Background: The voltage-gated proton channel Hv1 is specifically expressed in highly metastatic human breast tumor tissues and cell lines.
Results: Hv1 overexpression is significantly correlated with clinicopathological parameters and contributes to breast carcinogenesis.
Conclusion: High Hv1 expression is associated with poor progression and unfavorable clinical outcome of breast cancer.
Significance: Hv1 is a potential biomarker for prognosis of breast cancer and a potential target for anticancer drugs in therapy.
Keywords: Breast Cancer, Cell Proliferation, Matrix Metalloproteinase (MMP), Membrane Proteins, Proliferation, Metastasis, Overexpression, Prognosis, Voltage-gated Proton Channel Hv1
Abstract
In our previous work, we showed for the first time that the voltage-gated proton channel Hv1 is specifically expressed in highly metastatic human breast tumor tissues and cell lines. However, the contribution of Hv1 to breast carcinogenesis is not well known. In this study, we showed that Hv1 expression was significantly correlated with the tumor size (p = 0.001), tumor classification (p = 0.000), lymph node status (p = 0.000), clinical stage (p = 0.000), and Her-2 status (p = 0.045). High Hv1 expression was associated significantly with shorter overall (p = 0.000) and recurrence-free survival (p = 0.000). In vitro, knockdown of Hv1 expression in the highly metastatic MDA-MB-231 cells decreased the cell proliferation and invasiveness, inhibited the cell proton secretion and intracellular pH recovery, and blocked the cell capacity of acidifying extracellular milieu. Furthermore, the gelatinase activity in MDA-MB-231 cells that suppressed Hv1 was reduced. In vivo, the breast tumor size of the implantation of the MDA-MB-231 xenografts in nude mice that were knocked down by Hv1 was dramatically smaller than that in the control groups. The results demonstrated that the inhibition of Hv1 function via knockdown of Hv1 expression can effectively retard the cancer growth and suppress the cancer metastasis by the decrease of proton extrusion and the down-regulation of gelatinase activity. Based on these results, we came to the conclusion that Hv1 is a potential biomarker for prognosis of breast cancer and a potential target for anticancer drugs in breast cancer therapy.
Introduction
Tumor cells often exist in a hypoxic microenvironment with an acidic extracellular pH (pHo)4 compared with that of surrounding normal cells (1, 2). The glycolysis and proton secretion in tumor cells are proposed to contribute to the proliferation and invasion of cancer cells during the process of tumorigenesis and metastasis (3, 4). The high glycolytic activity and acidic metabolites in cancer cells result in the excessive production of intracellular acidity. The intracellular pH (pHi) is vital for all biological processes in cells, which influences cell proliferation, cell motility, tumorigenesis, metastasis, and apoptosis (5–9). To survive in this harsh environment, tumor cells must exhibit a dynamic cytosolic pH regulatory system.
Voltage-gated proton channel Hv1 is essential for proton transfer, which is mainly expressed in immune cells such as macrophages, neutrophils, and eosinophils (10, 11). Hv1 in mammalian phagocytes is proposed to be responsible for the proton-transporting pathway, which regulates intracellular pH during oxygen consumption associated with phagocytosis, called “respiratory burst” (12, 13). Hv1 is activated by depolarization and intracellular acidification, whose activities maintain neutral intracellular pH to keep the reactive oxygen species generation (14, 15). Hv1 not only regulates pH in cytoplasm but could also provide protons in the phagosome, a closed membrane compartment for killing and digestion of a pathogen (12). Hv1 is extremely selective for H+, with no detectable permeability to other cations (16, 17). The voltage activation relationship of Hv1 depends strongly on both pHi and pHo. Increasing pHo or lowering pHi promotes H+ channel opening by shifting the activation threshold to more negative potentials (12). Furthermore, Hv1 current is inhibited by submillimolar concentrations of Zn2+ and Cd2+ and other divalent cations (18).
Hv1 contains three predicted domains as follows: N-terminal acid and proline-rich domain, transmembrane voltage-sensor domain (VSD), and C-terminal domain. Voltage-gated K+ channels are composed of four subunits, each of which has a pore domain and a VSD. The four pore domains come together to form one single central pore, and four peripheral VSDs control the gate of the pore (19). In contrast to the voltage-gated K+ channels, the Hv1 contains a VSD but lacks the pore domain. Recent studies showed that Hv1 functions as a dimer in which the intracellular C-terminal domain is responsible for the dimeric architecture of the proteins, and each subunit contains its own pore (20–23). The intracellular C-terminal domain of Hv1 forms a dimer via a parallel α-helical coiled-coil and is essential for the protein localization (23).
Our previous work showed for the first time that Hv1 is specifically expressed in highly metastatic human breast tumor tissues and cell lines (24). However, the contribution of Hv1 to breast carcinogenesis is still unknown. Here, we investigated the clinicopathological and biological significance of Hv1 overexpression in breast cancer. Our results demonstrated that the inhibition of Hv1 function via knockdown of Hv1 expression using RNA-interfering technology can effectively retard the cancer growth and suppress the cancer metastasis by the decrease of proton extrusion and the down-regulation of gelatinase activity and suggested that Hv1 is a potential biomarker for prognosis of breast cancer and a potential target for anticancer drugs in breast cancer therapy.
EXPERIMENTAL PROCEDURES
Patients and Samples
Breast cancer tissue samples were obtained from patients who underwent routine curative surgery at the Department of Surgery, Tonghua Central Hospital, between 2001 and 2007. The patients were not pretreated with radiotherapy or chemotherapy prior to surgery. 105 breast cancer tissues and paired normal tissues that were taken from sites distant from the tumorous lesions, and 10 hyperplasia of breast tissues, were fixed in 10% formalin and then embedded in paraffin for immunohistochemical analysis. The clinicopathological features of these patients were shown in Table 1. Each patient's clinical status was classified according to the pathological tumor grade, tumor size, and lymph node status. Tumor differentiation was graded by the pathological Tumor-Node-Metastasis system (6th edition). This study was approved by the Ethics Committee of Tonghua Central Hospital, and informed consent was obtained from each patient.
TABLE 1.
Clinicopathological parameters
| Characteristics | No. of patients (%), n = 105 |
|---|---|
| Age (year) median 53.2 | |
| ≤50 | 48 (45.7) |
| >50 | 57 (54.3) |
| Primary site | |
| Left breast | 62 (59.0) |
| Right breast | 43 (41.0) |
| Tumor size (cm) | |
| ≤2 | 26 (24.8) |
| >2 | 79 (75.2) |
| Tumor classification | |
| T1 | 27 (25.7) |
| T2 | 64 (61.0) |
| T3 | 10 (9.5) |
| T4 | 4 (3.8) |
| Lymph node status | |
| Negative | 64 (61.0) |
| Positive | 41 (39.0) |
| Clinical stage | |
| I | 19 (18.1) |
| II | 45 (42.8) |
| III | 38 (36.2) |
| IV | 3 (2.9) |
| ER status | |
| −/+ | 85 (81.0) |
| ++/+++ | 20 (19.0) |
| PR status | |
| −/+ | 84 (80.0) |
| ++/+++ | 21 (20.0) |
| Her-2 status | |
| −/+ | 75 (71.4) |
| ++/+++ | 30 (28.6) |
Generation of a Polyclonal Antiserum
A polyclonal antiserum was generated against the C-terminal domain of Hv1 (24). The protein was purified to homogeneity after being expressed in Escherichia coli (25). The purified protein was injected into mice. HRP- and FITC-conjugated goat anti-mouse IgGs were purchased from Jackson ImmunoResearch (West Grove, PA).
