 | 10 | 10 | 10 | 40 |
| (25%) | (25%) | (25%) | (25%) | ||
| % Change | 10% | −60% | 100% | −50% |
TABLE 3.
Offspring distribution at 4 weeks of age; crossing of XwtXwt females and Xe2−Y males (paternal transmission of MeCP2_e2 null allele)
χ sum = 2.28, no significant difference. % Change = (% observed value − % expected value)/% expected value) × 100.
| XwtXe2− | XwtY | Total | |
|---|---|---|---|
| Observed | 50 | 55 | 105 |
| (48%) | (52%) | ||
| Estimated | 52.5 | 52.5 | 105 |
| (50%) | (50%) | ||
| % Change | −4% | 4% |
To further delineate the time period at which selection against embryos carrying maternal MeCP2_e2 null alleles occurred, we examined the genotype distribution at 13.5 dpc and observed similar trends (Tables 4 and 5). Moreover, we did not find any evidence of resorbed embryos at this time point (data not shown). We also performed morphological assessment of the uterus at preimplantation and postimplantation stages and found no abnormalities in preimplantation sites and the implantation process (data not shown). Nevertheless, these findings suggest that the reduced number of embryos carrying a mutant maternal MeCP2_e2 allele is due neither to a failure in implantation nor to embryo lethality at postimplantation but to reduced viability of the embryo prior to implantation or early embryonic lethality after implantation.
TABLE 4.
Offspring distribution at 13.5 dpc; crossing of XwtXe2− females and XwtY males (maternal transmission of MeCP2_e2 null allele)
χ sum = 13.25, p < 0.005. % Change = (% observed value − % expected value)/% expected value) × 100.
| XwtXwt | Xe2−Xwt | XwtY | Xe2−Y | Total | |
|---|---|---|---|---|---|
| Observed | 36 | 28 | 46 | 18 | 128 |
| (28%) | (22%) | (36%) | (14%) | ||
| Estimated | 32 | 32 | 32 | 32 | 128 |
| (25%) | (25%) | (25%) | (25%) | ||
| % Change | 13% | −13% | 44% | −44% |
TABLE 5.
Offspring distribution at 13.5 dpc; crossing of XwtXwt females and Xe2−Y males (paternal transmission of MeCP2_e2 null allele)
χ sum = 2.28, no significant difference. % Change = (% observed value − % expected value)/% expected value) × 100.
| XwtXe2− | XwtY | Total | |
|---|---|---|---|
| Observed | 27 | 17 | 44 |
| (61%) | (39%) | ||
| Estimated | 22 | 22 | 44 |
| (50%) | (50%) | ||
| % Change | 23% | −23% |
Maternally Transmitted MeCP2_e2 Null Allele Results in Apoptosis and Altered peg-1 Expression in Placenta
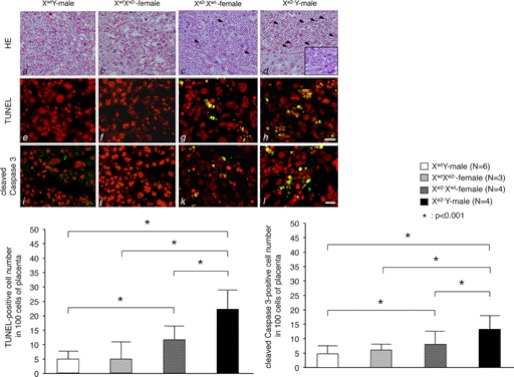
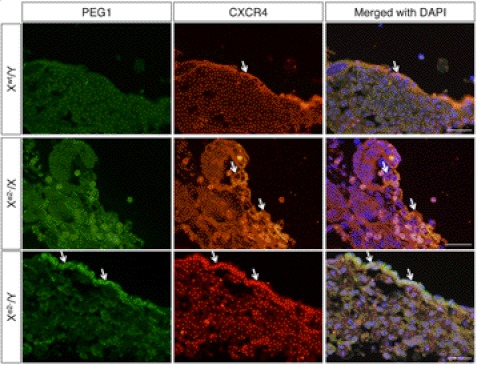
During early development of the female mammal, one of the two X chromosomes becomes transcriptionally inactive to allow dosage compensation of X-linked genes (19, 20). In mouse extraembryonic lineages, such as placenta, the paternally derived X chromosome undergoes preferential inactivation, a phenomenon called imprinted paternal X chromosome inactivation (XCI) (21, 22). Hence, we examined the effect of MeCP2_e2 deficiency in placenta tissue at 13.5 dpc. Interestingly, placentas of embryos carrying a maternal MeCP2_e2 null allele exhibited increased apoptosis, which was more notable in placentas of males (Fig. 3). These TUNEL-positive cells expressed peg-1 (supplemental Fig. 1), an imprinted gene known to function in placenta development (23, 24). In contrast, very few apoptotic cells were observed in the placenta of XwtXe2− embryos carrying a paternal MeCP2_e2 null allele (Fig. 3). In addition, immunostaining revealed increased Peg-1 levels in cells expressing CXCR4, a trophoblast marker (25), in the placenta of animals carrying a maternal MeCP2_e2 null allele (Fig. 4 and supplemental Fig. 1). Taken together, our results indicate that MeCP2_e2 is essential for the maintenance of peg-1 silencing in trophoblast cells and that elevated expression of peg-1 in the placenta has deleterious effects on cell survival.
