Abstract
The redox-active metal manganese plays a key role in cellular adaptation to oxidative stress. As a cofactor for manganese superoxide dismutase or through formation of non-proteinaceous manganese antioxidants, this metal can combat oxidative damage without deleterious side effects of Fenton chemistry. In either case, the antioxidant properties of manganese are vulnerable to iron. Cellular pools of iron can outcompete manganese for binding to manganese superoxide dismutase, and through Fenton chemistry, iron may counteract the benefits of non-proteinaceous manganese antioxidants. In this minireview, we highlight ways in which cells maximize the efficacy of manganese as an antioxidant in the midst of pro-oxidant iron.
Keywords: Antioxidants, Iron, Iron-Sulfur Protein, Manganese, Oxidative Stress, Oxygen Radicals, Superoxide Dismutase (SOD), Superoxide Ion
Introduction
In biology, iron and manganese play important roles in oxygen chemistry. Both metals are used in oxygen evolution through photosynthesis and can also serve as cofactors for enzymes that remove harmful byproducts of O2 metabolism such as superoxide (O2˙̄) and hydrogen peroxide, yet a major difference lies in the propensity of these metals to cause oxidative damage. Iron is well known for its reactivity with peroxide, generating the highly reactive hydroxyl radical through so-called Fenton chemistry. Manganese is less prone to such chemistry due to a higher reduction potential. Iron is often considered a pro-oxidant in biology under situations in which manganese is an antioxidant. Without the deleterious side effects of Fenton chemistry, manganese can safely operate as a cofactor for superoxide dismutase (SOD)2 enzymes and also provide oxidative stress resistance through formation of non-proteinaceous manganese-based antioxidants. Herein, we focus on these two biological roles of manganese in oxidative stress protection and the potential challenges faced by competing pools of iron.
Manganese Versus Iron as Cofactors for SOD
SOD enzymes fall into three distinct families that are unrelated in sequence but have converged in evolution to utilize a redox-active metal to disproportionate O2˙̄ into hydrogen peroxide and oxygen. These families are classified according to metal cofactor and include Cu,Zn-SODs, which use copper for catalysis and also bind a structural zinc atom; a rarer family of nickel-containing SODs (1); and an extensive Mn/Fe-SOD family that uses either manganese or iron.
Members of the Mn/Fe-SOD family are widespread in biology and are thought to have evolved from a common ancestor prior to the divergence of eubacteria, archaebacteria, and eukaryotes over 3 billion years ago (2, 3). The active sites of Mn- and Fe-SODs are virtually indistinguishable. The manganese or iron cofactor is coordinated to three histidines, one aspartate, and one solvent molecule in a distorted trigonal bipyramidal geometry. Outside the active site, the overall primary sequence and tertiary folds of Mn- and Fe-SODs are remarkably similar. Despite this striking conservation, these SODs are exquisitely metal-specific; for example, a Mn-SOD is active only with manganese and not iron. Rare exceptions include so-called cambialistic SODs, which are functional with either metal (4).
Mishaps in Metal Insertion into Mn-SOD
A key question in the field of metalloproteins regards cofactor specificity. How does a metalloenzyme find its correct cofactor among a sea of diverse metals in the cell? With Cu,Zn-SOD enzymes, metal insertion is facilitated by a helper protein known as the CCS copper chaperone (5, 6). However, to date, there have been no reports of analogous metal chaperones for the Mn/Fe-SOD family. The problem seems paramount with Mn-SODs because bacteria and eukaryotic cells tend to accumulate iron levels that are in vast excess over manganese (7–9).
Mn-SODs will readily bind iron with similar affinities and geometries as manganese (10–14), yet iron inactivates the enzyme. Iron binding can interfere with the substrate channel (4) and disturb the all-important redox potential of the catalytic site. When iron binds Mn-SODs, the mid-redox potential of the active site is lowered to a point that is incompatible with O2˙̄ disproportionation (15, 16). To make matters worse, studies with human Mn-SOD (Sod2) have shown that the iron-substituted enzyme gains peroxidase activity and has the potential to generate toxic oxygen radicals (13, 14). With such potent inhibition of Mn-SODs by iron, one would expect cells to prohibit iron interactions with the enzyme. Indeed, in eukaryotes, in which Sod2 is the only SOD of the mitochondrial matrix, misincorporation of iron is virtually nonexistent except in rare cases of mitochondrial defects (described below), yet the same sort of iron exclusion may not be as prevalent with bacteria.
Beyer and Fridovich (17) were the first to note misincorporation of iron into the Mn-SOD (SodA) of Escherichia coli. Aerobic cultures of E. coli naturally express a mixture of SodA enzymes that bind manganese or iron, but only the manganese-bound form is active. Iron inactivation of SodA is even more prevailing under anaerobic conditions (17, 18). Only in extreme cases of oxidative stress is there bulk metallation of E. coli SodA with the correct metal manganese (18).
Mn-SODs from various heterologous organisms have been expressed in E. coli for the purpose of producing recombinant protein, and not surprisingly, metal ion misincorporation is commonplace. For example, a tetrameric Mn-SOD from Thermophilus will indiscriminately acquire manganese or iron when expressed in E. coli (4, 19), and a cytosolic Mn-SOD from Candida albicans was seen to preferentially acquire iron in E. coli expression systems (20). Attempts to express human mitochondrial Sod2 in E. coli produced inactive enzyme unless cultures were supplemented with high levels of manganese (21). There is much competition between iron and manganese for binding to Mn-SOD molecules in E. coli.
