Abstract
The trace element copper is indispensable for all aerobic life forms. Its ability to cycle between two oxidation states, Cu1+ and Cu2+, has been harnessed by a wide array of metalloenzymes that catalyze electron transfer reactions. The metabolic needs for copper are sustained by a complex series of transporters and carrier proteins that regulate its intracellular accumulation and distribution in both pathogenic microbes and their animal hosts. However, copper is also potentially toxic due in part to its ability to generate reactive oxygen species. Recent studies suggest that the macrophage phagosome accumulates copper during bacterial infection, which may constitute an important mechanism of killing. Bacterial countermeasures include the up-regulation of copper export and detoxification genes during infection, which studies suggest are important determinants of virulence. In this minireview, we summarize recent developments that suggest an emerging role for copper as an unexpected component in determining the outcome of host-pathogen interactions.
Keywords: ATPases, Bacterial Pathogenesis, Copper Transport, Infectious Diseases, Macrophages, ATP7A, CTR1
History of Copper as a Bactericidal Agent
Copper has been used throughout the ages as an antimicrobial agent. The earliest recorded medicinal use of copper can be traced to the Edwin Smith Papyrus, an ancient Egyptian medical text that described the use of copper to sterilize chest wounds and drinking water (1). Copper was also widely used by the ancient Greeks and advocated by the Greek physician Hippocrates. Roman and Aztec civilizations similarly used copper compounds to treat common medical afflictions. Whether ingested, inhaled as a powder, or applied topically, copper was commonly used throughout much of human history to treat a wide variety of infectious conditions until the modern era of antibiotics (1).
Numerous applications of copper's antimicrobial activity are found in modern-day materials. For example, copper is used as a fungicidal application for plants (2) and as an electrolytic ionizer to combat Legionella in hospital drinking water (3), and in 2008, copper alloys became the first solid surfaces registered by the Environmental Protection Agency for marketing as an antimicrobial. This has led to renewed interest in using copper materials in the manufacture of frequently touched surfaces within healthcare facilities such as door handles and railings to combat the spread of nosocomial infections (4). Studies have shown that methicillin-resistant Staphylococcus aureus is killed on copper surfaces within 90 min compared with 72 h on stainless steel (5), and recent studies have documented the killing of several types of bacteria within minutes of contact with dry copper surfaces (6).
The ability of bacteria to survive in the presence of soluble copper salts or on solid copper surfaces is dependent on the expression of copper tolerance genes (7, 8). Copper tolerance in most bacteria involves the expression of at least one copper-exporting ATPase-type pump that is transcribed from an operon by a copper-responsive transcriptional regulator. Several recent studies suggest that these same pathways of copper tolerance within certain pathogenic bacteria are required to survive the innate immune response during infection. Although still in its infancy, this relatively new field of biometals research underscores a novel and unexpected role for copper in host immunity and emphasizes the medical importance of understanding copper homeostasis at the host-pathogen interface. Below is a brief review of current findings and future directions.
Mechanisms of Copper Toxicity
Several mechanisms have been ascribed to the antimicrobial properties of copper. These include its ability to accept and donate an electron as it cycles between Cu(I) and Cu(II) oxidation states. Under aerobic conditions, this redox property enables copper to catalyze the production of hydroxyl radicals via the Fenton and Haber-Weiss reactions (Reactions 1 and 2) (9).
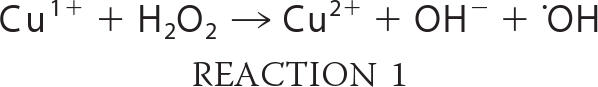 |
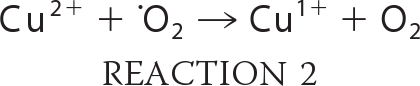 |
The hydroxyl radical is reactive with most types of macromolecules, resulting in damage to lipids, proteins, and nucleic acids. A second mechanism of copper toxicity is the disruption of protein structure. This may occur via disruption of protein structure through interactions with the polypeptide backbone (the biuret reaction) or through adventitious binding of copper to amino acids (e.g. Cys), which may exclude native metal cofactors from their ligands. This latter process is thought to be particularly damaging to iron-sulfur cluster proteins due to the high propensity of sulfur to form thiolate bonds with Cu(I) (10, 11).
Principles of Bacterial Copper Tolerance
The acquisition and insertion of copper into metalloenzymes of all organisms must be balanced by strict homeostatic mechanisms that prevent adventitious interactions of copper with cellular components. This is achieved in part by maintaining the cytoplasm essentially free of unbound copper by a sophisticated network of homeostatic proteins. In eukaryotes, this includes copper importers and exporters, as well as proteins that compartmentalize and sequester copper as it traffics within an organism. The reader is directed to excellent recent reviews on eukaryotic copper homeostasis (12, 13). Unlike eukaryotic cells, most bacteria have little need to import copper into their cytoplasm because bacterial copper-dependent enzymes are located within either the cytoplasmic membrane (e.g. cytochrome oxidase) or the periplasmic space. Accordingly, most microbes strive to exclude unligated copper within the cytoplasm. In Escherichia coli, the copper-responsive transcription factor CueR induces the expression of copper tolerance genes at 10−21 molar sensitivity, which translates to less than one free copper atom in the cytoplasm (14). In general, the avoidance of copper toxicity in bacteria is achieved by three principle mechanisms, including 1) copper export across the plasma membrane into the periplasmic space or the extracellular milieu, 2) copper sequestration within the cytoplasm or periplasm by copper-binding proteins, or 3) Cu(I) oxidation to generate the less toxic Cu(II) ion. Below is a general description of these mechanisms and their importance in bacterial virulence.