FIGURE 3.
MeCP2_e2 deficiency results in placenta abnormalities. The top panels (a–d) show placenta sections stained with hematoxylin and eosin. The inset shows the section at higher magnification. Arrows show apoptotic cells. The middle panels (e–h) show TUNEL staining of the same sections. Apoptotic nuclei appear as multiple spots (yellow), indicating DNA fragmentation. Propidium iodide was used as counterstain. The bottom panels (i–l) show cleaved caspase-3 immunostaining of the placenta. TUNEL-positive cells are indicated by arrows. Scale bar, 25 μm. An increase in the number of TUNEL-positive cells and cleaved caspase 3-positive cells was observed in the placentas of Xe2−Xwt and Xe2−Y embryos having a maternal MeCP2_e2 null allele (refer to bar graphs in lower panel for quantitation) *, p < 0.001; brackets and asterisks indicate significant differences. Error bars, S.D.
FIGURE 4.
Loss of maternal MeCP2_e2 results in failure to silence peg-1 expression in trophoblast cells. CXCR4 is a trophoblast cell marker. Xwt/Y and Xe2−Xwt placenta have minimal peg-1 expression, whereas Xe2−/Y placenta show elevated peg-1 levels in trophoblast cells (arrows). Scale bars, 50 μm.
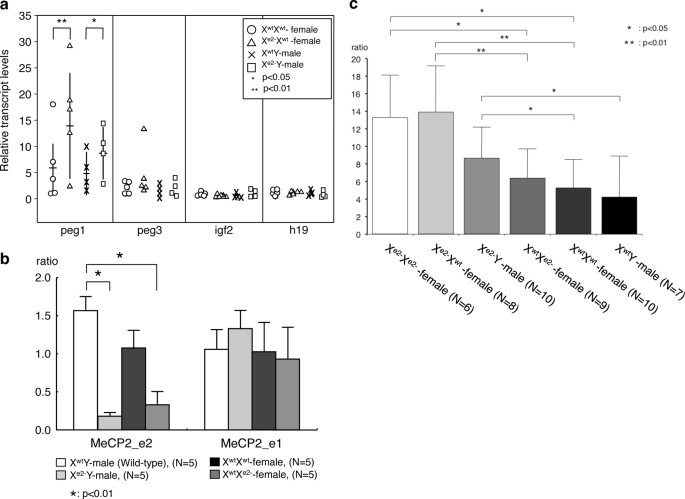
We also examined transcript levels of peg-1 and other imprinted genes involved in placenta function, such as peg-3, igf-2, and h19 (23). Among these four genes, peg-1 exhibited elevated transcript levels in the placenta of embryos carrying a maternal mutant allele (Fig. 5a), in concordance with our immunohistological findings. The mRNA levels of the other three genes were unchanged (Fig. 5a). In placentas of animals carrying the MeCP2 two-isoform knock-out allele, peg-1 expression was also elevated (Fig. 5b). The peg-1 transcript levels were not due to deregulation of imprinting in placenta because imprinted paternal XCI was found to be intact in these animals (Fig. 5c). Rather, elevated peg-1 transcript levels directly correlate with the loss of MeCP2_e2 expression effected by imprinted paternal XCI. These findings indicate that MeCP2_e2-specific transcriptional silencing activity is essential for the regulation of peg-1 expression and possibly of other genes in placenta.
FIGURE 5.
