Background: The P450 arachidonic acid metabolite, 20-HETE, is potently vasoactive and structurally related to known TRPV1 agonists.
Results: 20-HETE activates native murine and heterologously expressed human TRPV1, and sensitizes both wild-type and the hTRPV1 S502A mutant to stimulation by capsaicin and acidic pH.
Conclusion: 20-HETE is a novel potential endogenous activator of TRPV1.
Significance: The biological activity of 20-HETE may be partly mediated by its action on TRPV1.
Keywords: Arachidonic Acid, Neurons, Patch Clamp, Protein Expression, TRP Channels, Sensory Neuron
Abstract
TRPV1 is a member of the transient receptor potential ion channel family and is gated by capsaicin, the pungent component of chili pepper. It is expressed predominantly in small diameter peripheral nerve fibers and is activated by noxious temperatures >42 °C. 20-Hydroxyeicosatetraenoic acid (20-HETE) is a cytochrome P-450 4A/4F-derived metabolite of the membrane phospholipid arachidonic acid. It is a powerful vasoconstrictor and has structural similarities with other TRPV1 agonists, e.g. the hydroperoxyeicosatetraenoic acid 12-HPETE, and we hypothesized that it may be an endogenous ligand for TRPV1 in sensory neurons innervating the vasculature. Here, we demonstrate that 20-HETE both activates and sensitizes mouse and human TRPV1, in a kinase-dependent manner, involving the residue Ser502 in heterologously expressed hTRPV1, at physiologically relevant concentrations.
Introduction
The transient receptor potential vanilloid 1 channel (TRPV1)3 is a nonselective cation channel expressed in primary sensory neurons and implicated in thermal hyperalgesia (1, 2). It is a polymodal ion channel, gated by ligands, noxious heat, acid, and by voltage (3), and its responsiveness can be modulated by G protein-coupled receptor activation, e.g. bradykinin B2 (4, 5). Gating modulation is achieved through kinase-mediated phosphorylation of specific intracellular channel residues (4, 6, 7). Kinases that have been implicated in modulating TRPV1 include PKA, PKC, Ca2+/calmodulin-dependent kinase II, and Src kinase (for review, see Ref. 3). As with other kinase-dependent phenomena, recent observations suggest that the localization of this kinase activity at TRPV1 is facilitated by the formation of a complex involving the scaffolding protein AKAP79 (8).
The search for an endogenous chemical activator for TRPV1 has identified lipids as likely candidates because the molecules exhibit various degrees of structural similarity with capsaicin. The first such lipid proposed was anandamide (9). However, further assessment of other potential lipid candidates implicated the lipoxygenase-derived metabolites of the membrane phospholipid arachidonic acid, with hydroperoxyeicosatetraenoic acid 12-HPETE being the most potent activator of TRPV1 (10).
We have become interested in the possibility that a different arachidonic acid metabolite, generated by cytochrome P-450 4A/4F activity, 20-hydroxyeicosatetraenoic acid (20-HETE; supplemental Fig. 1) might be an endogenous activator of TRPV1. The reasons underlying this thesis is that 20-HETE exhibits potent vascular activity (11–15), and we have reported previously that constriction of mesenteric resistance arteries induced either by raised intraluminal pressure or by exposure to 20-HETE is blocked by capsazepine (16), a selective antagonist for TRPV1 (17). The effect of capsazepine is suggestive of a role for perivascular C-fibers in vascular reactivity and a direct activation of neuronally expressed TRPV1 by 20-HETE (16). However, whether this is the case or not has not been demonstrated previously. In this study, we show that 20-HETE is both an activator and sensitizer of TRPV1 and implicate protein kinase activity in the responses to this lipid. Our findings help provide a hitherto unsubstantiated link between TRPV1 channel activation and 20-HETE, hypothesized to explain a role for the metabolite in vascular responses.
EXPERIMENTAL PROCEDURES
Dorsal Root Ganglion (DRG) Cultures
Male and female wild-type (WT, C57BL/6) and TRPV1 knock-out (KO) mice (1) were used to generate primary neuronal cultures. Whole DRGs were dissected free from the spine, washed in sterile DMEM (PAA), and pooled in a Ca2+- and Mg2+-free HEPES-buffered dissociation solution for 20 min at 37 °C, containing 152 mm NaCl, 3 mm KCl, 10 mm hemiNa-HEPES, 1.5 mm Na2HPO4, and 10 mm glucose, 0.6 mg ml−1 collagenase type XI, and 1 mg ml−1 protease type IX. The sample was triturated and then washed in enzyme-free DMEM solution. Finally, the dissociated cells were plated onto poly-l-lysine-coated glass coverslips and maintained in normal heat-inactivated serum (10%, PAA) and antibiotic (penicillin-streptomycin, 200 units and 0.2 mg ml−1, respectively)-supplemented DMEM in a 37 °C, 5% CO2 incubator. Recording took place 24–48 h later. All reagents and materials were purchased from Sigma-Aldrich unless otherwise stated.
Cell Transfection
HEK293 cells were plated in 35-mm diameter Petri dishes and maintained in DMEM the day before transfection. cDNA of human TRPV1 (a gift from Professor David Julius; University of California, San Francisco) and fluorescent pEGFP-N3 (Clontech) vectors were transfected using Lipofectamine 2000 (Invitrogen) for 3 h at 37 °C. The Lipofectamine and DNA was then removed and replaced with normal culture medium. Recordings were made after 24 h.
