Background: Ascorbate biosynthesis in plants occurs mainly via the l-galactose pathway.
Results: Chlamydomonas reinhardtii VTC2 encodes a GDP-l-galactose phosphorylase whose transcript levels are induced in response to oxidative stress concurrent with increased ascorbate accumulation.
Conclusion: Increased oxidative stress in C. reinhardtii results in an enzymatic and non-enzymatic antioxidant response.
Significance: First characterization of C. reinhardtii ascorbate biosynthesis and recycling pathways.
Keywords: Algae, Oxidative Stress, Plant Biochemistry, Plant Molecular Biology, Vitamin C
Abstract
The l-galactose (Smirnoff-Wheeler) pathway represents the major route to l-ascorbic acid (vitamin C) biosynthesis in higher plants. Arabidopsis thaliana VTC2 and its paralogue VTC5 function as GDP-l-galactose phosphorylases converting GDP-l-galactose to l-galactose-1-P, thus catalyzing the first committed step in the biosynthesis of l-ascorbate. Here we report that the l-galactose pathway of ascorbate biosynthesis described in higher plants is conserved in green algae. The Chlamydomonas reinhardtii genome encodes all the enzymes required for vitamin C biosynthesis via the l-galactose pathway. We have characterized recombinant C. reinhardtii VTC2 as an active GDP-l-galactose phosphorylase. C. reinhardtii cells exposed to oxidative stress show increased VTC2 mRNA and l-ascorbate levels. Genes encoding enzymatic components of the ascorbate-glutathione system (e.g. ascorbate peroxidase, manganese superoxide dismutase, and dehydroascorbate reductase) are also up-regulated in response to increased oxidative stress. These results indicate that C. reinhardtii VTC2, like its plant homologs, is a highly regulated enzyme in ascorbate biosynthesis in green algae and that, together with the ascorbate recycling system, the l-galactose pathway represents the major route for providing protective levels of ascorbate in oxidatively stressed algal cells.
Introduction
l-Ascorbic acid plays an essential role in plants by protecting cells against oxidative damage. In addition to its antioxidant role, l-ascorbic acid is also an important enzyme cofactor, for example, in violaxanthin de-epoxidase, required for dissipation of excess excitation energy, and prolyl hydroxylases (1–3). In plants, several pathways have been proposed to function in l-ascorbic acid biosynthesis. The best described pathway, the Smirnoff-Wheeler pathway or the l-galactose pathway, involves 10 enzymatic steps to convert d-glucose to l-ascorbic acid via intermediate formation of GDP-d-mannose, GDP-l-galactose, l-galactose-1-P, l-galactose, and l-galactono-1,4-lactone (4). Whereas the initial six steps are also involved in cell wall/glycoprotein biosynthesis, GDP-l-galactose phosphorylase (VTC2/VTC5) catalyzes the first committed step in l-ascorbic acid biosynthesis forming l-galactose-1-P (5, 6). l-Galactose-1-P phosphatase (VTC4),3 l-galactose dehydrogenase (l-Gal-DH), and l-galactono-1,4-lactone dehydrogenase (GLDH) catalyze the final steps in the Smirnoff-Wheeler pathway in higher plants such as Arabidopsis thaliana (7–9).
The biosynthesis of l-ascorbic acid is not characterized in detail in the green algae. Unicellular green algae such as the chlorophytes Chlorella pyrenoidosa and Prototheca moriformis can synthesize l-ascorbate using the l-galactose pathway (10–12). Two other photosynthetic unicellular protists (Euglena gracilis and Ochromonas danica) (13, 14) and a diatom (Cyclotella cryptica) utilize the inversion pathway commonly found in animals (supplemental Fig. S1) (15). Here we provide evidence that the Smirnoff-Wheeler pathway is completely conserved in the green alga C. reinhardtii. The VTC2 protein from C. reinhardtii is highly similar to higher plant VTC2/VTC5, containing the HXHXH motif characteristic of members of the HIT protein superfamily of nucleotide hydrolases and transferases (16).
Higher plants facing increased oxidative stress exhibit, in addition to increased VTC2 mRNA and activity levels, elevated transcript abundance for all the enzymes of the vitamin C recycling pathway (ascorbate-glutathione system) in the chloroplast including ascorbate peroxidase (APX), monodehydroascorbate reductase (MDAR), dehydroascorbate reductase (DHAR), and glutathione reductase (GSHR) (2, 17). In this work, we found that Chlamydomonas reinhardtii cells facing oxidative stress have increased abundance of VTC2 transcripts and all the enzymes of the ascorbate-glutathione system, as well as higher total ascorbate content. This suggests that C. reinhardtii cells respond to oxidative stress by producing more l-ascorbic acid both via de novo synthesis through the l-galactose pathway and via increased recycling.
EXPERIMENTAL PROCEDURES
Materials
ADP-d-Glc, GDP-d-Glc, GDP-d-Man, UDP-d-Gal, UDP-d-Glc (all in the α-configuration), GDP-β-l-Fuc, and GDP were from Sigma. GDP-β-l-Gal, synthesized and purified as described (18) was provided by Prof. Shinichi Kitamura (Osaka Prefecture University). This preparation was further purified by the reversed-phase HPLC method as described in Ref. 5. Fractions containing GDP-l-Gal were lyophilized, resuspended in H2O, and stored at −20 °C. Hydrogen peroxide (30%) and tert-butyl hydroperoxide (tBuOOH) (70%) were purchased from Fisher and Lancaster Synthesis, Inc., respectively. Ascorbate oxidase from Cucurbita sp. (EC 1.10.3.3; A0157) was purchased from Sigma.
Strains and Culture Conditions
C. reinhardtii strains 2137 (CC1021) and CC425 were obtained from the Chlamydomonas culture collection (Duke University) and grown in Tris acetate-phosphate (TAP) medium (19) at 24 °C and 50–100 μmol m−2 s−1 light intensity.
Sequencing of C. reinhardtii VTC2
The VTC2 cDNA clone MXL096d05 (corresponding to EST BP098619) was completely sequenced. It contains the entire predicted VTC2 open reading frame, 1857 bp long, encoding a protein of 618 amino acids. The open reading frame is flanked by 499 nt of 5′ untranslated region and a 3′ untranslated region of 1396 nt followed by a 68-nt poly(A) tail. The complete VTC2 sequence has been deposited in NCBI (GenBank accession JQ246433).
VTC2 Cloning
The VTC2 expression construct was generated by nested PCR and the Gateway recombinational cloning system (Invitrogen) as described (20). Briefly, the coding sequence of VTC2 (amino acids D2-A618) was amplified with Phusion polymerase (New England Biolabs) from plasmid MXL096d05 using gene-specific primers with 5′ extensions encoding a TEV protease cleavage site in the forward primer (VTC2.D2) and a C-terminal hexahistidine tag followed by a stop codon in the reverse primer (VTC2.A628) (supplemental Table S4). The initial product was then amplified with a second set of primers to introduce AttB1 and AttB2 recombination sites (PE-277 and PE-278) (supplemental Table S4). Amplification products were gel purified and recombined into the donor vector pDONR201 and subsequently into expression vector pKM596 (20) to produce an N-terminal His-tagged maltose-binding protein (MBP) fusion using the Invitrogen protocol. DNA sequencing (Genewiz) was used to confirm the sequence of the expression construct.
VTC2 Expression and Purification
The expression plasmid was transformed into Escherichia coli BL21-Gold (DE3) cells (Novagen). Cells were grown in LB medium at 37 °C to an A600 nm of 0.6 at which point the temperature was shifted to 18 °C and protein expression was induced by the addition of isopropyl 1-thio-β-d-galactopyranoside to a concentration of 1 mm. Cell growth was continued overnight and the cells were collected by centrifugation the following day. The cell pellet was resuspended in wash buffer (20 mm Tris, pH 8.0, 300 mm NaCl, 10 mm imidazole, 0.2% Nonidet P-40, 10% glycerol) supplemented with protease inhibitor mixture (Sigma), PMSF (100 μm), DNase (20 μg ml−1), a few crystals of lysozyme, and 10 mm β-mercaptoethanol. Cells were lysed using a French press. The lysate was clarified by centrifugation (30 min at 35,000 × g) and the supernatant was incubated with nickel-nitrilotriacetic acid-agarose beads (Qiagen) for 60 min at 4 °C. The beads were washed extensively with wash buffer and bound protein was eluted with elution buffer (wash buffer containing 300 mm imidazole). VTC2 was further purified by size exclusion chromatography using a HiLoad Superdex S-200 column (GE Life Sciences) equilibrated in 20 mm Tris, pH 8.0, 300 mm NaCl, and 10% glycerol. Peak fractions were analyzed by SDS-PAGE and those containing VTC2 were pooled and concentrated. Two peaks containing VTC2 MBP fusion proteins were obtained by size exclusion chromatography. Both peaks contained pure MBP-VTC2 fusion protein and were pooled separately and concentrated. The fraction showing the highest activity was used for enzymatic analyses.