Quantitative PCR analysis of placenta. Shown are (a) placenta transcript levels of selected imprinted genes, peg-1, peg-3, igf-2, and h19, from 13.5 dpc embryos and (b) placenta transcript levels of peg-1 in MeCP2_e2 and MeCP2_e1 (two-isoform knockout) mutants. The horizontal and vertical bars of peg-1 transcripts (a) show averages and S.D. of each genotype, respectively. c, peg-1 expression in placentas of various genotypes. Maternally derived Xe2− allele up-regulated peg-1 expression. *, p < 0.05; **, p < 0.001. Brackets and asterisks indicate significant differences. Error bars, S.D.
The imprinted gene peg-1, located in murine chromosome 6, has been reported to play a role in angiogenesis in extraembryonic tissue (26). Mutations in peg-1 have also been implicated in placenta failure (24, 25) and embryonic growth retardation (27). One group has reported that paternally expressed transcripts are associated with premature placenta (28). Interestingly, paternal transmission of a peg-1 null allele in heterozygous mice results in diminished postnatal survival rates, whereas maternal transmission does not generate any remarkable phenotype (27, 29). It is clear from these reports that deregulation of peg-1 expression or imprinting status has deleterious consequences on embryo viability and placenta function. Our current study demonstrates that MeCP2_e2 is an essential regulator of peg-1 expression in extraembryonic tissue. As for how increased peg-1 expression correlates with observed placenta defects in carriers of a maternal MeCP2_e2 null allele, we propose a scenario wherein perturbations in peg-1 expression results in disruption of biological pathways that involve Peg-1, leading to enhanced apoptosis in placenta. Peg-1 is a membrane-bound protein that is predicted to have lipase or acyltransferase activity based on sequence homology with the α/β-hydrolase superfamily of proteins (30). Lipid metabolism is a very important biological process and is critical for the developing embryo and placenta. We propose that loss of MeCP2_e2 results in failure to transcriptionally silence peg-1 in extraembryonic tissue, leading to increased Peg-1 enzymatic activity, aberrant regulation of Peg-1 binding partners or downstream targets, and, ultimately, apoptosis.
We have earlier stated that we found the implantation process to be normal for these animals. Moreover, at 13.5 dpc, there was no evidence of resorbed embryos, and the skewed embryo genotypes resembled that from postnatal analysis. These results, taken together with the increased number of apoptotic trophoblast cells and elevated peg-1 expression in embryos carrying a maternal MeCP2_e2 null allele, suggest that the loss of MeCP2_e2 leads to trophoblast dysfunction during preimplantation through abnormal peg-1 expression. Furthermore, we view the increase in apoptotic trophoblast cells as a persisting phenotype brought about by early perturbation of placenta gene expression. In mice, placental development begins in the blastocyst at embryonic day 3.5 when the trophectoderm layer becomes distinct from the inner cell mass (32). The trophoblast that lines the blastocyst plays an important role during attachment to the endometrium and in the formation of the placenta (31, 32). It has been reported by other groups that trophoblast dysfunction leads to disruption of placenta formation and reduction of birth number (31, 33). In our current study, we have shown that loss of MeCP2_e2 results in a trophoblast defect that ultimately leads to reduced embryo viability.
Because some carriers of a mutant MeCP2_e2 allele are born and develop into healthy adults, we hypothesize that the placenta abnormalities in these animals may have been overcome by de novo MeCP2_e1 compensation or some other adaptation. In some types of extraembryonic cells, XCI can follow either a paternal or maternal pattern (34, 35). In somatic tissue, relaxation of imprinting occurs in certain pathological conditions (28, 36), and epigenetic heterogeneity at imprinted loci of autosomal chromosomes influences individual traits (37). The absence of MeCP2_e2 correlated with up-regulation of peg-1 expression, indicating a disturbance in regulation of downstream MeCP2 gene targets. Although increased apoptosis in placenta could be used to explain the decreased viability of Xe2−Y mice, this may also be interpreted as a way to eliminate functionally defective cells, thus contributing to the survival of some embryos.
The deleterious effects of MeCP2 mutations have been viewed mostly in the context of somatic XCI patterns. A number of studies have addressed the contribution of XCI to the pathogenesis of MeCP2 mutations (38, 39). It is suggested that XCI patterns may partly explain phenotypic variability in human RTT with MeCP2 mutations (38) and in mouse RTT models (39). Our findings indicate that this is not the full picture and that paternal X chromosome inactivation in the extraembryonic lineage also contributes to the deleterious consequences of MeCP2 mutations and, most likely, other X-linked gene mutations.
