Background: Currently, the only known treatment for scorpion envenomation is serotherapy with a heterologous polyclonal antibody displaying low specificity.
Results: The injection of a diabody mixture allowed mice to survive in a test that mimics severe envenomation with the crude venom.
Conclusion: The diabody mixture displayed better protective power than any other known remedy.
Significance: This could herald the next generation of antivenoms.
Keywords: Antibody Engineering, Immunotherapy, Neurotoxin, Protein Expression, Protein Purification, Diabody, Scorpion, Venom
Abstract
Androctonus australis is primarily involved in envenomations in North Africa, notably in Tunisia and Algeria, and constitutes a significant public health problem in this region. The toxicity of the venom is mainly due to various neurotoxins that belong to two distinct structural and immunological groups, group I (the AahI and AahIII toxins) and group II (AahII). Here, we report the use of a diabody mixture in which the molar ratio matches the characteristics of toxins and polymorphism of the venom. The mixture consists of the Db9C2 diabody (anti-group I) and the Db4C1op diabody (anti-AahII), the latter being modified to facilitate in vitro production and purification. The effectiveness of the antivenom was tested in vivo under conditions simulating scorpion envenomation. The intraperitoneal injection of 30 μg of the diabody mixture protected almost all the mice exposed to 3 LD50 s.c. of venom. We also show that the presence of both diabodies is necessary for the animals to survive. Our results are the first demonstration of the strong protective power of small quantities of antivenom used in the context of severe envenomation with crude venom.
Introduction
Scorpion envenomation is widespread in several countries, where it still constitutes a significant public health problem. Of the 1500 species described worldwide, 30 scorpions are considered to be potentially dangerous to human beings, and each year more than 1.2 million scorpion stings are reported and known to cause more than 3250 deaths (1, 2). The epidemiological pattern is fairly similar in many countries, but the incidence and severity of envenomation are closely correlated to the geographical area under consideration. Among the general population, the incidence varies from five scorpion stings per 100,000 inhabitants in the South of France, producing mild symptoms, to more than 1000 per 100,000 in Algeria, Tunisia, Mexico, and Israel (3). However, in these severely affected countries the mortality is often under-reported, because in many cases death does not occur in a hospital, and epidemiological data are scarce because of under-reporting and the small number of studies related to scorpion envenomation that have been reported. Like Leiurus quinquestratus and Androctonus mauretanicus, Androctonus australis hector is considered to be one of the most dangerous scorpions, responsible for serious and fatal events in humans (4). According to Adi-Bessalem et al. (5), the pathophysiological effects of A. australis hector venom lead to tissue damage and an inflammatory response. A. australis is mainly involved in envenomations in North Africa, especially in Tunisia and Algeria, where the annual incidence of scorpion stings is of 420 per 100,000 inhabitants and 120 per 100,000 inhabitants, respectively (6–8).
The active substances in scorpion venom are neurotoxic peptides. Although these peptides are only present in small amounts (<5% of venom dry weight), they are responsible for almost all fatal cases in mammals. The toxicity of A. australis hector venom is mainly due to three basic low molecular weight (∼7 kDa) neurotoxins that act on the voltage-gated sodium channels of excitable cells. These toxins belong to two distinct structural and immunological groups as follows: (i) group I contains AahI2 and AahIII, which display up to 80% sequence identity; and (ii) group II contains AahII, which shares only 44–45% sequence identity with AahI and AahIII (9, 10). No cross-antigenicity has been reported between these two groups of toxins (11). These toxins have been relatively well characterized for many years; their concentrations have been determined, and the variations observed reflect the polymorphism of scorpion toxins at an individual level. This is important because this functional and antigenic polymorphism is a problem in preparing an effective antivenom (11). The proportions of the three toxins differ in different venoms. The AahII toxin is the most abundant, followed by AahI, and then AahIII (12). AahII seems to be responsible for 70% of the toxicity and produces the greatest toxic effect, regardless of the route of injection (intracerebroventricular or subcutaneous (s.c.)) in mice. The LD50 of an s.c. injection in mice weighing 20 g is 180 ng for AahII versus 380 and 420 ng for AahI and AahIII, respectively (13).
At present, the only treatment for scorpion envenomations used worldwide is passive immunotherapy, which is based on the administration of antibodies produced by a hyperimmunized animal against the venom. However, the use of plasma antivenom serum therapy remains controversial (14). Plasma antivenom serum always has some disadvantages, such as serious undesirable side effects, including anaphylactic shock and serum sickness. Moreover, previous pharmacokinetic studies of venom components have shown that toxins diffuse rapidly from the bloodstream into the tissues, and various factors, such as the antibody form used and the route or the timing of administration of current anti-venom therapy, can limit the clinical efficacy of this treatment (15–18).
In recent years, antibodies have been engineered, and many studies have focused on novel anti-scorpion toxin antibody formats with improved pharmacokinetic properties (greater stability and faster tissue penetration), higher binding affinity, and neutralizing effects. In particular, the use of smaller recombinant antibody fragments, such as scFv or Fab and more recently VHH, has made it possible to achieve more efficient neutralization of scorpion venom toxins. Indeed, each of them has been shown to protect mice experimentally challenged with a single toxin (19–26). However, because of the absence of cross-antigenicity between the different toxins (AahII, AahI, and/or AahIII), the main drawback of these antibody fragments remains their failure to protect mice challenged with the whole venom.
In 2007, using the monoclonal antibodies 9C2 and 4C1, which neutralize the AahI and AahII toxins, respectively, we designed for the first time a bispecific tandem-scFv (T94H6), which is able to not only neutralize the most potent toxins in A. australis venom but also to protect experimentally envenomed mice against the overall toxicity of the venom (27). Other groups have subsequently confirmed the usefulness of this new generation of bispecific antivenoms with a bispecific nanobody (28).
