Background: RhoGDI affects biological activities of small Rho GTPases, leading to regulation of actin polymerization and cell motility.
Results: RhoGDI SUMOylation at Lys-138 was crucial for the function of RhoGDI in cell motility.
Conclusion: The regulation of SUMOylation of RhoGDI by XIAP plays a key role in regulating cancer cell Rho GTPase activation.
Significance: Our study reveals a molecular basis for XIAP mediation of cancer cell invasion and metastasis.
Keywords: Cancer Biology, Cell Motility, Cytoskeleton, Rho GTPases, XIAP, Rhogdi, Ring Domain, Sumoylation
Abstract
The Rho GDP dissociation inhibitor (RhoGDI) can bind to small GTPases and keep them in a biologically inactive state in cytoplasm, through which it affects actin polymerization and cell motility. However, mechanisms underlying how RhoGDI regulates Rho GTPase complex formation/membrane extraction/GTPase dissociation remain largely unexplored. Our previous studies reported that X-linked inhibitor of apoptosis protein (XIAP) interacted with RhoGDI via its RING domain and negatively modulated RhoGDI SUMOylation and HCT116 cancer cell migration. Here, we identified that RhoGDI SUMOylation specifically occurred at Lys-138, which was inhibited by XIAP domain. We further demonstrated that RhoGDI SUMOylation at Lys-138 was crucial for inhibiting actin polymerization and cytoskeleton formation as well as cancer cell motility. Moreover, SUMO-RhoGDI had a much higher binding affinity to small Rho GTPase compared with the un-SUMOylated form of RhoGDI. Taken together, our study demonstrated a novel modification of RhoGDI, SUMOylation at Lys-138, which played a key role in regulating Rho GTPase activation in cancer cells. The physiological regulation of RhoGDI SUMOylation by the RING domain of XIAP may account for modulation of cancer cell invasion and metastasis by XIAP.
Introduction
Small Rho GTPases are involved in the regulation of actin cytoskeleton events, including focal adhesions, formation of stress fibers, lamellipodia and filopodia, membrane ruffling, cell motility, and cell morphology (1). Rho GTPases cycle between the inactive GDP-bound form in the cytoplasm and active GTP-bound state in the plasma membrane (2). The Rho GDP dissociation inhibitor (RhoGDI)3 is a key down-regulator of the biological activities of small Rho GTPases (3). It is widely accepted that RhoGDI binds to the inactive GDP-bound form of small Rho GTPases, controlling GTPase partitioning between the cytosol and membrane compartments (4). It has been reported that phosphorylation of RhoA and Cdc42 promotes formation of the GTPase-RhoGDI complex and GTPase extraction from membrane (5, 6). Meanwhile, phosphorylation of RhoGDI facilitates Rho GTPase dissociation from RhoGDI (7, 8). However, a general mechanism for complex formation/membrane extraction/GTPase dissociation remains largely unexplored.
Small ubiquitin-like modifier (SUMO) is a reversible post-translational protein modifier. SUMOylation offers a range of functions for alteration of protein localization, interaction, stability, and activity (9). Our most recent studies have shown that X-linked inhibitor of apoptosis protein (XIAP) was able to interact with RhoGDI protein, inhibit RhoGDI SUMOylation, and subsequently regulate cytoskeleton formation and cell motilities in HCT116 cells (10). However, the specific SUMOylated residue(s) of RhoGDI and the impacts of RhoGDI SUMOylation on Rho GTPases activities are not yet explored. Here, we identified RhoGDI SUMOylation at Lys-138, which was crucial for its function in inhibiting Rho GTPases activities, cellular actin polymerization, and cell motility. We also demonstrated a novel biological function of the XIAP RING domain in attenuation of RhoGDI SUMOylation, which decreased RhoGDI function, and in turn facilitated actin polymerization, cytoskeleton reorganization, and cancer cell invasion. In conclusion, our studies revealed a novel mechanism accounting for XIAP regulation of cancer cell invasion and metastasis.
MATERIALS AND METHODS
Plasmids
The plasmids expressing HA-tagged XIAP, HA-tagged XIAP ΔRING, HA-tagged XIAP ΔBIR, and pEBB-HA expression empty vector were gifts from Dr. Colin S. Duckett (University of Texas at Austin, Austin, TX) (11). The pEGFP-C3/RhoGDI vector expressing green fluorescent protein (GFP)-tagged RhoGDI and Rac1/pcDNA3 were kindly provided by Dr. Mark R. Philips (New York University School of Medicine, New York). Three pairs of primers for mutations of SUMOylation sites in human RhoGDI protein are listed as follows: K105R, 5′-TCG TTT GTG CTG A Gg GAG GGT GTG GAG-3′ and 5′-CTC CAC ACC CTC CcT CAG CAC AAA CGA-3′; K138R, 5′-AGG AAA GGC GTC AgG ATT GAC AAG ACT-3′ and 5′-AGT CTT GTC AAT CcT GAC GCC TTT CCT-3′; K199R, 5′-AAT CTA ACC A TC AgG AAA GAA TGG AAA-3′ and 5′-TTT CCA TTC TTT CcT GAT GGT TAG A-3′. Mutations were introduced using QuikChange site-directed mutagenesis kit (Stratagene, San Diego). All constructs were confirmed by sequencing.
