Background: The trans-Golgi network (TGN) has been implicated in G protein-coupled receptor (GPCR) recycling and proteasomal degradation of endocytosed receptors.
Results: Endocytosed β1AR employs the TGN as a checkpoint for recycling and lysosomal degradation.
Conclusion: The TGN is an important organelle for determining the endocytic fate of β1AR.
Significance: The TGN not only regulates surface expression during GPCR export but also determines the fate of endocytosed GPCRs.
Keywords: G Protein-coupled Receptors (GPCR), Golgi, Receptor Endocytosis, Receptor Recycling, Receptor Regulation, β-Adrenergic Receptor, Cardiac Hypertrophy, Endocytic Trafficking, Lysosomal Degradation
Abstract
Receptor down-modulation is the key mechanism by which G protein-coupled receptors (GPCRs) prevent excessive receptor signaling in response to agonist stimulation. Recently, the trans-Golgi network (TGN) has been implicated as a key checkpoint for receptor endocytosis and degradation. Here, we investigated the involvement of the TGN in down-modulation of β1-adrenergic receptor in response to persistent isoprotenerol stimulation. Immunofluorescent staining showed that ∼50% of endocytosed β1AR colocalized with TGN-46 at 5 h. Disruption of the TGN by brefeldin A (BFA) led to the robust accumulation of endocytosed β1AR in Rab11+ recycling endosomes, inhibited β1AR entry into LAMP1+ lysosomes, and as a result enhanced β1AR recycling to the plasma membrane. The lysosomotropic agent, chloroquine, arrested the majority of endocytosed β1AR in the TGN by 4 h. Immunoblot analysis showed that either disruption of the TGN or blockage of the lysosome prevented β1AR degradation. Co-expression of GFP-arrestin-3 in β1AR cells increased the endocytosis of β1AR and facilitated its entry to the TGN but inhibited recycling to the plasma membrane. Arrestin-3-induced inhibition of β1AR recycling was reversed by BFA treatment, whereas chloroquine induced the accumulation of arrestin-3 with β1AR in the TGN. These results demonstrate for the first time that the TGN acts as a checkpoint for both the recycling and down-regulation of β1AR and that arrestin-3 not only mediates β1AR endocytosis but also its recycling through the TGN.
Introduction
Upon activation, G protein-coupled receptors (GPCR)2 initiate a variety of signaling cascades, and subsequently undergo receptor desensitization to avoid excessive signaling. Desensitization is typically initiated by the binding of arrestins to the phosphorylated carboxyl terminus of GPCRs through the catalization of G protein-coupled receptor kinases, which in turn causes the uncoupling of GPCRs from their G proteins and subsequent recruitment of receptors into clathrin-coated pits or caveolar microdormains (1–3). In general, internalized GPCRs face two endocytic trafficking pathways: either GPCRs are targeted to lysosomes for degradation via Rab7+ late endosomes, which results in complete termination of receptor signaling (down-modulation). Alternatively, GPCRs either recycle to the cell surface and re-couple with their G-proteins directly via Rab4+ and/or Rab5+ early endosomes (the “short cycle”), or slowly recycle to the cell surface via Rab11+ recycling endosomes (the “long cycle”), which results in restoration of functional receptors and signal recovery (resensitization)(2, 4, 5).
Recently, we have reported that G protein-coupled estrogen receptor-1 (GPER-1) undergoes arrestin-independent endocytosis, traffics in a retrograde manner to the trans-Golgi network (TGN), and suffers degradation via an ubiquitin-proteasomal pathway (6). Interestingly, two other GPCRs, i.e. somatostatin type 2A receptor (7, 8) and CC chemokine receptor 5 (9) also have been reported to traffic to the TGN through the early endosome after endocytosis. However, these two receptors are neither directed to proteasomes nor lysosomes for degradation, but rather recycle back to the cell surface after trafficking to the TGN. Thus, the TGN acts as a regulatory checkpoint in the retrograde trafficking route for either degradation or recycling of endocytosed GPCRs, which provides a third pathway for the endocytic trafficking of GPCRs.
β-Adrenergic receptors are comprised of 3 subtypes: β1 (β1AR), β2 (β2AR), and β3 (β3AR), which mediate epinephrine and norepinephrine-promoted cell signaling involved in regulating synaptic plasticity, memory formation, and cardiac function (10–14). β1AR couples to Gs proteins, whereas β2AR and β3AR couple to both Gs and Gi proteins, which confer to the three subtypes distinct signaling and differential physiological and pathological activities. β1AR is the predominant subtype in cardiac tissue and also the major pathologic mediator in heart failure (14). Multiple pathological conditions in the heart augment sympathetic nerve activity, which leads to persistent stimulation of β1AR and subsequent sustained signaling. Prolonged β1AR signaling disturbs cardiac function and facilitates the development of congestive heart failure (10, 14–16). A growing body of evidence has demonstrated that endocytosis, recycling, and degradation of β1AR play critical roles in spatiotemporal regulation of β1AR signaling that safeguards against cardiac hypertrophy and the progression of heart failure (14, 17, 18). Upon agonist stimulation, β1AR undergoes both arrestin- and dynamin-dependent endocytosis via clathrin-coated pits (19) or caveolar microdomains (3) and then resensitization through recycling back to the cell surface (19). Endocytosed β1AR is resistant to down-modulation with measured decay rates lasting more than 4 h, although ultimately it is targeted to the lysosome for degradation with persistent agonist stimulation (20, 21). Nevertheless, as compared with β2AR, β1AR has been less investigated, and the mechanisms mediating the endocytic trafficking and down-modulation of β1AR have yet to be fully elucidated.
Here we have explored the potential role of the TGN in mediating the recycling and degradation of endocytosed β1AR. Our results show that endocytosed β1AR re-enters the TGN in HEK-293 cells, and that the TGN acts as a regulatory checkpoint for both recycling and degradation of endocytosed β1AR, as disruption of the TGN enhances β1AR recycling and inhibits its degradation. Additionally, transient expression of arrestin-3 increases β1AR endocytosis but inhibits β1AR recycling back to the cell surface via the TGN.
EXPERIMENTAL PROCEDURES
Cell Lines and Culture Conditions
Human embryonal kidney 293 cells (HEK-293) were purchased from the American Tissue Culture Collection (Manassas, VA). HEK-293 cells that stably express amino-terminal tagged, hemagglutinin-β1 adrenergic receptor (HA-β1AR) were previously described (22). All cells were grown at 37 °C in phenol red-free DMEM/Ham's F-12 media (Invitrogen) supplemented with 5% fetal bovine serum and 50 μg/ml of gentamicin.
