Background: Mechanisms of γ-aminobutyric acid type A (GABAA) receptor anchorage remain elusive.
Results: The cell adhesion molecule neuroplastin-65 can be co-purified with GABAA receptors and co-localizes with α1, α2, and α5 but not α3 subunits.
Conclusion: Neuroplastin-65 interacts with distinct receptor subtypes at synaptic or extra-synaptic sites.
Significance: This interaction might contribute to a novel mechanism of GABAA receptor anchorage affecting receptor mobility and synaptic strength.
Keywords: Cell Adhesion, FRET, GABA Receptors, Neurotransmitter Receptors, Synaptic Plasticity, Cell Adhesion Molecule, Gephyrin, Inhibitory Synapse, Neuroplastin, Receptor Subtypes
Abstract
γ-Aminobutyric acid type A (GABAA) receptors are pentameric ligand-gated ion channels that mediate fast inhibition in the central nervous system. Depending on their subunit composition, these receptors exhibit distinct pharmacological properties and differ in their ability to interact with proteins involved in receptor anchoring at synaptic or extra-synaptic sites. Whereas GABAA receptors containing α1, α2, or α3 subunits are mainly located synaptically where they interact with the submembranous scaffolding protein gephyrin, receptors containing α5 subunits are predominantly found extra-synaptically and seem to interact with radixin for anchorage. Neuroplastin is a cell adhesion molecule of the immunoglobulin superfamily that is involved in hippocampal synaptic plasticity. Our results reveal that neuroplastin and GABAA receptors can be co-purified from rat brain and exhibit a direct physical interaction as demonstrated by co-precipitation and Förster resonance energy transfer (FRET) analysis in a heterologous expression system. The brain-specific isoform neuroplastin-65 co-localizes with GABAA receptors as shown in brain sections as well as in neuronal cultures, and such complexes can either contain gephyrin or be devoid of gephyrin. Neuroplastin-65 specifically co-localizes with α1 or α2 but not with α3 subunits at GABAergic synapses. In addition, neuroplastin-65 also co-localizes with GABAA receptor α5 subunits at extra-synaptic sites. Down-regulation of neuroplastin-65 by shRNA causes a loss of GABAA receptor α2 subunits at GABAergic synapses. These results suggest that neuroplastin-65 can co-localize with a subset of GABAA receptor subtypes and might contribute to anchoring and/or confining GABAA receptors to particular synaptic or extra-synaptic sites, thus affecting receptor mobility and synaptic strength.
Introduction
γ-Aminobutyric acid type A receptors (GABAA receptors) are the major inhibitory transmitter receptors in the central nervous system and the site of action of benzodiazepines, barbiturates, neurosteroids, anesthetics, and convulsants. They are ligand-gated ion channels composed of five subunits that can belong to different subunit classes, thus giving rise to a multiplicity of GABAA receptor subtypes. The majority of these receptors are composed of one γ, two α, and two β subunits (1). Clusters of these receptors can be found at inhibitory synapses mediating phasic inhibition but also at extra-synaptic locations where they mediate tonic inhibition (2, 3).
Synaptic strength can be correlated with GABAA receptor abundance and postsynaptic localization (4) and is regulated by the rate of receptor exo- and endocytosis (5–8). GABAA receptor clusters can be stabilized by the scaffolding protein gephyrin (9, 10) that contributes to the organization and assembly of inhibitory synapses (11). The direct interaction between GABAA receptors and gephyrin (12–14) allows the complex to be anchored to the underlying postsynaptic network causing a confined lateral diffusion of GABAA receptors (15, 16). An alternative clustering mechanism was suggested for GABAA receptor α5 subunits that can directly bind to the ezrin/radixin/moesin family protein radixin (17), which in turn anchors the receptor complex to the actin cytoskeleton. trans-Synaptic cell adhesion systems can also promote the organization of synapses, e.g. members of the immunoglobulin superfamily (18, 19) or neurexin-neuroligin cell adhesion molecules (20–22) induce postsynaptic clustering at glutamatergic synapses and seem also to be important for driving the postsynaptic assembly at inhibitory synapses (23–25).
Neuroplastin (np)2-65 and -55 (np65 and np55, respectively) are cell adhesion molecules of the immunoglobulin superfamily that contain three or two extracellular immunoglobulin domains, respectively (26), which are derived from alternative splicing from a single gene. These proteins also contain a single transmembrane and a short intracellular domain. Both np isoforms are enriched in rat brain membrane preparations, where np65 is highly enriched in forebrain postsynaptic density preparations, although np55 levels are reduced (27). Previous studies indicated that np65 may be important for synaptic plasticity because anti-np antibodies and recombinant np fragments block long term potentiation in rat brain slices (28). In this study, we show that np and GABAA receptors associate and that np is located at GABAergic synapses. We show that synaptically located np65 co-localizes with GABAA receptor α1 or α2 subunits, but not with α3 subunits, indicating a subtype-selective association. Down-regulation of np65 causes a mismatch of GABAA receptor α2 subunits and VIAAT at inhibitory synapses. Interestingly, we also find a small amount of synaptic clusters that contain GABAA receptors and np65 but are devoid of gephyrin. In addition, a significant amount of np65 appears not to be localized at synapses. This was supported by the finding that np65 can also co-localize with GABAA receptor α5 subunits, which are mainly found extra-synaptically. Taken together, these results suggest that np65 can associate with a subset of GABAA receptor subtypes and might contribute to a mechanism of receptor clustering or anchoring that is independent of gephyrin.
EXPERIMENTAL PROCEDURES
Plasmids
Wild-type GABAA receptor α1, β2, and γ2 subunits were cloned into the mammalian expression vector pCI (Promega, Madison, WI), as described previously (29), resulting in constructs α1-pCI, β2pCI, and γ2-pCI. Constructs α1-ECFP or α1-EYFP and β2-ECFP or β2-EYFP were subcloned into the pECFP-C1 or EYFP-C1 vectors (Clontech), by incorporating the fluorescence tags ECFP or EYFP into the intracellular loops of the α1 or β2 subunit, and further characterization of these constructs was described elsewhere (30). The cDNAs of np55 and np65 were cloned into the expression vectors pRC/CMV (Invitrogen) as described previously (26). The constructs np65-ECFP or np65-EYFP were generated by subcloning the cDNA of np65 into pECFP-N1 or EYFP-N1 vectors (Clontech), resulting in constructs containing the fluorescence tags at the C terminus of the proteins. The fidelity of all constructs was verified by DNA sequencing, and the expression was controlled by transient transfection of the constructs into HEK cells, followed by immunoprecipitation, SDS-PAGE, and Western blot analysis. The experiments were performed with each of these fluorescent constructs with similar results.
Antibodies
The antibodies against GABAA receptor subunits α1, α2, α3, α5, and β2 were generated and affinity-purified as described previously (31–33). Mouse monoclonal anti-β2/β3 antibodies against GABAA receptor β2 and β3 subunits were purchased from Abcam (Cambridge, UK), and rabbit polyclonal antibodies against GABAA receptor α1 subunits coupled with the fluorescence dye ATTO-488 were purchased from Alomone Labs (Jerusalem, Israel). Rabbit polyclonal antibodies against both isoforms of np (np55/65) as well as the isoform-specific antibodies against np65 were generated and used as described previously (28), and goat antibodies against np65 were purchased from R&D Systems (Minneapolis, MN), and mouse monoclonal antibodies against both np isoforms (SMgp65) were a gift from Prof. P. W. Beesley (Royal Holloway University of London, United Kingdom). Rabbit polyclonal antibodies against full-length EGFP protein were generated as described elsewhere (30). Mouse monoclonal antibodies against gephyrin and guinea pig polyclonal antibodies against VIAAT were purchased from Synaptic Systems (Göttingen, Germany). Secondary antibodies used were anti-rabbit Bodipy (goat), anti-mouse Cy3 (goat), and anti-guinea pig Cy5 (goat) or anti-rabbit FITC (donkey), anti-mouse Cy3 (donkey), and anti-goat Cy5 (donkey), all purchased from Jackson ImmunoResearch (West Grove, PA).
Co-purification of np and GABAA Receptor
To identify members of synaptic np-containing protein complexes, synaptosomes were prepared from rat forebrain as described elsewhere (34) and subsequently extracted with Tris-HCl-buffered saline (TBS) containing 1% Triton X-100. The np was completely extracted because it is only present in the supernatant after 100,000 × g ultracentrifugation for 1 h, and it was not detectable in the remaining pellet. This supernatant was the input material for immunoaffinity chromatography on an immobilized polyclonal rabbit antiserum raised against the intracellular domain of np or immobilized normal rabbit IgG (Sigma) as control. Bound proteins were eluted with 0.1 m glycine, 0.1% Triton X-100 and analyzed with SDS-PAGE. Bands exclusively appearing in anti-np fraction were selected for peptide mass fingerprint identification by matrix-assisted laser desorption/ionization-time-of-flight (MALDI-TOF) mass spectrometry (Reflex III, Bruker, Germany). A band corresponding to a molecular mass of ∼60 kDa was identified as GABAA receptor rβ2 subunit.
Preparation of GABAA Receptor Extracts
Adult Sprague-Dawley rats (6–8 weeks; Him:OFA/SPF, Himberg, Austria) were sacrificed by decapitation, and their brain was rapidly removed. Forebrains were homogenized in 5 volumes of ice-cold homogenization buffer (10 mm HEPES, pH 7.5, 1 mm EDTA, 1 mm benzamidine, 0.3 mm phenylmethylsulfonyl fluoride (PMSF), 300 mm sucrose). The homogenate was centrifuged for 10 min at 1,000 × g, and the pellet was discarded. The supernatant was centrifuged for 40 min at 10,000 × g. The pellet was homogenized in 5 volumes of washing buffer (homogenization buffer without sucrose) and centrifuged for 30 min at 45,000 × g. The pellet was suspended in washing buffer and stored in aliquots frozen at −80 °C. GABAA receptors were solubilized from rat forebrain membranes by using 5 ml/cerebrum of a deoxycholate (DOC) buffer (0.5% DOC, 0.05% phosphatidylcholine, 10 mm Tris-HCl, pH 8.5, 150 mm NaCl, 1 Complete Protease Inhibitor Mixture tablet (Roche Diagnostics) per 50 ml). The suspension was homogenized using an Ultra-Turrax and subsequently by pressing the suspension through a set of needles with increasingly smaller diameters using a syringe, followed by incubation under intensive stirring for 45 min at 4 °C.
