Background: Recent research has uncovered tumor-suppressive and oncogenic potential of miRNAs.
Results: miR-214 suppresses the proliferation, migration, and invasiveness of cervical cancer cells by targeting GALNT7.
Conclusion: Down-regulation of miR-214 results in overexpressed GALNT7, contributing to tumorigenesis of cervical cancer.
Significance: The identification of tumor suppressor miR-214 and its oncogenic target GALNT7 in cervical cancer cells is potentially valuable for cancer diagnosis and therapy.
Keywords: Cancer, Cell Growth, Cell Invasion, Glycosyltransferases, MicroRNA, GALNT7, Cervical Cancer Cells, Invasiveness, miR-214
Abstract
MicroRNAs are a class of small noncoding RNAs that function as key regulators of gene expression at the post-transcriptional level. In this study, we demonstrate that miR-214 is frequently down-regulated in cervical cancer, and its expression reduces the proliferation, migration, and invasiveness of cervical cancer cells, whereas inhibiting its expression results in enhanced proliferation, migration, and invasion. miR-214 binds to the 3′-UTR of UDP-N-acetyl-α-d-galactosamine:polypeptide N-acetylgalactosaminyltransferase 7 (GALNT7), thereby repressing GALNT7 expression. Furthermore, we are the first to show, using quantitative real-time PCR, that GALNT7 is frequently up-regulated in cervical cancer. The knockdown of GALNT7 markedly inhibits cervical cancer cell proliferation, migration, and invasion, whereas ectopic expression of GALNT7 significantly enhances these properties, indicating that GALNT7 might function as an oncogene in cervical cancer. The restoration of GALNT7 expression can counteract the effect of miR-214 on cell proliferation, migration, and invasiveness of cervical cancer cells. Together, these results indicate that miR-214 is a new regulator of GALNT7, and both miR-214 and GALNT7 play important roles in the pathogenesis of cervical cancer.
Introduction
Cervical carcinoma significantly affects the health of women worldwide, especially in developing countries (1), and currently ranks as the second leading cause of cancer mortality in women following breast cancer. Approximately 500,000 cases of cervical cancer are diagnosed per year, with nearly 40% of those resulting in death (2). Cervical cancer is a complex disease involving the abnormal expression of many oncogenes and tumor suppressor genes. Previous studies have revealed several genes associated with human cervical cancer. For example, pro-apoptotic protein Bax expression is often lost in carcinomas (3), and p53 plays a crucial role in the development of cervical cancer as well (3). Although focusing on known genes has yielded significant new information, previously unknown noncoding RNAs, such as miRNAs,3 may also provide insights into the biology of cervical cancer. miRNAs are a group of noncoding single-stranded RNAs, ∼22 nucleotides in length, that have emerged as an important class of short endogenous RNAs that regulate gene expression post-transcriptionally by base-paring with their target mRNA (4). Previous studies have identified cancer-specific miRNAs in many types of cancer, including melanoma (5, 6), leukemia (7), lung cancer (8, 9), breast cancer (10, 11), hepatocellular carcinoma (12, 13), and cervical cancer (14, 15). A recent study reported that miR-214 overexpression in melanoma cells resulted in increased invasive and metastatic behavior (6). Yang et al. (16) reported that miR-214 induces cell survival and cisplatin resistance through targeting the 3′-UTR of PTEN in human ovarian cancer cell lines. Xiong et al. (17) demonstrated that down-regulation of miR-214 induced G1 cell cycle arrest in gastric cancer cells by up-regulating PTEN. However, a new study indicated that reduced miR-214 levels may contribute to breast tumorigenesis by allowing abnormal accumulation of Ezh2, which resulted in unchecked cell proliferation and invasion (18). We previously demonstrated that miR-214 was down-regulated in cervical cancer tissue and could negatively regulate HeLa cell growth (14, 19). A new microarray analysis also indicated that miR-214 was down-regulated in cervical cancer tissue (20). Additionally, our previous cDNA microarray analysis indicated that GALNT7 increased up to 5.49-fold when miR-214 expression was suppressed in HeLa cells (19).
GALNT7 is one member of the UDP-N-acetyl-α-d-galactosamine:polypeptide N-acetylgalactosaminyltransferase (GalNAc-T or GALNT) family. GALNTs initiate mucin-type O-linked glycosylation in the Golgi apparatus by catalyzing the transfer of GalNAc to serine and threonine residues on target proteins (21). Because O-linked glycosylation proceeds stepwise (22), the addition of GalNAc to serine or threonine represents the first committed step in mucin biosynthesis. Despite this apparent simplicity, multiple GALNT family members appear to be necessary to fully glycosylate their protein substrates (21). Aberrant glycosylation is a well described hallmark of many human cancers and is associated with cell growth, differentiation, transformation, adhesion, metastasis, and tumor immune surveillance (23). Although the O-glycosylation of these glycoproteins is considered to play a key role in determining tumor properties, the regulation of GALNT7 expression in cancers remains largely unknown.
In this study, we determined by qRT-PCR that GALNT7 was overexpressed in human cervical cancer relative to adjacent normal tissues, and GALNT7 was identified as a direct target of miR-214. Knockdown of GALNT7 suppressed the growth and invasiveness of HeLa and C33A human cervical cancer cells. Taken together, our results indicate that GALNT7 may function as an oncogene and is a mediator of miR-214 in human cervical cancer.
EXPERIMENTAL PROCEDURES
miRNA Targets Prediction
The putative miRNA targets were predicted using the TargetScan, miRanda, and PicTar algorithms.
Plasmid Construction
Construction of the pcDNA3/pri-miR-214 (pri-miR-214) plasmid was described in our previous study (14). We also commercially synthesized a 2′-O-methyl-modified antisense oligonucleotide of miR-214 (ASO-miR-214) as an inhibitor of miR-214. The sequence is listed in Table 1.
TABLE 1.