Antibodies
Commercial antibodies included: rabbit anti-HA epitope monoclonal antibody (Cell Signaling, MA; 1:1000); mouse anti-LAMP1 (Santa Cruz Biotechnology; IgG1 at 1:40); FK2 anti-ubiquitin mAb (Millipore; 1:300); sheep anti-TGN-46 (AbD Serotec; 1:1000); mouse anti-Rab11 (1:200); rabbit anti-ubiquitin (Santa Cruz; 1:200); mouse anti-Rab-7 (Cell Signaling; 1:200); mouse anti-EEA-1 (1:500, Abcam); rabbit anti-calnexin (1:200, Cell Signaling); goat anti-rabbit Alexa Fluor 594 and anti-mouse Alexa Fluor 488 (Molecular Probes; 1:1000); donkey anti-sheep Alexa Fluor 647 and 555 (Molecular Probes; 1:1000); donkey anti-rabbit Alexa Fluor 555 and anti-mouse Alexa Fluor 488 (1:1000) and 647 (1:500) (Molecular Probes).
Plasmids
A molecular construct for GFP-arrestin-3 was kindly provided by Dr. Jeffrey L. Benovic (Thomas Jefferson University).
Endocytosis Analysis
HA-β1AR HEK-293 cells were seeded onto glass coverslips (0.3 × 106/35-mm dish) and washed with serum-free medium several times before use. In some instances, cells were transiently transfected with cDNAs encoding GFP-arrestin-3, using Lipofectamine 2000 (Invitrogen). Cells were chilled in cold medium and then incubated with rabbit anti-HA antibody or rabbit IgG alone in ice-cold serum-free media for 25 min. Cells were washed with cold serum-free media to remove excess antibody, and then treated in media prewarmed to 37 °C with vehicle or isoproterenol (ISO, 100 μm) and/or reagents (cycloheximide, 10–20 μg/ml; MG132, 10 μm; BFA, 5 μg/ml; chloroquine, 100 μm) and incubated at 37 °C for various times. Cells were fixed in 4% paraformaldehyde in PBS and then processed for immunofluorescence or ELISA analysis.
Immunofluorescene
Fixed cells were permeabilized in blocking buffer containing 0.1% Triton X-100, 3% BSA, and 3% normal goat or donkey serum in PBS (pH 7.4) or incubated in blocking buffer without Triton X-100 for 25 min at room temperature. Cells were then incubated for 1 h in primary antibodies diluted in blocking buffer. Unbound primary antibody was removed by washing in PBS. Cells were incubated at room temperature in secondary antibodies for 1 h, washed in PBS, and mounted in anti-quench mounting medium with DAPI (Vector Laboratories, Inc., Burlingame, CA). Immunofluorescent images were visualized with a Nikon Eclipse 80i microscope (Nikon, Inc., Melville, NY) equipped with a Nikon Plan Fluor 100X0.5–1.3 Oil Iris with differential interference contrast and epifluorescent capabilities using a QImaging Retiga 2000R digital camera and Nikon imaging software (NIS-Elements-BR 3.0), or with the Zeiss LSM 710 confocal laser scanning microscope with ZEN 2009 software (Zeiss). Captured images were processed with brightness/contrast adjustment using Photoshop CS2 (Adobe).
β1AR Recycling Assay
Surface HA-β1AR was prelabeled with rabbit HA mAb as described above. Cells were incubated at 37 °C with ISO and vehicle or BFA for the indicated time periods, and then chilled in ice-cold medium followed by several washes. To remove receptor-bound antibody at the plasma membrane, cells were incubated for 10 min with 0.1 m glycine-HCl (pH 3.0) on ice. After several washes with cold media, the cells were further incubated for 0.5 or 1 h at 37 ºC with warm medium containing vehicle or BFA alone, fixed, and processed for immunofluorescence or ELISA. To discriminate surface versus endocytosed β1AR, anti-rabbit Alexa 594 or -Alexa 488 antibodies were sequentially applied prior to permeabilization and post-permeabilization, respectively.
Quantitative Analysis of Colocalization and Measurement of the Pixel Intensity of Surface β1AR
Quantitative colocalization analysis was performed on raw data using a line scan in NIS-Elements-BR 3.0 software (Nikon, Japan). Line scan displays the plot of the pixel intensity in each channel versus the position along a straight line drawn across the image. A superimposition of the peaks of pixel intensity between channels indicates the extent of co-localization of two signals along the line. Line scan was also performed to measure the intensity of surface β1AR staining by showing the profile of pixel intensity of one channel along a line across the plasma membrane. Semiquantitation for percentage of endocytosed β1AR colocalized with organelle markers including TGN46, Rab11, and LAMP1 was performed by counting the number of endocytosed β1AR particles colocalized with various markers and total endocytosed β1AR particles.
ELISA
HA-β1AR cells were seeded onto poly-l-lysine-coated 6-well plates (1 × 106/well) and prelabeled with rabbit anti-HA antibody as described above. After removal of excess antibody, cells were incubated with ISO (100 μm) and vehicle or BFA for 4 h at 37 °C. Cells were fixed in 4% paraformaldehyde, incubated with or without 0.1% Triton X-100 in blocking buffer containing 3% BSA and 3% normal donkey serum in PBS for 30 min at room temperature, and then for 1 h with HRP-conjugated donkey anti-rabbit IgG (1:8000) diluted in 3% BSA in PBS at room temperature. After several rinses, cells were incubated with the HRP substrate 3,3′,5,5′-tetramethylbenzidine (Sigma) for 30 min. The reaction was stopped by the addition of hydrochloric acid and absorbance of the reaction product was measured at 450 nm. Nonspecific background was determined by omission of primary antibody and subtracted from raw scores.
Decay Rate Analysis
HA-β1AR cells were prelabeled with HA antibody as described above. Cells were then directly harvested (0 min) or incubated at 37 °C with cycloheximide (20 μg/ml) and vehicle or BFA (5 μg/ml) or chloroquine (100 μm) or MG132 (10 μm) for 5 h. Cells were harvested and solubilized in RIPA-buffered detergent supplemented with protease inhibitors as described previously (23). Insoluble proteins were clarified from detergent lysates by centrifugation at 12,000 × g for 20 min. Samples were prepared for electrophoresis by mixing 20–30 μg of detergent soluble protein in 2× reducing Laemmli sample buffer (Bio-Rad) for 10 min at room temperature prior to gel loading. The remainder of the lysate was incubated with protein A-coupled agarose beads overnight at 4 °C. Immunoabsorbed proteins were pelleted by centrifugation at 1,000 × g for 2 min and the supernatant was collected again for immunoenrichment of total receptor (below). Protein A beads containing surface-labeled receptors were washed 6 times with lysis buffer and then the captured proteins were eluted from the beads using 1× reducing Laemmli sample buffer for 10 min at room temperature.
Immunoblotting Analysis
Samples were resolved by 10% SDS-PAGE according to standard procedures (23). Equivalent amounts of total protein, as determined using the BCA protein assay, were resolved by electrophoresis, and proteins were transferred to nitrocellulose membrane. Membranes were blocked with 5% nonfat milk in TBS-T buffer for 30 min, and then the membranes were incubated overnight in primary antibody, diluted in 3 or 5% BSA in TBS-T buffer, at 4 °C. The membrane was washed three times with the same buffer and detected using HRP-conjugated donkey anti-rabbit IgG and enhanced chemiluminescence substrate (SuperSignal, Pierce).
