Background: The low-density lipoprotein receptor (LDLR) regulates Aβ levels in the mouse brain, but its effect on Aβ cellular uptake and degradation is unknown.
Results: Increasing LDLR levels enhanced Aβ uptake and degradation by astrocytes.
Conclusion: LDLR represents a pathway for Aβ uptake into astrocytes.
Significance: Identifying receptors involved in the cellular internalization of Aβ is important for understanding Alzheimer disease pathogenesis.
Keywords: Alzheimer Disease, Amyloid, ApoE, Astrocytes, Lipoprotein Receptor
Abstract
Accumulation of the amyloid β (Aβ) peptide within the brain is hypothesized to be one of the main causes underlying the pathogenic events that occur in Alzheimer disease (AD). Consequently, identifying pathways by which Aβ is cleared from the brain is crucial for better understanding of the disease pathogenesis and developing novel therapeutics. Cellular uptake and degradation by glial cells is one means by which Aβ may be cleared from the brain. In the current study, we demonstrate that modulating levels of the low-density lipoprotein receptor (LDLR), a cell surface receptor that regulates the amount of apolipoprotein E (apoE) in the brain, altered both the uptake and degradation of Aβ by astrocytes. Deletion of LDLR caused a decrease in Aβ uptake, whereas increasing LDLR levels significantly enhanced both the uptake and clearance of Aβ. Increasing LDLR levels also enhanced the cellular degradation of Aβ and facilitated the vesicular transport of Aβ to lysosomes. Despite the fact that LDLR regulated the uptake of apoE by astrocytes, we found that the effect of LDLR on Aβ uptake and clearance occurred in the absence of apoE. Finally, we provide evidence that Aβ can directly bind to LDLR, suggesting that an interaction between LDLR and Aβ could be responsible for LDLR-mediated Aβ uptake. Therefore, these results identify LDLR as a receptor that mediates Aβ uptake and clearance by astrocytes, and provide evidence that increasing glial LDLR levels may promote Aβ degradation within the brain.
Introduction
Alzheimer disease (AD),2 the most common cause of dementia, is characterized by the appearance of extracellular amyloid plaque deposition in the brain, intraneuronal neurofibrillary tangle formation, and marked neuronal and synaptic loss (1). Aggregation of the amyloid β (Aβ) peptide into oligomers and fibrils is hypothesized to lead to a pathological cascade resulting in synaptic dysfunction, neuronal loss, and ultimately cognitive decline (2). Aβ is produced by proteolytic processing of the amyloid precursor protein (APP) via proteases β- and γ-secretase, and is subsequently secreted into the extracellular space (3). Familial mutations in APP, presenilin 1 (PSEN1), and presenilin 2 (PSEN2) cause the rare early-onset form of AD primarily through altering the production of Aβ (4). However, Aβ production does not appear to be altered in the more common late-onset form of AD (1, 5). In fact, a recent study suggests that Aβ clearance from the central nervous system, and not production, may be impaired in individuals with late-onset AD (6). Therefore, better characterization of the mechanisms underlying Aβ elimination from the brain may lead to insights into the pathogenesis of the disease and reveal unique therapeutic targets.
Several clearance pathways for Aβ likely exist in the central nervous system, including cellular uptake and lysosomal degradation, transport across the blood-brain barrier, extracellular degradation by proteolytic enzymes, and bulk flow drainage of interstitial fluid and cerebrospinal fluid. Aβ clearance via the blood-brain barrier and degradation by extracellular enzymes has been extensively studied (5, 7, 8). However, the mechanisms regulating the process of cellular uptake and degradation of Aβ are less characterized. Current evidence suggests that astrocytes are one of the main cell types in the brain that play a central role in the clearance of Aβ. Astrocytes localize around Aβ plaques in AD brains (9–11), and have been shown to exhibit intracellular Aβ immunoreactivity in histological studies (12–15). Cell-based assays have shown that cultured astrocytes take up and degrade soluble Aβ and enhance the clearance of fibrillar Aβ from ex vivo brain slices (16–19). However, the receptors mediating Aβ uptake into astrocytes are currently unknown.
An isoform of apolipoprotein E (apoE4) is currently the strongest known genetic risk factor for AD. ApoE is a ligand that facilitates the receptor-mediated endocytosis of lipoprotein particles into cells (20). ApoE is hypothesized to play a central role in AD pathogenesis in large part through the regulation of Aβ deposition and clearance (21, 22). Murine studies have shown that the amount of apoE in the brain dramatically affects the extent of Aβ deposition, as deletion of apoE in APP transgenic mouse models significantly decreased brain amyloid levels (23, 24). Therefore, targeting proteins in the brain that modulate apoE levels represent an attractive pathway for decreasing amyloid deposition. The low-density lipoprotein receptor (LDLR) family of receptors is a group of proteins sharing similar structural characteristics that exhibit various important endocytic and signaling functions. Members of this family include LDLR, lipoprotein receptor related-protein 1 (LRP1), very-low density lipoprotein receptor, apolipoprotein E receptor 2 (apoER2), and megalin (LRP2) (25). LDLR plays a key role in cholesterol metabolism in the periphery through facilitating the removal of cholesterol-containing lipoprotein particles from the circulation (26). The uptake of lipoprotein particles occurs through the binding of apolipoprotein B-100 (apoB-100) or apoE to LDLR and subsequent clathrin-mediated endocytosis. In the central nervous system, the function of LDLR is less well characterized. Recently, we have shown that increasing LDLR levels in the brain significantly decreased apoE levels and markedly inhibited amyloid deposition in the APPswe/PSEN1ΔE9 (APP/PS1) transgenic mouse model (27). Using in vivo microdialysis, we also observed that LDLR overexpression decreased steady-state interstitial fluid Aβ levels and enhanced the clearance of Aβ from the brain extracellular space (27). These findings clearly demonstrated that LDLR is capable of regulating brain Aβ levels. However, the possibility that LDLR-mediated endocytosis represents a pathway for the cellular regulation of Aβ levels has yet to be analyzed.
In this study, we investigated how altering LDLR levels in primary astrocytes affects Aβ uptake and degradation. We provide evidence that both LDLR overexpression and deletion alters soluble Aβ uptake. We demonstrate that increasing levels of LDLR facilitates Aβ transport to lysosomes and enhances Aβ intracellular degradation. We also show that LDLR can modulate cellular Aβ uptake and clearance through a pathway that does not require the presence of apoE. Finally, we provide evidence that Aβ directly binds to LDLR. The findings from this study identify a specific receptor-mediated pathway for the uptake and clearance of Aβ by astrocytes, and suggest that enhancing LDLR levels in glial cells represents a potential approach to lowering Aβ levels in the brain.
EXPERIMENTAL PROCEDURES
Reagents
Aβ(1–40), Aβ(1–42), and Aβ(40–1) were purchased from American Peptide Company (Sunnyvale, CA). HiLyte Fluor 488-labeled Aβ42, TAMRA-labeled Aβ42, and Dutch/Iowa Aβ40 were purchased from Anaspec (Fremont, CA). Aβ peptides were reconstituted in dimethyl sulfoxide at a concentration of 200 μm and stored at −80 °C prior to use. 125I-Aβ(1–40) was purchased from PerkinElmer Life Sciences (Waltham, MA), reconstituted at a concentration of 22.7 nm in dimethyl sulfoxide, and stored at −20 °C prior to use. Recombinant mouse LDLR protein (extracellular domain, amino acids 1–790) was purchased from Sino Biological Inc. (catalog number 50305-M08H, Beijing, China), reconstituted in water at a concentration of 500 μg/ml, and dialyzed in PBS overnight at 4 °C. The dialyzed peptide was then stored at −80 °C prior to use. LysoTracker probe and DiI-LDL were purchased from Invitrogen. Recombinant receptor-associated protein (RAP) protein was purchased from EMD Biosciences (catalog number 553506). Recombinant mouse PCSK9 protein was purchased from Sino Biological Inc. (catalog number 50251-M08H, Beijing, China), reconstituted in water at a concentration of 500 μg/ml, and stored at −80 °C prior to use.
Primary Cultures
The generation and characterization of the LDLR transgenic (Tg) mice were described previously (27). LDLR−/− mice were purchased from Jackson Laboratory (catalog number 002207). For all experiments, wild type (WT) littermates were used as controls. All experimental protocols were approved by the Animal Studies Committee at Washington University, St. Louis, MO. Cortical primary murine astrocytes were cultured from postnatal day 2 mouse pups. Cortices were dissected from the brain and placed in Hanks' balanced salt solution. The brain tissue was then washed with Hanks' balanced salt solution and treated with 0.05% trypsin/EDTA for 15 min at 37 °C. Following trypsin digestion, the tissue was resuspended and triturated using fire-polished pipettes in growth media containing Dulbecco's modified Eagle's/F-12, 20% fetal bovine serum (FBS), 10 ng/ml of epidermal growth factor, 100 units/ml of penicillin/streptomycin, and 1 mm sodium pyruvate. The cell suspension was then passed through a 100-μm nylon filter and plated into T-75 flasks coated with poly-d-lysine. The medium of the mixed glial cultures was changed after 6 days, and every 3 days following the initial change. Once the cells reached confluence, they were shaken at 250 rpm for 3 h and the medium was aspirated to remove the less adherent microglial cells. The astrocyte-enriched cultures were then washed with PBS, detached from the plate using 0.05% trypsin/EDTA, and passaged into 6-, 12-, or 24-well plates for experiments.