Antibodies and Other Reagents
Antibodies (anti-HA, XIAP, and GFP) were purchased from Cell Signaling Technology, Inc. (Boston). Agarose-conjugated anti-HA antibody was obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Agarose-conjugated anti-Rac1 antibody and anti-RhoGDI (rabbit) antibody were from Millipore. Anti-FLAG antibody was obtained from Sigma. Antibody specific for GADPH was obtained from Cell Signaling Technology, Inc. (Boston) or Sungene Biotech (Tianjin, China). Oregon Green 488 phalloidin and Alexa Fluor 594 phalloidin were from Invitrogen.
Cell Culture and Transfection
Wild-type and XIAP−/− HCT116 cells (human colon cancer cell lines) were kind gifts from Dr. Bert Vogelstein (Howard Hughes Medical Institute and Sidney Kimmel Comprehensive Cancer Center, The Johns Hopkins Medical Institutions, Baltimore, MD) (12). They were cultured in McCoy's 5A medium (Invitrogen) supplemented with 10% fetal bovine serum (FBS, Nova-Tech, Grand Island, NE) and penicillin/streptomycin (Invitrogen). All cells were maintained in a humidified incubator at 37 °C in an incubator with 5% CO2 humidified atmosphere. Cell transfections were performed with Lipofectamine reagent (Invitrogen) or FuGENE® HD transfection reagent (Roche Applied Science). For stable transfection, cultures were subjected to hygromycin B or G418 or puromycin (Invitrogen.) drug selection, and cells surviving from the antibiotic selection were pooled as stable mass transfectants. These stable transfectants were then cultured in the selection antibiotic-free medium for at least two passages before use in experiments.
Wound Healing Assay
Cells were seeded into each well of 6-well plates and cultured until 80% confluence. Wounds were made by sterile pipette tips. Cells were washed with serum-free PBS and then cultured in normal medium for the various time points. Photos were taken every 24 h until the wound was healed in the parental cells (13). The wound area was quantified using the Cell Migration Analysis software (Muscale LLC, Scottsdale, AZ).
Cell Invasion Assay
A BD BioCoatTM MatrigelTM Invasion Chamber (BD Biosciences) was used for the invasion assay. Cells (2.5 × 104) were seeded per insert in triplicate in 500 μl of serum-free McCoy's 5A medium. Inserts were placed in wells containing 500 μl of medium with 5% FBS and 12-O-tetradecanoylphorbol-13-acetate (20 ng/ml). After 36 or 72 h, cells on the upper surface of the filters were completely removed by wiping with a cotton swab. The membrane was cut with a sharp scalpel and placed in a 96-well plate. The levels of invaded/migrated cells were determined by CellTiter-Glo® luminescent cell viability assay (Promega, Madison, WI). Invasion (%) = (ATP activity of invaded cells/ATP activity of migrated cells) × 100%.
Cell Proliferation Analysis
Viable cells (1 × 103) suspended in 100 μl of complete medium supplemented with 10% FBS were seeded in triplicate into 96-well plates. The plates were incubated at 37 °C in a humidified atmosphere of 5% CO2. The cells were extracted with 50 μl of lysis buffer at the various time points. The cell growth was measured by using a CellTiter-Glo® luminescent cell viability assay kit (Promega). The results are expressed as relative proliferation rate, which was calculated as follows: proliferation rate = ATP activity on nth day/ATP activity on 0 day.
Analysis of His-tagged SUMO1 Conjugates by Using Ni2+-NTA-Agarose Beads
This method for analysis of His-tagged SUMO1 was described in a previous study (14) and used with some modifications. Briefly, 293T or HCT116 cells were transfected with various constructs, together with FLAG-UBC9 and His6-SUMO1 (2 μg of each), using Lipofectamine 2000 (Invitrogen). Forty eight hours after transfection, the cells were collected, and 25% of cells were extracted with NEM-RIPA buffer to be used as input. The remaining 75% of cells were lysed in 3 ml of His-lysis buffer and incubated with 60 μl of Ni2+-NTA-agarose beads (Qiagen, Valencia, CA) with rotation at 4 °C overnight. The beads were washed for 5 min for each step at room temperature with 750 μl of each of the following buffers: Washing buffer 1, Washing buffer 2, Washing buffer 3, Washing buffer 4 (14). After the last wash, His6-tagged SUMOylated products were eluted by incubating the beads in 75 μl of Elution buffer for 20 min at room temperature. The eluates were analyzed by Western blotting.
Immunofluorescent Staining and Confocal Microscope
HCT116 and its transfectants were cultured on cover slides in 10% FBS McCoy's 5A medium for 48 h. The cells were fixed with 3.7% paraformaldehyde for 15 min and then permeabilized with 0.1% Triton X-100 in PBS for 15 min at room temperature. The cells were then blocked with 1% BSA/PBS for 30 min, incubated with Oregon-conjugated phalloidin for 30 min at room temperature, and then stained with 0.1 μg/ml DAPI for 1 min. The slides were washed three times with PBS and mounted with antifade reagent (Molecular Probes). The cells were observed under a confocal microscope (Leica DMI6000B).