Whole Cell Voltage Clamp
Recordings were made from small (<25-μm apparent diameter) primary sensory neurons or HEK293 cells using an Axopatch 1D amplifier (Molecular Devices). The solutions used for voltage clamp experiments were (intracellular): 143 mm KCl, 3 mm NaEGTA, 10 mm HEPES, 1.21 mm CaCl2, 1.21 mm MgCl2, 3 mm MgATP, and 0.5 mm Li GTP, and (extracellular): 140 mm NaCl, 10 mm HEPES, 2.1 mm CaCl2, 2.12 mm MgCl2, and 2.5 mm KCl. Both solutions were buffered to 7.2–3 using NaOH for all experiments except in those experiments where the effects of acidic pH were investigated. For acid stimuli the extracellular solution was modified with the equimolar substitution of HEPES with MES, and the pH buffered to 5.5. Microelectrodes were pulled from thin walled glass capillaries (Harvard Apparatus) and had initial resistances of 1.5–2.5 megohms when filled with intracellular solutions. Membrane currents were recorded at a holding potential of −60 mV, without leak subtraction, and acquired using a Digidata 1440A A-D converter (Molecular Devices) with pClamp 10.0 software (Molecular Devices) on a PC (Dell). Sweeps were typically of long duration >40 s and sampled at 1 KHz. Solutions containing drugs, e.g. capsaicin (Sigma-Aldrich), 12-hydroperoxyeicosatraenoic acid (12-HPETE Cayman Chemical), and 20-HETE (Cayman Chemical), and vehicle control solutions (all arachidonate-derived lipids were dissolved in saline) were applied to neurons undergoing patch clamp by local superfusion (0.5 ml min−1, for 10 s). Experimental solutions were changed using a stepping-motor driven rapid solution-exchange device (RSC-160, Bio-Logic SAS, France; tube to adjacent tube switching time 11 ms). The solution changer was driven by protocols in the acquisition program pClamp 10.0. Application of the protein kinase inhibitors at concentrations that exhibit relative selectivity was achieved using intracellular dialysis with H7 and H89 (Sigma-Aldrich) and PKCϵ translocation inhibitor peptide (iPKCϵ; Merck, Calbiochem). Control recordings were conducted in the presence of vehicle (0.02% dimethyl sulfoxide). All experiments were performed at room temperature (20–22 °C).
Unitary Current Recording
Recordings were made in voltage clamp mode from neuronal inside-out membrane patches, held at 60 mV. Symmetrical solutions were used, containing 140 mm sodium gluconate, 10 mm NaCl, 1 mm MgCl2, 10 mm HEPES, 1 mm NaEGTA, buffered to pH 7.3 with NaOH. 20-HETE was applied to the patch by superfusion, using the rapid solution exchanger.
Calcium Imaging
DRG neurons were loaded with Fluo-4 Direct (Molecular Probes) for 60 min at 37 °C. The dye was excited at 495/516 nm, and emitted fluorescence was filtered with a 480/520-nm bandpass filter, and subsequently time-lapse epifluorescence images were captured for single neurons. Fluorescence image (F) analysis was performed using Metamorph imaging software (Universal Imaging).
Site-directed Mutagenesis
The serine at amino acid position 502 of hTRPV1 was mutated to alanine (S502A). Point mutation was introduced into the pcDNA 3.0 plasmid containing human TRPV1 using the QuikChange II XL site-directed mutagenesis kit (Stratagene) according to the manufacturer's instructions and confirmed by DNA sequencing.
RESULTS
20-HETE Activates Human TRPV1
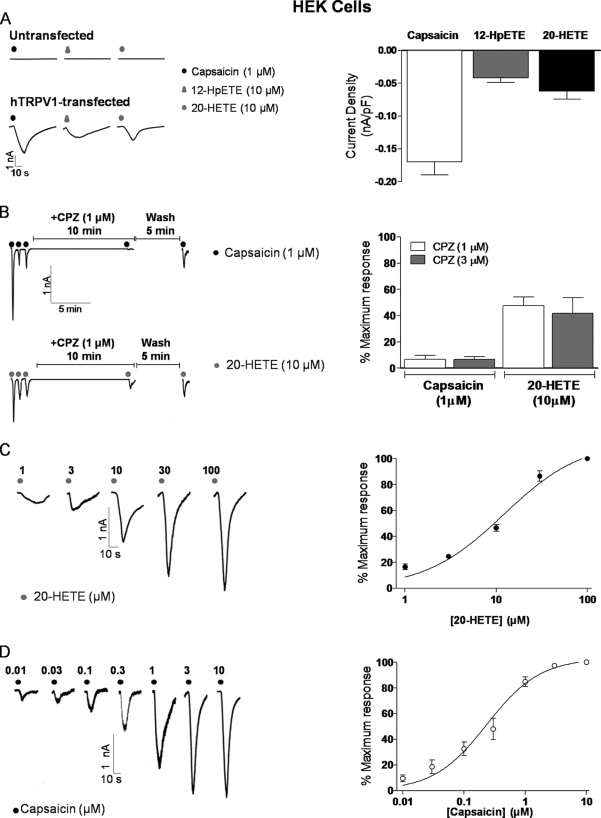
Human TRPV1 cDNA was transfected into HEK293 cells, and responses to capsaicin, 12-HPETE (the most potent endogenous TRPV1 activator identified to date) (10) and 20-HETE were measured by whole cell voltage clamping. 20-HETE (10 μm) application evoked an inward and slowly desensitizing current, as did both 1 μm capsaicin and 10 μm 12-HPETE (Fig. 1A). Repeated application of either 20-HETE or capsaicin resulted in apparent tachyphylaxis of the response, a phenomenon characteristic of TRPV1 activation (Fig. 1B). In contrast, no currents were observed in response to application of any ligand in untransfected HEK293 cells under the same conditions (Fig. 1A). Treatment of cells with capsazepine (1 and 3 μm, 10 min) significantly inhibited responses to both capsaicin (p < 0.001) and 20-HETE (p < 0.01 and <0.03) (Fig. 1B). The effect of 20-HETE was concentration-dependent with an EC50 value of 12.04 ± 1.47 μm (using a one-site binding equation giving an R2 = 0.950) which is ∼25 times higher than that found for capsaicin, EC50 0.23 ± 0.03 μm (using a one-site binding equation giving an R2 = 0.938) (Fig. 1, C and D).