Nucleic Acid Analysis
Total RNA was extracted from exponentially growing C. reinhardtii cells as previously described (21). RNA quality was assessed using an Agilent 2100 Bioanalyzer and RNA blot hybridization for CBLP as described (22). The probe used for detection was a 915-bp EcoRI fragment from the cDNA insert (encoding CBLP) in plasmid pcf8-13 (23).
Quantitative Real-time PCR on cDNA
cDNA synthesis and quantitative real-time PCR was performed on technical triplicates as described (22) using the gene-specific primers listed in supplemental Table S5. The data are presented as the fold-change in mRNA abundance, normalized to an endogenous reference transcript (CBLP or UBQ2), relative to the sample grown before 1 mm H2O2 or 0.1 mm tBuOOH treatment (time 0). The abundance of the two reference transcripts did not change under the conditions tested.
Ascorbate Measurements
C. reinhardtii cells were grown in TAP medium to a density of 3 × 106 cells ml−1, collected by centrifugation at 2,500 × g for 5 min, resuspended in extraction buffer containing 2% metaphosphoric acid, 2 mm EDTA, and 5 mm DTT and stored at −80 °C. To prepare extracts for vitamin C analysis, cells were lysed by freeze/thaw cycling and the soluble fractions were separated by centrifugation (16,100 × g, 10 min at 4 °C). Vitamin C content was measured by reversed-phase HPLC on an Econosphere C-18 column (5 μm bead size, 4.6 × 250 mm; Alltech Associates, Deerfield, IL) using a Hewlett Packard Series II 1090 liquid chromatograph. A mobile-phase gradient of 0–40% acetonitrile in 20 mm triethyl ammonium acetate, pH 6.0, was used at a flow rate of 1 ml min−1. The injection volume was 50–100 μl. Ascorbic acid was detected by monitoring the absorbance at 265 nm. The ascorbic acid peak was identified by comparison with the elution time of an l-ascorbate standard and by demonstrating a decrease of the peak area after the samples were treated with ascorbate oxidase. This treatment was performed by adding 2 units of ascorbate oxidase from Cucurbita sp. (EC 1.10.3.3) to 60 μl of the extract in a final concentration 0.12 m monosodium citrate for 1 h at 4 °C. The final pH of the reaction was about 5.6. The differences in the peak areas measured before and after addition of ascorbate oxidase were used to calculate ascorbic acid levels based on a standard curve. The cellular concentration of l-ascorbate was determined using a cell volume of 140 femtoliters (24).
HPLC-based Nucleoside Diphosphate (NDP)-Hexose Phosphorylase Assay
NDP-hexose phosphorylase activities of recombinant VTC2 enzyme were assayed by measuring NDP formation after incubation with NDP-hexose in a reaction mixture at pH 7.5 containing 50 mm Tris-HCl, 5 mm sodium phosphate, 10 mm NaCl, and 1 mm DTT. Reactions (26 °C) were initiated by enzyme addition and stopped after 5–10 min by heating at 98 °C for 5 min. After removal of precipitated protein by centrifugation, supernatants were analyzed by anion-exchange HPLC as described in Ref. 5. NDP and NDP-hexose concentrations were calculated by comparing the integrated peak areas with those of standard NDP or NDP-hexose solutions. GraphPad Prism (La Jolla, CA) was used to calculate Km and Vmax values.
RNA-Seq
Total RNA samples prepared from C. reinhardtii strain 2137 grown photoheterotrophically in the presence of 1 mm H2O2 for 30 and 60 min were sequenced on a GAIIx platform. cDNA libraries were made using the protocol from Illumina and sequenced as single-end 76-mers. Raw and processed sequence files are available at the NCBI Gene Expression Omnibus (accession number GSE34826). Sequence reads were aligned using Bowtie (25) in single-end mode and with a maximum tolerance of 3 mismatches to the Au10.2 transcript sequences corresponding to the version 4 assembly of the C. reinhardtii genome. Expression estimates were obtained for each individual run in units of RPKMs (reads per kilobase of mappable transcript length per million mapped reads) (26), after normalization by the number of aligned reads and transcript mappable length (27). Technical replicates were averaged to obtain per-sample expression estimates. Final expression estimates and fold-changes were obtained for each biological replicate.
Sequence and Phylogenetic Analyses
To search for orthologs/homologs of A. thaliana members of the l-galactose pathway in green algae, two BLAST searches were performed. In the first, protein sequences of the A. thaliana proteins were used as queries to search against algal genomes databases. Sequences of putative homologs were retrieved and used for the second query by performing BLASTp against the A. thaliana database. If in the second query the highest-scoring homolog in A. thaliana was exactly the original A. thaliana query sequence, that protein was considered an ortholog (mutual best hit). Phylogenetic relationships were inferred using the Maximum Likelihood method based on the Whelan and Goldman model (28). The tree with the highest log likelihood is shown. Branch lengths reflect the number of substitutions per site. All positions containing gaps and missing data were eliminated. Alignment of putative VTC2 homologs was performed using MUSCLE, and evolutionary analyses were conducted in MEGA5 (29).
RESULTS
The C. reinhardtii Genome Encodes a Homolog of Plant GDP-l-galactose Phosphorylase
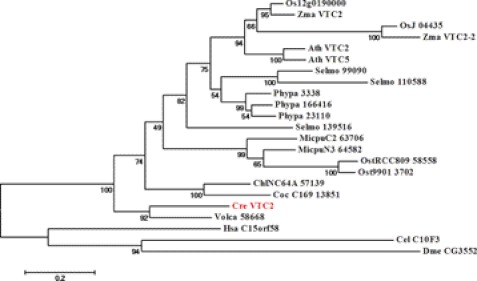
Biosynthesis of vitamin C in higher plants occurs mainly via the l-galactose pathway (9). In A. thaliana, the first committed step in the sequence of 10 enzymatic reactions from d-glucose to l-ascorbate is conversion of GDP-l-galactose to l-galactose-1-P, a reaction catalyzed by the GDP-l-galactose phosphorylase VTC2. Therefore, we were interested in finding homologs of VTC2 in C. reinhardtii and other green algae such as Volvox carteri, Chlorella sp. NC64A, Coccomyxa sp. C169, Micromonas sp. RCC299, and Ostreococcus RCC809. BLASTp searches identified a VTC2 homolog (Cre13.g588150) in C. reinhardtii (supplemental Fig. S2). Cre13.g588150 exhibits 46% amino acid sequence identity to A. thaliana VTC2. The A. thaliana genome encodes a VTC2 paralog, VTC5, which shows enzymatic properties similar to those of VTC2 (30, 31). The C. reinhardtii protein has 47% identity to VTC5 at the amino acid level. Because the C. reinhardtii genome encodes only a single protein highly similar to A. thaliana VTC2/VTC5, we termed this protein Cre13.g588150 VTC2. The amino acid sequence of the VTC2 protein from C. reinhardtii does not contain any transmembrane domains. Several subcellular localization prediction programs (ChloroP, TargetP, Psort, and PredSL) indicated that C. reinhardtii VTC2 does not possess obvious organellar targeting sequences, suggesting that, like the plant homologs, it is most likely a cytosolic protein. C. reinhardtii VTC2 contains a highly conserved histidine triad (HIT) motif (HXHXH, where X is a hydrophobic residue) (supplemental Fig. S2). C. reinhardtii VTC2 is more closely related to the Volvox VTC2 protein and among algal homologs it appears that the Micromonas sp. and Ostreococcus sp. proteins are more closely related to higher plant VTC2 proteins than to the animal VTC2 homologs (Fig. 1).
FIGURE 1.
Phylogenetic tree of VTC2-like proteins. Protein sequences homologous to C. reinhardtii VTC2 were used to build the phylogenetic tree as described under “Experimental Procedures.” Bootstrap values are shown below the branches. Bar, 0.2 amino acid substitutions per site. C. reinhardtii (Cre), Coccomyxa sp. C-169 (Coc_C169), Chlorella sp. NC64A (ChlNC64A), Volvox carteri f. nagariensis (Vca), O. lucimarinus (Ost9901), Ostreococcus sp. RCC809 (OstRCC809), M. pusilla (MicpuC2), Micromonas sp. RCC299 (MicpuN3), A. thaliana (Ath), Physcomitrella patens (Ppa), Ricinus communis (Rco), Oryza sativa (Osa), Selaginella moellendorffii (Selmo), D. melanogaster (Dme), C. elegans (Cel), and H. sapiens (Hsa).