Measurement of ApoE Levels by ELISA
Primary astrocytes were plated into 12-well plates and grown to confluence. The cell monolayers were then washed twice with serum-free media (SFM) (Dulbecco's modified Eagle's medium/F-12, N-2 growth supplement, 100 units/ml of penicillin/streptomycin, and 1 mm sodium pyruvate) and 500 μl of fresh SFM was added. The cells were then incubated at 37 °C for 3, 8, or 24 h. Following incubation, the medium was removed and the protease inhibitor was added (Complete protease inhibitor mixture, Roche Diagnostics). The cells were washed three times in PBS and lysed in 1% Triton X-100 lysis buffer (1% Triton X-100, 150 mm NaCl, 50 mm Tris-HCl, and Complete protease inhibitor mixture). The lysate was then cleared by centrifugation at 14,000 × g and the total protein content was measured by BCA assay. The apoE level in the medium was quantified using a sandwich ELISA for apoE. Mouse monoclonal antibody HJ6.2 was used as the capture antibody and mouse monoclonal antibody HJ6.3-biotin was used as the detection antibody (both antibodies were produced in-house with full-length astrocyte-derived mouse apoE-containing lipoproteins as the antigen). The pooled C57BL/6J plasma set at a concentration of 329 μg/ml was used as the standard for ELISA. 96-Well microtiter plates were coated overnight at 4 °C with HJ6.2 antibody (5 μg/ml). All washes were performed 5 times/well using a standard microplate washer. Coated plates were washed and blocked for 1 h at 37 °C in 1% milk in PBS. The plates were then washed again and samples and standards were loaded in 0.5% bovine serum albumin in PBS, 0.025% Tween 20 and incubated overnight at 4 °C. Then, plates were washed and incubated with HJ6.3-biotin antibody at a concentration of 400 ng/ml in 0.5% bovine serum albumin/PBS, 0.025% Tween 20 at 37 °C for 90 min. Plates were washed and horseradish peroxidase-conjugated streptavidin at a 1:5000 dilution was incubated for 90 min at room temperature. Plates were washed, tetramethylbenzidine substrate was added, and the absorbance was measured at 650 nm. All apoE values were normalized to total cell protein levels.
Aβ Uptake and Clearance Assays
Primary astrocytes were plated into either 12- or 24-well plates and grown to confluence. To measure Aβ uptake, the cells were first washed twice with SFM and fresh SFM was added to the cells. Soluble Aβ(1–40) or Aβ(1–42) were then added to the medium at a concentration of 2 μg/ml and the cells were incubated at 37 °C for 3 h. The medium was then removed and the cells were washed twice with PBS. To remove cell surface-bound Aβ, the cells were incubated with 0.05% trypsin/EDTA for 20 min. The cells were then pelleted by centrifugation and the pellet was washed twice with PBS. Following centrifugation, 1% Triton X-100 lysis buffer (1% Triton X-100, 150 mm NaCl, 50 mm Tris-HCl, and Complete protease inhibitor mixture) was added to the cell pellet and the cell pellet was incubated at 4 °C for 30 min. The cell lysates were then cleared by centrifugation at 14,000 × g. For the clearance assays, the cells were first washed twice with SFM and fresh SFM was then added to the cells. Soluble Aβ(1–40) or Aβ(1–42) were then added at a concentration of 2 μg/ml and the cells were incubated at 37 °C for 24 h. A control group was also included in which the Aβ was added directly to fresh SFM to calculate how much Aβ was initially added to the cells (0 h time point). The medium was then collected from the cells and Complete protease inhibitor (Roche) was added. The cell monolayer was then washed twice with PBS and 1% Triton X-100 lysis buffer was added to the cells and incubated at 4 °C for 30 min. The cell lysates were then cleared by centrifugation at 14,000 × g. Protein content was measured in all cell lysates using a BCA protein assay (Thermo Scientific). Aβ(x-40) and Aβ(x-42) specific sandwich ELISAs developed in our laboratory were used to quantify Aβ40 and Aβ42 levels, respectively, in the lysate or media. For the Aβ(x-40) assay, HJ2 (anti-Aβ35–40) was used as the capture antibody and HJ5.1-biotin (anti-Aβ13–28) as the detection antibody. For the Aβ(x-42) assay, HJ7.4 (anti-Aβ37–42) was used as the capture antibody and HJ5.1-biotin (anti-Aβ13–28) as the detection antibody.
125I-Aβ Degradation Assays
Primary astrocytes were plated into 12-well plates and grown to confluence. The cells were then washed twice with SFM and 0.25 nm 125I-Aβ(1–40) was added to the cells in 500 μl of SFM. The cells were then incubated for 3, 8, or 24 h at 37 °C. The medium was then collected and the cells were washed three times in PBS. The cells were then lysed in the plate by the addition of radioimmunoprecipitation assay (RIPA) buffer (1% Nonidet P-40, 1% sodium deoxycholate, 0.1% SDS, 25 mm Tris-HCl, 150 mm NaCl) (catalog number 89901, Thermo Scientific) and the lysate was cleared by centrifugation at 14,000 × g. Total protein content was measured by BCA assay. The medium was then subjected to trichloroacetic (TCA) acid precipitation. 50 mg/ml of BSA and 2% deoxycholate were added to the medium and the tubes were vortexed and incubated on ice for 30 min. TCA (20% of final volume) was added to the tubes and following a quick vortex, the tubes were incubated on ice for 1 h. All tubes were then spun down at 14,000 × g for 30 min. Counts/min in both the supernatant and pellet were measured by a γ counter. To measure Aβ degradation by astrocyte-conditioned medium, primary astrocytes were plated into 12-well plates and grown to confluence. The cells were then washed twice with SFM and fresh SFM was added to the cells. The cells were then incubated for 3, 8, or 24 h at 37 °C. The medium was then collected, and 125I-Aβ(1–40) was added to the astrocyte-conditioned medium for 3, 8, or 24 h at 37 °C. A TCA precipitation was then performed as described above. The cells from which the media was originally collected were also lysed as above, and the total protein content was measured with a BCA protein assay.
Fluorescent Aβ Uptake and Colocalization Analysis
Primary astrocytes were plated into 35-mm μ-dish chambers. For DiI-LDL imaging experiments, the cells were washed twice with SFM and HiLyte Fluor 488-labeled Aβ42 (3 μg/ml) was added to the cells in SFM. The cells were then incubated at 37 °C for 3 h prior to imaging. One hour prior to imaging, DiI-LDL was added to the cells (0.5 μg/ml). The cells were then washed twice with SFM, and fresh SFM was added for imaging. For the LysoTracker experiments, TAMRA-labeled Aβ42 (2 μg/ml) was added to the cells in SFM and the cells were incubated at 37 °C for 3 h prior to imaging. Fresh SFM was then added to the cells with 50 nm of the LysoTracker probe and the cells were incubated for another 15 min at 37 °C. Fresh SFM was then added to the cells prior to imaging. The cells were imaged using a Zeiss LSM5 Pascal system coupled to an Axiovert 200M microscope equipped with an argon 488 and He/Ne 543 laser. For the colocalization studies, Zeiss AIM software was used. Threshold quadrants were set using cells incubated only with either TAMRA-labeled Aβ42 or LysoTracker. Colocalization coefficients were calculated by summing the pixels in the colocalized quadrant and then dividing by the sum of pixels in the colocalized and noncolocalized quadrant. 2–3 cells were quantified in 5–6 regions of each dish for statistical analysis.
Construction of LDLR Lentivirus and Transduction of Astrocytes
The LDLR cDNA was subcloned from the pcDNA3.1 vector used to make the LDLR Tg mouse (27) into the FCIV (FM5) lentiviral vector (generous gift of Dr. Jeffrey Milbrandt, Washington University). This vector uses the ubiquitin promoter to express the gene of interest and also expresses the Venus protein via an internal ribosome entry site. Using PCR, the LDLR cDNA was amplified from the pcDNA3.1 vector with primers containing the AgeI and AscI restriction sites (forward primer, 5′-ACTGGTACCGGTGCCACCATGAGCACCGCGGATC-3′ and reverse primer, 5′GTACCAGGCGCGCCTCATGCCACATCGTCCTCCAGG-3′). Following digestion of both the LDLR PCR product and FCIV with AgeI and AscI, LDLR was ligated into the FCIV vector. The sequence and orientation of the insert was verified by complete sequencing. Lentivirus (FCIV-LDLR and FCIV) was produced and the titer calculated as described previously (28). Prior to transduction, primary astrocytes were plated in 24-well plates and grown to 60% confluence. Lentivirus was then added to the cells (multiplicity of infection of 1.5) and incubated at 37 °C for 48 h. Fresh medium was then added to the cells and the cells were cultured for 24 h. A second dose of lentivirus was then added to the cells (multiplicity of infection of 0.75) and the cells were incubated at 37 °C for 24 h. Fresh medium was then added and the cells were cultured for 8 to 10 days prior to performing experimental assays, changing the medium every 2–3 days. Lentivirus transduction was confirmed by both Venus expression and immunoblot for hemagglutinin (HA) and LDLR (see below).
Immunoblots
Primary astrocytes were lysed in either RIPA buffer (1% Nonidet P-40, 1% sodium deoxycholate, 0.1% SDS, 25 mm Tris-HCl, 150 mm NaCl) to measure LDLR, apoE, LRP1, and RAP levels or 1% Triton X-100 lysis buffer to measure Aβ levels. The lysates were spun down at 14,000 × g for 20 min and the supernatant was collected. Protein concentration was determined by a bicinchoninic acid (BCA) protein assay. Equal amounts of protein for each sample were run on 4–12% BisTris XT gels for apoE, LDLR, LRP1, and RAP and 16.5% Tris-Tricine gels for Aβ (Bio-Rad), and transferred to polyvinylidene fluoride membranes (0.45 μm pore size) and nitrocellulose membranes (0.2 μm pore size), respectively. Prior to blocking, the Aβ membranes were boiled for 10 min in PBS. All membranes were then blocked in 5% milk in TBS-T (Tris-buffered saline with 0.125% Tween 20). Blots were probed for LDLR (Novus catalog number NB110-57162 and MBL catalog number JM3839-100), HA (Covance), Aβ (82E1, IBL International), apoE (Calbiochem), LRP1 (generous gift of Dr. Guojun Bu, Mayo Clinic, Jacksonville, FA), RAP (R&D Systems catalog number AF4480), actin (Sigma), and tubulin (Sigma). The protein signal from the membranes was measured using a Lumigen TMA-6 ECL detection kit (Lumigen, USA) and quantified using ImageJ software (NIH).