FIGURE 1.
20-HETE is a novel activator for hTRPV1. A, left, currents evoked in response to 1 μm capsaicin, 10 μm 12-HPETE, and 10 μm 20-HETE in untransfected and hTRPV1-transfected HEK293 cells. A, right, summary of peak current densities evoked by 1 μm capsaicin (n = 38 cells of 13 individual experiments), 10 μm 12-HPETE (n = 10 cells of 4 experiments), and 10 μm 20-HETE (n = 35 cells of 14 experiments). B, effects of capsazepine on capsaicin and 20-HETE-evoked currents in hTRPV1-transfected HEK293 cells. 1–3 μm capsazepine decreases the response to 1 μm capsaicin (n = 6 cells of 3 experiments) and 10 μm 20-HETE (n = 5–6 cells of 3 experiments). C and D, concentration-peak current response curve for 0.01–10 μm capsaicin (C); responses normalized to response to 10 μm (n = 7 cells of 4 experiments) and 1–100 μm 20-HETE (D); responses normalized to the response to 100 μm (n = 7 cells of 4 experiments) in hTRPV1-transfected HEK293 cells. All summary data are shown as mean ± S.E. (error bars).
20-HETE Activates Native Mouse TRPV1
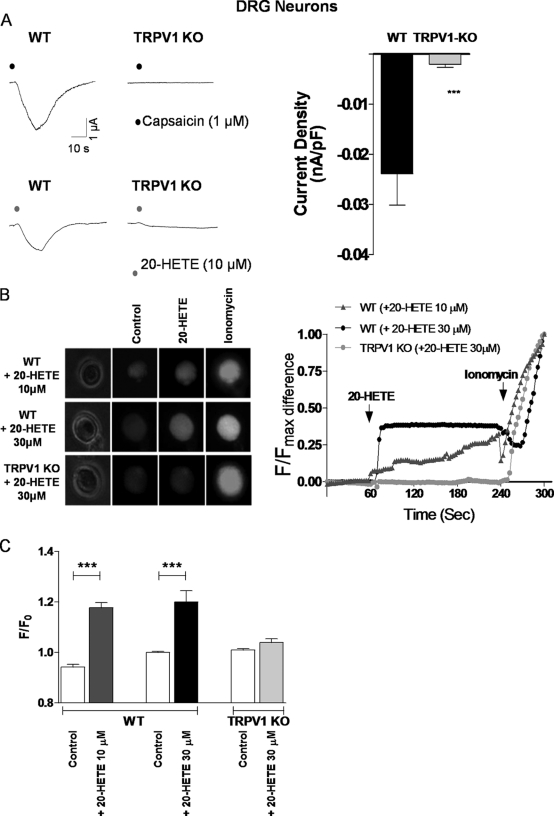
In whole cell voltage clamping experiments, with a holding potential of −60 mV, 10 μm 20-HETE generated inward currents in DRG neurons collected from WT mice, whereas responses in neurons from TRPV1 KO mice were significantly and substantially (≈90%) reduced (Fig. 2A). Furthermore, calcium imaging using the calcium sensitive dye, Fluo-4 AM, demonstrated that treatment of DRGs with 20-HETE (10–30 μm) (Fig. 2, B and C) or capsaicin (10 μm; ratio of F/F0 of WT = 1.21 ± 0.02 and TRPV1 KO = 1.02 ± 0.002 (n = 20 and 21, from 3 and 3 mice, respectively)) at 37 °C also elevated [Ca2+]i in cells isolated from WT but not TRPV1 KO mice. The profile of calcium elevation to either capsaicin or 20-HETE closely resembled that demonstrated using this technique by others in response to oleoylethanolamide (18) and selective vanilloid ligands (19). The response to the calcium ionophore, ionomycin, loaded into the bath at the end of each experiment as a positive control, was similar for both WT and TRPV1 KO neurons (ratio of F/F0 of WT = 2.4 ± 0.2 and TRPV1 KO = 2.1 ± 0.1, n = 40 from 4 mice, and n = 70 cells from 10 mice, respectively, Fig. 2D).
FIGURE 2.
20-Hete activates native murine TRPV1. A, representative traces of 1 μm capsaicin (upper) and 10 μm 20-HETE (lower)-evoked currents in voltage-clamped DRG neurons of WT and TRPV1 KO mice. Statistical significance was determined using unpaired Student's t test for n = 39 and 38 neurons of 10 and 9 mice, WT and KO, respectively, and is shown as ***, p < 0.001. B, Ca2+ imaging (Fluo-4 AM) of responses to 10–30 μm 20-HETE and 50 μm ionomycin in DRG neurons of WT and TRPV1 KO mice (left). Examples of fluorescence intensity (F/Fmax) measured against time are shown on the right. C, summary of F/F0 responses. Statistical significance was determined using unpaired Student's t test of n = 29, 28, and 16 neurons of 4, 4, and 3 mice, respectively, and shown as ***, p < 0.001). All summary data are shown as mean ± S.E. (error bars).