Primers and oligonucleotides used in this work
| Name | Sequence (5′–3′) |
|---|---|
| pri-miR-214-S | 5′-CATAGGATCCAGATCTGCTGAACTCTGACTACATG-3′ |
| pri-miR-214-A | 5′-GGGCGGAATTCTATTTCATAGGCACCACTC-3′ |
| ASO-miR-214 | 5′-ACTGCCTGTCTGTGCCTGCTGT-3′ |
| ASO-NC | 5′-TGACTGTACTGAGACTCGACTG-3′ |
| GALNT7–3′-UTR-S | 5′-CGCGGATCCTTTAGATCGCTCAGAGGTC-3′ |
| GALNT7–3′-UTR-A | 5′-CTGAATTCCAAACTGATCAGGAGGTTCCAG-3′ |
| GALNT7–3′-UTR-MS | 5′-CCGCCTGAAAGCAGGTCCAACTATTGTTATTAAC-3′ |
| GALNT7–3′-UTR-MA | 5′-GTTAATAACAATAGTTGGACCTGCTTTCAGGCGG-3′ |
| GALNT7-S-XhoI | 5′-GACTCCTCGAGACCATGAGGCTGAAGATTGG-3′ |
| GALNT7-A-XbaI | 5′-GCATCTAGAACACTATGGATGTTATTCATTTCCC3′ |
| GALNT7-siR-Top | 5′-GATCCGGACAGAACCATTTGCACTTTCAAGAGAAGTGCAAATGGTTCTGTCCTTTTTTGGAAA-3′ |
| GALNT7-siR-Bot | 5′-AGCTTTTCCAAAAAAGGACAGAACCATTTGCACTTCTCTTGAAAGTGCAAATGGTTCTGTCCG-3′ |
| β-Actin-S | 5′-CGTGACATTAAGGAGAAGCTG-3′ |
| β-Actin-A | 5′-CTAGAAGCATTTGCGGTGGAC-3′ |
| miR-214 RT | 5′-GTCGTATCCAGTGCAGGGTCCGAGGTGCACTGGATACGACACTGCCTG-3′ |
| miR-214 forward | 5′-TGCGGACAGCAGGCACAGAC-3′ |
| U6 RT | 5′-GTCGTATCCAGTGCAGGGTCCGAGGTGCACTGGATACGACAAAATATGG-3′ |
| U6 forward | 5′-TGCGGGTGCTCGCTTCGGCAGC-3′ |
| Reverse | 5′-CCAGTGCAGGGTCCGAGGT-3′ |
The enhanced green fluorescence protein (EGFP) expression vector, pcDNA3/EGFP, was constructed as described previously (24). The 3′-UTR fragment of the GALNT7 gene containing the predicted miR-214 binding site was amplified by PCR using the primers listed in Table 1. PCR products were cloned into the pcDNA3/EGFP plasmid between the BamHI and EcoRI sites. The resulting vector was named pcDNA3/EGFP-GALNT7 3′-UTR. Moreover, a mutant fragment of GALNT7 3′-UTR containing a mutated miR-214 binding site was amplified using PCR site-directed mutagenesis and cloned into the pcDNA3/EGFP plasmid between the same sites. All insertions were confirmed by sequencing.
To construct the pSilencer/shRNA-GALNT7 (siR-GALNT7) vector, a 70-bp double-strand fragment was obtained via an annealing reaction using two single strands listed in Table 1. The fragment was then cloned into the pSilencer2.1/neo vector (Ambion) between the BamHI and HindIII sites.
The pcDNA3 vector was used to generate a GALNT7 overexpression plasmid. The full-length human GALNT7 cDNA sequence (GenBankTM, NM_017423.2) was amplified using PCR from a cDNA clone vector and then cloned into the XhoI and XbaI restriction sites using primers listed in Table 1.
Cell Culture and Transfection
Two human cervical cancer cell lines, HeLa and C33A, were grown in RPMI 1640 supplemented with 10–20% heat-inactivated FBS, 100 IU/ml penicillin, and 100 μg/ml streptomycin in a humidified atmosphere of 95% air and 5% CO2 at 37 °C. Transfection was performed using the Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's protocol.
Human Tissue Samples and RNA Isolation
Seventeen pairs of clinical specimens, including 17 human cervical cancer tissue sections from patients with cervical cancer and corresponding adjacent normal tissues, were obtained from the Cancer Center of Sun Yat-sen University of Medical Sciences. All of the samples were obtained with the obtained with the informed consent of the patients and approved by the Ethics Committee of Sun Yat-sen University of Medical Sciences. RNA was isolated from tissue samples using the mirVana miRNA isolation kit (Ambion, Austin, TX) according to the manufacturer's protocol.
qRT-PCR
For the quantification of miR-214 expression, a stem-loop qRT-PCR was performed. Briefly, 2 μg of small RNA extracted from cells or tissue samples was reverse-transcribed to cDNA with the stem-loop reverse transcriptase primer using the M-MLV reverse transcriptase (Promega, Madison, WI). The cDNA was subsequently used for the amplification of miR-214 and an endogenous control, U6 snRNA, via PCR. PCR cycles were as follows: 94 °C for 3 min followed by 40 cycles of 94 °C for 30 s, 56 °C for 30 s, and 72 °C for 30 s.
To quantify GALNT7 gene expression, 5 μg of RNA extracted from cells or tissue samples was reverse-transcribed to cDNA using the M-MLV reverse transcriptase. The cDNA was used for PCR amplification of GALNT7 and an endogenous control gene, β-actin. PCR cycles were as follows: 94 °C for 3 min followed by 40 cycles of 94 °C for 30 s, 58 °C for 30 s, and 72 °C for 30 s.
The SYBR Premix Ex TaqTM kit (TaKaRa, Otsu, Shiga, Japan) was used to measure the amplified DNA, and qRT-PCR was performed using an iQ5 real-time PCR detection system (Bio-Rad). The relative gene expression levels were calculated using the 2−ΔΔCt method (25). All primers were purchased from AuGCT, Inc. (Beijing, China), and the sequences are shown in Table 1.
