Background: Many insects eat green leaves but excrete black feces using an unknown mechanism.
Results: Hindgut cells produce prophenoloxidase and secrete it into the hindgut content.
Conclusion: Prophenoloxidase induces the hindgut content and feces melanization by which bacteria flora are reduced.
Significance: This is a first-time disclosure of the enigma of insect black feces and its biological significance.
Keywords: Bacteria, Insect, Insect Immunity, Intestine, Metalloenzymes, Hindgut, Prophenoloxidase
Abstract
Many insects eat the green leaves of plants but excrete black feces in an as yet unknown mechanism. Insects cannot avoid ingesting pathogens with food that will be specifically detected by the midgut immune system. However, just as in mammals, many pathogens can still escape the insect midgut immune system and arrive in the hindgut, where they are excreted out with the feces. Here we show that the melanization of hindgut content induced by prophenoloxidase, a key enzyme that induces the production of melanin around invaders and at wound sites, is the last line of immune defense to clear bacteria before feces excretion. We used the silkworm Bombyx mori as a model and found that prophenoloxidase produced by hindgut cells is secreted into the hindgut contents. Several experiments were done to clearly demonstrate that the blackening of the insect feces was due to activated phenoloxidase, which served to regulate the number of bacteria in the hindgut. Our analysis of the silkworm hindgut prophenoloxidase discloses the natural secret of why the phytophagous insect feces is black and provides insight into hindgut innate immunity, which is still rather unclear in mammals.
Introduction
There are over 1011 microbe cells per gram content in the mammalian large intestine because it lacks Peyer's patches (1). Among those bacteria, fecal pathogens cause health problems throughout the world, particularly through water pollution (2). As in mammals, many pathogens can still escape insect midgut immune system and pass to the hindgut, which is an organ similar to the mammalian large intestine (3, 4). Because of similarities with mammals, the insect midgut immune system upon aging, stress, or infection has been extensively studied (4, 5). However, as yet, it is unclear whether there is an innate immunity system in mammalian large intestine and the insect hindgut that destroys pathogens before feces excretion.
Insects make up the largest group of animals on earth, and almost 45% of insects are herbivorous (6). Most herbivorous insects live on green leaves, and typical insects such as the silkworm eat green mulberry leaves although they excrete black feces. As yet, the mechanism of how feces are blackened is still unknown.
The insect gut is a digestion system that is divided into foregut, midgut, and hindgut. Insect hindgut is generally thought to re-absorb certain salts and amino acids from the content (before excretion as feces) to maintain the osmotic pressure in the hemolymph (7). Many symbiotic bacteria including un-killed pathogens cannot avoid being excreted with feces, and those microbes may further pollute insect feed and its habitat. Little is known whether insect hindgut has an immune system to deal with microbes in feces before excretion.
Prophenoloxidase (PPO)4 is a very important innate immunity protein that is produced by hemocytes in invertebrates (8–12). Activated phenoloxidase (PO) induces the production of melanin (melanization) around invading microorganisms to first seal them off from circulation and then kill them. Melanization is also induced around wounds to prevent additional infections. Activated PO produces melanin in the epidermis and cuticle and is therefore also responsible for insect body color and patterns (13, 14). Two PPO genes occur in the silkworm (8, 15). Because the feces of phytophagous insects is black, we hypothesized that PPO may be responsible for melanization of feces. However, like PPO, laccase and peroxidase also oxidize some phenols (10), and thus, these enzymes may also induce melanization of feces.
In this study, we used biochemical assays and in situ hybridization to show that cells in the hindgut of Bombyx mori produced PPO. PPO was also found in the hindgut contents. Melanization of the silkworm larvae feces was blocked, and bacterial number increased in hindgut when phenylthiourea (PTU) was introduced through feeding. Therefore, activated PO and PO-induced melanization of hindgut content helped reduce bacterial numbers, suggesting that PO is an important regulator of the bacterial flora in hindgut and feces.
EXPERIMENTAL PROCEDURES
Insect Feeding and Dissection
B. mori larvae (Nistari) were reared on mulberry leaves at 25 °C under a 12-h photoperiod. Larvae on day 3 of the fourth larval stage (IV-3), the fourth molting stage (IV-M), day 3 of the fifth larval stage (V-3), or at the wandering-stage (W) were used for experiments. To obtain samples for Western blot, immunostaining, or native gel assay, silkworms and other larval species were dissected in autoclaved 0.85% NaCl solution after bleeding. The dissected tissues were washed in fresh 0.85% NaCl solution three times to remove hemolymph. The gut was washed in 0.85% NaCl three times, and the corresponding gut parts were dried and then cut open to transfer contents to a new tube. Larvae were bled, and the hemolymph was transferred to a new tube after centrifuging at 10,000 × g for 5 min. The supernatant was the plasma. Dianemobius nigrofasciatus, Tribolium castaneum, Drosophila melanogaster (w1118), Coptotermes formosanus, Ostrinia furnaclis, Heliothis armigero, and Culex pipiens quinquefasciatus were fed as usual.
PPO, Laccase, and Peroxidase Enzymatic Assays
PPO, laccase, and peroxidase are a group of enzymes that may oxidize dopamine to produce melanin and metabolites (10). The activities of the three enzymes were compared in hindgut content using 3 μg of laccase (38429; Sigma), 1.67 ng of peroxidase (P719; Invitrogen), and 1.25 μg of purified recombinant Drosophila prophenoloxidase 1 (DmrPPO1) (16). A 200 μl aliquot of 2 mm ABTS (Sigma) for laccase and TMB solution for peroxidase was used (1:50; Invitrogen). 10 mm dopamine (Sigma) was used for DmrPPO1. DmrPPO1 needed to be activated with 30% ethanol before use (16). The inhibitors were incubated with each enzyme for 5 min before adding the substrate. 3 mm NaN3 (final concentration) was used for inhibition of laccase and peroxidase, and saturated PTU was used for inhibition of DmrPPO1. Gut content equal to feces wet weight was suspended in 200 μl of Tris buffer (10 mm, pH 7.4) and vortexed several times. The suspension (20 μl) was mixed with 200 μl of each substrate plus the corresponding enzyme inhibitor and/or Tris buffer to make the total volume the same. Because the inhibitors were used to detect whether they could inhibit different enzymes, each substrate with inhibitor added was treated as a blank. They were added in Tris buffer containing inhibitor alone, at the same volume as the blank, to determine HG1 and HG2 content. The solutions were incubated at room temperature for 8 min. The mixture was centrifuged at 10,000 × g for 1 min, and the absorbance of the supernatant was read at 10 min using the EXPERT 96 microplate reader (Biochrom, Holliston, MA). Absorbance was read at 490, 450, and 405 nm to detect PPO, laccase, and peroxidase activities, respectively. One unit of each enzyme activity was defined as ΔAλ/min = 0.001 (λ, wavelength).
