Background: Transcription factor FoxO1 is deacetylated in response to oxidative stress and hyperglycemia in diabetes.
Results: Mice bearing constitutively deacetylated alleles of Foxo1 develop larger atherosclerotic lesions despite improved plasma lipid levels in a bone marrow transplantation-independent manner.
Conclusion: FoxO1 deacetylation predisposes to atherosclerosis and vascular endothelial dysfunction.
Significance: The data identify a mechanism whereby oxidative stress, acting through FoxO1 deacetylation, promotes atherosclerosis in diabetic patients.
Keywords: Atherosclerosis, Genetics, Insulin Resistance, Mouse, Signal Transduction
Abstract
Complications of atherosclerosis are the leading cause of death of patients with type 2 (insulin-resistant) diabetes. Understanding the mechanisms by which insulin resistance and hyperglycemia contribute to atherogenesis in key target tissues (liver, vessel wall, hematopoietic cells) can assist in the design of therapeutic approaches. We have shown that hyperglycemia induces FoxO1 deacetylation and that targeted knock-in of alleles encoding constitutively deacetylated FoxO1 in mice (Foxo1KR/KR) improves hepatic lipid metabolism and decreases macrophage inflammation, setting the stage for a potential anti-atherogenic effect of this mutation. Surprisingly, we report here that when Foxo1KR/KR mice are intercrossed with low density lipoprotein receptor knock-out mice (Ldlr−/−), they develop larger aortic root atherosclerotic lesions than Ldlr−/− controls despite lower plasma cholesterol and triglyceride levels. The phenotype is unaffected by transplanting bone marrow from Ldlr−/− mice into Foxo1KR/KR mice, indicating that it is independent of hematopoietic cells and suggesting that the primary lesion in Foxo1KR/KR mice occurs in the vessel wall. Experiments in isolated endothelial cells from Foxo1KR/KR mice indicate that deacetylation favors FoxO1 nuclear accumulation and exerts target gene-specific effects, resulting in higher Icam1 and Tnfα expression and increased monocyte adhesion. The data indicate that FoxO1 deacetylation can promote vascular endothelial changes conducive to atherosclerotic plaque formation.
Introduction
Atherosclerosis and its complications represent a large unmet medical need in the treatment of type 2 diabetes (T2D)2 (1). In fact, the incidence and severity of atherosclerosis complications in T2D exceed those in the general population by factors of two and four, respectively, and account for nearly half of all diabetes-related expenditures, which in turn constitute one-third of medical insurance outlays by the United States government (2). In addition to its staggering costs, atherosclerotic cardiovascular disease in T2D is poorly responsive to tight glucose control (3, 4), raising the question of whether the predisposition of T2D patients to atherosclerosis reflects effects of insulin resistance, hyperglycemia, both, or neither (1).
Vascular endothelial dysfunction is the harbinger of atherosclerosis and is usually associated with hyperlipidemia, insulin resistance, or hyperglycemia (5). These factors affect endothelial cells in multiple ways, including altered nitric oxide metabolism, increased expression of cell adhesion molecules, and increased inflammation (6). In the presence of an altered lipoprotein milieu that includes increased production of oxidized low density lipoproteins (LDL), endothelial dysfunction leads to increased monocyte recruitment and their differentiation into macrophages that take up modified cholesterol-rich lipoproteins to form “foam cells” (7). This leads to the formation of atherosclerotic lesions and their stage-wise progression into increasingly complex thrombogenic lesions (8).
The role of insulin resistance in this process is well documented (6). In humans, insulin resistance and indeed hyperinsulinemia itself are associated with increased risk of cardiovascular atherosclerotic disease (9, 10). In studies of genetically engineered mice, targeted gene mutations affecting insulin signaling in endothelial cells increase atherosclerosis (11), as do generalized mutations of the insulin-regulated serine-threonine kinase, Akt1 (12). The latter effect is independent of hematopoietic cells, strongly implicating vascular insulin resistance as a causative factor (12).
The nutrient-sensitive transcription factor FoxO1 plays important roles in modulating insulin sensitivity (13). Among its functions are regulation of hepatic glucose production (14), β-cell response to oxidative stress (15), and bile acid metabolism (16). FoxO1 acts as a nutrient sensor not only for insulin and growth factors, but also for hyperglycemia-induced oxidative stress (15). FoxO1 activity is regulated by insulin- or growth factor-induced phosphorylation through Akt (17, 18). A second layer of regulation occurs through acetylation. FoxO1 is acetylated at multiple lysine residues and deacetylated by several deacetylases, including the NAD+-dependent deacetylase, SirT1 (15, 19). Deacetylation dampens the sensitivity of FoxO1 to insulin-induced phosphorylation, resulting in gain of function (20). Hyperglycemia-induced oxidative stress also causes FoxO1 deacetylation and nuclear translocation due to decreased sensitivity to insulin signaling, thus promoting endothelial dysfunction (21).
In this study, we employed mice homozygous for constitutively deacetylated FoxO1 alleles (Foxo1KR/KR) as a model mimicking the FoxO1 modifications associated with hyperglycemia (22) so that we could attempt to understand the contribution of hyperglycemia- (or oxidative stress-) induced FoxO1 modifications to atherosclerosis development in different organs.