Copper Exporters
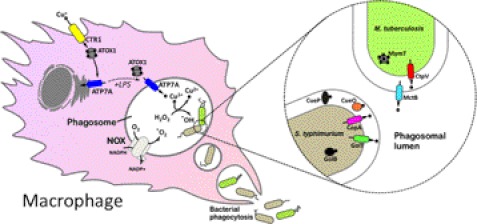
Analyses of archaeal and bacterial genomes indicates the presence of at least one P1B-type ATPase predicted to function in copper export across the plasma membrane (15). P1B-type ATPases are a ubiquitous subclass of heavy metal pumps belonging to the P-type ATPase superfamily of ATP-driven ion transporters. Like all P-type ATPases, the P1B-type ATPases share a conserved aspartic acid motif, DKTGT, which is phosphorylated during the reaction cycle via the γ-phosphate of ATP (16). Additional signature sequences present in the P1B-type ATPases include copper-binding Cys-X2-Cys or histidine-rich motifs at their cytoplasmic amino termini and a canonical His-Pro or Cys-Pro-(Cys/His) motif within the sixth membrane-spanning helix (17). P1B-type ATPases in Gram-positive bacteria such as S. aureus and Bacillus subtilis export Cu(I) out of the cytoplasm across the cell membrane (15), whereas in Gram-negative bacteria, Cu(I) is exported across the inner membrane to the periplasmic space (17). In certain bacteria, copper is driven from the periplasmic space across the outer membrane via additional transport systems. In E. coli, this occurs via the CusABC complex, a large multisubunit protein driven by the proton-motive force (18, 19). In Mycobacterium tuberculosis, the novel component MctB (mycobacterial copper transport protein B), located in the outer membrane, is postulated to function as a channel for copper export (Fig. 1) and, as discussed below, is a major determinant of virulence (20).
FIGURE 1.
Model of copper-mediated bacterial killing by the activated macrophage. Inflammatory agents (e.g. lipopolysaccharide) stimulate copper uptake by inducing the expression of the CTR1 copper importer at the plasma membrane. Cytoplasmic copper is delivered via the ATOX copper chaperone to the ATP7A copper pump, which undergoes trafficking to the phagolysosomal compartment, into which it loads copper. The NADPH oxidase (NOX) produces superoxide, which spontaneously generates hydrogen peroxide, the bactericidal potency of which is augmented by conversion to the hydroxyl radical via Cu(I)-catalyzed Fenton chemistry. Cu(I) may also function as a bactericidal agent by disruption of Fe-S clusters. Copper homeostasis proteins of S. typhimurium and M. tuberculosis are shown. Those in color are known to contribute to survival within cultured macrophages or in animal models of infection as described in the text.
Copper Sequestration
Other copper-detoxifying mechanisms in bacteria include copper sequestration via metallothionein-like proteins. MymT is a small metallothionein (4.9 kDa) in M. tuberculosis required for copper tolerance, and like the well described metallothioneins of eukaryotic cells, MymT contains cysteine-rich sequences capable of binding at least six copper atoms via copper-thiolate bonds (21). The small Cu(I)-binding protein CusF, located in the periplasm of E. coli, is thought to similarly function as a copper buffer either by preventing potentially toxic interactions with cellular components or by functioning as a copper delivery chaperone to the CusABC complex for copper export across the outer membrane (19). CueP is another recently identified protein in the periplasm of Salmonella typhimurium whose ability to bind copper is thought to protect against copper toxicity in this compartment (Fig. 1) (22, 23). Interestingly, cueP genes are found only in bacterial genomes lacking an E. coli Cus-like complex and may therefore be a functional surrogate for this system.
Copper Oxidation in the Bacterial Periplasm
As the major site of copper utilization in bacteria, the periplasm of bacteria contains several known copper-dependent enzymes. Notable examples include the copper/zinc-containing superoxide dismutases (SodC proteins), which protect certain pathogenic bacteria against superoxide anions generated by the respiratory burst of phagocytic cells (discussed below) (24, 25). Another cuproenzyme in the periplasm is CueO, a multi-copper oxidase found in both E. coli and S. typhimurium that is proposed to confer copper tolerance by oxidizing Cu(I) to the less toxic Cu(II) form under aerobic conditions (18, 26, 27); however, its definitive role is not completely understood. Periplasmic enzymes are sensitive to copper-induced damage in E. coli mutants of cueO (18, 27), consistent with studies suggesting that CueO functions in the oxidation of catecholate iron siderophores to yield compounds that chelate copper, thus limiting its toxicity within the periplasm (28). As noted below, CueO is also a necessary determinant of S. typhimurium virulence (Fig. 1) (29).
Regulation
The abundance of these bacterial copper defense systems is typically increased under excess copper concentrations via the action of copper-sensing transcription factors. In E. coli, this occurs via copper-induced transcription of cueO and copA genes by the CueR transcription factor (30, 31). S. typhimurium also contains a CueR homolog for copper-stimulated expression of cueO, copA, and cueP (32), as well as an additional transcription factor, GolS, which is responsive to both copper and gold and induces the expression of GolT, a second P1B-type ATPase involved in copper export into the periplasm (33, 34). In other bacteria such as M. tuberculosis and B. subtilis, copper inhibits transcriptional repressors such as CsoR, resulting in the increased expression of copper tolerance genes (35, 36). The mechanisms by which these transcriptional regulators acquire copper in some cases involve the exchange of copper with cytoplasmic copper chaperones (e.g. CopZ of Enterococcus hirae) (37), which may also serve as copper carriers to the copper-exporting P1B-type ATPases (23, 33).
Bacterial Copper Homeostasis as a Virulence Determinant
The successful bacterial pathogen must be able to rapidly respond to a changing hostile environment during infection of the host. The production of bactericidal toxins such as reactive oxygen and nitrogen species and the withdrawal of nutrients to starve the invading microbe are critical defense mechanisms of the innate immune system. Several recent studies suggest that exposure of bacterial pathogens to toxic copper concentrations within the host is a general defense mechanism of the innate immune system, and the ability of pathogenic bacteria to activate several of the aforementioned copper tolerance pathways influences their virulence. Below is a summary of these recent developments with a focus on the most well studied examples of the pathogenic bacteria M. tuberculosis and S. typhimurium.