Immunoprecipitation
Cells transfected with the indicated plasmids were lysed in cell lysis buffer (1% Triton X-100, 150 mm NaCl, 10 mm Tris, pH 7.4, 1 mm EDTA, 1 mm EGTA, 0.2 mm Na3VO4, 0.5% Nonidet P-40, and complete protein mixture inhibitors from Roche Applied Science) on ice. Protein concentrations were determined by the protein quantification assay kit (Bio-Rad). Lysate (0.5 mg) was precleared by incubation with Protein A/G plus-agarose (Santa Cruz Biotechnology, Inc.) and then incubated with anti-Rac1 antibody-conjugated agarose beads (Millipore, Temecula, CA) overnight. The immunoprecipitate was washed three times with the cell lysis buffer and subjected to the Western blotting assay.
RhoA/Rac1/Cdc42 Activity Assay
To measure endogenous small GTPase activity, Rho/Rac/Cdc42 activation assay combo kit was used (Cell Biolabs Inc., San Diego). Briefly, cells were lysed on ice in 1× Assay/Lysis Buffer for 10 min. Cell lysates were centrifuged for 10 min (14,000 × g, at 4 °C), and the supernatant was incubated with the Rhotekin RBD or PAK PBD-agarose beads at 4 °C for 1 h on a rotator. The beads were washed three times with 0.5 ml of 1× Assay Buffer. Protein samples were eluted with 1× SDS-PAGE sample buffer and processed for Western blotting with anti-RhoA, Rac1, and Cdc42 antibodies.
Western Blotting
Cell extracts were prepared with cell lysis buffer (10 mm Tris-HCl, pH 7.4, 1% SDS, and 1 mm Na3VO4), and protein concentrations were determined by the protein quantification assay kit (Bio-Rad). Thirty μg of proteins were resolved by SDS-PAGE and subsequently probed with the indicated primary antibodies and alkaline phosphatase-conjugated second antibody. Signals were detected by the enhanced chemifluorescence Western blotting system with scanning using the phosphorimager (model Storm 860, GE Healthcare) as described previously (15, 16). The densitometric analyses of the Western blotting bands were quantified by the software of TotalLab Quant.
Statistical Methods
Student's t test was utilized for determining the significance of differences of cell proliferation, wound healing, and cell invasion among various transfectants. The differences will be considered significant at p ≤ 0.05.
RESULTS
RhoGDI SUMOylation Occurred at Lys-138
The post-translational conjugation of SUMO exerts a wide variety of effects on the target proteins, including alterations of protein conformation, activity, localization, and protein-protein interactions (17). Our recent studies reported that XIAP bound to RhoGDI and had a potential effect on RhoGDI protein SUMOylation (10). To further identify SUMOylation acceptor site(s) in RhoGDI, we first analyzed the potential SUMOylated residues of RhoGDI using the Abgent SUMOplotTM program. As shown in Fig. 1A, SUMOylation sites of human RhoGDI protein were predicted at three lysine residues Lys-199, Lys-138, and Lys-105 (marked in bold and underlined). We mutated the three putative lysine residues to arginine and then transfected the point mutant RhoGDI constructs with His6-SUMO1 and FLAG-Ubc9 into 293T cells (Fig. 1B). We performed SUMOylation assays to determine the precise location of the SUMOylated site in RhoGDI using Ni2+-NTA beads to pull down proteins that were SUMOylated with His6-tagged SUMO1 as described in previous studies (14). As shown in Fig. 1B, in the presence of Ubc9 + SUMO1, the relative molecular mass of GFP-RhoGDI-WT shifted from 56 to 73 kDa, indicating that the band at ∼73 kDa might represent SUMO-GFP-RhoGDI. This shift of GFP-RhoGDI was completely absent in the co-transfectants of Ubc9 + SUMO1 with GFP-RhoGDI K138R mutant, although there was a clearly SUMOylated GFP-RhoGDI band in the co-transfectant of Ubc9 + SUMO1 with either GFP-RhoGDI K105R mutant or GFP-RhoGDI K199R mutant (Fig. 1B). These results clearly demonstrated that RhoGDI SUMOylation occurred at lysine 138.
FIGURE 1.
RhoGDI SUMOylation occurred at lysine 138. A, SUMOplot prediction of human RhoGDI protein. SUMO consensus contains the sequences ΨKX(E/D) (where Ψ is a large hydrophobic amino acid, K is the target lysine, X is any amino acid, and D or E is an acidic residue). SUMOylation sites of human RhoGDI protein were predicted by the program of Abgent SUMOplotTM. Three lysine residues at Lys-199, Lys-138, and Lys-105 with a high score are the putative SUMOylation sites (marked in bold and underlined). B, identification of SUMOylated site(s) of human RhoGDI. 293T cells were transiently transfected with constructs of His-SUMO1 and FLAG-Ubc9, together with either WT or mutants (K105R, K138R, or K199R) of GFP-RhoGDI. Cell extracts were used for nickel-nitrilotriacetic acid (Ni-NTA) precipitation, and pulled down proteins were identified by Western blotting. The data for input are shown in the lower five panels. IB, immunoblot; Endo, endogenous.