Enzymatic Components of l-Galactose Pathway to Vitamin C Biosynthesis Are Conserved in Green Algae
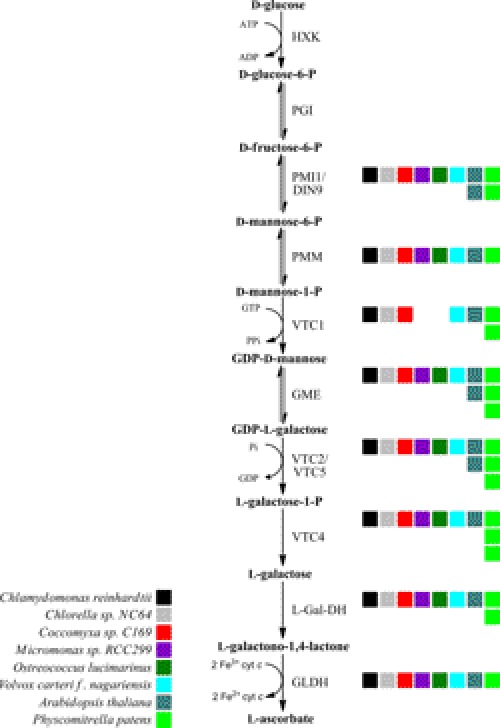
Because higher plant VTC2 has orthologs in C. reinhardtii and other green algae, we investigated whether the green algae encode the rest of the components of the Smirnoff-Wheeler pathway. BLASTp and tBLASTn searches identified orthologs (defined as mutual best BLAST hit) for almost all l-galactose pathway enzymes in six green algae (C. reinhardtii, V. carteri, Chlorella sp. NC64A, Coccomyxa sp. C169, Micromonas sp. RCC299, and Ostreococcus lucimarinus). Orthologs of A. thaliana phosphomannose isomerase (PMI1), phosphomannomutase (PMM), GDP-d-mannose 3″,5″-epimerase (GME1), l-galactose-1-P phosphatase (VTC4), l-Gal-DH, and GLDH are present in all six species (Fig. 2 and supplemental Table S1). Interestingly, our sequence analysis identified orthologs of GDP-d-mannose pyrophosphorylase (VTC1) in C. reinhardtii, V. carteri, Chlorella sp. NC64A, and Coccomyxa sp. C169, but not in Micromonas sp. (RCC299 and Micromonas pusilla) nor in Ostreococcus sp. (Ostreococcus tauri, Ostreococcus lucimarinus, and Ostreococcus RCC899) (Fig. 2 and supplemental Table S1). Micromonas sp. and Ostreococcus sp. both belong to the class Prasinophyceae, which diverged at the base of the algal lineage and are therefore more distantly related to the chlorophyte algae. It is possible that Micromonas spp. and Ostreococcus spp. synthesize GDP-d-mannose using a different pathway (see “Discussion”). Overall, we conclude that the l-galactose pathway to l-ascorbate biosynthesis is conserved in the green algae.
FIGURE 2.
The l-galactose pathway of ascorbic acid biosynthesis is conserved in green algae. Colored squares indicate the number of A. thaliana orthologs present in each organism. The enzymes catalyzing the successive steps are hexokinase (HXK), phosphoglucose isomerase (PGI), phosphomannose isomerase (PIM), phosphomannomutase (PMM), GDP-l-mannose pyrophosphorylase (VTC1), GDP-d-mannose-3′,5′-epimerase (GME), GDP-l-galactose phosphorylase (VTC2), l-galactose-1-P phosphatase (VTC4), l-Gal-DH, and GLDH.
Because alternative l-ascorbate biosynthetic pathways have been proposed (7, 32), we searched for orthologs/homologs of the enzymes catalyzing the proposed steps in these alternate pathways (supplemental Fig. S1). First, the proposed l-gulose pathway (33) involves the A. thaliana GDP-d-mannose 3″,5″-epimerase, which is orthologous to C. reinhardtii SNE1. This enzyme can form GDP-l-gulose, which, if converted to l-gulono-1,4-lactone, would provide a substrate for an oxidase reaction leading directly to l-ascorbate. Although C. reinhardtii GLDH demonstrates 30% amino acid sequence identity to the rat l-gulono-1,4-lactone dehydrogenase/oxidase (Gulo) (34), and two other similar proteins are present (Cre14.g611650 with 26% amino acid identity and Cre03.g177600 with 22% amino acid identity), these putative enzymes have not been characterized and no homologs of enzymes converting GDP-l-gulose to l-gulono-1,4-lactone have been found.
l-Gulono-1,4-lactone could also be potentially formed from myo-inositol via d-glucuronate (supplemental Fig. S1, animal-like pathway). C. reinhardtii codes for a potential myo-inositol oxidase (Cre01.g025850) that might be responsible for the formation of d-glucuronate and shows 31% amino acid identity to A. thaliana MIOX4 (supplemental Table S1). Conversion of d-glucuronate to l-gulonate would require the action of a glucuronate reductase, which has not been identified in plants. Formation of l-gulono-1,4-lactone from l-gulonate requires an aldonolactonase (gulonolactonase) (35). SMP30 (senescence marker protein 30) has been recently identified to function as a glucono/gulonolactonase (36). The C. reinhardtii genome does not encode a homolog to SMP30. Hence, it seems unlikely that C. reinhardtii would use this route as an alternate pathway to vitamin C biosynthesis.
It has also been proposed that biosynthesis of l-ascorbic acid could occur via the galacturonate or salvage pathway (7, 9) (supplemental Fig. S1). This pathway would involve conversion of methyl-d-galacturonate to d-galacturonate. The enzyme catalyzing this reaction has not yet been identified. Formation of l-galactonate from d-galacturonate is catalyzed in ripening strawberry fruits by an aldo-keto reductase specific for d-galacturonate (GalUR) (37). The C. reinhardtii genome encodes several aldo-keto reductases with homology to strawberry d-galacturonate reductase (supplemental Table S1), but none of them is an ortholog of the plant enzyme. On the other hand, orthologs of the strawberry GalUR are present in other algal species such as Chlorella sp. NC64A, Micromonas sp. RCC299, O. lucimarinus, or V. carteri (supplemental Table S1). The penultimate reaction in the galacturonate pathway (l-galactonate to l-galactono-1,4-lactone conversion) would require the function of an aldonolactonase, which has been recently characterized in the protist E. gracilis (38). BLASTp and tBLASTn searches did not identify any homologs to E. gracilis aldonolactonase in C. reinhardtii or V. carteri, but orthologs are present in Chlorella sp. NC64A, Coccomyxa sp. C169, Micromonas sp. RCC299, and O. lucimarinus (supplemental Table S1).
We conclude that essential components of the alternate pathways to l-ascorbate biosynthesis are missing in C. reinhardtii. We could identify homologs of l-gulono-1,4-lactone dehydrogenase for the l-gulose pathway and galacturonate reductase for the salvage pathway, but there are no orthologs to rat Gulo or strawberry GalUR and the sequence similarity is poor. On the other hand, we show that all components of the plant Smirnoff-Wheeler pathway have orthologs in C. reinhardtii and in other algal species. These results point to a conserved l-galactose pathway to l-ascorbate biosynthesis, which might represent the major route to l-ascorbate biosynthesis in algae, in particular C. reinhardtii and other Volvocales.
Recombinant C. reinhardtii VTC2 Is a GDP-l-galactose/GDP-d-glucose Phosphorylase
Previous studies demonstrated that A. thaliana VTC2 and VTC5 are GDP-l-galactose phosphorylases, converting GDP-l-galactose into l-galactose-1-P and GDP in the presence of Pi (5, 30, 31). To test whether C. reinhardtii VTC2 can catalyze this reaction, recombinant C. reinhardtii VTC2 was purified as a His- and MBP-tagged protein. Because A. thaliana VTC2 shows GDP-l-galactose and GDP-d-glucose phosphorylase activities, we first determined the activity of the C. reinhardtii enzyme on various sugar nucleotides in the presence of inorganic phosphate (Table 1). Similar activity was seen with GDP-l-Gal and GDP-d-Glc, whereas a 2-fold lower activity was found with GDP-l-Fuc. No significant phosphorylase activity was measured with GDP-d-Man, UDP-d-Glc, UDP-d-Gal, and ADP-d-Glc (Table 1). Thus, our data indicate that C. reinhardtii VTC2 possesses a similar nucleotide sugar substrate specificity as A. thaliana VTC2.
TABLE 1.