M. tuberculosis
As a pathogen of the mammalian respiratory system, M. tuberculosis infects the lungs and is the causative agent of tuberculosis. The ability to survive and replicate within alveolar macrophages is an important determinant of M. tuberculosis virulence (38). Infection of primary human macrophages by M. tuberculosis induces the expression of the P1B-type ATPase CtpV, which exports copper across the inner membrane (39). Because the ctpV gene is part of the copper-induced regulon that is controlled by the CsoR transcriptional regulator (36), these findings provided some of the first insights into the possibility that copper tolerance genes may play a role in virulence of M. tuberculosis, and recent studies are consistent with this hypothesis. The ΔctpV mutant was found to inflict less severe lung damage and lower rates of killing in a study of infected mice and guinea pigs compared with wild-type M. tuberculosis (40). However, because this same study found no difference in the lung bacterial load between ΔctpV and wild-type strains, it raised the possibility that other copper defense mechanisms might partially compensate for the loss of CtpV. Further insight came from studies of the putative outer membrane channel protein MctB (20). Loss of the mctB gene in M. tuberculosis or its homologous gene in Mycobacterium smegmatis resulted in hyperaccumulation of copper and a marked increase in the sensitivity to elevated concentrations of Cu(I), consistent with a role for MctB in copper export from the periplasmic space to the extracellular milieu (20). In a mouse model infected with aerosols of M. tuberculosis, the recovery of ΔmctB bacteria from the lung was reduced 10-fold in comparison with the wild type, and remarkably, this difference was increased to 100-fold if CuSO4 was added to the drinking water of the mice. These findings provided evidence not only that copper export is a critical determinant of virulence in M. tuberculosis but that this phenotype is subject to the level of copper intake by the host. Although MctB appears to play a dominant role in this process, several important questions are raised by these findings. What is the effect of combined mutations in copper tolerance pathways involving MymT, CtpV, and MctB, and are these proteins functionally interconnected? What is the importance of a newly identified copper-responsive regulon in M. tuberculosis that is regulated by RicR, a paralog of CsoR (41)? Do the copper-handling pathways of M. tuberculosis function effectively in the relatively aerobic environment of the exposed lung mucosa or the hypoxic environment of the granuloma, where much of the persistent infection resides? Clearly, there is much that needs to be done to fully understand the roles of copper in M. tuberculosis pathogenesis.
S. typhimurium
World wide, this Gram-negative bacterium is responsible for 93 million cases of gastrointestinal disease annually (42). During systemic infection, the ability of S. typhimurium to survive and replicate within the macrophage phagosome is a critical determinant of virulence, and like M. tuberculosis, the activities of copper tolerance genes appear to play important roles in this process. Two copper-exporting P1B-type ATPases in S. typhimurium, CopA and GolT, are functionally analogous to the M. tuberculosis CtpV protein and similarly contribute to copper tolerance by exporting copper out of the cytoplasm into the periplasmic space. Recent studies have explored the contribution of CopA and GolT to S. typhimurium virulence. The transcription of the copA gene was found to be induced between 8- and 12-fold upon phagocytosis by macrophage cells, thus providing further evidence that elevated phagosomal copper concentrations are a general feature of the activated macrophage during infection (23, 43). Although deletion of either copA or golT was found to have no impact on bacterial survival in cultured macrophages compared with the wild-type strain, the loss of both genes markedly reduced bacterial survival in this model (23). Thus, although copper export is important for S. typhimurium survival within the macrophage, it appears that there is some redundancy in the contributions of CopA and GolT. Interestingly, the ability of the ΔcopA/ΔgolT double mutant to colonize the livers and spleens of mice challenged with an oral gavage was no different from that of the wild-type strain. Although this underscores the potential differences between in vitro and in vivo models of infection, it also highlights the possibility that a more complete disruption of copper tolerance pathways may be required to fully sensitize the bacterium to the innate immune system in the whole animal.
In this context, it is interesting to note that CueO (also known as CuiD) appears to contribute to the virulence of S. typhimurium in a mouse model of systemic infection. Loss of cueO was found to impair colonization in the lungs and spleens of mice challenged with an oral gavage, but no differences were noted between the mutant and wild-type bacteria recovered from intestinal Peyer's patches or mesenteric lymph nodes (29). The implication of these findings is that the loss of CueO did not affect initial phases of infection but rather later stages once the bacteria had seeded the peripheral organs. Although this might suggest a defect in replicative ability within macrophages, no such differences between wild-type and mutant CueO bacteria were found, at least within a cultured macrophage model (29). Although these findings collectively point to the importance of several mechanisms of copper homeostasis in S. typhimurium for virulence, they also highlight the possibility that specific pathways of copper tolerance might offer a greater degree of protection at different stages of infection. Clearly, it will be important to generate individual and combined disruptions in the entire copper regulon to address such questions.
Is Copper Tolerance in Bacteria a General Determinant of Virulence?
The importance of copper resistance for virulence has been shown for several bacterial pathogens carrying mutations in P1B-ATPases. In Pseudomonas aeruginosa, a Gram-negative opportunistic pathogen that often infects individuals with a compromised immune system, deletion of the CopA1 ATPase (also known as CueA) decreased bacterial survival in murine spleen compared with the wild-type strain (44). Other studies have shown that a deletion mutant of the CtpA copper-exporting P-type ATPase in Listeria monocytogenes was cleared more rapidly from the livers of infected mice compare with the wild-type strain and was outcompeted by the wild-type strain in mixed infections (45). In a recent study, deletion of the CopA P1B-ATPase of Streptococcus pneumonia was found to reduce survival in the lungs of infected mice and, to a greater extent, the nasopharynx but surprisingly did not affect bacterial load in the blood (46). These findings highlight the possibility that bacterial copper homeostasis mechanisms play more important roles in the colonization of the host at initial sites of infection relative to subsequent events involving systemic infection. These studies reinforce the concept of copper homeostasis as a general mechanism of survival required by both Gram-positive and Gram-negative bacteria during infection of the host, the importance of which may vary according to the site or stage of infection.