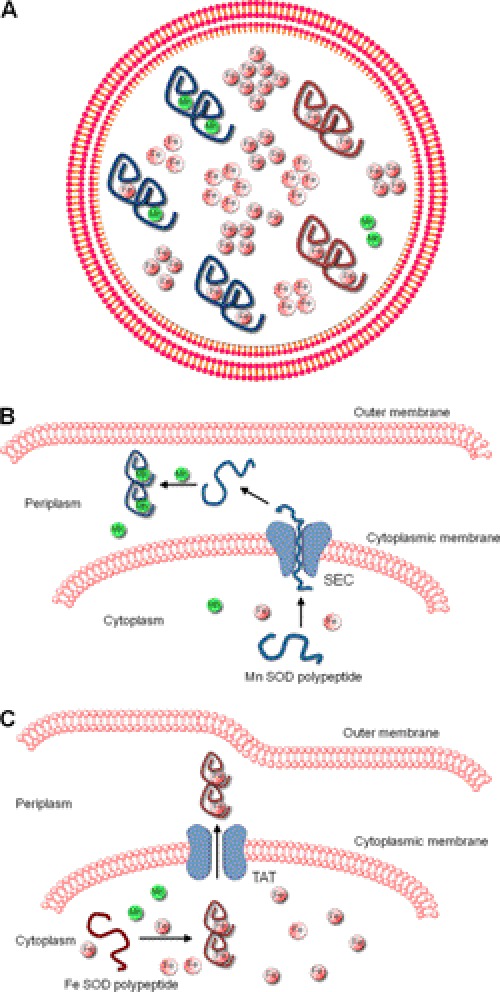
Why would E. coli allow misincorporation of iron into its Mn-SOD? The rationale is still unclear, but inactivation of SodA by iron may be of little consequence due to expression of a second SOD (SodB) in E. coli that uses iron. Nevertheless, there still exists some preferential binding of manganese to SodA when one considers total metal levels. On a per mole basis, E. coli accumulates 10–100 times more iron than manganese (7), yet a substantial fraction of SodA still acquires manganese (Fig. 1A). As proposed by Whittaker and co-workers (10), differential metal ion bioavailability may be key. The speciation of cellular manganese based on ligands and oxidation state of the metal may be more compatible for reactivity with Mn-SOD than the speciation of bulk iron, and when cells encounter oxidative stress, this differential bioavailability becomes even more pronounced, as iron is prevented from reacting with E. coli SodA (18).
FIGURE 1.
Models for metal selectivity of bacterial Mn-SOD and Fe-SOD enzymes. A, model of a Gram-negative E. coli cell showing Mn-SOD dimers in blue and Fe-SOD dimers in red. Under normal aerobic conditions, Mn-SOD molecules accumulate as mixed pools of all iron-, all manganese-, or iron- and manganese-containing dimers. Fe-SOD molecules are shown to accumulate only in the iron-bound state. Manganese (green) is far less abundant in E. coli than iron (pink). Depending on speciation, iron may exist in two states, only one of which is bioavailable (pink circles) to the SOD. B, shown is the Sec-driven export of the unfolded Mn-SOD polypeptide into the periplasmic space, where the enzyme may acquire its manganese without interference from iron. C, shown is the TAT-driven export of iron-bound and mature Fe-SOD into the periplasmic space. In this model, the Fe-SOD acquires its metal in the cytosol.
Certain bacteria may have overcome iron inactivation of Mn-SODs by subcellular compartmentalization. Although bacterial Mn/Fe-SODs are generally cytosolic, there are rare exceptions in which the enzyme is secreted into the periplasmic or extracellular space (22–26). Bacteria can export unfolded proteins through the Sec system or will use the twin-arginine translocation (TAT) system for exporting prefolded mature polypeptides (27). In the cases reported thus far, only Fe-SODs are exported in the fully metallated form through TAT. Mn-SODs tend to be exported via Sec as unfolded proteins and hence acquire their metal outside the cell, where competition with iron may not be an issue (Fig. 1, B and C) (22–26). Such a model for metal ion selectivity by cell compartmentalization has previously been described by Robinson and colleagues (28) for other periplasmic metalloproteins.
Role of Mitochondria in Dictating Metal Specificity to Manganese-Sod2
As with E. coli, eukaryotic mitochondria accumulate 10–100 times more total iron than manganese (29); however, mitochondrial Sod2 exclusively binds manganese. The polypeptide is intrinsically capable of iron binding, as shown through in vitro studies with recombinant Sod2 proteins (12–14). What then accounts for the high manganese selectivity inside mitochondria?
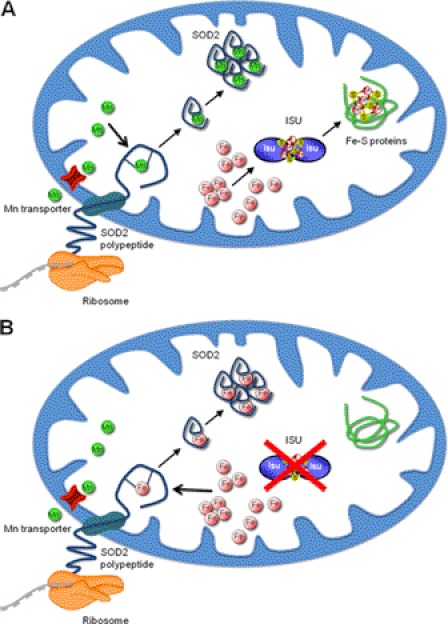
Using the bakers' yeast Saccharomyces cerevisiae as a model system, we observed that mitochondrial import of the polypeptide drives manganese insertion into the enzyme (30). Protein unfolding is an important prerequisite to metal insertion in Mn-SOD polypeptides (31–33), and what better way to unfold a protein than to translocate it across a biological membrane? An intriguing possibility is that a certain manganese transporter lies in close proximity to the site of Sod2 translocation to help drive manganese insertion over iron (Fig. 2A).
FIGURE 2.
Impact of the mitochondrial Fe-S pathway on manganese activation of Sod2. A, for eukaryotic Sod2, manganese is inserted into newly synthesized Sod2 polypeptides that are freshly imported into mitochondria. The Sod2 polypeptide is cotranslationally imported into mitochondria, and insertion of the manganese is coupled to Sod2 translocation across the mitochondrial inner membrane. Shown in red is a putative manganese transporter that may lie in close proximity to the site of Sod2 entry into mitochondria. Under normal conditions, much of the bioavailable iron is shielded from reacting with Sod2 by sequestration in the Fe-S pathway. Here, iron is used to assemble Fe-S scaffolds onto Isu, which are then transferred to Fe-S proteins. B, when the Fe-S pathway is blocked (indicated by red X on Isu), the iron for Fe-S clusters is diverted to Sod2. Iron binding to Sod2 precludes manganese binding, and the enzyme is inactive.
A second way mitochondria may facilitate manganese insertion is to funnel iron away from Sod2 into defined iron homeostasis pathways. Mitochondrial iron is used in the building of Fe-S cofactors, and evidence suggests that iron sequestered in the Fe-S pathway is blocked from reacting with Sod2 (as in Fig. 2A). This idea emerged from studies of S. cerevisiae mutants that exhibited a switch in Sod2 metal cofactor from manganese to iron (29, 34). All of these yeast mutants were affected in Fe-S biogenesis.