Despite the greatly increased protection they provide, we believe that bispecific fragments are not a completely appropriate response to provide effective protection against the whole venom. Indeed, in this context, one bispecific fragment is theoretically capable of neutralizing a single toxin in each group in a one to one ratio without taking into account the following: (i) the affinity of the scFvs against the toxin, (ii) the distinctive concentrations of toxins, or (iii) the degree of toxicity of each toxin.
To remedy this limitation of the current bispecific recombinants, we propose to develop a new strategy based on the use of a mixture of the following two diabodies: Db9C2 (29) directed against group I, and a new antibody fragment, Db4C1op, directed against group II, which includes the most poisonous scorpion venom toxins AahII. This approach allows us to adjust the quantities of the different antibodies, with a molar ratio in which Db4C1op dominates. To design Db4C1op, we optimized the scFv4C1 described by Mousli et al. (24); this allowed us to optimize its production and, especially, for the first time to purify it. Db4C1op was then characterized structurally and functionally before being used in a mixture with Db9C2 to protect mice challenged with whole venom under conditions that mimic natural envenomation. By this method, we obtained better protection of mice against whole A. australis venom than is provided by other strategies previously described in the literature.
EXPERIMENTAL PROCEDURES
Animals
Female C57BL/six mice (20 ± 2 g body weight) were obtained from Janvier (France). Animals were cared for in accordance with European Guidelines on animal welfare (2010/63/UE). The mice were housed in the conventional animal facilities of our laboratory and received water and food ad libitum before being used for the study.
Venom and Toxins
A. australis hector venom was collected in the Chellala area of Algeria before being water-extracted, freeze-dried, and stored at −20 °C until use (30). AahII toxin was isolated and purified from A. autralis hector venom as described previously (13). The toxin concentration of each solution was determined by ELISA. Two assays were designed for this purpose. AahI was measured using the 2G3 mAb as a capture antibody and the biotinylated 9C2 mAb as a detecting antibody (31). AahII was measured using polyclonal IgG anti-AahII and biotinylated 4C1 mAb (12).
Antitoxin Antibodies
The cDNA sequences of antibody 4C1/9C2 VH and VL are registered in the EMBL Data Bank (accession numbers Y17588 and Y17589 (24)/AJ278443 and AJ278442 (23)). Recombinant anti-AahI antibody fragment derived from hybridoma 9C2 ((scFv59C2)2 (also known as diabody Db9C2)) was produced and purified as described previously (29).
Oligonucleotides
All oligonucleotides were synthesized (Eurogentec), and their sequences are shown below. The restriction sites of interest (BamHI (GGATCC) and XhoI (CTCGAG)) are underlined: Link5R4C1, 5′-AGT GTC GGA TCC GAT GTT CAG ATG ACC CAG; VLF4C1, 5′- CTG TAG CTC GAG TTA TTT GAT TTC CAG TTT GGT GCC.
Plasmid Construction
The optimized synthetic gene scFv4C1op (anti-AahII antibody) fused to the mrc-ox74 epitope tag was assembled from synthetic oligonucleotides and PCR products. The fragment was cloned into pMA (AmpR) using the SacI and KpnI cloning sites (Invitrogen). The synthetic gene was then subcloned in-frame with the leader sequence pelB into the expression vector pSW1 using PstI and XhoI restriction enzymes, as described previously (29). Finally, the plasmid pSW1-4C1op was used as a template to create the diabody-4C1op gene (Db4C1op) in a PCR to modify the flanking regions of the gene encoding the 4C1op VL domain with the primers VLF4C1, to delete the MRC-OX74 sequence, and Link5R4C1, to generate subsequently a short rigid (Gly4-Ser) intramolecular linker. These primers carry unique restriction sites for BamHI and XhoI suitable for cloning in pSW1–4C1op.
Escherichia coli strain TG1 was used for all the cloning steps. The sequences for all newly constructed genes and plasmids were confirmed by DNA sequencing (Cogenics Online). All basic molecular biology procedures were carried out as described by Sambrook and Russel (32). Taq polymerase, restriction enzymes, calf intestinal phosphatase, and T4 DNA ligase were from Promega. All chemicals were of standard grade from Sigma or equivalent.
Protein Expression and Purification
For expression of functional recombinant antibody fragments in the bacterial periplasm, the plasmids pSW1-Db4C1op and pSW1-Db9C2 were first cloned into E. coli strain HB2151 (K12, ara, D(lac-pro), thi/F′ proA+B+, laclq lacZDM15). Transformed bacteria were grown at 37 °C to A600 nm = 1.2 in 2× YT medium (Sigma) containing 0.05 g/liter ampicillin (Euromedex). Isopropyl β-d-thiogalactoside (Euromedex) was then added to yield a final concentration of 0.1 and 0.84 mm for pSW1-Db4C1op and pSW1-Db9C2, respectively, and growth was then continued at 16 °C for 18 h. The bacterial cells were harvested by centrifuging at 4 °C (5000 × g, 20 min), and periplasmic extracts were prepared as described previously (29). All the soluble periplasmic proteins were extensively dialyzed against phosphate-buffered saline (PBS), pH 7.4, and finally centrifuged (10,000 × g, 4 °C, 30 min). The recombinant diabodies (Db4C1op and Db9C2) were purified by loading the periplasmic preparations extracted onto a protein l-agarose column (Thermo Fisher Scientific). After washing the gel with PBS, the adsorbed protein was eluted in different fractions with 0.1 m glycine, pH 3, and immediately neutralized with 1 m Tris, pH 8.9. The elution fractions containing the functional recombinant protein were pooled and dialyzed against PBS, pH 7.9, for buffer exchange, before being centrifuged (10,000 × g, 4 °C, 10 min), and then stored at −20 °C. The purification process was also checked using SDS-PAGE on homogeneous reduced 12% gel, Coomassie Brilliant Blue, and direct ELISA using either AahII toxin, as reported previously by Devaux et al. (23). Swiss Institute of Bioinformatics software (ProtParam tool) was used to determine the theoretical molecular weight of the recombinant diabodies and their extinction coefficients (33). Thus, the protein content of the solution was measured by UV spectrophotometry at A280 nm = 1.783 liter g−1 cm−1 for the Db4C1op (51705.4 Da) and A280 nm = 2.111 liter g−1cm−1 for Db9C2 (49858.8 Da).