After centrifugation at 150,000 × g, the clear supernatant was used for subsequent immunoprecipitation or immunoaffinity chromatography. For the determination of the solubilization efficiency of individual GABAA receptor subunits, the 150,000 × g pellet was redissolved in the same volume of DOC buffer. Aliquots of this suspension as well as aliquots from the clear supernatant of the 150,000 × g centrifugation were separately subjected to protein precipitation using the methanol/chloroform method (35). Precipitated protein was then subjected to SDS-PAGE and quantitative Western blot analysis. All experimental protocols with animals were performed according to local law and to the principles of laboratory animal care.
Immunoaffinity Chromatography
For investigating the specificity of the antibodies coupled to the columns, the antibodies were first tested against several other GABAA receptor subunits in Western blot analysis to exclude cross-reactivity, and next the antibodies were used for immunoprecipitation and subsequent radioligand binding assay to ensure precipitation of GABAA receptors. Immunoaffinity columns were prepared by coupling 3–5 mg of the purified rabbit polyclonal antibodies β2(351–405) or anti-EGFP antibodies to 1 ml of the ImmunoPure® Protein A IgG orientation kit (Pierce) according to the manufacturer's instructions. The columns were then washed once with PBS/NaCl (150 mm NaCl, 4 mm KH2PO4, 16 mm Na2HPO4, pH 7.5), then with glycine elution buffer, pH 2.45 (0.1 m glycine-HCl, pH 2.45, 150 mm NaCl, 0.1% Triton X-100), with IP low buffer for immunoprecipitation (IP low buffer (50 mm Tris-HCl, 0.5% Triton X-100, 150 mm NaCl, and 1 mm EDTA, pH 8.0)), with alkaline elution buffer (100 mm Na2HPO4, 150 mm NaCl, pH 11.5), and finally with IP low buffer. Columns were stored protected from light in IP low buffer containing sodium azide. Immunoaffinity chromatography was performed at 4 °C. The immunoaffinity columns were equilibrated in DOC extraction buffer. The extract was chromatographed slowly in three subsequent rounds of chromatography using three affinity columns containing the same kind of antibody to completely remove the respective subunit and its associated receptors from the extract. Receptors bound to the columns were then eluted from the column with glycine elution buffer, pH 2.45 (see above). The immunoaffinity columns were regenerated by washing with glycine elution buffer, pH 2.45, IP low buffer, antibody elution buffer, pH 11.5, and IP low buffer. The eluted proteins were precipitated by methanol/chloroform (35), dissolved in NuPAGE sample buffer (Invitrogen), and subjected to SDS-PAGE and Western blot analysis.
Cell Culture and Transfection
Transformed HEK 293 cells (CRL 1573; American Type Culture Collection, Manassas, VA) were grown in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% fetal calf serum (BioWhittaker, Lonza), 2 mm glutamine, 50 μm β-mercaptoethanol, 100 units/ml penicillin G, and 100 μg/ml streptomycin in 75-cm2 culture dishes using standard cell culture techniques. HEK cells (3 × 106) were transfected with a total amount of 20 μg of subunit cDNAs via the calcium phosphate precipitation method (36). For each co-transfection with four different DNAs, 5 μg of cDNA was used. The expression of GABAA receptors and np was kept constant by co-transfection with empty pCI, pECFP, or pEYFP vectors. Cells were harvested 48 h after transfection. For FRET experiments, 150,000 cells were plated over 15- or 24-mm coverslips (Paul Marienfeld GmbH) precoated with poly-d-lysine (Sigma) in a 6-well culture dish, respectively. Cells were imaged 24 h after transfection.
Co-immunoprecipitation of Total Receptors and Cell Surface Receptors
The culture medium was removed from transfected HEK cells, and cells from four culture dishes were extracted with 1 ml of a C12E10 extraction buffer (1% polyoxyethylene 10 lauryl ether (Sigma), 0.18% phosphatidylcholine (Sigma), 150 mm NaCl, 5 mm EDTA, and 50 mm Tris-HCl, pH 7.4, containing one Mini Complete Protease Inhibitor mixture tablet (Roche Diagnostics) per 10 ml of extraction buffer) for 8–12 h at 4 °C. The extract was centrifuged for 20 min at 45,000 rpm at 4 °C. The protein concentration of the supernatant was determined (Pierce, BCA protein assay kit) and then incubated for 4 h under gentle shaking with 15 μg of α1 subunit-specific antibodies or 15 μg of anti-np antibodies. After addition of Pansorbin (formalin-fixed Staphylococcus aureus cells, purchased from Calbiochem, EMD Bioscience Inc.) and 0.5% nonfat dry milk powder and shaking for an additional 2 h at 4 °C, the precipitate was washed three times with IP low buffer. Precipitated proteins were dissolved in sample buffer (NuPAGE LDS sample buffer, Invitrogen).
Co-immunoprecipitation of receptors expressed at the cell surface was performed according to a previously described protocol (29). Briefly, the culture medium was removed from transfected HEK cells, and the cells were washed twice with PBS (2.7 mm KCl, 1.5 mm KH2PO4, 140 mm NaCl, and 4.3 mm Na2HPO4, pH 7.3). Cells were then detached from the culture dishes by incubating with 2.5 ml of 5 mm EDTA in PBS for 5 min at room temperature. The resulting cell suspension was diluted in 6 ml of cold Dulbecco's modified Eagle's medium and centrifuged for 5 min at 1,500 rpm. The cell pellet from four dishes was incubated with α1 (35 μg) or np55/65 (35 μg) antibodies in 3 ml of the same medium for 45 min at 37 °C. Cells were again pelleted, and free antibodies were removed by washing three times with 6 ml of PBS buffer. The receptors were extracted with IP low buffer containing 1% Triton X-100 for 1 h under gentle shaking. Cell debris was removed by centrifugation (45,000 rpm, 20 min at 4 °C). Following protein concentration determination, Pansorbin and 0.5% nonfat dry milk powder was added and shaken for 2 h at 4 °C, and the precipitate was washed three times and dissolved in sample buffer (NuPAGE LDS sample buffer, Invitrogen).
To investigate a possible re-distribution of the antibodies during the extraction procedure, in other experiments HEK cells were transfected with wild-type α1, β3, and γ2 subunits as well as a truncated form of γ2 subunits. After cell surface labeling by α1(1–9) antibodies, the extracts containing the cell surface-labeled receptors were divided into two fractions. One fraction was kept at 4 °C for 2 h, and the other fraction was incubated with additional α1(1–9) antibodies at 4 °C for the same time period. Pansorbin was added to both fractions, and the resulting precipitates were centrifuged, washed, dissolved in sample buffer, and subjected to SDS-PAGE and Western blot analysis. In both precipitates, full-length subunits forming complete receptors could be detected, whereas truncated subunits could only be detected in the fraction where additional α1(1–9) antibodies had been added after cell lysis (32).
SDS-PAGE and Western Blot Analysis
SDS-PAGE was performed according to Neville and Glossmann (37) using 10% SDS-polyacrylamide gels in a discontinuous system. For estimation of the size of the proteins, pre-stained SDS-PAGE standards (Bio-Rad) were used in separate lanes. Proteins were blotted onto polyvinylidene difluoride (PVDF) membranes as described previously (38). For the detection of np, the mouse monoclonal antibody SMgp65 (28) was used. Alternatively, rabbit polyclonal antibodies against GABAA receptor β2 subunits were used, which were previously labeled with digoxigenin using the DIG protein labeling kit (Roche Diagnostics) according to the manufacturer's instructions. As secondary antibodies, either anti-mouse coupled to alkaline phosphatase (Jackson ImmunoResearch, Suffolk, UK) or anti-digoxigenin-alkaline phosphatase Fab fragments (Roche Diagnostics) were used. The reaction of alkaline phosphatase was visualized with CDP-Star (Applied Biosystems, Bedford, MA) according to the manufacturer's instructions. For quantitative Western blot analysis, immunoblots were exposed to the Fluor-S MultiImager (Bio-Rad) and evaluated using the Quantity One quantitation software (Bio-Rad) and Prism (Graph Pad Software Inc., San Diego). The linear range of the detection system was established by measuring the antibody-generated signal to a range of antigen concentrations. Under the experimental conditions used, the immunoreactivities were within the linear range, and this permitted a direct comparison of the amount of antigen per gel lane between samples. Statistical significance was tested using the unpaired Student's t test.
FRET Imaging
FRET was measured as described previously (39–41). Briefly, FRET was performed using an epifluorescence microscope (Carl Zeiss Axiovert 200) using the “three-filter method” (42). The images were taken using a ×63 oil immersion objective and Ludl filter wheels to allow for rapid switching between the fluorescence excitation and emission filters for CFP (ICFP; excitation, 436 nm; emission, 480 nm, and dichroic mirror, 455 nm), YFP (IYFP; excitation, 500 nm; emission, 535 nm, and dichroic mirror, 515 nm), and FRET (IFRET; excitation, 436 nm; emission, 535 nm, and dichroic mirror, 455 nm). Images were captured by a CCD camera (Kappa GmbH, Gleichen, Germany), and regions of interest were selected manually expressing both FRET partners. Intensity (I) from the three filter sets was obtained after background subtraction. NFRET was calculated as shown in Equation 1,
where a = 23%, which is the percentage of CFP contribution to FRET intensity, and b = 67% which is the percentage of YFP contributing to FRET intensity.
Isolation of Primary Hippocampal Neurons
Primary neurons from 17-day-old embryonic (E17) rat brains were isolated as described previously (43). Briefly, dissected E17 hippocampi were incubated in a trypsin/EDTA solution (Biochrom, Berlin, Germany) at 37 °C for 10 min, washed twice with a modified Hanks' balanced salt solution (HBSS: 137 mm NaCl, 7 mm HEPES, 4.2 mm NaHCO3, 0.35 mm Na2HPO4, 0.45 mm KH2PO4, 5.4 mm KCl, 1.3 mm CaCl2, 0.8 mm MgSO4, 5.5 mm d-glucose, 100 units/ml penicillin, 100 mg/ml streptomycin in double distilled H2O; pH adjusted to 7.3 with 37% HCl or 10 m NaOH) all from Merck)), and dissociated by gentle trituration. Cell counts of dissociated neurons were subsequently determined in a Neubauer hemocytometer (Brand, Wertheim, Germany). The neuronal cell suspension was seeded onto poly-l-lysine-coated glass coverslips equilibrated (37 °C; 5% CO2) and placed on a layer of feeder cells in neuronal tissue culture medium (1× modified Eagle's medium (Invitrogen) containing 26 mm NaHCO3 (Merck), 1 mm sodium pyruvate (Sigma), 2 mm stable l-glutamine (PromoCell, Heidelberg, Germany), 33 mm d-glucose (Merck), and 2% (v/v) B-27 supplement (Invitrogen) in double distilled H2O). Neurons were then incubated for up to 21 days at 37 °C, 5% CO2, and expression of np and GABAA receptor was followed from days in vitro (DIV) 1–21. GABAergic synapses were detectable from DIV6–7 onward.