20-HETE Activation of TRPV1 Requires Ser502 Residue
In hTRPV1-transfected HEK cells responses to both 20-HETE and capsaicin underwent desensitization, a phenomenon consistent with the known activity of capsaicin and other agents that directly gate channel opening (3, 20). Assessment of 20-HETE effects in excised patches demonstrated an apparent delayed 20-HETE-evoked channel opening (supplemental Fig. 2) with characteristics reminiscent of that demonstrated in response to other structurally related lipids (18). These findings are suggestive of direct activation of TRPV1 by 20-HETE, although the apparent delay in activity may indicate a requirement of additional signaling prior to channel activation. Further investigation and identification of possible binding sites for 20-HETE are warranted.
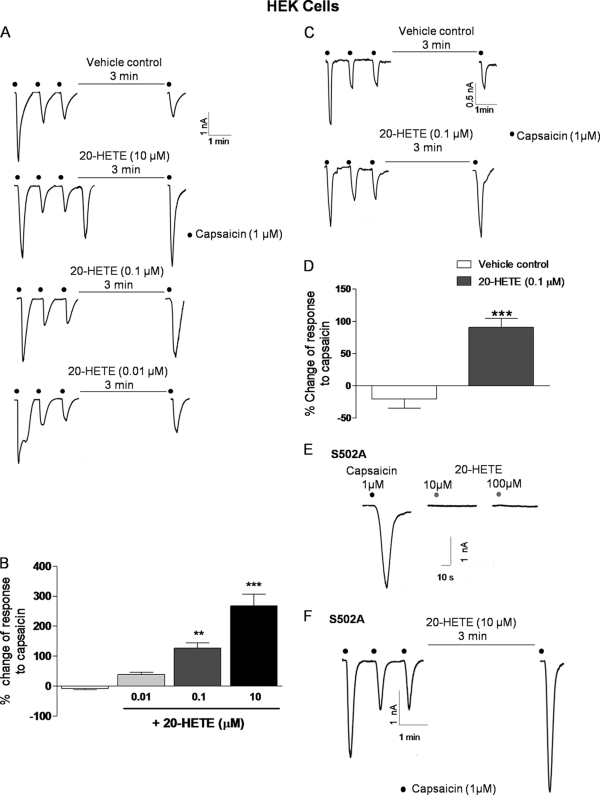
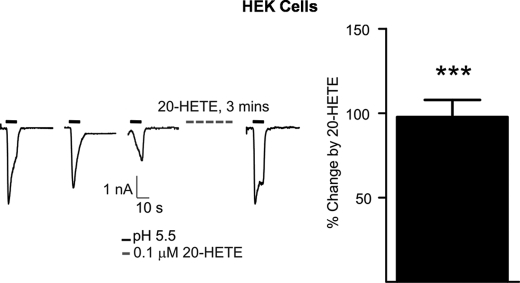
In addition to direct activation, our findings demonstrate that 20-HETE acts as a “modulator” of TRPV1 activity. A 3-min exposure to 10 μm 20-HETE, following capsaicin desensitization, induced a substantial inward current but also potentiated the response to a subsequent exposure to 1 μm capsaicin (Fig. 3A). This subsequent potentiation was not related to direct TRPV1 gating by 20-HETE because lower nm concentrations of 20-HETE, which did not stimulate channel opening, also enhanced responses to capsaicin. This effect was concentration-dependent (Fig. 3, A and B) and similarly evident in DRG neurons (Fig. 3, C and D). To determine whether these sensitizing effects of 20-HETE might also occur in response to stimuli encountered in vivo we investigated the effects of this same low concentration of 20-HETE on acidic pH-induced channel activation. Exposure of cells to pH 5.5 resulted in typical TRPV1 channel gating as shown by others that suffered desensitization with repeat exposure. Incubation of cells for 3 min with 0.1 μm 20-HETE significantly (n = 5 cells from three independent experiments) enhanced the subsequent response to acid by ∼100% (Fig. 4).
FIGURE 3.
20-HETE potentiates the response to capsaicin, and the action of 20-HETE on hTPRV1 requires Ser502. A, 1 μm capsaicin-evoked currents in WT hTRPV1-transfected HEK293 cells under control conditions (top panel) and following exposure to 0.01–10 μm 20-HETE for 3 min. B, summary data demonstrating concentration-dependent sensitization of capsaicin responses by 20-HETE (control, n = 11 cells of 8 experiments) and 0.01, 0.1, and 10 μm 20-HETE (n = 6 cells of 3 experiments, n = 8 cells of 4 experiments, and n = 13 cells of 6 experiments, respectively). Statistical significance was determined using one-way ANOVA with Dunnett's post tests and is shown as **, p < 0.01, and ***, p < 0.001. Error bars, S.E. C, representative traces of currents evoked by 1 μm capsaicin on DRG neurons of WT mice under control conditions (upper) and 0.1 μm 20-HETE (lower) treatment for 3 min. D, summary of the potentiating effects of 0.1 μm 20-HETE on DRG neurons from WT mice. Statistical significance was determined using Student's t test and is shown as ***, p < 0.001, n = 6 neurons of 3 mice. E, example traces of 1 μm capsaicin and 10–100 μm 20-HETE (upper)-evoked currents in S502A mutant. Effects of a 3-min 10 μm 20-HETE treatment on the response to 1 μm capsaicin in S502A mutant are shown in lower panel (n = 8 cells of 5 experiments). All summary data are shown as mean ± S.E. (error bars).