Immunohistochemistry
Histological diagnoses of tumorous and nontumorous formalin-fixed and paraffin-embedded tissues were confirmed on hematoxylin and eosin-stained sections. Immunohistochemistry was performed with the polyclonal antiserum of Hv1. The anti-Hv1 serum was diluted 100-fold with PBST (phosphate-buffered saline containing 0.1% Tween 20) containing 1% (w/v) BSA. The paraffin-embedded sections filled with 10 mm EDTA buffer, pH 8.0, were heated in a microwave oven for 12 min. After cooling, the sections were treated with 0.5% Triton X-100 in PBS for 10 min and exposed to 3% (v/v) hydrogen peroxide (H2O2) for 10 min to inhibit endogenous peroxidase activity. Subsequently, the sections were incubated in PBST containing 5% fetal bovine serum and 2% BSA for 30 min to reduce nonspecific binding. Incubation with primary antibody was performed overnight at 4 °C in a humidified chamber. HRP-coupled anti-mouse secondary antibody was used at a final dilution of 1:400 for 1 h. Finally, the visualization signal was developed with diaminobenzidine, and the slides were counterstained in hematoxylin. Negative control was performed to treat with nonimmune mouse serum as the primary antibody instead of anti-Hv1 serum.
Stained sections were evaluated in a blinded manner without prior knowledge of the clinical information using the German immunoreactive score, “ImmunoReactive Score” (IRS). Briefly, the IRS assigns sub-scores for immunoreactive distribution (0–4) and intensity (0–3) and then multiplies them to yield the IRS score. The percent positivity was scored as “0” (<5%), “1” (5–25%), “2” (25–50%), “3” (50–75%), and “4” (>75%). The staining intensity was scored according to the area of Hv1-positive staining cells as “0, −” (negative, <5%), “1, +” (weakly positive, 5–25%), “2, ++” (positive, 25–50%), and “3, +++” (strongly positive, >50%). The final Hv1 expression score was calculated with the value of percent positivity score plus staining intensity score, which ranged from 0 to 12. We estimated IRS by averaging the values in eight fields at ×500 magnification for each specimen. Intratumoral Hv1 expression was defined as follows: low expression (score 0–3) and high expression (score 4–12). Immunohistochemical analysis and scoring were performed by two independent experienced pathologists.
Cell Culture
Human breast cancer cell lines MCF-7 and MDA-MB-231 were cultured in RPMI 1640 medium (Invitrogen) containing 10% fetal bovine serum (FBS) in a humidified 5% CO2 atmosphere at 37 °C.
Lentivirus Vectors for Hv1 Small Hairpin RNA
Small hairpin RNA (shRNA) of Hv1 lentivirus gene transfer vector encoding green fluorescent protein (GFP) sequence was constructed by Genechem Co., Ltd., Shanghai, China. The targeting sequence of the shRNA was 5′-CGCCAAGATTCAACACCTT-3′ and the random sense sequence was 5′-TTCTCCGAACGTGTCACGT-3′, both of which were confirmed by sequencing. The recombinant lentivirus of small interference RNA targeting Hv1 (Hv1-siRNA) and the control lentivirus (Hv1-scr) were prepared and titered to 109 transfection units/ml.
Lentivirus siRNA Gene Transfection
MCF-7 and MDA-MB-231 cells were plated in 24-well plates (5 × 104 cells/well) overnight. The lentiviruses were diluted in 0.25 ml (108 transfection units/ml) of serum-free medium containing Polybrene (5 mg/ml) and added to the cells for 5 h of incubation at 37 °C, followed by incubation in 0.25 ml of fresh RPMI 1640 medium containing 10% FBS for another 5 h, which was replaced with fresh RPMI 1640 medium, and the cells were cultured for next 48 h.
Cell Proliferation Assay
Cells were prepared at a concentration of 5 × 103 cells/100 μl and then distributed in 96-well plates at 100 μl/well and cultured overnight. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay was performed every day for up to 6 days. 10 μl of 5 mg/ml 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (Sigma) was added to each well; the plates were incubated at 37 °C for 4 h, and the supernatants were removed carefully; 150 μl of dimethyl sulfoxide (DMSO) was added to each well, and the plates were agitated on a shaker for 5 min. The optical density was measured with a microplate reader (Bio-Rad) at 570 nm. Experiments were performed in triplicate.
Invasion Assay
In vitro invasion assays were performed to assess the effects of Hv1 on the invasive ability of the highly metastatic MDA-MB-231 and poorly metastatic MCF-7 cells, as described in our previous work (24). Briefly, transwells with 8-μm pore size filters (Millipore) were covered with Matrigel (BD Biosciences) and inserted into a 24-well plate. RPMI 1640 medium (500 μl) containing 10% FBS was added to the lower chamber, and 200 μl of a cell suspension (5 × 104 cells) was placed in the upper chamber. The plate was incubated at 37 °C in a humidified atmosphere containing 5% CO2 for 24 h. The penetrated cells were stained with crystal violet solution and photographed. Each experiment was conducted four times.
Immunofluorescence Cytochemistry
MDA-MB-231 cells grown on glass coverslips at confluence in 6-well tissue culture plates were fixed with 4% (w/v) paraformaldehyde in PBS at room temperature for 30 min, washed in PBS, treated with 0.5% Triton X-100 in PBS for 20 min, exposed to 3% (v/v) hydrogen peroxide (H2O2) for 15 min, and blocked with 5% fetal bovine serum and 2% BSA in PBS for 20 min at room temperature. The blocked coverslips were incubated with anti-Hv1 mouse serum at a dilution of 1:100 with PBST containing 1% (w/v) BSA at 37 °C for 1 h. After washing three times in PBS, the coverslips were further incubated with FITC-coupled goat anti-mouse secondary antibody at a final dilution of 1:400 for 1 h. The cell nuclei were stained with DAPI. The images of the cells on coverslips were recorded on a Leica TCS SP5 confocal microscopy (LEICA, Germany) with the FITC-filter set for enhanced GFP and the DAPI filter set for the nuclear DAPI dye. The images were later processed by Adobe Photoshop software.
Zymography
The activities of gelatinase in the supernatants of the cultured MCF-7 and MDA-MB-231 cells were measured, as described in our previous work (24). Briefly, the cells were plated in a 24-well plate (1.5 × 105 per well) and cultured for 24 h in RPMI 1640 medium containing 10% FBS at 37 °C in 5% CO2. After the cells were washed three times with PBS and 0.5 ml of serum-free RPMI 1640 medium was added into each well, the cells were incubated at 37 °C in 5% CO2 for 24 h. The medium was collected and run in 10% SDS-PAGE (containing 0.1% gelatin). The protein concentrations of the medium were determined using BCA method and to each well was added 20 μg of protein. After electrophoresis, gels were soaked for 0.5 h in the renaturing buffer (50 mm Tris-HCl, pH 7.5, containing 2.5% Triton X-100, and 5 mm CaCl2) and incubated in the developing buffer (50 mm Tris-HCl, pH 7.5, containing 1% Triton X-100 and 5 mm CaCl2) for 20 h. The gels were stained with Coomassie Brilliant Blue.
Activity of Hv1 Channel
The activity of Hv1 in MDA-MB-231 cells was assessed as a change in pHi in response to membrane depolarization by BCECF fluorescence (11). The cells were incubated with 3.0 μm BCECF-AM (Molecular Probe) in serum-free RPMI 1640 medium for 30 min, respectively, and washed three times with PSS solution (140 mm NaCl, 5 mm KCl, 5 mm glucose, 1 mm CaCl2, 1 mm MgCl2, 20 mm Tris, pH 7.5). Cells were incubated with NH4Cl/N-methyl d-glucamine solution (100 mm N-methyl d-glucamine, 40 mm NH4Cl, 5 mm KCl, 5 mm glucose, 1 mm CaCl2, 1 mm MgCl2, 20 mm Tris, pH 7.3) for 20 min and washed by ammonium-free solution (140 mm N-methyl d-glucamine, 5 mm KCl, 5 mm glucose, 1 mm CaCl2, 1 mm MgCl2, 20 mm Tris, pH 7.4), rapidly inducing intracellular acidification (5). Membrane depolarization was achieved by loading high K+ solution (145 mm KCl, 5 mm glucose, 1 mm CaCl2, 1 mm MgCl2, 20 mm Tris, pH 7.5). Intracellular pH-changed acid-loaded cells were detected by fluorescent probe BCECF at an excitation wavelength of 488 nm and an emission wavelength range of 500–540 nm under membrane depolarizing conditions using a confocal microscope (Leica TCS SP5, Germany).