The purified diabody preparations were resolved by size-exclusion FPLC on a Superdex 75 HR 10/30 column (molecular mass range 3000–70,000) (Amersham Biosciences) calibrated with standards from Roche Applied Science. The column was loaded with 20 μg of the sample to be analyzed. Proteins were eluted with PBS at a rate of 0.5 ml/min and detected with a UV detector at 280 nm.
Antigen-binding Analysis by FPLC
To form immunocomplexes, 9.03 μg (193 pmol) of the Db4C1op was incubated with 0–2.53 μg (0–387 pmol) of toxin AahII in 125 μl of PBS for 1 h at 37 °C. Samples were analyzed by FPLC gel filtration using the Superdex 75 HR 10/30 column (Amersham Biosciences).
Protective Activity after Experimental Envenomation
Female C57BL/six mice weighing ± 20 g were used for in vivo protection assays. First, the whole A. australis hector venom was titrated to determine the LD50 in mice by subcutaneous injection. A variable quantity of the A. australis venom was then injected in a volume of 200 μl into mice by subcutaneous route. Immediately afterward or several minutes later (as indicated), each diabody singly or the mixture of diabodies (Db4C1op and Db9C2) was intraperitoneally injected into mice in a volume of 200 μl. Eight animals were used for each test condition; the clinical signs and survival ratio were recorded for 24 h after injection. In control experiments, the mixture of diabodies was replaced by 0.1% BSA.
RESULTS
Design and Construction of Recombinant scFvs
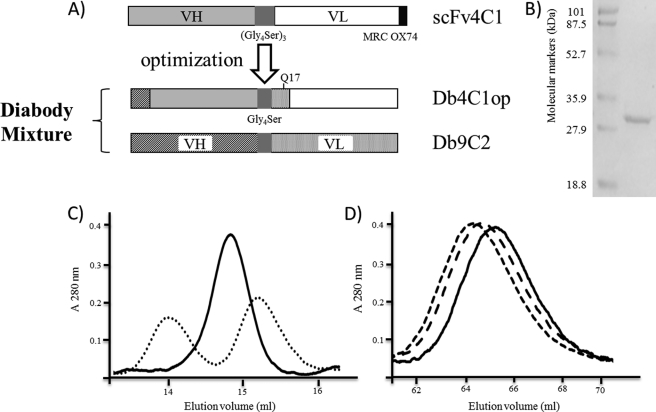
To increase the scFv4C1op yield and to obtain a purified product, the following changes were made (Fig. 1A). First, the scFv4C1op amino acid sequence was engineered to produce the following changes. The VH (EVHLVE) and VL (DVLMTQSPLSLPVSLGDQASIS) regions of scFv4C1 were replaced by the N-terminal regions of Db9C2 (QVQLQQ and DVQMTQSPASLSVSGQTVTIT, respectively), plus the E17Q substitution in the VL framework 1 allowing the promotion of PpL binding activity (34). Second, from the previous amino acid sequence, a synthetic gene was performed according to GeneArt. This gene was optimized for the prokaryotic expression system before being inserted into the bacterial expression vector pSW1 consisting of the pelB signal sequence, which was expected to direct the nascent recombinant protein to the periplasm of the bacteria. To generate Db4C1op, the pSW1-Db4C1op expression vector was designed after constructing a coding sequence and then cloned into pSW1–4C1op as a BamHI/XhoI insert. The genetic construction consisted of a PCR amplification of a cassette encoding part of the (Gly4-Ser) linker and the entire light-chain variable region of antibody 4C1 with primers VLF4C1 and Link5R4C1. Following restriction with BamHI and XhoI, this gene was inserted into the pSW1–4C1op vector restricted in the same way. The vector constructed was designated pSW1-Db4C1op. The recombinant clones carrying the plasmid containing the insert fused in-frame with the PelB sequence were selected by PCR screening, and DNA sequencing was performed to verify that no mutation appeared (Fig. 1A). pSW1-Db4C1op encodes diabody 4C1op, free of tag, in which the VH C-terminal Ser-128 is joined to the VL N-terminal D1 according to IMGT® by the short rigid (Gly4-Ser) intramolecular linker. This linker creates a steric constraint that is predicted for the association between two molecules of Db4C1op.
FIGURE 1.
Engineering of recombinant antibody fragment Db4C1, purification, and characterization of the Db4C1op (SDS-PAGE and size-exclusion chromatography on a calibrated Superdex 75 HR 10/30 column of the protein l-agarose). A, schematic representation of the optimized scFv4C1 with a peptide linker of 15 residues between the VH and VL cassettes and peptide flag MRC OX74. The VH and VL N-terminal regions of scFv4C1 were replaced by the N-terminal regions of the Db9C2, conferring a protein-L recognizing capacity to the scFv4C1 fragment. A five-residue linker between the VH and VL sequences was used to obtain the diabody format (Db4C1), a bivalent structure. B, SDS-PAGE of the Db4C1op preparation stained with Coomassie Brilliant Blue. C, chromatography of affinity-purified Db4C1op (solid line) and scFv4C1 eluted as monomers or dimers (dotted line). D, chromatography of affinity-purified Db4C1op complexed with AahII in a molar ratio of 1:2 (fine dashed line), 2:1 (broad dashed line), 1:0 (solid line).
Bacterial Expression and Purification and Structural Characterization of Db4C1op
Because the structural properties of Db9C2 have already been extensively characterized as described in Aubrey et al. (29), in this part of the study we focused on the new antibody fragment format, Db4C1op.