Coimmunoprecipitation of Aβ and LDLR
His-tag purified recombinant LDLR (5 μg/ml, extracellular domain) was incubated with Aβ40 (400 nm) for 4 h at 37 °C in binding buffer (50 mm NaCl, 50 mm Tris-HCl, 2 mm CaCl2, pH 7.4). For the competition experiments, LDLR was preincubated with RAP, PCSK9, or Aβ(40–1) for 2 h at room temperature in binding buffer. For the immunoprecipitation, the LDLR-Aβ samples were diluted 1:1 in binding buffer with 0.1% Triton X-100. 50 μl of anti-His microbeads (Miltenyi Biotec catalog number 130-091-124, Auburn, CA) were then added to each sample followed by a 30-min incubation with rotation at 4 °C. The samples were then applied to μ-columns (Miltenyi Biotec, catalog number 130-042-701) and the beads were washed 5 times with wash buffer (binding buffer with 1% Triton X-100 and 0.25% sodium deoxycholate) and once with binding buffer. Pre-heated elution buffer was then applied to the columns and the eluate was collected and analyzed by SDS-PAGE (16.5% Tris-Tricine). LDLR was detected using an anti-His antibody (Santa Cruz, catalog number sc-8036HRP) and Aβ was detected using the 82E1 antibody (IBL International).
Surface Plasmon Resonance (SPR)
Sensor chips were purchased from GE Healthcare-BIAcore. All SPR experiments were carried out on a BIAcore 2000 instrument at 25 °C. Lyophilized Aβ(1–40) and Aβ(1–42) peptides were resuspended in trifluoroacetic acid and incubated at room temperature for 15 min. The peptides were then dried under nitrogen gas and resuspended in hexafluoroisopropanol. The hexafluoroisopropanol was then dried under nitrogen gas, resuspended in nitrogen gas, aliquoted into separate tubes, and dried under nitrogen gas. The dry Aβ film was then stored at −80 °C. Prior to use, the Aβ film was dissolved in dimethyl sulfoxide. Aβ(1–40) and Aβ(1–42) were immobilized onto a CM5 sensor chip surface at densities of ∼4–5 fmol/mm2 by amine coupling with sodium citrate buffer (pH 4.75), in accordance with the manufacturer's instructions (BIAcore AB). One flow cell was activated and blocked with 1 m ethanolamine without any protein and used as a control surface to normalize SPR signal from Aβ immobilized on the flow cells. Experiments were conducted in PBS (pH 7.4) and the analyte was injected at a flow rate of 30 μl/min. Dissociation was followed in the same buffer for 6 min. After each run, the sensor chip was regenerated using 2 m guanidine-HCl, 10 mm Tris-HCl (pH 8.0) and washed with running buffer for 5–10 min prior to the next injection. Data analysis was performed using Scrubber2 (Center for Bimolecular Interaction, Utah University) and BIAevaluation software (GE Healthcare-BIAcore), and dissociation constants were calculated using a single-site binding model in GraphPad Prism software. Data are based on 3 independent measurements using 6 different concentrations for each measurement. KD values are presented as mean ± S.D.
Statistics
All data are presented as mean ± S.E. unless otherwise noted. Statistical significance (*, p < 0.05; **, p < 0.01; ***, p < 0.001) was determined using GraphPad Prism software. For the comparison of two means with one independent variable (genotype), a two-tailed Student's t test was used. For the comparison of multiple means with one independent variable (genotype), a one-way analysis of variance followed by a Tukey post-test was used. For the comparison of multiple means with two independent variables (genotype and time, genotype and lentivirus transduction), a two-way analysis of variance followed by a Bonferroni post-test was used. Additional “Experimental Procedures” are found in the supplemental materials.
RESULTS
LDLR Overexpression in Primary Astrocytes Increases Aβ Uptake and Clearance
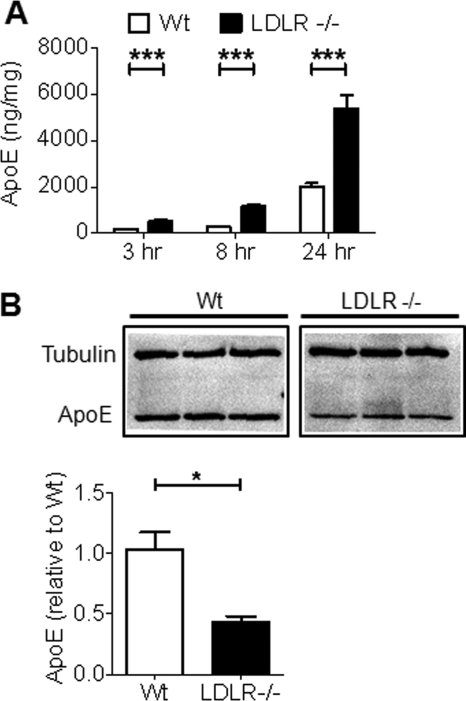
To determine whether LDLR mediates the uptake and clearance of Aβ by astrocytes, we first cultured primary astrocytes from the cortices of Tg mice that overexpress mouse LDLR under control of the mouse prion promoter (27). The LDLR transgene in these mice contains an HA tag to facilitate detection of LDLR protein levels. Immunoblots were performed to measure the amount of LDLR overexpression in these cells. Consistent with our previous study, LDLR Tg astrocytes expressed about 8-fold higher LDLR levels than WT cells (Fig. 1A). To assess the functional effect of increasing LDLR levels in astrocytes, we measured the extra- and intracellular levels of apoE. Astrocytes endogenously secrete lipoprotein particles with discoidal HDL structure and size that contain apoE (29). Because LDLR overexpression dramatically decreased apoE levels in brain tissue (27), we hypothesized that increasing the LDLR levels in astrocytes would promote apoE uptake and consequently lead to decreased apoE levels outside of the cells. WT and LDLR Tg primary astrocytes were cultured in serum-free conditions and the amount of apoE in the medium was measured at several time points. Serum-free conditions were used so that the majority of the lipoproteins present in the media were produced by astrocytes. The media from LDLR Tg astrocytes had significantly decreased apoE levels at all time points measured, with a maximum 80% decrease observed after 24 h (Fig. 1B). The amount of intracellular apoE was also measured after 24 h by immunoblot, and LDLR Tg cells had increased levels of apoE in comparison to WT cells (Fig. 1C). To confirm that the changes in apoE distribution in the LDLR Tg cells were due to an alteration in uptake rather than apoE production, apoE mRNA levels were measured by quantitative PCR. No differences were observed between WT and LDLR Tg cells (supplemental Fig. S1). Therefore, the decrease in extracellular apoE levels and increase in intracellular apoE levels in LDLR-overexpressing astrocytes are likely due to enhanced uptake of apoE-containing lipoprotein particles.
FIGURE 1.
Increased LDLR levels alter the extracellular and intracellular levels of apoE in primary astrocytes. Primary astrocytes were cultured from the cortices of both WT and LDLR transgenic mice. The LDLR transgene is expressed under control of the mouse prion promoter and also contains a hemagglutinin (HA) tag. A, LDLR and HA levels in the cells were measured by immunoblot. Unglycosylated LDLR migrates at 90 kDa and several glycosylated species of the protein migrate between 100 and 150 kDa. Representative images are shown. B, the functional effect of increased LDLR levels on apoE uptake was assessed by measuring the levels of endogenously produced apoE in the culture media. Primary astrocytes were incubated for the indicated time points in serum-free medium and the amount of apoE was measured by ELISA. Mean ± S.E. (n = 4), * denotes p < 0.05, ** denotes p < 0.01, *** denotes p < 0.001. C, the amount of cell-associated apoE was also measured by immunoblot of the cell lysates obtained after a 24-h incubation. A representative image is shown.
Previous studies have shown that cultured astrocytes are capable of taking up and clearing soluble Aβ from the media (16, 17, 19). Given the dramatic effect that LDLR overexpression has on lowering Aβ levels in the brain (27), we hypothesized that increasing the LDLR levels in astrocytes would enhance Aβ uptake and clearance. Aβ uptake was assessed by the addition of soluble Aβ40 (2 μg/ml) or Aβ42 (2 μg/ml) to the media of WT and LDLR Tg astrocytes for 3 h at 37 °C. Trypsin was added to the cells to remove Aβ bound to the extracellular cell surface and the amount of cell-internalized Aβ was measured by ELISA. LDLR overexpression enhanced the amount of intracellular Aβ40 and Aβ42 by 3.1- and 2.2-fold, respectively (Fig. 2, A and B). The differences in intracellular Aβ levels were also confirmed by immunoblot (Fig. 2C). These results suggest that increasing LDLR levels enhances Aβ uptake into primary astrocytes. To measure the effect of increasing LDLR levels on Aβ clearance from the medium, soluble Aβ40 (2 μg/ml) or Aβ42 (2 μg/ml) were added to WT and LDLR Tg astrocytes media for 24 h at 37 °C. The amount of Aβ remaining was then measured by ELISA. After 24 h, 71% Aβ40 remained in the WT astrocytes medium, whereas only 30% Aβ40 remained in the LDLR Tg cells medium (Fig. 2D). For Aβ42, 43% remained in the WT astrocytes medium, whereas only 17% remained in the LDLR Tg cells medium after 24 h (Fig. 2E). Therefore, the amount of Aβ remaining after 24 h was less for LDLR Tg cells in comparison to WT cells, with a decrease of 58% for Aβ40 and a decrease of 61% for Aβ42.
FIGURE 2.