Immunocytochemistry of Dissociated Primary Neurons, Image Acquisition, and Analysis
DIV11–21-old neurons were fixed for 15 min in 4% (w/v) paraformaldehyde in PBS. Cells were then incubated for 30 min in 5% (w/v) bovine serum albumin (BSA, Sigma) to block nonspecific staining with or without 0.1% Triton X-100 and then incubated for 2 h with primary antibodies in 5% BSA. Antibodies used were as follows: mouse monoclonal antibodies directed against the extracellular domains of GABAA receptor β2 and β3 subunits (1:100) or rabbit polyclonal antibodies directed against GABAA receptor α2, α3, or α5 subunits (all 5 μg/ml); polyclonal antibodies against the extracellular immunoglobulin domains of np55 and np65 (5 μg/ml); goat polyclonal antibodies against the extracellular immunoglobulin domain specific to np65 (1:500); mouse monoclonal antibodies against gephyrin (1:500); guinea pig polyclonal antibodies against VIAAT (1:1000), and rabbit polyclonal antibodies against GABAA receptor α1 subunits coupled to ATTO-488 (1:50). After washing, cells were incubated for 45 min with secondary antibodies conjugated to appropriate fluorophores. Secondary antibodies used were as follows: Bodipy-conjugated goat anti-rabbit (1:100); Cy3-conjugated goat anti-mouse (1:500); Cy5-conjugated goat anti-guinea pig (1:100); FITC-conjugated donkey anti-rabbit (1:500); Cy3-conjugated donkey anti-mouse (1:500), and Cy5-conjugated donkey anti-goat (1:500). Fluorescent images were acquired under identical conditions using a Leica TCS SP5 II confocal microscope using 496 nm laser (Ar, 65 milliwatt), 543 nm laser (HeNe, 1 milliwatt), and 633 nm laser (HeNe, 10 milliwatt). Images were visualized using Leica software application, then subjected to 10–20 cycles of three-dimensional deconvolution using AutoQuant X software (Media Cybernetics, Roper Industries Ltd.), and processed using Imaris software (Bitplane).
Immunocytochemistry of Hippocampal Sections
Adult male Sprague-Dawley rats (6–8 weeks; Him:OFA/SPF, Himberg, Austria) were injected with a lethal dose of thiopental (150 mg/kg, intraperitoneally), transcardially perfused through the ascending aorta with 0.9% saline solution, and followed by 200 ml of chilled 4% paraformaldehyde and 15% saturated picrinic acid in PBS over 20 min using a peristaltic pump. The brains were removed from the skulls and transferred in 0.1 m phosphate buffer (PB) with 0.05% sodium azide, where they were kept until cutting. Coronal sections (50 μm) of the hippocampus were prepared on a Vibratome and stored in 0.1 m PB with 0.05% sodium azide until staining. Free-floating sections of three different levels were used for staining. The sections were rinsed in 0.1 m PB and transferred in TBS containing 0.1% Triton X-100. The nonspecific binding was blocked by incubation in 20% normal horse serum for 2 h at room temperature. The primary antibodies were diluted in 1% normal horse serum in TBS with 0.1% Triton X-100, and the sections were incubated for 48–72 h at 4 °C. After extensive washing, sections were incubated with donkey anti-rabbit, donkey anti-mouse, and donkey anti-guinea pig secondary antibodies for 4 h at room temperature. Sections were mounted with Vectashield (Vector Laboratories Ltd., Peterborough, UK) and examined and photographed with a confocal laser-scanning microscope (Leica TCS SP5 II).
Generation of shRNA Constructs and Transfection into HEK Cells and Hippocampal Neurons
Different regions of the np immunoglobulin sequence were selected to generate shRNA constructs using the pSuper RNAi system (OligoEngine, Seattle). Small hairpin RNA (shRNA) constructs were transiently transfected into HEK cells together with np65 constructs. HEK cells were harvested 48 h after transfection; proteins were extracted; np65 was precipitated as described above, and the immunoprecipitates were subjected to SDS-PAGE and Western blot analysis. The construct shRNA1 that specifically recognized a sequence within the first immunoglobulin domain specific for np65 (5′-AACGGAATGACTTGAGGCA-3′) efficiently down-regulated overexpressed np65. This construct was chosen for transfection into primary rat hippocampal neurons. At DIV5, neurons were transfected with construct shRNA1 and pEGFP vector using the Ca2+-phosphate/DNA co-precipitate-based method (44).
RESULTS
Neuroplastin and GABAA Receptors Associate with Each Other
The glycoprotein np was reported to be located at synaptic specializations, but its general function at post-synaptic termini has not been fully elucidated. To investigate putative interaction partners of np, rat brain extracts were subjected to affinity chromatography using either a rabbit polyclonal antiserum against the intracellular domain of np or rabbit normal IgG. Eluted proteins were then subjected to SDS-PAGE and protein staining. Protein bands that were present in the samples purified over np-antibody columns but not in those containing normal IgG were identified, excised, and subsequently subjected to mass spectrometry. Data from mass spectrometry indicated a sequence that was identical to GABAA receptor β2 subunits (gi|6978869 NP_037089.1) with an apparent molecular mass of about 60 kDa,3 which was somewhat higher than the reported molecular mass of 50–53 for GABAA receptor β2 subunits. This demonstrated that np and β2 subunits could be co-purified from rat brains and suggested that np and GABAA receptors might be present in the same protein complex. This finding prompted us to confirm the association of np with GABAA receptors in a reciprocal experiment. To this end, rat forebrain membranes were extracted with DOC buffer, and the cleared extract was loaded on immunoaffinity columns containing antibodies directed against GABAA receptor β2 subunits. Extract, efflux, and eluate were subjected to SDS-PAGE and Western blot analysis using digoxygenin-labeled β2 antibodies. GABAA receptor β2 subunits were present in all samples, migrated as protein bands of about 50–53 kDa, and were significantly enriched in the affinity column eluate (Fig. 1, upper panel). Western blot analysis of the same samples using monoclonal SMgp65 antibodies indicated that np migrated as two distinct bands at 55 and 65 kDa representing the two described np isoforms (26) and could be retained by and co-eluted from GABAA receptor β2 columns (Fig. 1, lower panel). The β2 antibodies used were specific for GABAA receptor β2 subunits as shown in control experiments using brain membranes of knock-out mice that were deficient for β2 subunits (supplemental Fig. 1A). In addition, β2 antibodies were unable to directly precipitate np in heterologous expression systems (supplemental Fig. 1B), whereas np antibodies could not precipitate GABAA receptor β2 subunits (supplemental Fig. 1C). Finally, immunoaffinity columns containing antibodies directed against EGFP could not retain β2 subunits (supplemental Fig. 1D). Taken together, these results indicate that np was retained by the immunoaffinity column because it was associated with GABAA receptors containing β2 subunits.
FIGURE 1.

Co-purification of neuroplastin with GABAA receptor β2 subunits from rat brain extracts. Western blot analysis indicates the presence of np in brain membrane extracts as well as in β2(351–405) immunoaffinity column efflux and eluates. Aliquots of each fraction were subjected to SDS-PAGE and Western blot analysis using digoxigenin (DIG)-labeled β2(351–405) antibodies (upper panel) or monoclonal mouse SMgp65 antibodies (lower panel). The latter antibodies detected both np isoforms, np55 and np65, that migrated as protein bands of 55 and 65 kDa, respectively (26). This is a typical experiment performed two times with comparable results.
Interestingly, only about 15% of np was found to be associated with GABAA receptors. This suggested that either the interaction between np and GABAA receptors was only transient and/or weak, was sensitive to extraction methods, or that only a small fraction of np is associated with GABAA receptors.
To confirm the interaction of GABAA receptors with np in a heterologous expression system np55 or np65 expression constructs were co-transfected with GABAA receptor α1, β2, and γ2 subunits in HEK 293 cells. Proteins were extracted and immunoprecipitated using antibodies against both np isoforms (np55/65) (Fig. 2, two left panels). The precipitated complexes were subjected to SDS-PAGE and Western blot analysis using SMgp65 antibodies or digoxigenin-labeled β2 antibodies. np55 and np65 were expressed to a similar extent (Fig. 2, left panel), and np55/65 antibodies could precipitate GABAA receptors as indicated by the presence of the β2 subunit in the precipitate (Fig. 2, 2nd panel from left). In other experiments, the same HEK cell extracts were immunoprecipitated by antibodies recognizing GABAA receptor α1 subunits (Fig, 2, 3rd panel from left) and subjected to Western blot analysis using SMgp65 antibodies. Conversely, α1 antibodies that did not exhibit any cross-reactivity with other GABAA receptor subunits or np (data not shown), precipitated np55, and np65 (Fig. 2, 3rd panel from left). In similar experiments, we aimed at investigating whether np-GABAA receptor complexes were found at the cell surface. To this end, appropriately transfected HEK cells were incubated with antibodies directed against the extracellular domain of GABAA receptor α1 subunits, and the labeled antibody-receptor complexes were extracted and subjected to immunoprecipitation. Precipitates were analyzed by SDS-PAGE and Western blot analysis. A possible redistribution of the antibodies during the extraction procedure could be excluded by an experiment performed analogously to that described by Klausberger et al. (32). Thus, we demonstrate that np associates with GABAA receptors at the cell surface of transfected HEK cells (Fig. 2, right panel). Interestingly, quantification of the Western blot data indicated that three times more np65 than np55 could be co-precipitated with GABAA receptors from the cell surface. Therefore, for further experiments we focused on the interaction of np65 with GABAA receptors.
FIGURE 2.