Western Blotting
Western blotting was performed to determine GALNT7 protein expression. All proteins were resolved on an 8% SDS-denatured polyacrylamide gel and were then transferred onto a nitrocellulose membrane. Membranes were incubated with blocking buffer for 90 min at room temperature and then incubated with an antibody against GALNT7 or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) with Blotto overnight at 4 °C. The membranes were washed and incubated with a horseradish peroxidase (HRP)-conjugated secondary antibody. Protein expression was assessed by enhanced chemiluminescence and exposure to chemiluminescent film. The LabWorks image acquisition and analysis software (UVP, LLC) was used to quantify band intensities. All antibodies were purchased from Saier Biotechnology (Tianjin, China).
Fluorescent Reporter Assay
HeLa cells were co-transfected with pri-miR-214, or ASO-miR-214 in a 48-well plate followed by the pcDNA3/EGFP-GALNT7 3′-UTR reporter vector or the pcDNA3/EGFP-GALNT7 3′-UTR mutant the next day. The RFP expression vector, pDsRed2-N1 (Clontech), was used for normalization. The cells were lysed 72 h later, and the proteins were harvested. EGFP and RFP fluorescence intensity was determined using an F-4500 fluorescence spectrophotometer (HITACHI, Tokyo, Japan).
Detection of Cell Viability and Proliferative Capacity
To determine cell viability and proliferative capacity, cells were examined using the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) and colony formation assays as described previously (24, 26). Cells were seeded in 96-well plates at either 8,000 cells/well (HeLa cells) or 13,000 cells/well (C33A cells) and tested using the MTT assay at different time points. For the colony formation assay, the number of viable cell colonies was determined after either 10 (HeLa cells) or 15 days (C33A cells) after inoculation of 150 cells/well in triplicate in 12-well plates. The cells were stained with crystal violet. The ability to form colonies was evaluated by determining the colony formation number.
Cell Migration and Invasion Assays
For the Transwell migration assay, 5 × 104 HeLa cells or 1.3 × 105 C33A cells in 200 μl of RPMI 1640 without FBS were seeded into the upper part of each Transwell chamber (pore size of 8 μm; Corning) containing a non-coated membrane. For the invasion assay, 5 × 104 HeLa cells or 1.3 × 105 C33A cells were placed on the upper chamber of each insert coated with 40 μl of 2 mg/ml Matrigel (growth factor reduced BD MatrigelTM matrix), and 600 μl of RPMI 1640 with 20% FBS was added to the lower part of the chamber. After incubating for several hours (18 and 30 h for the HeLa and C33A cells, respectively, in the migration assay; 24 and 48 h for the HeLa and C33A cells, respectively, in the invasion assay), the chambers were disassembled, and the membranes were stained with a 2% crystal violet solution for 10 min and placed on a glass slide. Then, cells that had migrated across the membrane were counted in five random visual fields using a light microscope. All assays were performed three independent times in triplicate.
Immunohistochemistry
Immunohistochemistry was performed according to the methods described previously (27). The sections were pretreated with microwave irradiation, blocked, and incubated using polyclonal rabbit anti-human GALNT7 (Saier Biotechnology). Staining intensity was assessed.
Statistical Analysis
Statistical significance was determined using a Student's t test. Data are expressed as means ± S.D. A p value less than 0.05 was considered as statistically significant.
RESULTS
GALNT7 Is Directly Targeted by miR-214
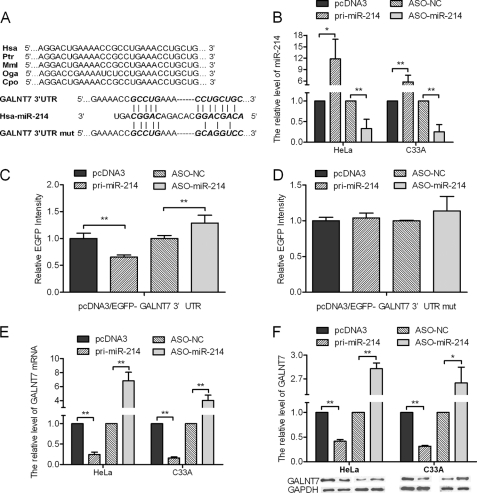
Our previous study demonstrated that blocking miR-214 resulted in an up-regulation of GALNT7 (19). To determine whether miR-214 represses GALNT7 expression by binding directly to its 3′-UTR, we first used algorithm programs to predict miR-214 binding sites in the 3′-UTR of GALNT7, which are conserved among species (Fig. 1A). The free energy of the hybrids is −25.6 kcal/mol as predicted by the PicTar algorithm. Next, the effect of miR-214 on GALNT7 expression was validated by miR-214 gain and loss of functions. In both HeLa and C33A cells, qRT-PCR was performed to validate the miR-214 overexpression construct or miR-214 ASO, with pcDNA3 or ASO-NC to be the respective controls (Fig. 1B). Also, we performed absolute qRT-PCR to confirm the alteration of miR-214 and to indicate the miR-214 copy number per cell in the parental and transfected cervical cancer cell lines (supplemental Fig. 1). HeLa cells were co-transfected with the pcDNA3/EGFP-GALNT7 3′-UTR report vector and pri-miR-214 or ASO-miR-214. As shown in Fig. 1C, the intensity of EGFP fluorescence in the pri-miR-214 group was significantly reduced, whereas that in the ASO-miR-214 group increased significantly at 48 h after transfection. To determine the function of the miR-214 binding site, we constructed an additional EGFP reporter vector containing the GALNT7 3′-UTR with a mutant miR-214 binding site. As a result, neither overexpression nor blocking of miR-214 had any effect on the intensity of EGFP fluorescence in cells transfected with the 3′-UTR mutant vector (Fig. 1D). Together, these results demonstrate that miR-214 binds directly to the 3′-UTR of GALNT7 to repress gene expression.
FIGURE 1.