LDLR overexpression enhances the uptake and clearance of Aβ by primary astrocytes. Primary astrocytes from either WT or LDLR transgenic mice were incubated with soluble (A) Aβ40 or (B) Aβ42 (2 μg/ml) for 3 h at 37 °C. The cells were then washed with PBS, incubated with trypsin to remove cell surface bound Aβ, and lysed in Triton X-100 lysis buffer. The cell-internalized Aβ was then assessed by ELISA. Mean ± S.E. (n ≥ 4), *** denotes p < 0.001. C, immunoblot analysis for Aβ was also performed on the cell lysates. Representative images are shown. Aβ clearance was assessed by the addition of either (D) Aβ40 or (E) Aβ42 (2 μg/ml) to the media of primary astrocytes. After 24 h, the levels of Aβ remaining in the medium along with the starting amount of Aβ were measured by ELISA. Mean ± S.E. (n ≥ 4), * denotes p < 0.05, *** denotes p < 0.001.
LRP1, another member of the LDL receptor family, has also been shown to promote the internalization of Aβ into neuronal cells (30). To determine whether increasing LDLR levels alter LRP1 levels, the amount of LRP1 in WT and LDLR Tg astrocytes was analyzed by immunoblot (supplemental Fig. S2A). LRP1 levels were actually decreased in LDLR overexpressing astrocytes, suggesting that the increase in Aβ uptake and clearance in these cells is not due to increased LRP1 levels. The levels of RAP, a chaperone for the LDL receptors, were also measured in LDLR Tg and WT astrocytes (supplemental Fig. 2B). RAP has been shown to bind to Aβ and regulate its uptake into cells (31). LDLR overexpression did not significantly change RAP levels in astrocytes. In summary, these results demonstrate that increasing LDLR levels in primary astrocytes enhanced both the uptake and clearance of soluble Aβ.
Increasing LDLR Levels in Primary Astrocytes Promote Cellular Degradation of Aβ
To verify that the increased Aβ uptake by LDLR-overexpressing astrocytes resulted in enhanced degradation of the peptide, we directly assessed Aβ degradation using 125I-Aβ(1–40). 125I-Aβ(1–40) was incubated with WT and LDLR Tg cells at 37 °C for several time points and a TCA precipitation was then performed. In this assay, degraded Aβ peptide was not precipitated and therefore cannot be efficiently pelleted with centrifugation. The amount of Aβ that has been degraded can be directly quantified by measuring the radioactive counts in the supernatant following centrifugation (Fig. 3A). We observed that LDLR Tg cells degraded significantly more Aβ than WT cells at all time points analyzed. The amount of intact Aβ measured from the LDLR Tg cells was also lower for all time points (Fig. 3B). After 24 h of incubation, LDLR Tg cells degraded 80% of the Aβ, whereas WT cells degraded 53% of the Aβ that was initially added (Fig. 3C). These results demonstrate that LDLR overexpression enhances Aβ degradation by primary astrocytes.
FIGURE 3.
LDLR overexpression increases the cellular degradation of Aβ by primary astrocytes. A, schematic diagram of the experiments used to measure degradation of 125I-Aβ by primary astrocytes. 125I-Aβ was added to primary astrocytes from either WT or LDLR Tg mice at the indicated time points. After each time point, media was collected and a TCA precipitation was performed to detect degraded Aβ. B, the supernatant (sup) and pellet counts/min are plotted as a function of time. Representative data from one experiment is shown. Experiment was repeated three times with similar results. C, degraded Aβ was quantified by calculating the percent of Aβ degraded as a percent of the total intact Aβ added. Mean ± S.E., * denotes p < 0.05, ** denotes p < 0.01. D, to measure the ability of astrocyte-conditioned media to degrade Aβ, media was collected from either WT or LDLR Tg primary astrocytes. 125I-Aβ was then added to the astrocyte-conditioned medium at the indicated time points and a TCA precipitation was performed. The supernatant (sup) and pellet counts/min are plotted as a function of time. Representative data from one experiment is shown. The experiment was repeated two times with similar results. E, degraded Aβ was quantified by calculating the percent of Aβ degraded as a percent of the total intact Aβ added. Mean ± S.E., * denotes p < 0.05, n.s., not significant.
Previously it has been shown that astrocytes secrete proteases that are capable of degrading Aβ, including insulin degrading enzyme and matrix metalloproteinase (32, 33). Therefore, to determine whether the effect of LDLR on Aβ degradation is due to intracellular or extracellular degradation we analyzed the ability of astrocyte-conditioned medium from WT and LDLR Tg astrocytes to degrade Aβ in the absence of cells. Media from WT and LDLR Tg primary astrocytes was collected and then incubated with 125I-Aβ for the indicated time points. Aβ degradation was then assessed by TCA precipitation. Media from both WT and LDLR Tg astrocytes was capable of degrading Aβ, but to a lesser extent than when cells were present (compare Fig. 3, B and D). After 8 h, LDLR Tg astrocyte medium degraded significantly more Aβ than the medium from WT cells. After 24 h, we observed that media from LDLR Tg astrocytes degraded significantly less Aβ than WT (Fig. 3E). Taken together, the difference in the effect of LDLR on Aβ degradation with and without cells after 24 h indicates that LDLR is capable of promoting cellular Aβ degradation.
LDLR Facilitates Vesicular Trafficking of Aβ to Lysosomes
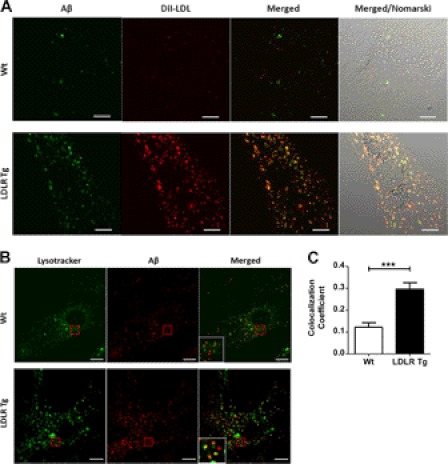
To determine whether LDLR promotes the vesicular uptake of Aβ, we incubated primary astrocytes with fluorescently labeled Aβ42 and DiI-LDL. LDL is internalized by receptor-mediated endocytosis through LDLR, and thus serves as an endocytic marker (34, 35). WT and LDLR Tg primary astrocytes were incubated with fluorescently labeled Aβ42 and DiI-LDL for 3 h at 37 °C. Microscopic visualization demonstrated a punctate pattern for both the DiI-LDL and Aβ42, demonstrating the uptake of both molecules into vesicular compartments. Notably, we observed that there was more DiI-LDL and Aβ42 endocytosed by the LDLR Tg cells (Fig. 4A). There was also considerable overlap between the DiI-LDL and Aβ42 signal in the LDLR Tg cells, demonstrating that LDLR overexpression increased the amount of Aβ in endocytic vesicles. To determine the intracellular fate of the internalized Aβ, we incubated primary astrocytes with fluorescently labeled Aβ42 for 3 h at 37 °C and then added LysoTracker to stain the lysosomes (Fig. 4B). LDLR Tg astrocytes displayed significantly increased colocalization of Aβ with the lysosome signal in comparison to WT cells, with 30% of the Aβ signal colocalized in the LDLR Tg cells and only 12% colocalized in WT cells (Fig. 4C). Therefore, increasing the LDLR levels in astrocytes enhanced the endocytic transport of Aβ to lysosomes.
FIGURE 4.
LDLR facilitates Aβ trafficking to lysosomes through a similar pathway as lipoprotein particles. A, to demonstrate that increasing LDLR levels promotes the transport of Aβ in similar vesicles as lipoprotein particles, WT and LDLR Tg primary astrocytes were incubated with fluorescent Aβ42 (3 μg/ml) and DiI-LDL (0.5 μg/ml) for 3 h at 37 °C. The cells were then washed and imaged using confocal microscopy. Overlap of Aβ and the DiI-LDL signal was observed in the LDLR Tg cells. B, to observe Aβ uptake into lysosomal compartments, WT and LDLR Tg primary astrocytes were incubated with fluorescent Aβ42 (2 μg/ml) for 3 h at 37 °C. The cells were then washed and 50 nm LysoTracker was added to the cells for 15 min. The cells were then washed again and imaged using confocal microscopy. C, colocalization of the Aβ and LysoTracker signal was analyzed and quantified. Mean ± S.E., *** denotes p < 0.001. Error bar represents 10 μm.
LDLR Deletion Decreases Uptake and Clearance of Aβ by Primary Astrocytes
To further determine the role of LDLR in the cellular metabolism of Aβ, we analyzed whether endogenous LDLR levels in primary astrocytes participate in Aβ uptake and clearance. Primary astrocytes were cultured from the cortices of WT and LDLR−/− mice. Immunoblot analysis of the cell lysates confirmed that the LDLR protein was not expressed in LDLR−/− astrocytes (supplemental Fig. S3A). Previous studies have shown that LDLR deletion significantly increased apoE levels in the mouse brain, likely due to impaired uptake and clearance of apoE-containing lipoprotein particles (36, 37). To determine whether LDLR deletion affects apoE uptake and clearance by astrocytes, WT and LDLR−/− primary astrocytes were cultured in serum-free conditions and the amount of endogenously produced apoE was measured after several time points. The medium from LDLR−/− cells had significantly increased apoE levels at all time points measured, with a maximum 77% increase observed after 8 h and a 62% increase observed after 24 h (Fig. 5A). The amount of apoE in the cell lysates was also measured by immunoblot after 24 h. LDLR−/− astrocytes had decreased apoE levels in comparison to WT cells (Fig. 5B). Because we could not rule out that the changes in apoE levels in the media and cell lysate were due to changes in protein expression, we measured the amount of apoE mRNA in LDLR−/− and WT cells. LDLR−/− astrocytes had elevated apoE mRNA amounts in comparison to WT cells (supplemental Fig. S1). It is possible that increased apoE production could play a role in the elevation of apoE levels in LDLR−/− astrocyte media. However, the known role of LDLR in the uptake of lipoproteins combined with the observation that the LDLR−/− astrocytes contained less intracellular apoE than WT cells suggest that the increase in apoE levels in LDLR−/− cells is primarily due to decreased uptake.