Gut Staining, Gut Content, and Feces Collection
The dissected gut of the silkworms was incubated in TMB, ABTS, or dopamine containing 30% ethanol or not for staining after food contents were removed. Dopamine is a PPO substrate, and ethanol can be used to activate PPO (8). NaN3 or PTU was added to monitor the change in melanization when dopamine (with ethanol) was used for staining.
Different parts of the gut were opened, and the contents were transferred to pre-weighed tubes to measure the wet weight of the contents. Gut contents were suspended in either 10 mm Tris buffer (pH 7.4) (for PO activity assay or PPO degradation observation) or 500 mm NaCl and 5 mm EDTA (for Western blot assay). The tubes were vortexed several times, centrifuged at 10,000 × g at 4 °C for 5 min, and the supernatant was transferred to a new tube. The supernatant was incubated at room temperature for different times to determine whether PPO was degradable.
To check whether there is PPO in feces, 20 feces samples excreted for 0 or 60 min (V-3 black feces and wandering-stage green feces, respectively) were suspended in 10 mm Tris buffer (pH 7.4) containing 500 mm NaCl and 5 mm EDTA and concentrated to ∼40 μl by ultrafiltration. 15 μl of the concentrated solution were loaded for Western blot assay.
Fluorescent Bead Injection and Tracing
The injected fluorescent beads phagocytosed by insect hemocytes were observed under a fluorescent microscope, to monitor hemocyte movement (17). In the same way, fluorescent beads (1.0 mm 4, Red (580/605); Molecular Probes) were injected into the silkworm larvae (on V-3) for at least 6 h (17). The phagocytosed beads inside hemocytes were first observed under a fluorescent microscope. The dissected hindguts were fixed, sectioned, and deparaffinized as described (17) to examine under the fluorescent microscope whether there were phagocytosed fluorescent beads brought by circulating hemocytes.
Immune Challenge and Lysozyme Detection
V-3 silkworm larvae were injected with 5 × 106 formalin-killed Escherichia coli cells suspended in sterilized 0.85% NaCl solution or 0.85% NaCl alone for immune challenge for 12 h. Then the silkworm larvae were bled to obtain plasma. HG1 and HG2 and its contents were also sampled. These samples were used for detecting lysozyme by Western blot.
Bacterial Culture
Gut content (equal to the weight of feces) and feces from V-3 and wandering-stage larvae were suspended in 1 ml of LB medium, and the suspended solutions with gut content fragments (25 μl plus 75 μl of fresh LB medium) were spread on LB plates cultured at 37 °C overnight. Colonies of bacteria were counted and calculated.
Phenylthiourea (PTU) Feeding
PTU is a strong PO inhibitor (18). It was dissolved in autoclaved water, and saturated PTU was filtered to remove bacteria. The PTU solution was spread on mulberry leaves and dried before feeding to the silkworm larvae. The change in the color of feces was observed continuously. HG1 and HG2 contents from PTU-fed larvae were removed for Western blot as described above. After feeding the PTU, the excreted green feces were collected immediately for bacterial culture, as described above.
Tissue Culture and Native Gel Analysis
Midgut and hindgut from V-3 larvae were cultured in Grace medium containing 10% fetal bovine serum. The silk gland was cultured as a control. The culture medium was sampled at scheduled times for the native gel assay as described previously (19).
SDS-PAGE and Western Blot Analysis
Different parts of the silkworm larvae gut were dissected. Tissues were homogenized and sonicated in 10 mm Tris-HCl (pH 7.4), and centrifuged at 10,000 × g at 4 °C for 5 min. The supernatant was collected, and total protein concentration was determined with bovine serum albumin as the standard. Approximately 10 μg of protein was loaded per lane, and SDS-PAGE and Western blot assay were performed. Antibody against the silkworm PPO (a gift from Dr. T. Asano; 1:5,000) (20), lysozyme (a gift from Dr. K. Suzuki; 1:5,000) (21), or Manduca sexta laccase (a gift from Dr. M. Kanost; 1:2,000) (22) was used as the first antibody, and the AP-conjugated goat anti-rabbit IgG (1:5,000) was used as the second antibody. EasySee Western Marker (DM201; TransGen) was used as the protein marker.
LC-MS/MS
The EttanTM MDLC system (GE Healthcare, Piscataway, NJ) was used for desalting and separating tryptic peptide mixtures. In this system, samples were desalted on reverse phase (RP) trap columns (Zorbax 300 SB C18, Agilent Technologies, Santa Clara, CA), and then separated on an RP column (150 μm i.d., 100 mm length, Column Technology Inc., Fremont, CA). Mobile phase A (0.1% formic acid in high performance liquid chromatography grade water) and mobile phase B (0.1% formic acid in acetonitrile) were selected. The tryptic peptide mixture (20 μg) was loaded onto the columns, and separation was performed at a flow rate of 2 μl/min using a linear gradient of 4–50% B for 120 min. A FinniganTM LTQTM linear ion trap MS (Thermo Electron Corp. Rockford, IL) equipped with an electrospray interface was connected to the LC setup to detect the eluted peptides. Data-dependent MS/MS spectra were obtained simultaneously. Each scan cycle consisted of one full MS scan in profile mode followed by five MS/MS scans in centroid mode with the following Dynamic ExclusionTM settings: repeat count 2, repeat duration 30 s, exclusion duration 90 s. Each sample was analyzed in triplicate. The silkworm protein sequence database used in this analysis was downloaded from the NCBI website using the keyword Bombyx mori.