Substrate specificity of recombinant C. reinhardtii VTC2
Various sugar nucleotides (50 μm) were incubated in the presence of C. reinhardtii VTC2 recombinant enzyme (0.025 μg ml−1) for 10 min at 26 °C. With GDP-l-Gal as a substrate, the specific activity of the C. reinhardtii VTC2 enzyme was 25.4 ± 5.1 μmol min−1 mg protein−1 (mean ± S.D., n = 3); this value was taken as 100%. The phosphorylase activities found with the other sugar nucleotides are given as a percentage of the activity found with GDP-l-Gal ± S.D. Values represent the means of 2–3 individual experiments for each substrate.
| Substrate | Relative activity of recombinant C. reinhardtii VTC2 |
|---|---|
| % ± S.D. | |
| GDP-l-Gal | 100 |
| GDP-d-Glc | 87.4 ± 29.2 |
| GDP-l-Fuc | 51.4 ± 15.1 |
| GDP-d-Man | 0 ± 0 |
| UDP-d-Glc | 0.5 ± 0.8 |
| UDP-d-Gal | 2.5 ± 4.1 |
| ADP-d-Glc | 3.7 ± 5.8 |
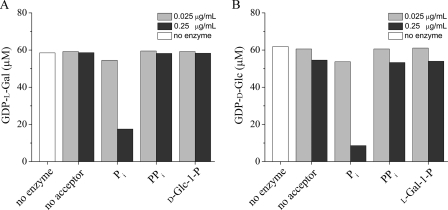
The conserved HIT motif (HXHXH) in C. reinhardtii VTC2 is typical of HIT hydrolases, whereas plant VTC2 proteins contain a HIT motif typical for HIT transferases/phosphorylases (HXHXQ) (16). Therefore, we tested the acceptor specificity of C. reinhardtii VTC2 by measuring the GDP-l-Gal or GDP-d-Glc consumption after incubation of the recombinant VTC2 enzyme with different possible acceptors. When recombinant C. reinhardtii VTC2 was incubated in the absence of Pi, we detected no hydrolytic activity (Fig. 3). However, in the presence of Pi, we observed a dramatic increase in GDP-l-Gal and GDP-d-Glc consumption (Fig. 3). Incubation of the enzyme with pyrophosphate (PPi), d-Glc-1-P (in the presence of GDP-l-Gal), or l-Gal-1-P (in the presence of GDP-d-Glc) did not result in any significant nucleotide sugar substrate consumption (Fig. 3). Additionally, we did not detect the formation of GMP or GTP, the expected products of hydrolase or pyrophosphorylase activity, under any of these conditions (data not shown). These data clearly indicate that C. reinhardtii VTC2 is a phosphorylase like the Arabidopsis enzyme.
FIGURE 3.
Acceptor specificity of C. reinhardtii recombinant VTC2. GDP-l-Gal and GDP-d-Glc consumption was measured by the HPLC assay described under “Experimental Procedures.” GDP-l-Gal or GDP-d-Glc were added to the reaction mixtures at a final concentration of 50 μm. The consumption of GDP-l-Gal (A) and GDP-d-Glc (B) was measured with a final enzyme concentration of 0.025 μg/ml (light gray bars) or 0.25 μg/ml (dark gray bars).
C. reinhardtii VTC2 has similar, low micromolar, Michaelis constants for both GDP-l-Gal and GDP-d-Glc (Table 2). Interestingly, with C. reinhardtii VTC2 we have found at least 10 times higher kcat values for both substrates compared with the A. thaliana VTC2 recombinant enzyme, leading to about 10 times higher catalytic efficiencies for the former than for the latter enzyme (Table 2).
TABLE 2.
Characterization of the GDP-hexose phosphorylase activities of the recombinant A. thaliana and C. reinhardtii proteins
Values for the A. thaliana enzyme were taken from Linster et al. (5). Km and Vmax values for the C. reinhardtii VTC2 homolog were obtained by fitting the initial rate data to the Michaelis-Menten equation using the GraphPad Prism program. Enzymatic turnover numbers were derived from the Vmax values by using a molecular mass of 110 kDa for His-MBP-tagged C. reinhardtii enzyme with the assumption that the enzyme preparations were pure. Incubation times and enzyme concentrations were adjusted to obtain initial velocity data. Enzymatic activities were measured by the HPLC assay as described under “Experimental Procedures.” Values are the mean ± S.D. calculated from 2–3 individual experiments for each substrate.
| Substrate |
kcat |
Km |
kcat/Km |
|||
|---|---|---|---|---|---|---|
| C. reinhardtii | A. thaliana | C. reinhardtii | A. thaliana | C. reinhardtii | A. thaliana | |
| s−1 | mm | s−1m−1 | ||||
| GDP-l-galactose | 615 ± 3 | 64 ± 8 | 0.008 ± 0.001 | 0.010 ± 0.001 | 9.2 ± 1.3 × 107 | 6.3 ± 0.9 × 106 |
| GDP-d-glucose | 813 ± 277 | 23 ± 3 | 0.0088 ± 0.0029 | 0.0044 ± 0.0016 | 8.0 ± 1.3 × 107 | 5.7 ± 2.3 × 106 |
VTC2 Transcript Levels and Ascorbate Levels Are Increased in Response to Oxidative Stress
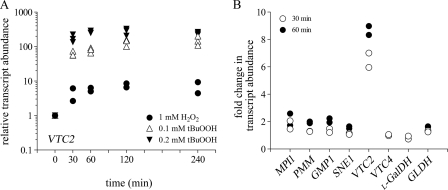
Previous studies have indicated that A. thaliana VTC2 mRNA levels are increased in leaves subjected to high light (30) and in seedlings grown in light compared with those grown in the dark (39). Therefore, we tested whether transcript levels of C. reinhardtii VTC2 respond to oxidative stress. C. reinhardtii was grown photoheterotrophically to 2 × 106 cells ml−1, then challenged with 1 mm H2O2 or 0.1 and 0.2 mm tBuOOH for 30, 60, 120, and 240 min. Both H2O2 and tBuOOH (an organic peroxide capable of inducing lipid peroxidation) treatments enhance intracellular reactive oxygen species production. The concentrations of H2O2 and tBuOOH and time points used in this study were previously shown to have no effect on cell growth in C. reinhardtii and yet were high enough to induce the antioxidant defense mechanisms (40–43). A lower concentration of tBuOOH was used because it was more stable than H2O2 under our culture conditions (supplemental Fig. S3). VTC2 transcript abundance was assessed by real-time PCR. We found that VTC2 mRNA transcript abundance increased 4-fold after 30 min and reached a maximum of 7-fold induction after 120 min exposure to 1 mm H2O2 (Fig. 4A). When C. reinhardtii cells were exposed to 0.1 mm tBuOOH, we observed a more dramatic induction in the VTC2 transcript levels (50-fold increase after 30 min with the highest induction of 155-fold after 240 min). Increasing the tBuOOH concentration to 0.2 mm resulted in an even higher increase in the VTC2 mRNA abundance (150-fold after 30 min and 250-fold after 120 min) (Fig. 4A).
FIGURE 4.
VTC2 transcript levels are increased in response to oxidative stress. A, fold-change (log10) of VTC2 transcript assessed by real-time PCR in C. reinhardtii cells grown photoheterotrophically in the presence of 1 mm H2O2 (filled circles), 0.1 mm tBuOOH (open triangles), or 0.2 mm tBuOOH (filled inverted triangles) for the indicated times. Each data point represents the average of three technical triplicates for quantitative PCR from one biological replicate of treated cells. The symbol for the untreated cultures (time 0) represents the overlap of all three symbols used. B, transcript abundance of genes encoding enzymes of the l-galactose pathway quantified by RNA-Seq in C. reinhardtii cells grown in the presence of 1 mm H2O2 for 30 min (open circles) or 60 min (filled circles). Fold-changes were calculated relative to the transcript abundances in untreated cells for both quantitative PCR and RNA-Seq experiments.
To assess the overall impact of peroxide stress on the Smirnoff-Wheeler pathway, we quantified the abundance of transcripts for each gene in the pathway in H2O2-treated versus untreated cells. Changes in the VTC2 transcript levels after H2O2 exposure observed by RNA-Seq are very similar to those observed by real-time PCR (6.4-fold induction after 30 min and 8.6-fold induction after 60 min) (Fig. 4B). Other components of the pathway including MPI1, PMM, and GMP1 showed at best a 2-fold increase in their transcript abundance after 60 min of exposure to H2O2. The transcript levels of SNE1, VTC4, l-GalDH, and GLDH did not change significantly (Fig. 4B and supplemental Table S2) in response to H2O2 treatment. Together, the combined real-time PCR and RNA-Seq analyses demonstrated that VTC2 mRNA levels are highly and selectively induced by oxidative stress, indicating that the GDP-l-galactose phosphorylase step is potentially the key regulatory point of the l-ascorbate biosynthetic pathway in C. reinhardtii.
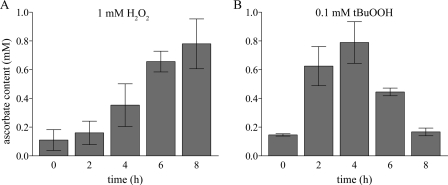
Next, we asked the question whether the increased VTC2 mRNA levels correlate with a change in ascorbate content. Total ascorbate levels were measured in cell extracts from C. reinhardtii grown under 1 mm H2O2 or 0.1 mm tBuOOH stress for 2, 4, 6, and 8 h. Total ascorbate content increased progressively after addition of H2O2, showing a slight increase after 2 h and reaching a maximum after 8 h, where we measured 7-fold higher ascorbate concentrations than in untreated cells (Fig. 5A). On the other hand, cells treated with 0.1 mm tBuOOH displayed a 4-fold higher ascorbate content 2 h after addition of tBuOOH, with a further increase after 4 h (5-fold). In contrast to C. reinhardtii cells exposed to H2O2, after 6 h of tBuOOH treatment we noticed a drop in the total ascorbate levels (3-fold more compared with untreated cells), which decreased even further after 8 h to levels similar to those observed for untreated cells (Fig. 5B). The observation that tBuOOH treatment depletes cellular ascorbate has been made previously in rat hepatocytes (44) and rat astrocytes (45). Altogether, our results indicate a correlation between the VTC2 mRNA levels and l-ascorbic acid content in C. reinhardtii cells exposed to oxidative stress.