FIGURE 5.
Deletion of LDLR alters the extracellular and intracellular levels of apoE. Primary astrocytes were cultured from the cortices of WT and LDLR−/− mice. A, to show that LDLR deletion alters lipoprotein levels in astrocytes, apoE uptake was assessed by measuring the levels of endogenously produced apoE in the culture media. Primary astrocytes were incubated for the indicated time points in serum-free medium and the amount of apoE in the medium was measured by ELISA. Mean ± S.E. (n ≥ 4). *** denotes p < 0.001. B, the amount of cell-associated apoE was also measured by immunoblot of the cell lysates obtained after the 24-h incubation. Quantification of the apoE band intensity normalized to tubulin intensity is shown below the image.
The effect of LDLR deletion on Aβ uptake was assessed by the addition of soluble Aβ40 (2 μg/ml) to LDLR−/− and WT primary astrocytes for 3 h. Quantification of Aβ ELISA showed that cellular Aβ levels decreased by 43% in LDLR−/− astrocytes compared with WT cells (Fig. 6A). The difference in internalization was qualitatively confirmed by immunoblot analysis of the cell lysates (Fig. 6B). To confirm that the decrease in Aβ uptake in LDLR−/− astrocytes was due to lack of LDLR rather than a nonspecific alteration in cellular function, we increased the LDLR function by transducing the LDLR−/− astrocytes with an LDLR-expressing lentivirus. Immunoblot analysis confirmed that the lentiviral-transduced astrocytes expressed LDLR (supplemental Fig. 3B). Cell-internalized Aβ was increased by 1.4-fold in the LDLR-lentiviral-transduced cells in comparison to cells transduced with an empty-virus control (Fig. 6C). Finally, to measure the effect of LDLR deletion on the clearance of Aβ from the media, soluble Aβ40 (2 μg/ml) was added to the media of WT and LDLR Tg astrocytes for 24 h at 37 °C. The amount of Aβ remaining was then measured by ELISA. LDLR−/− astrocytes cleared less Aβ in comparison to WT cells, however, the difference was not significant (Fig. 6D).
FIGURE 6.
Lack of LDLR impairs Aβ uptake in astrocytes. To assess the effect of LDLR deletion on Aβ uptake, WT and LDLR−/− astrocytes were incubated with Aβ40 (2 μg/ml) for 3 h. The cells were then washed with PBS, incubated with trypsin to remove cell surface-bound Aβ, and lysed in Triton X-100 lysis buffer. The amount of Aβ in the cell lysate was then assessed by ELISA (A) and immunoblot (B). For the immunoblot, a representative image is shown. Mean ± S.E. (n ≥ 4). *** denotes p < 0.001. C, to verify the effect of LDLR deletion on Aβ uptake, LDLR function was restored in the LDLR−/− astrocytes by transduction with an LDLR lentivirus. Aβ uptake was then assessed as in A and compared with the level of uptake by LDLR−/− cells transduced with control lentivirus and WT cells. Mean ± S.E. (n ≥ 4). ** denotes p < 0.01. D, the effect of LDLR deletion on Aβ clearance was assessed by the addition of Aβ40 (2 μg/ml) to the WT and LDLR−/− astrocytes media. After 24 h, the amount of Aβ remaining was measured by ELISA and compared with the starting amount. Mean ± S.E. (n ≥ 4). * denotes p < 0.05; n.s., not significant.
As measured with the astrocytes that overexpress LDLR, the effect of LDLR deletion on LRP1 and RAP levels was assessed by immunoblot (supplemental Fig. S2, A and B). Deletion of LDLR resulted in a significant decrease in LRP1 levels, but did not affect RAP levels. As a result, we cannot rule out the possibility that a decrease in LRP1 plays a role in the effect of LDLR deletion on Aβ uptake. However, the LDLR overexpression data convincingly demonstrates that LDLR has an effect on Aβ uptake and clearance that is independent of LRP1. In summary, this data demonstrates that endogenous LDLR may represent a pathway of Aβ uptake in primary astrocytes, although this effect may also be mediated by compensatory decreases in LRP1 levels.
LDLR Effect on Aβ Uptake and Clearance Does Not Require ApoE
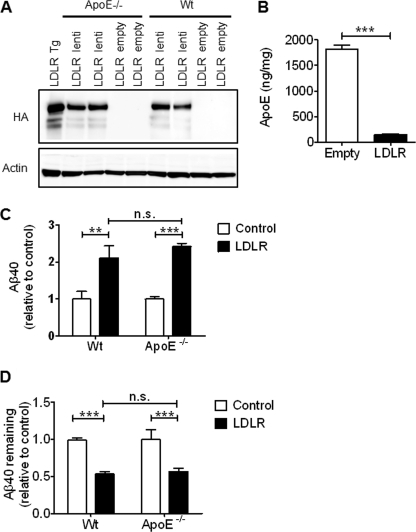
ApoE has previously been shown to bind to Aβ (38–40), and is capable of enhancing the cellular degradation of Aβ by primary astrocytes and microglia (18, 33). The effect of LDLR on Aβ uptake and clearance may therefore depend upon LDLR modulation of astrocyte apoE levels, or may occur due to direct binding of an apoE-Aβ complex to LDLR. To determine whether the effect of LDLR on Aβ uptake is dependent upon the presence of apoE, we overexpressed HA-tagged LDLR in apoE−/− primary astrocytes through lentiviral transduction. Immunoblot detection of the HA tag showed that the amount of LDLR overexpressed in WT and apoE−/− astrocytes was comparable (Fig. 7A). To determine whether the LDLR expressed by the lentivirus had a functional effect in the cells, extracellular apoE levels were measured in LDLR lentiviral-transduced WT astrocytes. Overexpression decreased apoE levels by 92% after 24 h, confirming that the LDLR expressed via the lentivirus was functional (Fig. 7B). Aβ uptake was assessed by the addition of soluble Aβ40 (2 μg/ml) to WT and apoE−/− primary astrocytes transduced with LDLR lentivirus. LDLR overexpression increased the Aβ uptake in WT cells and apoE−/− astrocytes to a similar extent, with a 2.1-fold increase in WT cells and a 2.4-fold increase in apoE−/− cells (Fig. 7C). The effect of LDLR overexpression on Aβ clearance in the absence of apoE was also measured by determining the amount of Aβ remaining in the WT and apoE−/− astrocyte medium transduced with LDLR after a 24-h incubation. LDLR overexpression decreased the amount of Aβ remaining in WT cells by 40% and in apoE−/− cells by 43% (Fig. 7D). Therefore, the presence of apoE is not necessary for LDLR to modulate both Aβ uptake and clearance by primary astrocytes.
FIGURE 7.
The effect of LDLR on Aβ uptake and clearance is not dependent on apoE. A, to determine whether the effect of LDLR on Aβ uptake and clearance requires the presence of apoE, LDLR was expressed in apoE−/− and WT primary astrocytes via lentiviral transduction. LDLR expression was confirmed by immunoblot for HA. LDLR Tg astrocyte lysate is shown for comparison. B, to confirm that the LDLR protein expressed after lentiviral transduction was functional, WT cells were transduced and the amount of endogenously produced apoE was measured by ELISA in the cell medium after a 24-h incubation. Mean ± S.E. (n ≥ 4). ** denotes p < 0.01. C, Aβ uptake was measured in WT and apoE−/− primary astrocytes transduced with LDLR lentivirus. Aβ40 (2 μg/ml) was incubated with the cells for 3 h. The cells were then washed with PBS, treated with trypsin to remove cell surface-bound Aβ, and lysed in Triton X-100 lysis buffer. The cell-internalized Aβ was then measured by ELISA. Control samples were transduced with the empty lentivirus. Mean ± S.E. (n ≥ 4). ** denotes p < 0.01; *** denotes p < 0.001; n.s., not significant. D, Aβ clearance was assessed by the addition of Aβ40 (2 μg/ml) to the media of WT and ApoE−/− astrocytes transduced with the LDLR lentivirus. After 24 h, the amount of Aβ remaining was measured by ELISA and compared with cells transduced with empty lentivirus. Mean ± S.E. (n ≥ 4). *** denotes p < 0.001; n.s., not significant.
LDLR Binds Directly to Aβ in an in Vitro Setting
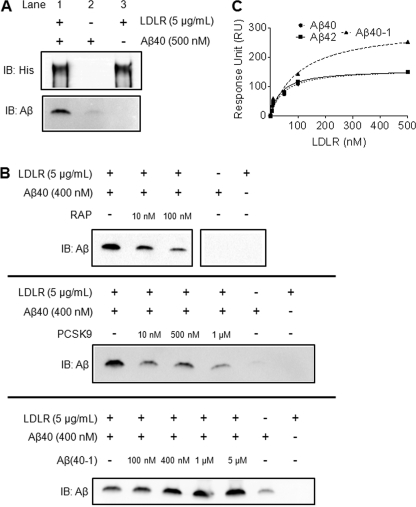
Because apoE was not required for the effect of LDLR on Aβ internalization, we investigated whether LDLR may directly interact with the Aβ peptide. Coimmunoprecipitation experiments were carried out using Aβ and the extracellular domain of LDLR. Both Aβ40 and LDLR were incubated together for 4 h at 37 °C, and LDLR was immunoprecipitated using anti-His beads. LDLR was efficiently pulled down by the anti-His antibody, as shown in Fig. 8A (lanes 1 and 3). A significant amount of Aβ40 was also pulled down with LDLR (lane 1), which was not due to nonspecific binding of Aβ40 to the anti-His beads (compare lanes 1 and 2). Ligand blotting also verified the direct interaction between LDLR and Aβ40 (supplemental Fig. S4). To demonstrate the specificity of the interaction between Aβ40 and LDLR, the immunoprecipitation experiment was repeated with the addition of increasing concentrations of either RAP or proprotein convertase subtlisin/kexin type 9 (PCSK9), two established ligands for LDLR. Both RAP and PCSK9 decreased the amount of Aβ bound to LDLR in a dose-dependent manner (Fig. 8B). Addition of Aβ(40–1) did not impair binding between Aβ40 and LDLR, and interestingly led to an apparent increased binding (Fig. 8B). Taken together these results demonstrate that Aβ directly binds to LDLR through an interaction that can be blocked using known ligands to LDLR.