In Situ Hybridization
B. mori PPO1 and PPO2 mRNA were detected in hindgut by in situ hybridization following described methods (23, 24). The antisense and sense RNA probe was labeled with digoxigenin by in vitro transcription with T7 RNA polymerase in a reaction containing digoxigenin-UTP (DIG RNA Labeling Kit; Boehringer, Ingelheim am Rhein, Germany). The primers for cloning PPO1 and PPO2 and those for synthesizing antisense and sense RNA probes are listed in supplemental Table S1.
Immunohistochemistry
Different parts of the silkworm gut were dissected as described above and fixed overnight at 4 °C in Bouin's fluid (23). Samples were sectioned and deparaffinized as described (17). To detect PPO, a polyclonal antibody against the silkworm PPO (1:1,000) was used as the first antibody (20), and rhodamine-conjugated goat anti-rabbit IgG (1:200) was used as the second antibody. To detect melanin in the hindgut tissue, a monoclonal antibody 6D2 (IgM) (1:200) against melanin derived from Coccidioides posadasii was used as the first antibody (a gift from Dr. Garry T. Cole) (25), and FITC-conjugated goat anti-mouse IgM (1:1000) was the second antibody. All other procedures were followed as described (26), and DAPI was used to counterstain nuclei. All pictures were taken under a fluorescent microscope (Olympus BX51) under differential interference contrast using the appropriate filter.
RESULTS
Black Feces Excreted by the Feeding Stage Silkworm: What Is the Secret Behind It?
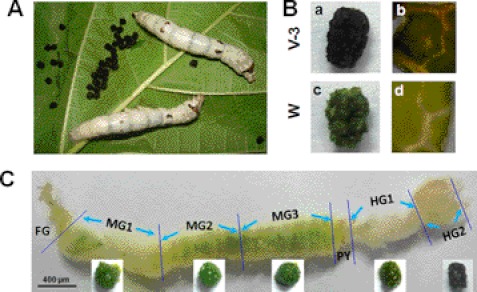
Silkworm larvae that are fed on green mulberry leaves excreted black feces in which the leaf fragments were also black (Fig. 1, A, B-a, and B-b). However, during the wandering-stage, the last feces and mulberry leaf fragments were green (Fig. 1, B-c and B-d). After dissecting a silkworm larva on day 3 of the fifth larval stage (V-3), we found that the contents in different parts of the midgut (MG) were all green (Fig. 1C, bottom). The hindgut (HG) is divided into two parts, and in the first part of the hindgut (HG1), the content was always green. When the content passed to the second part of the hindgut (HG2), it became black (Fig. 1C). Feces excreted by larvae before the wandering-stage are always black.
FIGURE 1.
Black feces excreted by silkworm larvae. A, V-3 silkworm larvae, black feces, and mulberry leaves. B, morphology of black feces excreted by feeding-stage larvae (V-3) and that of green feces excreted by wandering-stage (W) larvae. The mulberry leaf fragments were black (b) or green (d) in the corresponding black (a) or green (c) feces. C, morphology of the gut dissected from a V-3 silkworm larva, and its gut content from the corresponding location. The midgut is equally divided into three parts (MG1, MG2, and MG3). The hindgut is divided into two parts due to its different morphology (HG1 and HG2). The HG2 content was black. FG, foregut; MG, midgut; HG, hindgut; PY, pylorus.
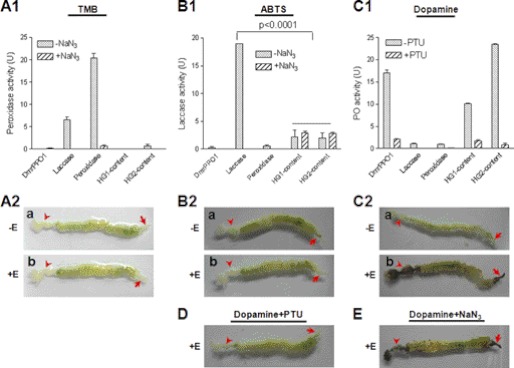
PPO is a key enzyme in the silkworm that induces melanization around wounds and invading foreign particles (8). Similar to PPO, laccase and peroxidase also oxidize some phenols (10); thus, it was necessary to investigate which of these redox enzymes is responsible for the melanization of feces. When diluted 3,3′,5,5;-tetraMethylBenzidine (TMB) solution (1:50), which is a peroxidase substrate, was incubated with a commercial preparation of peroxidase (1.67 ng), it resulted in very high enzyme activity (Fig. 2A1). Laccase (3 μg) also oxidized TMB, but the activity was significantly lower than 1.67 ng of peroxidase. Purified recombinant Drosophila PPO1 (DmrPPO1, 1.25 μg) did not oxidize TMB after the PPO had been activated by ethanol (16). No peroxidase activity was present in HG1 and HG2 of the hindgut (Fig. 2A1). When ABTS solution (a laccase substrate) was added to DmrPPO1 or peroxidase, neither of them could oxidize this substrate (Fig. 2B1). Significant low laccase-like enzyme activity was observed in HG1 and HG2, but when NaN3 was added, they had almost the same activity as the group that did not receive NaN3, suggesting that ABTS oxidization was not due to laccase, as NaN3 is a potent inhibitor of laccase activity (27). Further, a Western blot assay using M. sexta laccase antibody did not detect any positive band in the hindgut content (supplemental Fig. S1). When dopamine, a very good PPO substrate, was added to different samples, DmrPPO1 oxidized the substrate very efficiently (Fig. 2C1). However, laccase and peroxidase minimally oxidized dopamine.
FIGURE 2.
Comparison of the enzyme activity of three oxidases and gut staining. DmrPPO1 was activated by ethanol (E) to have PO activity (16). A1 and A2, peroxidase activity detection. Peroxidase (1.67 ng) and laccase (3 μg) but not DmrPPO1 (1.25 μg) oxidized TMB. No peroxidase activity was detected in HG1 or HG2 content (A1) or in different parts of gut (A2). B1 and B2, laccase activity detection. DmrPPO1 and peroxidase only minimally oxidized ABTS. Some activities were detected in the HG1 and HG2 contents, but they were not inhibited by NaN3 (B1). The real laccase activity was significantly inhibited by NaN3. No laccase activity was detected in the gut (B2). C1 and C2, PO activity detection. Laccase and peroxidase minimally oxidized dopamine. HG1 and HG2 content had obviously high PO activity (C1). The foregut and hindgut stained black only if ethanol was used for activation (C2-b was imaged at 30 min). D and E, effects of laccase and peroxidase inhibitor NaN3 and PO inhibitor PTU on gut staining. PTU significantly inhibited melanization in the foregut and hindgut (D), whereas NaN3 did not (E). Columns represent the mean of individual measurements ± S.E. (n = 3). Significant differences were calculated with an unpaired t test program.