The Db4C1op gene was expressed by growing the recombinant HB2151 bacteria induced with isopropyl β-d-thiogalactoside. The experimental conditions described under “Experimental Procedures” were selected after testing several induction conditions. As related previously, there are no standard conditions suitable for the periplasmic production of all kinds of periplasmic scFv in bacteria. Soluble proteins were extracted from the periplasm of induced bacteria by EDTA treatment combined with osmotic shock, and the purification of Db4C1op fragments was achieved by affinity chromatography on a protein l-agarose gel column, which had previously been shown to be effective for the purification of other scFv and Fab molecules. Yields of 0.3–0.85 mg/liter of bacterial culture of soluble affinity-purified Db4C1op were normally obtained. The Db4C1op preparation was then analyzed by SDS-PAGE and size-exclusion chromatography. The electrophoresis of the Db4C1op preparation under reducing conditions led to the observation of a homogeneous and pure Db4C1op, with a single protein band corresponding to an apparent molecular mass of slightly less than 31 kDa (Fig. 1B). No minor bands were detected, demonstrating the high purity of the preparations. To characterize the formation of multimeric structures, purified scFv4C1op and Db4C1op were submitted to size-exclusion chromatography on a calibrated Superdex 75 column (Fig. 1C). The analysis of the scFv4C1op, used here as a control, on the same column revealed the presence of a main peak (60%) eluted at 15.2 ml preceded by a minor peak (40%) eluted at 14 ml. These two peaks correspond to monomeric and dimeric structures, respectively. Gel filtration of the Db4C1op preparation revealed the presence of a single peak eluting at 14.8 ml corresponding to the Db4C1op homodimer. No monomeric Db4C1op fraction was eluted from the column. Both findings suggest a major trend to form dimers. This experiment was repeated several times after prolonged storage at 4 °C or at −20 °C. On each occasion, this resulted in a unique elution profile corresponding to the homodimer completely free of any other molecular mass species and no additional degradation products were observed, demonstrating the stability of the Db4C1op dimeric structure.
Antigen Binding Activity of Db4C1op
The active purified Db4C1op was controlled by a direct ELISA, revealing the Db4C1op-AahII complex by protein l-peroxidase-conjugated (Pierce, data not shown). This demonstrated that the purified Db4C1op had been correctly processed and recognized the AahII toxin in a dose-dependent manner. To find out whether the affinity-purified Db4C1op protein was functional and bivalent, a size-exclusion chromatography of the immunocomplexes formed by Db4C1op molecules and increasing amounts of toxin AahII was performed (Fig. 1D). This resulted in an increasing fraction of shifted Db4C1op due to antibody-antigen complex formation; when the molar quantity of AahII toxin added was doubled, the elution peak from the Superdex column was completely shifted to the left, and a single peak with intermediate mobility was observed when samples resulting from the incubation of Db4C1op with AahII in a molar ratio of 2 to 1 had been loaded onto the column. We concluded that both binding sites of the Db4C1op were active and that the preparation contained no detectable amount of inactive or partially active protein as Db9C2 (29). All the results seemed to show that none of the changes made previously on the scFv4C1 sequence to obtain Db4C1op had altered the original binding activity.
In Vivo Protection against the Whole Venom by the Mixture of Diabodies
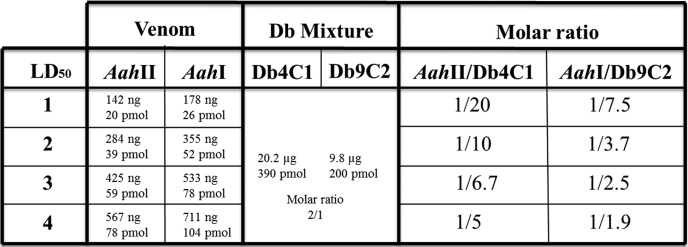
Before studying the biological activity of a mixture of two diabodies against experimental envenomation, monoclonal antibodies were used to determine by ELISA the concentrations of the two major toxins (AahI and AahII) in the A. australis hector venom according to Devaux et al. (12, 31). The concentrations of AahI and AahII were very similar, 250 and 200 μg/ml, respectively. The toxicity of the whole venom was measured after s.c. injection in C57BL/six mice weighing 20 ± 2 g to provide an accurate determination of the LD50. We found that a dose of venom corresponding to one LD50 contained 142 ng of AahII and 178 ng of AahI (Table 1). These values are lower than the known LD50 values for each individual toxin (180 ng for AahII and 380 ng for AahI).
TABLE 1.
Representation of molar ratios, taking into account the estimated amount of the AahI and AahII toxins in the venom according to Devaux et al. (12)
The mixture used was the mixture of two pure, homogeneous, and functional diabodies in the following proportions: (i) the molar ratio between the two diabodies was 2:1 (Db4C1op:Db9C2), and (ii) the amounts of antibody fragments were also adjusted compared with the proportion of AahII in 4 LD50 of whole venom to obtain a final molar ratio of 5:1 (Db4C1op:AahII). It is essential to neutralize AahII, the most toxic fraction of venom, which is recognized by Db4C1op. Thus 20.2 μg of Db4C1op were added to 9.8 μg of Db9C2 (Table 1).