Recently, it has been reported that transgenic expression of either the MeCP2_e1 or MeCP2_e2 splice variant prevents the development of RTT-like neuronal phenotypic manifestations in a mouse model lacking MeCP2. This finding indicates that either MeCP2 splice variant is sufficient to fulfill MeCP2 function in the mouse brain (40). Our findings reveal a novel mechanism for the pathogenesis of MeCP2 mutations in extraembryonic tissue, wherein maternally inherited MeCP2_e2 mutations result in placenta abnormalities that ultimately lead to a survival disadvantage for carriers of this mutant allele. Our study also provides an explanation for the absence of reports on MeCP2_e2-specific exon 2 mutations in RTT. It is conceivable that MeCP2_e2 mutations in humans may result in a phenotype that evades a diagnosis of RTT. Moreover, the possible link between a novel genetic disorder characterized by reduced embryo viability and MeCP2 exon 2 mutations is a concept that merits further exploration. In summary, we have demonstrated that MeCP2_e2 is dispensable for RTT-associated neurological phenotypes. We have also discovered a novel requirement for MeCP2_e2 in placenta and embryo viability and have provided proof of existence of isoform-specific functions for two MeCP2 splicing variants.
Supplementary Material
Acknowledgments
We thank Dr. S. Kudo for the MeCP2 antibody and helpful suggestions and S. Kumagai and N. Tomimatsu for help with some of the experiments.
This work was supported by Ministries of Health, Labor, and Welfare Grants 15B-3, 18A-3, H21-Nanchi-Ippan-110, and H22-Nanchi-Ippan-133 and by Ministries of Education, Culture, Science, Sports, and Technology of Japan Grant 18390304.

This article contains supplemental Fig. 1.
A. Otsuki and A. Kurimasa, unpublished results.
- RTT
- Rett syndrome
- TRE
- tetracycline-responsive promoter
- tTA
- tetracycline transactivator
- XCI
- X chromosome inactivation
- PGK
- phosphoglycerate kinase.
REFERENCES
- 1. Amir R. E., Van den Veyver I. B., Wan M., Tran C. Q., Francke U., Zoghbi H. Y. (1999) Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 23, 185–188 [DOI] [PubMed] [Google Scholar]
- 2. Rett A. (1966) [On an unusual brain atrophy syndrome in hyperammonemia in childhood]. Wien Med. Wochenschr. 116, 723–726 [PubMed] [Google Scholar]
- 3. Hagberg B., Aicardi J., Dias K., Ramos O. (1983) A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls. Rett's syndrome. Report of 35 cases. Ann. Neurol. 14, 471–479 [DOI] [PubMed] [Google Scholar]
- 4. Lewis J. D., Meehan R. R., Henzel W. J., Maurer-Fogy I., Jeppesen P., Klein F., Bird A. (1992) Purification, sequence, and cellular localization of a novel chromosomal protein that binds to methylated DNA. Cell 69, 905–914 [DOI] [PubMed] [Google Scholar]
- 5. Meehan R. R., Lewis J. D., Bird A. P. (1992) Characterization of MeCP2, a vertebrate DNA-binding protein with affinity for methylated DNA. Nucleic Acids Res. 20, 5085–5092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nan X., Ng H. H., Johnson C. A., Laherty C. D., Turner B. M., Eisenman R. N., Bird A. (1998) Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature 393, 386–389 [DOI] [PubMed] [Google Scholar]
- 7. Jones P. L., Veenstra G. J., Wade P. A., Vermaak D., Kass S. U., Landsberger N., Strouboulis J., Wolffe A.P. (1998) Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat. Genet. 2, 187–191 [DOI] [PubMed] [Google Scholar]
- 8. Harikrishnan K. N., Chow M. Z., Baker E. K., Pal S., Bassal S., Brasacchio D., Wang L., Craig J. M., Jones P. L., Sif S., El-Osta A. (2005) Brahma links the SWI/SNF chromatin-remodeling complex with MeCP2-dependent transcriptional silencing. Nat. Genet. 37, 254–264 [DOI] [PubMed] [Google Scholar]