Copper Homeostasis in the Mammalian Host
It has been known for decades that marked changes in micronutrient homeostasis in the host are accompanied by infection or inflammation. The adaptive changes in iron homeostasis of both host and pathogen during infection are among the most intensively studied examples in this area (47, 48). Although the invading pathogen expresses an array of iron acquisition pathways needed for replication and survival, this is countered by a complex series of iron-withholding mechanisms in the host. In contrast to iron, it is well documented that copper levels in the serum are significantly elevated in response to inflammation (49–60). Copper accumulates at sites of inflammation (61), within granulomatous lesions of lungs infected with M. tuberculosis (20), and within the exudates of wounds and burns relative to serum (62, 63). The form of copper at these inflammatory sites is unknown; however, a candidate is the copper-containing protein ceruloplasmin, a serum protein containing 85% of circulating copper that is secreted from the liver during the acute-phase inflammatory response (64). Although the mechanisms by which copper homeostasis is altered within specific organs in response to infection are unknown, these changes may form adaptive responses of the innate immune system to mobilize copper toward sites of infection to aid in bacterial killing by macrophages. Consistent with this concept, various studies have shown that copper deficiency increases the susceptibility to various pathogens, including Candida albicans (65, 66), Pasteurella haemolytica (67), Trypanosoma lewisi (68), S. typhimurium (69), and coxsackievirus B3 (70). Conversely, other studies have shown that copper supplementation is protective against E. coli-induced mastitis in dairy cattle (71) and M. tuberculosis in mice (20). Although the underlying mechanisms by which the copper status of the host impacts susceptibility to infection are unknown, it is likely that the bactericidal activity of phagocytic cells of the innate immune system is partially responsible because the respiratory burst capacity of and the bacterial killing by these cells are reduced by copper deficiency (72–74). Elemental analysis using x-ray microprobe approaches has demonstrated that although the abundance of some elements declines within the mycobacterium-containing phagosome, the copper abundance markedly increases up to several hundred micromolar (75), a concentration well within the range known to induce expression of copper exporters in M. tuberculosis and S. typhimurium and to kill mutants lacking such transporters (23, 36).
Importance of Mammalian Copper Transporters in Macrophage Bactericidal Activity
Bacterial phagocytosis by the macrophage occurs via plasma membrane invagination, budding, and fusion events, which result in the formation of the membrane-enclosed phagosome containing the microbe (76). The phagosome then undergoes a series of maturation steps, acquiring bactericidal enzymes and toxins as it acidifies and fuses with lysosomes to become the phagolysosomal compartment. A critical event in pathogen killing is the assembly of the NADPH oxidase on the membrane of the phagosome, which catalyzes the one-electron reduction of oxygen to produce superoxide (77). This so-called respiratory burst is a front line defense against invading microbes, and its importance in innate immunity is underscored in patients with X-linked granulomatous disease, in which lack of a functional NADPH oxidase increases the susceptibility to infection (78). Recent studies from the author's (P. J. P.) laboratory have demonstrated a link between the copper homeostasis machinery of the macrophage and its ability to use copper as a bactericidal agent. Proinflammatory factors lipopolysaccharide (a component of the Gram-negative bacterial cell wall) and interferon-γ were found to stimulate copper uptake in RAW 267.4 macrophage cells by increasing the expression of the copper importer CTR1 (79, 80). Significantly, these same proinflammatory conditions led to an increase in the expression of the ATP7A protein, a P1B-type ATPase that is normally located in the Golgi complex, into which it transports copper to various metalloenzymes in the secretory pathway. Moreover, these inflammatory conditions induced the trafficking of the ATP7A protein into cytoplasmic vesicles and the phagolysosome, thus providing a possible mechanism by which bactericidal levels of copper may be delivered into this compartment during infection (Fig. 1) (79). Consistent with this model, the killing of E. coli was suppressed in RAW 264.7 macrophages in which ATP7A expression was silenced, suggesting that copper delivery to the phagosomal lumen via ATP7A augments its bactericidal activity (79). Further support for this model came from the finding that a copper-sensitive mutant of E. coli lacking the CopA copper-exporting P1B-type ATPase was not only killed more efficiently by RAW 264.7 macrophages compared with the wild-type strain, but this hypersensitivity was abrogated by knockdown of ATP7A in macrophages (79). Whether CTR1 and ATP7A play critical roles in killing other bacterial pathogens such as M. tuberculosis and S. typhimurium in vitro and in vivo is the subject of ongoing investigations. However, consistent with a role for ATP7A in immune defense, it is notable that patients with Menkes disease who lack a functional ATP7A copper transporter have increased susceptibility to infection of the bladder and lungs (81–84).
Possible Mechanism of Copper-dependent Bacterial Killing by Macrophages
Although the above studies shed light on the transporters underlying copper-dependent killing by macrophages, the question of mechanism remains unanswered. It is reasonable to speculate that the toxic potential of copper may be exploited by the macrophage to augment bacterial killing by the respiratory burst (Fig. 1). The superoxide radical is short-lived in the phagosome and converts to H2O2 non-enzymatically. H2O2 is highly diffusible and relatively unreactive; however, in the presence of a reduced transition metal such as Cu(I), H2O2 can produce, via the Fenton reaction, the highly reactive hydroxyl radical, which is able to oxidatively damage surrounding molecules encountered (Fig. 1) (85, 86). It is also possible that copper may indirectly contribute to iron-mediated Fenton chemistry by releasing iron from labile sites such as iron-sulfur cluster proteins (10). In these scenarios, it is readily apparent how bacterial copper detoxification mechanisms might confer increased survival in the macrophage.
It is worth noting that in some bacteria such as M. tuberculosis and S. typhimurium, the presence of copper-containing superoxide dismutases in the periplasm contributes to virulence by protection against superoxide (24, 25). However, under conditions in which Cu(I) levels are elevated in the phagosome, one can envisage the activity of these superoxide dismutases as a double-edged sword that contributes to H2O2 production and thus Fenton chemistry, which may be exacerbated by bacterial copper exporters into the periplasm. It will therefore be of interest to determine how the function of ATP7A-mediated copper delivery into the phagosome, as well as bacterial exporters of copper into the periplasmic space, affects the contribution of SodC proteins to virulence in organisms such as M. tuberculosis and S. typhimurium.