Larval guts were stained using the above substrates with or without addition of ethanol. When TMB (Fig. 2A2) and ABTS (Fig. 2B2) were used, neither peroxidase nor laccase activity were detected in gut tissues. No staining occurred when dopamine was used if ethanol was absent (Fig. 2, C2-a). However, the foregut and hindgut were stained black if ethanol was added (Fig. 2, C2-b). When phenylthiourea (PTU) (strong PO activity inhibitor) but not NaN3 (laccase and peroxidase inhibitor) was added, melanization of the foregut and hindgut was clearly inhibited (Fig. 2, D and E). Taken together, these observations demonstrate that hindgut content melanization is a result of PPO activity, and accordingly that PPO might be present in the hindgut.
Typical insect larvae of different Orders including Diptera (Drosophila melanogaster and Culex pipiens quinquefasciatus), Isoptera (Coptotermes formosanus), Orthoptera (Dianemobius nigrofasciatus), Coleoptera (Tribolium castaneum), and Lepidoptera (Ostrinia furnaclis and Heliothis armigero) were dissected and stained as shown in Fig. 2, C2-b. Both the foregut and hindgut of all different species were stained black (supplemental Fig. S2), suggesting that PPO is present in the guts (foregut and hindgut) of other insect species as well.
PPO in the Silkworm Hindgut
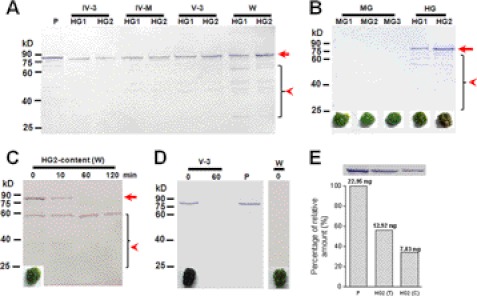
The larval hindgut (HG1 and HG2) was dissected out from larvae of different stages and then used for Western blot assay to detect PPO. The PPO protein was detected in hindgut tissues, and the band was at the corresponding position as plasma PPO (Fig. 3A). In wandering-stage larvae, many bands smaller than PPO appeared (<65 kDa). When sections of the hindgut were cultured in vitro, PPO was released into the culture medium according to native gel assay (supplemental Fig. S3). No PPO was found when sections of the midgut and silk gland were cultured.
FIGURE 3.
Western blot analysis of PPO in the hindgut and hindgut content of silkworm larvae. A, PPO was detected in the hindgut (HG1 and HG2) from larvae at different developmental stages. B, PPO was present in the contents of both hindgut regions, but was a little degraded during preparation. Corresponding gut content is shown under each lane. C, quick degradation of PPO in HG2 contents from larvae on wandering-stage. HG2 contents were suspended and incubated at room temperature for different times. PPO was almost degraded within 10 min. The arrow indicates PPO. The arrowhead indicates degraded PPO bands. D, PPO detected in feces. PPO was detected in freshly excreted feces from larvae on V-3 (black feces) but not on wandering-stage (green feces) by Western blot. E, comparison of PPO in plasma (0.5 μl), HG2 tissue supernatant (HG2(T), 10 μg), and HG2 content (HG2(C), collected from five larvae, with 10% of the suspension solution loaded). Band intensities were normalized to the amount of PPO in plasma in lane 1. About 45.9 ng of purified PPO was found in 1 μl of plasma (28). There was 12.92 ng of PPO in 10 μg of HG2 tissue supernatant. The amount of PPO in H2 content was 15.7 ng (7.83 ng × 10 ÷ 5) on average. P: plasma (V-3).
Midgut and hindgut contents (of V-3 larvae) were suspended in Tris buffer for a Western blot assay. We found a PPO signal in the hindgut contents, and many smaller bands were detected in HG2 (Fig. 3B). PPO from V-3 larvae was found to be degraded very slowly (supplemental Fig. S4), whereas that in HG2 content from wandering-stage larvae degraded very rapidly (Fig. 3C). No PPO was detected in the midgut content. Feces excreted by V-3 larvae were collected immediately (0 min) and 60 min later. The Western blot results showed a PPO band when feces were collected at 0 min but not at 60 min after excretion (Fig. 3D), suggesting that activated PO had already been bound to other proteins to form a large complex that cannot be easily extracted from feces and, thus, was not detected. Green feces excreted by wandering-larvae were immediately collected, but no PPO band was detected (Fig. 3D). PPO in the HG2 part of the hindgut during the wandering-stage may also be degraded quickly in vivo. PPO in plasma, HG2 tissue lysate, and HG2 content (on V-3) were compared by Western blot (Fig. 3E). Plasma contained 45.9 ng/μl PPO after purification (28). The amount of PPO in HG2 content (equal to a feces) was about 15.7 ng on average.
PPO Is Produced by Hindgut Cells but Not Contamination from Hemolymph
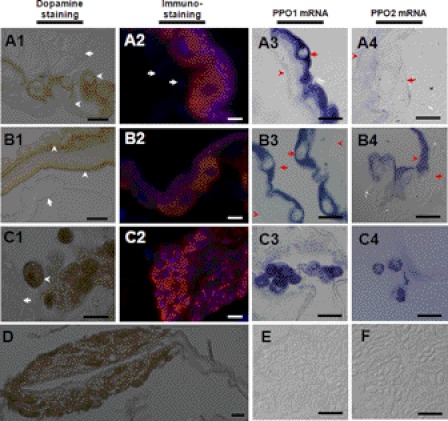
Hindguts of the silkworm (Fig. 2, C2-b) and other insects (supplemental Fig. S2) were stained black by a mixture of dopamine and ethanol, indicating that PPO is produced in those tissues. Large epidemical cells in the HG1 and HG2 were positively stained with dopamine after ethanol activation (Fig. 4, A1 and B1). Many small cells (Fig. 4, C1) and some cyst-like tissues in HG2 (Fig. 4D) were also positively stained by dopamine. The small cells in HG2 (Fig. 4, C1) might be released from the cyst-like tissue. However, tissues such as muscle (Fig. 4, A1) and Malpighian tubules (Fig. 4, B1 and C1) were not positively stained in HG1 and HG2. The immunostaining results also indicated that PPO was localized to some large and small cells in the hindgut but not in muscle cells (Fig. 4, A2, B2, and C2). In situ assays showed strong PPO1 mRNA signals in large cells of HG1 and HG2 (Fig. 4, A3 and B3). However, the signal for PPO2 was extremely weak in large epidemical cells (Fig. 4, A4 and B4). PPO1 and PPO2 mRNA were clearly identified in small cells in HG2 (Fig. 4, C3 and C4) but were not detected in the midgut as a control (Fig. 4, E and F). No signal was observed when sense RNA probe was used (data not shown).