EXPERIMENTAL PROCEDURES
Animal Studies
Foxo1KR/KR mice have been described (15, 21, 22). They were intercrossed with Ldlr−/− mice to generate Foxo1KR/KR:Ldlr−/− mice that were further backcrossed onto Ldlr−/− mice on the C57BL background for 5–6 generations prior to analysis. Animals were maintained on normal chow diet at ambient temperature on a 12-h dark/light cycle. The Western-type diet (WTD) was purchased from Harlan Laboratories (TD88137, 42% milk fat, 0.15% cholesterol). Atherosclerotic lesions and metabolic parameters were analyzed as described (23, 24). For bone marrow transplantation, 6-week-old male Foxo1KR/KR:Ldlr−/− mice and Ldlr−/− controls were irradiated at a dose of 6.5 grays twice at a 4-h interval and then received isolated bone marrow cells from donor mice (1 donor to 2.5 recipients) by retinal vein injection. Mice were allowed to recover for 2 weeks, during which time they were provided with antibiotics in the drinking water (enrofloxacin 0.125 g/liter) and then placed on WTD for 11 weeks for atherosclerosis studies. The Columbia University Animal Care and Utilization Committee approved all animal experimentation.
Determination of Hepatic Lipid Content
We homogenized ∼100 mg of liver in 3 ml of PBS and added 12 ml of chloroform-methanol (2:1). We resuspended the mixture and centrifuged it at 3,000 rpm for 10 min. We transferred the organic (bottom) phase into 20-cc scintillation vials and re-extracted the aqueous phase by adding 10 ml of chloroform-methanol-water (86:14:1) and repeating the centrifugation. We combined the organic layers from the two extractions, dried them using N2 gas, and dissolved them into 1 ml of 15% Triton X-100 in chloroform. After evaporation with N2 gas, we solubilized the lipids into 1 ml of H2O and measured the lipid content.
Hepatic Lipid Secretion Assays
We fasted mice for 5 h prior to injection of P407 (Sigma-Aldrich) at a dose of 1 g/kg of body weight. We drew blood and separated serum at various time points from the tail to measure triglycerides and cholesterol levels.
Peritoneal Macrophage Apoptosis Assays
We harvested peritoneal macrophages from WT or Foxo1KR/KR mice by peritoneal lavage 3 days after intraperitoneal injection of 4% thioglycolate and cultured them in 5.5 mm glucose DMEM supplemented with 10% fetal bovine serum (FBS) and 20% L929 cell-conditioned medium (25). Cells were treated with a combination of 58035 (10 μg/ml) and acetylated LDL (100 μg/ml) (FC), 7-ketocholesterol (30 μm), or thapsigargin (0.5 μm) for 18 h. Apoptosis was detected with Alexa Fluor 488 annexin V and phosphatidylinositol.
Primary Lung Endothelial Cell Culture
We performed lung endothelial cell isolation and culture as described (11). We incubated minced lung for 1 h at 37 °C in digestion medium, filtered the cells through a 40-μm strainer, and centrifuged them at 350 × g. We plated tissue digest from one mouse on a collagen-coated 10-cm dish. We cultured cells with DMEM containing with 5.5 mm glucose, 10% (v/v) FBS, 25 mm HEPES, 100 units/ml penicillin, 0.1 mg/ml streptomycin, 0.05 mg/ml endothelial mitogen, and 0.2 mg/ml heparin. After trypsin treatment during the first and second passage, we sorted cells as described above. We used cells at passages 2–4 after serum starvation for 16 h in DMEM supplemented with 5 mm glucose and 0.25% (v/v) bovine serum albumin (BSA). FoxO1 immunocytochemistry was performed as described previously (26).
Monocyte Adhesion Assay
Lung endothelial cells were grown in a 48-well plate to confluence. After stimulation with 100 μg/ml oxidized LDL (oxLDL) or 20 ng/ml TNF-α for 6 h, they were co-cultured with WEHI-274.1 cells (5 × 105 cells/well) for 1 h at 37 °C. After washing twice with DMEM, adhered WEHI-274.1 cells were counted under a microscope.
RNA Analysis
We used TRIzol reagents (Invitrogen) with glycogen precipitation to isolate total RNA from primary cells and High-Capacity cDNA reverse transcription kits (Applied Biosystems) to carry out reverse transcription. We performed quantitative real-time PCR with goTaq quantitative real-time PCR master mix (Promega) on a Bio-Rad CFX96 real-time PCR system. The relative gene expression levels were determined by the ΔΔCt method using cyclophilin A as a reference gene. The primer sequences are: cyclophilin A, forward 5′-TAT CTG CAC TGC CAA GAC TGA GTG-3′ and reverse 5′-CTT CTT GCT GGT CTT GCC ATT CC-3′; iNOS, forward 5′-GCT GTT CTC AGC CCA ACA AT-3′ and reverse 5′-GTC GAT GTC ACA TGC AGC TT-3′; Icam1, forward 5′-TGG CCC CTG GTC ACC GTT GTG AT-3′ and reverse 5′-AAC AGT TCA CCT GCA CGG ACC CA-3′; Vcam1, forward 5′-GGT CTT GGG AGC CTC AAC GGT-3′ and reverse 5′-AGG GCC ATG GAG TCA CCG ATT T-3′; Il-1α, forward 5′-TCA ACC AAA CTA TAT ATC AGG ATG TGG-3′ and reverse 5′-CGA GTA GGC ATA CAT GTC AAA TTT TAC-3′; Ccr1, forward 5′-GTG TTC ATC ATT GGA GTG GTG G-3′ and reverse 5′-GGT TGA ACA GGT AGA TGC TGG TC-3′; Tnfα, forward 5′-AGG TGT GGA CCT CGT TTC TG-3′ and reverse 5′-CGG ACT CCG CAA AGT CTA AG-3′.