Measurements of Intracellular pH
Intracellular pH was measured in the monolayers using the pH-sensitive fluorescent probe BCECF-AM. Cells were incubated with 3.0 μm of BCECF-AM (Molecular Probe) in bicarbonate-free RPMI 1640 medium at 37 °C for 30 min. After loading, the cells were washed three times with HEPES buffer to remove the extracellular dye and made to remain in the identical buffer. The HEPES buffer contained 140 mm NaCl, 5 mm KCl, 1 mm MgSO4, 1 mm CaCl2, 1 mm NaH2PO4, 5.5 mm glucose, and 20 mm HEPES, pH 7.4. The fluorescence at excitation wavelengths of 488 and 458 nm was recorded at an emission wavelength range of 500–540 nm using a confocal microscope (Leica TCS SP5, Germany). Calibration of fluorescence versus pH was performed by equilibration of external and internal pH with nigericin (10 μm) in a high K+ buffer with a range of pH from 6.0 to 7.6. The high K+ buffer contained 145 mm KCl, 5 mm glucose, 1 mm CaCl2, 1 mm MgCl2, and 20 mm HEPES (or MES). The relative fluorescence ratio values were plotted against corresponding pHi values, which allowed determination of the unknown pHi.
Measurement of Acid Production
To examine the role of Hv1 in intracellular proton extrusion, the pH values of the extracellular milieu of the high metastatic (MDA-MB-231) and poor metastatic (MCF-7) cells were measured. All cells were cultured in RPMI 1640 medium in the presence of 10% FBS at confluence and then changed into unbuffered DMEM (Dulbecco's modified Eagle's medium, Invitrogen) (without sodium bicarbonate, initial pH 7.2) for 24 h at 37 °C in a water-saturated atmosphere devoid of CO2. The medium pH values were measured using pH meter (METTLER TOLEDO). Each experiment was conducted three times.
Quantitative Real Time PCR
The mRNA expression levels of Hv1 in MCF-7 and MDA-MB-231 cells were evaluated by quantitative real time PCR using ABI PRISM 7000 sequence detection system (Applied Biosystems, Foster City, CA). Total RNAs were extracted using RNAiso reagent (Takara) and reverse-transcribed by Moloney murine leukemia virus super transcriptase. Real time quantitative reverse transcription-PCR was performed with SYBR PrimeScriptTM RT-PCR kit (Takara, Tokyo, Japan) according to the manufacturer's instructions. The primers were as follows: Hv1, 5′-CAGGTCATCATCATCTGCTTG-3′ (forward) and 5′-CCGTTCTGAACGTGTCTTAAC-3′ (reverse); GAPDH, 5′-CCAAGGTCATCCATGACAAC-3′ (forward) and 5′-AGAGGCAGGGATGATGTTCT-3′ (reverse). PCR thermal condition used was s follows: 94 °C for 30 s; annealing, 52 °C for 30 s; extension, 72 °C for 30 s. Relative mRNA expression levels of proteins were calculated according to the following equation: 2−ΔΔCT, in which ΔΔCT = (CTHv1 − CTGAPDH) − (CTHv1 − CTGAPDH)MDA-MB-231. All experiments were performed in triplicate.
Tumor Xenograft
Cells were suspended in serum-free RPMI 1640 medium at a density of 1 × 107 cells/ml. Each mouse (4–6 weeks of age, female, BALB/c nu+/nu+, from Academy of Military Science, Beijing, China) was s.c. injected into the left upper flank region on day 0. The shortest and longest diameters of the tumor were measured with calipers at 2-day intervals, and the volume of each tumor (mm3) was calculated according to length × width2/2. When the tumor volume reached ∼500 mm3, mice were sacrificed at day 30. Isolated tumors were fixed in formalin and embedded in paraffin. Five-micrometer sections were stained with hematoxylin/eosin and analyzed for Ki-67 and Hv1 expression. These studies were approved by the Animal Use Committee of Institute of Hematology (Chinese Academy of Medical Sciences, Tianjin), which approved all protocols for treating animals.
Statistical Analysis
All statistics were performed using SPSS16.0 software. Measurement data were represented as mean ± S.D. Comparison of the mean between groups was performed by t test. p values <0.05 were considered significant. Survival analysis was assessed using the Kaplan-Meier method, and survival rate was compared by log-rank test.
RESULTS
Increased Expression Levels of Hv1 in Breast Cancer
Hv1 in immune cells is proposed to be responsible for the proton-transporting pathway, which regulates intracellular pH during oxygen consumption associated with phagocytosis, called respiratory burst (12, 13), but its function in tumorigenesis has not been identified. To investigate Hv1 for use as a potential biomarker and therapeutic target for breast cancer, Hv1 expression levels in breast cancer and normal tissues were detected by immunohistochemistry. As shown in Fig. 1 and Table 2, Hv1 staining was mainly moderate or strongly positive in invasive ductal carcinoma tissues but not in normal breast tissues. Hv1 was expressed in both the nucleus and the plasma membrane of tumor cells (Fig. 1). In hyperplasia of breast and ductal carcinoma in situ, the staining was negative or weakly positive (Fig. 1, B and C, and Table 2). Hv1 in invasive ductal carcinoma tissues was significantly expressed compared with that in normal breast, hyperplasia of breast, and ductal carcinoma in situ tissues (Fig. 1 and Table 2), suggesting that Hv1 may be involved in breast tumorigenesis. Overall, 61 of the 105 cases (58.1%) showed high expression Hv1 in the tumor tissues (IRS over 4), whereas 44 (41.9%) of the cases showed low expression (IRS 0–3). Generally, Hv1 staining density was significantly higher in invasive ductal carcinoma tissues than in ductal carcinoma in situ tissues (6.2 ± 3.7 versus 3.4 ± 1.6, p < 0.001) (Table 2).
FIGURE 1.
Up-regulation of voltage-gated proton channel Hv1 protein is illustrated in tissues from patients with breast cancer. Representative immunohistochemical staining of Hv1 is shown (A–I). Tissue samples from patients with breast cancer were stained with anti-Hv1 antibody. Hv1 staining was negative or weak in normal breast (A) and hyperplasia of breast tissues (B and C), but there was moderate or strong Hv1 staining in breast cancer (D–I). The Hv1 is stained in brown, and the background nuclei are in blue. A, B, D, F, and H, 200; C, E, G, and I, 500. A negative control was performed to treat with nonimmune mouse serum as the primary antibody instead of anti-Hv1 antibody.
TABLE 2.
Average density of immunohistochemistry staining
The abbreviations used are as follows: PT, peritumoral tissues; HB, hyperplasia of breast; DCIS, ductal carcinoma in situ; IDC, invasive ductal carcinoma. *, 0.001; **, 0.001; and ***, 0.001 were compared with PT.
| Type of tissues | Staining density |
p | ||
|---|---|---|---|---|
| Mean | Standard deviation | Score range | ||
| PT | 0 | 0.0 | 0 | <0.001 |
| HB | 0.4 | 0.5 | 0–1 | <0.001* |
| DCIS | 3.4 | 1.6 | 1–8 | <0.001** |
| IDC | 6.2 | 3.7 | 1–12 | <0.001*** |
High Hv1 Expression Is Associated with Poor Prognosis
The correlations between Hv1 expression and clinicopathological characteristics are summarized in Table 3. There were significant associations for the tumor size (p = 0.001), tumor classification (p = 0.000), lymph node status (p = 0.000), clinical stage (p = 0.000), and Her-2 status (p = 0.045) in patients who had high Hv1 expression compared with patients who had low Hv1 expression. There was no significant association between Hv1 expression and the other clinical features, such as age and primary site of tumors. In addition, the correlations of the expression levels of ER, PR, GST-π, and P-gp with clinicopathological characteristics did not have statistical significance, except Her-2, Ki-67, and TopoII, in which Her-2 expression is significantly associated with tumor classification (p = 0.015), lymph node status (p = 0.058), and clinical stage (p = 0.029), Ki-67 with tumor classification (p = 0.012), and TopoII with tumor size (p = 0.005) (Table 4).