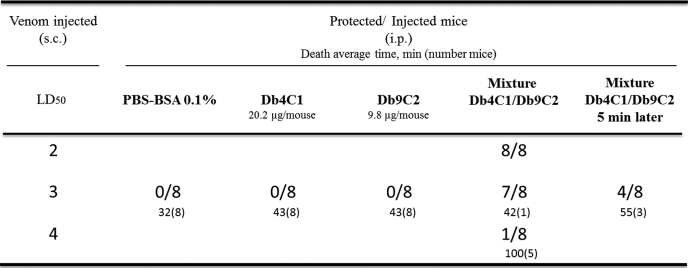
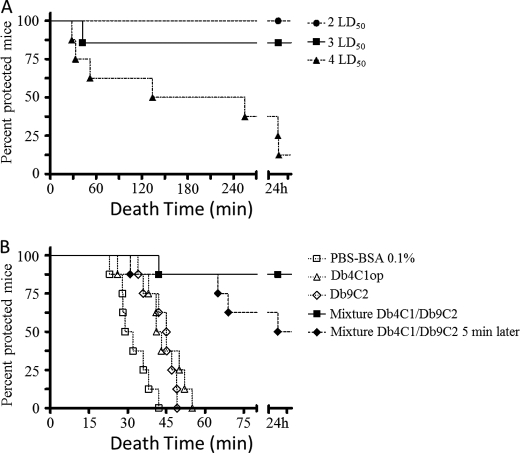
To test the protection provided by the mixture of diabodies under conditions that closely mimic those of accidental envenomation, mice were injected via the subcutaneous route with doses above the LD50 of freshly calibrated venom. Immediately afterward, the mice were given an intraperitoneal injection of our therapy mixture. The in vivo interaction of the mixture of diabodies with whole venom was investigated by determining the end point of the protective capacity. 100% of the mice given the mixture survived injection with 2 LD50 of venom. The mixture also protected, although to a lesser extent, mice injected with 3 LD50 of venom, and although only one of the eight animals challenged died after 42 min, all the others were still alive 24 h later. Finally, although the envenoming of mice with 4 LD50 markedly reduced the survival rate to 12.5%, the animals died significantly later as follows: five and two of the eight mice died after 100 ± 96 min and at least 5 h after challenge, respectively (Table 2 and Fig. 2A). Under these conditions, the protective capacity of the mixture was found to be between 66 and 100 LD50/mg for 2 and 3 LD50 doses of venom, respectively.
TABLE 2.
In vivo protection of mice experimentally envenomed by the subcutaneous injection of purified A. australis venom and treated by intraperitoneal injection of antibody fragments
x/y indicates the ratio of protected/injected mice. Mice were observed for 24 h. z(w) indicates the average time of death (min) of the dead mice, excluding those that died after more than 5 h.
FIGURE 2.
In vivo protection of experimentally envenomed mice. A, mice were injected by the subcutaneous route with doses greater than the LD50 of freshly calibrated venom (2 LD50, 3 LD50, or 4 LD50). B, mice were treated after the s.c. injection of 3 LD50 by intraperitoneal injection with PBS-BSA 0.1%, Db4C1op, Db9C2, mixture Db4C1/Db9C2, or mixture Db4C1/Db9C2 injected 5 min after the venom.
For 3 LD50, the end point protective capacity, when the mixture was replaced either by 0.1% BSA (used here as a control) or by only one of the diabodies at the same concentration as in the mixture, no protection was observed. Nevertheless, death seemed to occur slightly later in the diabody-treated mice, 43 ± 9 and 43 ± 6 min for Db4C1op and Db9C2, respectively, versus 32 ± 7 min for mice treated with 0.1% BSA (Table 2 and Fig. 2B). Finally, when the mixture was injected 5 min after envenomation of the mice, only 50% of the mice survived, but death occurred later in those that did die: 55 ± 21 min for three mice and more than 5 h for the last mouse (Table 2 and Fig. 2B). In conclusion, at 3 LD50, the dose that mimics a lethal scorpion sting, the results showed first that neither diabody used alone was sufficient to protect the mice against whole venom, and second, although no significant delay in the onset of symptoms of poisoning was observed, regardless of the experimental conditions, the mixture of diabodies provided better protection over time, highlighting its effectiveness and the need to include both antibodies to achieve full protection.
DISCUSSION
Over the last decade, the development of antibody therapy has advanced considerably. Many antibody fragments have been described and have been shown to be of real interest as a potential antivenom therapy instead of the conventional, albeit controversial, immunotherapy based on the use of equine F(ab′)2.
In our study, making use of recent advances in the expression and production of recombinant protein, we investigated using a mixture of homodimeric diabodies to protect against the lethal effect of the whole venom of A. autralis hector. The diabody mixture was preferred to an scFv mixture because dimers of diabodies (50 kDa) are significantly larger than scFv monomers and are similar in size to Fab, which are currently considered to be one the most suitable formats for antibody fragments in antivenom therapy, due to its tissue penetration, bioavailability, and detoxifying potential (35, 36).
Starting from the scFv4C1 fragment described by Mousli et al. (24), we have engineered Db4C1op, a new antibody fragment. As described previously, several modifications were necessary to allow us to produce a pure and homogeneous protein with a yield of 0.3–0.85 mg of protein/liter of culture. In previous attempts, the amounts of scFv4C1 obtained were low, and most of the proteins produced were located in the bacterial cytoplasm, mainly as insoluble inclusion bodies. With our new construct, the production yield is similar to that of Db9C2, described by Aubrey et al. (29). The beneficial effect of the modifications is not easily evaluated, and the codon optimization for prokaryotic expression does not seem to be sufficient. Nevertheless, the replacement of some parts of the VL scFv4C1 N-terminal region by those of scFv9C2 made it possible for the first time to purify the Db4C1op fragment in a pure and homogeneous manner thanks to the protein L affinity. In addition, the flag peptide was suppressed to reduce the immunogenicity of the fragment, which is critical for therapeutic applications (34). The beginning of the VH scFv4C1 domain was also modified because a different expression vector (pSW1) was used in place of the original one (pHEN). Indeed, no expression of Db4C1op, when cloned in the pHEN vector, was observed (data not shown). Our results clearly show that the 5-amino acid residue linker between the variable domains of Db4C1op promotes the formation of very stable homodimers. Furthermore, FPLC has shown that the oligomeric Db4C1op was never observed, in contrast to Db9C2, suggesting a lack of flexibility, which is probably due to the nature of certain amino acids on the exposed surface of Db4C1op. Moreover, the dimeric Db4C1op fragment appears to be compact and more like monomeric scFv4C1op than its dimeric forms.