FIGURE 4.
20-HETE potentiates the response of hTRPV1 to low extracellular pH (5. 5) in transfected HEK293 cells. 20-HETE (0.1 μm for 3 min) exposure increases the amplitude of the acid evoked current (left) by 97.68 ± 8.29%, ***, p = 0.001, one-sample t test, n = 5 (right).
Desensitization of TRPV1 is thought to be a process dependent upon a number of factors, including surface channel expression, intracellular calcium levels, and the phosphorylation state of the channel (3). Phosphorylation also plays a major role in determining the enhanced sensitivity of TRPV1 in inflammatory processes (for review, see Ref. 3). A key phosphorylation site for TRPV1 channel sensitization is residue Ser502, which has been identified as a common target for phosphorylation by PKA, PKC, and Ca2+/calmodulin-dependent kinase II (3). To test whether the effects of 20-HETE might be related to an effect at this site we investigated the effect of substitution of Ser502 with a nonpolar amino acid alanine on responses in transfected HEK293 cells heterologously expressing this mutant. The maximum response to capsaicin remained unchanged compared with cells expressing WT hTRPV1, in agreement with recently published findings (21) (peak current density response to 1 μm capsaicin in Ser502 mutant of −0.15 ± 0.05 nA/pf, n = 14 in four experiments, compared with WT, −0.17 ± 0.02 nA/pf, n = 38 in 13 experiments, p = 0.621). In contrast, direct channel gating by 20-HETE in the Ser502 mutant was entirely absent (Fig. 3E), although 20-HETE-induced potentiation of capsaicin was unaffected by this mutation. These findings implicate the Ser502 residue in the direct responses to 20-HETE but not 20-HETE-induced sensitization.
Protein Kinase Inhibitors Prevent 20-HETE-induced Gating and Sensitization of TRPV1
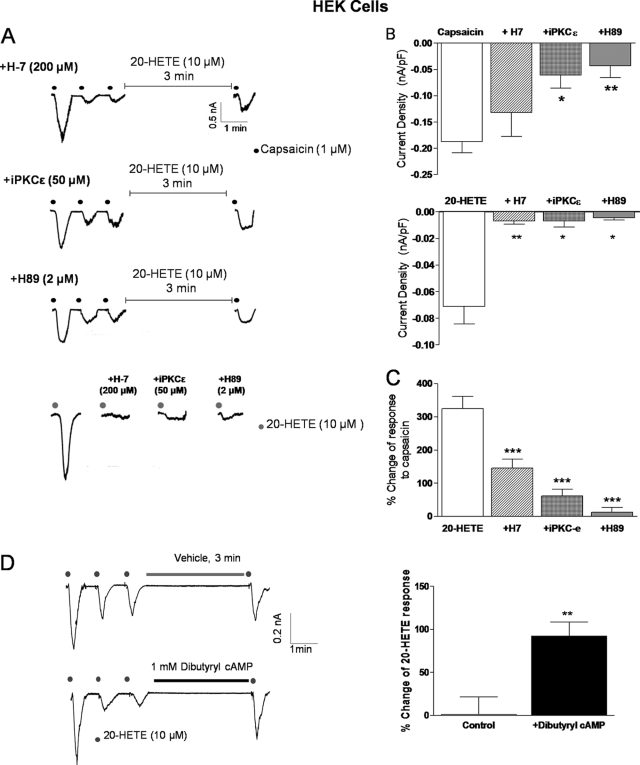
Alteration of responses to 20-HETE following mutation of a key site for phosphorylation implicates a protein kinase-dependent pathway. To test this possibility further we investigated the effects of three protein kinase inhibitors at concentrations previously demonstrated to express selectivity for their specific targets. These agents have also been used previously in demonstrating the importance of TRPV1 phosphorylation pathways; H-7, a broad band protein kinase inhibitor for PKA and PKC (200 μm), PKC-ϵ translocation inhibitor peptide (iPKCϵ, 50 μm) (22, 23) because this isoform has been implicated particularly in TRPV1 sensitization (4, 24) and H-89 (2 μm), a potent selective PKA inhibitor that binds competitively to the ATP pocket of the kinase catalytic subunit (25). These inhibitors were added to the internal voltage clamp recording solution. H-7, iPKC-ϵ, and H-89 all substantially attenuated direct gating of the channel by 20-HETE (10 μm, p < 0.01, <0.05, and <0.05, respectively, Fig. 5, A and B). In addition, 20-HETE-induced sensitization of responses to capsaicin was also attenuated (1 μm, all p < 0.001; Fig. 5C), with the greatest apparent inhibitory activity exhibited by H-89 (Fig. 5C). Comparison of 1 μm capsaicin-induced currents in vehicle control and inhibitor-treated cells suggests that protein kinase activity, and therefore likely phosphorylation, is important for capsaicin-induced TRPV1 activation (Fig. 5B) in support of recent observations (21). In addition, further support for a role for PKA-induced alteration of 20-HETE activity is provided by the observation that treatment with the cell-permeant cAMP analog dibutyryl-cAMP significantly enhanced responses to 20-HETE (Fig. 5D).Thus, our results are consistent with activation of PKA and, albeit to a lesser extent, PKC, in mediation of 20-HETE-induced direct channel gating and sensitization.
FIGURE 5.