RESULTS
Colocalization of Endocytosed β1AR with TGN-46
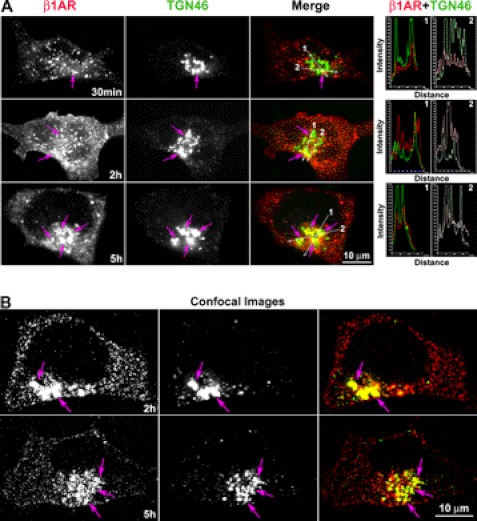
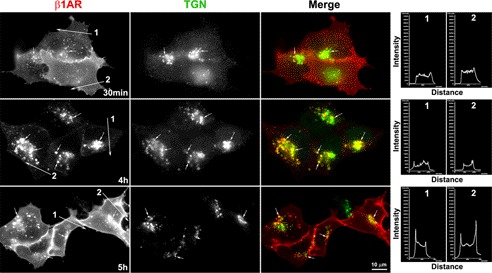
Prior studies demonstrate that β1AR undergoes prolonged down-regulation (>4 h) with lysosomal degradation in response to persistent ligand stimulation (6, 20, 21). To investigate whether endocytosed β1AR traffics to the TGN with persistent agonist stimulation, we employed the broadly applied procedure of monitoring endocytosed GPCRs by surface labeling with antibody (6). HEK-293 cells stably expressing amino-terminally tagged HA-β1AR (HA-β1AR cells) were prelabeled with HA antibody at 4 °C, incubated with ISO at 37 °C after removal of excess antibody, and then fixed at various time points. Cells were then permeabilized and immunostained with antibodies specific for TGN-46, a marker for the TGN. ISO treatment resulted in surface-labeled β1AR appearing in small- to medium-sized vesicles that redistributed throughout the cytoplasm from the cell surface by 30 min (Fig. 1A). Endocytosed β1AR tended to traffic toward, and be concentrated in, the perinuclear area by 1 h. By 5 h, most β1AR was accumulated in the perinuclear compartment (Fig. 1A). Although a few endocytosed β1AR colocalized with TGN-46 at early time points (e.g. 5–30 min), a small portion of the receptors (∼5%) colocalized with the TGN-46 at 1 h after agonist treatment. However, persistent agonist stimulation for 5 h caused approximately 50% of the endocytosed β1AR to colocalize with TGN-46 (Fig. 1A). Quantitative analysis of these epifluorescent images by measuring pixel intensity profiles showed precise coincidence of intensity peaks for β1AR and TGN-46 (Fig. 1A).
FIGURE 1.
Endocytosed β1AR colocalizes with the TGN over time course. HA-β1AR cells were prelabeled with HA antibody at 4 °C. After removal of excess antibody, cells were incubated with ISO (100 μm) at 37 °C and fixed at indicated time points. The cells were permeabilized and incubated with sheep anti-TGN antibody. Surface-labeled HA-β1AR and the TGN were visualized with donkey anti-rabbit Alexa 555 (red) and anti-sheep Alexa 488 (green) antibodies, respectively. Images were captured by epifluorescence (A) and confocal scanning microscopy (B). Colocalization of HA-β1AR with TGN46 (arrows) is shown in yellow (arrows) in the merged images. The right panels in A show the intensity profiles versus the position alone with two lines drawn across the merged images (arrows show the direction of scanning). The superposition of peaks for each channel indicates the degree of colocalization between the two channels. Nuclei were detected with DAPI (blue). Results are representative of 200 cells from 5 independent experiments.
To ensure that co-localization of HA-β1AR and TGN-46 is not due to overlap in the captured epifluorescent images, confocal scanning images were also captured at 0.8-μm thick optical sections (Fig. 1B). The results using confocal microscopy are consistent with those obtained by epifluorescence, and clearly show that β1AR concentrates in the TGN over time in cells that receive persistent isoprotenerol stimulation.
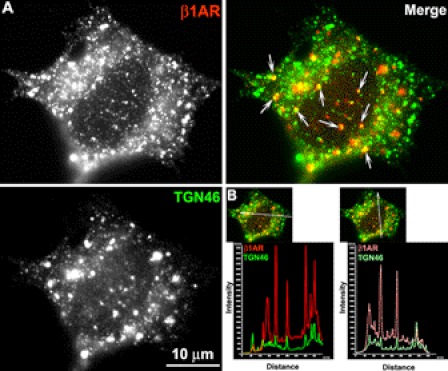
Given that many endosomal organelles are concentrated in the perinuclear compartment, the possibility that endocytosed β1AR might be colocalized with other endosomal organelles that overlap with TGN-46 was considered. To exclude this possibility, cells were treated with the microtubule disruptor, nocodazole, a strategy previously employed to disperse endosomes and Golgi membranes from the perinuclear compartment (9). Surface-labeled β1AR cells were treated with nocodazole at 37 °C for 1 h after a 3-h ligand stimulation. Co-immunostaining showed that nocodazole treatment resulted in diffuse distribution of TGN-46 throughout the cytoplasm (Fig. 2), which is in contrast to the compact distribution of TGN-46 in untreated cells (Fig. 1). Intensity profiles showed co-alignment of pixel peaks for β1AR and TGN-46 signals after nocodazole treatment (Fig. 2), demonstrating that β1AR and TGN-46 are coassociated.
FIGURE 2.
Co-distribution of β1AR with TGN46 after nocodazole treatment. HA-β1AR cells were prelabeled with rabbit HA mAb at 4 °C, incubated at 37 °C with ISO for 3 h and then for another 1 h with nocodazole (10 μm) and ISO. A, permeabilized cells were immunostained for TGN46 (green) and surface-labeled β1AR (red). Co-localization of HA-β1AR with TGN46 is shown in yellow (arrows) in the merged images. B, quantitative analysis of co-localization of HA-β1AR with TGN46 using a line scan. Results are representative of 52 cells from 2 independent experiments.