Statistical Analysis
We evaluated statistical significance by performing unpaired two-tailed Student's t test. We used the customary threshold of p < 0.05 to declare statistical significance. All data are presented as means ± S.E.
RESULTS
Lower Plasma TG Levels in Chow-fed Foxo1KR/KR Mice
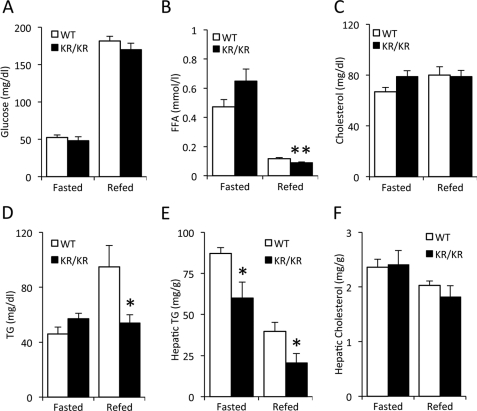
We assessed glucose and lipid levels in adult Foxo1KR/KR mice. Unlike mice harboring the same mutation on the outbred C57BL/6J × 129sv background (22), they displayed normal fasting and re-fed glucose (Fig. 1A) and insulin levels (not shown). These differences are likely the result of backcrossing onto the insulin-sensitive C57BL/6J strain, as we have seen in other mutations affecting insulin signaling (23, 27, 28). FFA levels showed a small decrease in the re-fed state, whereas total cholesterol levels were unchanged (Fig. 1, B and C). In contrast, the rise of plasma triglyceride (TG) levels after re-feeding was blunted in Foxo1KR/KR mutants (Fig. 1D). The decrease in plasma TG was associated with decreased hepatic TG content in fasted and re-fed animals (Fig. 1E), without significant changes in hepatic lipogenic gene expression (data not shown) or total cholesterol content (Fig. 1F).
FIGURE 1.
Metabolic analysis in chow-fed mice. A–D, plasma glucose, FFA, cholesterol, and TG in 14–16-month-old male Foxo1KR/KR (KR/KR) mice and control littermates (WT) fasted for 48 h and re-fed 24 h. E and F, hepatic cholesterol and TG content in re-fed Foxo1KR/KR (KR/KR) mice and control littermates (WT). *, p < 0.05, **, p < 0.01, n = 5–6. Data are presented as data as means ± S.E.
Reduced Plasma TG in Foxo1KR/KR Mice following WTD Feeding
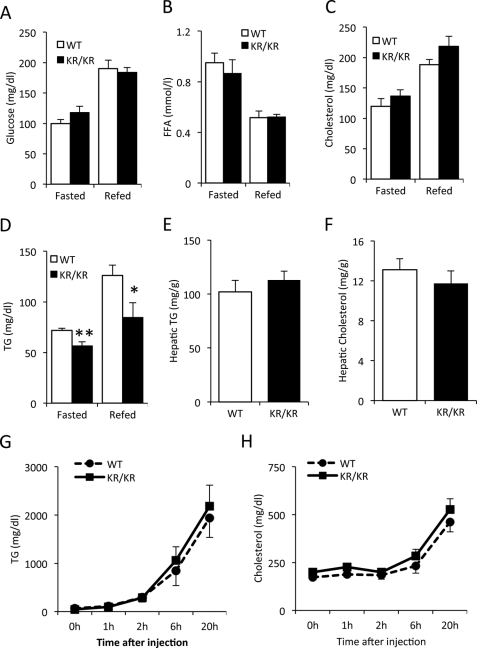
We used a standard cholesterol-rich, high lipid diet (so-called Western-type diet) (22) to probe the predisposition of Foxo1KR/KR mice to abnormalities of lipid metabolism. WTD feeding for 3 weeks increased plasma glucose, FFA, and cholesterol levels in Foxo1KR/KR mice to the same extent as in control littermates (Fig. 2, A–C). In contrast, plasma TG levels remained lower in Foxo1KR/KR mice in both fasted and re-fed conditions (Fig. 2D). Hepatic cholesterol and TG content in Foxo1KR/KR were similar to control mice (Fig. 2, E and F), providing evidence that decreased plasma TG levels were not secondary to decreased liver TG content. Likewise, the lower TG levels of Foxo1KR/KR mice were not due to defects in hepatic lipid secretion because lipid secretion rates, assessed by measuring plasma lipid levels following P407 injection to block clearance, were similar to WT controls (Fig. 2, G and H). These data indicate that FoxO1 deacetylation protects from the rise in postprandial TG levels following short term WTD.
FIGURE 2.
Metabolic analysis in WTD-fed mice. A–D, plasma glucose, FFA, cholesterol, and TG in 14–16-month-old male Foxo1KR/KR (KR/KR) mice and control littermates (WT) fasted for 48 h and re-fed 24 h. E and F, hepatic cholesterol and TG content in re-fed Foxo1KR/KR (KR/KR) mice and control littermates (WT). G and H, plasma TG and cholesterol levels in 14–16-month-old male Foxo1KR/KR (KR/KR) mice and control littermates (WT) after injection of P407. *, p < 0.05, **, p < 0.01, n = 6. Data are presented as means ± S.E.