The biogenesis of Fe-S cofactors begins with the assembly of Fe-S scaffolds onto yeast mitochondrial Isu1 and Isu2 proteins (referred collectively herein as Isu). These scaffolds are then transferred to Fe-S proteins through the aid of a molecular chaperone (yeast Ssq1) and a glutaredoxin (yeast Grx5) (35, 36). We observed that blockage of Fe-S assembly by ssq1 or grx5 mutations drives iron into Sod2, and the same is true when yeast cells lack Mtm1, a mitochondrial transporter that plays an unknown role in Fe-S maturation (29, 34, 37). Such derailing of iron from the Fe-S pathway to Sod2 can be prevented by expressing a dominant-negative Isu protein that binds Fe-S clusters but cannot release them (34). Hence, iron derived from either Isu or sources upstream of Isu becomes available to Sod2 when the Fe-S pathway is blocked (Fig. 2B). As long as the Fe-S pathway remains intact, Sod2 is safeguarded from iron inactivation.
Although the mitochondrial environment is clearly important for cofactor selection in Sod2, features inherent to the Sod2 polypeptide may also contribute. Our preliminary studies indicate that Mn-SOD molecules that have evolved in an iron-rich environment (e.g. eukaryotic mitochondria) are less likely to bind cellular iron than Mn-SODs that have evolved in an iron-poor environment (e.g. Borrelia burgdorferi, an organism that is reportedly devoid of iron) (38). The Sod2 polypeptide of mitochondria has devised ways to coexist with abundant iron.
Non-SOD Manganese-based Antioxidants
The role of manganese as a cofactor for SOD is not the only means by which this metal can guard against damage from O2˙̄ and other reactive oxygen species. In a large variety of organisms, non-proteinaceous complexes of manganese have been shown to protect against oxidative stress and provide a backup for SOD enzymes. In this minireview, we collectively refer to such complexes as “manganese-antioxidants.”
The existence of manganese-antioxidants was first reported by Archibald and Fridovich (39) in 1981 when it was discovered that strains of Lactobaccilus plantarum that lacked SOD enzymes were nevertheless resistant to O2˙̄ due to accumulation of vast quantities of manganese. L. plantarum accumulates up to 20 mm manganese compared with the low μm levels of manganese typical of other organisms (7–9, 39). This high level of manganese was essential for the aerobic survival of L. plantarum and correlated with the presence of a superoxide-scavenging activity in cell lysates that was non-proteinaceous but manganese-dependent in nature (39). The existence of such manganese-antioxidants seemed logical for an organism like L. plantarum that naturally evolved without SOD, yet manganese-antioxidants are characteristic of various SOD-expressing organisms as well.
In strains of E. coli, Neisseria, Bacillus, and the yeast S. cerevisiae genetically engineered to lack SOD enzymes, oxidative damage can be reversed by supplementing the growth medium with high manganese (40–44). Furthermore, in our early genetic screens for yeast suppressors of SOD deficiency, virtually all of the genes and mutants isolated were seen to affect manganese uptake and accumulation (45–49). Either by supplementing the growth medium with manganese or by genetic augmentation of manganese uptake, high non-physiological levels of manganese can substitute for SOD enzymes. However, physiological (≈μm) levels of manganese are also important because yeast or Bacillus strains that lack SOD cannot grow in air when starved of manganese (42, 50).
In addition to serving as a substitute for SOD, manganese-antioxidants can boost oxidative stress resistance in organisms with active SOD enzymes. Moreover, the efficacy of manganese in this regard is reliant on a high manganese/iron ratio in the cell. In the elegant work of Daly et al. (51), the extreme radiation resistance of Deinococcus radiodurans was found to result from non-SOD manganese-based antioxidants and a high intracellular manganese/iron ratio. Similar results correlating radiation resistance with manganese-antioxidants have been reported for extreme halophilic and desiccation-resistant microbes (52, 53). With ionizing radiation, the radiolysis of water can result in the production of OH•, O2˙̄, and H2O2. Iron can augment this oxidative damage through Fenton chemistry conversion production of OH•, whereas manganese can promote O2˙̄ scavenging without OH• production (54, 55). Hence, manganese-antioxidant activity is best served in a cellular environment that has low iron. Interestingly, Daly et al. (54, 56) have shown that manganese protects proteins rather than DNA from the deleterious effects of radiation.
Although manganese-antioxidants have been widely characterized in bacteria and yeast, their contribution to oxidative stress resistance in multicellular organisms is less well understood. Nevertheless, a high accumulation of manganese in the nematode Caenorhabditis elegans either through genetic disruption of manganese-trafficking pathways (57) or through supplementation with manganese salts (58) can rescue oxidative stress and enhance life span. It is therefore likely that non-SOD complexes of manganese can promote oxidative stress resistance in higher organisms as well.
Complex Mixtures of Manganese Can Serve as Non-SOD Antioxidants
What is the chemical makeup of the manganese-antioxidant? Seminal studies by Archibald and Fridovich (39, 59) identified the manganese-antioxidant as a superoxide-scavenging activity in extracts of L. plantarum that was dialyzable and heat- and protease-resistant but susceptible to metal chelation by EDTA. Similar findings have since been reported for cell-free lysates of E. coli (41), D. radiodurans (60), Bacillus subtilis (42), and the bakers' yeast S. cerevisiae (43, 61). The hexaquo Mn2+ cation is a weak scavenger of superoxide, but when liganded to small molecules such as orthophosphate or carboxylates (e.g. lactate, succinate, and malate), manganese can efficiently scavenge O2˙̄ (62, 63). With L. plantarum, lactate complexes with manganese were proposed to represent the major antioxidant based on the abundance of lactate in this microbe (62). In other organisms, manganese-phosphate may remove superoxide (63), and indeed, oxidative stress resistance in S. cerevisiae strongly correlates with cellular [Mn-Pi] as determined by electron nuclear double resonance spectroscopy (64). The rate of superoxide disproportion by manganese-phosphate is 2 orders of magnitude slower compared with Mn-SOD (63), but with the abundance of manganese and phosphate in the cell, this appears sufficient to guard against oxidative damage.