Endocytosed β1AR Returns to TGN through the Recycling Endosome
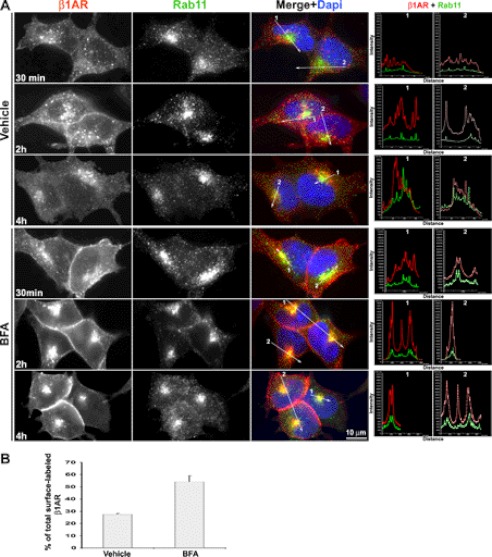
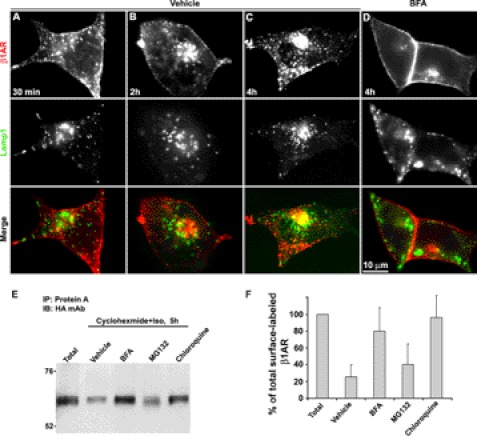
Prior studies have shown that endocytosed β1AR recycles back to the plasma membrane upon removal of agonist (19). Here, we hypothesized that the Rab11+ recycling endosomal compartment in the perinuclear area is the most likely site from which endocytosed β1AR traffics to the TGN as reported for GPER-1 (6). To test this idea, surface-labeled β1AR was followed over time after disruption of the TGN using BFA, and cells were co-immunostained for endocytosed β1AR and Rab11. Co-immunostaining showed that BFA treatment preferentially destroys the structure of TGN as the compact TGN-46 staining compartments was destroyed by BFA (supplemental Fig. S1). No apparent difference was observed in receptor internalization between BFA- and vehicle-treated cells prior to 30 min, suggesting that BFA does not influence β1AR endocytosis (supplemental Fig. S2). In vehicle-treated cells, a small portion of endocytosed β1AR colocalized with Rab11 between 30 min and 5 h. However, in BFA-treated cells most of the endocytosed β1AR accumulated in the perinuclear area and colocalized with Rab11, which became readily apparent at 2 and 4 h. A high degree of colocalization of β1AR with Rab11 in BFA-treated cells was also confirmed by quantitative colocalization analysis using line scan (Fig. 3A).
FIGURE 3.
Disruption of the TGN results in the accumulation of endocytosed HA-β1AR in the recycling endosome. A, HA-β1AR cells were prelabeled with rabbit HA antibody at 4 °C, incubated with ISO and vehicle or BFA (5 μg/ml) at 37 °C, and fixed at the indicated time points. Cells were then permeabilized and immunostained for HA-β1AR (red) and Rab11 (green). Co-localization of HA-β1AR with Rab11 was shown in yellow in the merged images. The right panels in A show the intensity profiles versus the position alone with two lines drawn across the merged images (arrows show the direction of scanning). Nuclei were detected with DAPI (blue). Results are representative of 98 cells in 3 independent experiments. B, ELISA showing the amount of surface-labeled β1AR remaining in BFA- (55.2 ± 4.9%) versus vehicle-treated (27.5 ± 0.67%) cells at 4 h. The data were presented as mean ± S.E. (n = 3).
To exclude the possibility that endocytosed β1AR might be colocalized in other endosomal organelles that overlap with Rab11 in the perinuclear area after BFA treatment, surface-labeled β1AR and markers for perinuclear endosomes and organelles, including EEA-1 (early endosome), or Rab7 (late endosome), or calnexin (endoplasmic reticulum), were co-immunostained. Epifluorescence and intensity profile analyses revealed that BFA treatment influenced neither the distribution patterns of all three markers nor caused the accumulation of β1AR in the late endosome or endoplasmic reticulum (supplemental Figs. S3–S5). In addition, BFA treatment did not influence the co-localization of endocytosed β1AR with EEA-1 (supplemental Fig. S3), which also supports the above observation that BFA does not impact β1AR endocytosis.
By comparison, a 4-h BFA treatment resulted in higher levels of both intracellular and surface β1AR than observed in control cells (Fig. 3A). ELISA revealed approximately twice as much surface-labeled β1AR remaining in BFA-treated cells as compared with control cells (Fig. 3B). Together, these data demonstrate that endocytosed β1AR preferentially accumulates in Rab11+ recycling endosomes after TGN uncoupling, and that TGN disruption prevents endocytosed β1AR from degradation.
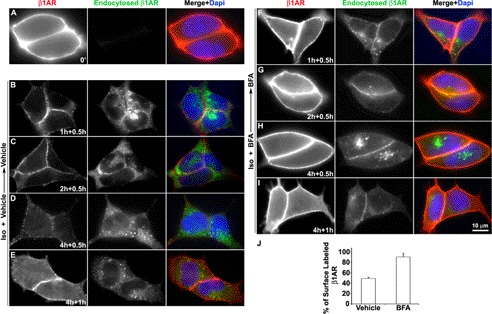
To further determine whether β1AR that accumulates in Rab11 endosomes recycles back to the plasma membrane after removal of the agonist, surface-labeled HA-β1AR cells were stimulated with agonist in the absence or presence of BFA for 4 h. After removal of receptor-bound antibodies at the cell surface by glycine acid washes, cells were further incubated at 37 °C with warm media containing vehicle or BFA alone for 0.5 h or 1 h. Surface recycled and endocytosed receptors were distinguished by sequentially applying goat anti-rabbit Alexa 594 antibodies prior to permeabilizaton and anti-rabbit Alexa 488 antibodies post-permeabilization, respectively. Negligible amounts of surface antibody-bound receptors were detected after glycine acid washing (data not shown). Prior to warming, immunostaining was observed only at the cell surface but not in the cytoplasm, indicating that dissociation of primary antibody and subsequent nonspecific binding in the cytoplasm did not occur during the staining procedure (Fig. 4A).
FIGURE 4.
Disruption of the TGN regulates the recycling of HA-β1AR to the plasma membrane after removal of isoprotenerol. Cells were prelabeled with rabbit HA mAb at 4 °C, and incubated at 37 °C with ISO and vehicle or BFA for 0, 1, 2, and 4 h. After agonist and surface receptor-bound antibody were removed, cells were further incubated with vehicle or BFA for 0.5 h (B–D and F–H) or 1 h (E and I). A–I, fixed cells were incubated with goat anti-rabbit Alexa 594 for visualizing total surface-labeled HA-β1AR (A, red) or recycled HA-β1AR (B–I, red) prior to permeabilization, and then incubated with goat anti-rabbit Alexa 488 for visualizing endocytosed HA-β1AR (green) post-permeabilization. Results are representatives from 3 independent experiments. J, ELISA showing the quantitative results of experiments similar to D and H.