Co-precipitation of recombinant neuroplastin and GABAA receptors in HEK cells. HEK cells were transfected as indicated. Proteins were extracted, immunoprecipitated by np (np55/65) antibodies or GABAA receptor α1(1–9) antibodies, and subjected to SDS-PAGE and Western blot analysis using monoclonal mouse SMgp65 antibodies for the identification of np55 and np65 (1st panel on the left) or digoxigenin (DIG)-labeled β2 antibodies for detection of GABAA receptor β2 subunits. Precipitation by np55/65 antibodies revealed a co-precipitation of GABAA receptor β2 subunits (2nd panel from left), and conversely, precipitation by α1(1–9) antibodies revealed a co-precipitation of np55 and np65 (3rd panel from left). In parallel, GABAA receptors expressed at the surface of appropriately transfected HEK cells were immunolabeled by incubation with α1(1–9) antibodies and extracted under conditions where the interaction with the antibody was not impaired (29). The antibody-receptor complexes were then precipitated by adding immunoprecipitin and subjected to SDS-PAGE and Western blot analysis using SMgp65 antibodies. Results indicated that np55 as well as np65 could be co-precipitated with GABAA receptors from the cell surface (right panel).
Neuroplastin-65 Directly Interacts with GABAA Receptors
To investigate whether the association of np65 with GABAA receptors is due to a direct physical interaction or is mediated by linker proteins, FRET studies were performed. To do so, we generated fluorescence-tagged np65-ECFP or np65-EYFP fusion proteins, as well as tagged GABAA α1-EYFP or β2-EYFP receptor subunit constructs (30). Different combinations of constructs were transfected into HEK cells (Table 1), and their expression was investigated using an epifluorescence microscope, and distinct regions where both fluorescence-tagged proteins were clearly present were chosen to calculate their NFRET values (see under “Experimental Procedures”). FRET analysis of cells co-expressing np65-ECFP and np65-EYFP indicated that these fusion proteins interacted to a high extent (Table 1), probably by forming homo-oligomers.
TABLE 1.
NFRET values indicate an interaction between GABAA receptor subunits and neuroplastin
HEK cells were transfected with the fluorescence-tagged or untagged constructs as indicated. NFRET values obtained from either fluorescence-tagged np65 homo-oligomers or from fluorescence-tagged np65 and GABAA receptor α1 or β2 subunits forming hetero-oligomers were significantly higher than NFRET values from fluorescence-tagged np65 and P2X2 receptors (the latter combination served as a putative negative control in these experiments, indicated as +/− interaction in the table). NFRET values are given as mean values ± S.E. Data are from one representative experiment investigating 1–4 regions of interest from 32 cells. A strong FRET signal (NFRET) was found for np65-ECFP-np65-EYFP complexes (indicated as +++ interaction in the table) as well as for complexes of np65-ECFP with complete GABAA receptors containing α1-EYFP or β2-EYFP subunits (indicated as ++ interaction in the table). FRET analyses were performed three times with comparable results, each time using transfected HEK cells from independent experiments.
| Construct 1 | Construct 2 | Construct 3 | Construct 4 | NFRET | Interaction |
|---|---|---|---|---|---|
| np65-ECFP | np65-EYFP | 0.408 ± 0.07 | +++ | ||
| np65-ECFP | α1-EYFP | WT β2 | WT γ2 | 0.319 ± 0.03 | ++ |
| np65-ECFP | β2-EYFP | WT α1 | WT γ2 | 0.353 ± 0.08 | ++ |
| np65-ECFP | P2X2-EYFP | 0.153 ± 0.04 | +/− | ||
| ECFP | EYFP | 0.023 ± 0.01 | − |
In addition, when np65-ECFP was co-expressed with α1-EYFP, wild-type β2 and γ2 subunits, significant NFRET values were observed (Fig. 3 and Table 1). Significant FRET was also observed when np65-ECFP was co-expressed with β2-EYFP, wild-type α1, and γ2 subunits. Another unrelated fluorescence-tagged receptor (P2X2-EYFP; see Ref. 30) showed significantly lower NFRET values, and empty vectors expressing only the fluorescence proteins ECFP and EYFP did not yield any significant FRET. These results indicated that np65 and GABAA receptors associate and are in direct physical contact.
FIGURE 3.
FRET imaging of recombinant neuroplastin and GABAA receptors in HEK cells. HEK cells were transfected with the respective fluorescence-tagged constructs (see also Table 1). Pictures are taken from one representative experiment in which HEK cells were transfected with fluorescence-tagged np65-ECFP and α1-EYFP as well as untagged β2 and γ2 subunits. Images are shown in pseudo-colors representing data obtained from epifluorescence microscopy using CFP, YFP, and FRET channels. Three consecutive FRET analyses using three independent transfection experiments yielded comparable results. Scale bar, 20 μm.
Neuroplastin Co-localizes with GABAA Receptors in Dissociated Primary Hippocampal Neurons
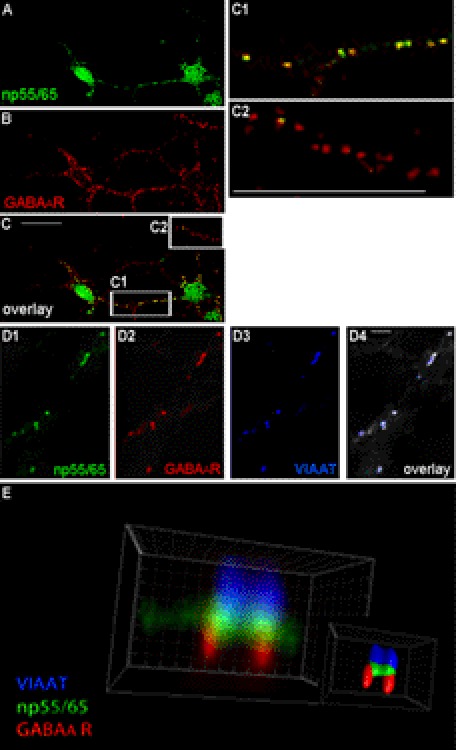
To investigate a possible co-localization of np and GABAA receptors in hippocampal neurons, primary neurons from 17-day-old embryonic (E17) rat brains were isolated and seeded onto poly-l-lysine-coated glass coverslips and then incubated for 0–21 DIV to allow GABAergic synapse formation to occur. A time course following the expression of np and GABAA receptors indicated a peak of co-localization between np and GABAA receptors from DIV8 to DIV18 (data not shown). Dissociated hippocampal neurons were stained at DIV13, using polyclonal rabbit antibodies against np55 and np65 (np55/65) as well as the monoclonal mouse antibody β2/3, directed against GABAA receptor β2 and β3 subunits. Because both antibodies recognized extracellular epitopes of the respective proteins and the experiment was performed in the absence of detergents, staining indicated that GABAA receptors containing β2 or β3 subunits co-localize with np (Fig. 4, A–C) to a significant extent at the cell surface. Interestingly, although some neurites display significant amounts of distinct puncta of co-localization (Fig. 4C1), others show little co-localization between GABAA receptors and np (Fig. 4C2). Quantification of these data suggested that about 26.6% of all GABAA receptor clusters in dissociated hippocampal neurons are co-localized with np clusters. Further quantification of the same data revealed that 17.6% of np co-localize with GABAA receptor clusters in dissociated primary neurons. These results indicate that np is most likely not only localized at GABAergic synapses but also elsewhere, e.g. at glutamatergic synapses. This is not very surprising because previous results indicated a role of np65 for α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor trafficking (45). To investigate whether these clusters are found at the synapse, we repeated these experiments (this time in the presence of detergents) by triple staining, including antibodies directed against the VIAAT that supposedly label all GABAergic synapses. As shown in Fig. 4, D1–D4, we find regions with distinct clusters of np, GABA receptor, and VIAAT, indicating that these clusters are most likely synaptically localized. The finding that only about a fourth of GABAA receptors co-localize with np suggests that np55 and/or np65 are present at only a subset of GABAergic synapses.
FIGURE 4.
Co-localization of neuroplastin and GABAA receptors in dissociated hippocampal neurons in culture. A–C, co-immunostaining in the absence of detergents was performed on dissociated hippocampal neurons in culture at DIV13 using polyclonal rabbit antibodies against np55 and np65 (green, top panel) and monoclonal mouse antibodies against GABAA receptor β2/3 subunits (red, 2nd panel from top). Overlaid images from both channels are depicted in the 3rd panel from the top (overlay). Clusters containing GABAA receptors and np55/65 are shown in a section of a dendrite (boxed region C1). Other dendrites contained mainly GABAA receptors and no np55/65 (boxed region C2). The boxed regions from the 3rd panel (C, overlay) are enlarged and shown in the two panels on the right (C1 and C2). Scale bars, 20 μm. D1–4, co-immunostaining in the presence of detergents was performed as above but included polyclonal guinea pig antibodies against VIAAT (blue, D3). Overlaid images from all three channels are depicted in the rightmost panel (D4, overlay). The images selected represent a section of a dendrite with a high amount of co-localization between these three proteins and clearly indicate synaptically localized clusters of GABAA receptors and np. Scale bar, 3 μm. E, modeling of individual GABAergic synapses. Triple immunostaining was performed on dissociated hippocampal neurons in culture at DIV13 using polyclonal rabbit antibodies against np (green), monoclonal mouse antibodies against GABAA receptor β2/3 subunits (red), and polyclonal guinea pig antibodies against VIAAT (blue). Data were subjected to 20 cycles of three-dimensional deconvolution (AutoQuant), and image processing of cropped data displaying two individual synapses was performed using Imaris software. The big picture (left) displays fluorescence signals after deconvolution, and the small picture shows surface modeling of the two synapses using these fluorescence signals.
To study the co-localization of the np, GABAA receptor, and VIAAT in more detail, we subjected z-stacks of confocal images to three-dimensional deconvolution. The fluorescence signals derived from secondary antibodies recognizing the protein complex were used for remodeling individual synapses using Imaris software. As shown in Fig. 4E, all three proteins are most likely localized at the same synapse. The distinct signals of the interaction partners of this complex suggest that np is located adjacent to the presynaptic marker VIAAT and the postsynaptic GABAA receptors and appears to mediate the contact between the pre- and postsynapse.