However, the story appears more complex than simple superoxide scavenging by Mn-Pi or manganese-lactate. Mixtures of manganese, phosphate, peptides, and nucleoside bases isolated from radioresistant organisms such as D. radiodurans have been shown to protect proteins against radiation/oxidative damage (52, 60). In spores of B. subtilis that accumulate high levels of dipicolinic acid, Mn2+-dipicolinic acid complexes were shown to protect against protein oxidative damage (65), and in desiccation-resistant strains of cyanobacteria that accumulate high trehalose, manganese complexes with trehalose are proposed to guard against DNA oxidative damage (52, 66). Most likely, numerous biological complexes with manganese can act chemically in cells to promote the superoxide-scavenging activity of this metal, including certain as of yet unidentified complexes of manganese. In this regard, various manganese-based porphyrin compounds have been chemically synthesized and used as antioxidants (67), and it is possible that similar compounds are produced in certain organisms as part of an oxidative stress defense.
Direct removal of superoxide is not the only means by which non-SOD-based manganese-antioxidants can provide protection against oxidative damage. At physiological pH and in bicarbonate, manganese can also disproportionate H2O2 (68), and this reactivity has been proposed to contribute to the high radiation resistance of D. radiodurans (56). Most recently, Imlay and colleagues (69, 70) have put forth a novel mechanism for manganese-based oxidative stress resistance. Specifically, manganese is proposed to replace iron in the active site of mononuclear iron-containing enzymes. Because of the avid reactivity of iron with peroxide, the active sites of key Fe-S and mononuclear iron-containing enzymes are prime targets for damage by reactive oxygen. During oxidative stress, E. coli cells shift from an iron- to a manganese-centered metabolism, and mononuclear iron enzymes such as ribulose-5-phosphate 3-epimerase switch to using manganese as a cofactor (69, 70).
Cellular Control of Manganese-Antioxidants in a Eukaryotic Model
How physiological is the formation of manganese-antioxidants? Are the reactive manganese complexes with phosphate, lactate, etc., simply formed as part of a passive process, or are manganese-antioxidants tightly regulated according to cellular need? It was previously proposed that formation of manganese-antioxidants may be constitutive, requiring little energy input from the cell, and that only the enzymes that remove reactive oxygen (e.g. SOD, peroxidases, and catalases) are regulated during oxidative stress (71). In our recent studies with S. cerevisiae, we provided evidence to the contrary: like their enzymatic counterparts, the formation of non-proteinaceous manganese-antioxidants is tightly regulated according to cellular need (61). In particular, we found that conserved nutrient-sensing and nutrient-signaling pathways control the efficacy of intracellular manganese as a non-SOD antioxidant (61).
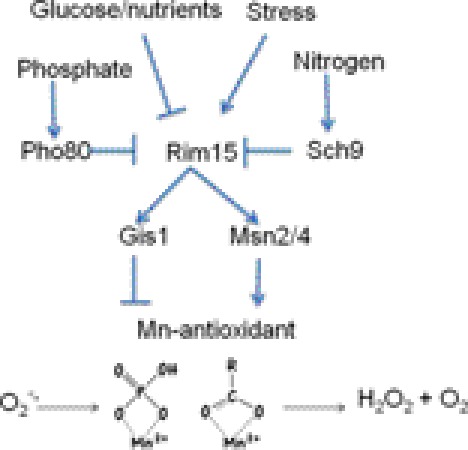
The response to stress and nutrients in yeast involves the transcription factors Msn2, Msn4, and Gis1, which regulate stress proteins and factors for metabolism and cell cycle control (72–75). These transcription factors themselves are regulated by a series of upstream kinases that sense and respond to environmental signals such as changes in phosphate (the Pho80/Pho85 kinase) and nitrogen (the Sch9 kinase). The signals from these environment-sensing kinases are relayed to the Rim15 kinase, which in turn regulates Msn2, Msn4, and Gis1 (Fig. 3) (72–75). We observed that this intricate signaling system for responding to stress and nutrients also regulates manganese-antioxidant activity in S. cerevisiae. Specifically, the manganese-antioxidant was promoted under conditions in which Msn2 and Msn4 were activated and was repressed through activation of Gis1 (61). Msn2, Msn4, and Gis1 modulate the activity of manganese-antioxidants without changing the levels of intracellular manganese and are proposed to control the assembly of manganese complexes that remove superoxide (61). Hence, formation of manganese-antioxidants is not necessarily a passive constitutive process but is part of the cell's tightly regulated battery for responding and adapting to stress.
FIGURE 3.
Nutrient- and stress-signaling pathways regulate the manganese-antioxidant in yeast cells. The activity of the S. cerevisiae Rim15 kinase is controlled by a host of environmental signals, including stress, glucose, phosphate, and nitrogen. These environmental signals are sensed and relayed to the Rim15 kinase through upstream response kinases, including Pho80 for phosphate and Sch9 for nitrogen. Rim15 in turn activates the Gis1 and Msn2/Msn4 transcription factors, which work in opposite fashion to control the activity of non-proteinaceous manganese-antioxidants, including manganese-phosphate and manganese complexes with carboxylates, as illustrated. These compounds can promote oxidative stress resistance by removing O2˙̄ in a SOD-like reaction.
Manganese-Antioxidants in Pathogenesis?
Manganese uptake is essential for the virulence of many bacterial pathogens (76–80). The activation of Mn-SOD enzymes and the formation of non-proteinaceous manganese-antioxidants may become critical as pathogens face the oxidative burst of the host immune response. An emerging theme in host immunity is the concept of “nutritional immunity,” where pathogens are starved of essential iron, zinc, and manganese ions (81). Manganese starvation can be accomplished through macrophage Nramp transporters (82–84) or by chelating manganese through calprotectin. As recently uncovered by Skaar and colleagues, neutrophils secrete calprotectin at sites of infection to starve pathogens of manganese and zinc; calprotectin was effective in killing Staphylococcus aureus mutants lacking SOD enzymes and was proposed to deplete the pathogen of manganese-antioxidants (81, 85, 86). Manganese-antioxidants may therefore provide an important measure of oxidative stress resistance for pathogens.