Future Directions
The antimicrobial properties of copper have played important roles in medicine for millennia; however, its role in the innate immune system is a relatively recent discovery. Accordingly, there are many unanswered questions to be addressed in the coming years. What is the mechanism by which copper kills bacteria within the macrophage? Can drugs that are designed to inhibit copper tolerance proteins of bacterial pathogens give rise to a new class of antibiotics? What are the transporters and regulatory mechanisms in the host responsible for systemic mobilization of copper into the bloodstream during infection, and does this process play a role in delivering copper to sites of infection? Does the macrophage use copper against other pathogenic agents such as fungi, viruses, helminths, and protists? Do other professional phagocytes such as dendritic cells, microglia, and neutrophils similarly use copper as a weapon against bacteria? The answers to such questions will require careful exploration of copper-handling pathways within the host and pathogen at the levels of biochemistry, cell biology, and physiology and how each of these pathways contributes to the struggle to control this essential yet toxic nutrient at the host-pathogen interface.
Acknowledgments
We thank members of the Petris laboratory for critical analysis of the manuscript.
Footnotes
This work was supported, in whole or in part, by National Institutes of Health Grants DK59893 and DK093866. This is the sixth article in the Thematic Minireview Series on Metals in Biology 2012.
REFERENCES
- 1. Dollwet H. H. A., Sorenson J. R. J. (1985) History uses of copper compounds in medicine. Trace Elements Med. 2, 80–87 [Google Scholar]
- 2. Borkow G., Gabbay J. (2005) Copper as a biocidal tool. Curr. Med. Chem. 12, 2163–2175 [DOI] [PubMed] [Google Scholar]
- 3. Lin Y. E., Stout J. E., Yu V. L. (2011) Controlling Legionella in hospital drinking water: an evidence-based review of disinfection methods. Infect. Control Hosp. Epidemiol. 32, 166–173 [DOI] [PubMed] [Google Scholar]
- 4. Casey A. L., Adams D., Karpanen T. J., Lambert P. A., Cookson B. D., Nightingale P., Miruszenko L., Shillam R., Christian P., Elliott T. S. (2010) Role of copper in reducing hospital environment contamination. J. Hosp. Infect. 74, 72–77 [DOI] [PubMed] [Google Scholar]
- 5. Noyce J. O., Michels H., Keevil C. W. (2006) Potential use of copper surfaces to reduce survival of epidemic meticillin-resistant Staphylococcus aureus in the healthcare environment. J. Hosp. Infect. 63, 289–297 [DOI] [PubMed] [Google Scholar]
- 6. Espírito Santo C., Lam E. W., Elowsky C. G., Quaranta D., Domaille D. W., Chang C. J., Grass G. (2011) Bacterial killing by dry metallic copper surfaces. J. Appl. Microbiol. 77, 794–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Elguindi J., Wagner J., Rensing C. (2009) Genes involved in copper resistance influence survival of Pseudomonas aeruginosa on copper surfaces. J. Appl. Microbiol. 106, 1448–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Espírito Santo C., Taudte N., Nies D. H., Grass G. (2008) Contribution of copper ion resistance to survival of Escherichia coli on metallic copper surfaces. Appl. Environ. Microbiol. 74, 977–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liochev S. I., Fridovich I. (2002) The Haber-Weiss cycle–70 years later: an alternative view. Redox Rep. 7, 55–57; author reply 59–60) [DOI] [PubMed] [Google Scholar]
- 10. Macomber L., Imlay J. A. (2009) The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc. Natl. Acad. Sci. U.S.A. 106, 8344–8349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Keyer K., Imlay J. A. (1996) Superoxide accelerates DNA damage by elevating free iron levels. Proc. Natl. Acad. Sci. U.S.A. 93, 13635–13640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim B. E., Nevitt T., Thiele D. J. (2008) Mechanisms for copper acquisition, distribution, and regulation. Nat. Chem. Biol. 4, 176–185 [DOI] [PubMed] [Google Scholar]
- 13. Gupta A., Lutsenko S. (2009) Human copper transporters: mechanism, role in human diseases, and therapeutic potential. Future Med. Chem. 1, 1125–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Changela A., Chen K., Xue Y., Holschen J., Outten C. E., O'Halloran T. V., Mondragón A. (2003) Molecular basis of metal ion selectivity and zeptomolar sensitivity by CueR. Science 301, 1383–1387 [DOI] [PubMed] [Google Scholar]
- 15. Solioz M., Abicht H. K., Mermod M., Mancini S. (2010) Response of Gram-positive bacteria to copper stress. J. Biol. Inorg. Chem. 15, 3–14 [DOI] [PubMed] [Google Scholar]
- 16. Lutsenko S., Barnes N. L., Bartee M. Y., Dmitriev O. Y. (2007) Function and regulation of human copper-transporting ATPases. Physiol. Rev. 87, 1011–1046 [DOI] [PubMed] [Google Scholar]
- 17. Argüello J. M., Eren E., González-Guerrero M. (2007) The structure and function of heavy metal transport P1B-ATPases. Biometals 20, 233–248 [DOI] [PubMed] [Google Scholar]
- 18. Outten F. W., Huffman D. L., Hale J. A., O'Halloran T. V. (2001) The independent cue and cus systems confer copper tolerance during aerobic and anaerobic growth in Escherichia coli. J. Biol. Chem. 276, 30670–30677 [DOI] [PubMed] [Google Scholar]