FIGURE 8.
Direct interaction between Aβ and LDLR. A, to assess whether Aβ could directly associate with LDLR, Aβ40 (500 nm) and recombinant extracellular LDLR (5 μg/ml) were incubated together and immunoprecipitated using anti-His beads to pull down LDLR. The isolated proteins were then eluted from the beads and subjected to SDS-PAGE and immunoblot analysis for LDLR (His) and Aβ. Control experiments included incubating Aβ40 alone with anti-His beads and immunoprecipitating LDLR without the addition of Aβ40. B, the specificity of the binding of Aβ to LDLR was determined by performing competition experiments with known LDLR ligands. Increasing amounts of either RAP or PCSK9 were preincubated with recombinant extracellular LDLR for 2 h, and Aβ40 was then added to the protein mixture and incubated at 37 °C for 4 h. LDLR was then immunoprecipitated using anti-His beads, and the eluted samples were subjected to SDS-PAGE and immunoblot analysis for Aβ. The experiment was also repeated using Aβ(40-1) as a competing peptide. C, surface plasmon resonance was used to measure the interaction between the extracellular domain of LDLR and Aβ. Aβ40, Aβ42, or Aβ(40-1) were immobilized on the SPR chip and various concentrations of LDLR were flown over the surface. To calculate the dissociation constant for the interaction (KD), we plotted the resonance units as a function of LDLR concentration.
We used SPR to quantify the affinity of the interaction between LDLR and Aβ. Soluble Aβ40 and Aβ42 were immobilized on the sensor chip, and binding to LDLR was measured by flow of the extracellular LDLR domain over the immobilized Aβ peptides. A dose-dependent interaction between LDLR and both Aβ40 and Aβ42 was detected (representative sensograms are shown in supplemental Fig. S5B). We then plotted the SPR response units for each concentration of LDLR tested to calculate the thermodynamic dissociation constants (KD) of the interactions (Fig. 8C). The KD values were 47.4 ± 9.9 nm for Aβ40 and 37.4 ± 8.0 nm for Aβ42. The interaction between LDLR and the reverse Aβ peptide (Aβ40-1) was also measured (Fig. 8C). Although Aβ40-1 still associated with LDLR, the interaction was weaker than that of Aβ40 and Aβ42, with a KD value of 106.7 ± 36.1 nm. Finally, the binding of a mutant form of Aβ (Dutch/Iowa Aβ40, DIAβ40) to LDLR was assessed. Interestingly, the binding of mutant Aβ was stronger than that of Aβ40 and Aβ42, with a KD of 4.54 ± 0.7 nm (supplemental Fig. S5A).
DISCUSSION
Previously we have shown that LDLR overexpression in the mouse brain markedly decreased the levels of Aβ and extent of plaque deposition in the APP/PS1 transgenic mouse model brain (27). In this study, we analyzed how LDLR regulates the cellular uptake and metabolism of Aβ by astrocytes. Overexpression of LDLR significantly increased the uptake and clearance of both Aβ40 and Aβ42 by astrocytes, whereas deletion of LDLR had the opposite effect. Increasing the LDLR levels also enhanced cellular degradation of Aβ through facilitating intracellular trafficking of Aβ to the lysosome. Despite the observation that increasing LDLR levels in astrocytes led to a decrease in extracellular apoE levels and increase in intracellular apoE levels, the effect of LDLR on Aβ uptake and clearance did not require apoE. Finally, we show that Aβ is capable of directly binding to LDLR. Overall, these results identify LDLR as a novel pathway for Aβ uptake into astrocytes and suggest that increasing glial levels of LDLR may be a feasible therapeutic strategy for promoting Aβ clearance from the extracellular space.
Several cell types in the brain are capable of internalizing both fibrillar and soluble Aβ, including astrocytes (17–19, 41), microglia (33, 41), neurons (42), and endothelial cells (43, 44). The ability of microglia and astrocytes to degrade soluble Aβ suggests that both of these cell types play a role in Aβ clearance from the brain. Several pathways and receptors regulate Aβ clearance by microglia, including scavenger receptors, Toll-like receptors, and fluid phase macropinocytosis (for a review, see Ref. 45). However, the cellular pathways that facilitate Aβ uptake and clearance by astrocytes have not been extensively characterized. Previously it has been shown that primary astrocytes grown in culture were capable of degrading soluble Aβ and fibrillar Aβ present in the plaques of murine brain sections (17, 18). ApoE appears to play an important role in this process, as astrocytes cultured from apoE−/− mice were not capable of degrading Aβ in tissue sections. Furthermore, co-incubation of primary astrocytes with RAP, a protein that antagonizes ligand binding to receptors of the LDLR family, inhibited the ability of astrocytes to degrade Aβ (18). These results suggest that both apoE and a receptor from the LDLR family function in regulating the clearance of Aβ by astrocytes. In our current study, we extend these previous findings by highlighting the importance of LDLR in regulating both the uptake and clearance of soluble Aβ by astrocytes.
The ϵ4 allele of the APOE gene is currently the strongest genetic risk factor for late-onset AD (22). Data from human studies and animal models suggest that apoE primarily influences AD pathogenesis through altering the aggregation of Aβ and its clearance from the brain (21, 22, 46, 47). Altering the amount of apoE in the brain influences amyloid deposition and clearance (23, 24, 48). For this reason, recent attention has been devoted to identifying receptors in the brain that regulate apoE levels. In mouse studies, modulation of LDLR protein levels in the brain altered apoE amounts. LDLR−/− mice had significantly elevated amounts of apoE in the brain and cerebrospinal fluid (36, 37, 49, 50), whereas mice overexpressing LDLR in the brain had lower levels of apoE (27). In the current study, we demonstrate that modulation of LDLR levels in astrocytes similarly alters apoE levels. Astrocytes that overexpress LDLR have decreased apoE levels in the media and increased levels within the cell, whereas LDLR−/− astrocytes have elevated apoE levels in the media and decreased intracellular levels of apoE. Notably, we observed a statistically significant increase in apoE mRNA levels in LDLR−/− astrocytes. The reason for this increase is unclear, but it may be a compensatory response of the cells to the decrease in intracellular apoE and cholesterol. Despite the increase in apoE mRNA, total intracellular apoE levels in the LDLR−/− cells were lower than WT cells. Therefore, this data strongly suggests that LDLR regulates the uptake of apoE-lipoprotein particles from the media. Although several cell types in the brain likely mediate the effect of LDLR on apoE levels in vivo, these in vitro results provide evidence that astrocytes may contribute to the differences in apoE amount observed in the mouse brain following LDLR deletion or overexpression.
The effect of LDLR on the amount of Aβ in the brain has been studied through genetic modulation of LDLR levels in transgenic mouse models of human Aβ deposition. Although several groups have analyzed the effect of LDLR deletion on Aβ deposition, the results have been inconsistent. In Tg 2576 APP transgenic mice and 5XFAD APP/PS1 transgenic mice, LDLR deletion caused an increase in human amyloid deposition (37, 50). However, in PDAPP mice crossed to LDLR−/− mice there was no significant change in human Aβ levels, although there was a trend toward increased Aβ deposition in mice lacking LDLR (36). A different group looking at the effect of LDLR deletion on mouse Aβ levels found no changes in comparison to WT mice (49). Our studies have found that LDLR overexpression in the mouse brain dramatically decreased Aβ deposition in APP/PS1 transgenic mice. Furthermore, we observed that the clearance of Aβ from the interstitial fluid was significantly increased in LDLR transgenic mice (27). Several mechanisms could be responsible for this effect, including increased cellular catabolism of Aβ or increased transport of Aβ across the blood-brain barrier into the plasma where it is rapidly degraded. In these mice, one of the primary cell types expressing the transgene was astrocytes. In the current study, we provide a potential cellular mechanism for the effect of LDLR overexpression on Aβ levels in the brain. LDLR overexpression in primary astrocytes by expression of an LDLR transgene or through LDLR lentiviral transduction significantly increased Aβ uptake and enhanced Aβ clearance from the media. LDLR−/− astrocytes internalized less Aβ in comparison to WT cells and exhibited less Aβ clearance from the media, although the effect on clearance was not statistically significant. Taken together, these results suggest that LDLR is an important mediator of Aβ uptake and clearance in astrocytes, and differences in astrocyte-mediated clearance of Aβ may explain the decrease in extracellular Aβ levels observed in the LDLR Tg mouse brain. However, LDLR could also influence other pathways of Aβ clearance from the brain, including transport across the blood-brain barrier or clearance by other cell types. Also, we observed that altering LDLR levels changes LRP1 levels in primary astrocytes, another LDL receptor that has been shown to regulate Aβ levels. However, LDLR overexpression actually led to a decrease in LRP1 levels, suggesting the increase in Aβ uptake and clearance is due to LDLR rather than LRP1 in these cells. In future studies, it will be important to determine whether LDLR alters other modes of Aβ clearance and to better characterize the interaction between LDLR and LRP1-mediated Aβ uptake.