FIGURE 4.
PPO is produced by hindgut cells. (A1, A2, A3, A4) HG1; (B1, B2, B3, B4, C1, C2, C3, C4) HG2. (A1, B1, C1, D) hindguts were sectioned for morphological observation after dopamine staining. Large epidemical cells in HG1 and HG2 stained brown, indicating activated PO oxidation of substrates. Some positively stained small cells were found in HG2 (C1), which were probably released from cyst-like tissue (D). Arrows indicate negatively stained muscle (A1), Malpighian tubules (B1 and C1). Arrowheads indicate positively stained large epidemical cells and membrane-like structures. (A2, B2, C2) immunostained cells in hindguts. Arrows indicate muscle cell that were not labeled by PPO antibody. Many small cells in the cyst were also positively stained (C2). (A3, B3, C3, A4, B4, C4, E, F) PPO1 and PPO2 mRNA levels in hindguts (A3, B3, C3, A4, B4, C4) and midguts (E, F) by in situ hybridization. PPO1 mRNA was observed in the arrow-indicated HG1 (A3) and HG2 large epidemical cells (B3). The PPO2 signal was extremely weak in large epidemical cells (A4, B4) (arrow-indicated). PPO1 (C3) and PPO2 (C4) mRNA was observed in small cells in HG2. No PPO1 (E) and PPO2 mRNA (F) was observed in the midgut. In A3, A4, B3, B4, the arrows indicate membrane-like structures. This work was repeated at least three times with similar results. Bars: C1, A2, B2, C2: 10 μm; all others: 20 μm.
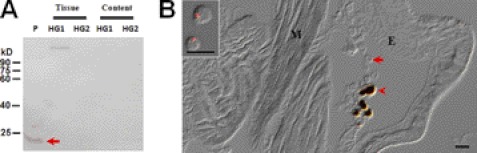
Lysozyme is a 14-kDa plasma protein that can be induced by prior immunization (29). We used this protein as a probe to monitor whether the dissection could cause plasma protein contamination and whether the hemolymph materials can freely enter the hindgut. Samples from plasma, HG1 and HG2 tissues, and its contents were separated by Western blot after V-3 silkworm larvae were injected with E. coli. The results showed lysozyme in plasma but not in hindgut tissues or its contents (Fig. 5A), indicating no contamination and no direct physical connection between hemolymph and HG1 or HG2. When hemocytes were labeled with injected fluorescent beads, none of the beads phagocytosed by hemocytes were detected inside the HG2 small cells (Fig. 5B), suggesting that the small cells were other cells than hemocytes. In Drosophila, no hemocytes were detected in hindgut epithelium through a transgenic analysis (30), which provides some evidence that the small cells observed in the silkworm HG2 were not hemocytes.
FIGURE 5.
PPO in hindgut is not from hemolymph contamination. A, lysozyme was used as a probe to detect whether there is a physical connection between the hemolymph and hindgut. V-3 silkworm larvae were injected with dead E. coli. The hindguts and its contents were sampled after 12 h and treated as in Fig. 3. Lysozyme was found in plasma but not in the hindguts or its contents by Western blot assay. B, small cells inside HG2 were not labeled by the injected fluorescent beads. The phagocytosed fluorescent beads by hemocytes were used as probes to monitor hemocytes movement (17). After injection of fluorescent beads as previously described (17), circulating hemocytes were observed to have the phagocytosed beads (inset). However, no signal was detected inside the small cells of HG2. The arrow indicates a small cell inside HG2. Some cells became auto-melanized (arrowhead) during the preparation because of PPO activation. The images were merged from those taken using red filter and DIC optics. P: plasma; HG, hindgut; M: muscle; E: large epidemical cells. Bar: 20 μm.
When dsRNA against Tribolium TcPPO1 and TcPPO2 was injected into larvae, hindgut staining was significantly blocked (supplemental Fig. S6), indicating that those PPOs are expressed in the hindgut. Furthermore, when hemocytes were ablated in Drosophila mutant MK2, in which the source of plasma PPO is eliminated because crystal cells produce and release PPO into plasma through cell rupture (8), melanization of enterocytes was still observed in the hindgut (30), suggesting again that hindgut cells are responsible for producing PPO. Taken together, these results suggest that insect hindgut cells in addition to hemocytes produce PPO.
PPO in the Hindgut Is the Key Factor That Turns Feces Black
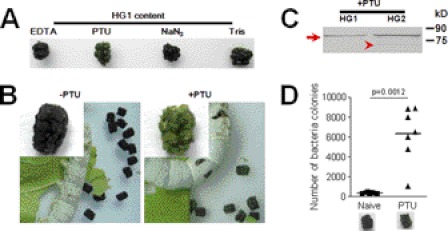
Several lines of evidence from the present study suggest that hindgut PPO is the key factor that turns feces black. First, silkworm larvae at the wandering-stage excreted green feces (Fig. 1, B-c) because the PPO in HG2 content was degraded very rapidly (Fig. 3C) and hence could not melanize the feces. We did not detect any PPO in green feces collected immediately after excretion (Fig. 3D). This was a natural control to show the importance of PPO in feces melanization. Second, HG2 proteins from V-3 larvae were identified by liquid chromatography-mass spectroscopy (LC-MS/MS), and no other enzymes that oxidize substrates to induce melanization were present except PPO (supplemental Table S2). Notably, PPO was not present in HG2 content of wandering-stage larvae (supplemental Table S3), which was due to quick degradation in vitro (Fig. 3C). Third, when HG1 contents were removed and dipped in different solutions the contents easily became black in solutions with EDTA, NaN3, and Tris buffer but not in that with PTU (Fig. 6A). Thus PTU, a strong PO inhibitor (18), obviously prevented the feces from turning black. When silkworm larvae were placed on ice after bleeding, HG1 content became black within 20 min in vivo. When PTU, NaN3, ethanol, or water was injected into larvae after bleeding, PTU alone inhibited HG1 content from becoming black. Finally, when saturated PTU was added to mulberry leaves that were fed to silkworm larvae, much of the feces excreted by PTU-fed larvae became green later (Fig. 6B), whereas larvae fed on leaves soaked with water still excreted black feces. PPO in HG1 and HG2 contents was detected when PTU was fed to silkworm larvae (Fig. 6C). Furthermore, a weak band indicating PO in HG2 was also detected.