Concluding Statements
The redox-active metals copper, iron, and manganese all serve as catalytic cofactors for SOD. Cu,Zn-SOD does have a rather well defined “peroxidase-like” activity (87), and the same could be true for Fe-SOD. Unlike these two enzymes, the same peroxidase-like activity has not been reported for Mn-SOD. Manganese appears to be the ideal metal for oxidative stress protection. Whether manganese is acting as a cofactor for SOD or as a non-proteinaceous manganese-antioxidant, the metal is at battle with iron. On a per mole basis, cellular iron is often in vast excess over cellular manganese and can readily compete with manganese for binding to Mn-SOD enzymes. Nevertheless, evolutionary adaptations have favored manganese insertion into Mn-SOD, e.g. through enhancements in manganese ion bioavailability via chemical speciation of the metal or by subcellular compartmentalization of manganese in the case of secreted Mn-SODs. In eukaryotic mitochondria, in which iron binding to Sod2 is virtually nonexistent, the sequestration of iron in the Fe-S pathway may additionally promote manganese selectivity for Mn-SOD. Small non-proteinaceous complexes of manganese can also act as antioxidants, and manganese-antioxidant activity is best served in a cellular environment with low iron. Without having to counteract the pro-oxidant effects of iron, manganese-antioxidants can effectively shield proteins from radiation and oxidative damage. Manganese can also enhance oxidative stress resistance by substituting as a cofactor for iron in certain enzymes susceptible to oxidative attack. The broad role of manganese as an antioxidant may be particularly relevant in the microbial response to environmental stress and in pathogenesis. It is likely that multicellular organisms have evolved with analogous and even more complex ways to exploit this metal to promote life in oxygen.
This work was supported in part by National Institutes of Health Grant R01 ES08996 (to V. C. C.). This is the fifth article in the Thematic Minireview Series on Metals in Biology 2012.
- SOD
- superoxide dismutase
- TAT
- twin-arginine translocation.
REFERENCES
- 1. Dupont C. L., Neupane K., Shearer J., Palenik B. (2008) Diversity, function, and evolution of genes coding for putative nickel-containing superoxide dismutases. Environ. Microbiol. 10, 1831–1843 [DOI] [PubMed] [Google Scholar]
- 2. May B. P., Dennis P. P. (1989) Evolution and regulation of the gene encoding superoxide dismutase from the archaebacterium Halobacterium cutirubrum. J. Biol. Chem. 264, 12253–12258 [PubMed] [Google Scholar]
- 3. Wintjens R., Noël C., May A. C., Gerbod D., Dufernez F., Capron M., Viscogliosi E., Rooman M. (2004) Specificity and phenetic relationships of iron- and manganese-containing superoxide dismutases on the basis of structure and sequence comparisons. J. Biol. Chem. 279, 9248–9254 [DOI] [PubMed] [Google Scholar]
- 4. Whittaker J. W. (2003) The irony of manganese superoxide dismutase. Biochem. Soc. Trans. 31, 1318–1321 [DOI] [PubMed] [Google Scholar]
- 5. Culotta V. C., Klomp L. W., Strain J., Casareno R. L., Krems B., Gitlin J. D. (1997) The copper chaperone for superoxide dismutase. J. Biol. Chem. 272, 23469–23472 [DOI] [PubMed] [Google Scholar]
- 6. Leitch J. M., Yick P. J., Culotta V. C. (2009) The right to choose: multiple pathways for activating copper,zinc superoxide dismutase. J. Biol. Chem. 284, 24679–24683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Outten C. E., O'Halloran T. V. (2001) Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science 292, 2488–2492 [DOI] [PubMed] [Google Scholar]
- 8. Rosenfeld L., Reddi A. R., Leung E., Aranda K., Jensen L. T., Culotta V. C. (2010) The effect of phosphate accumulation on metal ion homeostasis in Saccharomyces cerevisiae. J. Biol. Inorg. Chem. 15, 1051–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eide D. J., Clark S., Nair T. M., Gehl M., Gribskov M., Guerinot M. L., Harper J. F. (2005) Characterization of the yeast ionome: a genome-wide analysis of nutrient mineral and trace element homeostasis in Saccharomyces cerevisiae. Genome Biol. 6, R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mizuno K., Whittaker M. M., Bächinger H. P., Whittaker J. W. (2004) Calorimetric studies on the tight-binding metal interactions of Escherichia coli manganese superoxide dismutase. J. Biol. Chem. 279, 27339–27344 [DOI] [PubMed] [Google Scholar]
- 11. Iranzo O. (2011) Manganese complexes displaying superoxide dismutase activity: a balance between different factors. Bioorg. Chem. 39, 73–87 [DOI] [PubMed] [Google Scholar]
- 12. Kang Y., He Y. X., Zhao M. X., Li W. F. (2011) Structures of native and iron-substituted SOD2 from Saccharomyces cerevisiae. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 67, 1173–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yamakura F., Kawasaki H. (2010) Post-translational modifications of superoxide dismutase. Biochim. Biophys. Acta 1804, 318–325 [DOI] [PubMed] [Google Scholar]
- 14. Yamakura F., Kobayashi K., Furukawa S., Suzuki Y. (2007) In vitro preparation of iron-substituted human manganese superoxide dismutase: possible toxic properties for mitochondria. Free Radic. Biol. Med. 43, 423–430 [DOI] [PubMed] [Google Scholar]
- 15. Vance C. K., Miller A. F. (1998) A simple proposal that can explain the inactivity of metal-substituted superoxide dismutases. J. Am. Chem. Soc. 120, 461–467 [Google Scholar]
- 16. Jackson T. A., Brunold T. C. (2004) Combined spectroscopic/computational studies on iron- and manganese-dependent superoxide dismutases: insights into second-sphere tuning of active site properties. Acc. Chem. Res. 37, 461–470 [DOI] [PubMed] [Google Scholar]
- 17. Beyer W. F., Jr., Fridovich I. (1991) In vivo competition between iron and manganese for occupancy of the active site region of the manganese superoxide dismutase of Escherichia coli. J. Biol. Chem. 266, 303–308 [PubMed] [Google Scholar]