Role of protein kinases in 20-HETE-induced TRPV1 activation. A, example currents evoked by 1 μm capsaicin in hTRPV1-transfected HEK293 cells before and after treatment with 10 μm 20-HETE (3 min) in the presence of continuous intracellular dialysis with vehicle (0.05% dimethyl sulfoxide) or protein kinase inhibitors H-7 (200 μm), PKCϵ translocation inhibitor peptide (iPKCϵ, 50 μm) or H-89 (2 μm). B, summary of effects of inhibition of protein kinase activity on the peak amplitude response to 1 μm capsaicin (upper, n = 33, 19, 9, and 10 cells of 13, 4, 3, and 3 experiments) and 10 μm 20-HETE (lower, n = 36, 13, 8, and 8 cells of 14, 4, 3, and 3 experiments for control and in the presence of inhibitors, respectively). Statistical significance was determined using one-way ANOVA followed by Dunnett's post test and is shown as **, p < 0.01 and *, p < 0.05. C, summary of effects of protein kinase inhibitors on 10 μm 20-HETE-induced potentiation of 1 μm capsaicin-evoked currents (n = 11, 12, 8, and 8 cells of n = 5, 4, 3, and 3 experiments, respectively). Statistical significance was determined using one-way ANOVA followed by Dunnett's post test and is shown as ***, p < 0.001. D, example currents evoked by 10 μm 20-HETE and summary data (right) in hTRPV1-transfected HEK293 cells before and after treatment with 1 mm dibutyryl-cAMP (3 min, n = 9 cells of 3 independent experiments). All summary data are shown as mean ± S.E. (error bars).
DISCUSSION
Here, we demonstrate that the arachidonate metabolite 20-HETE activates TRPV1 and thereby identify a novel potential endogenous activator of neuronal TRPV1. Our findings suggest that 20-HETE acts in two distinct ways on TRPV1. First, we have shown that exposing TRPV1 to 20-HETE gates the channel, possibly by a direct interaction. Our work implicates Ser502 in this effect because mutation of this site renders the channel inoperable by 20-HETE, while retaining sensitivity to capsaicin. Furthermore, block of either PKC or PKA using relatively selective inhibitors reduces the amplitude of currents evoked by 20-HETE applications, suggesting that protein kinase activity (and therefore possibly phosphorylation) is required for 20-HETE-induced interaction and gating of the channel. In particular H-89 produced the greatest inhibition, implicating PKA predominantly in the effects evidenced in our experiments. An important caveat to consider, however, is the suggestion that H-89 exhibits off-target effects including inhibition of both the nuclear kinases S6K1 and MSK-1 with Ki values equal or lower than that for PKA (26). Although to date there is no evidence suggesting that these kinases influence membrane-bound TRPV1 activity in the literature. In addition, a recent report suggests that H-89 may interact directly with KV1.3 channels (27). However, it is unlikely that an effect at this channel underlies the effects seen in this study because, although HEK cells do express this channel, gating only occurs at potentials (−20 mV and above) substantially more positive than the −60-mV holding potential used in our experiments (28).
Interestingly, the inhibitory effects of the broad spectrum protein kinase inhibitor were not as substantial as the more selective inhibitors for PKCϵ and PKA. This reduced activity of H-7 might intimate that there may be some, as of yet unreported, protein kinase activity resulting in inhibition of channel activity. Further investigations specifically exploring this possibility are warranted.
Whether 20-HETE directly binds to and activates the channel is at present uncertain although binding sites on intracellular domains of TRPV1 for other arachidonate-derived direct activators (12-HPETE) have been proposed (10). Moreover, the profile of channel activity of inside-out patches in response to 20-HETE demonstrates a delayed activation (supplemental Fig. 2) similar to that evident for 12-HPETE (10). An alternative explanation for our findings is that protein kinase activity per se results in channel gating, i.e. 20-HETE stimulates PKA or PKC and that the activity of these kinases, likely causing phosphorylation, results in channel gating in the absence of ligand binding, as has been suggested to occur with other TRPV1 activators. However, this is a controversial issue with evidence both for and against (6, 7) such a possibility. Dependence of TRPV1 activity upon resting phosphorylation is a characteristic that has been proposed previously for other TRPV1 activators including the satiety factor oleoylethanolamide (18) and even more recently capsaicin itself, although the latter being dependent upon phosphorylation at sites distinct from Ser502 (21).
Second, our data indicate a modulatory action on the channel, characterized by a potentiation of responses to capsaicin or acidic pH. This effect occurs at concentrations 2–3 orders of magnitude lower than those necessary to gate the channel and can occur even in the Ser502 mutant where 20-HETE-induced changes in current are absent. This finding suggests that 20-HETE exerts its effects on TRPV1 by either interacting with or altering the function of distinct sites within the channel. Likely possible candidates besides Ser502 include the residue Ser116 which has been identified as critical for PKA-induced alterations of TRPV1 activation (7). Our studies also show that inhibitors of protein kinase alter capsaicin-induced gating of TRPV1 under resting conditions, as shown by others recently (21). However, unlike the response to 20-HETE this effect did not involve the Ser502 residue because capsaicin-induced responses were intact in cells expressing the mutant channel, again in agreement with previous findings. The reason for the apparent difference is not entirely clear, but ligand-specific binding characteristics may be implicated.