No obvious difference in the recycling of endocytosed β1AR between vehicle- and BFA-treated cells was detected prior to 30 min (data not shown). However, a striking difference in β1AR recycling was observed by 1 h between vehicle- and BFA-treated cells (Fig. 4, B–I). At 4 h, the majority of β1AR was recycled back to the cell surface in BFA-treated cells 30 min after removal of agonist, as the tuft of endocytosed β1AR became smaller in the cytoplasm, and the intensity of surface β1AR became stronger (Fig. 4, H and I). In contrast, most of the β1AR remained in the cytoplasm in vehicle-treated cells with only few receptors recycling back to the cell surface during 30 min after agonist wash-out (Fig. 4C). At 4 h, more recycled receptors were observed after allowing receptor recycling for a longer time (from 0.5 to 1 h) (Fig. 4, D, E, H, and I). Quantitative analysis by ELISA revealed that at 4 h nearly twice as much β1AR was recycled to the cell surface in BFA-treated cells (90.4 ± 7.2%) as compared with control cells (48.8 ± 2.6%) during 0.5 h after removal of agonist (Fig. 4J). These results imply that β1AR, which accumulates after TGN disruption, is capable of recycling back to the cell surface from the recycling endosomes upon removal of agonist, which may also explain why high levels of surface β1AR are detected in BFA-treated cells at 4 h (Fig. 3A).
TGN Acts as Checkpoint for HA-β1AR Lysosomal Degradation
The above experiments show that disruption of the TGN results in the accumulation of endocytosed β1AR in the recycling endosome, suggesting a role for TGN in the down-modulation of endocytosed β1AR. To further address this issue, it was determined whether blockage of the lysosome arrests endocytosed β1AR in the lysosome or its upstream organelle, TGN, because prior studies have identified the lysosome as a major degradation organelle for β1AR (6, 20, 21). Surface-labeled HA-β1AR cells were treated with the lysosomotropic agent, chloroquine, in the presence of ISO for various lengths of time and cells were then immunostained for LAMP1 or TGN-46 (Fig. 5). Colocalization of endocytosed β1AR with LAMP1 was not observed, whereas the integrity of LAMP1-marked lysosome was destroyed by chloroquine (data not shown). In contrast, ∼30% of endocytosed β1AR colocalized with TGN-46 by 30 min and the majority of the receptors with TGN-46 at 4 h. Interestingly, by 5 h, only a small portion of β1AR colocalized with TGN-46, instead a large number of receptors appeared at the plasma membrane as confirmed by the intensity profile measurement, suggesting that long-term blockage of the lysosome forces accumulated receptors from the TGN to recycle back to the plasma membrane (Fig. 5). Next, it was examined whether disruption of the TGN inhibited endocytosed β1AR entry to the lysosome. Surface-labeled HA-β1AR cells were treated with ISO and BFA or vehicle for various lengths of time and immunostained for LAMP1. Co-immunostaining results showed that less than 5% of the surface-labeled β1AR colocalized with LAMP1 at 30 min, but receptor colocalization with LAMP1 was increased at later time points. In vehicle-treated control cells, by 4 h, about 30% of β1AR was observed to colocalize with LAMP1 (Fig. 6, A–C). In contrast, in BFA-treated cells, colocalization of β1AR with LAMP1 was not easily detected at 4 h with a large number of the receptors appearing at the plasma membrane, reflecting an increase in β1AR recycling after TGN disruption (Fig. 6D). Similar results were also observed when surface-labeled β1AR and Rab7-marked late endosomes were co-immunostained (supplemental Fig. S4).
FIGURE 5.
Lysosomal inhibition induces the accumulation of endocytosed β1AR in the TGN. Surface-labeled HA-β1AR cells were incubated with ISO and chloroquine, fixed at various time points, and immunostained for TGN-46. Surface-labeled HA-β1AR and TGN-46 were visualized with donkey anti-rabbit Alexa 594 (red) and anti-sheep Alexa 488 (green), respectively. Co-localization of HA-β1AR with TGN-46 (arrows) is shown in yellow in the merged images. Nuclei were detected with DAPI (blue). Note: whereas the majority of endocytosed HA-β1AR co-localizes with the TGN-46 at 4 h, less HA-β1AR co-localizes with the TGN-46 but more HA-β1AR appears at the plasma membrane at 5 h. The right panels show the pixel intensity profiles of red channels (β1AR staining) along the lines across the plasma membrane. Results are representative of 120 cells from 3 independent experiments.
FIGURE 6.
Brefeldin A treatment prevents HA-β1AR from entering the lysosome for degradation. Surface-labeled HA-β1AR cells were incubated with ISO and vehicle or BFA, chloroquine, or MG132 and fixed or harvested at the indicated time points. A–D, cells were immunostained for LAMP1, a specific marker of the lysosome. Surface-labeled HA-β1AR and LAMP1 were visualized with goat anti-rabbit Alexa 594 (red) and anti-mouse Alexa 488 (green), respectively. Colocalization of HA-β1AR with LAMP1 is shown in yellow in the merged image. E, Western blotting showing that treatment of BFA or chloroquine inhibits the degradation of HA-β1AR. The immunoprecipitates prepared by pull-down with protein A-agarose beads were subjected to 10% SDS-PAGE separation. Results are representatives from 3 independent experiments. F, quantitative analysis from 3 independent Western blots by measuring the band density with ImageJ (NIH) indicated that only 26.7 ± 14.2% of surface-labeled β1AR for vehicle treatment remained in contrast to 41.4 ± 24.1% for MG132 treatment, 80.3 ± 27.4% for BFA treatment, and 96.1 ± 25.5% for chloroquine treatment. The results shown represent the mean ± S.E. of 3 independent experiments.
Finally, we tested the hypothesis that disruption of the TGN might directly protect the degradation of endocytosed β1AR in the lysosome by immunoblot analysis (Fig. 6E). Surface-labeled HA-β1AR cells were treated for 5 h with ISO and vehicle, or BFA, chloroquine, or MG132 (a specific inhibitor of proteasome) under inhibition of protein synthesis using cyclohexmide. Probing the immunoprecipitates of surface-labeled receptor proteins prepared by pull-down with protein A beads revealed that after persistent agonist treatment for 5 h less than one-fifth of the surface-labeled β1AR remained. Treatment with chloroquine or BFA blocked the degradation of endocytosed receptor with greater than 95 and 80%, respectively, of endocytosed receptor measured relative to control. MG132 imparted only a slight protective effective on the degradation of endocytosed β1AR (Fig. 6F). Reprobing the same blots with FK2 anti-ubiquitin mAb showed negative results, suggesting that β1AR is not directly ubiquitinated (data not shown), which is in agreement with a prior finding (20). These results indicate that most of endocytosed β1AR is degraded in the lysosome and disruption of the TGN similarly blocks its degradation, which is consistent with the results from immunofluorescent and the ELISA shown in Fig. 3.