GALNT7 is directly repressed by miR-214. A, sequence alignment of miR-214 with the wild-type and mutant (mut) 3′-UTR of GALNT7. B, the expression level of miR-214 in HeLa and C33A cells was significantly altered following transfection with either pri-miR-214 or ASO-miR-214 expression constructs as determined by qRT-PCR using U6 snRNA for normalization. C, the intensity of EGFP fluorescence in HeLa cells transfected with pri-miR-214 was decreased after 48 h and increased following transfection with ASO-miR-214. D, pri-miR-214 and ASO-miR-214 had no effect on the intensity of EGFP fluorescence in cells transfected with the 3′-UTR mutant vector. E and F, the mRNA (E) or protein (F) levels of GALNT7 in HeLa and C33A cells decreased or increased when compared with the control group when pri-miR-214 was overexpressed or blocked, respectively (*, p < 0.05, **, p < 0.005). Hsa, Homo sapiens; ptr, Pan troglodytes; Mml, Macaca mulatta; Oga, Otolemur garnettii; Cpo, Cavia porcellus.
Additionally, to determine whether miR-214 also suppresses endogenous GALNT7 expression at the post-transcriptional level, we analyzed the effect of miR-214 on endogenous GALNT7 mRNA and protein levels using qRT-PCR analysis and Western blotting, respectively. In HeLa cells, overexpression of miR-214 resulted in a 76% decrease in GALNT7 mRNA levels (Fig. 1E) and a 59% decrease in protein expression (Fig. 1F). Furthermore, GALNT7 mRNA and protein levels increased 5.8- and 1.8-fold, respectively, in HeLa cells transfected with ASO-miR-214 (Fig. 1, E and F). We observed similar results in the C33A cell line (Fig. 1, E and F). These data indicate that miR-214 negatively regulates endogenous GALNT7 protein expression through mRNA degradation and translational repression.
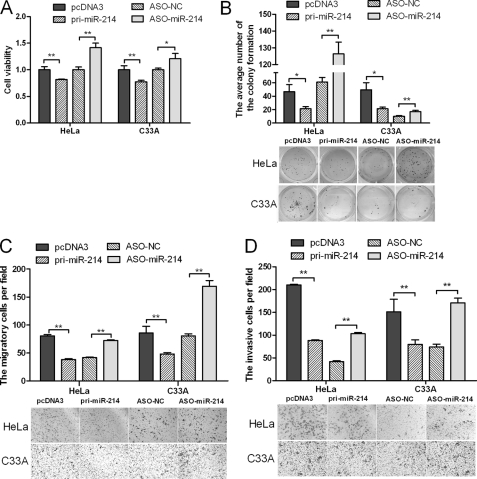
miR-214 Represses Proliferation, Migration, and Invasiveness of HeLa and C33A Cells
We performed MTT, colony formation, cell migration, and invasiveness assays using HeLa and C33A cells transfected with either pri-miR-214 or ASO-miR-214 plasmids to determine the effects of miR-214 expression in vitro. MTT and colony formation assays demonstrated a statistically significant reduction in the cell viability and proliferation of the pri-miR-214-transfected cells relative to the control group, whereas ASO-miR-214 obviously increased these properties in HeLa cells (Fig. 2, A and B). Transwell assay without Matrigel (Fig. 2C) demonstrated that miR-214 overexpression reduced migration in HeLa cells by 53%, and transfection of ASO-miR-214 increased migration to 1.74-fold when compared with the control cells. Furthermore, overexpression of miR-214 resulted in a 59% reduction in the invasive potential of HeLa cells when compared with control cells in Transwell assay with Matrigel, and cells transfected with ASO-miR-214 had an approximately 1.5-fold increase in their invasive potential (Fig. 2D). Similar results were obtained with the C33A cell line (Fig. 2, A–D). These data suggest that miR-214 inhibits cell proliferation, migration, and invasiveness in HeLa and C33A cells in vitro.
FIGURE 2.
miR-214 represses cell proliferation, migration, and invasiveness. Cervical cancer cells were transfected with either pri-miR-214 or ASO-miR-214. Cell viability was determined at 24, 48, and 72 h after seeding in 96-well plates using the MTT assay. A, the histogram shows the data at the time point of 48 h. All three data points showed a significant difference. B, cervical cancer cells were transfected with pri-miR-214 or ASO-miR-214 and then seeded in 12-well plates. For the colony formation assay, the cells were stained with 2% crystal violet solution, and a representative image is shown. C and D, migration and invasion assays were performed with HeLa and C33A cells transfected with either pri-miR-214 or ASO-miR-214. Representative images and randomly selected fields are shown (*, p < 0.05, **, p < 0.005).
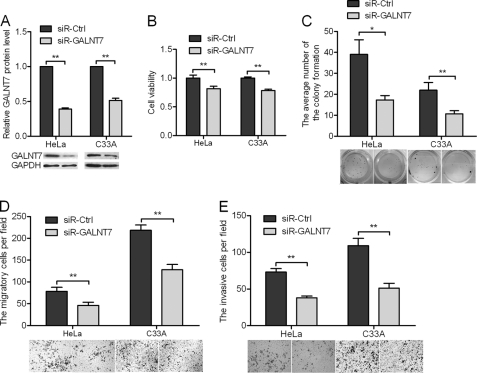
Knockdown of GALNT7 Inhibits Proliferation, Migration, and Invasiveness of HeLa and C33A Cells
HeLa and C33A cells were transfected with specific small interfering RNA (siRNA) targeting GALNT7. Western blotting was used to evaluate the effect of siRNA on GALNT7 protein inhibition. As shown in Fig. 3A, siR-GALNT7 caused a statistically significant reduction of GALNT7 protein levels, up to 60% in HeLa cells and 47% in C33A cells. Inhibition of GALNT7 expression decreased viability (Fig. 3B) and colony formation (Fig. 3C) of cervical cancer cells when compared with control cells. Furthermore, knockdown of GALNT7 expression resulted in a significant decrease in the rate of cell migration (Fig. 3D) and invasion (Fig. 3E) toward a high serum gradient. These findings demonstrate the effect of GALNT7 knockdown on cell proliferation, migration, and invasion, which are consistent with the effect of miR-214 overexpression in both HeLa and C33A cells.