Larger Atherosclerotic Lesions in Foxo1KR/KR:Ldlr−/− Mice
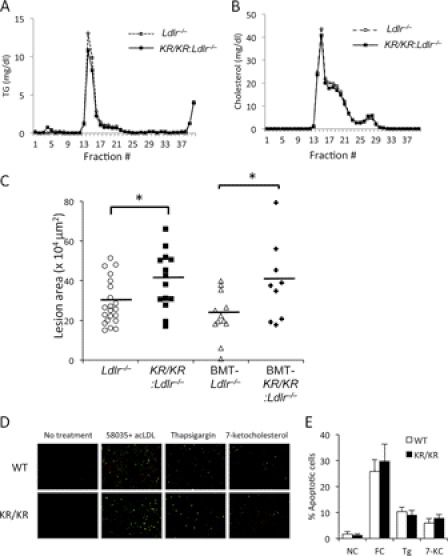
To study the effect of FoxO1 deacetylation on atherosclerosis, we intercrossed Foxo1KR/KR with Ldlr−/− mice and then subjected the mutant mice to WTD for 12 weeks. Foxo1KR/KR:Ldlr−/− mice maintained lower TG levels than controls and also demonstrated a significant decrease of total cholesterol levels (Table 1). FPLC analysis of TG and cholesterol content of plasma lipoproteins indicated modest decreases of VLDL TG (Fig. 3A) and LDL cholesterol (Fig. 3B), without changes to glucose and insulin levels (Table 1).
TABLE 1.
Metabolic characteristics of Foxo1KR/KR mice on Ldlr−/− background
Male Foxo1KR/KR:Ldlr−/−mice and control Ldlr−/− littermates (WT) were fed WTD for 12 weeks. Mice were fasted for 5–6 h before analysis. We present data as means ± S.E. NS = not significant.
| Genotype | Ldlr−/− (n = 21) | Foxo1KR/KR:Ldlr−/− (n = 14) | p (two-tailed) |
|---|---|---|---|
| Body weight (g) | 33.8 ± 0.9 | 32.1 ± 1.3 | NS |
| Glucose (mg/dl) | 145 ± 7 | 145 ± 8 | NS |
| Insulin (ng/ml) | 1.21 ± 0.15 | 1.47 ± 0.26 | NS |
| Aortic root lesion area (μm2) | 287,782 ± 24,767 | 399,271 ± 38706 | p < 0.05 |
| TG (mg/dl) | 523 ± 52 | 370 ± 38 | p < 0.05 |
| Total cholesterol (mg/dl) | 1,206 ± 101 | 907 ± 102 | p < 0.05 |
| HDL-C (mg/dl) | 60 ± 6 | 53 ± 7 | NS |
| FFA (mmol/l) | 0.5 ± 0.03 | 0.5 ± 0.03 | NS |
| Ketones (mm) | 1.1 ± 0.1 | 0.9 ± 0.1 | NS |
FIGURE 3.
Metabolic and aortic root lesion analyses in WTD-fed Foxo1KR/KR:Ldlr−/− mice. A and B, FPLC analysis of TG and cholesterol content in lipoprotein fractions of pooled serum from Foxo1KR/KR:Ldlr−/− and control Ldlr−/− mice fasted for 6 h. C, aortic root lesion size in Foxo1KR/KR:Ldlr−/− and control Ldlr−/− mice without or following bone marrow transplantation (BMT). Data are presented as means ± S.E. *, p < 0.05, n = 9–21. D, apoptosis measurements in thioglycolate-elicited peritoneal macrophages isolated from WTD-fed male Foxo1KR/KR:Ldlr−/− mice (KR/KR) and control Ldlr−/− mice (WT). A representative experiment is shown. E, quantification of apoptosis cells in D, n = 4. Data are presented as means ± S.E. Tg, thapsigargin; 7-KC, 7-ketocholesterol. NC, no challenge; FC, free cholesterol.
We conducted a formal assessment of atherosclerotic lesions by histomorphometric measurements of aortic root lesion size. Based on the reduced plasma TG and cholesterol, we predicted that we would find either smaller lesions or no effect on lesion size of the Foxo1KR/KR mutation. Surprisingly, we detected an ∼40% increase in lesion size in Foxo1KR/KR:Ldlr−/− mice (Table 1 and Fig. 3C). Because the mutant Foxo1KR allele is expressed in all tissues, we conclude that the benefits of reduced plasma lipids and normal hepatic lipid secretion in Foxo1KR/KR:Ldlr−/− mice are offset by effects of the mutation at other sites.
Unaltered Apoptosis in Macrophages Isolated from Foxo1KR/KR:Ldlr−/− Mice
We have previously shown that macrophages isolated from Foxo1KR/KR mice have reduced inflammation in response to free cholesterol loading, without changes to apoptosis induced by a variety of stimuli (25). Given the role of macrophage apoptosis in atherosclerotic plaque formation and progression (8), we examined whether macrophages from the double mutant Foxo1KR/KR:Ldlr−/− mice showed altered apoptosis. However, similar to our previous study in Foxo1KR/KR mice (25), peritoneal macrophages isolated from WTD-fed Foxo1KR/KR:Ldlr−/− mice showed the same apoptotic response to free cholesterol loading, thapsigargin (an inducer of the unfolded protein response), and 7-ketocholesterol as macrophages isolated from control Ldlr−/− mice (Fig. 3, D and E).