We also provide evidence in this study that LDLR overexpression in astrocytes directly promotes the cellular degradation of Aβ. Quantification of 125I-Aβ degradation via TCA precipitation showed that LDLR-overexpressing astrocytes degraded significantly more Aβ than WT cells. Secreted extracellular proteases were not responsible for the effect of LDLR on Aβ degradation. The medium from LDLR-overexpressing astrocytes degraded even less Aβ than WT cells after a 24-h incubation. Regardless of genotype, we observed that the extent of Aβ degradation by astrocyte-conditioned medium was minor in comparison to the Aβ degradation that occurred in the presence of primary astrocytes. Previous groups have demonstrated a significant Aβ clearance in the presence of astrocyte-conditioned medium due to the presence of extracellular proteases, such as metalloproteinases and insulin degrading enzyme (32, 33). The reason for the difference between our findings and these previous studies is not clear, but may be due to methodological differences in how Aβ degradation was measured. Previous studies described the degradation of Aβ by measuring the disappearance of full-length Aβ as detected by ELISA or immunoblot, or the appearance of large proteolytic fragments (32, 33). However, our study quantified Aβ degradation products that were too small to be precipitated by TCA, and likely represent complete digestion of the Aβ peptide. Despite the lack of significant extracellular Aβ degradation by astrocyte-conditioned medium, our results show that increasing the levels of LDLR in astrocytes enhances intracellular Aβ degradation. The increased degradation likely occurs through the lysosomal pathway, as LDLR promoted the intracellular trafficking of Aβ to the lysosome. It is important to point out that Aβ in the brain exists in several different aggregation states, including oligomers and fibrils (1). Because our study focused on the degradation of soluble Aβ, it will be important in the future to determine whether LDLR enhances the ability of astrocytes to degrade higher-order species of Aβ associated with amyloid plaques.
The effect of LDLR on Aβ uptake and clearance does not appear to be dependent upon apoE. Several studies have shown that apoE is capable of binding to Aβ (38–40). Therefore, we hypothesized that apoE may facilitate the uptake of Aβ via LDLR through binding of an apoE-Aβ complex to LDLR. However, we found that LDLR was capable of promoting the uptake of Aβ into primary astrocytes even in apoE−/− cells. Therefore, it is likely that LDLR regulates the internalization of apoE and Aβ through independent processes, although we cannot rule out that a small amount of Aβ is taken up as a complex with apoE. ApoE can also enhance the ability of both astrocytes and microglia to degrade Aβ (18, 33). Despite the increased intracellular apoE levels in LDLR-overexpressing astrocytes that could promote intracellular Aβ degradation, apoE was not required for the effect of LDLR on Aβ clearance. Support also exists in vivo that LDLR can regulate Aβ levels independently of apoE. A recent study demonstrated that deletion of LDLR increases the level of amyloid and Aβ deposition in 5XFAD APP/PS1 transgenic mouse brains, even in the brains of mice lacking apoE (50). 5XFAD/LDLR−/− mice had decreased astrocytosis regardless of whether apoE was present, suggesting LDLR may function in the astrocytic response to Aβ deposition in vivo (50). Therefore, although apoE may regulate Aβ uptake and clearance by astrocytes, the effect of LDLR and apoE on these processes appears to be independent. In the future, it will be of interest to determine whether LDLR overexpression can decrease plaque deposition in the brain in the absence of apoE.
Because apoE did not appear to regulate the uptake of Aβ via LDLR, we analyzed whether Aβ could directly bind to LDLR in vitro. We showed via immunoprecipitation and surface plasmon resonance that both Aβ40 and Aβ42 can bind to the extracellular domain of LDLR with KD values of 47.4 and 37.4 nm, respectively. Competition experiments using both PCSK9 and RAP demonstrated that these LDLR ligands impaired Aβ binding to LDLR. These results suggest that Aβ may interact with the domains of LDLR that bind PCSK9 and RAP. Future studies will be necessary to define the exact Aβ-binding site on LDLR. Another member of the LDLR receptor family, LRP1, has also been shown to bind directly to Aβ with KD values in the low nanomolar range (43). Despite the fact that the binding we observe between LDLR and Aβ is slightly weaker than the binding of Aβ to LRP1 a direct interaction with LDLR may still be relevant for Aβ internalization. Furthermore, we cannot rule out the possibility that Aβ binds to the cell surface through another protein that potentially functions as a co-receptor with LDLR, and LDLR then subsequently facilitates Aβ uptake after it binds to the cell surface. Such a cooperative process has recently been proposed for Aβ uptake into neuronal cells via LRP1 and heparan sulfate proteoglycan (30).
In summary, we identified LDLR as a novel pathway of Aβ uptake and degradation in primary astrocytes. We also show that the ability of LDLR to facilitate Aβ uptake and clearance is not dependent upon apoE. Finally, we have identified a potential interaction between Aβ and LDLR that may play a role in the ability of LDLR to regulate Aβ internalization into cells. Regulating glial levels of LDLR appears to be a potential approach toward lowering brain Aβ levels. Therefore, it will be important in the future to better characterize how brain LDLR levels can be regulated from both a molecular and pharmaceutical perspective to identify unique therapeutic targets to treat AD.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants AG13956, NS034467 (to D. M. H.), and P30NS069329 (to J. K.), an American Health Assistance Foundation AHAF centennial award (to D. M. H), and Neuroscience Blueprint Core Grant P30 NS057105 (to Washington University).

This article contains supplemental ”Experimental Procedures“ and Figs. S1–S5.
- AD
- Alzheimer disease
- Aβ
- amyloid β
- LDLR
- low-density lipoprotein receptor
- apoE
- apolipoprotein E
- SPR
- surface plasmon resonance
- APP
- amyloid precursor protein
- RAP
- receptor-associated protein
- PCSK9
- proprotein convertase subtlisin/kexin type 9
- LRP1
- lipoprotein receptor related-protein 1
- Tg
- transgenic
- SFM
- serum-free medium
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- Tricine
- N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine
- TAMRA
- carboxytetramethylrhodamine.
REFERENCES
- 1. Holtzman D. M., Morris J. C., Goate A. M. (2011) Alzheimer disease, the challenge of the second century. Sci. Transl. Med. 3, 77sr1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hardy J., Selkoe D. J. (2002) The amyloid hypothesis of Alzheimer disease. Progress and problems on the road to therapeutics. Science 297, 353–356 [DOI] [PubMed] [Google Scholar]
- 3. De Strooper B. (2010) Proteases and proteolysis in Alzheimer disease. A multifactorial view on the disease process. Physiol. Rev. 90, 465–494 [DOI] [PubMed] [Google Scholar]
- 4. Hardy J. (2006) A hundred years of Alzheimer disease research. Neuron 52, 3–13 [DOI] [PubMed] [Google Scholar]
- 5. Selkoe D. J. (2001) Clearing the brain's amyloid cobwebs. Neuron 32, 177–180 [DOI] [PubMed] [Google Scholar]
- 6. Mawuenyega K. G., Sigurdson W., Ovod V., Munsell L., Kasten T., Morris J. C., Yarasheski K. E., Bateman R. J. (2010) Decreased clearance of CNS β-amyloid in Alzheimer disease. Science 330, 1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tanzi R. E., Moir R. D., Wagner S. L. (2004) Clearance of Alzheimer Aβ peptide. The many roads to perdition. Neuron 43, 605–608 [DOI] [PubMed] [Google Scholar]
- 8. Zlokovic B. V. (2008) The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 57, 178–201 [DOI] [PubMed] [Google Scholar]
- 9. Mandybur T. I., Chuirazzi C. C. (1990) Astrocytes and the plaques of Alzheimer disease. Neurology 40, 635–639 [DOI] [PubMed] [Google Scholar]
- 10. Pike C. J., Cummings B. J., Cotman C. W. (1995) Early association of reactive astrocytes with senile plaques in Alzheimer disease. Exp. Neurol. 132, 172–179 [DOI] [PubMed] [Google Scholar]
- 11. Itagaki S., McGeer P. L., Akiyama H., Zhu S., Selkoe D. (1989) Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. J. Neuroimmunol. 24, 173–182 [DOI] [PubMed] [Google Scholar]
- 12. Nagele R. G., D'Andrea M. R., Lee H., Venkataraman V., Wang H. Y. (2003) Astrocytes accumulate Aβ42 and give rise to astrocytic amyloid plaques in Alzheimer disease brains. Brain Res. 971, 197–209 [DOI] [PubMed] [Google Scholar]
- 13. Funato H., Yoshimura M., Yamazaki T., Saido T. C., Ito Y., Yokofujita J., Okeda R., Ihara Y. (1998) Astrocytes containing amyloid β-protein (Aβ)-positive granules are associated with Aβ40-positive diffuse plaques in the aged human brain. Am. J. Pathol. 152, 983–992 [PMC free article] [PubMed] [Google Scholar]
- 14. Thal D. R., Schultz C., Dehghani F., Yamaguchi H., Braak H., Braak E. (2000) Amyloid β-protein (Aβ)-containing astrocytes are located preferentially near N-terminal-truncated Aβ deposits in the human entorhinal cortex. Acta Neuropathol. 100, 608–617 [DOI] [PubMed] [Google Scholar]
- 15. Thal D. R., Härtig W., Schober R. (1999) Diffuse plaques in the molecular layer show intracellular Aβ(8–17)-immunoreactive deposits in subpial astrocytes. Clin. Neuropathol. 18, 226–231 [PubMed] [Google Scholar]