FIGURE 6.
Melanization of silkworm feces is inhibited by the PO inhibitor PTU. A, PTU inhibited melanization of HG1 content. HG1 content was removed and dipped into different solutions. Then the contents were placed on new parafilm, and the extra solution was absorbed. HG1 content became black within 20 min after being dipped into EDTA, NaN3, and Tris solution. PTU inhibited HG1 content melanization. B, PTU solution was spread on mulberry leaves fed to the silkworm larvae. One day later, feces excreted by PTU-fed silkworm larvae were green. C, PTU did not inhibit PPO production or secretion. The arrow and arrowhead indicate PPO and PO, respectively, detected in green feces. D, feeding PTU increased the number of bacterial colonies in green feces. The excreted green feces was suspended for bacterial culture after PTU feeding. Each dot corresponds to the colonies of bacteria suspended from gut content or feces from one silkworm larva. The average for each group is indicated by a horizontal black bar (n = 7). Significant differences were calculated with an unpaired t test program.
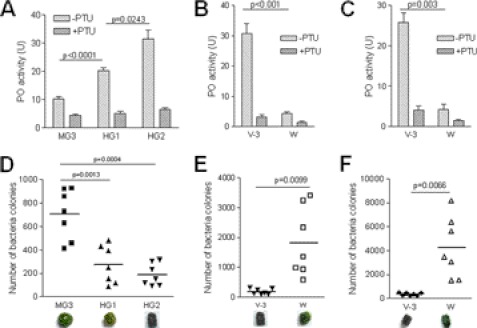
PO Activity Regulates Bacterial Number in Hindgut
Reactive compounds produced during PPO activation have broad-spectrum antibacterial activities (31). PO activity in the hindgut was significantly higher than that in the midgut (Fig. 7A). When gut bacteria were cultured, significantly fewer bacteria were found in the gut content after entering HG1 and HG2 (Fig. 7D). The PPO in HG2 content and green feces of wandering larvae degraded (Fig. 3, C and D), and the HG2 content had very low PO activity (Fig. 7B). There was significantly higher number of bacteria in HG2 content and green feces of wandering-stage larvae than in those of V-3 larvae (Fig. 7E). The same results were also observed with feces excreted by V-3 and wandering-stage larvae. Green feces excreted by wandering-stage larvae had significantly lower PO activity (Fig. 7C) but had significantly higher bacteria number (Fig. 7F) than the black feces excreted by V-3 larvae. These results indicate that PO and PO-induced melanization in the gut content and feces are advantageous for regulating the number of bacteria. When PTU was fed to silkworm larvae, there were significantly more bacteria in green feces than in black feces from naïve larvae (Fig. 6D). PTU alone had no effect on cultured bacteria.
FIGURE 7.
PO activity regulating bacteria number in hindgut and feces. A–C, PO activities were compared among different parts of gut contents from V-3 larvae (A) as well as among HG2 contents (B) and fresh feces (C) from V-3 and wandering-stage larvae, respectively. PO activities in the gut contents of V-3 larvae are: HG2>HG1>MG3 (A). PO activities in the hindgut contents (B) and fresh feces (C) of V-3 larvae were higher than in those of wandering-stage larvae, respectively. Columns represent the mean of individual animal measurements ± S.E. (n = 5). D–F, bacteria number in the gut contents and fresh feces. Bacteria colonies in the gut contents and fresh feces from V-3 and wandering-stage larvae as shown in A–C, were counted after being cultured. The bacterial number was significantly lower when PO activity was high in gut contents (D, E) and fresh feces (F). Each dot corresponds to the colonies of bacteria suspended from gut content or feces from one silkworm larva. The average for each group is indicated by a horizontal black bar (n = 7). Significant differences were calculated using an unpaired t test program.
Our analyses show that PPO in the hindgut of phytophagous insects is a key factor turning feces black, and PO-induced melanization of hindgut content effectively eliminates bacteria and controls the bacterial flora in the insect hindgut and feces. Thus, PPO produced by hindgut cells can effectively maintain the hindgut innate immunity to regulate bacteria number in hindgut and feces of the phytophagous insects.
DISCUSSION
Most insects are consuming feed that may contain pathogens. When the ingested pathogens come to the intestine and to the midgut, they are detected, eliminated, and secreted mostly without affecting and damaging the commensal and mutualist flora (4). In mammalian gut, the Toll-like and Nod-like receptors assist in recognizing and responding to pathogens that may cause gut disease and inflammation (32). However, many pathogens can still escape detection and elimination by the immune system in the small intestine and are transferred to large intestine where there are over 1011 microbe cells per gram contents (1). It is well known that mammalian fecal pathogens cause water pollution over the world and induce a lot of health problems (2). However, in contrast to the small intestine, the large intestine has no Peyer's patches (1), and it is not fully clear whether any innate immune system to defend against pathogens are present there before the feces are excreted.
Black feces excreted by insects is a very common phenomenon. Most of these insects are agricultural pests living on green leaves. Pathogens ingested via feed intake may escape the midgut innate immune system and come to the hindgut and are excreted out with the feces. Many commensal and mutualist bacteria occur in the insect midgut (3) and some are inevitably excreted with feces. Commensal and mutualist bacteria and the escaped pathogens from the midgut that enter the feces would probably pollute the larva's immediate environment including its food and cause further infection to other organisms (3). Using the silkworm B. mori as a model, we determined that PO in the hindgut plays an important role in the process that turns feces black, through which fecal pathogens and possible commensal and mutualist bacteria are eliminated to prevent such contamination.