Atherosclerotic Lesion Size in Foxo1KR/KR:Ldlr−/− Mice Is Unaffected by Bone Marrow Transfer
To probe further the role of the hematopoietic compartment in the pathogenesis of increased atherosclerosis in Foxo1KR/KR:Ldlr−/− mice, we performed bone marrow transplants. Following x-ray irradiation to suppress the endogenous marrow, we transferred bone marrow from Foxo1KR/KR:Ldlr−/− mice into Ldlr−/− mice and vice versa and then subjected mice to WTD for 11 weeks. Foxo1KR/KR:Ldlr−/− mice tended to have lower plasma cholesterol and TG levels than control Ldlr−/− mice (Table 2), although the differences did not reach statistical significance in this instance. However, morphometric analysis of the aortic root showed a persistent increase of lesion size in Foxo1KR/KR:Ldlr−/− mice when compared with Ldlr−/− mice (Fig. 3C), suggesting that atherosclerosis development is unaffected by hematopoietic-derived cell types, including macrophages. Thus, by exclusion, it appears that the proatherogenic abnormalities in Foxo1KR/KR:Ldlr−/− mice can be accounted for by vessel wall abnormalities.
TABLE 2.
Metabolic characteristics of Foxo1KR/KRmice on Ldlr−/− background following bone marrow transplantation
Mice were fed WTD for 11 weeks and fasted for 5–6 h before analysis. We present data as means ± S.E. NS = not significant.
| Genotype | Ldlr−/− (n = 12) | Foxo1KR/KR:Ldlr−/− (n = 9) | p (two-tailed) |
|---|---|---|---|
| Body weight (g) | 26.9 ± 0.9 | 29.6 ± 0.9 | NS |
| Glucose (mg/dl) | 133 ± 4 | 127 ± 10 | NS |
| TG (mg/dl) | 278 ± 31 | 235 ± 35 | NS |
| Total cholesterol (mg/dl) | 1031 ± 82 | 887 ± 51 | NS |
| FFA (mmol/l) | 0.62 ± 0.05 | 0.7 ± 0.04 | NS |
| Aortic root lesion area (μm2) | 224888 ± 33998 | 388130 ± 66267 | <0.05 |
Deacetylated FoxO1 Affects Vascular Endothelial Cell Gene Expression
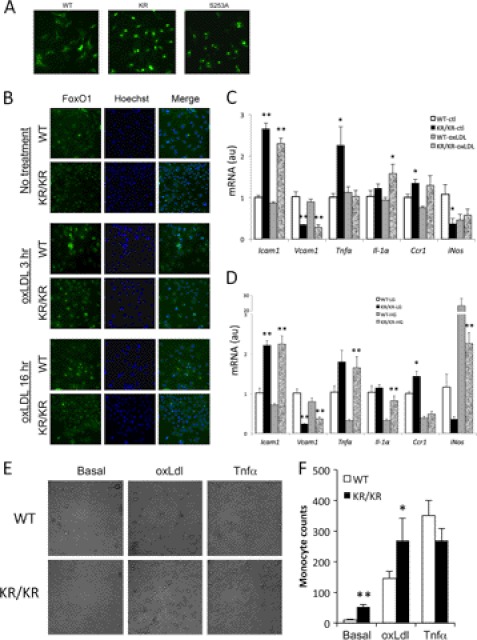
Multiple cell types in the vessel wall contribute to atherosclerosis progression, but the inciting lesion is generally assumed to occur in endothelial cells (29). Therefore, we focused our investigations on cellular mechanisms whereby the deacetylated FoxO1 might predispose to endothelial dysfunction. To document that deacetylation results in gain of function in vascular endothelial cells, we first monitored FoxO1 subcellular localization in the presence of serum, a condition known to cause FoxO1 localization to the cytoplasm (30). In this set of experiments, we used a GFP-tagged FoxO1 to facilitate detection. In human aortic endothelial cells, expression of GFP-FoxO1-WT resulted in nuclear FoxO1 localization in only 8% of transfected cells, whereas 69% of cells expressing GFP-FoxO1-KR showed nuclear localization. As a positive control, 92% of cells transfected with the phosphorylation-defective, constitutively nuclear FoxO1 S253A (in which the key Akt phosphorylation site has been mutated) (31) showed nuclear localization (Fig. 4A). These data confirm data in other cell types indicating that deacetylation promotes FoxO1 nuclear retention (15, 21, 22).
FIGURE 4.
Deacetylation of FoxO1 induced dysfunctions in vascular endothelial cells. A, human aortic endothelial cells were transduced with adenoviruses encoding GFP-tagged FoxO1 wild type (WT), Foxo1KR/KR (KR), or S253A mutant at multiplicity of infection = 20, and FoxO1 localization was determined by fluorescence microscopy in cells cultured in the presence of 10% FBS. B, isolated lung endothelial cells were treated with oxLDL for 3 or 16 h and then stained with FoxO1 (green) and Hoechst (nuclei). C and D, gene expression analysis in mRNA isolated from lung endothelial cells derived from wild type (WT) or Foxo1KR/KR mice (KR/KR). Cells were incubated in low glucose (LG, 5.5 mm) or high glucose (HG, 25 mm) for 11 h (C) or with oxLDL (0.1 mg/ml) for 7 h (D) prior to isolating RNA. *, p < 0.05, **, p < 0.01 versus untreated cells, n = 4 for each treatment condition. ctl, control; au, arbitrary units. E, monocyte adhesion assay on primary lung endothelial cells. Cells were pretreated with oxLDL (0.1 mg/ml) or TNFα (20 ng/ml) for 6 h before co-culture with WEHI-274.1 cells. F, quantification of adhering monocytes in E. *, p < 0.05, **, p < 0.01 versus EC from WT mice, n = 4. Data are presented as means ± S.E.