TABLE 3.
Association between clinicopathological characteristics and Hv1 expression in the 105 patients with breast cancer
| Characteristics | No. of patients | Hv1 expression |
p value | |
|---|---|---|---|---|
| High | Low | |||
| Age (years) | ||||
| ≤50 | 48 (45.7) | 28 (58.3) | 20 (41.7) | 0.964 |
| >50 | 57 (54.3) | 33 (57.9) | 24 (42.1) | |
| Primary site | ||||
| Left breast | 62 (59.0) | 37 (59.7) | 25 (40.3) | 0.693 |
| Right breast | 43 (41.0) | 24 (55.8) | 19 (44.2) | |
| Tumor size (cm) | ||||
| ≤2 | 26 (24.8) | 8 (30.8) | 18 (69.2) | 0.001 |
| >2 | 79 (75.2) | 53 (67.1) | 26 (32.9) | |
| Tumor classification | ||||
| T1 | 27 (25.7) | 5 (18.5) | 22 (81.5) | 0.000 |
| T2 | 64 (61.0) | 44 (68.7) | 20 (31.3) | |
| T3 | 10 (9.5) | 8 (80.0) | 2 (20.0) | |
| T4 | 4 (3.8) | 4 (100.0) | 0 (0.0) | |
| Lymph node status | ||||
| Negative | 64 (61.0) | 28 (43.7) | 36 (56.3) | 0.000 |
| Positive | 41 (39.0) | 33 (80.5) | 8(19.5) | |
| Clinical stage | ||||
| I | 19 (18.1) | 4 (21.1) | 15 (78.9) | 0.000 |
| II | 45 (42.8) | 21 (46.7) | 24 (53.3) | |
| III | 38 (36.2) | 33 (86.8) | 5 (13.2) | |
| IV | 3 (2.9) | 3 (100.0) | 0 (0.0) | |
| ER status | ||||
| −/+ | 85 (81.0) | 49 (57.6) | 36 (42.4) | 0.848 |
| ++/+++ | 20 (19.0) | 12 (60.0) | 8 (40.0) | |
| PR status | ||||
| −/+ | 84 (80.0) | 46 (54.8) | 38 (45.2) | 0.166 |
| ++/+++ | 21 (20.0) | 15 (71.4) | 6 (28.6) | |
| Her-2 status | ||||
| −/+ | 75 (71.4) | 39 (52.0) | 36 (48.0) | 0.045 |
| ++/+++ | 30 (28.6) | 22 (73.3) | 8 (26.7) | |
| Ki-67 status | ||||
| ≤25% | 78 (74.3) | 47 (60.3) | 31 (39.7) | 0.446 |
| >25% | 27 (25.7) | 14 (51.9) | 13 (48.1) | |
| TopoII status | ||||
| −/+ | 74 (70.5) | 47 (63.5) | 27 (36.5) | 0.082 |
| ++ | 31 (29.5) | 14 (45.2) | 17 (54.8) | |
| GST-π status | ||||
| −/+ | 61 (58.1) | 32 (52.5) | 29(47.5) | 0.168 |
| ++/+++ | 44 (41.9) | 29 (65.9) | 15(34.1) | |
| P-gp status | ||||
| −/+ | 87 (82.9) | 52 (59.8) | 35 (40.2) | 0.444 |
| ++/+++ | 18 (17.1) | 9 (50.0) | 9 (50.0) | |
TABLE 4.
Correlation between ER, PR, Her-2, Ki-67, TopoII, GST-π and P-gp expression and clinicopathological parameters in breast cancer
For Ki-67, ≤25%, as a low expression; >25%, as a high expression. For ER, PR, Her-2, TopoII, GST-π and P-gp, −/+, as a low expression; >+, as a high expression.
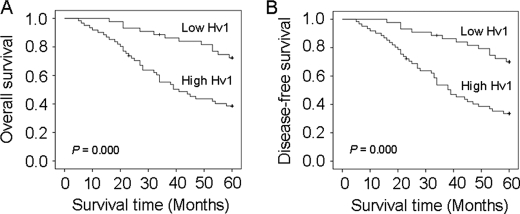
Kaplan-Meier survival curves showed that patients who had high Hv1 expression were more likely to have a shorter overall survival (p = 0.000, Fig. 2A) and recurrence-free survival (p = 0.000, Fig. 2B) compared with patients who had low Hv1 expression, suggesting that Hv1 overexpression may be associated with a poor clinical prognosis. Patients who had high Hv1 expression had a poor recurrence-free survival (p = 0.000) compared with patients who had low Hv1 expression (univariate analysis) (Table 5). Overall survival examined by Cox univariate analysis also indicated that high expression of Hv1 was significantly associated with shorter survival (p = 0.000). Univariate Cox regression analyses showed that tumor size, lymph node status, and Hv1 expression level were significantly associated with recurrence-free and overall survival, whereas other clinical characteristics lost their predictive significance. Furthermore, multivariate Cox regression analyses revealed that high expression of Hv1 was an independent risk factor for overall survival (relative risk = 0.448, p = 0.029) and recurrence-free survival (relative risk = 0.404, p = 0.011) (Table 6). Patients who had high expression of Hv1 were prone to have an early recurrence compared with patients who had low expression of Hv1 (39.0 ± 4.7 versus 53.7 ± 3.6, p < 0.000)) (Table 5).
FIGURE 2.
Kaplan-Meier overall and disease-free survival curves were calculated according to voltage-gated proton channel Hv1 expression levels. A, differences in cumulative overall survival are observed between patients with high and low levels of Hv1 expression. B, differences in cumulative recurrence-free survival are observed between patients with high and low levels of Hv1 expression. p values were obtained using the log-rank test.
TABLE 5.
Univariate logistic regression analysis of Hv1 expression
| Variable | Recurrence-free survival |
Overall survival |
||||
|---|---|---|---|---|---|---|
| No. of patients | Median (95% CIa) | p | No. of patients | Median (95% CIa) | p | |
| Hv1 expression | ||||||
| High | 61 | 39.0 (34.3–43.7) | 0.000 | 61 | 40.1 (35.4–45.0) | 0.000 |
| Low | 44 | 53.7 (50.1–57.4) | 44 | 54.1 (50.5–57.7) | ||
| Age (years) | ||||||
| ≤50 | 48 | 48.7 (44.0–53.3) | 48 | 0.129 | 48.8 (44.3–53.4) | 0.373 |
| >50 | 57 | 42.2 (37.4–47.1) | 57 | 43.6 (38.7–48.6) | ||
| Primary site | ||||||
| Left breast | 62 | 44.8 (40.2–49.4) | 0.731 | 62 | 46.0 (41.4–50.7) | 0.853 |
| Right breast | 43 | 45.8 (40.7–50.9) | 43 | 46.1 (41.1–51.1) | ||
| Tumor size (cm) | ||||||
| ≤2 | 26 | 51.5 (45.7–57.3) | 0.024 | 26 | 53.0 (47.3–58.7) | 0.009 |
| >2 | 79 | 43.1 (39.1–47.1) | 79 | 43.7 (39.7–47.8) | ||
| Lymph node status | ||||||
| Negative | 64 | 50.5 (46.8–54.3) | 0.000 | 64 | 51.3 (47.6–54.9) | 0.000 |
| Positive | 41 | 36.7 (31.1–42.3) | 41 | 37.8 (32.0–43.6) | ||
a CI indicates confidence interval.
TABLE 6.