RhoGDI SUMOylation at Lys-138 Was Crucial for RhoGDI Inhibition of Cancer Cell Migration, Invasion, and Actin Polymerization
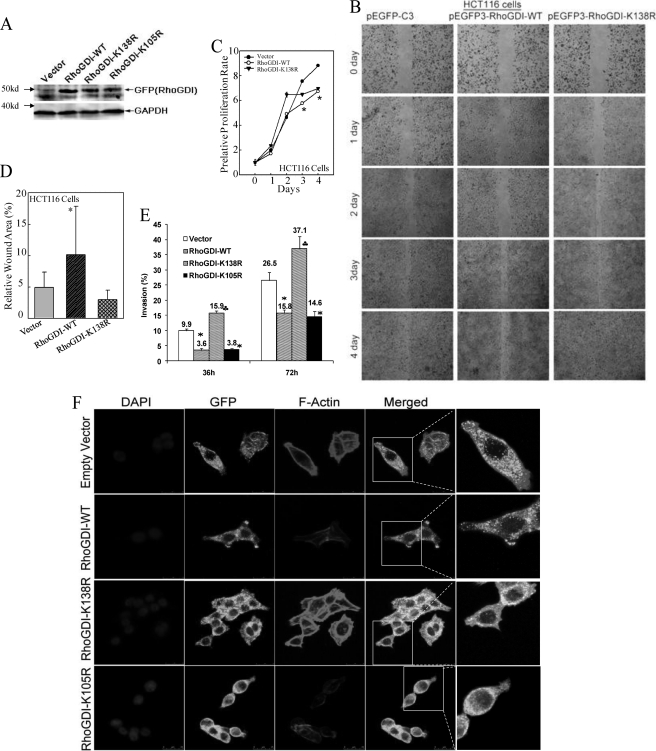
Above results strongly indicated that human RhoGDI was SUMOylated at the position of Lys-138. To determine whether SUMO-RhoGDI was crucial for function of RhoGDI in regulating cell migration and invasion, we performed wound healing assays with stable transfectants containing the empty vector pEGFP-C3, RhoGDI-WT, and RhoGDI-K138R (Fig. 2A). We found that expression of RhoGDI-WT obviously inhibited spontaneous wound closure as compared with that in empty vector transfectants at day 2–4 in wound healing assay, whereas mutation of K138R failed to provide an inhibitory effect as observed in RhoGDI-WT transfectants at all time points tested (Fig. 2B). To exclude the possibility that impact of RhoGDI on cell migration was due to inhibition of cell proliferation, we compared cell proliferation rate among three transfectants (Fig. 2C). The results showed that during the first 2 days when overexpression of either RhoGDI-WT or RhoGDI-K138R did not show a significant inhibition on cell proliferation (Fig. 2C), a defect in cell migration caused by overexpression of RhoGDI-WT, but not K138R mutation, was ready to be observed in comparison with that in vector transfectants (Fig. 2B). Because a partial inhibition (∼25%) of cell proliferation was observed on day 4 in RhoGDI-WT transfectants (Fig. 2C), we normalized wound closure rate over the proliferation rate, and we still found significant attenuation of cell migration in RhoGDI-WT transfectants as compared with that in vector transfectants (Fig. 2D). Furthermore, we also observed that expression of RhoGDI-WT or RhoGDI-K105R decreased cell invasion, whereas RhoGDI-K138R expression robustly enhanced cell invasion (Fig. 2E, p < 0.01). Then we compared actin polymerization and cell skeleton morphological alterations. As shown in Fig. 2F, expression of RhoGDI-K138R significantly induced F-actin enrichment and more protrusions and membrane ruffles, whereas expression of RhoGDI-WT or RhoGDI-K105R showed a decrease in actin polymerization as compared with that in the empty vector. Thus, these results provided direct evidence that RhoGDI SUMOylation at Lys-138 was crucial for the inhibitory effect of RhoGDI in cell actin polymerization, migration, and invasion.
FIGURE 2.
RhoGDI SUMOylation at Lys-138 was crucial for RhoGDI inhibition of cancer cell migration, invasion, and actin polymerization. A, stable transfectants of GFP-RhoGDI-WT, GFP-RhoGDI-K138R, GFP-RhoGDI-K105R, and the empty vector in HCT116 cells were identified by Western blotting assay. B, wound healing capabilities were compared among the indicated transfectants of HCT116 cells. C, proliferation rates were determined in the indicated cell lines by a CellTiter-Glo® luminescent cell viability assay kit. Results were represented as the means ± S.D. of the triplicate wells. The asterisk indicates a significant decrease in proliferation rate in GFP-RhoGDI-WT transfectants compared with that in the vector transfectant (p < 0.05). D, wound area on the 4th day was quantified using Cell Migration Analysis software and normalized over the proliferation rate on 4th day as shown in C. The quantitative data were shown as relative wound area (%, error bar represent S.D., n = 3). The asterisk indicates a significant increase in wound area in GFP-RhoGDI-WT transfectants compared with that in the vector transfectant (p < 0.05). E, indicated transfectants were used for comparison of their invasive capabilities using BD BioCoatTM MatrigelTM invasion chamber. Results were represented as the means ± S.D. of invasive cells from at least three independent experiments with duplicate wells for each experiment. The asterisk indicates a significant decrease in comparison with HCT116(vector) cells (p < 0.01), and the (♣) indicates a significant increase in comparison with HCT116(vector) cells (p < 0.01). F, indicated transfectants were used to evaluate cell actin polymerization under confocal microscopy as indicated: GFP-RhoGDI, F-actin, and DAPI.