Several candidate compounds have been proposed to act as endogenous TRPV1 channel ligands. In particular, products of lipoxygenase-dependent metabolism of the membrane phospholipid, arachidonic acid, including 12- and 15-(S)-HPETE, 5-(S)- and 5-(R)-hydroxyeicosatetraenoic acid (5-(S)-HETE and 5-(R)-HETE), 12-(S)-HETE, and leukotriene B4 have all been found to directly activate the channel in inside-out membrane patches (10). Our investigations clearly demonstrate that the structurally related 20-HETE also activates TRPV1 both in hTRPV1-transfected HEK293 and in murine DRG. Both TRPV1 antagonism and the use of cells from TRPV1 KO mice produced substantially reduced 20-HETE current amplitudes (up to ∼90% suppression). The small but measurable remaining response suggests a minor role for other ion channels. It is possible that these effects are related to TRPC activation because both HEK 293 cells (29) and murine DRG neurons (30) basally express various isoforms of TRPC channel. Perhaps of particular relevance to the current study is the TRPC6 isoform, which is basally expressed in both cells and has been implicated previously in 20-HETE activity both in terms of inducing current but also with respect to vasoconstriction of blood vessels (31).
Thus, in sum, the results from this study demonstrate that 20-HETE is an activator and modulator of TRPV1 activity at concentrations that are thought to occur in vivo. Our data suggest that 20-HETE likely binds with TRPV1 to result in direct gating. However, in addition our findings support the view that 20-HETE elevates kinase activity, particularly PKA, and that this underlies enhanced sensitivity of the channel to activators including capsaicin and acidic pH. We believe that our findings support investigation of the role of TRPV1 activation in physiological and pathological situations where alterations in 20-HETE synthesis and bioactivity have been implicated, such as for example hypertension (32, 33).
Supplementary Material
This work was supported by The Wellcome Trust (to H. W., J. Ö., K. J. B., and C. P.).

This article contains supplemental Figs. 1 and 2.
- TRPV1
- transient receptor potential vanilloid 1
- DRG
- dorsal root ganglion
- 20-HETE
- 20-hydroxyeicosatetraenoic acid
- 12-HPETE
- hydroperoxyeicosatetraenoic acid.
REFERENCES
- 1. Caterina M. J., Leffler A., Malmberg A. B., Martin W. J., Trafton J., Petersen-Zeitz K. R., Koltzenburg M., Basbaum A. I., Julius D. (2000) Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288, 306–313 [DOI] [PubMed] [Google Scholar]
- 2. Davis J. B., Gray J., Gunthorpe M. J., Hatcher J. P., Davey P. T., Overend P., Harries M. H., Latcham J., Clapham C., Atkinson K., Hughes S. A., Rance K., Grau E., Harper A. J., Pugh P. L., Rogers D. C., Bingham S., Randall A., Sheardown S. A. (2000) Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature 405, 183–187 [DOI] [PubMed] [Google Scholar]
- 3. Tominaga M., Tominaga T. (2005) Structure and function of TRPV1. Pflugers Arch. Eur. J. Physiol. 451, 143–150 [DOI] [PubMed] [Google Scholar]
- 4. Cesare P., Dekker L. V., Sardini A., Parker P. J., McNaughton P. A. (1999) Specific involvement of PKC-ϵ in sensitization of the neuronal response to painful heat. Neuron 23, 617–624 [DOI] [PubMed] [Google Scholar]
- 5. Cesare P., McNaughton P. (1996) A novel heat-activated current in nociceptive neurons and its sensitization by bradykinin. Proc. Natl. Acad. Sci. U.S.A. 93, 15435–15439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bhave G., Hu H. J., Glauner K. S., Zhu W., Wang H., Brasier D. J., Oxford G. S., Gereau R. W. (2003) Protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1). Proc. Natl. Acad. Sci. U.S.A. 100, 12480–12485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bhave G., Zhu W., Wang H., Brasier D. J., Oxford G. S., Gereau R. W. (2002) cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron 35, 721–731 [DOI] [PubMed] [Google Scholar]
- 8. Zhang X., Li L., McNaughton P. A. (2008) Proinflammatory mediators modulate the heat-activated ion channel TRPV1 via the scaffolding protein AKAP79/150. Neuron 59, 450–461 [DOI] [PubMed] [Google Scholar]
- 9. Zygmunt P. M., Petersson J., Andersson D. A., Chuang H., Sørgård M., Di Marzo V., Julius D., Högestätt E. D. (1999) Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 400, 452–457 [DOI] [PubMed] [Google Scholar]
- 10. Hwang S. W., Cho H., Kwak J., Lee S. Y., Kang C. J., Jung J., Cho S., Min K. H., Suh Y. G., Kim D., Oh U. (2000) Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc. Natl. Acad. Sci. U.S.A. 97, 6155–6160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gebremedhin D., Lange A. R., Lowry T. F., Taheri M. R., Birks E. K., Hudetz A. G., Narayanan J., Falck J. R., Okamoto H., Roman R. J., Nithipatikom K., Campbell W. B., Harder D. R. (2000) Production of 20-HETE and its role in autoregulation of cerebral blood flow. Circ. Res. 87, 60–65 [DOI] [PubMed] [Google Scholar]
- 12. Harder D. R. (1984) Pressure-dependent membrane depolarization in cat middle cerebral artery. Circ. Res. 55, 197–202 [DOI] [PubMed] [Google Scholar]