Insect PPO is considered to be mainly produced by hemocytes (8). However, we found that PPO can be transcribed and expressed in some hindgut cells that were shown by using in situ hybridization and immunostaining analysis. PPO was also detected in the content of the hindgut, which suggests that PPO produced by hindgut cells is secreted, although the exact mechanism for this secretion is unknown. We also found that transcription of important enzymes in the plasma PPO activation pathway (e.g. PPAE and BAEE) (8) occurred in silkworm foregut and hindgut tissues (supplemental Fig. S7) but the corresponding proteins were not found in the gut content suspension (supplemental Tables S2 and S3), which suggests that the protein level was too low to be detected. In the hindguts of Drosophila mutants with developmental defects, some cells were found to be melanized upon infection or stress (30, 33), which also provides some support to the existence of PPO in Drosophila hindgut.
PPO is a very important immune protein in many invertebrate animal and when it is knocked-down by RNAi in crayfish or shrimp (34), the number of bacteria in hemolymph increases resulting in mortality. Reactive compounds produced by activated PO during melanization can kill many types of bacteria (31). During dissection and treatment, some cells in hindgut became auto-melanized (Fig. 5B and supplemental Fig. S5, A and C). When monoclonal antibody against melanin was used for immunostaining, the signal for melanin was detected in those epidemical cells (supplemental Fig. S5). A decrease in the number of bacteria in hindgut contents and feces was correlated with the PO activity and darkening. Along with the change in color may also be dehydration, which could partially be attributed to the incorporation of polymerized hydrophobic polyphenols into the feces. In addition to any toxic properties of oxidized products of phenols by PO, the dehydration might contribute to decreasing the bacterial load. This is probably the reason why there is not any hindgut specific pathogen for studying the relationship between insect fitness and hindgut PO activity. Therefore, PPO in the hindgut is a very important complement to other intestinal innate immune processes.
Many herbivorous mammalians such as sheep, cattle, and elephants also excrete black feces. Mammalian animals also have tyrosinase, which belongs to the same group of proteins as insect PPO (8). We do not know whether the mechanism is the same or if it is biologically significant. However, the hindgut innate immunity provided by the silkworm PPO for clearing bacteria in the hindgut indicates that innate immunity might occur in the mammalian large intestine.
Supplementary Material
Acknowledgment
We thank Lynn M. Riddiford for helpful comments.
This work was supported by the National Natural Science Foundation of China (30970408), National Basic Research Program of China (2012CB114605), Chinese Academy of Sciences (KSCX2-EW-J-12 and 2009OHTP05), and the Swedish Science Research Council (319-2010-6250).

This article contains supplemental Tables S1–S3 and Figs. S1–S7.
- PPO
- prophenoloxidase
- PO
- phenoloxidase
- FG
- foregut
- MG
- midgut
- HG
- hindgut
- PY
- pylorus
- PTU
- phenylthiourea
- TMB
- 3,3′,5,5;-tetramethylbenzidine.
REFERENCES
- 1. Walter J., Ley R. (2011) The human gut microbiome: ecology and recent evolutionary changes. Annu. Rev. Microbiol. 65, 411–429 [DOI] [PubMed] [Google Scholar]
- 2. Field K. G., Samadpour M. (2007) Fecal source tracking, the indicator paradigm, and managing water quality. Water Res. 41, 3517–3538 [DOI] [PubMed] [Google Scholar]
- 3. Dillon R. J., Dillon V. M. (2004) The gut bacteria of insects: nonpathogenic interactions. Annu. Rev. Entomol. 49, 71–92 [DOI] [PubMed] [Google Scholar]
- 4. Apidianakis Y., Rahme L. G. (2011) Drosophila melanogaster as a model for human intestinal infection and pathology. Dis. Model. Mech. 4, 21–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hakim R. S., Baldwin K., Smagghe G. (2010) Regulation of midgut growth, development, and metamorphosis. Annu. Rev. Entomol. 55, 593–608 [DOI] [PubMed] [Google Scholar]
- 6. Schoonhoven L. M., van Loon J. J. A., Dicke M. (2005), Insect-Plant Biology, Chapman & Hall, London [Google Scholar]
- 7. Lehane M. J., Billingsley P. B. (1996) Biology of the Insect Midgut, Chapman & Hall, London [Google Scholar]
- 8. Ashida M., Brey P. (1998) Recent Advances on the Research of the Insect Prophenoloxidase Cascade, Chapman & Hall, London [Google Scholar]
- 9. Cerenius L., Kawabata S., Lee B. L., Nonaka M., Söderhäll K. (2010) Proteolytic cascades and their involvement in invertebrate immunity. Trends Biochem. Sci. 35, 575–583 [DOI] [PubMed] [Google Scholar]
- 10. Kanost M. R., Gorman M. J. (2008) Phenoloxidases in Insect Immunity, Academic Press & Elsevier, San Diego [Google Scholar]
- 11. Kanost M. R., Jiang H., Yu X. Q. (2004) Innate immune responses of a lepidopteran insect, Manduca sexta. Immunol. Rev. 198, 97–105 [DOI] [PubMed] [Google Scholar]
- 12. Strand M. R. (2008) The insect cellular immune response. Insect Science 15, 1–14 [Google Scholar]
- 13. Wittkopp P. J., Beldade P. (2009) Development and evolution of insect pigmentation: genetic mechanisms and the potential consequences of pleiotropy. Semin. Cell Dev. Biol. 20, 65–71 [DOI] [PubMed] [Google Scholar]
- 14. Hiruma K., Riddiford L. M. (2009) The molecular mechanisms of cuticular melanization: the ecdysone cascade leading to dopa decarboxylase expression in Manduca sexta. Insect Biochem. Mol. Biol. 39, 245–253 [DOI] [PubMed] [Google Scholar]