Arrestin-3 Co-trafficks with β1AR to TGN
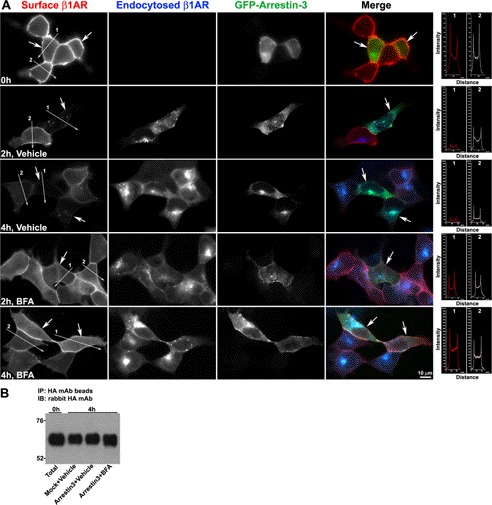
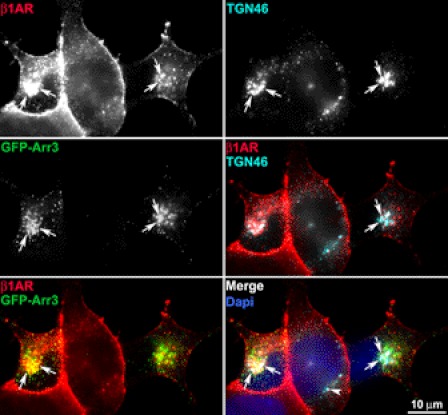
Arrestins play pivotal roles in endocytic trafficking and down-modulation of many GPCRs (reviewed in Refs. 1, 2, and 28). Prior studies have shown that arrestins are required for endocytosis of β1AR and are involved in the degradation of β2AR (19). To explore the role of arrestin in the sorting of endocytosed β1AR to the TGN and β1AR degradation, β1AR endocytosis was monitored in cells that were transiently transfected with GFP-arrestin-3 in the presence of persistent agonist stimulation. Although in unstimulated cells surface-labeled β1AR and arrestin-3 were uniformly distributed at the cell surface and throughout the cytoplasm, respectively, upon persistent ISO stimulation GFP-arrestin-3 exhibited a punctuate staining pattern throughout the cytoplasm with GFP-arrestin-3 concentrated in the perinuclear area (supplemental Fig. S6). This agonist-induced redistribution of arrestin was observed prior to β1AR endocytosis as early as 2 min after stimulation (supplemental Fig. S6). At 2 min, surface-labeled β1AR only colocalized with arrestin-3 at the cell surface, reflecting β1AR recruitment into clathrin-coated pits. By 30 min, most of the endocytosed β1AR colocalized with GFP-arrestin-3, although some GFP-arrestin-3 was clustered in the absence of receptor in the cytoplasm, which likely represents internalized unlabeled surface receptors (supplemental Fig. S6). Co-expression of GFP-arrestin-3 did not alter the surface expression of β1AR before agonist stimulation, as the surface levels of β1AR were similar between nontransfected versus transfected cells. However, GFP-arrestin-3 increased the disappearance of β1AR from the cell surface, especially at 2 h with ligand stimulation, suggesting that arrestin-3 overexpression increases the endocytosis of β1AR and/or inhibits β1AR recycling back to the cell surface (Fig. 7A). Intriguingly, disruption of TGN by BFA blocked the effect of arrestin-3 on the surface disappearance of β1AR, as no apparent difference in the levels of surface or endocytosed β1AR was observed between nontransfected and transfected cells between 1 and 5 h (Fig. 7A). Additionally, immunoblot analysis revealed that overexpression of arrestin-3 did not alter ligand-induced degradation of endocytosed β1AR despite the increase in β1AR endocytosis (Fig. 7B). To determine whether arrestin-3-induced β1AR endocytosis facilitates β1AR entry to the TGN, β1AR trafficking to the TGN was measured in arrestin-3 transfected cells. As shown in Fig. 8, forced expression of arrestin-3 slightly enhanced the colocalization of endocytosed HA-β1AR with TGN-46 at 30 min. Interestingly, a number of β1AR- and GFP-arrestin-3-labeled vesicles were easily observed to colocalize with TGN-46, suggesting that arrestin-3 co-traffics with β1AR to the TGN. To further validate this concept, the capacity of β1AR- or arrestin-3-labeled vesicles to accumulate in the TGN after blockage of the lysosome using chloroquine was examined. Indeed, triple labeling experiments indicated that chloroquine treatment resulted in the robust colocalization of both β1AR and arrestin-3 with TGN-46 (Fig. 9).
FIGURE 7.
GFP-arrestin-3 increases the endocytosis of β1AR, but does not facilitate the degradation of β1AR. HA-β1AR cells were mock transfected or transfected with cDNA encoding GFP-arrestin-3, surface-labeled with rabbit HA antibody, incubated at 37 ºC with ISO and vehicle or BFA and then fixed (A) or lysed for Western blotting (B) at 0, 2, and 4 h. A, surface-labeled (red) and endocytosed HA-β1AR (blue) were discriminated by incubation of the cells with goat anti-rabbit Alexa 594 prior to permeabilization and goat anti-rabbit Alexa 488 post-permeabilization, respectively. Arrows indicate GFP-arrestin-3-expressing cells (green). The right panels in A show the pixel intensity profiles of red channels (β1AR staining) along the lines across the plasma membrane. Lines 1 and 2 were drawn across the plasma membrane of arrestin-expressing cells and nontransfected cells, respectively. Results are representatives of 128 cells from 3 independent experiments. B, Western blotting (IB) for β1AR. Solublized samples were immunoprecipitated (IP) with mouse HA mAb-conjugated agarose beads, subjected to 10% SDS-PAGE separation, and then probed with rabbit HA mAb. Results are representative of 210 cells from 3 independent experiments.
FIGURE 8.
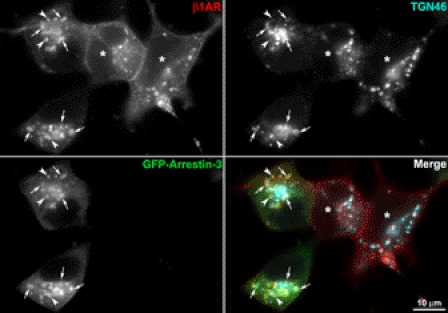
GFP-arrestin-3 has a modest effect on entry of endocytosed β1AR into the TGN. HA-β1AR cells were transfected with GFP-arrestin-3 (GFP-Arr3), surface-labeled with HA mAb, incubated at 37 ºC with ISO, and fixed at 30 min. Surface-labeled β1AR (red) and TGN-46 (cyan) were visualized with donkey anti-rabbit Alexa 555 and anti-sheep Alexa 647, respectively. Arrows indicate the colocalization of β1AR with GFP-Arr3 and TGN-46. Arrowheads indicates the nontransfected cell. Results are representatives of 146 cells from 3 independent experiments.