Neuroplastin Is Located at a Subset of GABAergic Synapses in Dissociated Primary Hippocampal Neurons
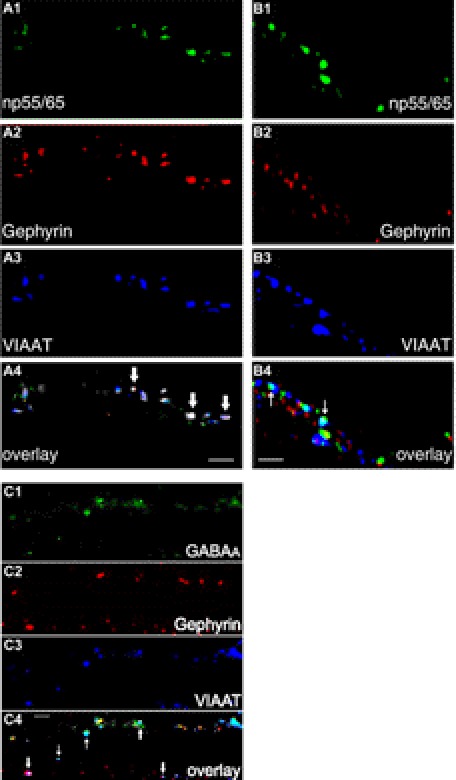
GABAA receptors expressed in hippocampal neurons co-localize with the scaffolding protein gephyrin at inhibitory postsynapses (46–48). Furthermore, gephyrin appears to be a critical determinant of GABAA receptor clustering. It was therefore interesting to investigate whether np could be found at synapses that were labeled by antibodies against gephyrin. For this purpose, dissociated hippocampal neurons were stained at DIV13, using polyclonal rabbit antibodies against np55 and np65 (np55/65), monoclonal mouse antibodies against gephyrin, and polyclonal guinea pig antibodies against VIAAT. Triple staining indicated the presence of distinct clusters containing np and gephyrin at inhibitory synapses (Fig. 5, A1–A4). Interestingly, quantification analysis revealed that only 8.5% of gephyrin clusters co-localized with np clusters, and only about 5.8% of np clusters co-localized with gephyrin clusters, indicating a partial overlap of np and gephyrin at GABAergic synapses.
FIGURE 5.
Co-localization of a subset of neuroplastin clusters with gephyrin at GABAergic synapses in dissociated hippocampal neurons. Co-immunostaining was performed on dissociated hippocampal neurons in culture at DIV13 using polyclonal rabbit antibodies against np (green), monoclonal mouse antibodies against gephyrin (red), and polyclonal guinea pig antibodies against VIAAT (blue). A1–A4, a section of a triple-stained dendrite is shown. The bold arrows in the overlay panel indicate clusters where np (A1), gephyrin (A2), and VIAAT (A3) are co-localized (A4, white). B1–B4, another section of a different neurite is shown in which the thin arrows in the overlay panel indicate clusters where np (B1) and VIAAT (B3) are co-localized (B4, cyan), but these clusters are devoid of gephyrin (B2). C1–C4, some GABAergic synapses are devoid of gephyrin in dissociated hippocampal neurons in culture. Co-immunostaining was performed on dissociated hippocampal neurons in culture at DIV14 using ATTO-488-coupled rabbit antibodies against GABAA receptor α1 subunits (C1, green), monoclonal mouse antibodies against gephyrin (C2, red), and polyclonal guinea pig antibodies against VIAAT (C3, blue). Bold arrows in the overlay panels indicate clusters containing GABAA receptor α1 subunits, gephyrin, and VIAAT (C4, white), and thin arrows indicate clusters containing GABAA receptor α1 subunits and VIAAT (C4, cyan), which are devoid of gephyrin. Scale bar, 2 μm.
Interestingly, when other neurites of the culture dish shown in Fig. 5A were investigated, it could be demonstrated that some np clusters co-localized with VIAAT but were devoid of gephyrin (Fig. 5, B1–B4). This is in agreement with previous findings that some GABAergic synapses might be independent of gephyrin interaction (49, 50). To further investigate this assumption, we labeled hippocampal neurons using fluorescence (ATTO-488)-coupled antibodies directed against the N terminus of GABAA receptor α1 subunits as well as antibodies against gephyrin and VIAAT (Fig. 5, C1–C4). Usually, in rat brains α1 subunits are expressed mainly postnatally, and in dissociated hippocampal neurons, they are only found after DIV10–12 to a significant extent.4 As shown in Fig. 5C, some synaptic GABAA receptor clusters contained α1 subunits as well as gephyrin (bold arrows), whereas other synaptic GABAA receptor clusters contained α1 subunits but were devoid of gephyrin (thin arrows). It is thus possible that some synaptic GABAA receptors interact with np and form clusters also in the absence of gephyrin.
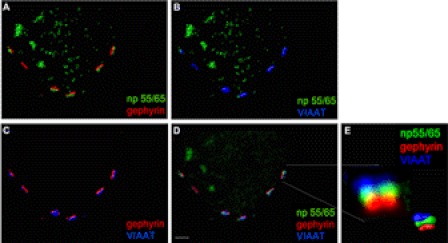
In an experiment analogous to that described for Fig. 4E, primary hippocampal neurons were stained using antibodies against np65, gephyrin, and VIAAT (Fig. 6D), and synapses were modeled using Imaris software (Fig. 6, A–C). The modeled individual synapses (Fig. 6E) revealed a similar topology of the three proteins np, VIAAT, and gephyrin as seen with antibodies that labeled np, VIAAT, and GABAA receptors (Fig. 4E), again suggesting that the three proteins np55/65, gephyrin, and VIAAT were most likely localized at the same synapse.
FIGURE 6.
Co-localization and synaptic modeling of neuroplastin and gephyrin at inhibitory synapses. Co-immunostaining was performed on dissociated hippocampal neurons in culture at DIV13 using polyclonal rabbit antibodies against np (green), monoclonal mouse antibodies against gephyrin (red), and polyclonal guinea pig antibodies against VIAAT (blue). Data were subjected to 20 cycles of three-dimensional deconvolution (AutoQuant), and image processing of cropped data displaying individual synapses was performed using Imaris software. A–D show the cell body of a neuron with several GABAergic synapses. A–C show surface modeling of the synapses by Imaris highlighting only two of the respective proteins investigated, and D displays fluorescence signals after deconvolution before image processing. An individual synapse was singled out for magnification as indicated by two lines as section markers. E, magnification of the marked individual synapse showing fluorescence signals after deconvolution (left picture) and the small picture (inset at the right corner) shows surface modeling of the synapse using these fluorescence signals and confirming the localization of np at GABAergic synapses. Scale bar, 2 μm.
Neuroplastin Co-localizes with GABAA Receptor α1, α2, and α5 Subunits
The observation that only about 26% of GABAA receptors co-localize with np prompted us to investigate whether there is a distinct co-localization of np with certain GABAA receptor subtypes. Because of the recent availability of an antibody that specifically stained only the np65 isoform, we used this antibody for immunolabeling. Thus, hippocampal neurons in culture were stained using antibodies against np65, VIAAT, and GABAA receptor subtype-specific antibodies directed against either α1 or α2 or α3 or α5 subunits. Results indicated that about 4% of GABAA receptor α1 subunits co-localize with np65 (Fig. 7A, bold arrow) at the synapse as indicated by co-labeling with antibodies against VIAAT. This value, however, probably under-represents the extent of co-localization between np65 and GABAA receptor α1 subunits due to the background of this commercially purchased ATTO-488 α1 antibody. In addition, 7.2% of GABAA receptor α2 subunits were found to co-localize at inhibitory synapses (Fig. 7B, bold arrow). Interestingly, a small number of clusters containing np65 and GABAA receptor α2 subunits could also be found that were not stained by VIAAT antibodies (Fig. 7B, thin arrow) indicating that there might be a minor population of GABAA receptors containing α2 subunits that are located extra-synaptically and can co-localize with np65. In contrast, np65 and GABAA receptor α3 subunits co-localize only to a negligible extent (Fig. 7C). Finally, 7.8% of GABAA receptor α5 subunits co-localize with np65, but these clusters almost entirely lacked VIAAT staining and were thus most likely extra-synaptically located (Fig. 7D, thin arrow). In addition, a small number of clusters contained GABAA receptor α5 subunits, np65, and VIAAT thus indicating their synaptic localization (Fig. 7D, bold arrow).
FIGURE 7.
Co-localization of neuroplastin and GABAA receptor subtypes in dissociated hippocampal neurons in culture. Co-immunostaining was performed on dissociated hippocampal neurons in culture at DIV13 using monoclonal mouse antibodies against VIAAT (red), polyclonal goat antibodies against np65 (blue), and polyclonal rabbit antibodies directed against individual GABAA receptor subunits (green) as indicated in the figure. A, co-staining using antibodies against GABAA receptor subunit α1. B, co-staining using antibodies against GABAA receptor subunit α2. C, co-staining using antibodies against GABAA receptor subunit α3. D, co-staining using antibodies against GABAA receptor subunit α5. All images were taken by confocal microscopy and were subjected to 20 cycles of three-dimensional deconvolution (AutoQuant), and image processing of cropped data displaying individual synapses was performed using Imaris software. Bold arrows generally indicate synaptic localization of receptor clusters, and thin arrows suggest extra-synaptic localization of clusters.
Neuroplastin Is Localized at GABAergic Synapses in Hippocampal Sections
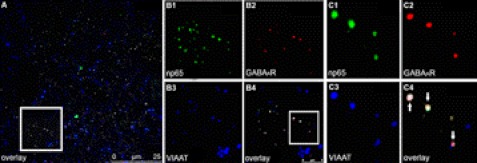
Results from dissociated primary hippocampal neurons strongly indicated that np65 is localized at GABAergic synapses. To verify that this assumption is also valid for GABAergic synapses in brain tissue, we performed immunohistochemical studies of hippocampal sections of adult rats. Immunostained sections were subjected to confocal microscopy. Sections selected showed a strong and distinct staining of np65 as well as GABAA receptors, and z-stacks of these sections were then analyzed individually. Fig. 8 shows a distinct area of the dentate gyrus where there was clear co-localization of np65, GABAA receptor β2/3 subunits, and VIAAT (arrows in Fig. 8C4), supporting our co-localization data from hippocampal neurons in culture. In agreement with our data from immunostaining of dissociated hippocampal neurons, which indicated either a strong or a weak co-localization in different dendrites (Fig. 4C), immunostaining of other sections of the brain tissue revealed a co-localization to a lesser extent.4
FIGURE 8.
Localization of neuroplastin at GABAergic synapses in hippocampal tissue sections. A–C, immunohistochemical staining of free-floating hippocampal brain sections from the dentate gyrus of adult male rats using rabbit antibodies that recognized np65 (B1 and C1, green), monoclonal mouse antibodies against GABAA receptor β2/3 subunits (B2 and C2, red), and polyclonal guinea pig antibodies against VIAAT (B3 and C3, blue). All pictures were taken by confocal microscopy. The box in the left panel (A) indicates the section that is enlarged in the four middle panels (B1–B4). The boxed area in one of the middle panels (B4, bottom) indicates the section that is further enlarged in the four panels to the right (C1–C4). Clusters in which all three proteins, np65, GABAA receptor β2/3 subunits, and VIAAT, are co-localized are indicated by arrows (C4). Scale bars, 25 μm (A) or 5 μm (B4) as indicated.