FIGURE 3.
Knockdown of GALNT7 suppresses proliferation, migration, and invasiveness of cervical cancer cells. A, GALNT7 protein level was measured by Western blotting 48 h after transfection of siR-GALNT7 into HeLa and C33A cells. GAPDH was used as loading/transfer control (Ctrl) and for normalization of values. B and C, the effects of GALNT7 knockdown on cell viability (B) were determined using the MTT assay, and cell proliferation was determined using the colony formation assay (C). D and E, changes in cell migration and invasiveness induced by siR-GALNT7 were determined by migration and invasion assays. *, p < 0.05, **, p < 0.005.
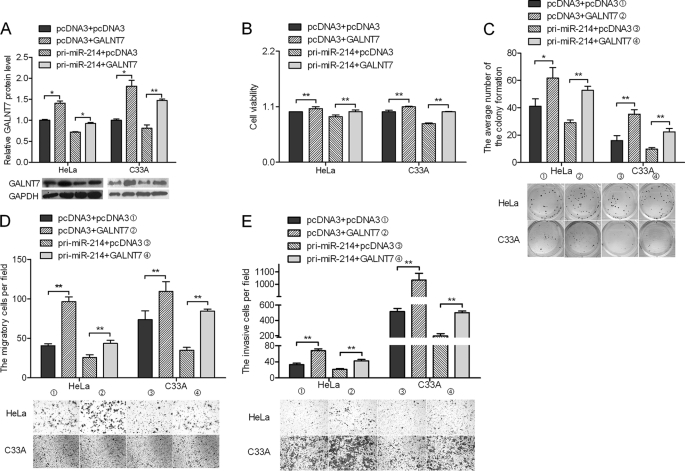
Restoration of GALNT7 Counteracts Effects of miR-214 Expression
To confirm that the effects of miR-214 on the proliferation, migration, and invasiveness of HeLa and C33A cells are mediated through GALNT7, we constructed a pcDNA3/GALNT7 vector containing the GALNT7 ORF without the 3′-UTR to avoid the influence of miRNAs. Transfection of HeLa and C33A cervical cancer cells with this GALNT7 ORF expression construct reversed the negative effects of miR-214 on GALNT7 protein levels (Fig. 4A). The inhibition of cell proliferation (Fig. 4B), colony formation (Fig. 4C), migration (Fig. 4D), and invasiveness (Fig. 4E) caused by pri-miR-214 was abrogated in cells co-transfected with the pcDNA3/GALNT7 vector. The overexpression of GALNT7 countered the effect of miR-214 on cell proliferation, migration, and invasiveness of HeLa and C33A cells.
FIGURE 4.
GALNT7 rescues miR-214-induced cellular phenotypes in cervical cancer cells. A, cells were co-transfected with the pcDNA3/GALNT7 vector, which did not contain the 3′-UTR of GALNT7, with or without pri-miR-214 vector. B–E, at 48 h after transfection, the GALNT7 protein level was measured by Western blotting. MTT assay (B), colony formation assay (C), Transwell assays without Matrigel (D), or Transwell assays with Matrigel (E) were used to evaluate the cell viability, growth capacity, and potential for cell migration and invasion, respectively. *, p < 0.05, **, p < 0.005.
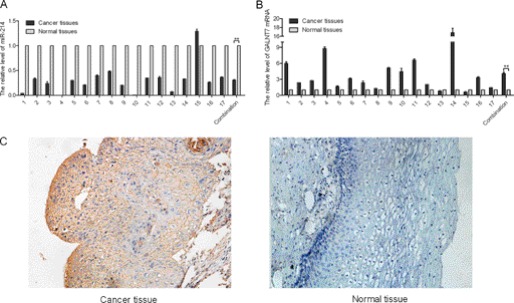
Expression of miR-214 and GALNT7 in Cervical Cancer and Normal Tissues
To detect the expression of miR-214 in human cervical cancer tissues, stem-loop qRT-PCR assay was performed on 17 pairs of cervical cancer and adjacent normal tissues. miR-214 was expressed at a lower level (Fig. 5A), whereas GALNT7 was expressed at a higher level in the tumor tissues when compared with the corresponding normal tissues (Fig. 5B). To further detect the level of GALNG7 in human cervical cancer tissues, we utilized an immunohistochemistry assay to detect the GALNT7 expression level in human cervical cancer tissues and adjacent normal tissues. As shown in Fig. 5C, the expression levels of GALNT7 were significantly higher than those in adjacent normal tissues, further supporting that miR-214 negatively regulates GALNT7.
FIGURE 5.
Differential expression of miR-214 and GALNT7 in cervical cancer tissues and adjacent normal tissues. A and B, the relative expression of miR-214 (A) and GALNT7 (B) in the 17 pairs, including cervical cancer tissues and matched normal tissues, was determined using qRT-PCR. C, expression of GALNT7 in cervical cancer tissue and normal tissues by immunohistochemistry (n = 10). **, p < 0.005.
DISCUSSION
Over the past few years, hundreds of miRNAs have been shown to play important roles in regulating gene expression through degradation of mRNA or repression of translation in a variety of model systems (28, 29). Evidence suggests that miRNAs may function as a novel class of both tumorigenic and tumor-suppressing genes (30). For example, miR-17–92 is significantly increased and plays a key role in tumorigenesis in both small cell lung cancers and human B-cell lymphomas (31, 32). Let-7 can directly regulate multiple cell cycle-associated tumorigenic proteins, including CDK6, CDC25a, and CCND2, and thus potentially acts as a tumor suppressor gene (33, 34). However, it has been reported that miR-214 is overexpressed in some cancers and may play an oncogenic role by targeting the FAP2C and PTEN tumor suppressors (6, 16, 17). Interestingly, miR-214 is down-regulated in human breast cancer tissues (14, 18–20), which is consistent with our results. One possible explanation is that biological molecules have different influences in different tumor cells. For example, KLF4 was found to be an oncogene in breast cancer (35), but Guan et al. (36) reported KLF4 to be a tumor suppressor in B-cell non-Hodgkin lymphoma and in classic Hodgkin lymphoma. Additionally, miRNAs have different functions in different tissues. For instance, miR-9 is up-regulated in breast cancer cells (37), but down-regulated in human ovarian cancer (38). miR-155 is significantly up-regulated in diffuse large B-cell lymphoma (39) and is down-regulated in human breast cancer (40). Depending on which factors drive tumorigenesis in the specific cellular milieu, the same miRNA may act as a tumor suppressor in some cancers and as a tumorigenic agent in others. Therefore, we speculate that cell-specific environments may account for the differences observed between miR-214 functions in cervical cancer when compared with other cancers.