- 19. Franke S., Grass G., Rensing C., Nies D. H. (2003) Molecular analysis of the copper-transporting efflux system CusCFBA of Escherichia coli. J. Bacteriol. 185, 3804–3812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wolschendorf F., Ackart D., Shrestha T. B., Hascall-Dove L., Nolan S., Lamichhane G., Wang Y., Bossmann S. H., Basaraba R. J., Niederweis M. (2011) Copper resistance is essential for virulence of Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U.S.A. 108, 1621–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gold B., Deng H., Bryk R., Vargas D., Eliezer D., Roberts J., Jiang X., Nathan C. (2008) Identification of a copper-binding metallothionein in pathogenic mycobacteria. Nat. Chem. Biol. 4, 609–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pontel L. B., Soncini F. C. (2009) Alternative periplasmic copper resistance mechanisms in Gram-negative bacteria. Mol. Microbiol. 73, 212–225 [DOI] [PubMed] [Google Scholar]
- 23. Osman D., Waldron K. J., Denton H., Taylor C. M., Grant A. J., Mastroeni P., Robinson N. J., Cavet J. S. (2010) Copper homeostasis in Salmonella is atypical and copper-CueP is a major periplasmic metal complex. J. Biol. Chem. 285, 25259–25268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fang F. C., DeGroote M. A., Foster J. W., Bäumler A. J., Ochsner U., Testerman T., Bearson S., Giárd J. C., Xu Y., Campbell G., Laessig T. (1999) Virulent Salmonella typhimurium has two periplasmic Cu,Zn-superoxide dismutases. Proc. Natl. Acad. Sci. U.S.A. 96, 7502–7507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Piddington D. L., Fang F. C., Laessig T., Cooper A. M., Orme I. M., Buchmeier N. A. (2001) Cu,Zn-superoxide dismutase of Mycobacterium tuberculosis contributes to survival in activated macrophages that are generating an oxidative burst. Infect. Immun. 69, 4980–4987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Singh S. K., Roberts S. A., McDevitt S. F., Weichsel A., Wildner G. F., Grass G. B., Rensing C., Montfort W. R. (2011) Crystal structures of multicopper oxidase CueO bound to copper(I) and silver(I): functional role of a methionine-rich sequence. J. Biol. Chem. 286, 37849–37857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grass G., Rensing C. (2001) CueO is a multi-copper oxidase that confers copper tolerance in Escherichia coli. Biochem. Biophys. Res. Commun. 286, 902–908 [DOI] [PubMed] [Google Scholar]
- 28. Grass G., Thakali K., Klebba P. E., Thieme D., Müller A., Wildner G. F., Rensing C. (2004) Linkage between catecholate siderophores and the multicopper oxidase CueO in Escherichia coli. J. Bacteriol. 186, 5826–5833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Achard M. E., Tree J. J., Holden J. A., Simpfendorfer K. R., Wijburg O. L., Strugnell R. A., Schembri M. A., Sweet M. J., Jennings M. P., McEwan A. G. (2010) The multi-copper ion oxidase CueO of Salmonella enterica serovar Typhimurium is required for systemic virulence. Infect. Immun. 78, 2312–2319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Outten F. W., Outten C. E., Hale J., O'Halloran T. V. (2000) Transcriptional activation of an Escherichia coli copper efflux regulon by the chromosomal MerR homolog, CueR. J. Biol. Chem. 275, 31024–31029 [DOI] [PubMed] [Google Scholar]
- 31. Stoyanov J. V., Hobman J. L., Brown N. L. (2001) CueR (YbbI) of Escherichia coli is a MerR family regulator controlling expression of the copper exporter CopA. Mol. Microbiol. 39, 502–511 [DOI] [PubMed] [Google Scholar]
- 32. Kim J. S., Kim M. H., Joe M. H., Song S. S., Lee I. S., Choi S. Y. (2002) The sctR of Salmonella enterica serovar Typhimurium encoding a homolog of MerR protein is involved in the copper-responsive regulation of cuiD. FEMS Microbiol. Lett. 210, 99–103 [DOI] [PubMed] [Google Scholar]
- 33. Checa S. K., Espariz M., Audero M. E., Botta P. E., Spinelli S. V., Soncini F. C. (2007) Bacterial sensing of and resistance to gold salts. Mol. Microbiol. 63, 1307–1318 [DOI] [PubMed] [Google Scholar]
- 34. Pontel L. B., Audero M. E., Espariz M., Checa S. K., Soncini F. C. (2007) GolS controls the response to gold by the hierarchical induction of Salmonella-specific genes that include a CBA efflux-coding operon. Mol. Microbiol. 66, 814–825 [DOI] [PubMed] [Google Scholar]
- 35. Smaldone G. T., Helmann J. D. (2007) CsoR regulates the copper efflux operon copZA in Bacillus subtilis. Microbiology 153, 4123–4128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu T., Ramesh A., Ma Z., Ward S. K., Zhang L., George G. N., Talaat A. M., Sacchettini J. C., Giedroc D. P. (2007) CsoR is a novel Mycobacterium tuberculosis copper-sensing transcriptional regulator. Nat. Chem. Biol. 3, 60–68 [DOI] [PubMed] [Google Scholar]
- 37. Cobine P., Wickramasinghe W. A., Harrison M. D., Weber T., Solioz M., Dameron C. T. (1999) The Enterococcus hirae copper chaperone CopZ delivers copper(I) to the CopY repressor. FEBS Lett. 445, 27–30 [DOI] [PubMed] [Google Scholar]
- 38. Flannagan R. S., Cosío G., Grinstein S. (2009) Antimicrobial mechanisms of phagocytes and bacterial evasion strategies. Nat. Rev. Microbiol. 7, 355–366 [DOI] [PubMed] [Google Scholar]