- 9. Kriaucionis S., Bird A. (2004) The major form of MeCP2 has a novel N terminus generated by alternative splicing. Nucleic Acids Res. 32, 1818–1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mnatzakanian G. N., Lohi H., Munteanu I., Alfred S. E., Yamada T., MacLeod P. J., Jones J. R., Scherer S. W., Schanen N. C., Friez M. J., Vincent J. B., Minassian B. A. (2004) A previously unidentified MECP2 open reading frame defines a new protein isoform relevant to Rett syndrome. Nat. Genet. 36, 339–341 [DOI] [PubMed] [Google Scholar]
- 11. Bienvenu T., Chelly J. (2006) Molecular genetics of Rett syndrome. When DNA methylation goes unrecognized. Nat. Rev. Genet. 7, 415–426 [DOI] [PubMed] [Google Scholar]
- 12. Guy J., Hendrich B., Holmes M., Martin J. E., Bird A. (2001) A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat. Genet. 27, 322–326 [DOI] [PubMed] [Google Scholar]
- 13. Chen R. Z., Akbarian S., Tudor M., Jaenisch R. (2001) Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat. Genet. 27, 327–331 [DOI] [PubMed] [Google Scholar]
- 14. Fukuda T., Itoh M., Ichikawa T., Washiyama K., Goto Y. (2005) Delayed maturation of neuronal architecture and synaptogenesis in cerebral cortex of Mecp2-deficient mice. J. Neuropathol. Exp. Neurol. 64, 537–544 [DOI] [PubMed] [Google Scholar]
- 15. Dragich J. M., Kim Y. H., Arnold A. P., Schanen N. C. (2007) Differential distribution of the MeCP2 splice variants in the postnatal mouse brain. J. Comp. Neurol. 501, 526–542 [DOI] [PubMed] [Google Scholar]
- 16. Chang Y. S., Wang L., Suh Y. A., Mao L., Karpen S. J., Khuri F. R., Hong W. K., Lee H. Y. (2004) Mechanisms underlying lack of insulin-like growth factor-binding protein-3 expression in non-small-cell lung cancer. Oncogene 23, 6569–6580 [DOI] [PubMed] [Google Scholar]
- 17. Itoh M., Ide S., Takashima S., Kudo S., Nomura Y., Segawa M., Kubota T., Mori H., Tanaka S., Horie H., Tanabe Y., Goto Y. (2007) Methyl CpG-binding protein 2 (a mutation of which causes Rett syndrome) directly regulates insulin-like growth factor binding protein 3 in mouse and human brains. J. Neuropathol. Exp. Neurol. 66, 117–123 [DOI] [PubMed] [Google Scholar]
- 18. Chen W. G., Chang Q., Lin Y., Meissner A., West A. E., Griffith E. C., Jaenisch R., Greenberg M. E. (2003) Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science 302, 885–889 [DOI] [PubMed] [Google Scholar]
- 19. Mak W., Nesterova T. B., de Napoles M., Appanah R., Yamanaka S., Otte A. P., Brockdorff N. (2004) Reactivation of the paternal X chromosome in early mouse embryos. Science 303, 666–669 [DOI] [PubMed] [Google Scholar]
- 20. Sado T., Ferguson-Smith A. C. (2005) Imprinted X inactivation and reprogramming in the preimplantation mouse embryo. Hum. Mol. Genet. 14, R59–64 [DOI] [PubMed] [Google Scholar]
- 21. Takagi N., Sasaki M. (1975) Preferential inactivation of the paternally derived X chromosome in the extraembryonic membranes of the mouse. Nature 256, 640–642 [DOI] [PubMed] [Google Scholar]
- 22. Harper M. I., Fosten M., Monk M. (1982) Preferential paternal X inactivation in extraembryonic tissues of early mouse embryos. J. Embryol. Exp. Morphol. 67, 127–135 [PubMed] [Google Scholar]
- 23. Obata Y., Kaneko-Ishino T., Koide T., Takai Y., Ueda T., Domeki I., Shiroishi T., Ishino F., Kono T. (1998) Disruption of primary imprinting during oocyte growth leads to the modified expression of imprinted genes during embryogenesis. Development 125, 1553–1560 [DOI] [PubMed] [Google Scholar]
- 24. Coan P. M., Burton G. J., Ferguson-Smith A. C. (2005) Imprinted genes in the placenta. A review. Placenta 26, S10–S20 [DOI] [PubMed] [Google Scholar]