Next, we isolated and cultured primary endothelial cells from Foxo1KR/KR and littermate control mice and assessed endogenous FoxO1 subcellular localization. Similar to the transfected protein, endogenous FoxO1 in endothelial cells isolated from Foxo1KR/KR mice was primarily nuclear when compared with cells isolated from WT mice in basal conditions and following stimulation with oxidized LDL (Fig. 4B).
We measured gene expression in response to oxidized LDL, elevated glucose (25 mm), or lipopolysaccharide. We (38) and others (32) have shown that Icam and Vcam are FoxO1 targets. In primary endothelial cells derived from Foxo1KR/KR mice, we observed increased expression of Icam1 and decreased expression of Vcam1, consistent with our previous observations that the effects of the FoxO1 deacetylated mutant are target gene-specific (15) (Fig. 4C). We observed similar changes to Icam1 and Vcam1 in glucose-treated cells (Fig. 4D). In addition, we observed increased expression of pro-inflammatory cytokines Tnfα and Il-1α, but reduced iNos under basal conditions (Fig. 4, C and D) and following treatment with 25 mm glucose (Fig. 4D). These data indicate that the main pro-atherogenic change in cultured endothelial cells in response to FoxO1 deacetylation is increased Icam1 expression, raising the possibility that altered endothelial/monocyte interactions underlie the pathogenesis of increased atherosclerosis in Foxo1KR/KR:Ldlr−/− mice (33).
To investigate the latter point, we performed ex vivo cell adhesion assays by co-culturing primary endothelial cells derived from Foxo1KR/KR and WT mice with WEHI-274.1 cells following incubation with oxidized LDL or TNFα. We observed increased adhesion of monocytes to endothelial cells under basal conditions and following incubation with oxLDL, but not TNFα (Fig. 4, E and F).
DISCUSSION
T2D worsens atherosclerosis. Paradoxically, tight glycemia control, either by insulin or by insulin-sensitizing drugs, fails to reduce atherosclerosis complications in T2D (4, 34). In this study, we employed Foxo1KR/KR knock-in mice to mimic the effect of oxidative stress- (or hyperglycemia-) induced FoxO1 deacetylation on atherosclerosis and demonstrate that deacetylation of FoxO1 induces a small reduction of plasma TG and LDL cholesterol levels. Despite these potentially beneficial changes vis à vis atherogenesis, homozygosity for the deacetylated Foxo1 allele in the Ldlr−/− background increased atherosclerosis in a bone marrow transplantation-independent manner. Experiments with isolated vascular endothelial cells indicate that deacetylated FoxO1 affects their gene expression response to a variety of pathogenic stimuli and increases monocyte/endothelial interactions, suggesting that the primary atherogenic abnormality in these mice occurs in the vascular endothelium.
Several lines of evidence support the contention that altered FoxO1 function in vascular endothelial cells is responsible for the observed worsening of lesions. First, similar to our findings, ubiquitous loss of function of Akt1 worsens atherosclerosis in LDL receptor- or ApoE-deficient mice in a bone marrow transplantation-independent manner, suggesting that the main effect of Akt1 on atherosclerosis is through its vascular endothelial cell functions (12). Because FoxO1 is inhibited by Akt, it is likely that FoxO1 gain of function phenocopies Akt loss of function. Second, a different type of FoxO1 gain of function by way of a phosphorylation-defective mutant that mimics the effects of insulin resistance also causes endothelial cell dysfunction by promoting reactive oxygen species formation, and elevated glucose is permissive for this effect (21). Third, targeted inactivation of insulin receptor in vascular endothelial cells promotes atherosclerosis and can reasonably be expected to increase FoxO1 activity (11). Fourth, inactivation of the three genes encoding FoxO isoforms (1, 3a, and 4) in vascular endothelial cells protects Ldlr−/− mice against atherosclerosis (38). We are mindful that the Foxo1KR/KR mutation is ubiquitous and that this limitation prevents us from definitively concluding that its untoward effects on atherosclerosis are due to vascular endothelial cells, as opposed to, for example, vascular smooth muscle cells. In this regard, it is noteworthy that Akt1 mutations affect both endothelial and vascular smooth muscle cells (35). Therefore, any conclusions as to the relative role of endothelial dysfunction in this model will have to be documented by further studies targeting the deacetylated mutant FoxO1 to vascular endothelial cells.
The pathogenesis of atherosclerosis is complex, with contributions from several mechanisms (inflammation, tissue damage, altered metabolism) in different tissues (29). Similarly, FoxO1 exerts tissue-specific effects that are modulated by hyperglycemia via oxidative stress. In the liver, hyperglycemia increases FoxO1-mediated gluconeogenesis, further increasing insulin resistance through glucose toxicity (36). FoxO1 could potentially also affect adipose tissue, as we have shown that Foxo1KR/KR mice have lower rates of lipolysis (22). In the macrophage, FoxO1 represses inflammatory responses but does not affect apoptosis, with negligible overall effects on atherogenesis (25, 37). The present data indicate that the effects of FoxO1 gain of function through deacetylation in vascular endothelial cells trump its beneficial effects to lower plasma TG and cholesterol levels in WTD-fed Ldlr−/− mice. Our data are also consistent with the observation that liver-specific Foxo1 ablation fails to affect atherosclerosis development despite a mild elevation of plasma and hepatic lipid levels (16).
In conclusion, our study demonstrates that oxidative stress- (or hyperglycemia-) mediated FoxO1 deacetylation in vascular endothelial cells contributes to the increased risk of atherosclerosis in diabetic patients. In this regard, our data suggest that treatment options should aim to lower FoxO1 activity in this cell type to reduce atherosclerosis in diabetes.