- 18. Privalle C. T., Fridovich I. (1992) Transcriptional and maturational effects of manganese and iron on the biosynthesis of manganese superoxide dismutase in Escherichia coli. J. Biol. Chem. 267, 9140–9145 [PubMed] [Google Scholar]
- 19. Whittaker M. M., Whittaker J. W. (1999) Thermally triggered metal binding by recombinant Thermus thermophilus manganese superoxide dismutase, expressed as the apoenzyme. J. Biol. Chem. 274, 34751–34757 [DOI] [PubMed] [Google Scholar]
- 20. Lamarre C., LeMay J. D., Deslauriers N., Bourbonnais Y. (2001) Candida albicans expresses an unusual cytoplasmic manganese-containing superoxide dismutase (SOD3 gene product) upon the entry and during the stationary phase. J. Biol. Chem. 276, 43784–43791 [DOI] [PubMed] [Google Scholar]
- 21. Borgstahl G. E., Parge H. E., Hickey M. J., Beyer W. F., Jr., Hallewell R. A., Tainer J. A. (1992) The structure of human mitochondrial manganese superoxide dismutase reveals a novel tetrameric interface of two 4-helix bundles. Cell 71, 107–118 [DOI] [PubMed] [Google Scholar]
- 22. Krehenbrink M., Edwards A., Downie J. A. (2011) The superoxide dismutase SodA is targeted to the periplasm in a SecA-dependent manner by a novel mechanism. Mol. Microbiol. 82, 164–179 [DOI] [PubMed] [Google Scholar]
- 23. Leclère V., Chotteau-Lelièvre A., Gancel F., Imbert M., Blondeau R. (2001) Occurrence of two superoxide dismutases in Aeromonas hydrophila: molecular cloning and differential expression of the sodA and sodB genes. Microbiology 147, 3105–3111 [DOI] [PubMed] [Google Scholar]
- 24. Geissdörfer W., Ratajczak A., Hillen W. (1997) Nucleotide sequence of a putative periplasmic manganese superoxide dismutase from Acinetobacter calcoaceticus ADP1. Gene 186, 305–308 [DOI] [PubMed] [Google Scholar]
- 25. Saenkham P., Eiamphungporn W., Farrand S. K., Vattanaviboon P., Mongkolsuk S. (2007) Multiple superoxide dismutases in Agrobacterium tumefaciens: functional analysis, gene regulation, and influence on tumorigenesis. J. Bacteriol. 189, 8807–8817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Braunstein M., Espinosa B. J., Chan J., Belisle J. T., Jacobs W. R., Jr. (2003) SecA2 functions in the secretion of superoxide dismutase A and in the virulence of Mycobacterium tuberculosis. Mol. Microbiol. 48, 453–464 [DOI] [PubMed] [Google Scholar]
- 27. du Plessis D. J., Nouwen N., Driessen A. J. (2011) The Sec translocase. Biochim. Biophys. Acta 1808, 851–865 [DOI] [PubMed] [Google Scholar]
- 28. Tottey S., Waldron K. J., Firbank S. J., Reale B., Bessant C., Sato K., Cheek T. R., Gray J., Banfield M. J., Dennison C., Robinson N. J. (2008) Protein-folding location can regulate manganese binding versus copper or zinc binding. Nature 455, 1138–1142 [DOI] [PubMed] [Google Scholar]
- 29. Yang M., Cobine P. A., Molik S., Naranuntarat A., Lill R., Winge D. R., Culotta V. C. (2006) The effects of mitochondrial iron homeostasis on cofactor specificity of superoxide dismutase 2. EMBO J. 25, 1775–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Luk E., Yang M., Jensen L. T., Bourbonnais Y., Culotta V. C. (2005) Manganese activation of superoxide dismutase 2 in the mitochondria of Saccharomyces cerevisiae. J. Biol. Chem. 280, 22715–22720 [DOI] [PubMed] [Google Scholar]
- 31. Whittaker M. M., Lerch T. F., Kirillova O., Chapman M. S., Whittaker J. W. (2011) Subunit dissociation and metal binding by Escherichia coli apo-manganese superoxide dismutase. Arch. Biochem. Biophys. 505, 213–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Whittaker M. M., Whittaker J. W. (2009) In vitro metal uptake by recombinant human manganese superoxide dismutase. Arch. Biochem. Biophys. 491, 69–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Whittaker M. M., Mizuno K., Bächinger H. P., Whittaker J. W. (2006) Kinetic analysis of the metal-binding mechanism of Escherichia coli manganese superoxide dismutase. Biophys. J. 90, 598–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Naranuntarat A., Jensen L. T., Pazicni S., Penner-Hahn J. E., Culotta V. C. (2009) The interaction of mitochondrial iron with manganese superoxide dismutase. J. Biol. Chem. 284, 22633–22640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lill R., Mühlenhoff U. (2005) Iron-sulfur protein biogenesis in eukaryotes. Trends Biochem. Sci. 30, 133–141 [DOI] [PubMed] [Google Scholar]
- 36. Rouault T. A., Tong W. H. (2005) Iron-sulfur cluster biogenesis and mitochondrial iron homeostasis. Nat. Rev. Mol. Cell Biol. 6, 345–351 [DOI] [PubMed] [Google Scholar]
- 37. Nilsson R., Schultz I. J., Pierce E. L., Soltis K. A., Naranuntarat A., Ward D. M., Baughman J. M., Paradkar P. N., Kingsley P. D., Culotta V. C., Kaplan J., Palis J., Paw B. H., Mootha V. K. (2009) Discovery of genes essential for heme biosynthesis through large-scale gene expression analysis. Cell Metab. 10, 119–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Posey J. E., Gherardini F. C. (2000) Lack of a role for iron in the Lyme disease pathogen. Science 288, 1651–1653 [DOI] [PubMed] [Google Scholar]
- 39. Archibald F. S., Fridovich I. (1981) Manganese and defenses against oxygen toxicity in Lactobacillus plantarum. J. Bacteriol. 145, 442–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tseng H. J., Srikhanta Y., McEwan A. G., Jennings M. P. (2001) Accumulation of manganese in Neisseria gonorrhoeae correlates with resistance to oxidative killing by superoxide anion and is independent of superoxide dismutase activity. Mol. Microbiol. 40, 1175–1186 [DOI] [PubMed] [Google Scholar]
- 41. Al-Maghrebi M., Fridovich I., Benov L. (2002) Manganese supplementation relieves the phenotypic deficits seen in superoxide dismutase-null Escherichia coli. Arch. Biochem. Biophys. 402, 104–109 [DOI] [PubMed] [Google Scholar]