- 16. Shaffer L. M., Dority M. D., Gupta-Bansal R., Frederickson R. C., Younkin S. G., Brunden K. R. (1995) Amyloid β protein (Aβ) removal by neuroglial cells in culture. Neurobiol. Aging 16, 737–745 [DOI] [PubMed] [Google Scholar]
- 17. Wyss-Coray T., Loike J. D., Brionne T. C., Lu E., Anankov R., Yan F., Silverstein S. C., Husemann J. (2003) Adult mouse astrocytes degrade amyloid-β in vitro and in situ. Nat. Med. 9, 453–457 [DOI] [PubMed] [Google Scholar]
- 18. Koistinaho M., Lin S., Wu X., Esterman M., Koger D., Hanson J., Higgs R., Liu F., Malkani S., Bales K. R., Paul S. M. (2004) Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-β peptides. Nat. Med. 10, 719–726 [DOI] [PubMed] [Google Scholar]
- 19. Nielsen H. M., Veerhuis R., Holmqvist B., Janciauskiene S. (2009) Binding and uptake of Aβ(1–42) by primary human astrocytes in vitro. Glia 57, 978–988 [DOI] [PubMed] [Google Scholar]
- 20. Mahley R. W. (1988) Apolipoprotein E, cholesterol transport protein with expanding role in cell biology. Science 240, 622–630 [DOI] [PubMed] [Google Scholar]
- 21. Verghese P. B., Castellano J. M., Holtzman D. M. (2011) Apolipoprotein E in Alzheimer disease and other neurological disorders. Lancet Neurol. 10, 241–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim J., Basak J. M., Holtzman D. M. (2009) The role of apolipoprotein E in Alzheimer disease. Neuron 63, 287–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bales K. R., Verina T., Dodel R. C., Du Y., Altstiel L., Bender M., Hyslop P., Johnstone E. M., Little S. P., Cummins D. J., Piccardo P., Ghetti B., Paul S. M. (1997) Lack of apolipoprotein E dramatically reduces amyloid β-peptide deposition. Nat. Genet. 17, 263–264 [DOI] [PubMed] [Google Scholar]
- 24. Bales K. R., Verina T., Cummins D. J., Du Y., Dodel R. C., Saura J., Fishman C. E., DeLong C. A., Piccardo P., Petegnief V., Ghetti B., Paul S. M. (1999) Apolipoprotein E is essential for amyloid deposition in the APP(V717F) transgenic mouse model of Alzheimer disease. Proc. Natl. Acad. Sci. U.S.A. 96, 15233–15238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Herz J., Bock H. H. (2002) Lipoprotein receptors in the nervous system. Annu. Rev. Biochem. 71, 405–434 [DOI] [PubMed] [Google Scholar]
- 26. Brown M. S., Goldstein J. L. (1986) A receptor-mediated pathway for cholesterol homeostasis. Science 232, 34–47 [DOI] [PubMed] [Google Scholar]
- 27. Kim J., Castellano J. M., Jiang H., Basak J. M., Parsadanian M., Pham V., Mason S. M., Paul S. M., Holtzman D. M. (2009) Overexpression of low-density lipoprotein receptor in the brain markedly inhibits amyloid deposition and increases extracellular Aβ clearance. Neuron 64, 632–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li M., Husic N., Lin Y., Christensen H., Malik I., McIver S., Daniels C. M., Harris D. A., Kotzbauer P. T., Goldberg M. P., Snider B. J. (2010) Optimal promoter usage for lentiviral vector-mediated transduction of cultured central nervous system cells. J. Neurosci. Methods 189, 56–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fagan A. M., Holtzman D. M., Munson G., Mathur T., Schneider D., Chang L. K., Getz G. S., Reardon C. A., Lukens J., Shah J. A., LaDu M. J. (1999) Unique lipoproteins secreted by primary astrocytes from wild type, apoE−/−, and human apoE transgenic mice. J. Biol. Chem. 274, 30001–30007 [DOI] [PubMed] [Google Scholar]
- 30. Kanekiyo T., Zhang J., Liu Q., Liu C. C., Zhang L., Bu G. (2011) Heparan sulfate proteoglycan and the low-density lipoprotein receptor-related protein 1 constitute major pathways for neuronal amyloid-β uptake. J. Neurosci. 31, 1644–1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kanekiyo T., Bu G. (2009) Receptor-associated protein interacts with amyloid-β peptide and promotes its cellular uptake. J. Biol. Chem. 284, 33352–33359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yin K. J., Cirrito J. R., Yan P., Hu X., Xiao Q., Pan X., Bateman R., Song H., Hsu F. F., Turk J., Xu J., Hsu C. Y., Mills J. C., Holtzman D. M., Lee J. M. (2006) Matrix metalloproteinases expressed by astrocytes mediate extracellular amyloid-β peptide catabolism. J. Neurosci. 26, 10939–10948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jiang Q., Lee C. Y., Mandrekar S., Wilkinson B., Cramer P., Zelcer N., Mann K., Lamb B., Willson T. M., Collins J. L., Richardson J. C., Smith J. D., Comery T. A., Riddell D., Holtzman D. M., Tontonoz P., Landreth G. E. (2008) ApoE promotes the proteolytic degradation of Aβ. Neuron 58, 681–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Goldstein J. L., Brown M. S., Anderson R. G., Russell D. W., Schneider W. J. (1985) Receptor-mediated endocytosis. Concepts emerging from the LDL receptor system. Annu. Rev. Cell Biol. 1, 1–39 [DOI] [PubMed] [Google Scholar]
- 35. Dunn K. W., Maxfield F. R. (1992) Delivery of ligands from sorting endosomes to late endosomes occurs by maturation of sorting endosomes. J. Cell Biol. 117, 301–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fryer J. D., Demattos R. B., McCormick L. M., O'Dell M. A., Spinner M. L., Bales K. R., Paul S. M., Sullivan P. M., Parsadanian M., Bu G., Holtzman D. M. (2005) The low-density lipoprotein receptor regulates the level of central nervous system human and murine apolipoprotein E but does not modify amyloid plaque pathology in PDAPP mice. J. Biol. Chem. 280, 25754–25759 [DOI] [PubMed] [Google Scholar]
- 37. Cao D., Fukuchi K., Wan H., Kim H., Li L. (2006) Lack of LDL receptor aggravates learning deficits and amyloid deposits in Alzheimer transgenic mice. Neurobiol. Aging 27, 1632–1643 [DOI] [PubMed] [Google Scholar]
- 38. LaDu M. J., Falduto M. T., Manelli A. M., Reardon C. A., Getz G. S., Frail D. E. (1994) Isoform-specific binding of apolipoprotein E to β-amyloid. J. Biol. Chem. 269, 23403–23406 [PubMed] [Google Scholar]
- 39. Tokuda T., Calero M., Matsubara E., Vidal R., Kumar A., Permanne B., Zlokovic B., Smith J. D., Ladu M. J., Rostagno A., Frangione B., Ghiso J. (2000) Lipidation of apolipoprotein E influences its isoform-specific interaction with Alzheimer amyloid β peptides. Biochem. J. 348, 359–365 [PMC free article] [PubMed] [Google Scholar]
- 40. Strittmatter W. J., Weisgraber K. H., Huang D. Y., Dong L. M., Salvesen G. S., Pericak-Vance M., Schmechel D., Saunders A. M., Goldgaber D., Roses A. D. (1993) Binding of human apolipoprotein E to synthetic amyloid β peptide. Isoform-specific effects and implications for late-onset Alzheimer disease. Proc. Natl. Acad. Sci. U.S.A. 90, 8098–8102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mandrekar S., Jiang Q., Lee C. Y., Koenigsknecht-Talboo J., Holtzman D. M., Landreth G. E. (2009) Microglia mediate the clearance of soluble Aβ through fluid phase macropinocytosis. J. Neurosci. 29, 4252–4262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Saavedra L., Mohamed A., Ma V., Kar S., de Chaves E. P. (2007) Internalization of β-amyloid peptide by primary neurons in the absence of apolipoprotein E. J. Biol. Chem. 282, 35722–35732 [DOI] [PubMed] [Google Scholar]
- 43. Deane R., Wu Z., Sagare A., Davis J., Du Yan S., Hamm K., Xu F., Parisi M., LaRue B., Hu H. W., Spijkers P., Guo H., Song X., Lenting P. J., Van Nostrand W. E., Zlokovic B. V. (2004) LRP/amyloid β-peptide interaction mediates differential brain efflux of Aβ isoforms. Neuron 43, 333–344 [DOI] [PubMed] [Google Scholar]
- 44. Yamada K., Hashimoto T., Yabuki C., Nagae Y., Tachikawa M., Strickland D. K., Liu Q., Bu G., Basak J. M., Holtzman D. M., Ohtsuki S., Terasaki T., Iwatsubo T. (2008) The low-density lipoprotein receptor-related protein 1 mediates uptake of amyloid β peptides in an in vitro model of the blood-brain barrier cells. J. Biol. Chem. 283, 34554–34562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lee C. Y., Landreth G. E. (2010) The role of microglia in amyloid clearance from the AD brain. J. Neural. Transm. 117, 949–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Deane R., Sagare A., Hamm K., Parisi M., Lane S., Finn M. B., Holtzman D. M., Zlokovic B. V. (2008) ApoE-isoform specific disruption of amyloid β-peptide clearance from mouse brain. J. Clin. Invest. 118, 4002–4013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Castellano J. M., Kim J., Stewart F. R., Jiang H., DeMattos R. B., Patterson B. W., Fagan A. M., Morris J. C., Mawuenyega K. G., Cruchaga C., Goate A. M., Bales K. R., Paul S. M., Bateman R. J., Holtzman D. M. (2011) Human apoE isoforms differentially regulate brain amyloid-β peptide clearance. Sci. Transl. Med. 3, 89ra57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. DeMattos R. B., Cirrito J. R., Parsadanian M., May P. C., O'Dell M. A., Taylor J. W., Harmony J. A., Aronow B. J., Bales K. R., Paul S. M., Holtzman D. M. (2004) ApoE and clusterin cooperatively suppress Aβ levels and deposition. Evidence that ApoE regulates extracellular Aβ metabolism in vivo. Neuron 41, 193–202 [DOI] [PubMed] [Google Scholar]
- 49. Elder G. A., Cho J. Y., English D. F., Franciosi S., Schmeidler J., Sosa M. A., Gasperi R. D., Fisher E. A., Mathews P. M., Haroutunian V., Buxbaum J. D. (2007) Elevated plasma cholesterol does not affect brain Aβ in mice lacking the low-density lipoprotein receptor. J. Neurochem. 102, 1220–1231 [DOI] [PubMed] [Google Scholar]
- 50. Katsouri L., Georgopoulos S. (2011) Lack of LDL receptor enhances amyloid deposition and decreases glial response in an Alzheimer disease mouse model. PLoS One 6, e21880. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.