FIGURE 5.
Ascorbate levels are elevated in response to oxidative stress. Vitamin C concentration was measured in extracts of C. reinhardtii cells exposed to 1 mm H2O2 (A) or 0.1 mm tBuOOH (B) for 2, 4, 6, and 8 h. Error bars represent the S.D. from three biological replicates.
Genes Encoding Components of Ascorbate-glutathione Scavenging System Are Induced in Response to Oxidative Stress
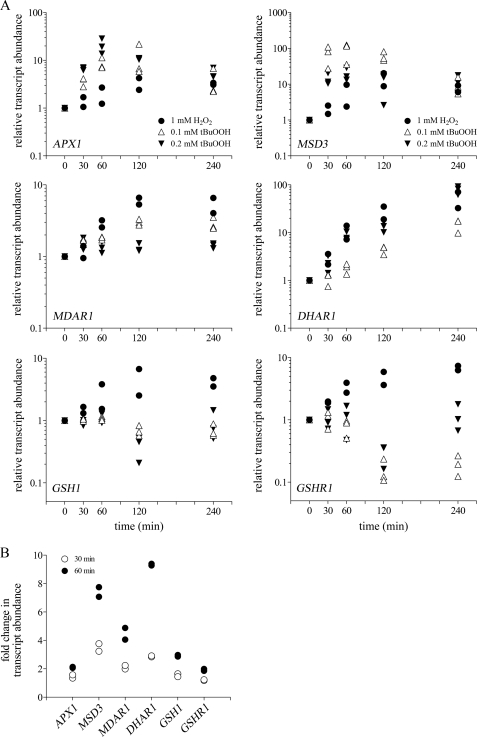
The ascorbate-glutathione cycle is a well known mechanism to scavenge H2O2 in various cell compartments (2), particularly in plants (46) (and see Fig. 7). Therefore, we were interested in expression profiles of the genes encoding the ascorbate-glutathione system components in C. reinhardtii cells exposed to 1 mm H2O2 or 0.1–0.2 mm tBuOOH for 30, 60, 120, or 240 min. In plants, and most likely also in C. reinhardtii, Photosystem I is the major site for superoxide anion production (O2˙̄) (17), which is disproportionated to H2O2 by the action of one or several superoxide dismutases. Here we found that in C. reinhardtii, MSD3 transcript levels (encoding plastid-localized MnSOD3) are highly induced in response to peroxide treatment (Fig. 6A). Treatment of C. reinhardtii cells with 1 mm H2O2 resulted in a 2–15-fold induction of this gene over the 4-h exposure period. An even higher level of up-regulation (100-fold after 60 min) was reached when C. reinhardtii cells were exposed to 0.1 mm tBuOOH. H2O2 produced by MnSOD3 is reduced to H2O by ascorbate in a reaction catalyzed by APX1. The mRNA abundance of C. reinhardtii APX1 was induced 2–4-fold after exposure to 1 mm H2O2, whereas 0.1 mm tBuOOH treatment resulted in a 10–15-fold induction of APX1 transcript levels (Fig. 6A). Ascorbate peroxidase oxidizes ascorbate to monodehydroascorbate, which is either reduced to ascorbate by the action of MDAR1, or spontaneously disproportionates to dehydroascorbate. MDAR1 mRNA abundance was induced in response to 1 mm H2O2 (5–6-fold after 120 min), whereas 0.1 mm tBuOOH treatment resulted in a more subtle 2–3-fold up-regulation of this gene. Dehydroascorbate can be reduced back to ascorbate by DHAR1. The reaction requires reduced GSH. The resulting oxidized GSSG is converted back to GSH by glutathione reductases (GSHR1/2 in C. reinhardtii). DHAR1 transcript abundance was progressively up-regulated after exposure to 1 mm H2O2 (from 2–3-fold after 30 min to 50-fold after 240 min). A similar trend of DHAR1 overexpression was observed under tBuOOH treatment (Fig. 6A). The transcript levels of the key enzyme involved in glutathione synthesis, γ-glutamylcysteine synthetase (GSH1), and GSHR1 were induced only in response to 1 mm H2O2 (Fig. 6A). Interestingly, neither of those transcripts changed in abundance during the first 60 min after 0.1 or 0.2 mm tBuOOH addition and in fact they even decreased after 120 min (Fig. 6A). RNA-Seq analysis of C. reinhardtii cells exposed to 1 mm H2O2 for 30 and 60 min indicated up-regulation of all the genes encoding the enzymes of the ascorbate-glutathione cycle (Fig. 6B and supplemental Table S3). The increase in their transcript abundance was higher after 60 min and, in agreement with the real-time PCR results, MSD3 and DHAR1 were the most highly induced genes. We conclude, based on the transcript abundance changes observed in this study in response to peroxide stress, that the ascorbate-glutathione system plays an important role in the oxidative stress response in C. reinhardtii.
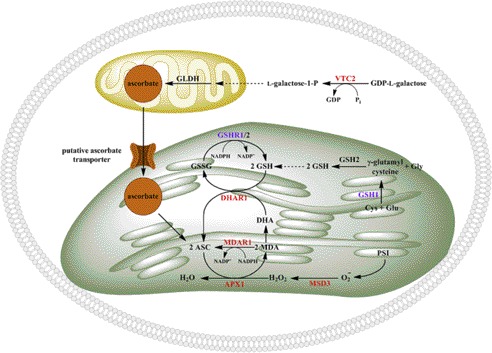
FIGURE 7.
Putative regulatory sites for ascorbate biosynthesis and ascorbate recycling in C. reinhardtii. Enzymes depicted in red indicate the proteins whose mRNA levels are increased in response to oxidative stress (1 mm H2O2 and 0.1 mm tBuOOH) and those in magenta that show changes in mRNA levels only in response to H2O2 treatment. APX1 (ascorbate peroxidase), MSD3 (Mn superoxide dismutase), MDAR1 (monodehydroascorbate reductase), DHAR1 (dehydroascorbate reductase), GSH1 (γ-glutamylcysteine synthetase), GSH2 (glutathione synthetase), GSHR1/2 (glutathione reductase), GLDH (l-galactono-1,4-lactone dehydrogenase), VTC2 (GDP-l-galactose phosphorylase), ASC (ascorbate), MDA (monodehydroascorbate), and DHA (dehydroascorbate) are indicated.
FIGURE 6.
Oxidative stress conditions result in up-regulation of GSH-ascorbate cycle enzymes. A, relative transcript levels of genes coding for ascorbate-glutathione cycle components were quantified by real-time PCR in C. reinhardtii cells grown in the presence of 1 mm H2O2 (filled circles), 0.1 mm tBuOOH (open triangles), or 0.2 mm tBuOOH (filled inverted triangles) for 30, 60, 120, and 240 min. Fold-changes were calculated relative to the transcripts abundance in untreated cells and data are represented in the log10 scale. Relative transcript abundance was calculated as described in the legend of Fig. 4A. Each data point represents the average of three technical triplicates from one biological replicate. B, RNA-Seq was also used to measure transcript levels of genes encoding for enzymes of the ascorbate-glutathione system in C. reinhardtii cells exposed to 1 mm H2O2 for 30 and 60 min.
DISCUSSION
Higher plants synthesize l-ascorbic acid using primarily the Smirnoff-Wheeler pathway (4, 9), in which VTC2 catalyzes a rate-limiting step by converting GDP-l-galactose to l-galactose-1-P (5, 8). Here we provide evidence that C. reinhardtii and other green algal genomes encode functional plant VTC2 homologs. Our sequence analyses identified orthologs of all the Smirnoff-Wheeler pathway enzymes in C. reinhardtii. Moreover, with the exception of GDP-d-mannose pyrophosphorylase (VTC1), which appears to be missing in Prasinophyceae like Micromonas spp. or Ostreococcus spp., all other enzymes of the l-galactose pathway are conserved in divergent green algae. The absence of VTC1 in Prasinophyceae might be compensated by the operation, in those species, of VTC2 cycles such as those proposed by Laing et al. (6) or Wolucka and Van Montagu (47), where l-galactose-1-P would be formed by a GDP-l-galactose transferase activity of VTC2 (using d-Man-1-P or d-Glc-1-P as guanylyl acceptors instead of Pi) and GDP-d-mannose formation would be ensured by a hypothetical 2′-epimerase from GDP-d-glucose (8).