Multivariate Cox proportional hazards analysis for recurrence-free survival and overall survival according to Hv1 expression
| Variable | Recurrence-free survival |
Overall survival |
||||
|---|---|---|---|---|---|---|
| No. of patients | RRa (95% CIb) | pc | No. of patients | RRa (95% CIb) | pc | |
| Hv1 expression | ||||||
| High | 61 | 1.000 | 0.011 | 61 | 1.000 | 0.029 |
| Low | 44 | 0.404 (0.201–0.813) | 44 | 0.448 (0.218–0.920) | ||
| Age (yr) | ||||||
| ≤50 | 48 | 1.000 | 0.163 | 48 | 1.000 | 0.527 |
| >50 | 57 | 1.487 (0.852–2.596) | 57 | 1.203 (0.679–2.129) | ||
| Primary site | ||||||
| Left breast | 62 | 1.000 | 0.974 | 62 | 1.000 | 0.553 |
| Right breast | 43 | 1.009 (0.572–1.781) | 43 | 1.195 (0.664–2.148) | ||
| Tumor size (cm) | ||||||
| ≤2 | 26 | 1.000 | 0.397 | 26 | 1.000 | 0.185 |
| >2 | 79 | 1.426 (0.627–3.242) | 79 | 1.862 (0.743–4.670) | ||
| Lymph node status | ||||||
| Negative | 64 | 1.000 | 0.001 | 64 | 1.000 | 0.001 |
| Positive | 41 | 2.642 (1.499–4.654) | 41 | 2.657 (1.473–4.793) | ||
a RR indicates relative risk.
b CI indicates confidence interval.
c p values were obtained by Cox proportional hazards analysis modeled for high and low/negative levels of Hv1 expression.
Hv1 Contributes to Breast Cancer Cell Proliferation and Invasiveness
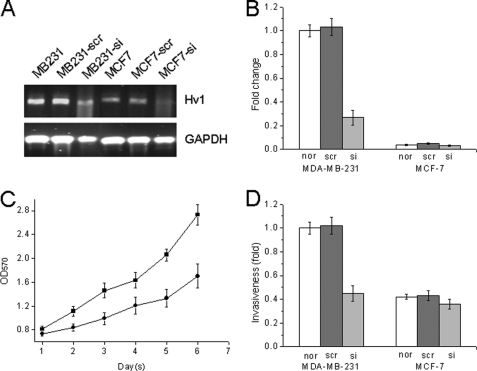
In our previous work, we detected Hv1 expression levels in human breast cancer cell lines, MCF-7, MDA-MB-231, MDA-MB-468, MDA-MB-453, T-47D, and SK-BR-3, and we found that Hv1 is expressed at a high level in the highly metastatic MDA-MB-231 cell line but at a very low level in the MCF-7 and T-47D cell lines (24, 26). To determine the effect of Hv1 on tumor progression in breast cancer, we examined the proliferation and invasiveness of MDA-MB-231 and MCF-7 cells. We down-regulated the expression of Hv1 in the highly metastatic human breast cancer MDA-MB-231 cells by infecting the cells with Hv1-siRNA lentivirus. As shown in Fig. 3, A and B, the mRNA expression level of Hv1 in the MDA-MB-231 cells was decreased to about 70%. The cell growth was inhibited (40% decrease) in MDA-MB-231 cells that were infected with Hv1-siRNA lentivirus compared with the cells that were infected with a nonspecific Hv1-scr control lentivirus (Fig. 3C), although the growth rate in MDA-MB-231 and MCF-7 cells was unchanged (data not shown). MDA-MB-231 cells that were infected with Hv1-siRNA lentivirus had decreased invasive activities (40–50% inhibition) compared with the control (invasion assay) (Fig. 3D).
FIGURE 3.
Decreased cell proliferation and invasiveness are illustrated in voltage-gated proton channel Hv1-suppressed cells. MDA-MB-231 and MCF-7 cells were infected with Hv1-siRNA lentivirus and nonspecific Hv1-scr control lentivirus, respectively. A and B, quantitative real time PCR analysis was conducted in Hv1-suppressed breast cancer cells. Values are means ± S.D. (n = 3). p < 0.05, compared with normal MDA-MB-231 cells (nor). C, proliferation of Hv1-suppressed cells was decreased. ●, MDA-MB-231 cells infected with Hv1-siRNA lentivirus; ■, MDA-MB-231 cells infected with nonspecific Hv1-scr control lentivirus. Values are means ± S.D. (n = 5). p < 0.05, compared with MDA-MB-231-scr cells. D, invasiveness was significantly inhibited by Hv1-siRNA in the highly (MDA-MB-231) metastatic cells. Values are means ± S.D. (n = 3). p < 0.05, compared with normal MDA-MB-231 cells (nor).
Hv1 Regulates Breast Cancer Cytosolic pH
The Hv channels have been described in the plasma or phagosome membranes of many blood cells, including macrophage, neutrophils, and eosinophils, that undergo phagocytosis. However, in our previous work, the Hv1 is localized in both intracellular compartment and plasma membranes in HeLa cells (23). To determine the localization of Hv1 in breast cancer cells, the localization of Hv1 in MDA-MB-231 cells was detected by immunofluorescence cytochemistry. As shown in Fig. 4A, Hv1 is also expressed both in plasma membrane and intracellular sites.
FIGURE 4.
Voltage-gated proton channel Hv1 regulates breast cancer cytosolic pH. A, Hv1 is mainly expressed in plasma membrane in MDA-MB-231 cells. Panel a, observation for FITC fluorescence (green); panel b, DAPI stain to visualize the nuclei (blue); panel c, images merged the fluorescence of FITC and DAPI. B, H+ channel activity of Hv1. The proton secretion activity of Hv1 in breast cancer cells was inhibited by down-regulation Hv1 expression. Panel a, membrane depolarization induces proton extrusion in MDA-MB-231 cells; panel b, inhibition of Hv1 expression by Hv1-siRNA notably suppresses the proton extrusion. The arrows in panels a and b indicate the time when the high K+ solution was added into the culture dish, without interruptions in the recordings. C, Hv1 regulates intracellular pH of breast cancer cells. Suppression of Hv1 by Hv1-siRNA notably induces a decrease in intracellular pH in the highly metastatic MDA-MB-231 cells. Values are means ± S.D. (n = 3). p < 0.05, compared with MDA-MB-231 cells (nor) for MDA-MB-231 and MCF-7 cells (nor) for MCF-7. D, highly metastatic MDA-MB-231 cells have a high capacity to acidify the extracellular milieu, whereas down-regulation of Hv1 expression in the cells obviously inhibited acidifying the extracellular milieu. MCF-7 and MDA-MB-231 cells infected with Hv1-siRNA lentivirus and nonspecific Hv1-scr control lentivirus, respectively, were grown at a confluence, and then changed into unbuffered DMEM for 24 h at 37 °C in a water-saturated atmosphere devoid of CO2. Values are means ± S.D. (n = 3). ▴, MCF7-scr; ●, MCF7-si; ■, MDA-MB-231-scr; ▾, MDA-MB-231-si. E, activity of gelatinase was apparently reduced in the supernatant of MDA-MB-231 cells suppressed Hv1 by Hv1-siRNA compared with MDA-MB-231 cells nonsuppressed Hv1.
The H+ channel activity of Hv1 in breast cancer cells was measured with the pH-sensitive probe BCECF. BCECF is a widely used pH indicator for estimating pHi (27–29). Its fluorescence intensity at maximum emission wavelength is pH-dependent as follows: a fall in pH with a decrease in fluorescence intensity and a rise in pH with an increase in fluorescence intensity. We acid-preloaded and exposed MDA-MB-231 cells with nonsuppressed and suppressed Hv1 to an outward-acting proton force (in high-K+ medium) that will drive an efflux of H+ ions. As shown in Fig. 4B, the sharp increase on the fluorescence intensity of BCECF at membrane depolarization was observed for the nonsuppressed Hv1 of the cells (Fig. 4B, panel a), indicating that an increase in pHi occurred. In contrast to the nonsuppressed Hv1 of the cells, an intense decrease of the fluorescence intensity of BCECF at membrane depolarization was observed for the suppressed Hv1 of the cells, indicating a decrease in pHi in the cells occurred (Fig. 4B, panel b). The inhibition of Hv1 expression in MDA-MB-231 cells notably suppressed proton extrusion. The results revealed that the pHi recovery was due to active Hv1.