In this study, we investigated the combination of two freshly purified diabodies (Db9C2 and Db4C1op). These antibody fragments conserve the affinity of the monoclonal antibodies from which they are derived. The most important advance in our strategy, compared with the use of two single molecules in tandem (scFv tandem or VHH tandem), is that it makes it possible to administer two different antibodies in differing molar ratios. The amounts administered can be adjusted according to various important factors such as the following: (i) the affinity of antibodies (the affinities of IgG4C1 and IgG9C2 differ considerably, 4 × 10−10 and 0.2 × 10−10 m, respectively); (ii) the characteristics of the venom, the amounts of each of the toxins (AahI, AahII, and AahIII), and their toxicities may vary depending on the venom considered. Pharmacokinetic characteristics are also a substantial point in envenomation. In many cases, the characteristics of the venom tend to resemble those of the AahII toxin. Even though this toxin disappears from the bloodstream more rapidly than the AahI toxin, it is the most abundant and seems to be responsible for almost all of the lethal effects (12). For all these reasons, it is essential to use more Db4C1op than Db9C2 to neutralize and protect against the whole venom.
It is interesting to note that when neither diabody or only one of them is injected intraperitoneally, after 3 LD50 envenomation, all the mice died quickly, within a short interval of time. The neutralization of the AahII toxin or the immunologic group I toxins delayed the onset of death in a similar way, suggesting that the two immunologic groups have similar toxicity. Administering 3 LD50 was more significant than administering 1 LD100 or 2 LD50, because even when the half of the toxicity of the venom has been neutralized, the remaining half was still greater than 1 LD100.
The amounts of the two diabodies appear to be correctly adjusted to the amount of toxins in the venom. Indeed, the mice died after a different interval depending on the circulating toxins. The AahII toxin has the highest affinity reported for site 3 of the voltage-sensitive sodium channels of excitable cells (13). Thus, when the experimental conditions included Db9C2 treatment, the time interval during which the animals died was the shortest (6 min, immunologic group I toxin-free), although it was longer when Db4C1op was used (9 min, AahII-free). If any toxin was neutralized, as shown in the control experiment, the mortality period was intermediate between those for the other two conditions, suggesting competition occurred between the different toxins to access site 3. However, when the mixture did not allow any animals to survive, there was greater disparity (100 min ± 96 (4 LD50) and 55 min ± 21 (3 LD50 and a 5-min delay)) considering only mice died within 5 h; it is probably due to the sensitivity of the mice and the fact that the whole venom was used instead of purified toxins.
We must emphasize that we studied the diabody mixture under conditions that closely reflected natural envenomation. We did not evaluate the in vivo neutralization of toxins or of venom preincubated with the antibody mixture because the formation of the antigen-antibody immunocomplex, before injecting the animals, reflects the neutralizing titer of the antivenom but not its protective capacity. For this reason, the injection of venom and antibodies via different routes is necessary to allow the venom to spread throughout the entire body of the animal. Our in vivo assays clearly indicate that this mixture can protect animals challenged with 2 LD50 of freshly calibrated venom and also protect seven of eight mice when they are challenged with 3 LD50. It is important to point out that the protective capacity of the mixture was estimated to be between 66 and 100 LD50 of venom/mg of protein. This is a significant finding, and the effective diabody mixture even appears to be much more effective than the bispecific nanobodies described by Hmila et al. (28), which had a protective capacity estimated to be 23.5 LD50/mg for full protection at 2 LD50 with 85 μg of bispecific NbF12–10 injected i.v. If the mixture was injected 5 min after the venom, only 50% of the mice survived, but the average survival time was considerably greater, three of them died after 55 min and one at least 5 h after envenomation.
With regard to the protective capacity, two major parameters must be considered. First is the pharmacokinetics of the protagonists, which varies depending on the toxin, antibody, or antibody fragments involved. To date, few studies have been performed investigating the pharmacokinetic antibody fragments. Second, the format of the recombinant antibody plays a crucial rule, and the notion that the more closely the format of the antibody fragment is related to the antigen, the better the protection it provides is certainly distorted by differences in affinity and epitope. The protective capacity depends on the above parameters and can be overestimated if large amounts of antibodies are injected. We are aware that the immunogenicity of our antibody fragment, Db9C2 and Db4C1op, is open to criticism because of their murine origin. They could elicit an anti-mouse antibody response in humans, which would dramatically reduce the efficacy of the therapy. Although there are some techniques available to reduce immunogenicity and increase the tolerability of antibody fragments, such as CRD grafting, we are convinced that their use in humans is less problematic than is generally admitted. Indeed, the humanization of antibody fragments is often accompanied by a decrease in their affinity and therefore in their neutralizing capacity. Compared with the human VH and VL domains, Db4C1op and Db9C2 FRs display high sequence homologies of 81.5 and 70.5%, respectively, and it is of interest that Db4C1op VH shares high sequence identity with the human family III VH, the most widespread VH family (37). This is similar to other VHHs described (26, 38, 39). In short, in the treatment of scorpion envenomation, these antibodies are intended to be injected only once, and not repeatedly like some antibodies for the treatment of chronic diseases or those intended for use in a prophylactic protocol. Finally, for many years, the Food and Drug Administration has been approving the human use of recombinant antibodies containing murine sequences.
In conclusion, the Db4C1op antibody fragment is now easier to produce and to purify than the original scFv4C1 (24). The use of the Db4C1op and Db9C2, in a molar ratio 2:1 and with a small amount of protein (30 μg), demonstrates significant in vivo protection of mice experimentally poisoned by the crude venom. It is now possible to continue to improve the affinity of the 4C1 and to modulate its specificity so that it recognizes BotIII toxin, the most toxic protein in Buthus occitanus venom (the sequence of which differs from that of AahII by only three amino acid residues) (40). Polyvalent antivenoms could now be designed and created to protect against the venoms of both A. australis and B. occitanus, two dangerous scorpions found in the same regions.
Footnotes
- AahI
- A. australis hector group I
- AahII
- A. australis hector group II.