FIGURE 9.
Blockage of the lysosome leads to accumulation of arrestin-3 and β1AR in the TGN. HA-β1AR cells were transfected with cDNA encoding GFP-arrestin-3, surface-labeled with rabbit HA antibody, and then incubated at 37 °C with ISO and chloroquine for 4 h. Cells were immunostained for surface and endocytosed β1AR and TGN-46. Arrows point to colocalization of β1AR, arrestin-3, and TGN-46. Arrowheads point to colocalization of β1AR and arrestin-3. Asterisks indicate nontransfected cells.
Arrestin-3 Inhibits Recycling of Endocytosed β1AR via TGN
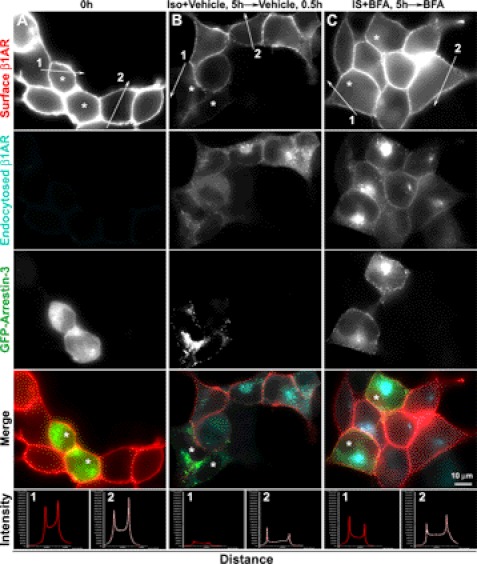
To further clarify whether arrestin-3 inhibits β1AR recycling back to the plasma membrane, the levels of recycled β1AR at the cell surface of arrestin-3-transfected versus mock transfected cells were monitored after removal of ISO at 4 h. Recycled and endocytosed β1AR were distinguished by sequential incubation of cells with Alexa 555 antibody prior to permeabilization and with Alexa 647 antibody post-permeabilization, respectively. With vehicle treatment, only a few β1AR are recycled back to the cell surface of arrestin-3-expressing cells as compared with nontransfected cells on the same coverslips. In contrast, BFA treatment restored the capability of β1AR recycling in arrestin-3-expressing cells to a similar extend to that in nontransfected cells on the same slides (Fig. 10). These data suggest a role for arrestin-3 in the inhibition of β1AR recycling back to the plasma membrane via the TGN after β1AR endocytosis.
FIGURE 10.
Brefeldin A blocks arrestin-induced inhibition of β1AR recycling. HA-β1AR cells were transfected with cDNA encoding GFP-arrestin-3 and surface-labeled with rabbit HA antibody. Cells were either fixed at 0 (A) or incubated at 37 °C with ISO and vehicle or BFA for 4 h and with vehicle or BFA alone for 30 min after removal of ISO, and then fixed (B and C). Recycled and endocytosed receptors were discriminated by goat anti-rabbit Alexa 555 (red) and goat anti-mouse Alexa 647 (cyan), respectively. The bottom panels show the pixel intensity profiles of red channels (β1AR staining) along the lines across the plasma membrane. Lines 1 and 2 were drawn across the plasma membrane of arrestin-expressing cells and nontransfected cells, respectively. Asterisks indicate the cells expressing GFP-arrestin-3. Results are representative of 187 cells from 3 independent experiments.
DISCUSSION
In this study, we report a novel endocytic trafficking route for β1AR, in which the TGN acts as a checkpoint for modulating both degradation and recycling of endocytosed β1AR. We further show that arrestin-3 regulates β1AR endocytosis and its recycling via the TGN.
To date, the molecular events regulating the endocytosis, recycling, and degradation of β1AR are not yet fully understood. Liang and colleagues (19–21) compared the endocytic trafficking and degradation between β1AR and β2AR during persistent ISO stimulation in a series of studies and showed that both subtypes undergo agonist-promoted endocytosis, which is dependent upon recruitment of both arrestin and dynamin into clathrin-coated pits. These studies showed that both subtypes traffic to distinct endosomal compartments at early time intervals following persistent agonist stimulation with β1AR concentrating in small, peripheral vesicles, and β2AR accumulating in the perinuclear area (19). In our study, we monitored agonist-promoted trafficking of β1AR over 5 h. We found a redistribution pattern of endocytosed β1AR that was similar to that reported by Liang et al. (19) at early time points. However, by 5 h endocytosed β1AR also accumulated in the perinuclear area, with 40–50% of the receptors colocalized with TGN-46, indicating that endocytosed β1AR returns to the TGN. This finding was further reinforced by the following evidence: (i) endocytosed β1AR still remained colocalized with TGN-46 after dispersing endosomal compartments in the perinuclear area; (ii) blockage of the lysosome induced the arrest of the bulk of endocytosed β1AR in the TGN; and (iii) co-expression of arrestin-3 facilitated β1AR entry to the TGN.
Immunostaining, ELISA, and immunoblotting experiments demonstrated that disruption of the TGN inhibited the entry of endocytosed β1AR into the late endosomes or lysosomes, and prevented β1AR degradation. These results indicate that endocytosed β1AR is mainly sorted to the lysosome for degradation via the TGN at late time points of agonist stimulation. Prior studies have demonstrated the resistance of β1AR to degradation with persistent agonist stimulation in several cell lines and identified the cytoplasmic tail of β1AR as the determinant for this resistance (21). Prolonged colocalization of β1AR with the TGN and lysosome observed in the present study may be associated with the mechanisms mediating prolonged degradation of β1AR. Interestingly, the Gs-coupled receptor, GPER-1, also utilizes the TGN as a checkpoint for degradation but undergoes rapid degradation in the proteasome (6) rather than the slow degradation times that we report here for β1AR in the lysosome. At present, it is unclear why there is a significant difference in the decay rate of endocytosed GPER-1 versus β1AR. However, GPER-1 internalization from clathrin-coated vesicles was not influenced by dominant-negative versions of arrestin-2 that lacked β-adaptin and/or clathrin binding sites (6). Together these data may suggest that fundamentally distinct mechanisms may employ the TGN as a common node for receptor sorting to distinct destinations for degradation. Additionally, it should be noted that previous studies and our current work failed to detect agonist-promoted ubiquitination of β1AR, suggesting the existence of chaperone proteins that may be associated with β1AR in response to agonist stimulation and subsequently envoy β1AR to the lysosome for degradation.