XIAP RING Domain Inhibited RhoGDI SUMOylation at Lys-138
To further verify the inhibition of SUMO-RhoGDI by XIAP, we transfected GFP-RhoGDI with His6-SUMO1 + FLAG-Ubc9 constructs into WT, XIAP−/−, and XIAP−/−(HA-XIAP) HCT116 cells. As shown in Fig. 3A, the 73-kDa bands of SUMO-GFP-RhoGDI protein were observed in the presence of SUMO1 + Ubc9 in WT cells. As expected, SUMO-GFP-RhoGDI was significantly increased in XIAP−/− cells when compared with that in WT cells, whereas restoration of HA-XIAP into XIAP−/− cells, XIAP−/−(HA-XIAP), obviously reduced the expression of SUMO-GFP-RhoGDI to a similar level as seen in WT HCT116 cells (Fig. 3A). These results indicated that XIAP did have an inhibitory effect on SUMO-RhoGDI. Our recent results showed that XIAP could interact with RhoGDI via its RING domain (10). To test whether the RING domain was responsible for the inhibitory role of XIAP in SUMO-RhoGDI, we transfected GFP-RhoGDI, His6-SUMO1, and FLAG-Ubc9, together with one of the constructs containing HA-XIAP, HA-XIAP ΔRING, and HA-XIAP ΔBIR, into 293T cells, and evaluated the status of RhoGDI SUMOylation. SUMO-GFP-RhoGDI was shown at comparable levels in the transfectants of either HA-XIAP ΔRING or HA-vector, whereas SUMO-GFP-RhoGDI was barely detected in the transfectants of either HA-XIAP or HA-XIAP ΔBIR (Fig. 3B). The identification of overexpression of HA-XIAP, HA-XIAP ΔRING, and HA-XIAP ΔBIR in their stable transfectants is shown in the Input panel of Fig. 3B using anti-HA-specific antibodies. Those results strongly indicated that the RING domain was crucial for the role of XIAP in inhibiting RhoGDI SUMOylation at Lys-138.
FIGURE 3.
XIAP RING domain was crucial for XIAP inhibiting RhoGDI SUMOylation. WT, XIAP−/−, and XIAP−/−(HA-XIAP) HCT116 cells (A) and 293T cells (B) were transfected with GFP-RhoGDI, together with His-SUMO1 and FLAG-Ubc9 (A) and various constructs as indicated (B). Cell extracts were used for nickel-nitrilotriacetic acid (Ni-NTA) precipitation, and pulled down proteins were identified by Western blotting. IB, immunoblot.
RhoGDI SUMOylation at Lys-138 Played an Important Role in Inhibiting Small Rho GTPase Activity
Small Rho GTPases are best known for their ability to activate actin polymerization, and RhoGDI is a key negative regulator for Rho GTPase activation. To explore whether there is any effect of SUMO-RhoGDI on Rho GTPase activity in HCT116 cells, we used the Rho/Rac/Cdc42 activation assay combo kit to monitor the small Rho GTPase activation. As shown in Fig. 4A, RhoGDI-WT expression attenuated small Rho GTPase activation (except Cdc42) as compared with those in the empty vector, whereas RhoGDI-K138R expression increased the activation of RhoA, Cdc42, and Rac1. The quantitative data were shown under a Western blotting band (Fig. 4A). These results indicated that RhoGDI SUMOylation at Lys-138 facilitated the function of RhoGDI as an inhibitor against the activation of small GTPases.
FIGURE 4.
XIAP RING domain played an important role in inhibiting Rho GTPase activity. A–C, lysates from HCT116 cells stably transfected with GFP-RhoGDI-K138R or GFP-RhoGDI-WT or the empty vector (A) or from WT, XIAP−/−, and XIAP−/−(HA-XIAP) (B), or from XIAP−/−(HA-ΔRING) and XIAP−/−(HA-ΔBIRs) HCT116 cells (C) were used for small GTPase activity assay following the manufacturer's instructions. GTPγS and GDP were used for positive and negative controls, respectively. The densitometric analyses of the active (RBD/PBD-bound) proteins are determined as relative ratio to the GTPγS-bound form proteins. Results shown were representative of three independent experiments. D and E, XIAP−/− cells were transfected with Rac1 expression vector and then infected with SUMO1 overexpression virus (Ad-SUMO1 Q94P) or control virus (Ad-LacZ). Immunoprecipitation was carried out using anti-Rac antibody-conjugated agarose beads. The immune complex was examined by Western blotting assay (D), and the densitometric analyses were carried to show the ratio of SUMO-RhoGDI protein to un-SUMOylated RhoGDI in the immunocomplex and in Input (E).