To determine whether Hv1 regulates pHi of breast cancer cell, the intracellular pH values in both MDA-MB-231 and MCF-7 cells were evaluated by BCECF fluorescence. As shown in Fig. 4C, the down-regulation of Hv1 expression in MDA-MB-231 cells significantly increased acidity of intracellular pH from 7.45 to 7.32 but not a remarkable change in MCF-7 cells. The finding showed that the inhibition of Hv1 expression in MDA-MB-231 cells notably suppressed proton extrusion. These results clearly showed that Hv1 regulates intracellular pH of high metastatic breast cancer cells.
Hv1 Plays a Dominant Role in Acidifying Extracellular Milieu
To further assess the role of Hv1 in intracellular proton extrusion, we measured the pH values of the extracellular milieu of MDA-MB-231, MCF-7, and MDA-MB-231 cell-mediated knockdown of Hv1 expression. As shown in Fig. 4D, the highly metastatic MDA-MB-231 cells were more active in acidifying a nonbuffered medium than the poorly metastatic MCF-7 cells. The high capacity of MDA-MB-231 cells to acidify extracellular milieu was blocked by Hv-siRNA-mediated knockdown of Hv1 expression (Fig. 4D). This result also indicated that Hv1 plays a dominant role in intracellular proton extrusion.
Hv1 Promotes Gelatinase Activity
The secretion and activation of some ECM-degenerating proteases are pH-regulated. We examined the activity of MMP-2/MMP-9, which is closely related to cancer metastasis according to the previous reports (30). The supernatants of the cultured MCF-7 and MDA-MB-231 cells were collected, and the gelatinase activities were assayed. Zymography showed that the activity of MMP-2 was apparently reduced in the supernatant of MDA-MB-231 cell-suppressed Hv1 compared with the control MDA-MB-231 cells, whereas the activity of MMP-9 was almost independent of Hv1 expression, as shown in Fig. 4E, suggesting that Hv1 contributes to cellular invasiveness.
Silencing Hv1 Reduces the Rate of Xenograft Tumor Growth
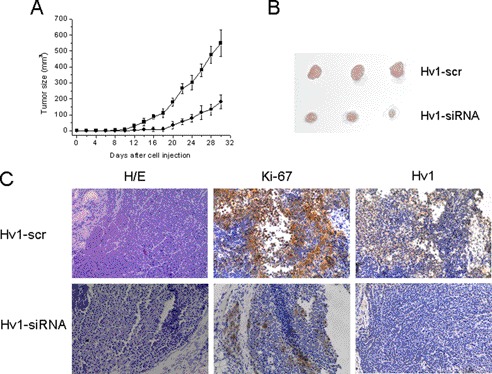
To investigate the in vivo functional consequences of Hv1 expression on tumor growth, nude mice were injected s.c. with MDA-MB-231 cells, MDA-MB-231 cells infected with Hv1-siRNA lentivirus (MDA-MB-231-si), and MDA-MB-231 cells infected with Hv1-scr lentivirus (MDA-MB-231-scr). At the 30th day, we found that the average size of xenografts in the MDA-MB-231-si group was dramatically smaller than that of the MDA-MB-231 and MDA-MB-231-scr groups (Fig. 5). However, no difference was observed between the MDA-MB-231 and scr-MDA-MB-231 groups (data not shown). Immunohistochemical analysis of tumor sections from mice injected with si-MDA-MB-231 (Fig. 5C) showed diminished levels of Ki-67 in cells for Hv1-silenced mice, indicating that the proliferation of tumor cells was decreased. Thus, these results showed that invalidation of Hv1 brings about a dramatic decrease in tumor xenograft cell growth.
FIGURE 5.
Down-regulation of Hv1 expression reduces the rate of xenograft tumor growth. A and B, average size of xenografts in MDA-MB-231-si group was dramatically smaller than that of the MDA-MB-231-scr group. ●, MDA-MB-231 cells infected with Hv1-siRNA lentivirus; ■, MDA-MB-231 cells infected with nonspecific Hv1-scr control lentivirus. C, immunohistochemical analysis of tumor sections from mice injected with MDA-MB-231-si and MDA-MB-231-scr by H&E, Ki-67, and Hv1 staining.
DISCUSSION
In this study, our data revealed that Hv1 expression levels were significantly higher in breast cancer tissues than in the corresponding nontumor tissues, which were significantly associated with tumor recurrence and metastasis. Patients who had high expression of Hv1 were remarkably poorly recurrence-free and overall survival compared with patients who had low expression of Hv1. Our results clearly demonstrated that high Hv1 expression is associated with poor progression and unfavorable clinical outcome of breast cancer. In vivo and in vitro results revealed that Hv1 functions in breast tumor growth and invasiveness by promoting the activity of matrix metalloproteinases such as MMP-2, suggesting that Hv1 in the highly metastatic human is essential to tumor progression and metastasis through the regulation of the intracellular pH of breast cancer cells.
The cytosolic pH (pHi) is a vital parameter for all biological processes in cells. Tumor cells often exist in a hypoxic microenvironment with a lower pHo value than that of surrounding normal cells (1, 31, 32). The high glycolytic metabolite in cancer cells results in an excessive production of intracellular acidity, and overly extruded acid induces extracellular acidification (3, 4). To overcome the hypoxic microenvironment and prevent the intracellular accumulation of the increased acidic metabolites, tumor cells must be enhanced by the ability to dispose of the increased intracellular protons. Some pHi regulatory mechanisms in tumor cells have been described, such as Na+/H+ exchangers, bicarbonate (HCO3−) transporters, proton-lactate symporters, and proton pumps (8, 26, 33–36).
Recently, vacuolar H+-ATPases (V-ATPases) have been extensively studied as a novel and important intracellular pH regulatory system in some cancer cells (34, 35, 36). The importance of V-ATPases in cancer malignancy has been repeatedly demonstrated in several human cancer tumors and cell lines, including hepatocellular carcinoma, B-cell tumors, and melanoma (37–39). Inhibition of V-ATPase function via knockdown of the protein subunit ATP6L expression using RNA-interfering technology can effectively retard the growth and metastasis of human hepatocellular carcinoma xenografts (37). Inhibition of V-ATPase activity can also be achieved by treatment with proton pump inhibitors. Recent data showed that proton pump inhibitors can induce apoptosis of human B-cell tumors and inhibit the growth of human melanoma (38, 39). Moreover, proton pump inhibitors were shown to selectively induce apoptosis of gastric cancer cells (40). The use of these proton pump inhibitors has been proposed as a potential new strategy against cancer (35, 36). Sennoune et al. (26) proposed that the poorly metastatic breast cancer MCF-7 cells preferentially utilize Na+/H+ exchanger and HCO3−-based H+-transporting mechanisms, whereas the highly metastatic MDA-MB-231 cells used plasma membrane V-ATPases. The highly metastatic cells were more invasive and migratory than the poorly metastatic cells.
In our case, we showed that Hv1 function relates to breast tumor growth and metastasis through proton extrusion and the up-regulation of gelatinase activity. The role of Hv1 in breast cancer development, progression, and metastasis may relate to other proton pumps, such as V-ATPases. Montcourrier et al. (41) showed that the highly metastatic MDA-MB-231 human breast cancer cells were more active in acidifying a nonbuffered balanced salt solution than the poorly metastatic MCF-7 cells. Bafilomycin A1, a specific inhibitor of V-ATPases, had none or only a weak effect on acid extrusion when tested in the presence of glucose, and they proposed that further characterization of the breast cancer cell pump was required. The breast cancer cell pump candidate may be the voltage-gated proton channel Hv1, as demonstrated in this study.