Glycosyltransferases are a group of enzymes that catalyze the addition of monosaccharides to core proteins, and glycosylation is known to play a role in carcinogenesis and metastasis in many common cancers (41). For example, GALNT3 is overexpressed in human pancreatic ductal adenocarcinoma tissues and correlates with oncogenic activity (42). A recent study revealed that ectopic expression of miR-30b/30d promoted the metastatic behavior of melanoma cells by directly targeting GALNT7, which resulted in increased synthesis of the immunosuppressive cytokine IL-10, in addition to reduced immune cell activation and recruitment (5). miR-378 regulates nephronectin expression to modulate osteoblast differentiation by targeting GALNT7 (43). Here we show that GALNT7 is up-regulated in cervical cancer when compared with normal cervical tissues. GALNT7 promotes cell proliferation, migration, and invasiveness of both HeLa and C33A cervical cancer cells. These results suggest that GALNT7 may function as an oncogene in cervical cancer.
We identified GALNT7 as a target gene of miR-214. Our results can be summarized by seven major findings. (a) Previous microarray analysis demonstrated that GALNT7 expression was elevated 5.49-fold when miR-214 was suppressed using antisense oligonucleotides (19). (b) We used bioinformatic software, including TargetScan, miRanda, and PicTar algorithms, to predict that GALNT7 is a candidate target of miR-214. (c) We found that miR-214 is down-regulated, whereas GALNT7 is up-regulated in cervical cancer tissues when compared with adjacent normal tissues. (d) miR-214 negatively regulates GALNT7 at both mRNA and protein levels. (e) Expression of an EGFP reporter containing the 3′-UTR of GALNT7 was inhibited when miR-214 was overexpressed and activated when ASO-miR-214 was used. (f) miR-214 suppresses and GALNT7 promotes the proliferation, migration, and invasiveness of HeLa and C33A cells. (g) Restoration of GALNT7 counteracts the effects of miR-214. Accordingly, we conclude that miR-214 targets GALNT7. Although miR-30b/30d can target the GALNT7 transcript, nucleotides 171–208 in the 3′-UTR binding site are different from and do not overlap with the miR-214 binding site, nucleotides 71–77 of the 3′-UTR. Whether miR-214 and miR-30b/30d can simultaneously regulate GALNT7 in cervical cancer cells remains to be elucidated.
In conclusion, miR-214 is expressed at a low level in cervical cancer when compared with normal cervical tissues, and overexpression of miR-214 inhibits cell growth and invasion. A new target gene of miR-214, GALNT7, was found to be up-regulated in cervical cancer tissues. These findings indicate that inhibition of miR-214 in cervical cancer may contribute to the malignant phenotype by maintaining a high level of GALNT7. Thus, the identification of miR-214 and its target gene, GALNT7, in cervical cancer may help us to understand potential molecular mechanisms of tumorigenesis and may provide new prognostic markers for the management of cervical cancer in the future.
Supplementary Material
This work was supported by the National Natural Science Foundation of China (Grants 30873017 and 91029714, 31071191) and the Natural Science Foundation of Tianjin (Grants 08JCZDJC23300 and 09JCZDJC17500).

This article contains supplemental Fig. 1.
- miRNA
- microRNA
- miR
- microRNA
- pri-miR
- primary microRNA
- GALNT7
- UDP-N-acetyl-α-d-galactosamine:polypeptide N-acetylgalactosaminyltransferase 7
- ASO
- antisense oligonucleotide
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- EGFP
- enhanced green fluorescence protein
- qRT-PCR
- quantitative real-time polymerase chain reaction
- PTEN
- phosphatase and tensin homolog.