RING Domain Was Crucial for XIAP Inhibiting Small Rho GTPases Activation
To evaluate role of XIAP in regulating small GTPases activation, we monitored Rho GTPase activation in WT and XIAP−/− cells. As shown in Fig. 4B, the levels of activated RhoA, Cdc42, and Rac1 observed in XIAP−/− HCT116 cells were dramatically reduced in comparison with those from WT cells. Restoration of XIAP expression in XIAP−/− cells, XIAP−/−(HA-XIAP), led to re-gaining the small Rho GTPase activation to comparable levels of WT cells (Fig. 4B). Those results indicated that the increased levels of SUMO-RhoGDI by depletion of XIAP facilitated the inhibitory effect of RhoGDI on GTPase activity, which consistently supported our conclusion that RhoGDI SUMOylation was responsible for up-regulation of the function of RhoGDI. Moreover, we compared the Rho GTPase activity between XIAP−/−(HA-ΔBIRs) and XIAP−/−(HA-ΔRING) transfectants. The results showed that the RING domain of XIAP played an important role in up-regulation of Rho GTPases activity, although baculovirus IAP repeat domains were not required (Fig. 4C). To further test whether SUMO-RhoGDI increased RhoGDI binding activity to Rho GTPase, we transfected Rac1 into XIAP−/− cells followed by infection with Ad-SUMO1 Q94P, a virus that expresses a dual His-S-SUMO1-Q94P/IRES/HA-Ubc9 protein and is able to increase the global SUMOylation of proteins (18). We then carried out the co-immunoprecipitation assay using anti-Rac1 antibody-conjugated agarose resins and compared the protein levels of SUMOylated and unmodified RhoGDI in the immunocomplex. As shown in Fig. 4D, Rac1 could pull down RhoGDI as expected. More importantly, the SUMO-RhoGDI was also found in the immunocomplex with a much higher ratio to the unmodified form of RhoGDI when compared with those in the input (Fig. 4E). These data suggest that the SUMO-RhoGDI protein had a much higher binding affinity to small Rho GTPase than the unmodified RhoGDI protein, which might be responsible for high activity of SUMO-RhoGDI, although it only represented a small fraction. It should be noted that other forms of protein modifications on RhoGDI, in addition to SUMO-RhoGDI, might also be anticipated to bind with Rac1, because some shifted protein bands were observed in the Rac1 pulldown assay when probed with anti-RhoGDI antibody (Fig. 4D). Collectively, our results strongly demonstrated that the RING domain was critical for the inhibitory effect of XIAP on RhoGDI SUMOylation, by which XIAP increased Rho GTPase activity, and in turn promoted actin polymerization and subsequently cancer cell motility (Fig. 5).
FIGURE 5.
A model for the XIAP-regulated RhoGDI SUMOylation signaling pathway in modulation of cell motility. SUMO-RhoGDI down-regulates Rho GTPase (RhoA, Rac1, and Cdc42) activity. XIAP binds to RhoGDI through its RING domain and inhibits RhoGDI SUMOylation at Lys-138, resulting in activation of GTPases, through which XIAP promotes actin polymerization and facilitates cell migration, invasion, and metastasis.
DISCUSSION
In this study, we for the first time identified RhoGDI SUMOylation at Lys-138, which positively regulated the function of RhoGDI in controlling cell motility. In addition, we consistently showed that the RING domain of XIAP provided an inhibitory effect on RhoGDI SUMOylation at Lys-138 via direct binding. In summary, we concluded that RhoGDI SUMOylation at Lys-138 was a major mediator for the function of XIAP in regulating cancer cell migration and invasion.
Hundreds of SUMOylation substrates have been identified, most of which are nuclear and perinuclear proteins (9, 19). Recently, some cytoplasmic proteins have also been reported to exist in a SUMOylated form (20, 21). RhoGDI regulates the function of Rho GTPases by controlling their cytosol-membrane cycling. Based on our results, the level of endogenous SUMO-RhoGDI was as low as 5% of total RhoGDI proteins under normal growth conditions (10), which was consistent with the notion that most SUMO-modified proteins occupied a small percentage at a steady state (9). Nevertheless, the small pool of SUMOylated protein gives rise to strong biological effects, because SUMOylation is a very quick, reversible, and highly dynamic modification, and consequently influences conformational change or protein-protein interactions in a timely fashion. Impairment of RhoGDI SUMOylation by the point mutation of K138R facilitated cell migration, invasion and actin polymerization, and cytoskeleton formation in HCT116 cells. Moreover, SUMO-RhoGDI preferentially bound to Rho GTPase and down-regulated the activities of Rho GTPase and cell motility in comparison with those of un-SUMOylated RhoGDI. Thus, we concluded that SUMO-RhoGDI was an active form of RhoGDI that was responsible for the regulatory role of RhoGDI in cell motility.
Malliri and co-workers (22) found that Rac1, the Rho-like GTPase, could be SUMOylationally modified by SUMO E3 ligase, PIAS3 (protein inhibitor of activated STAT3), based on the results from both in vivo and in vitro assays. The putative SUMOylation sites of human Rac1 were mapped to Lys-188, Lys-183, and Lys-184 or Lys-186 via mass spectrometry. Replacement of these residues to arginines caused reductions in GTP binding and in lamellipodia-ruffle formation as well as in cell migration (22). Therefore, the authors concluded that although SUMOylated Rac1 only represented a small fraction of total Rac1, it made great contribution to maintaining Rac1 in activated GTP-bound form resulting from either increased binding to a GEF or disassociating from a GTPase-activation protein, which finally facilitated Rac1 function in cell migration and invasion (22). There are two common findings in our study and the study by Malliri and co-workers (22). First, we both agree that proteins in the SUMOylated form are among the fraction that contribute most to their biological functions despite the relatively small occupancy of this type of modification in the total protein pool. Second, we both anticipate that SUMOylational conjugation affects protein function through either structural alterations or changing protein-protein interaction.