HIT proteins are members of a superfamily of nucleotide hydrolases and transferases, which, based on sequence, substrate specificity, structure, evolution and mechanism, are classified into the Hint, Fhit, Aprataxin, scavenger decapping protein, and GalT branches (16). The first four branches, characterized by a HXHXH motif, contain nucleotide hydrolases, whereas GalT branch members, generally possessing a HXHXQ motif, are nucleotide phosphorylases or transferases. The best-characterized member of the GalT branch is galactose-1-phosphate uridylyltransferase, which represents the second enzyme in the Leloir pathway of galactose utilization. The HIT motif in A. thaliana and other plant VTC2 proteins (HXHXQ) and the enzymatic properties of A. thaliana VTC2, which is a GDP-l-Gal/GDP-d-Glc phosphorylase, would place this protein in the GalT branch of the HIT superfamily. Interestingly, C. reinhardtii VTC2 possesses the HXHXH motif found in members of the hydrolase branches of the HIT superfamily (16). Animal homologs of plant VTC2 also have the HXHXH motif and have been shown to act as specific GDP-d-glucose phosphorylases needed for quality control of the nucleoside diphosphate sugar pool (48). This work provides an additional example to suggest that the HXHXH versus HXHXQ motifs do not always predict the biochemical reaction catalyzed by the corresponding HIT enzyme (48–50). In this study we indeed showed that the recombinant C. reinhardtii VTC2 enzyme has a GDP-l-Gal/GDP-d-Glc phosphorylase activity as do the land plant homologs. The algal enzyme can use both GDP-l-Gal and GDP-d-Glc as substrates and requires inorganic phosphate as acceptor. The recombinant purified enzyme displayed an about 10-fold higher catalytic efficiency with both nucleotide sugar substrates relative to A. thaliana VTC2. The latter was previously found to exhibit some transferase activity (31), and we also detected a minor GDP-l-galactose transferase activity with d-Glc-1-P as a guanylyl acceptor for recombinant C. reinhardtii VTC2. This transferase activity was at least 100-fold lower than its phosphorylase activity (data not shown).
l-Ascorbic acid is a major antioxidant in plants and animals (46). In plants, cellular l-ascorbic levels are increased in response to environmental stresses such as high light (1, 51), high temperature (52), and exposure to UV radiation (53, 54) or ozone (55, 56). l-Ascorbic acid plays an important role in photosynthesis where it acts by scavenging superoxide and H2O2, participates in regeneration of α-tocopheryl radicals produced by α-tochopherol during reduction of lipid peroxyl radicals, and functions as cofactor for violaxanthin de-epoxidase (1) and prolyl hydroxylases (2, 3).
Here we provide evidence suggesting a role of l-ascorbic acid in protecting C. reinhardtii cells against oxidative stress. Reactive oxygen species-inducing chemicals like H2O2 and tBuOOH resulted in increased VTC2 mRNA levels, which are 10–15 times more abundant after exposure to tBuOOH compared with H2O2 treatment. This might be explained by the fact that tBuOOH persists for a longer time than does H2O2 in liquid cultures. In addition, H2O2 can produce highly reactive hydroxyl radicals, whereas tBuOOH can decompose to other alkoxyl and peroxyl radicals. Pro-oxidant effects of H2O2 treatment resulted in persistent elevated levels of total ascorbate, whereas, after an initial increase, the total ascorbate levels dropped back to wild-type levels after exposure to tBuOOH for 8 h. This is not surprising because exposure of astrocytes, hepatocytes, or Hep2G cells to tBuOOH had previously been shown to lead to decreased levels of intracellular l-ascorbic acid and GSH (44, 45, 57). An A. thaliana line (ppr40-1) that has impaired electron flow at complex III showed decreased levels of total ascorbate and enhanced activity of GLDH and ascorbate-glutathione cycle enzymes (58). Similarly, inhibition of mitochondrial respiratory electron transport at the levels of complex I, complex II, or complex IV resulted in a 50% decrease in total ascorbate levels in A. thaliana (59). It is well known that plant mitochondria are the place where the last step of vitamin C biosynthesis occurs in plants. GLDH is an inner membrane mitochondrial flavin enzyme that uses oxidized cytochrome c as an electron acceptor (60) and recently has been shown to be required for accumulation of complex I in A. thaliana (61). On the other hand, tBuOOH has been shown to inhibit mitochondrial respiratory chain enzymes in rat hepatocytes (62). Therefore the higher VTC2 transcript levels and depletion of intracellular ascorbate content in C. reinhardtii exposed for longer times to tBuOOH might at least in part be a result of oxidatively damaged mitochondria and impaired respiratory electron transport.
Our RNA-Seq analysis of H2O2 stressed C. reinhardtii cells indicates a significant increase in mRNA levels only for VTC2 and only a small increase (1.5–2-fold) for the other components of the l-galactose pathway. A similar pattern of expression for all genes encoding l-galactose pathway enzymes has been observed in A. thaliana exposed to high light (30). Our results suggest that VTC2 might be the regulatory point controlling l-ascorbate biosynthesis in C. reinhardtii. Supporting evidence for this also comes from studies in A. thaliana where it has been demonstrated that supplementation with l-ascorbate decreases VTC2 mRNA abundance, possibly indicating a feedback inhibition at the transcriptional level (30). Moreover, the increased l-ascorbate content after exposure to high-light resulted in higher GDP-l-galactose phosphorylase activity (30).
The ascorbate-glutathione cycle is the major H2O2 scavenging system in photosynthetic organisms (2, 17, 46) (Fig. 7). In C. reinhardtii the superoxide anion (O2˙̄) formed at the site of Photosystem I is converted to H2O2 by superoxide dismutases MnSOD3 and FeSOD. The H2O2 is reduced to water by ascorbate in a reaction catalyzed by APX1 (63). Oxidation of ascorbate produces monodehydroascorbate, which either can be reduced to ascorbate by MDAR1 or can spontaneously disproportionate to dehydroascorbate. DHAR1 uses GSH to regenerate ascorbate from dehydroascorbate and GSHR1/2 regenerates GSH from GSSG. It has been demonstrated that overexpression of A. thaliana or tomato (Lycopersicon esculentum Mill) monodehydroascorbate reductase (64, 65) results in increased ascorbate levels. Similarly, overexpression of dehydroascorbate reductase had the same effect in enhancing the plant vitamin C content, conferring increased tolerance to oxidative stress (66, 67). In this study, oxidatively stressed C. reinhardtii cells showed enhanced mRNA abundance for all transcripts encoding the ascorbate-glutathione components. An interesting observation was that exposure of C. reinhardtii cells to tBuOOH did not induce glutathione synthesis (GSH1) or GSSG reduction (GSHR1), suggesting that under these conditions, another (glutathione-independent) mechanism is required for dehydroascorbate reduction. A similar mechanism has been observed to be functional in rat liver where a selenoenzyme thioredoxin reductase reduces dehydroascorbate to ascorbate (68). C. reinhardtii, unlike land plants, has selenoenzymes, including a thioredoxin reductase prototype (69, 70).
Supplementary Material
Acknowledgments
We thank Prof. Shinichi Kitamura (Osaka Prefecture University) for kindly providing GDP-l-galactose and Annie Shin for help with the expression and purification of recombinant VTC2.
This work was supported, in whole or in part, by National Institutes of Health Grant GM026020 (to S. G. C.), the Division of Chemical Sciences, Geosciences and Biosciences, Office of Basic Energy Sciences of the United States Department of Energy Grant DE-FD02-04ER15529 (to S. S. M.), an Ellison Medical Foundation Senior Scholar Award (to S. G. C.), and UCLA Philip Whitcome and Graduate Division Dissertation Year Fellowships (to L. N. A.). The preparation of recombinant VTC2 enzyme was supported by the Department of Energy Grant DE-FC03-02ER63421 (to David Eisenberg).

This article contains supplemental Figs. S1–S3 and Tables S1–S5.
The nucleotide sequence(s) reported in this paper has been submitted to the GenBankTM/EBI Data Bank with accession number(s) JQ246433.
- VTC4
- l-galactose-1-P phosphatase
- l-Gal-DH
- l-galactose dehydrogenase
- GLDH
- l-galactono-1,4-lactone dehydrogenase
- APX
- ascorbate peroxidase
- MDAR
- monodehydroascorbate reductase
- DHAR
- dehydroascorbate reductase
- GSHR
- glutathione reductase
- MBP
- maltose-binding protein
- NDP
- nucleoside diphosphate
- HIT
- histidine triad
- tBuOOH
- tert-butyl-hydroperoxide.