Acknowledgments
We thank I. Tabas, A. Tall, and members of the Accili laboratory for helpful discussions and Ana Flete for technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants HL87123, DK57539, and DK63608 (to D. A.).
- T2D
- type 2 diabetes
- TG
- triglyceride
- oxLDL
- oxidized low density lipoproteins
- WTD
- Western-type diet.
REFERENCES
- 1. Bornfeldt K. E., Tabas I. (2011) Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. 14, 575–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Caro J. J., Ward A. J., O'Brien J. A. (2002) Lifetime costs of complications resulting from type 2 diabetes in the U.S. Diabetes Care 25, 476–481 [DOI] [PubMed] [Google Scholar]
- 3. Holman R. R., Paul S. K., Bethel M. A., Matthews D. R., Neil H. A. (2008) 10-Year follow-up of intensive glucose control in type 2 diabetes. N. Engl. J. Med. 359, 1577–1589 [DOI] [PubMed] [Google Scholar]
- 4. Duckworth W., Abraira C., Moritz T., Reda D., Emanuele N., Reaven P. D., Zieve F. J., Marks J., Davis S. N., Hayward R., Warren S. R., Goldman S., McCarren M., Vitek M. E., Henderson W. G., Huang G. D. (2009) Glucose control and vascular complications in veterans with type 2 diabetes. N. Engl. J. Med. 360, 129–139 [DOI] [PubMed] [Google Scholar]
- 5. Rask-Madsen C., King G. L. (2007) Mechanisms of disease: endothelial dysfunction in insulin resistance and diabetes. Nat. Clin. Pract. Endocrinol. Metab. 3, 46–56 [DOI] [PubMed] [Google Scholar]
- 6. Kim J. A., Montagnani M., Koh K. K., Quon M. J. (2006) Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation 113, 1888–1904 [DOI] [PubMed] [Google Scholar]
- 7. Tabas I., Tall A., Accili D. (2010) The impact of macrophage insulin resistance on advanced atherosclerotic plaque progression. Circ. Res. 106, 58–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tabas I. (2010) Macrophage death and defective inflammation resolution in atherosclerosis. Nat. Rev. Immunol. 10, 36–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Després J. P., Lamarche B., Mauriège P., Cantin B., Dagenais G. R., Moorjani S., Lupien P. J. (1996) Hyperinsulinemia as an independent risk factor for ischemic heart disease. N. Engl. J. Med. 334, 952–957 [DOI] [PubMed] [Google Scholar]
- 10. Howard G., O'Leary D. H., Zaccaro D., Haffner S., Rewers M., Hamman R., Selby J. V., Saad M. F., Savage P., Bergman R. (1996) Insulin sensitivity and atherosclerosis: the Insulin Resistance Atherosclerosis Study (IRAS) investigators. Circulation 93, 1809–1817 [DOI] [PubMed] [Google Scholar]
- 11. Rask-Madsen C., Li Q., Freund B., Feather D., Abramov R., Wu I. H., Chen K., Yamamoto-Hiraoka J., Goldenbogen J., Sotiropoulos K. B., Clermont A., Geraldes P., Dall'Osso C., Wagers A. J., Huang P. L., Rekhter M., Scalia R., Kahn C. R., King G. L. (2010) Loss of insulin signaling in vascular endothelial cells accelerates atherosclerosis in apolipoprotein E null mice. Cell Metab. 11, 379–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fernández-Hernando C., Ackah E., Yu J., Suárez Y., Murata T., Iwakiri Y., Prendergast J., Miao R. Q., Birnbaum M. J., Sessa W. C. (2007) Loss of Akt1 leads to severe atherosclerosis and occlusive coronary artery disease. Cell Metab. 6, 446–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Accili D., Arden K. C. (2004) FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell 117, 421–426 [DOI] [PubMed] [Google Scholar]
- 14. Lin H. V., Accili D. (2011) Hormonal regulation of hepatic glucose production in health and disease. Cell Metab. 14, 9–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kitamura Y. I., Kitamura T., Kruse J. P., Raum J. C., Stein R., Gu W., Accili D. (2005) FoxO1 protects against pancreatic beta cell failure through NeuroD and MafA induction. Cell Metab. 2, 153–163 [DOI] [PubMed] [Google Scholar]
- 16. Haeusler R. A., Pratt-Hyatt M., Welch C. L., Klaassen C. D., Accili D. (2012) Impaired generation of 12-hydroxylated bile acids links hepatic insulin signaling with dyslipidemia. Cell Metab. 15, 65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brunet A., Bonni A., Zigmond M. J., Lin M. Z., Juo P., Hu L. S., Anderson M. J., Arden K. C., Blenis J., Greenberg M. E. (1999) Akt promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell 96, 857–868 [DOI] [PubMed] [Google Scholar]
- 18. Nakae J., Park B. C., Accili D. (1999) Insulin stimulates phosphorylation of the forkhead transcription factor FKHR on serine 253 through a wortmannin-sensitive pathway. J. Biol. Chem. 274, 15982–15985 [DOI] [PubMed] [Google Scholar]
- 19. Brunet A., Sweeney L. B., Sturgill J. F., Chua K. F., Greer P. L., Lin Y., Tran H., Ross S. E., Mostoslavsky R., Cohen H. Y., Hu L. S., Cheng H. L., Jedrychowski M. P., Gygi S. P., Sinclair D. A., Alt F. W., Greenberg M. E. (2004) Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303, 2011–2015 [DOI] [PubMed] [Google Scholar]