- 13. Huang A., Sun D., Yan C., Falck J. R., Kaley G. (2005) Contribution of 20-HETE to augmented myogenic constriction in coronary arteries of endothelial NO synthase knockout mice. Hypertension 46, 607–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Frisbee J. C., Roman R. J., Falck J. R., Krishna U. M., Lombard J. H. (2001) 20-HETE contributes to myogenic activation of skeletal muscle resistance arteries in Brown Norway and Sprague-Dawley rats. Microcirculation 8, 45–55 [PubMed] [Google Scholar]
- 15. Wang M. H., Zhang F., Marji J., Zand B. A., Nasjletti A., Laniado-Schwartzman M. (2001) CYP4A1 antisense oligonucleotide reduces mesenteric vascular reactivity and blood pressure in SHR. Am. J. Physiol. Regul. Integr. Comp. Physiol. 280, R255–261 [DOI] [PubMed] [Google Scholar]
- 16. Scotland R. S., Chauhan S., Davis C., De Felipe C., Hunt S., Kabir J., Kotsonis P., Oh U., Ahluwalia A. (2004) Vanilloid receptor TRPV1, sensory C-fibers, and vascular autoregulation: a novel mechanism involved in myogenic constriction. Circ. Res. 95, 1027–1034 [DOI] [PubMed] [Google Scholar]
- 17. Bevan S., Hothi S., Hughes G., James I. F., Rang H. P., Shah K., Walpole C. S., Yeats J. C. (1992) Capsazepine: a competitive antagonist of the sensory neuron excitant capsaicin. Br. J. Pharmacol. 107, 544–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ahern G. P. (2003) Activation of TRPV1 by the satiety factor oleoylethanolamide. J. Biol. Chem. 278, 30429–30434 [DOI] [PubMed] [Google Scholar]
- 19. Zhao J., Gover T. D., Muralidharan S., Auston D. A., Weinreich D., Kao J. P. (2006) Caged vanilloid ligands for activation of TRPV1 receptors by 1- and 2-photon excitation. Biochemistry 45, 4915–4926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jara-Oseguera A., Simon S. A., Rosenbaum T. (2008) TRPV1: on the road to pain relief. Curr. Mol. Pharmacol. 1, 255–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Studer M., McNaughton P. A. (2010) Modulation of single-channel properties of TRPV1 by phosphorylation. J. Physiol. 588, 3743–3756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Johnson J. A., Gray M. O., Chen C. H., Mochly-Rosen D. (1996) A protein kinase C translocation inhibitor as an isozyme-selective antagonist of cardiac function. J. Biol. Chem. 271, 24962–24966 [DOI] [PubMed] [Google Scholar]
- 23. Hu K., Mochly-Rosen D., Boutjdir M. (2000) Evidence for functional role of ϵPKC isozyme in the regulation of cardiac Ca2+ channels. Am. J. Physiol. Heart Circ. Physiol. 279, H2658–2664 [DOI] [PubMed] [Google Scholar]
- 24. Mandadi S., Tominaga T., Numazaki M., Murayama N., Saito N., Armati P. J., Roufogalis B. D., Tominaga M. (2006) Increased sensitivity of desensitized TRPV1 by PMA occurs through PKCϵ-mediated phosphorylation at Ser800. Pain 123, 106–116 [DOI] [PubMed] [Google Scholar]
- 25. Engh R. A., Girod A., Kinzel V., Huber R., Bossemeyer D. (1996) Crystal structures of catalytic subunit of cAMP-dependent protein kinase in complex with isoquinolinesulfonyl protein kinase inhibitors H7, H8, and H89: structural implications for selectivity. J. Biol. Chem. 271, 26157–26164 [DOI] [PubMed] [Google Scholar]
- 26. Lochner A., Moolman J. A. (2006) The many faces of H89: a review. Cardiovasc. Drug Rev. 24, 261–274 [DOI] [PubMed] [Google Scholar]
- 27. Choi J., Choi B. H., Hahn S. J., Yoon S. H., Min D. S., Jo Y., Kim M. (2001) Inhibition of Kv1.3 channels by H-89 (N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide) independent of protein kinase A. Biochem. Pharmacol. 61, 1029–1032 [DOI] [PubMed] [Google Scholar]
- 28. Jiang B., Sun X., Cao K., Wang R. (2002) Endogenous KV channels in human embryonic kidney (HEK-293) cells. Mol. Cell. Biochem. 238, 69–79 [DOI] [PubMed] [Google Scholar]
- 29. Zagranichnaya T. K., Wu X., Villereal M. L. (2005) Endogenous TRPC1, TRPC3, and TRPC7 proteins combine to form native store-operated channels in HEK-293 cells. J. Biol. Chem. 280, 29559–29569 [DOI] [PubMed] [Google Scholar]
- 30. Elg S., Marmigere F., Mattsson J. P., Ernfors P. (2007) Cellular subtype distribution and developmental regulation of TRPC channel members in the mouse dorsal root ganglion. J. Comp. Neurol. 503, 35–46 [DOI] [PubMed] [Google Scholar]
- 31. Inoue R., Jensen L. J., Jian Z., Shi J., Hai L., Lurie A. I., Henriksen F. H., Salomonsson M., Morita H., Kawarabayashi Y., Mori M., Mori Y., Ito Y. (2009) Synergistic activation of vascular TRPC6 channel by receptor and mechanical stimulation via phospholipase C/diacylglycerol and phospholipase A2/ω-hydroxylase/20-HETE pathways. Circ. Res. 104, 1399–1409 [DOI] [PubMed] [Google Scholar]
- 32. Miyata N., Roman R. J. (2005) Role of 20-hydroxyeicosatetraenoic acid (20-HETE) in vascular system. J. Smooth Muscle Res. 41, 175–193 [DOI] [PubMed] [Google Scholar]
- 33. Laffer C. L., Laniado-Schwartzman M., Nasjletti A., Elijovich F. (2004) 20-HETE and circulating insulin in essential hypertension with obesity. Hypertension 43, 388–392 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.