Neuroplastin Down-regulation Causes Impaired Localization of GABAA Receptor α2 Subunits at Inhibitory Synapses
The co-localization between np65 and α2 subunits at GABAergic synapses prompted us to evaluate the impact of down-regulation of np65 in primary hippocampal neurons. To this end, we generated several shRNA constructs directed against sequences within the immunoglobulin domain of np. To determine which construct causes an efficient down-regulation of np65, we first tested the constructs in a heterologous expression system (supplemental Fig. 2). The shRNA1 efficiently down-regulated overexpressed np65 and was therefore chosen for transfection of hippocampal neurons in culture at DIV5 together with an EGFP construct as transfection control. Neurons were checked for expression of EGFP and co-labeled using antibodies against GABAA receptor α2 subunits and VIAAT. After immunostaining, GABAergic synapses were inspected using confocal microscopy. Results indicated that in EGFP- and shRNA1-transfected neurons in which endogenous np65 was presumably down-regulated, GABAA receptor α2 subunit staining was more diffuse, and in some dendrites there was a significant mismatch between α2 subunits and VIAAT (Fig. 9, A and B, arrows). Importantly, neurons on the same coverslip that were not transfected exhibited a clear match between well concentrated postsynaptic GABAA receptor α2 subunits and presynaptic VIAAT (Fig. 9, A and B, asterisks) as expected.
FIGURE 9.
Down-regulation of np65 causes a loss of co-localization between GABAA receptor α2 subunits and VIAAT. Primary hippocampal neurons were transfected with shRNA directed against np65 together with pEGFP (upper, left panel, green). Neurons were stained 48 h after transfection with antibodies against GABAA receptor α2 subunits (upper, middle panel, red) and VIAAT (upper, right panel, blue). Boxed regions (A and B) show dendritic sections of a transfected and an untransfected neuron next to each other. Boxed regions are enlarged (bottom panels A and B). Arrows indicate mismatches between GABAA receptor α2 subunits and VIAAT in dendrites of transfected neurons. Asterisks indicate a clear co-localization of α2 subunits and VIAAT in dendrites of untransfected neurons on the same coverslip.
DISCUSSION
Cell Adhesion Molecule Neuroplastin-65 Interacts with GABAA Receptors
The cell adhesion molecule neuroplastin, a member of the immunoglobulin superfamily, is enriched at postsynaptic densities (28) and was previously found to play a role at glutamatergic synapses (45). In this study, we demonstrate that np can also interact with GABAA receptors. This conclusion is supported by several lines of evidence. First, GABAA receptor β2 subunits co-purify with np, and the np isoforms np65 and np55 could be co-purified with GABAA receptors from rat brain extracts. Second, np as well as GABAA receptors transiently transfected into HEK cells could be co-precipitated from cell extracts as well as from the cell surface using antibodies against np or GABAA receptor subunits. Interestingly, GABAA receptors displayed a three times higher association with the brain-specific isoform np65 at the cell surface than with np55. Third, FRET analysis using fluorescence-tagged np65 and GABAA receptor subunit proteins expressed in HEK cells suggested a direct interaction between GABAA receptors and np65. Fourth, GABAA receptors and np65 co-localized in embryonic hippocampal neurons in culture as well as in hippocampal sections from adult rat brains. Taken together, these results strongly indicate that np65 is a novel interaction partner of GABAA receptors. Whether interaction between these proteins already occurs intracellularly, resulting in co-trafficking of the associated proteins to the cell surface, or whether interaction between GABAA receptors and np65 exclusively occurs at the cell surface was not investigated in this study. Further experiments will have to distinguish between these possibilities.
Clusters Containing Neuroplastin-65 as Well as GABAA Receptors Are Found at Synaptic Sites
Triple immunostaining of cultured neurons using antibodies against np, GABAA receptors, and the presynaptic marker VIAAT indicated that these clusters were located at synaptic sites. This finding was supported by further processing of these immunocytochemical images by deconvolution, increasing magnification, and surface modeling of the fluorescence signals derived from the staining antibodies, which suggested that these proteins indeed formed distinct complexes at synapses. Interestingly, np seemed to be located adjacent to VIAAT as well as GABAA receptors, as expected from cell adhesion molecules that are usually found at synaptic sites where they probably contribute to the developmental recognition and alignment of pre- and postsynapses. Immunolabeling studies determining the co-localization of np65 with different GABAA receptor subtypes supported the synaptic localization of np65. There, np65 co-localized with GABAA receptor α1 and α2 subunits that are predominantly found at synaptic sites and showed extensive overlap when co-stained with antibodies against VIAAT. Finally, the synaptic localization of np65 together with GABAA receptors and VIAAT was not only demonstrated in neuronal cells in culture but also confirmed using sections from adult rat brains. These results thus suggest that np65 is a cell adhesion molecule that provides an important and novel link between the pre- and postsynaptic side at GABAergic synapses.
Some Neuroplastin-65 Clusters Located at GABAergic Synapses Are Devoid of Gephyrin
The co-localization of GABAA receptors with np65 at GABAergic synapses raises the question on the extent of co-localization with gephyrin. Gephyrin, by directly interacting with GABAA receptor α2 or α3 subunits (12–14), has been demonstrated to link receptor complexes to the cytoskeleton. Our data from primary hippocampal neurons indicate that 17.6% of the np clusters associate with GABAA receptors, but only 5.8% of np clusters associate with gephyrin. This leads to the conclusion that some clusters of np and GABAA receptors are devoid of gephyrin. These clusters could be located synaptically or extra-synaptically. We could indeed demonstrate that some clusters that are located synaptically, because they were labeled by antibodies against np65 and VIAAT, did not contain gephyrin. Analogously, we found some clusters containing VIAAT and GABAA receptors that were devoid of gephyrin. These data are consistent with previous results indicating that postsynaptic GABAA receptor clusters can form gephyrin independently in neurons (51). We therefore conclude that the interaction between GABAA receptors and np65 might reflect a novel mechanism how receptors are confined to particular synaptic sites without the compulsory presence of the submembranous scaffold protein gephyrin. This confinement might contribute to receptor diffusion properties and synaptic strength. Recently, an interaction between GABAA receptors and the cell adhesion molecule neurexin was demonstrated to affect synaptic transmission (52) by possibly interfering with GABAA receptor mobility or stabilization at the surface during synapse maturation. Whether a similar mechanism also holds true for clusters containing GABAA receptors and np65 will have to be elucidated.
Clusters Containing Neuroplastin-65 and GABAA Receptors Are Also Found at Extra-synaptic Sites
In addition to the clusters that were stained by antibodies against np65, GABAA receptor subunits, and VIAAT, we detected clusters of np65 and GABAA receptor subunits that were devoid of VIAAT indicating an extra-synaptic location. Among the different GABAA receptor subtypes, the ones containing GABAA receptor α5 subunits are mainly located extra-synaptically (17, 53) and only to a lesser extent at synaptic sites (17, 54). Both receptor populations containing α5 subunits appeared in clustered formations at the neuronal surface. Consistent with this observation, we demonstrated that np65 can co-localize with GABAA receptor α5 subunits and is thus most likely also located extra-synaptically.
Previous data indicated that receptors containing α5 subunits are not clustered by gephyrin but by the actin-binding protein radixin. Radixin is a member of the ezrin/radixin/moesin family that directly interacts with GABAA receptor α5 subunits and anchors the receptors to the cytoskeleton (17). Future experiments will have to investigate whether np65 can be found co-associated with radixin and α5 subunits. Interestingly, data on other cell adhesion molecules of the immunoglobulin superfamily indicate that they can bind to cytoskeletal adaptor proteins of the ezrin/radixin/moesin family (55, 56) or ankyrin (57). It is thus tempting to speculate that np65 could also be involved in linking extra-synaptic GABAA receptors to the cytoskeleton by binding to cytoskeletal adaptor proteins such as radixin, thus modulating receptor mobility. Taken together, np65 might contribute to the anchorage of either extra-synaptic or synaptic receptor clusters. This assumption is further supported by the observation that pattern search using the Eukaryote Linear Motif server (58) revealed an amino acid sequence motif in the intracellular C-terminal region of np65, which can be recognized by proteins containing Src homology domain 3 binding domains. Such Src homology domain 3 domains that mediate assembly of protein complexes can often be found in scaffolding proteins or kinases.
Possible Importance of the Interaction between Neuroplastin-65 and GABAA Receptors
FRET experiments between differently tagged np65 indicate that np65 displays homophilic interactions in cis (i.e. at the same cell). This is consistent with data from basigin, the closest related homolog of np55/65, which also forms homo-oligomers in a cis-dependent manner (59). Furthermore, FRET experiments as well as co-immunoprecipitation experiments from the surface of HEK cells indicate that np65 additionally displays heterophilic interactions in cis with GABAA receptors. A similar heterophilic association of np55 with fibroblast growth factor receptor 1 has been previously demonstrated (60). Other data indicate that np65 can also undergo homophilic interactions in trans, i.e. between opposing molecules at the surface of adjacent cells (28). This was supported in this study by confocal images of triple immunostained hippocampal neurons in culture as well as by the deconvolution analysis of the synaptic clusters that indicated a topological location of np across the synaptic cleft adjacent to both GABAA receptors and VIAAT. All these observations are consistent with the hypothesis that np65 as a cell adhesion molecule is most likely involved in stabilizing and confining the apposition and alignment of pre- and postsynapses. In fact, down-regulation of np65 and the concomitant loss of co-localization of GABAA receptor α2 subunits with VIAAT supports a role for np65 in the matching fidelity of postsynaptic proteins with their presynaptic counterparts.
These multiple forms of interactions of np65 probably reflect higher order complex formation common in trans-synaptic cell adhesion systems as it was shown for the related neural cell adhesion molecule (NCAM) that can undergo homophilic trans-interactions as well as cis-interactions (61). In addition, heterophilic interactions of cell adhesion molecules with neurotransmitter receptors also seem common (20, 62–65). It is thus tempting to speculate that such supramolecular structures provided by trans-synaptic cell adhesion molecules function to generate a platform for concentrating neurotransmitter receptors and associated proteins. At such platforms, cell adhesion proteins can convey information from the cell exterior to intracellular signaling proteins (e.g. kinases or phosphatases), which in turn can then regulate receptor trafficking. This speculation is supported by previous findings that extracellular activation (in trans) of np65 induced p38 MAPK (45, 66) leading to AMPA receptor endocytosis. Thus, np and other cell adhesion molecules might trigger intracellular cascades regulating neurotransmitter receptor abundance at the cell surface and thus synaptic strength. Extending this line of arguments, Tyagarajan and Fritschy (67) postulated that homeostasis depends on signaling cascades regulating in parallel the efficacy of glutamatergic and GABAergic transmission, and changes in excitability at glutamatergic synapses have to be paralleled by corresponding changes at GABAergic synapses to avoid hyperexcitation (or silencing) of the network. Further experiments will have to investigate the possibility that np65 might be involved in a homeostatic regulation of GABAA receptor trafficking. Such a mechanism would be highly important because a perturbation of GABAA receptor homeostasis is found under pathological conditions, including anxiety, epilepsy, schizophrenia, and insomnia (68–71).