In addition, we consistently showed that RING domain played an important role of XIAP in inhibiting RhoGDI SUMOylation at Lys-138 based on the observations that RING domain (HA-ΔBIRs) was enough for restoration of Rho GTPase activity in XIAP−/− cells, whereas RING domain deletion (HA-ΔRING) did not show such restoration. These data, together with our previous finding that RhoGDI bound with XIAP via XIAP RING domain (10), suggested that signaling cross-talk existed between the XIAP RING domain and Rho GTPases. Protein SUMOylation is a complicated process balanced by SUMOylation-conjugation enzymes and de-SUMOylating enzymes (10, 17). Abundance and stability of de-SUMOylating enzymes result in the highly unstable and transient nature of this kind of protein modifications (10, 17). Although our studies reported here provided clear evidence for the XIAP negative regulation of SUMO-RhoGDI, we had no direct evidence showing whether the XIAP-RhoGDI interaction results in an increase of the de-SUMOylation process or a decrease of SUMOylation conjugation of RhoGDI. This issue is currently under investigation in our laboratory.
RING domains often function as modulates that confer ubiquitin protein ligase (E3) activity (23). The findings from Rajalingam and co-workers (24, 25) showed that depletion of XIAP or c-IAPs led to stabilization of C-RAF and Rac1 as well as cell migration in a panel of tumor cell lines. However, in our experimental systems, we did not observe obvious reduction in Rac1 expression in HCT116 XIAP−/− cells. The differential effects of XIAP in regulation of small GTPase expression might be due to different cell lines used in the various studies. This anticipation has been supported by the data obtained from the same study (25). Rajalingam and co-workers (25) reported that XIAP-deficient mouse embryo fibroblasts only gave rise to a robust increase in Rac1 in the early passages, whereas in the later passages the phenomenon was not reproducible possibly due to up-regulation of c-IAP1 or by the activation of chaperone machinery.
It has been reported that conjugation of ubiquitin and SUMO prefers the lysine residues, and in some instances they do compete with each other for modification on the same lysine residues (26). In our studies, we did not detect any obvious reduction of RhoGDI ubiquitination between WT and HCT116 XIAP−/− cells,4 indicating that XIAP may not be implicated in up-regulating RhoGDI ubiquitination. However, we cannot exclude the potential RhoGDI ubiquitination at Lys-138.
Three important novel findings are described in this paper. 1) RhoGDI could be SUMOylated at lysine 138; XIAP RING domain attenuates RhoGDI SUMOylation at Lys-138. 2) SUMO-RhoGDI increases its negative regulation on small Rho GTPases, ultimately influencing actin polymerization, cancer cell migration, and invasion. 3) SUMO-RhoGDI, but not un-SUMOylated RhoGDI, preferentially binds to Rho GTPase and down-regulates the activities of GTPases. In summary, our studies demonstrate a new molecular mechanism responsible for XIAP RING domain to function in the modulation of Rho GTPase activation, tumor cell migration, and invasion.
Acknowledgments
We thank Drs. Colin S. Duckett and Mark R. Philips for the generous gifts of plasmids, Dr. Germán Rosas-Acosta for providing SUMO1 Q94P virus, and Dr. Bert Vogelstein for the gift of wild-type as well as XIAP−/− HCT116 cells.
This work was supported, in whole or in part, by National Institutes of Health Grant CA112557 from NCI and Grants ES000260, ES010344 from NIEHS. This work was also supported by National Science Foundation of China Grants NSFC30928023 and NSFC30971516.
J. Liu, D. Zhang, W. Luo, J. Yu, X. Zhang, J. Chen, X. Wu, and C. Huang, unpublished data.
- RhoGDI
- Rho GDP dissociation inhibitor
- SUMO
- small ubiquitin-like modifier
- XIAP
- X-linked inhibitor of apoptosis protein
- GTPγS
- guanosine 5′-3-O-(thio)triphosphate.