- 42. Inaoka T., Matsumura Y., Tsuchido T. (1999) SodA and manganese are essential for resistance to oxidative stress in growing and sporulating cells of Bacillus subtilis. J. Bacteriol. 181, 1939–1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chang E. C., Kosman D. J. (1989) Intracellular Mn(II)-associated superoxide-scavenging activity protects Cu,Zn-superoxide dismutase-deficient Saccharomyces cerevisiae against dioxygen stress. J. Biol. Chem. 264, 12172–12178 [PubMed] [Google Scholar]
- 44. Sanchez R. J., Srinivasan C., Munroe W. H., Wallace M. A., Martins J., Kao T. Y., Le K., Gralla E. B., Valentine J. S. (2005) Exogenous manganous ion at millimolar levels rescues all known dioxygen-sensitive phenotypes of yeast lacking Cu,ZnSOD. J. Biol. Inorg. Chem. 10, 913–923 [DOI] [PubMed] [Google Scholar]
- 45. Liu X. F., Elashvili I., Gralla E. B., Valentine J. S., Lapinskas P., Culotta V. C. (1992) Yeast lacking superoxide dismutase. Isolation of genetic suppressors. J. Biol. Chem. 267, 18298–18302 [PubMed] [Google Scholar]
- 46. Liu X. F., Culotta V. C. (1994) The requirement for yeast superoxide dismutase is bypassed through mutations in BSD2, a novel metal homeostasis gene. Mol. Cell. Biol. 14, 7037–7045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lapinskas P. J., Cunningham K. W., Liu X. F., Fink G. R., Culotta V. C. (1995) Mutations in PMR1 suppress oxidative damage in yeast cells lacking superoxide dismutase. Mol. Cell. Biol. 15, 1382–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lin S. J., Culotta V. C. (1996) Suppression of oxidative damage by Saccharomyces cerevisiae ATX2, which encodes a manganese-trafficking protein that localizes to Golgi-like vesicles. Mol. Cell. Biol. 16, 6303–6312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liu X. F., Supek F., Nelson N., Culotta V. C. (1997) Negative control of heavy metal uptake by the Saccharomyces cerevisiae BSD2 gene. J. Biol. Chem. 272, 11763–11769 [DOI] [PubMed] [Google Scholar]
- 50. Reddi A. R., Jensen L. T., Naranuntarat A., Rosenfeld L., Leung E., Shah R., Culotta V. C. (2009) The overlapping roles of manganese and Cu,ZnSOD in oxidative stress protection. Free Radic. Biol. Med. 46, 154–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Daly M. J., Gaidamakova E. K., Matrosova V. Y., Vasilenko A., Zhai M., Venkateswaran A., Hess M., Omelchenko M. V., Kostandarithes H. M., Makarova K. S., Wackett L. P., Fredrickson J. K., Ghosal D. (2004) Accumulation of Mn(II) in Deinococcus radiodurans facilitates γ-radiation resistance. Science 306, 1025–1028 [DOI] [PubMed] [Google Scholar]
- 52. Robinson C. K., Webb K., Kaur A., Jaruga P., Dizdaroglu M., Baliga N. S., Place A., Diruggiero J. (2011) A major role for non-enzymatic antioxidant processes in the radioresistance of Halobacterium salinarum. J. Bacteriol. 193, 1653–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fredrickson J. K., Li S. M., Gaidamakova E. K., Matrosova V. Y., Zhai M., Sulloway H. M., Scholten J. C., Brown M. G., Balkwill D. L., Daly M. J. (2008) Protein oxidation: key to bacterial desiccation resistance? ISME J. 2, 393–403 [DOI] [PubMed] [Google Scholar]
- 54. Daly M. J. (2009) A new perspective on radiation resistance based on Deinococcus radiodurans. Nat. Rev. Microbiol. 7, 237–245 [DOI] [PubMed] [Google Scholar]
- 55. Daly M. J. (2006) Modulating radiation resistance: insights based on defenses against reactive oxygen species in the radioresistant bacterium Deinococcus radiodurans. Clin. Lab. Med. 26, 491–504 [DOI] [PubMed] [Google Scholar]
- 56. Daly M. J., Gaidamakova E. K., Matrosova V. Y., Vasilenko A., Zhai M., Leapman R. D., Lai B., Ravel B., Li S. M., Kemner K. M., Fredrickson J. K. (2007) Protein oxidation implicated as the primary determinant of bacterial radioresistance. PLoS Biol. 5, e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cho J. H., Ko K. M., Singaravelu G., Ahnn J. (2005) Caenorhabditis elegans PMR1, a P-type calcium ATPase, is important for calcium/manganese homeostasis and oxidative stress response. FEBS Lett. 579, 778–782 [DOI] [PubMed] [Google Scholar]
- 58. Lin Y. T., Hoang H., Hsieh S. I., Rangel N., Foster A. L., Sampayo J. N., Lithgow G. J., Srinivasan C. (2006) Manganous ion supplementation accelerates wild-type development, enhances stress resistance, and rescues the life span of a short-lived Caenorhabditis elegans mutant. Free Radic. Biol. Med. 40, 1185–1193 [DOI] [PubMed] [Google Scholar]
- 59. Archibald F. S., Fridovich I. (1982) Investigations of the state of the manganese in Lactobacillus plantarum. Arch. Biochem. Biophys. 215, 589–596 [DOI] [PubMed] [Google Scholar]
- 60. Daly M. J., Gaidamakova E. K., Matrosova V. Y., Kiang J. G., Fukumoto R., Lee D. Y., Wehr N. B., Viteri G. A., Berlett B. S., Levine R. L. (2010) Small-molecule antioxidant proteome shields in Deinococcus radiodurans. PLoS ONE 5, e12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Reddi A. R., Culotta V. C. (2011) Regulation of manganese antioxidants by nutrient-sensing pathways in Saccharomyces cerevisiae. Genetics 189, 1261–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Archibald F. S., Fridovich I. (1982) The scavenging of superoxide radical by manganous complexes: in vitro. Arch. Biochem. Biophys. 214, 452–463 [DOI] [PubMed] [Google Scholar]
- 63. Barnese K., Gralla E. B., Cabelli D. E., Valentine J. S. (2008) Manganous phosphate acts as a superoxide dismutase. J. Am. Chem. Soc. 130, 4604–4606 [DOI] [PubMed] [Google Scholar]