- 39. Graham J. E., Clark-Curtiss J. E. (1999) Identification of Mycobacterium tuberculosis RNAs synthesized in response to phagocytosis by human macrophages by selective capture of transcribed sequences (SCOTS). Proc. Natl. Acad. Sci. U.S.A. 96, 11554–11559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ward S. K., Abomoelak B., Hoye E. A., Steinberg H., Talaat A. M. (2010) CtpV: a putative copper exporter required for full virulence of Mycobacterium tuberculosis. Mol. Microbiol. 77, 1096–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Festa R. A., Jones M. B., Butler-Wu S., Sinsimer D., Gerads R., Bishai W. R., Peterson S. N., Darwin K. H. (2011) A novel copper-responsive regulon in Mycobacterium tuberculosis. Mol. Microbiol. 79, 133–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Majowicz S. E., Musto J., Scallan E., Angulo F. J., Kirk M., O'Brien S. J., Jones T. F., Fazil A., Hoekstra R. M. (2010) The global burden of nontyphoidal Salmonella gastroenteritis. Clin. Infect. Dis. 50, 882–889 [DOI] [PubMed] [Google Scholar]
- 43. Heithoff D. M., Conner C. P., Hanna P. C., Julio S. M., Hentschel U., Mahan M. J. (1997) Bacterial infection as assessed by in vivo gene expression. Proc. Natl. Acad. Sci. U.S.A. 94, 934–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schwan W. R., Warrener P., Keunz E., Stover C. K., Folger K. R. (2005) Mutations in the cueA gene encoding a copper homeostasis P-type ATPase reduce the pathogenicity of Pseudomonas aeruginosa in mice. Int. J. Med. Microbiol. 295, 237–242 [DOI] [PubMed] [Google Scholar]
- 45. Francis M. S., Thomas C. J. (1997) Mutants in the CtpA copper-transporting P-type ATPase reduce virulence of Listeria monocytogenes. Microb. Pathog. 22, 67–78 [DOI] [PubMed] [Google Scholar]
- 46. Shafeeq S., Yesilkaya H., Kloosterman T. G., Narayanan G., Wandel M., Andrew P. W., Kuipers O. P., Morrissey J. A. (2011) The cop operon is required for copper homeostasis and contributes to virulence in Streptococcus pneumoniae. Mol. Microbiol. 81, 1255–1270 [DOI] [PubMed] [Google Scholar]
- 47. Nevitt T. (2011) War-Fe-re: iron at the core of fungal virulence and host immunity. Biometals 24, 547–558 [DOI] [PubMed] [Google Scholar]
- 48. Ganz T. (2011) Hepcidin and iron regulation, 10 years later. Blood 117, 4425–4433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Auer D. E., Ng J. C., Thompson H. L., Inglis S., Seawright A. A. (1989) Acute-phase response in horses: changes in plasma cation concentrations after localized tissue injury. Vet. Rec. 124, 235–239 [DOI] [PubMed] [Google Scholar]
- 50. Morimoto A., Sakata Y., Watanabe T., Murakami N. (1989) Characteristics of fever and acute-phase response induced in rabbits by IL-1 and TNF. Am. J. Physiol. 256, R35–R41 [DOI] [PubMed] [Google Scholar]
- 51. Akçil E., Yavuz G., Koçak M. (2003) Effects of inflammation and anti-inflammatory treatment on serum trace element concentrations. Biol. Trace Elem. Res. 93, 95–104 [DOI] [PubMed] [Google Scholar]
- 52. Milanino R., Marrella M., Gasperini R., Pasqualicchio M., Velo G. (1993) Copper and zinc body levels in inflammation: an overview of the data obtained from animal and human studies. Agents Actions 39, 195–209 [DOI] [PubMed] [Google Scholar]
- 53. Ward W. F., Molteni A., Ts'ao C., Ischiropoulos H. (1989) Serum copper concentration as an index of experimental lung injury. Adv. Exp. Med. Biol. 258, 287–302 [DOI] [PubMed] [Google Scholar]
- 54. Shah I., Lewkow L. M., Khilanani U. (1983) Correlation of hypercupremia with other acute-phase reactants in malignant lymphoma. Cancer 51, 851–854 [DOI] [PubMed] [Google Scholar]
- 55. Yilmaz A., Sari R. A., Gundogdu M., Kose N., Dag E. (2005) Trace elements and some extracellular antioxidant proteins levels in serum of patients with systemic lupus erythematosus. Clin. Rheumatol. 24, 331–335 [DOI] [PubMed] [Google Scholar]
- 56. Koyanagi A., Kuffó D., Gresely L., Shenkin A., Cuevas L. E. (2004) Relationships between serum concentrations of C-reactive protein and micronutrients in patients with tuberculosis. Ann. Trop. Med. Parasitol. 98, 391–399 [DOI] [PubMed] [Google Scholar]
- 57. Srinivas U., Braconier J. H., Jeppsson B., Abdulla M., Akesson B., Ockerman P. A. (1988) Trace element alterations in infectious diseases. Scand. J. Clin. Lab. Invest. 48, 495–500 [DOI] [PubMed] [Google Scholar]
- 58. Hällgren R., Feltelius N., Lindh U. (1987) Redistribution of minerals and trace elements in chronic inflammation–a study on isolated blood cells from patients with ankylosing spondylitis. J. Rheumatol. 14, 548–553 [PubMed] [Google Scholar]
- 59. Conforti A., Franco L., Milanino R., Totorizzo A., Velo G. P. (1983) Copper metabolism during acute inflammation: studies on liver and serum copper concentrations in normal and inflamed rats. Br. J. Pharmacol. 79, 45–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Seyrek A., Kocyigit A., Erel O. (2005) Essential trace elements selenium, zinc, copper, and iron concentrations and their related acute-phase proteins in patients with vivax malaria. Biol. Trace Elem. Res. 106, 107–115 [DOI] [PubMed] [Google Scholar]
- 61. Beveridge S. J., Garrett I. R., Whitehouse M. W., Vernon-Roberts B., Brooks P. M. (1985) Biodistribution of 64Cu in inflamed rats following administration of two anti-inflammatory copper complexes. Agents Actions 17, 104–111 [DOI] [PubMed] [Google Scholar]