REFERENCES
- 1. Chippaux J. P., Goyffon M. (2008) Epidemiology of scorpionism. A global appraisal. Acta Trop. 107, 71–79 [DOI] [PubMed] [Google Scholar]
- 2. Prendini L., Wheeler W. C. (2005) Scorpion higher phylogeny and classification, taxonomic anarchy, and standards for peer review in on-line publishing. Cladistics 21, 446–494 [DOI] [PubMed] [Google Scholar]
- 3. Chowell G., Díaz-Dueñas P., Bustos-Saldaña R., Mireles A. A., Fet V. (2006) Epidemiological and clinical characteristics of scorpionism in Colima, Mexico (2000–2001). Toxicon 47, 753–758 [DOI] [PubMed] [Google Scholar]
- 4. Bouaziz M., Bahloul M., Kallel H., Samet M., Ksibi H., Dammak H., Ahmed M. N., Chtara K., Chelly H., Hamida C. B., Rekik N. (2008) Epidemiological, clinical characteristics, and outcome of severe scorpion envenomation in South Tunisia. Multivariate analysis of 951 cases. Toxicon 52, 918–926 [DOI] [PubMed] [Google Scholar]
- 5. Adi-Bessalem S., Hammoudi-Triki D., Laraba-Djebari F. (2008) Pathophysiological effects of Androctonus australis hector scorpion venom. Tissue damages and inflammatory response. Exp. Toxicol. Pathol. 60, 373–380 [DOI] [PubMed] [Google Scholar]
- 6. Benguedda A. C., Laraba-Djébari F., Ouahdi M., Hellal H., Griene L., Guerenik M., Laid Y., and Comité National de Lutte Contre l'Envenimation Scorpionique (CNLES) (2002) Fifteen years' experience in scorpion envenomation control in Algeria. Bull. Soc. Pathol. Exot. 95, 205–208 [PubMed] [Google Scholar]
- 7. Njah M., Ben Abdelaziz A., Abdouli M., Zaher M., Garaoui A. (2001) Health program and use of community health workers. The example of scorpion envenomation in Tunisia. Sante 11, 57–62 [PubMed] [Google Scholar]
- 8. Mansour N. (2001) Delay and characteristics of scorpion bite management in the Sidi-Bouzid region. Arch. Inst. Pasteur Tunis 78, 25–31 [PubMed] [Google Scholar]
- 9. el Ayeb M., Rochat H. (1985) Polymorphism and quantitative variations of toxins in the venom of the scorpion Androctonus australis hector. Toxicon 23, 755–760 [DOI] [PubMed] [Google Scholar]
- 10. el Ayeb M., Bahraoui E. M., Granier C., Rochat H. (1986) Use of antibodies specific to defined regions of scorpion α-toxin to study its interaction with its receptor site on the sodium channel. Biochemistry 25, 6671–6678 [DOI] [PubMed] [Google Scholar]
- 11. Delori P., Van Rietschoten J., Rochat H. (1981) Scorpion venoms and neurotoxins. An immunological study. Toxicon 19, 393–407 [DOI] [PubMed] [Google Scholar]
- 12. Devaux C., Jouirou B., Naceur Krifi M., Clot-Faybesse O., El Ayeb M., Rochat H. (2004) Quantitative variability in the biodistribution and in toxinokinetic studies of the three main α toxins from the Androctonus australis hector scorpion venom. Toxicon 43, 661–669 [DOI] [PubMed] [Google Scholar]
- 13. Martin M. F., Rochat H. (1986) Large scale purification of toxins from the venom of the scorpion Androctonus australis Hector. Toxicon 24, 1131–1139 [DOI] [PubMed] [Google Scholar]
- 14. Abroug F., ElAtrous S., Nouira S., Haguiga H., Touzi N., Bouchoucha S. (1999) Serotherapy in scorpion envenomation. A randomized controlled trial. Lancet 354, 906–909 [DOI] [PubMed] [Google Scholar]
- 15. Krifi M. N., Savin S., Debray M., Bon C., El Ayeb M., Choumet V. (2005) Pharmacokinetic studies of scorpion venom before and after antivenom immunotherapy. Toxicon 45, 187–198 [DOI] [PubMed] [Google Scholar]
- 16. Hammoudi-Triki D., Lefort J., Rougeot C., Robbe-Vincent A., Bon C., Laraba-Djebari F., Choumet V. (2007) Toxicokinetic and toxicodynamic analyses of Androctonus australis hector venom in rats. Optimization of antivenom therapy. Toxicol. Appl. Pharmacol. 218, 205–214 [DOI] [PubMed] [Google Scholar]
- 17. Krifi M. N., Miled K., Abderrazek M., El Ayeb M. (2001) Effects of antivenom on Buthus occitanus tunetanus (Bot) scorpion venom pharmacokinetics. Toward an optimization of antivenom immunotherapy in a rabbit model. Toxicon 39, 1317–1326 [DOI] [PubMed] [Google Scholar]
- 18. Ismail M., Abd-Elsalam M. A. (1998) Pharmacokinetics of 125I-labeled IgG, F(ab′)2, and Fab fractions of scorpion and snake antivenins. Merits and potential for therapeutic use. Toxicon 36, 1523–1528 [DOI] [PubMed] [Google Scholar]
- 19. Hmila I., Abdallah R B. A., Saerens D., Benlasfar Z., Conrath K., Ayeb M. E., Muyldermans S., Bouhaouala-Zahar B. (2008) VHH, bivalent domains, and chimeric heavy chain-only antibodies with high neutralizing efficacy for scorpion toxin AahI. Mol. Immunol. 45, 3847–3856 [DOI] [PubMed] [Google Scholar]
- 20. Abderrazek R. B., Hmila I., Vincke C., Benlasfar Z., Pellis M., Dabbek H., Saerens D., El Ayeb M., Muyldermans S., Bouhaouala-Zahar B. (2009) Identification of potent nanobodies to neutralize the most poisonous polypeptide from scorpion venom. Biochem. J. 424, 263–272 [DOI] [PubMed] [Google Scholar]
- 21. Meddeb-Mouelhi F., Bouhaouala-Zahar B., Benlasfar Z., Hammadi M., Mejri T., Moslah M., Karoui H., Khorchani T., El Ayeb M. (2003) Immunized camel sera and derived immunoglobulin subclasses neutralizing Androctonus australis hector scorpion toxins. Toxicon 42, 785–791 [DOI] [PubMed] [Google Scholar]