Acknowledgments
We thank Martina Veit, Elisabeth Doegl, and Mirjana Stojanovic for excellent technical support. We are also indebted to Prof. P. W. Beesley (Royal Holloway University of London, United Kingdom) for supplying us with monoclonal antibodies against neuroplastin. We thank Thomas Rosahl (Merck Sharp & Dohme, Harlow, Essex, United Kingdom) for providing us with brains from the GABAA receptor β2−/− KO mouse strain.
This work was supported by Deutsche Forschungsgemeinschaft Grant SFB 779 (to T. K. and E. D. G.), the Pakt für Forschung und Innovation of the Leibniz Society (Wissensgemeinschaft Gottfried Wilhelm Leibniz (WGL)) (to E. D. G.), European Union Structural Funds 2007–2013 (to K. H. S.), and grants from The Medical University Vienna, Center for Brain Research (to I. S.-J., W. S., M. A. K., and S. T.).

This article contains supplemental Figs. 1 and 2.
K.-H. Smalla, P. Klemmer, T. Kaehne, and E. D. Gundelfinger, unpublished observations.
I. Sarto-Jackson, unpublished observations.
- np
- neuroplastin
- ECFP
- enhanced cyan fluorescence protein
- EYFP
- enhanced yellow fluorescence protein
- DOC
- deoxycholate
- IP low buffer
- low salt immunoprecipitation buffer
- DIV
- days in vitro
- sh
- small hairpin
- PB
- phosphate buffer
- VIAAT
- vesicular inhibitory amino acid transporter.
REFERENCES
- 1. Olsen R. W., Sieghart W. (2008) International Union of Pharmacology. LXX. Subtypes of γ-aminobutyric acid type A receptors. Classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol. Rev. 60, 243–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Farrant M., Nusser Z. (2005) Variations on an inhibitory theme. Phasic and tonic activation of GABAA receptors. Nat. Rev. Neurosci. 6, 215–229 [DOI] [PubMed] [Google Scholar]
- 3. Lévi S., Schweizer C., Bannai H., Pascual O., Charrier C., Triller A. (2008) Homeostatic regulation of synaptic GlyR numbers driven by lateral diffusion. Neuron 59, 261–273 [DOI] [PubMed] [Google Scholar]
- 4. Nusser Z., Cull-Candy S., Farrant M. (1997) Differences in synaptic GABAA receptor number underlie variation in GABA mini amplitude. Neuron 19, 697–709 [DOI] [PubMed] [Google Scholar]
- 5. Lüscher B., Keller C. A. (2004) Regulation of GABAA receptor trafficking, channel activity, and functional plasticity of inhibitory synapses. Pharmacol. Ther. 102, 195–221 [DOI] [PubMed] [Google Scholar]
- 6. Bogdanov Y., Michels G., Armstrong-Gold C., Haydon P. G., Lindstrom J., Pangalos M., Moss S. J. (2006) Synaptic GABAA receptors are directly recruited from their extrasynaptic counterparts. EMBO J. 25, 4381–4389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jacob T. C., Moss S. J., Jurd R. (2008) GABAA receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat. Rev. Neurosci. 9, 331–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tretter V., Moss S. J. (2008) GABAA receptor dynamics and constructing GABAergic synapses. Front. Mol. Neurosci. 1, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Essrich C., Lorez M., Benson J. A., Fritschy J. M., Lüscher B. (1998) Postsynaptic clustering of major GABAA receptor subtypes requires the γ2 subunit and gephyrin. Nat. Neurosci. 1, 563–571 [DOI] [PubMed] [Google Scholar]
- 10. Kneussel M., Brandstätter J. H., Laube B., Stahl S., Müller U., Betz H. (1999) Loss of postsynaptic GABAA receptor clustering in gephyrin-deficient mice. J. Neurosci. 19, 9289–9297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dobie F. A., Craig A. M. (2011) Inhibitory synapse dynamics. Coordinated presynaptic and postsynaptic mobility and the major contribution of recycled vesicles to new synapse formation. J. Neurosci. 31, 10481–10493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tretter V., Jacob T. C., Mukherjee J., Fritschy J. M., Pangalos M. N., Moss S. J. (2008) The clustering of GABAA receptor subtypes at inhibitory synapses is facilitated via the direct binding of receptor α2 subunits to gephyrin. J. Neurosci. 28, 1356–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tretter V., Kerschner B., Milenkovic I., Ramsden S. L., Ramerstorfer J., Saiepour L., Maric H. M, Moss S. J., Schindelin H., Harvey R. J., Sieghart W., Harvey K. (2011) Molecular basis of the γ-aminobutyric acid A receptor α3 subunit interaction with the clustering protein gephyrin. J. Biol. Chem. 286, 37702–37711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Saiepour L., Fuchs C., Patrizi A., Sassoè-Pognetto M., Harvey R. J., Harvey K. (2010) Complex role of collybistin and gephyrin in GABAA receptor clustering. J. Biol. Chem. 285, 29623–29631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jacob T. C., Bogdanov Y. D., Magnus C., Saliba R. S., Kittler J. T., Haydon P. G., Moss S. J. (2005) Gephyrin regulates the cell surface dynamics of synaptic GABAA receptors. J. Neurosci. 25, 10469–10478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bannai H., Lévi S., Schweizer C., Inoue T., Launey T., Racine V., Sibarita J. B., Mikoshiba K., Triller A. (2009) Activity-dependent tuning of inhibitory neurotransmission based on GABAAR diffusion dynamics. Neuron 62, 670–682 [DOI] [PubMed] [Google Scholar]
- 17. Loebrich S., Bähring R., Katsuno T., Tsukita S., Kneussel M. (2006) Activated radixin is essential for GABAA receptor α5 subunit anchoring at the actin cytoskeleton. EMBO J. 25, 987–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ko J., Kim S., Chung H. S., Kim K., Han K., Kim H., Jun H., Kaang B. K., Kim E. (2006) SALM synaptic cell adhesion-like molecules regulate the differentiation of excitatory synapses. Neuron 50, 233–245 [DOI] [PubMed] [Google Scholar]
- 19. Uryu K., Butler A. K., Chesselet M. F. (1999) Synaptogenesis and ultrastructural localization of the polysialylated neural cell adhesion molecule in the developing striatum. J. Comp. Neurol. 405, 216–232 [DOI] [PubMed] [Google Scholar]
- 20. Chih B., Engelman H., Scheiffele P. (2005) Control of excitatory and inhibitory synapse formation by neuroligins. Science 307, 1324–1328 [DOI] [PubMed] [Google Scholar]
- 21. Varoqueaux F., Aramuni G., Rawson R. L., Mohrmann R., Missler M., Gottmann K., Zhang W., Südhof T. C., Brose N. (2006) Neuroligins determine synapse maturation and function. Neuron 51, 741–754 [DOI] [PubMed] [Google Scholar]
- 22. Craig A. M., Kang Y. (2007) Neurexin-neuroligin signaling in synapse development. Curr. Opin. Neurobiol. 17, 43–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Graf E. R., Zhang X., Jin S. X., Linhoff M. W., Craig A. M. (2004) Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell 119, 1013–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Varoqueaux F., Jamain S., Brose N. (2004) Neuroligin 2 is exclusively localized to inhibitory synapses. Eur. J. Cell Biol. 83, 449–456 [DOI] [PubMed] [Google Scholar]
- 25. Kang Y., Zhang X., Dobie F., Wu H., Craig A. M. (2008) Induction of GABAergic postsynaptic differentiation by α-neurexins. J. Biol. Chem. 283, 2323–2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Langnaese K., Beesley P. W., Gundelfinger E. D. (1997) Synaptic membrane glycoproteins gp65 and gp55 are new members of the immunoglobulin superfamily. J. Biol. Chem. 272, 821–827 [DOI] [PubMed] [Google Scholar]
- 27. Hill I. E., Selkirk C. P., Hawkes R. B., Beesley P. W. (1988) Characterization of novel glycoprotein components of synaptic membranes and postsynaptic densities, gp65 and gp55, with a monoclonal antibody. Brain Res. 461, 27–43 [DOI] [PubMed] [Google Scholar]
- 28. Smalla K. H., Matthies H., Langnäse K., Shabir S., Böckers T. M., Wyneken U., Staak S., Krug M., Beesley P. W., Gundelfinger E. D. (2000) The synaptic glycoprotein neuroplastin is involved in long term potentiation at hippocampal CA1 synapses. Proc. Natl. Acad. Sci. U.S.A. 97, 4327–4332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sarto-Jackson I., Furtmueller R., Ernst M., Huck S., Sieghart W. (2007) Spontaneous cross-link of mutated α1 subunits during GABAA receptor assembly. J. Biol. Chem. 282, 4354–4363 [DOI] [PubMed] [Google Scholar]
- 30. Shrivastava A. N., Triller A., Sieghart W., Sarto-Jackson I. (2011) Regulation of GABAA receptor dynamics by interaction with purinergic P2X2 receptors. J. Biol. Chem. 286, 14455–14468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tretter V., Ehya N., Fuchs K., Sieghart W. (1997) Stoichiometry and assembly of a recombinant GABAA receptor subtype. J. Neurosci. 17, 2728–2737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Klausberger T., Fuchs K., Mayer B., Ehya N., Sieghart W. (2000) GABAA receptor assembly. Identification and structure of γ2 sequences forming the intersubunit contacts with α1 and β3 subunits. J. Biol. Chem. 275, 8921–8928 [DOI] [PubMed] [Google Scholar]
- 33. Sarto I., Klausberger T., Ehya N., Mayer B., Fuchs K., Sieghart W. (2002) A novel site on γ3 subunits important for assembly of GABAA receptors. J. Biol. Chem. 277, 30656–30664 [DOI] [PubMed] [Google Scholar]
- 34. Carlin R. K., Grab D. J., Cohen R. S., Siekevitz P. (1980) Isolation and characterization of postsynaptic densities from various brain regions. Enrichment of different types of postsynaptic densities. J. Cell Biol. 86, 831–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wessel D., Flügge U. I. (1984) A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal. Biochem. 138, 141–143 [DOI] [PubMed] [Google Scholar]