REFERENCES
- 1. Smirnoff N. (2000) Ascorbate biosynthesis and function in photoprotection. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355, 1455–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gill S. S., Tuteja N. (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 48, 909–930 [DOI] [PubMed] [Google Scholar]
- 3. Smirnoff N., Wheeler G. L. (2000) Ascorbic acid in plants. Biosynthesis and function. Crit. Rev. Biochem. Mol. Biol. 35, 291–314 [DOI] [PubMed] [Google Scholar]
- 4. Wheeler G. L., Jones M. A., Smirnoff N. (1998) The biosynthetic pathway of vitamin C in higher plants. Nature 393, 365–369 [DOI] [PubMed] [Google Scholar]
- 5. Linster C. L., Gomez T. A., Christensen K. C., Adler L. N., Young B. D., Brenner C., Clarke S. G. (2007) Arabidopsis VTC2 encodes a GDP-l-galactose phosphorylase, the last unknown enzyme in the Smirnoff-Wheeler pathway to ascorbic acid in plants. J. Biol. Chem. 282, 18879–18885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Laing W. A., Wright M. A., Cooney J., Bulley S. M. (2007) The missing step of the l-galactose pathway of ascorbate biosynthesis in plants, an l-galactose guanyltransferase, increases leaf ascorbate content. Proc. Natl. Acad. Sci. U.S.A. 104, 9534–9539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hancock R. D., Viola R. (2005) Biosynthesis and catabolism in l-ascorbic acid in plants. Crit. Rev. Plant Sci. 24, 167–188 [Google Scholar]
- 8. Linster C. L., Clarke S. G. (2008) l-Ascorbate biosynthesis in higher plants. The role of VTC2. Trends Plant Sci. 13, 567–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smirnoff N., Conklin P. L., Loewus F. A. (2001) Biosynthesis of ascorbic acid in plants. A renaissance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52, 437–467 [DOI] [PubMed] [Google Scholar]
- 10. Di Matteo A., Hancock R. D., Ross H. A., Frusciante L., Viola R. (2003) Characterization of Chlorella pyrenoidosa l-ascorbic acid accumulating mutants. Identification of an enhanced biosynthetic enzyme activity and cloning of the putative gene from Arabidopsis thaliana. Comp. Biochem. Physiol. A 134, S155 [Google Scholar]
- 11. Renstrøm B., Grün M., Loewus F. (1982) Biosynthesis of l-ascorbic acid in Chlorella pyrenoidosa. Plant Cell Lett. 28, 299–305 [Google Scholar]
- 12. Running J. A., Burlingame R. P., Berry A. (2003) The pathway of l-ascorbic acid biosynthesis in the colourless microalga Prototheca moriformis. J. Exp. Bot. 54, 1841–1849 [DOI] [PubMed] [Google Scholar]
- 13. Helsper J. P., Kagan L., Hilby C. L., Maynard T. M., Loewus F. A. (1982) l-Ascorbic acid biosynthesis in Ochromonas danica. Plant Physiol. 69, 465–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shigeoka S., Nakano Y., Kitaoka S. (1979) The biosynthetic pathway of l-ascorbic acid in Euglena gracilis Z. J. Nutr. Sci. Vitaminol. 25, 299–307 [DOI] [PubMed] [Google Scholar]
- 15. Grün M., Loewus F. A. (1984) l-Ascorbic acid biosynthesis in the euryhaline diatom Cyclotella cryptica. Planta 160, 6–11 [DOI] [PubMed] [Google Scholar]
- 16. Brenner C. (2007) in Encyclopedia of Life Sciences, pp. 1–6, John Wiley & Sons, Ltd., Chichester, UK [Google Scholar]
- 17. Asada K. (1999) The water-water cycle in chloroplasts. Scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 601–639 [DOI] [PubMed] [Google Scholar]
- 18. Watanabe K., Suzuki K., Kitamura S. (2006) Characterization of a GDP-d-mannose 3″,5″-epimerase from rice. Phytochemistry 67, 338–346 [DOI] [PubMed] [Google Scholar]
- 19. Harris E. H. (2009) The Chlamydomonas Sourcebook, A Comprehensive Guide to Biology and Laboratory Use, 2nd Ed., Academic Press, San Diego: [DOI] [PubMed] [Google Scholar]
- 20. Fox J. D., Waugh D. S. (2003) Maltose-binding protein as a solubility enhancer. Methods Mol. Biol. 205, 99–117 [DOI] [PubMed] [Google Scholar]
- 21. Quinn J. M., Merchant S. (1998) Copper-responsive gene expression during adaptation to copper deficiency. Methods Enzymol. 297, 263–279 [DOI] [PubMed] [Google Scholar]
- 22. Allen M. D., del Campo J. A., Kropat J., Merchant S. S. (2007) FEA1, FEA2, and FRE1, encoding two homologous secreted proteins and a candidate ferrireductase, are expressed coordinately with FOX1 and FTR1 in iron-deficient Chlamydomonas reinhardtii. Eukaryot. Cell 6, 1841–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schloss J. A. (1990) A Chlamydomonas gene encodes a G protein β subunit-like polypeptide. Mol. Gen. Genet. 221, 443–452 [DOI] [PubMed] [Google Scholar]
- 24. Craigie R. A., Cavalier-Smith T. (1982) Cell volume and the control of the Chlamydomonas cell cycle. J. Cell Sci. 54, 173–191 [Google Scholar]
- 25. Langmead B., Trapnell C., Pop M., Salzberg S. L. (2009) Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mortazavi A., Williams B. A., McCue K., Schaeffer L., Wold B. (2008) Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 5, 621–628 [DOI] [PubMed] [Google Scholar]
- 27. Castruita M., Casero D., Karpowicz S. J., Kropat J., Vieler A., Hsieh S. I., Yan W., Cokus S., Loo J. A., Benning C., Pellegrini M., Merchant S. S. (2011) Systems biology approach in Chlamydomonas reveals connections between copper nutrition and multiple metabolic steps. Plant Cell 23, 1273–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Whelan S., Goldman N. (2001) A general empirical model of protein evolution derived from multiple protein families using a maximum-likelihood approach. Mol. Biol. Evol. 18, 691–699 [DOI] [PubMed] [Google Scholar]
- 29. Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. (2011) MEGA5. Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28, 2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dowdle J., Ishikawa T., Gatzek S., Rolinski S., Smirnoff N. (2007) Two genes in Arabidopsis thaliana encoding GDP-l-galactose phosphorylase are required for ascorbate biosynthesis and seedling viability. Plant J. 52, 673–689 [DOI] [PubMed] [Google Scholar]
- 31. Linster C. L., Adler L. N., Webb K., Christensen K. C., Brenner C., Clarke S. G. (2008) A second GDP-l-galactose phosphorylase in Arabidopsis en route to vitamin C. Covalent intermediate and substrate requirements for the conserved reaction. J. Biol. Chem. 283, 18483–18492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ishikawa T., Shigeoka S. (2008) Recent advances in ascorbate biosynthesis and the physiological significance of ascorbate peroxidase in photosynthesizing organisms. Biosci. Biotechnol. Biochem. 72, 1143–1154 [DOI] [PubMed] [Google Scholar]
- 33. Wolucka B. A., Van Montagu M. (2003) GDP-mannose 3′,5′-epimerase forms GDP-l-gulose, a putative intermediate for the de novo biosynthesis of vitamin C in plants. J. Biol. Chem. 278, 47483–47490 [DOI] [PubMed] [Google Scholar]
- 34. Radzio J. A., Lorence A., Chevone B. I., Nessler C. L. (2003) l-Gulono-1,4-lactone oxidase expression rescues vitamin C-deficient Arabidopsis (vtc) mutants. Plant Mol. Biol. 53, 837–844 [DOI] [PubMed] [Google Scholar]
- 35. Linster C. L., Van Schaftingen E. (2007) Vitamin C. Biosynthesis, recycling, and degradation in mammals. FEBS J. 274, 1–22 [DOI] [PubMed] [Google Scholar]
- 36. Kondo Y., Inai Y., Sato Y., Handa S., Kubo S., Shimokado K., Goto S., Nishikimi M., Maruyama N., Ishigami A. (2006) Senescence marker protein 30 functions as gluconolactonase in l-ascorbic acid biosynthesis, and its knockout mice are prone to scurvy. Proc. Natl. Acad. Sci. U.S.A. 103, 5723–5728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Agius F., González-Lamothe R., Caballero J. L., Muñoz-Blanco J., Botella M. A., Valpuesta V. (2003) Engineering increased vitamin C levels in plants by overexpression of a d-galacturonic acid reductase. Nat. Biotechnol. 21, 177–181 [DOI] [PubMed] [Google Scholar]
- 38. Ishikawa T., Nishikawa H., Gao Y., Sawa Y., Shibata H., Yabuta Y., Maruta T., Shigeoka S. (2008) The pathway via d-galacturonate/l-galactonate is significant for ascorbate biosynthesis in Euglena gracilis. Identification and functional characterization of aldonolactonase. J. Biol. Chem. 283, 31133–31141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Müller-Moulé P. (2008) An expression analysis of the ascorbate biosynthesis enzyme VTC2. Plant Mol. Biol. 68, 31–41 [DOI] [PubMed] [Google Scholar]