- 22. Aubrey N., Muzard J., Christophe Peter J., Rochat H., Goyffon M., Devaux C., Billiald P. (2004) Engineering of a recombinant Fab from a neutralizing IgG directed against scorpion neurotoxin AahI, and functional evaluation versus other antibody fragments. Toxicon 43, 233–241 [DOI] [PubMed] [Google Scholar]
- 23. Devaux C., Moreau E., Goyffon M., Rochat H., Billiald P. (2001) Construction and functional evaluation of a single-chain antibody fragment that neutralizes toxin AahI from the venom of the scorpion Androctonus australis hector. Eur. J. Biochem. 268, 694–702 [DOI] [PubMed] [Google Scholar]
- 24. Mousli M., Devaux C., Rochat H., Goyffon M., Billiald P. (1999) A recombinant single-chain antibody fragment that neutralizes toxin II from the venom of the scorpion Androctonus australis hector. FEBS Lett. 442, 183–188 [DOI] [PubMed] [Google Scholar]
- 25. Juárez-González V. R., Riaño-Umbarila L., Quintero-Hernández V., Olamendi-Portugal T., Ortiz-León M., Ortíz E., Possani L. D., Becerril B. (2005) Directed evolution, phage display, and combination of evolved mutants. A strategy to recover the neutralization properties of the scFv version of BCF2, a neutralizing monoclonal antibody specific to scorpion toxin Cn2. J. Mol. Biol. 346, 1287–1297 [DOI] [PubMed] [Google Scholar]
- 26. Holliger P., Hudson P. J. (2005) Engineered antibody fragments and the rise of single domains. Nat. Biotechnol. 23, 1126–1136 [DOI] [PubMed] [Google Scholar]
- 27. Juste M., Martin-Eauclaire M. F., Devaux C., Billiald P., Aubrey N. (2007) Using a recombinant bispecific antibody to block Na+-channel toxins protects against experimental scorpion envenoming. Cell. Mol. Life Sci. 64, 206–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hmila I., Saerens D., Ben Abderrazek R., Vincke C., Abidi N., Benlasfar Z., Govaert J., El Ayeb M., Bouhaouala-Zahar B., Muyldermans S. (2010) A bispecific nanobody to provide full protection against lethal scorpion envenoming. FASEB J. 24, 3479–3489 [DOI] [PubMed] [Google Scholar]
- 29. Aubrey N., Devaux C., Sizaret P. Y., Rochat H., Goyffon M., Billiald P. (2003) Design and evaluation of a diabody to improve protection against a potent scorpion neurotoxin. Cell. Mol. Life Sci. 60, 617–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Miranda F., Kupeyan C., Rochat H., Rochat C., Lissitzky S. (1970) Purification of animal neurotoxins. Isolation and characterization of 11 neurotoxins from the venoms of the scorpions Androctonus australis hector, Buthus occitanus tunetanus, and Leiurus quinquestriatus quinquestriatus. Eur. J. Biochem. 16, 514–523 [DOI] [PubMed] [Google Scholar]
- 31. Devaux C., Clot-Faybesse O., Pugnière M., Mani J. C., Rochat H., Granier C. (2002) A strategy for inducing an immune response against Androctonus australis scorpion venom toxin I in mice. Production of high affinity monoclonal antibodies and their use in a sensitive two-site immunometric assay. J. Immunol. Methods 271, 37–46 [DOI] [PubMed] [Google Scholar]
- 32. Sambrook J., R. D. W. (2001) in Molecular Cloning: A Laboratory Manual (Sambrook J., Russel D. W., eds) Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 33. Appel R. D., Bairoch A., Hochstrasser D. F. (1994) A new generation of information retrieval tools for biologists. The example of the ExPASy WWW server. Trends Biochem. Sci. 19, 258–260 [DOI] [PubMed] [Google Scholar]
- 34. Muzard J., Adi-Bessalem S., Juste M., Laraba-Djebari F., Aubrey N., Billiald P. (2009) Grafting of protein L binding activity onto recombinant antibody fragments. Anal. Biochem. 388, 331–338 [DOI] [PubMed] [Google Scholar]
- 35. Chippaux J. P., Goyffon M. (1998) Venoms, antivenoms, and immunotherapy. Toxicon 36, 823–846 [DOI] [PubMed] [Google Scholar]
- 36. Dart R. C., McNally J. (2001) Efficacy, safety, and use of snake antivenoms in the United States. Ann. Emerg. Med. 37, 181–188 [DOI] [PubMed] [Google Scholar]
- 37. Tomlinson I. M., Walter G., Marks J. D., Llewelyn M. B., Winter G. (1992) The repertoire of human germ line VH sequences reveals about 50 groups of VH segments with different hypervariable loops. J. Mol. Biol. 227, 776–798 [DOI] [PubMed] [Google Scholar]
- 38. Muyldermans S. (2001) Single domain camel antibodies. Current status. J. Biotechnol. 74, 277–302 [DOI] [PubMed] [Google Scholar]
- 39. Harmsen M. M., De Haard H. J. (2007) Properties, production, and applications of camelid single-domain antibody fragments. Appl. Microbiol. Biotechnol. 77, 13–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Benkhadir K., Kharrat R., Cestèle S., Mosbah A., Rochat H., El Ayeb M., Karoui H. (2004) Molecular cloning and functional expression of the α-scorpion toxin BotIII. Pivotal role of the C-terminal region for its interaction with voltage-dependent sodium channels. Peptides 25, 151–161 [DOI] [PubMed] [Google Scholar]