- 20. Qiang L., Banks A. S., Accili D. (2010) Uncoupling of acetylation from phosphorylation regulates FoxO1 function independent of its subcellular localization. J. Biol. Chem. 285, 27396–27401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tanaka J., Qiang L., Banks A. S., Welch C. L., Matsumoto M., Kitamura T., Ido-Kitamura Y., DePinho R. A., Accili D. (2009) Foxo1 links hyperglycemia to LDL oxidation and endothelial nitric oxide synthase dysfunction in vascular endothelial cells. Diabetes 58, 2344–2354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Banks A. S., Kim-Muller J. Y., Mastracci T. L., Kofler N. M., Qiang L., Haeusler R. A., Jurczak M. J., Laznik D., Heinrich G., Samuel V. T., Shulman G. I., Papaioannou V. E., Accili D. (2011) Dissociation of the glucose and lipid regulatory functions of FoxO1 by targeted knock-in of acetylation-defective alleles in mice. Cell Metab. 14, 587–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Han S., Liang C. P., Westerterp M., Senokuchi T., Welch C. L., Wang Q., Matsumoto M., Accili D., Tall A. R. (2009) Hepatic insulin signaling regulates VLDL secretion and atherogenesis in mice. J. Clin. Invest. 119, 1029–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Qiang L., Lin H. V., Kim-Muller J. Y., Welch C. L., Gu W., Accili D. (2011) Proatherogenic abnormalities of lipid metabolism in SirT1 transgenic mice are mediated through Creb deacetylation. Cell Metab. 14, 758–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tsuchiya K., Banks A. S., Liang C. P., Tabas I., Tall A. R., Accili D. (2011) Homozygosity for an allele encoding deacetylated FoxO1 protects macrophages from cholesterol-induced inflammation without increasing apoptosis. Arterioscler. Thromb. Vasc. Biol. 31, 2920–2928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kitamura T., Kitamura Y. I., Kobayashi M., Kikuchi O., Sasaki T., Depinho R. A., Accili D. (2009) Regulation of pancreatic juxtaductal endocrine cell formation by FoxO1. Mol. Cell Biol. 29, 4417–4430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kido Y., Philippe N., Schäffer A. A., Accili D. (2000) Genetic modifiers of the insulin resistance phenotype in mice. Diabetes 49, 589–596 [DOI] [PubMed] [Google Scholar]
- 28. Han S., Liang C. P., DeVries-Seimon T., Ranalletta M., Welch C. L., Collins-Fletcher K., Accili D., Tabas I., Tall A. R. (2006) Macrophage insulin receptor deficiency increases ER stress-induced apoptosis and necrotic core formation in advanced atherosclerotic lesions. Cell Metab. 3, 257–266 [DOI] [PubMed] [Google Scholar]
- 29. Glass C. K., Witztum J. L. (2001) Atherosclerosis: the road ahead. Cell 104, 503–516 [DOI] [PubMed] [Google Scholar]
- 30. Frescas D., Valenti L., Accili D. (2005) Nuclear trapping of the forkhead transcription factor FoxO1 via Sirt-dependent deacetylation promotes expression of glucogenetic genes. J. Biol. Chem. 280, 20589–20595 [DOI] [PubMed] [Google Scholar]
- 31. Nakae J., Kitamura T., Kitamura Y., Biggs W. H., 3rd, Arden K. C., Accili D. (2003) The forkhead transcription factor Foxo1 regulates adipocyte differentiation. Dev. Cell 4, 119–129 [DOI] [PubMed] [Google Scholar]
- 32. Lee J. W., Chen H., Pullikotil P., Quon M. J. (2011) Protein kinase A-α directly phosphorylates FoxO1 in vascular endothelial cells to regulate expression of vascular cellular adhesion molecule-1 mRNA. J. Biol. Chem. 286, 6423–6432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kevil C. G., Patel R. P., Bullard D. C. (2001) Essential role of ICAM-1 in mediating monocyte adhesion to aortic endothelial cells. Am. J. Physiol. Cell Physiol. 281, C1442–C1447 [DOI] [PubMed] [Google Scholar]
- 34. Boyle P. J. (2007) Diabetes mellitus and macrovascular disease: mechanisms and mediators. Am. J. Med. 120, Suppl. 2, S12–S17 [DOI] [PubMed] [Google Scholar]
- 35. Fernández-Hernando C., József L., Jenkins D., Di Lorenzo A., Sessa W. C. (2009) Absence of Akt1 reduces vascular smooth muscle cell migration and survival and induces features of plaque vulnerability and cardiac dysfunction during atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 29, 2033–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Haeusler R. A., Han S., Accili D. (2010) Hepatic FoxO1 ablation exacerbates lipid abnormalities during hyperglycemia. J. Biol. Chem. 285, 26861–26868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Senokuchi T., Liang C. P., Seimon T. A., Han S., Matsumoto M., Banks A. S., Paik J. H., DePinho R. A., Accili D., Tabas I., Tall A. R. (2008) Forkhead transcription factors (FoxOs) promote apoptosis of insulin-resistant macrophages during cholesterol-induced endoplasmic reticulum stress. Diabetes 57, 2967–2976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tsuchiya K., Tanaka J., Shuiqing Y., Welch C. L., Depinho R. A., Tabas I., Tall A. R., Goldberg I. J., Accili D. (2012) FoxOs integrate pleiotropic actions of insulin in vascular endothelium to protect mice from atherosclerosis. Cell Metab. 15, 372–381 [DOI] [PMC free article] [PubMed] [Google Scholar]