- 25. Wu X., Li D. J., Yuan M. M., Zhu Y., Wang M. Y. (2004) The expression of CXCR4/CXCL12 in first-trimester human trophoblast cells. Biol. Reprod. 70, 1877–1885 [DOI] [PubMed] [Google Scholar]
- 26. Mayer W., Hemberger M., Frank H. G., Grümmer R., Winterhager E., Kaufmann P., Fundele R. (2000) Expression of the imprinted genes MEST/Mest in human and murine placenta suggests a role in angiogenesis. Dev. Dyn. 217, 1–10 [DOI] [PubMed] [Google Scholar]
- 27. Lefebvre L., Viville S., Barton S. C., Ishino F., Keverne E. B., Surani M. A. (1998) Abnormal maternal behavior and growth retardation associated with loss of the imprinted gene Mest. Nat. Genet. 20, 163–169 [DOI] [PubMed] [Google Scholar]
- 28. Looijenga L. H., Gillis A. J., Verkerk A. J., van Putten W. L., Oosterhuis J. W. (1999) Heterogeneous X inactivation in trophoblastic cells of human full-term female placentas. Am. J. Hum. Genet. 64, 1445–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Beechey C. V. (2000) Peg1/Mest locates distal to the currently defined imprinting region on mouse proximal chromosome 6 and identifies a new imprinting region affecting growth. Cytogenet. Cell Genet. 90, 309–314 [DOI] [PubMed] [Google Scholar]
- 30. Nikonova L., Koza R. A., Mendoza T., Chao P. M., Curley J. P., Kozak L. P. (2008) Mesoderm-specific transcript is associated with fat mass expansion in response to a positive energy balance. FASEB J. 22, 3925–3937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee K. Y., Jeong J. W., Tsai S. Y., Lydon J. P., DeMayo F. J. (2007) Mouse models of implantation. Trends Endcrinol. Metab. 18, 234–239 [DOI] [PubMed] [Google Scholar]
- 32. Watson E. D., Cross J. C. (2005) Development of structures and transport functions in the mouse placenta. Physiology 20, 180–193 [DOI] [PubMed] [Google Scholar]
- 33. Chaddha V., Viero S., Huppertz B., Kingdom J. (2004) Developmental biology of the placenta and the origins of placental insufficiency. Semin. Fetal Neonatal Med. 9, 357–369 [DOI] [PubMed] [Google Scholar]
- 34. Migeon B. R., Wolf S. F., Axelman J., Kaslow D. C., Schmidt M. (1985) Incomplete X chromosome dosage compensation in chorionic villi of human placenta. Proc. Natl. Acad. Sci. U.S.A. 82, 3390–3394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Coutinho-Camillo C. M., Brentani M. M., Butugan O., Torloni H., Nagai M. A. (2003) Relaxation of imprinting of IGFII gene in juvenile nasopharyngeal angiofibromas. Diagn. Mol. Pathol. 12, 57–62 [DOI] [PubMed] [Google Scholar]
- 36. Sakatani T., Wei M., Katoh M., Okita C., Wada D., Mitsuya K., Meguro M., Ikeguchi M., Ito H., Tycko B., Oshimura M. (2001) Epigenetic heterogeneity at imprinted loci in normal populations. Biochem. Biophys. Res. Commun. 283, 1124–1130 [DOI] [PubMed] [Google Scholar]
- 37. Bourdon V., Philippe C., Martin D., Verloès A., Grandemenge A., Jonveaux P. (2003) MECP2 mutations or polymorphisms in mentally retarded boys. Diagnostic implications. Mol. Diagn. 7, 3–7 [DOI] [PubMed] [Google Scholar]
- 38. Shahbazian M. D., Sun Y., Zoghbi H. Y. (2002) Balanced X chromosome inactivation patterns in the Rett syndrome brain. Am. J. Med. Genet. 111, 164–168 [DOI] [PubMed] [Google Scholar]
- 39. Young J. I., Zoghbi H. Y. (2004) X-chromosome inactivation patterns are unbalanced and affect the phenotypic outcome in a mouse model of rett syndrome. Am. J. Hum. Genet. 74, 511–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kerr B., Soto C. J., Saez M., Abrams A., Walz K., Young J. I. (2012) Transgenic complementation of MeCP2 deficiency. Phenotypic rescue of Mecp2-null mice by isoform-specific transgenes. Eur. J. Hum. Genet. 20, 69–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.