The intracellular pH such as the pH of internal vesicles and the cytosolic pH, and pH gradients in tumor cells have been analyzed (35, 36). The level of cytosolic pH is extremely important for tumor cells, inasmuch as a decrease of cytosolic pH possibly stops tumor cell metabolism and induces cell death. An alkaline cytosolic pH and an acidic extracellular pH resulting in high glycolytic activity and acidic metabolites are characteristics of tumor cells. The aberrant pH gradient between the alkaline cytosol and the acidic extracellular environment is involved in tumor progression and malignancy, which are maintained by up-regulated activity of V-ATPases that extrude protons outside the cell and acidify intracellular vesicles (35, 36).
The low pHo is one of hallmarks of malignant cells and optimal for cancer cells in solid tumors; on the contrary, it is toxic to normal cells (1, 31). The low pHo increases ECM digestion through increased secretion and activation of proteases and remolding of ECM, induces apoptosis of adjacent normal cells, promotes angiogenesis, inhibits the host immune system, and thus contributes to cancer invasion and metastasis (4, 42–46). The activities of proteases needing a low extracellular pH to optimize their activation include cathepsin (cathepsin B and D), matrix metalloproteinase (MMP-2, MMP-9, and MMP-3), bone morphogenetic protein-1-type metalloproteinases, tissue serine proteases, and adamalysin-related membrane proteases. Among them, the matrix metalloproteinase family is essentially involved in degradation and remolding of ECM, because of their ability to collectively degrade all the structural components of the ECM (35, 36, 42–46).
Hv1 is mainly expressed in plasma membrane of breast tumor cells, which is correlated with the metastatic potential of these cells. The mechanism that Hv1 promotes the growth and metastasis of breast tumor may rely on its cytosolic and extracellular pH regulation activity, which is related to the activation, secretion, and cellular distribution of many proteases involved in the digestion of ECM, such as matrix metalloproteinases (45, 46). The acidic pH of the extracellular microenvironment can induce the increase of the secretion of these proteases and enhance the activation of the relevant proteases either in intracellular vesicles or in extracellular environment (42–46). The reduction in the activity of gelatinase/metalloproteinase is induced by Hv1 activity inhibition. This means that inhibition of Hv1, as well as all the other proton pumps overexpressed by malignant tumors, may lead to a strong inhibition of lytic enzymes in the extracellular environment, thus impairing the invasiveness of tumor cells. Therefore, we could postulate that the Hv1 would affect the pHi and pHo, and consequently influence metastatic potential in breast cancer.
In conclusion, we demonstrated here that Hv1 is overexpressed in patients with breast cancer, and high Hv1 expression is correlated with the disease progression and poor clinical outcome in breast cancer. The preferential expression of Hv1 in breast cancer is essential for the acquisition of tumor development, progression, and metastasis. Therefore, it appears that Hv1 is a novel molecular target in the strategies for the prediction of tumor recurrence and prognosis or treatment of breast cancer and is a potential target in breast cancer therapy and an excellent candidate for anticancer drugs.
Acknowledgment
We are deeply grateful to Prof. Wenxin Qin, National Laboratory for Oncogenes and Related Genes, WHO Collaborating Center for Research on Cancer, Shanghai Cancer Institute, Shanghai Jiao Tong University, for supporting and encouraging the research and many useful discussions on the manuscript.
This work was supported by National Natural Science Foundation of China Grant 30970579.
- pHo
- extracellular pH
- pHi
- intracellular pH
- ECM
- extracellular matrix
- VSD
- voltage-sensor domain
- BCECF
- 2′,7′-bis(carboxyethyl)-5,6-carboxyfluorescein
- IRS
- ImmunoReactive Score
- V-ATPase
- vacuolar H+-ATPase
- ER
- estrogen receptor
- PR
- progesterone receptor
- P-gp
- P-glycoprotein.
REFERENCES
- 1. Griffiths J. R. (1991) Are cancer cells acidic? Br. J. Cancer 64, 425–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gillies R. J., Liu Z., Bhujwalla Z. (1994) 31P MRS measurements of extracellular pH of tumors using 3-aminopropylphosphonate. Am. J. Physiol. 267, C195–C203 [DOI] [PubMed] [Google Scholar]
- 3. Racker E. (1972) Bioenergetics and the problem of tumor growth. Am. Sci. 60, 56–63 [PubMed] [Google Scholar]
- 4. Gatenby R. A., Gawlinski E. T. (2003) The glycolytic phenotype in carcinogenesis and tumor invasion. Insights through mathematical models. Cancer Res. 63, 3847–3854 [PubMed] [Google Scholar]
- 5. Roos A., Boron W. F. (1981) Intracellular pH. Physiol. Rev. 61, 296–434 [DOI] [PubMed] [Google Scholar]
- 6. Chambard J. C., Pouyssegur J. (1986) Intracellular pH controls growth factor-induced ribosomal protein S6 phosphorylation and protein synthesis in the G0-G1 transition of fibroblasts. Exp. Cell Res. 164, 282–294 [DOI] [PubMed] [Google Scholar]
- 7. Perona R., Serrano R. (1988) Increased pH and tumorigenicity of fibroblasts expressing a yeast proton pump. Nature 334, 438–440 [DOI] [PubMed] [Google Scholar]
- 8. Schlappack O. K., Zimmermann A., Hill R. P. (1991) Glucose starvation and acidosis. Effect on experimental metastatic potential, DNA content, and MTX resistance of murine tumor cells. Br. J. Cancer 64, 663–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gottlieb R. A., Giesing H. A., Zhu J. Y., Engler R. L., Babior B. M. (1995) Cell acidification in apoptosis. Granulocyte colony-stimulating factor delays programmed cell death in neutrophils by up-regulating the vacuolar H+-ATPase. Proc. Natl. Acad. Sci. U.S.A. 92, 5965–5968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ramsey I. S., Moran M. M., Chong J. A., Clapham D. E. (2006) A voltage-gated proton-selective channel lacking the pore domain. Nature 440, 1213–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sasaki M., Takagi M., Okamura Y. (2006) A voltage sensor-domain protein is a voltage-gated proton channel. Science 312, 589–592 [DOI] [PubMed] [Google Scholar]
- 12. Decoursey T. E. (2003) Voltage-gated proton channels and other proton transfer pathways. Physiol. Rev. 83, 475–579 [DOI] [PubMed] [Google Scholar]
- 13. Henderson L. M., Chappell J. B., Jones O. T. (1987) The superoxide-generating NADPH oxidase of human neutrophils is electrogenic and associated with an H+ channel. Biochem. J. 246, 325–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clark R. A., Leidal K. G., Pearson D. W., Nauseef W. M. (1987) NADPH oxidase of human neutrophils. Subcellular localization and characterization of an arachidonate-activatable superoxide-generating system. J. Biol. Chem. 262, 4065–4074 [PubMed] [Google Scholar]
- 15. Morgan D., Cherny V. V., Murphy R., Katz B. Z., DeCoursey T. E. (2005) The pH dependence of NADPH oxidase in human eosinophils. J. Physiol. 569, 419–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. DeCoursey T. E., Cherny V. V. (1994) Voltage-activated hydrogen ion currents. J. Membr. Biol. 141, 203–223 [DOI] [PubMed] [Google Scholar]