REFERENCES
- 1. Kaibuchi K., Kuroda S., Amano M. (1999) Regulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells. Annu. Rev. Biochem. 68, 459–486 [DOI] [PubMed] [Google Scholar]
- 2. Olofsson B. (1999) Rho guanine dissociation inhibitors. Pivotal molecules in cellular signaling. Cell. Signal. 11, 545–554 [DOI] [PubMed] [Google Scholar]
- 3. Etienne-Manneville S., Hall A. (2002) Rho GTPases in cell biology. Nature 420, 629–635 [DOI] [PubMed] [Google Scholar]
- 4. Dovas A., Couchman J. R. (2005) RhoGDI. Multiple functions in the regulation of Rho family GTPase activities. Biochem. J. 390, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Forget M. A., Desrosiers R. R., Gingras D., Béliveau R. (2002) Phosphorylation states of Cdc42 and RhoA regulate their interactions with Rho GDP dissociation inhibitor and their extraction from biological membranes. Biochem. J. 361, 243–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tu S., Wu W. J., Wang J., Cerione R. A. (2003) Epidermal growth factor-dependent regulation of Cdc42 is mediated by the Src tyrosine kinase. J. Biol. Chem. 278, 49293–49300 [DOI] [PubMed] [Google Scholar]
- 7. Price L. S., Langeslag M., ten Klooster J. P., Hordijk P. L., Jalink K., Collard J. G. (2003) Calcium signaling regulates translocation and activation of Rac. J. Biol. Chem. 278, 39413–39421 [DOI] [PubMed] [Google Scholar]
- 8. DerMardirossian C., Rocklin G., Seo J. Y., Bokoch G. M. (2006) Phosphorylation of RhoGDI by Src regulates Rho GTPase binding and cytosol-membrane cycling. Mol. Biol. Cell 17, 4760–4768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Geiss-Friedlander R., Melchior F. (2007) Concepts in sumoylation. A decade on. Nat. Rev. Mol. Cell Biol. 8, 947–956 [DOI] [PubMed] [Google Scholar]
- 10. Liu J., Zhang D., Luo W., Yu Y., Yu J., Li J., Zhang X., Zhang B., Chen J., Wu X. R., Rosas-Acosta G., Huang C. (2011) X-linked inhibitor of apoptosis protein (XIAP) mediates cancer cell motility via Rho GDP dissociation inhibitor (RhoGDI)-dependent regulation of the cytoskeleton. J. Biol. Chem. 286, 15630–15640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lewis J., Burstein E., Reffey S. B., Bratton S. B., Roberts A. B., Duckett C. S. (2004) Uncoupling of the signaling and caspase-inhibitory properties of X-linked inhibitor of apoptosis. J. Biol. Chem. 279, 9023–9029 [DOI] [PubMed] [Google Scholar]
- 12. Cummins J. M., Kohli M., Rago C., Kinzler K. W., Vogelstein B., Bunz F. (2004) X-linked inhibitor of apoptosis protein (XIAP) is a nonredundant modulator of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis in human cancer cells. Cancer Res. 64, 3006–3008 [DOI] [PubMed] [Google Scholar]
- 13. Shan D., Chen L., Njardarson J. T., Gaul C., Ma X., Danishefsky S. J., Huang X. Y. (2005) Synthetic analogues of migrastatin that inhibit mammary tumor metastasis in mice. Proc. Natl. Acad. Sci. U.S.A. 102, 3772–3776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yu J., Zhang S. S., Saito K., Williams S., Arimura Y., Ma Y., Ke Y., Baron V., Mercola D., Feng G. S., Adamson E., Mustelin T. (2009) PTEN regulation by Akt-EGR1-ARF-PTEN axis. EMBO J. 28, 21–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Song L., Li J., Zhang D., Liu Z. G., Ye J., Zhan Q., Shen H. M., Whiteman M., Huang C. (2006) IKKβ programs to turn on the GADD45α-MKK4-JNK apoptotic cascade specifically via p50 NF-κB in arsenite response. J. Cell Biol. 175, 607–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Song L., Li J., Ye J., Yu G., Ding J., Zhang D., Ouyang W., Dong Z., Kim S. O., Huang C. (2007) p85α acts as a novel signal transducer for mediation of cellular apoptotic response to UV radiation. Mol. Cell. Biol. 27, 2713–2731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rosas-Acosta G., Russell W. K., Deyrieux A., Russell D. H., Wilson V. G. (2005) A universal strategy for proteomic studies of SUMO and other ubiquitin-like modifiers. Mol. Cell. Proteomics 4, 56–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pal S., Rosas J. M., Rosas-Acosta G. (2010) Identification of the nonstructural influenza A viral protein NS1A as a bona fide target of the small ubiquitin-like modifier by the use of dicistronic expression constructs. J. Virol. Methods 163, 498–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hay R. T. (2005) SUMO. A history of modification. Mol. Cell 18, 1–12 [DOI] [PubMed] [Google Scholar]
- 20. Watts F. Z. (2004) SUMO modification of proteins other than transcription factors. Semin. Cell Dev. Biol. 15, 211–220 [DOI] [PubMed] [Google Scholar]
- 21. Wilson V. G., Rosas-Acosta G. (2005) Wrestling with SUMO in a new arena. Sci. STKE 2005, pe32. [DOI] [PubMed] [Google Scholar]
- 22. Castillo-Lluva S., Tatham M. H., Jones R. C., Jaffray E. G., Edmondson R. D., Hay R. T., Malliri A. (2010) SUMOylation of the GTPase Rac1 is required for optimal cell migration. Nat. Cell Biol. 12, 1078–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Srinivasula S. M., Ashwell J. D. (2008) IAPs. What's in a name? Mol. Cell 30, 123–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dogan T., Harms G. S., Hekman M., Karreman C., Oberoi T. K., Alnemri E. S., Rapp U. R., Rajalingam K. (2008) X-linked and cellular IAPs modulate the stability of c-RAF kinase and cell motility. Nat. Cell Biol. 10, 1447–1455 [DOI] [PubMed] [Google Scholar]
- 25. Oberoi T. K., Dogan T., Hocking J. C., Scholz R. P., Mooz J., Anderson C. L., Karreman C., Meyer zu Heringdorf D., Schmidt G., Ruonala M., Namikawa K., Harms G. S., Carpy A., Macek B., Köster R. W., Rajalingam K. (2012) IAPs regulate the plasticity of cell migration by directly targeting Rac1 for degradation. EMBO J. 31, 14–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gill G. (2004) SUMO and ubiquitin in the nucleus. Different functions, similar mechanisms? Genes Dev. 18, 2046–2059 [DOI] [PubMed] [Google Scholar]