- 64. McNaughton R. L., Reddi A. R., Clement M. H., Sharma A., Barnese K., Rosenfeld L., Gralla E. B., Valentine J. S., Culotta V. C., Hoffman B. M. (2010) Probing in vivo Mn2+ speciation and oxidative stress resistance in yeast cells with electron nuclear double resonance spectroscopy. Proc. Natl. Acad. Sci. U.S.A. 107, 15335–15339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Granger A. C., Gaidamakova E. K., Matrosova V. Y., Daly M. J., Setlow P. (2011) Effects of manganese and iron levels on Bacillus subtilis spore resistance and effects of Mn2+, other divalent cations, orthophosphate, and dipicolinic acid on protein resistance to ionizing radiation. Appl. Environ. Microbiol. 77, 32–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shirkey B., McMaster N. J., Smith S. C., Wright D. J., Rodriguez H., Jaruga P., Birincioglu M., Helm R. F., Potts M. (2003) Genomic DNA of Nostoc commune (Cyanobacteria) becomes covalently modified during long-term (decades) desiccation but is protected from oxidative damage and degradation. Nucleic Acids Res. 31, 2995–3005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Batinić-Haberle I., Rebouças J. S., Spasojević I. (2010) Superoxide dismutase mimics: chemistry, pharmacology, and therapeutic potential. Antioxid. Redox Signal. 13, 877–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Stadtman E. R., Berlett B. S., Chock P. B. (1990) Manganese-dependent disproportionation of hydrogen peroxide in bicarbonate buffer. Proc. Natl. Acad. Sci. U.S.A. 87, 384–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sobota J. M., Imlay J. A. (2011) Iron enzyme ribulose-5-phosphate 3-epimerase in Escherichia coli is rapidly damaged by hydrogen peroxide but can be protected by manganese. Proc. Natl. Acad. Sci. U.S.A. 108, 5402–5407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Anjem A., Varghese S., Imlay J. A. (2009) Manganese import is a key element of the OxyR response to hydrogen peroxide in Escherichia coli. Mol. Microbiol. 72, 844–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Horsburgh M. J., Wharton S. J., Karavolos M., Foster S. J. (2002) Manganese: elemental defense for a life with oxygen. Trends Microbiol. 10, 496–501 [DOI] [PubMed] [Google Scholar]
- 72. Swinnen E., Wanke V., Roosen J., Smets B., Dubouloz F., Pedruzzi I., Cameroni E., De Virgilio C., Winderickx J. (2006) Rim15 and the crossroads of nutrient-signaling pathways in Saccharomyces cerevisiae. Cell Div. 1, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Pedruzzi I., Dubouloz F., Cameroni E., Wanke V., Roosen J., Winderickx J., De Virgilio C. (2003) TOR and PKA signaling pathways converge on the protein kinase Rim15 to control entry into G0. Mol. Cell 12, 1607–1613 [DOI] [PubMed] [Google Scholar]
- 74. Cameroni E., Hulo N., Roosen J., Winderickx J., De Virgilio C. (2004) The novel yeast PAS kinase Rim15 orchestrates G0-associated antioxidant defense mechanisms. Cell Cycle 3, 462–468 [PubMed] [Google Scholar]
- 75. Zhang N., Wu J., Oliver S. G. (2009) Gis1 is required for transcriptional reprogramming of carbon metabolism and the stress response during transition into stationary phase in yeast. Microbiology 155, 1690–1698 [DOI] [PubMed] [Google Scholar]
- 76. Kehres D. G., Maguire M. E. (2003) Emerging themes in manganese transport, biochemistry, and pathogenesis in bacteria. FEMS Microbiol. Rev. 27, 263–290 [DOI] [PubMed] [Google Scholar]
- 77. Dintilhac A., Alloing G., Granadel C., Claverys J. P. (1997) Competence and virulence of Streptococcus pneumoniae: Adc and PsaA mutants exhibit a requirement for zinc and manganese resulting from inactivation of putative ABC metal permeases. Mol. Microbiol. 25, 727–739 [DOI] [PubMed] [Google Scholar]
- 78. Ouyang Z., He M., Oman T., Yang X. F., Norgard M. V. (2009) A manganese transporter, BB0219 (BmtA), is required for virulence by the Lyme disease spirochete, Borrelia burgdorferi. Proc. Natl. Acad. Sci. U.S.A. 106, 3449–3454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Champion O. L., Karlyshev A., Cooper I. A., Ford D. C., Wren B. W., Duffield M., Oyston P. C., Titball R. W. (2011) Yersinia pseudotuberculosis mntH functions in intracellular manganese accumulation, which is essential for virulence and survival in cells expressing functional Nramp1. Microbiology 157, 1115–1122 [DOI] [PubMed] [Google Scholar]
- 80. Anderson E. S., Paulley J. T., Gaines J. M., Valderas M. W., Martin D. W., Menscher E., Brown T. D., Burns C. S., Roop R. M., 2nd (2009) The manganese transporter MntH is a critical virulence determinant for Brucella abortus 2308 in experimentally infected mice. Infect. Immun. 77, 3466–3474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kehl-Fie T. E., Skaar E. P. (2010) Nutritional immunity beyond iron: a role for manganese and zinc. Curr. Opin. Chem. Biol. 14, 218–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Govoni G., Vidal S., Gauthier S., Skamene E., Malo D., Gros P. (1996) The Bcg/Ity/Lsh locus: genetic transfer of resistance to infections in C57BL/6J mice transgenic for the Nramp1 Gly-169 allele. Infect. Immun. 64, 2923–2929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Cellier M., Shustik C., Dalton W., Rich E., Hu J., Malo D., Schurr E., Gros P. (1997) Expression of the human NRAMP1 gene in professional primary phagocytes: studies in blood cells and in HL-60 promyelocytic leukemia. J. Leukocyte Biol. 61, 96–105 [DOI] [PubMed] [Google Scholar]
- 84. Jabado N., Jankowski A., Dougaparsad S., Picard V., Grinstein S., Gros P. (2000) Natural resistance to intracellular infections: natural resistance-associated macrophage protein 1 (Nramp1) functions as a pH-dependent manganese transporter at the phagosomal membrane. J. Exp. Med. 192, 1237–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Corbin B. D., Seeley E. H., Raab A., Feldmann J., Miller M. R., Torres V. J., Anderson K. L., Dattilo B. M., Dunman P. M., Gerads R., Caprioli R. M., Nacken W., Chazin W. J., Skaar E. P. (2008) Metal chelation and inhibition of bacterial growth in tissue abscesses. Science 319, 962–965 [DOI] [PubMed] [Google Scholar]
- 86. Kehl-Fie T. E., Chitayat S., Hood M. I., Damo S., Restrepo N., Garcia C., Munro K. A., Chazin W. J., Skaar E. P. (2011) Nutrient metal sequestration by calprotectin inhibits bacterial superoxide defense, enhancing neutrophil killing of Staphylococcus aureus. Cell Host Microbe 10, 158–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Liochev S. I., Fridovich I. (2010) Mechanism of the peroxidase activity of Cu,Zn-superoxide dismutase. Free Radic. Biol. Med. 48, 1565–1569 [DOI] [PubMed] [Google Scholar]