REFERENCES
- 1. Noordhuis M. G., Eijsink J. J., Roossink F., de Graeff P., Pras E., Schuuring E., Wisman G. B., de Bock G. H., van der Zee A. G. (2011) Prognostic cell biological markers in cervical cancer patients primarily treated with (chemo)radiation: a systematic review. Int. J. Radiat. Oncol. Biol. Phys. 79, 325–334 [DOI] [PubMed] [Google Scholar]
- 2. Ellenson L. H., Wu T. C. (2004) Focus on endometrial and cervical cancer. Cancer Cell 5, 533–538 [DOI] [PubMed] [Google Scholar]
- 3. Karlidag T., Cobanoglu B., Keles E., Alpay H. C., Ozercan I., Kaygusuz I., Yalcin S., Sakallioglu O. (2007) Expression of Bax, p53, and p27/kip in patients with papillary thyroid carcinoma with or without cervical nodal metastasis. Am. J. Otolaryngol. 28, 31–36 [DOI] [PubMed] [Google Scholar]
- 4. Lim L. P., Lau N. C., Weinstein E. G., Abdelhakim A., Yekta S., Rhoades M. W., Burge C. B., Bartel D. P. (2003) The microRNAs of Caenorhabditis elegans. Genes Dev. 17, 991–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gaziel-Sovran A., Segura M. F., Di Micco R., Collins M. K., Hanniford D., Vega-Saenz de Miera E., Rakus J. F., Dankert J. F., Shang S., Kerbel R. S., Bhardwaj N., Shao Y., Darvishian F., Zavadil J., Erlebacher A., Mahal L. K., Osman I., Hernando E. (2011) miR-30b/30d regulation of GalNAc transferases enhances invasion and immunosuppression during metastasis. Cancer Cell 20, 104–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Penna E., Orso F., Cimino D., Tenaglia E., Lembo A., Quaglino E., Poliseno L., Haimovic A., Osella-Abate S., De Pittà C., Pinatel E., Stadler M. B., Provero P., Bernengo M. G., Osman I., Taverna D. (2011) MicroRNA-214 contributes to melanoma tumor progression through suppression of TFAP2C. EMBO J. 30, 1990–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gao X. N., Lin J., Li Y. H., Gao L., Wang X. R., Wang W., Kang H. Y., Yan G. T., Wang L. L., Yu L. (2011) MicroRNA-193a represses c-kit expression and functions as a methylation-silenced tumor suppressor in acute myeloid leukemia. Oncogene 30, 3416–3428 [DOI] [PubMed] [Google Scholar]
- 8. Yanaihara N., Caplen N., Bowman E., Seike M., Kumamoto K., Yi M., Stephens R. M., Okamoto A., Yokota J., Tanaka T., Calin G. A., Liu C. G., Croce C. M., Harris C. C. (2006) Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell 9, 189–198 [DOI] [PubMed] [Google Scholar]
- 9. Wang R., Wang Z. X., Yang J. S., Pan X., De W., Chen L. B. (2011) MicroRNA-451 functions as a tumor suppressor in human non-small cell lung cancer by targeting ras-related protein 14 (RAB14). Oncogene 30, 2644–2658 [DOI] [PubMed] [Google Scholar]
- 10. Rao X., Di Leva G., Li M., Fang F., Devlin C., Hartman-Frey C., Burow M. E., Ivan M., Croce C. M., Nephew K. P. (2011) MicroRNA-221/222 confers breast cancer fulvestrant resistance by regulating multiple signaling pathways. Oncogene 30, 1082–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Patel J. B., Appaiah H. N., Burnett R. M., Bhat-Nakshatri P., Wang G., Mehta R., Badve S., Thomson M. J., Hammond S., Steeg P., Liu Y., Nakshatri H. (2011) Control of EVI-1 oncogene expression in metastatic breast cancer cells through microRNA miR-22. Oncogene 30, 1290–1301 [DOI] [PubMed] [Google Scholar]
- 12. Yao J., Liang L., Huang S., Ding J., Tan N., Zhao Y., Yan M., Ge C., Zhang Z., Chen T., Wan D., Yao M., Li J., Gu J., He X. (2010) MicroRNA-30d promotes tumor invasion and metastasis by targeting Gαi2 in hepatocellular carcinoma. Hepatology 51, 846–856 [DOI] [PubMed] [Google Scholar]
- 13. Fang J. H., Zhou H. C., Zeng C., Yang J., Liu Y., Huang X., Zhang J. P., Guan X. Y., Zhuang S. M. (2011) MicroRNA-29b suppresses tumor angiogenesis, invasion, and metastasis by regulating matrix metalloproteinase 2 expression. Hepatology 54, 1729–1740 [DOI] [PubMed] [Google Scholar]
- 14. Qiang R., Wang F., Shi L. Y., Liu M., Chen S., Wan H. Y., Li Y. X., Li X., Gao S. Y., Sun B. C., Tang H. (2011) Plexin-B1 is a target of miR-214 in cervical cancer and promotes the growth and invasion of HeLa cells. Int. J. Biochem. Cell Biol. 43, 632–641 [DOI] [PubMed] [Google Scholar]
- 15. Au Yeung C. L., Tsang T. Y., Yau P. L., Kwok T. T. (2011) Human papillomavirus type 16 E6 induces cervical cancer cell migration through the p53/microRNA-23b/urokinase-type plasminogen activator pathway. Oncogene 30, 2401–2410 [DOI] [PubMed] [Google Scholar]
- 16. Yang H., Kong W., He L., Zhao J. J., O'Donnell J. D., Wang J., Wenham R. M., Coppola D., Kruk P. A., Nicosia S. V., Cheng J. Q. (2008) MicroRNA expression profiling in human ovarian cancer: miR-214 induces cell survival and cisplatin resistance by targeting PTEN. Cancer Res. 68, 425–433 [DOI] [PubMed] [Google Scholar]
- 17. Xiong X., Ren H. Z., Li M. H., Mei J. H., Wen J. F., Zheng C. L. (2011) Down-regulated miRNA-214 induces a cell cycle G1 arrest in gastric cancer cells by up-regulating the PTEN protein. Pathol. Oncol. Res. 17, 931–937 [DOI] [PubMed] [Google Scholar]
- 18. Derfoul A., Juan A. H., Difilippantonio M. J., Palanisamy N., Ried T., Sartorelli V. (2011) Decreased microRNA-214 levels in breast cancer cells coincides with increased cell proliferation, invasion, and accumulation of the Polycomb Ezh2 methyltransferase. Carcinogenesis 32, 1607–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yang Z., Chen S., Luan X., Li Y., Liu M., Li X., Liu T., Tang H. (2009) MicroRNA-214 is aberrantly expressed in cervical cancers and inhibits the growth of HeLa cells. IUBMB Life 61, 1075–1082 [DOI] [PubMed] [Google Scholar]