- 17. Cherny V. V., Markin V. S., DeCoursey T. E. (1995) The voltage-activated hydrogen ion conductance in rat alveolar epithelial cells is determined by the pH gradient. J. Gen. Physiol. 105, 861–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cherny V. V., DeCoursey T. E. (1999) pH-dependent inhibition of voltage-gated H+ currents in rat alveolar epithelial cells by Zn2+ and other divalent cations. J. Gen. Physiol. 114, 819–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Long S. B., Campbell E. B., Mackinnon R. (2005) Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science 309, 897–903 [DOI] [PubMed] [Google Scholar]
- 20. Koch H. P., Kurokawa T., Okochi Y., Sasaki M., Okamura Y., Larsson H. P. (2008) Multimeric nature of voltage-gated proton channels. Proc. Natl. Acad. Sci. U.S.A. 105, 9111–9116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tombola F., Ulbrich M. H., Isacoff E. Y. (2008) The voltage-gated proton channel Hv1 has two pores, each controlled by one voltage sensor. Neuron 58, 546–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee S. Y., Letts J. A., Mackinnon R. (2008) Dimeric subunit stoichiometry of the human voltage-dependent proton channel Hv1. Proc. Natl. Acad. Sci. U.S.A. 105, 7692–7695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li S. J., Zhao Q., Zhou Q., Unno H., Zhai Y., Sun F. (2010) The role and structure of the carboxyl-terminal domain of the human voltage-gated proton channel Hv1. J. Biol. Chem. 285, 12047–12054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang Y., Li S. J., Pan J., Che Y., Yin J., Zhao Q. (2011) Specific expression of the human voltage-gated proton channel Hv1 in highly metastatic breast cancer cells promotes tumor progression and metastasis. Biochem. Biophys. Res. Commun. 412, 353–359 [DOI] [PubMed] [Google Scholar]
- 25. Li S. J., Zhao Q., Zhou Q., Zhai Y. (2009) Expression, purification, crystallization, and preliminary crystallographic study of the carboxyl-terminal domain of the human voltage-gated proton channel Hv1. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 65, 279–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sennoune S. R., Bakunts K., Martínez G. M., Chua-Tuan J. L., Kebir Y., Attaya M. N., Martínez-Zaguilán R. (2004) Vacuolar H+-ATPase in human breast cancer cells with distinct metastatic potential. Distribution and functional activity. Am. J. Physiol. Cell Physiol. 286, C1443–C1452 [DOI] [PubMed] [Google Scholar]
- 27. Henderson L. M., Thomas S., Banting G., Chappell J. B. (1997) The arachidonate-activatable, NADPH oxidase-associated H+ channel is contained within the multimembrane-spanning N-terminal region of gp91-phox. Biochem. J. 325, 701–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nilsson C., Kågedal K., Johansson U., Ollinger K. (2003) Analysis of cytosolic and lysosomal pH in apoptotic cells by flow cytometry. Methods Cell. Sci. 25, 185–194 [DOI] [PubMed] [Google Scholar]
- 29. Chiche J., Ilc K., Laferrière J., Trottier E., Dayan F., Mazure N. M., Brahimi-Horn M. C., Pouysségur J. (2009) Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell growth by counteracting acidosis through the regulation of the intracellular pH. Cancer Res. 69, 358–368 [DOI] [PubMed] [Google Scholar]
- 30. Giannelli G., Antonaci S. (2002) Gelatinases and their inhibitors in tumor metastasis. From biological research to medical applications. Histol. Histopathol. 17, 339–345 [DOI] [PubMed] [Google Scholar]
- 31. Gillies R. J., Liu Z., Bhujwalla Z. (1994) 31P-MRS measurements of extracellular pH of tumors using 3-aminopropylphosphonate. Am. J. Physiol. 267, C195–C203 [DOI] [PubMed] [Google Scholar]
- 32. Raghunand N., Martínez-Zaguilán R., Wright S. H., Gillies R. J. (1999) pH and drug resistance. II. Turnover of acidic vesicles and resistance to weakly basic chemotherapeutic drugs. Biochem. Pharmacol. 57, 1047–1058 [DOI] [PubMed] [Google Scholar]
- 33. Pouysségur J., Sardet C., Franchi A., L'Allemain G., Paris S. (1984) A specific mutation abolishing Na+/H+ antiport activity in hamster fibroblasts precludes growth at neutral and acidic pH. Proc. Natl. Acad. Sci. U.S.A. 81, 4833–4837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gillies R. J., Martinez-Zaguilan R. (1991) Regulation of intracellular pH in BALB/c 3T3 cells. Bicarbonate raises pH via NaHCO3/HCl exchange and attenuates the activation of Na+/H+ exchange by serum. J. Biol. Chem. 266, 1551–1556 [PubMed] [Google Scholar]
- 35. Fais S., De Milito A., You H., Qin W. (2007) Targeting vacuolar H+-ATPases as a new strategy against cancer. Cancer Res. 67, 10627–10630 [DOI] [PubMed] [Google Scholar]
- 36. Fais S. (2010) Proton pump inhibitor-induced tumor cell death by inhibition of a detoxification mechanism. J. Intern. Med. 267, 515–525 [DOI] [PubMed] [Google Scholar]
- 37. Lu X., Qin W., Li J., Tan N., Pan D., Zhang H., Xie L., Yao G., Shu H., Yao M., Wan D., Gu J., Yang S. (2005) The growth and metastasis of human hepatocellular carcinoma xenografts are inhibited by small interfering RNA targeting to the subunit ATP6L of proton pump. Cancer Res. 65, 6843–6849 [DOI] [PubMed] [Google Scholar]
- 38. De Milito A., Iessi E., Logozzi M., Lozupone F., Spada M., Marino M. L., Federici C., Perdicchio M., Matarrese P., Lugini L., Nilsson A., Fais S. (2007) Proton pump inhibitors induce apoptosis of human B-cell tumors through a caspase-independent mechanism involving reactive oxygen species. Cancer Res. 67, 5408–5417 [DOI] [PubMed] [Google Scholar]
- 39. De Milito A., Canese R., Marino M. L., Borghi M., Iero M., Villa A., Venturi G., Lozupone F., Iessi E., Logozzi M., Della Mina P., Santinami M., Rodolfo M., Podo F., Rivoltini L., Fais S. (2010) pH-dependent antitumor activity of proton pump inhibitors against human melanoma is mediated by inhibition of tumor acidity. Int. J. Cancer 127, 207–219 [DOI] [PubMed] [Google Scholar]
- 40. Yeo M., Kim D. K., Kim Y. B., Oh T. Y., Lee J. E., Cho S. W., Kim H. C., Hahm K. B. (2004) Selective induction of apoptosis with proton pump inhibitor in gastric cancer cells. Clin. Cancer Res. 10, 8687–8696 [DOI] [PubMed] [Google Scholar]
- 41. Montcourrier P., Silver I., Farnoud R., Bird I., Rochefort H. (1997) Breast cancer cells have a high capacity to acidify extracellular milieu by a dual mechanism. Clin. Exp. Metastasis 15, 382–392 [DOI] [PubMed] [Google Scholar]
- 42. Rozhin J., Sameni M., Ziegler G., Sloane B. F. (1994) Pericellular pH affects distribution and secretion of cathepsin B in malignant cells. Cancer Res. 54, 6517–6525 [PubMed] [Google Scholar]
- 43. Montcourrier P., Mangeat P. H., Valembois C., Salazar G., Sahuquet A., Duperray C., Rochefort H. (1994) Characterization of very acidic phagosomes in breast cancer cells and their association with invasion. J. Cell Sci. 107, 2381–2391 [DOI] [PubMed] [Google Scholar]
- 44. Martínez-Zaguilán R., Seftor E. A., Seftor R. E., Chu Y. W., Gillies R. J., Hendrix M. J. (1996) Acidic pH enhances the invasive behavior of human melanoma cells. Clin. Exp. Metastasis 14, 176–186 [DOI] [PubMed] [Google Scholar]
- 45. Fasciglione G. F., Marini S., D'Alessio S., Politi V., Coletta M. (2000) pH and temperature dependence of functional modulation in metalloproteinases. A comparison between neutrophil collagenase and gelatinases A and B. Biophys. J. 79, 2138–2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Johnson L. L., Pavlovsky A. G., Johnson A. R., Janowicz J. A., Man C. F., Ortwine D. F., Purchase C. F., 2nd, White A. D., Hupe D. J. (2000) A rationalization of the acidic pH dependence for stromelysin-1 (matrix metalloproteinase-3) catalysis and inhibition. J. Biol. Chem. 275, 11026–11033 [DOI] [PubMed] [Google Scholar]