- 62. Voruganti V. S., Klein G. L., Lu H. X., Thomas S., Freeland-Graves J. H., Herndon D. N. (2005) Impaired zinc and copper status in children with burn injuries: need to reassess nutritional requirements. Burns 31, 711–716 [DOI] [PubMed] [Google Scholar]
- 63. Jones P. W., Taylor D. M., Williams D. R., Finney M., Iorwerth A., Webster D., Harding K. G. (2001) Using wound fluid analyses to identify trace element requirements for efficient healing. J. Wound Care 10, 205–208 [DOI] [PubMed] [Google Scholar]
- 64. Fox P. L., Mukhopadhyay C., Ehrenwald E. (1995) Structure, oxidant activity, and cardiovascular mechanisms of human ceruloplasmin. Life Sci. 56, 1749–1758 [DOI] [PubMed] [Google Scholar]
- 65. Jones D. G., Suttle N. F. (1981) Some effects of copper deficiency on leukocyte function in sheep and cattle. Res. Vet. Sci. 31, 151–156 [PubMed] [Google Scholar]
- 66. Boyne R., Arthur J. R. (1981) Effects of selenium and copper deficiency on neutrophil function in cattle. J. Comp. Pathol. 91, 271–276 [DOI] [PubMed] [Google Scholar]
- 67. Jones D. G., Suttle N. F. (1983) The effect of copper deficiency on the resistance of mice to infection with Pasteurella haemolytica. J. Comp. Pathol. 93, 143–149 [DOI] [PubMed] [Google Scholar]
- 68. Crocker A., Lee C., Aboko-Cole G., Durham C. (1992) Interaction of nutrition and infection: effect of copper deficiency on resistance to Trypanosoma lewisi. J. Natl. Med. Assoc. 84, 697–706 [PMC free article] [PubMed] [Google Scholar]
- 69. Newberne P. M., Hunt C. E., Young V. R. (1968) The role of diet and the reticuloendothelial system in the response of rats to Salmonella typhimurium infection. Br. J. Exp. Pathol. 49, 448–457 [PMC free article] [PubMed] [Google Scholar]
- 70. Smith A. D., Botero S., Levander O. A. (2008) Copper deficiency increases the virulence of amyocarditic and myocarditic strains of coxsackievirus B3 in mice. J. Nutr. 138, 849–855 [DOI] [PubMed] [Google Scholar]
- 71. Scaletti R. W., Trammell D. S., Smith B. A., Harmon R. J. (2003) Role of dietary copper in enhancing resistance to Escherichia coli mastitis. J. Dairy Sci. 86, 1240–1249 [DOI] [PubMed] [Google Scholar]
- 72. Babu U., Failla M. L. (1990) Respiratory burst and candidacidal activity of peritoneal macrophages are impaired in copper-deficient rats. J. Nutr. 120, 1692–1699 [DOI] [PubMed] [Google Scholar]
- 73. Babu U., Failla M. L. (1990) Copper status and function of neutrophils are reversibly depressed in marginally and severely copper-deficient rats. J. Nutr. 120, 1700–1709 [DOI] [PubMed] [Google Scholar]
- 74. Torre P. M., Harmon R. J., Sordillo L. M., Boissonneault G. A., Hemken R. W., Trammell D. S., Clark T. W. (1995) Modulation of bovine mononuclear cell proliferation and cytokine production by dietary copper insufficiency.. J. Nutr. Immunol. 3, 3–20 [Google Scholar]
- 75. Wagner D., Maser J., Lai B., Cai Z., Barry C. E., 3rd, Höner Zu Bentrup K., Russell D. G., Bermudez L. E. (2005) Elemental analysis of Mycobacterium avium-, Mycobacterium tuberculosis-, and Mycobacterium smegmatis-containing phagosomes indicates pathogen-induced microenvironments within the host cell's endosomal system. J. Immunol. 174, 1491–1500 [DOI] [PubMed] [Google Scholar]
- 76. Pieters J. (2008) Mycobacterium tuberculosis and the macrophage: maintaining a balance. Cell Host Microbe 3, 399–407 [DOI] [PubMed] [Google Scholar]
- 77. Sumimoto H. (2008) Structure, regulation, and evolution of Nox family NADPH oxidases that produce reactive oxygen species. FEBS J. 275, 3249–3277 [DOI] [PubMed] [Google Scholar]
- 78. Rosenzweig S. D. (2008) Inflammatory manifestations in chronic granulomatous disease (CGD). J. Clin. Immunol. 28, S67–S72 [DOI] [PubMed] [Google Scholar]
- 79. White C., Lee J., Kambe T., Fritsche K., Petris M. J. (2009) A role for the ATP7A copper-transporting ATPase in macrophage bactericidal activity. J. Biol. Chem. 284, 33949–33956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. White C., Kambe T., Fulcher Y. G., Sachdev S. W., Bush A. I., Fritsche K., Lee J., Quinn T. P., Petris M. J. (2009) Copper transport into the secretory pathway is regulated by oxygen in macrophages. J. Cell Sci. 122, 1315–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kreuder J., Otten A., Fuder H., Tümer Z., Tønnesen T., Horn N., Dralle D. (1993) Clinical and biochemical consequences of copper-histidine therapy in Menkes disease. Eur. J. Pediatr. 152, 828–832 [DOI] [PubMed] [Google Scholar]
- 82. Gunn T. R., Macfarlane S., Phillips L. I. (1984) Difficulties in the neonatal diagnosis of Menkes kinky hair syndrome–Trichopoliodystrophy. Clin. Pediatr. 23, 514–516 [DOI] [PubMed] [Google Scholar]
- 83. Uno H., Arya S. (1987) Neuronal and vascular disorders of the brain and spinal cord in Menkes kinky hair disease. Am. J. Med. Genet. Suppl. 3, 367–377 [DOI] [PubMed] [Google Scholar]
- 84. Agertt F., Crippa A. C., Lorenzoni P. J., Scola R. H., Bruck I., Paola L., Silvado C. E., Werneck L. C. (2007) Menkes disease: case report. Arq. Neuropsiquiatr. 65, 157–160 [DOI] [PubMed] [Google Scholar]
- 85. Wardman P., Candeias L. P. (1996) Fenton chemistry: an introduction. Radiat. Res. 145, 523–531 [PubMed] [Google Scholar]
- 86. Kadiiska M. B., Mason R. P. (2002) In vivo copper-mediated free radical production: an ESR spin-trapping study. Spectrochim. Acta A Mol. Biomol. Spectrosc. 58, 1227–1239 [DOI] [PubMed] [Google Scholar]