- 40. Allen M. D., Kropat J., Tottey S., Del Campo J. A., Merchant S. S. (2007) Manganese deficiency in Chlamydomonas results in loss of photosystem II and MnSOD function, sensitivity to peroxides, and secondary phosphorus and iron deficiency. Plant Physiol. 143, 263–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fischer B. B., Eggen R. I., Niyogi K. K. (2010) Characterization of single oxygen-accumulating mutants isolated in a screen for altered oxidative stress response in Chlamydomonas reinhardtii. BMC Plant Biol. 10, 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ledford H. K., Chin B. L., Niyogi K. K. (2007) Acclimation to singlet oxygen stress in Chlamydomonas reinhardtii. Eukaryot. Cell 6, 919–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Long J. C., Merchant S. S. (2008) Photooxidative stress impacts the expression of genes encoding iron metabolism components in Chlamydomonas. Photochem. Photobiol. 84, 1395–1403 [DOI] [PubMed] [Google Scholar]
- 44. Glascott P. A., Jr., Gilfor E., Farber J. L. (1995) Relationship of the metabolism of vitamins C and E in cultured hepatocytes treated with tert-butyl hydroperoxide. Mol. Pharmacol. 48, 80–88 [PubMed] [Google Scholar]
- 45. Daskalopoulos R., Korcok J., Tao L., Wilson J. X. (2002) Accumulation of intracellular ascorbate from dehydroascorbic acid by astrocytes is decreased after oxidative stress and restored by propofol. Glia 39, 124–132 [DOI] [PubMed] [Google Scholar]
- 46. Foyer C. H., Noctor G. (2011) Ascorbate and glutathione. The heart of the redox hub. Plant Physiol. 155, 2–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wolucka B. A., Van Montagu M. (2007) The VTC2 cycle and the de novo biosynthesis pathways for vitamin C in plants. An opinion. Phytochemistry 68, 2602–2613 [DOI] [PubMed] [Google Scholar]
- 48. Adler L. N., Gomez T. A., Clarke S. G., Linster C. L. (2011) A novel GDP-d-glucose phosphorylase involved in quality control of the nucleoside diphosphate sugar pool in Caenorhabditis elegans and mammals. J. Biol. Chem. 286, 21511–21523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Guranowski A., Wojdyła A. M., Zimny J., Wypijewska A., Kowalska J., Jemielity J., Davis R. E., Bieganowski P. (2010) Dual activity of certain HIT-proteins. A. thaliana Hint4 and C. elegans DcpS act on adenosine 5′-phosphosulfate as hydrolases (forming AMP) and as phosphorylases (forming ADP). FEBS Lett. 584, 93–98 [DOI] [PubMed] [Google Scholar]
- 50. Mori S., Shibayama K., Wachino J., Arakawa Y. (2010) Purification and molecular characterization of a novel diadenosine 5′,5‴-P(1),P(4)-tetraphosphate phosphorylase from Mycobacterium tuberculosis H37Rv. Protein Expr. Purif. 69, 99–105 [DOI] [PubMed] [Google Scholar]
- 51. Müller-Moulé P., Golan T., Niyogi K. K. (2004) Ascorbate-deficient mutants of Arabidopsis grow in high light despite chronic photooxidative stress. Plant Physiol. 134, 1163–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Larkindale J., Hall J. D., Knight M. R., Vierling E. (2005) Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance. Plant Physiol. 138, 882–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Conklin P. L., Williams E. H., Last R. L. (1996) Environmental stress sensitivity of an ascorbic acid-deficient Arabidopsis mutant. Proc. Natl. Acad. Sci. U.S.A. 93, 9970–9974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Filkowski J., Kovalchuk O., Kovalchuk I. (2004) Genome stability of vtc1, tt4, and tt5 Arabidopsis thaliana mutants impaired in protection against oxidative stress. Plant J. 38, 60–69 [DOI] [PubMed] [Google Scholar]
- 55. Chen Z., Gallie D. R. (2005) Increasing tolerance to ozone by elevating foliar ascorbic acid confers greater protection against ozone than increasing avoidance. Plant Physiol. 138, 1673–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sanmartin M., Drogoudi P. A., Lyons T., Pateraki I., Barnes J., Kanellis A. K. (2003) Overexpression of ascorbate oxidase in the apoplast of transgenic tobacco results in altered ascorbate and glutathione redox states and increased sensitivity to ozone. Planta 216, 918–928 [DOI] [PubMed] [Google Scholar]
- 57. Alía M., Ramos S., Mateos R., Bravo L., Goya L. (2005) Response of the antioxidant defense system to tert-butyl hydroperoxide and hydrogen peroxide in a human hepatoma cell line (HepG2). J. Biochem. Mol. Toxicol. 19, 119–128 [DOI] [PubMed] [Google Scholar]
- 58. Zsigmond L., Tomasskovics B., Deák V., Rigó G., Szabados L., Bánhegyi G., Szarka A. (2011) Enhanced activity of galactono-1,4-lactone dehydrogenase and ascorbate-glutathione cycle in mitochondria from complex III-deficient Arabidopsis. Plant Physiol. Biochem. 49, 809–815 [DOI] [PubMed] [Google Scholar]
- 59. Yabuta Y., Maruta T., Nakamura A., Mieda T., Yoshimura K., Ishikawa T., Shigeoka S. (2008) Conversion of l-galactono-1,4-lactone to l-ascorbate is regulated by the photosynthetic electron transport chain in Arabidopsis. Biosci. Biotechnol. Biochem. 72, 2598–2607 [DOI] [PubMed] [Google Scholar]
- 60. Leferink N. G., van den Berg W. A., van Berkel W. J. (2008) l-Galactono-γ-lactone dehydrogenase from Arabidopsis thaliana, a flavoprotein involved in vitamin C biosynthesis. FEBS J. 275, 713–726 [DOI] [PubMed] [Google Scholar]
- 61. Pineau B., Layoune O., Danon A., De Paepe R. (2008) l-Galactono-1,4-lactone dehydrogenase is required for the accumulation of plant respiratory complex I. J. Biol. Chem. 283, 32500–32505 [DOI] [PubMed] [Google Scholar]
- 62. Drahota Z., Kriváková P., Cervinková Z., Kmonícková E., Lotková H., Kucera O., Houstek J. (2005) tert-Butyl hydroperoxide selectively inhibits mitochondrial respiratory chain enzymes in isolated rat hepatocytes. Physiol. Res. 54, 67–72 [DOI] [PubMed] [Google Scholar]
- 63. Takeda T., Ishikawa T., Shigeoka S. (1997) Metabolism of hydrogen peroxide by the scavenging system in Chlamydomonas reinhardtii. Physiol. Plant 99, 49–55 [Google Scholar]
- 64. Eltayeb A. E., Kawano N., Badawi G. H., Kaminaka H., Sanekata T., Shibahara T., Inanaga S., Tanaka K. (2007) Overexpression of monodehydroascorbate reductase in transgenic tobacco confers enhanced tolerance to ozone, salt and polyethylene glycol stresses. Planta 225, 1255–1264 [DOI] [PubMed] [Google Scholar]
- 65. Li F., Wu Q. Y., Sun Y. L., Wang L. Y., Yang X. H., Meng Q. W. (2010) Overexpression of chloroplastic monodehydroascorbate reductase enhanced tolerance to temperature and methyl viologen-mediated oxidative stresses. Physiol. Plant 139, 421–434 [DOI] [PubMed] [Google Scholar]
- 66. Chen Z., Young T. E., Ling J., Chang S. C., Gallie D. R. (2003) Increasing vitamin C content of plants through enhanced ascorbate recycling. Proc. Natl. Acad. Sci. U.S.A. 100, 3525–3530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang Z., Xiao Y., Chen W., Tang K., Zhang L. (2010) Increased vitamin C content accompanied by an enhanced recycling pathway confers oxidative stress tolerance in Arabidopsis. J. Integr. Plant Biol. 52, 400–409 [DOI] [PubMed] [Google Scholar]
- 68. May J. M., Mendiratta S., Hill K. E., Burk R. F. (1997) Reduction of dehydroascorbate to ascorbate by the selenoenzyme thioredoxin reductase. J. Biol. Chem. 272, 22607–22610 [DOI] [PubMed] [Google Scholar]
- 69. Novoselov S. V., Gladyshev V. N. (2003) Non-animal origin of animal thioredoxin reductases. Implications for selenocysteine evolution and evolution of protein function through carboxyl-terminal extensions. Protein Sci. 12, 372–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Novoselov S. V., Rao M., Onoshko N. V., Zhi H., Kryukov G. V., Xiang Y., Weeks D. P., Hatfield D. L., Gladyshev V. N. (2002) Selenoproteins and selenocysteine insertion system in the model plant cell system, Chlamydomonas reinhardtii. EMBO J. 21, 3681–3693 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.