- 36. Chen C. A., Okayama H. (1988) Calcium phosphate-mediated gene transfer. A highly efficient transfection system for stably transforming cells with plasmid DNA. BioTechniques 6, 632–638 [PubMed] [Google Scholar]
- 37. Neville D. M., Jr., Glossmann H. (1974) Molecular weight determination of membrane protein and glycoprotein subunits by discontinuous gel electrophoresis in dodecyl sulfate. Methods Enzymol. 32, 92–102 [DOI] [PubMed] [Google Scholar]
- 38. Ehya N., Sarto I., Wabnegger L., Sieghart W. (2003) Identification of an amino acid sequence within GABAA receptor β3 subunits that is important for receptor assembly. J. Neurochem. 84, 127–135 [DOI] [PubMed] [Google Scholar]
- 39. Bartholomäus I., Milan-Lobo L., Nicke A., Dutertre S., Hastrup H., Jha A., Gether U., Sitte H. H., Betz H., Eulenburg V. (2008) Glycine transporter dimers. Evidence for occurrence in the plasma membrane. J. Biol. Chem. 283, 10978–10991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Milan-Lobo L., Gsandtner I., Gaubitzer E., Rünzler D., Buchmayer F., Köhler G., Bonci A., Freissmuth M., Sitte H. H. (2009) Subtype-specific differences in corticotropin-releasing factor receptor complexes detected by fluorescence spectroscopy. Mol. Pharmacol. 76, 1196–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schicker K., Hussl S., Chandaka G. K., Kosenburger K., Yang J. W., Waldhoer M., Sitte H. H., Boehm S. (2009) A membrane network of receptors and enzymes for adenine nucleotides and nucleosides. Biochim. Biophys. Acta 1793, 325–334 [DOI] [PubMed] [Google Scholar]
- 42. Xia Z., Liu Y. (2001) Reliable and global measurement of fluorescence resonance energy transfer using fluorescence microscopes. Biophys. J. 81, 2395–2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zeitelhofer M., Vessey J. P., Xie Y., Tübing F., Thomas S., Kiebler M., Dahm R. (2007) High efficiency transfection of mammalian neurons via nucleofection. Nat. Protoc. 2, 1692–1704 [DOI] [PubMed] [Google Scholar]
- 44. Dahm R., Zeitelhofer M., Götze B., Kiebler M. A., Macchi P. (2008) Visualizing mRNA localization and local protein translation in neurons. Methods Cell Biol. 85, 293–327 [DOI] [PubMed] [Google Scholar]
- 45. Empson R. M., Buckby L. E., Kraus M., Bates K. J., Crompton M. R., Gundelfinger E. D., Beesley P. W. (2006) The cell adhesion molecule neuroplastin-65 inhibits hippocampal long term potentiation via a mitogen-activated protein kinase p38-dependent reduction in surface expression of GluR1-containing glutamate receptors. J. Neurochem. 99, 850–860 [DOI] [PubMed] [Google Scholar]
- 46. Craig A. M., Banker G., Chang W., McGrath M. E., Serpinskaya A. S. (1996) Clustering of gephyrin at GABAergic but not glutamatergic synapses in cultured rat hippocampal neurons. J. Neurosci. 16, 3166–3177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kneussel M., Betz H. (2000) Receptors, gephyrin, and gephyrin-associated proteins. Novel insights into the assembly of inhibitory postsynaptic membrane specializations. J. Physiol. 525, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sassoè-Pognetto M., Panzanelli P., Sieghart W., Fritschy J. M. (2000) Co-localization of multiple GABAA receptor subtypes with gephyrin at postsynaptic sites. J. Comp. Neurol. 420, 481–498 [DOI] [PubMed] [Google Scholar]
- 49. Panzanelli P., Perazzini A. Z., Fritschy J. M., Sassoè-Pognetto M. (2005) Heterogeneity of γ-aminobutyric acid type A receptors in mitral and tufted cells of the rat main olfactory bulb. J. Comp. Neurol. 484, 121–131 [DOI] [PubMed] [Google Scholar]
- 50. Fritschy J. M., Harvey R. J., Schwarz G. (2008) Gephyrin. Where do we stand, where do we go? Trends Neurosci. 31, 257–264 [DOI] [PubMed] [Google Scholar]
- 51. Danglot L., Triller A., Bessis A. (2003) Association of gephyrin with synaptic and extrasynaptic GABAA receptors varies during development in cultured hippocampal neurons. Mol. Cell. Neurosci. 23, 264–278 [DOI] [PubMed] [Google Scholar]
- 52. Zhang C., Atasoy D., Araç D., Yang X., Fucillo M. V., Robison A. J., Ko J., Brunger A. T., Südhof T. C. (2010) Neurexins physically and functionally interact with GABAA receptors. Neuron 66, 403–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Brünig I., Scotti E., Sidler C., Fritschy J. M. (2002) Intact sorting, targeting, and clustering of γ-aminobutyric acid A receptor subtypes in hippocampal neurons in vitro. J. Comp. Neurol. 443, 43–55 [DOI] [PubMed] [Google Scholar]
- 54. Christie S. B., de Blas A. L. (2002) α5 subunit-containing GABAA receptors form clusters at GABAergic synapses in hippocampal cultures. Neuroreport 13, 2355–2358 [DOI] [PubMed] [Google Scholar]
- 55. Dickson T. C., Mintz C. D., Benson D. L., Salton S. R. (2002) Functional binding interaction identified between the axonal CAM L1 and members of the ERM family. J. Cell Biol. 157, 1105–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Furutani Y., Matsuno H., Kawasaki M., Sasaki T., Mori K., Yoshihara Y. (2007) Interaction between telencephalin and ERM family proteins mediates dendritic filopodia formation. J. Neurosci. 27, 8866–8876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nishimura K., Yoshihara F., Tojima T., Ooashi N., Yoon W., Mikoshiba K., Bennett V., Kamiguchi H. (2003) L1-dependent neuritogenesis involves ankyrinB that mediates L1-CAM coupling with retrograde actin flow. J. Cell Biol. 163, 1077–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Puntervoll P., Linding R., Gemünd C., Chabanis-Davidson S., Mattingsdal M., Cameron S., Martin D. M., Ausiello G., Brannetti B., Costantini A., Ferrè F., Maselli V., Via A., Cesareni G., Diella F., Superti-Furga G., Wyrwicz L., Ramu C., McGuigan C., Gudavalli R., Letunic I., Bork P., Rychlewski L., Küster B., Helmer-Citterich M., Hunter W. N., Aasland R., Gibson T. J. (2003) ELM server. A new resource for investigating short functional sites in modular eukaryotic proteins. Nucleic Acids Res. 31, 3625–3630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yoshida S., Shibata M., Yamamoto S., Hagihara M., Asai N., Takahashi M., Mizutani S., Muramatsu T., Kadomatsu K. (2000) Homo-oligomer formation by basigin, an immunoglobulin superfamily member, via its N terminal immunoglobulin domain. Eur. J. Biochem. 267, 4372–4380 [DOI] [PubMed] [Google Scholar]
- 60. Owczarek S., Kiryushko D., Larsen M. H., Kastrup J. S., Gajhede M., Sandi C., Berezin V., Bock E., Soroka V. (2010) Neuroplastin-55 binds to and signals through the fibroblast growth factor receptor. FASEB J. 24, 1139–1150 [DOI] [PubMed] [Google Scholar]
- 61. Soroka V., Kolkova K., Kastrup J. S., Diederichs K., Breed J., Kiselyov V. V., Poulsen F. M., Larsen I. K., Welte W., Berezin V., Bock E., Kasper C. (2003) Structure and interactions of NCAM Ig1–2-3 suggest a novel zipper mechanism for homophilic adhesion. Structure 11, 1291–1301 [DOI] [PubMed] [Google Scholar]
- 62. Stork O., Welzl H., Wotjak C. T., Hoyer D., Delling M., Cremer H., Schachner M. (1999) Anxiety and increased 5-HT1A receptor response in NCAM null mutant mice. J. Neurobiol. 40, 343–355 [DOI] [PubMed] [Google Scholar]
- 63. Kohsaka H., Takasu E., Nose A. (2007) In vivo induction of postsynaptic molecular assembly by the cell adhesion molecule fasciclin2. J. Cell Biol. 179, 1289–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Li H. L., Huang B. S., Vishwasrao H., Sutedja N., Chen W., Jin I., Hawkins R. D., Bailey C. H., Kandel E. R. (2009) Dscam mediates remodeling of glutamate receptors in Aplysia during de novo and learning-related synapse formation. Neuron 61, 527–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Xiao M. F., Xu J. C., Tereshchenko Y., Novak D., Schachner M., Kleene R. (2009) Neural cell adhesion molecule modulates dopaminergic signaling and behavior by regulating dopamine D2 receptor internalization. J. Neurosci. 29, 14752–14763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Owczarek S., Soroka V., Kiryushko D., Larsen M. H., Yuan Q., Sandi C., Berezin V., Bock E. (2011) Neuroplastin-65 and a mimetic peptide derived from its homophilic binding site modulate neuritogenesis and neuronal plasticity. J. Neurochem. 117, 984–994 [DOI] [PubMed] [Google Scholar]
- 67. Tyagarajan S. K., Fritschy J. M. (2010) GABAA receptors, gephyrin, and homeostatic synaptic plasticity. J. Physiol. 588, 101–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dani V. S., Chang Q., Maffei A., Turrigiano G. G., Jaenisch R., Nelson S. B. (2005) Reduced cortical activity due to a shift in the balance between excitation and inhibition in a mouse model of Rett syndrome. Proc. Natl. Acad. Sci. U.S.A. 102, 12560–12565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Möhler H. (2006) GABAA receptors in central nervous system disease. Anxiety, epilepsy, and insomnia. J. Recept. Signal. Transduct. Res. 26, 731–740 [DOI] [PubMed] [Google Scholar]
- 70. Fritschy J. M. (2008) Epilepsy, E/I balance, and GABAA receptor plasticity. Front. Mol. Neurosci. 1, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Südhof T. C. (2008) Neuroligins and neurexins link synaptic function to cognitive disease. Nature 455, 903–911 [DOI] [PMC free article] [PubMed] [Google Scholar]