- 15. Xia Q., Zhou Z., Lu C., Cheng D., Dai F., Li B., Zhao P., Zha X., Cheng T., Chai C., Pan G., Xu J., Liu C., Lin Y., Qian J., Hou Y., Wu Z., Li G., Pan M., Li C., Shen Y., Lan X., Yuan L., Li T., Xu H., Yang G., Wan Y., Zhu Y., Yu M., Shen W., Wu D., Xiang Z., group G. a., Yu J., Wang J., Li R., Shi J., Li H., Li G., Su J., Wang X., Li G., Zhang Z., Wu Q., Li J., Zhang Q., Wei N., Xu J., Sun H., Dong L., Liu D., Zhao S., Zhao X., Meng Q., Lan F., Huang X., Li Y., Fang L., Li C., Li D., Sun Y., Zhang Z., Yang Z., Huang Y., Xi Y., Qi Q., He D., Huang H., Zhang X., Wang Z., Li W., Cao Y., Yu Y., Yu H., Li J., Ye J., Chen H., Zhou Y., Liu B., Wang J., Ye J., Ji H., Li S., Ni P., Zhang J., Zhang Y., Zheng H., Mao B., Wang W., Ye C., Li S., Wang J., Wong G. K.-S., Yang H. (2004) A draft sequence for the genome of the domesticated silkworm (Bombyx mori). Science 306, 1937–1940 [DOI] [PubMed] [Google Scholar]
- 16. Li X., Ma M., Liu F., Chen Y., Lu A., Ling Q. Z., Li J., Beerntsen B. T., Yu X. Q., Liu C., Ling E. (2012) Properties of Drosophila melanogaster prophenoloxidases expressed in Escherichia coli. Dev. Comp. Immunol. 36, 648–656 [DOI] [PubMed] [Google Scholar]
- 17. Ling E., Shirai K., Kanekatsu R., Kiguchi K., Kobayashi Y., Funayama T., Watanabe H. (2006) Contribution of circulating hemocytes to the regeneration of heavy ion beams 12C5+ irradiated hematopoietic organs in the silkworm, Bombyx mori, through the way of phagocytosis of injured cells after invasion. Dev. Comp. Immunol. 30, 531–543 [DOI] [PubMed] [Google Scholar]
- 18. Ryazanova A. D., Alekseev A. A., Slepneva I. A. (2012) The phenylthiourea is a competitive inhibitor of the enzymatic oxidation of DOPA by phenoloxidase. J. Enzym. Inhib. Med. Chem. 27, 78–83 [DOI] [PubMed] [Google Scholar]
- 19. Wang Z., Lu A., Li X., Shao Q., Beerntsen B. T., Liu C., Ma Y., Huang Y., Zhu H., Ling E. (2011) A systematic study on hemocyte identification and plasma prophenoloxidase from Culex pipiens quinquefasciatus at different developmental stages. Exp. Parasitol. 127, 135–141 [DOI] [PubMed] [Google Scholar]
- 20. Asano T., Takebuchi K. (2009) Identification of the gene encoding pro-phenoloxidase A(3) in the fruitfly, Drosophila melanogaster. Insect Mol. Biol. 18, 223–232 [DOI] [PubMed] [Google Scholar]
- 21. Tan A., Tanaka H., Sato N., Yaguchi M., Nagata M., Suzuki K. (2003) Identification of novel tissue-specific proteins in the suboesophageal body of the silkworm, Bombyx mori. J. Insect Biotechnol. Sericol. 72, 41–50 [Google Scholar]
- 22. Dittmer N. T., Gorman M. J., Kanost M. R. (2009) Characterization of endogenous and recombinant forms of laccase-2, a multicopper oxidase from the tobacco hornworm, Manduca sexta. Insect Biochem. Mol. Biol. 39, 596–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fouda M. M., Hiragaki S., Tufail M., Shao Q. M., Takeda M. (2010) Precursor structure, distribution, and possible functions of pigment-dispersing hormone (PDH) in the terrestrial isopod Armadillidium vulgare (Latreille). J. Insect Physiol. 56, 1728–1737 [DOI] [PubMed] [Google Scholar]
- 24. Jiang H., Wang Y., Ma C., Kanost M. R. (1997) Subunit composition of pro-phenol oxidase from Manduca sexta: molecular cloning of subunit ProPO-P1. Insect Biochem. Mol .Biol. 27, 835–850 [DOI] [PubMed] [Google Scholar]
- 25. Nosanchuk J. D., Yu J. J., Hung C. Y., Casadevall A., Cole G. T. (2007) Coccidioides posadasii produces melanin in vitro and during infection. Fungal Genet. Biol. 44, 517–520 [DOI] [PubMed] [Google Scholar]
- 26. Ling E., Ao J., Yu X. Q. (2008) Nuclear translocation of immulectin-3 stimulates hemocyte proliferation. Mol Immunol. 45, 2598–2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dittmer N. T., Kanost M. R. (2010) Insect multicopper oxidases: diversity, properties, and physiological roles. Insect Biochem. Mol. Biol. 40, 179–188 [DOI] [PubMed] [Google Scholar]
- 28. Ashida M. (1971) Purification and characterization of pre-phenoloxidase from hemolymph of the silkworm Bombyx mori. Arch. Biochem. Biophys. 144, 749–762 [DOI] [PubMed] [Google Scholar]
- 29. Gillespie and J. P., Kanost M. R., Trenczek T. (1997) Biological mediators of insect immunity. Annu. Rev. Entomol. 42, 611–643 [DOI] [PubMed] [Google Scholar]
- 30. Seisenbacher G., Hafen E., Stocker H. (2011) MK2-dependent p38b signaling protects Drosophila hindgut enterocytes against JNK-induced apoptosis under chronic stress. PLoS Genet. 7, e1002168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhao P., Li J., Wang Y., Jiang H. (2007) Broad-spectrum antimicrobial activity of the reactive compounds generated in vitro by Manduca sexta phenoloxidase. Insect Mol. Biol. 37, 952–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Carvalho F. A., Aitken J. D., Vijay-Kumar M., Gewirtz A. T. (2012) Annu. Rev. Physiol. 74, 177–198 [DOI] [PubMed] [Google Scholar]
- 33. Chen J., Xie C., Tian L., Hong L., Wu X., Han J. (2010) Participation of the p38 pathway in Drosophila host defense against pathogenic bacteria and fungi. Proc. Natl. Acad. Sci. U.S.A. 107, 20774–20779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu H., Jiravanichpaisal P., Cerenius L., Lee B. L., Söderhäll I., Söderhäll K. (2007) Phenoloxidase is an important component of the defense against Aeromonas hydrophila Infection in a crustacean, Pacifastacus leniusculus. J. Biol. Chem. 282, 33593–33598 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.