- 20. Rao Q., Zhou H., Peng Y., Li J., Lin Z. (2011) Aberrant microRNA expression in human cervical carcinomas. Med. Oncol. doi: 10.1007/s12032-011-9830-2 [DOI] [PubMed] [Google Scholar]
- 21. Ten Hagen K. G., Hagen F. K., Balys M. M., Beres T. M., Van Wuyckhuyse B., Tabak L. A. (1998) Cloning and expression of a novel, tissue specifically expressed member of the UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase family. J. Biol. Chem. 273, 27749–27754 [DOI] [PubMed] [Google Scholar]
- 22. Strous G. J. (1979) Initial glycosylation of proteins with acetylgalactosaminylserine linkages. Proc. Natl. Acad. Sci. U.S.A. 76, 2694–2698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brockhausen I. (1999) Pathways of O-glycan biosynthesis in cancer cells. Biochim. Biophys. Acta 1473, 67–95 [DOI] [PubMed] [Google Scholar]
- 24. Liu T., Tang H., Lang Y., Liu M., Li X. (2009) MicroRNA-27a functions as an oncogene in gastric adenocarcinoma by targeting prohibitin. Cancer Lett. 273, 233–242 [DOI] [PubMed] [Google Scholar]
- 25. Schmittgen T. D., Livak K. J. (2008) Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 3, 1101–1108 [DOI] [PubMed] [Google Scholar]
- 26. Tang H., Tang X. Y., Liu M., Li X. (2008) Targeting α-fetoprotein represses the proliferation of hepatoma cells via regulation of the cell cycle. Clin. Chim. Acta. 394, 81–88 [DOI] [PubMed] [Google Scholar]
- 27. Lai K. W., Koh K. X., Loh M., Tada K., Subramaniam M. M., Lim X. Y., Vaithilingam A., Salto-Tellez M., Iacopetta B., Ito Y., Soong R. (2010) MicroRNA-130b regulates the tumor suppressor RUNX3 in gastric cancer. Eur. J. Cancer 46, 1456–1463 [DOI] [PubMed] [Google Scholar]
- 28. Bartel D. P. (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281–297 [DOI] [PubMed] [Google Scholar]
- 29. Lagos-Quintana M., Rauhut R., Lendeckel W., Tuschl T. (2001) Identification of novel genes coding for small expressed RNAs. Science 294, 853–858 [DOI] [PubMed] [Google Scholar]
- 30. Zhang B., Pan X., Cobb G. P., Anderson T. A. (2007) MicroRNAs as oncogenes and tumor suppressors. Dev. Biol. 302, 1–12 [DOI] [PubMed] [Google Scholar]
- 31. Hayashita Y., Osada H., Tatematsu Y., Yamada H., Yanagisawa K., Tomida S., Yatabe Y., Kawahara K., Sekido Y., Takahashi T. (2005) A polycistronic microRNA cluster, miR-17–92, is overexpressed in human lung cancers and enhances cell proliferation. Cancer Res. 65, 9628–9632 [DOI] [PubMed] [Google Scholar]
- 32. He L., Thomson J. M., Hemann M. T., Hernando-Monge E., Mu D., Goodson S., Powers S., Cordon-Cardo C., Lowe S. W., Hannon G. J., Hammond S. M. (2005) A microRNA polycistron as a potential human oncogene. Nature 435, 828–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Johnson S. M., Grosshans H., Shingara J., Byrom M., Jarvis R., Cheng A., Labourier E., Reinert K. L., Brown D., Slack F. J. (2005) RAS is regulated by the let-7 microRNA family. Cell 120, 635–647 [DOI] [PubMed] [Google Scholar]
- 34. Johnson C. D., Esquela-Kerscher A., Stefani G., Byrom M., Kelnar K., Ovcharenko D., Wilson M., Wang X., Shelton J., Shingara J., Chin L., Brown D., Slack F. J. (2007) The let-7 microRNA represses cell proliferation pathways in human cells. Cancer Res. 67, 7713–7722 [DOI] [PubMed] [Google Scholar]
- 35. Al-Nedawi K., Meehan B., Micallef J., Lhotak V., May L., Guha A., Rak J. (2008) Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumor cells. Nat. Cell Biol. 10, 619–624 [DOI] [PubMed] [Google Scholar]
- 36. Guan H., Xie L., Leithäuser F., Flossbach L., Möller P., Wirth T., Ushmorov A. (2010) KLF4 is a tumor suppressor in B-cell non-Hodgkin lymphoma and in classic Hodgkin lymphoma. Blood 116, 1469–1478 [DOI] [PubMed] [Google Scholar]
- 37. Ma L., Young J., Prabhala H., Pan E., Mestdagh P., Muth D., Teruya-Feldstein J., Reinhardt F., Onder T. T., Valastyan S., Westermann F., Speleman F., Vandesompele J., Weinberg R. A. (2010) miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat. Cell Biol. 12, 247–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li J., Wang C. Y. (2008) TBL1-TBLR1 and β-catenin recruit each other to Wnt target-gene promoter for transcription activation and oncogenesis. Nat. Cell Biol. 10, 160–169 [DOI] [PubMed] [Google Scholar]
- 39. Eis P. S., Tam W., Sun L., Chadburn A., Li Z., Gomez M. F., Lund E., Dahlberg J. E. (2005) Accumulation of miR-155 and BIC RNA in human B-cell lymphomas. Proc. Natl. Acad. Sci. U.S.A. 102, 3627–3632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Iorio M. V., Ferracin M., Liu C. G., Veronese A., Spizzo R., Sabbioni S., Magri E., Pedriali M., Fabbri M., Campiglio M., Ménard S., Palazzo J. P., Rosenberg A., Musiani P., Volinia S., Nenci I., Calin G. A., Querzoli P., Negrini M., Croce C. M. (2005) MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 65, 7065–7070 [DOI] [PubMed] [Google Scholar]
- 41. Casey R. C., Oegema T. R., Jr., Skubitz K. M., Pambuccian S. E., Grindle S. M., Skubitz A. P. (2003) Cell membrane glycosylation mediates the adhesion, migration, and invasion of ovarian carcinoma cells. Clin. Exp. Metastasis 20, 143–152 [DOI] [PubMed] [Google Scholar]
- 42. Taniuchi K., Cerny R. L., Tanouchi A., Kohno K., Kotani N., Honke K., Saibara T., Hollingsworth M. A. (2011) Overexpression of GalNAc-transferase GalNAc-T3 promotes pancreatic cancer cell growth. Oncogene 30, 4843–4854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kahai S., Lee S. C., Lee D. Y., Yang J., Li M., Wang C. H., Jiang Z., Zhang Y., Peng C., Yang B. B. (2009) MicroRNA miR-378 regulates nephronectin expression modulating osteoblast differentiation by targeting GalNT-7. PLoS One 4, e7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.