Further study showed that endocytosed β1AR return to the TGN through Rab11+ recycling endosomes, as evidenced by the findings that disruption of the TGN resulted in the perinuclear accumulation of endocytosed β1AR preferentially in Rab11+ recycling endosomes, but not in other organelles including the early and late endosomes, lysosomes, and endoplasmic reticulum. Analysis of recycled receptors revealed that β1AR that accumulated in the recycling endosomes due to TGN disruption was capable of returning to the cell surface. These results imply that TGN may also influence β1AR recycling by down-regulation. Prior work has reported that overexpression of Rab4 in HL-1 cardiac myocytes increases the recycling of β1AR back to the cell surface (17). Here, we speculated that overexpression of Rab4 may facilitate the trafficking of β1AR through Rab4-containing rapid recycling endosomes to Rab11-containing slow recycling endosomes, from which β1AR recycles back to the cell surface. This also supports a previous finding that β1AR undergoes a slower recycling time than β2AR (the latter via rapid recycling endosomes) (19). Interestingly, at 5 h after blockage of the lysosome, β1AR was largely observed to disassociate with TGN-46 and recycle back to the cell surface with persistent agonist stimulation (Fig. 5), which suggests that β1AR that accumulates in the TGN due to blockage of the lysosome alternatively recycles back to the cell surface through the TGN. This may, in part, explain why the endosomal recycling inhibitor, monesin, blocked β2AR recycling but not β1AR recycling as reported elsewhere (19).
Arrestins play multiple roles in mediating the endocytosis, recycling, ubiquitination, and degradation of GPCRs, and as well as G-protein-independent signaling (26–31). Here, we found that arrestin-3 overexpression increased β1AR endocytosis and subsequently facilitated the entry of β1AR with arrestin-3 to the TGN after agonist stimulation. However, consistent with prior work (21), immunoblot analysis revealed no difference in the rate of β1AR degradation between arrestin-3 and control cells. Intriguingly, in untreated cells, arrestin-3 overexpression resulted in very little recycling of β1AR to the cell surface relative to control cells. In contrast, disruption of the TGN blocked the arrestin-3-induced inhibition of β1AR recycling. These data suggest that arrestin-3 inhibits β1AR recycling through the TGN-associated mechanism. This finding is at odds with prior work, which has shown that arrestin-2 and -3 increase the recycling of the formyl peptide receptor (26, 27), and may suggest that arrestins regulate the recycling of GPCRs in a receptor-specific fashion.
Studies by others have shown that CC chemokine receptor 5 (9) and somatostatin type 2A (7, 8) receptor also return to the TGN from the cell surface and recycle back to the cell surface via the TGN rather than undergoing proteasomal destruction. It is noteworthy that endocytosed CC chemokine receptor 5 was initially thought to accumulate in the perinuclear region (9), but the TGN was identified later as the perinuclear organelle where CC chemokine receptor 5 retrotranslocated (9). Interestingly, other GPCRs such as β2AR (19), CXCR2 (C-X-C chemokine receptor type 2 (24), somatostatin receptor 3 (25), N-formyl peptide receptor (26), and vasopressin 2A receptor (5) also accumulate in the perinuclear area after endocytosis. Thus, it is possible that these receptors might also return to the TGN after desensitization.
Taken together, retrotranslocation of GPCRs via the trans-Golgi network plays a previously unappreciated role in receptor down-modulation and/or recycling. Our data presented here provides a broader appreciation of the role of the TGN in GPCR trafficking and shows that β1AR also employs the TGN as a regulatory checkpoint for mediating its lysosomal down-modulation and as a result, influencing its recycling. Thus, the TGN not only regulates surface expression of GPCRs during exocytosis but also plays a role in determining their endocytic fate. Dysfunctional regulation of endocytosis, recycling, and degradation of β1AR is associated with cardiac hypertrophy and heart failure (14, 17, 18). The existence of a novel endocytic pathway through the TGN for β1AR may provide alternative targets for developing new strategies to treat β1AR-related disorders such as cardiac hypertrophy.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grant R01 CA119165-01A2 (to E. J. F.).

This article contains supplemental Figs. S1–S6.
- GPCRs
- G protein-coupled receptors
- GPER-1
- G protein-coupled estrogen receptors
- TGN
- trans-Golgi network
- β1AR
- β1-adrenergic receptor
- β2AR
- β2-adrenergic receptor
- LAMP1
- lysosomal-associated membrane protein 1
- BFA
- brefeldin A
- ISO
- isoproterenol.
REFERENCES
- 1. Marchese A., Chen C., Kim Y. M., Benovic J. L. (2003) The ins and outs of G protein-coupled receptor trafficking. Trends Biochem. Sci. 28, 369–376 [DOI] [PubMed] [Google Scholar]
- 2. Marchese A., Paing M. M., Temple B. R., Trejo J. (2008) G protein-coupled receptor sorting to endosomes and lysosomes. Annu. Rev. Pharmacol. Toxicol. 48, 601–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rapacciuolo A., Suvarna S., Barki-Harrington L., Luttrell L. M., Cong M., Lefkowitz R. J., Rockman H. A. (2003) Protein kinase A and G protein-coupled receptor kinase phosphorylation mediates β1-adrenergic receptor endocytosis through different pathways. J. Biol. Chem. 278, 35403–35411 [DOI] [PubMed] [Google Scholar]
- 4. Jean-Alphonse F., Hanyaloglu A. C. (2011) Regulation of GPCR signal networks via membrane trafficking. Mol. Cell. Endocrinol. 331, 205–214 [DOI] [PubMed] [Google Scholar]
- 5. Innamorati G., Le Gouill C., Balamotis M., Birnbaumer M. (2001) The long and the short cycle. Alternative intracellular routes for trafficking of G-protein-coupled receptors. J. Biol. Chem. 276, 13096–13103 [DOI] [PubMed] [Google Scholar]
- 6. Cheng S. B., Quinn J. A., Graeber C. T., Filardo E. J. (2011) Down-modulation of the G-protein-coupled estrogen receptor, GPER, from the cell surface occurs via a trans-Golgi-proteasome pathway. J. Biol. Chem. 286, 22441–22455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lelouvier B., Tamagno G., Kaindl A. M., Roland A., Lelievre V., Le Verche V., Loudes C., Gressens P., Faivre-Baumann A., Lenkei Z., Dournaud P. (2008) Dynamics of somatostatin type 2A receptor cargoes in living hippocampal neurons. J. Neurosci. 28, 4336–4349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Csaba Z., Lelouvier B., Viollet C., El Ghouzzi V., Toyama K., Videau C., Bernard V., Dournaud P. (2007) Activated somatostatin type 2 receptors traffic in vivo in central neurons from dendrites to the trans-Golgi before recycling. Traffic 8, 820–834 [DOI] [PubMed] [Google Scholar]
- 9. Escola J. M., Kuenzi G., Gaertner H., Foti M., Hartley O. (2010) CC chemokine receptor 5 (CCR5) desensitization. Cycling receptors accumulate in the trans-Golgi network. J. Biol. Chem. 285, 41772–41780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Port J. D., Bristow M. R. (2001) Altered β-adrenergic receptor gene regulation and signaling in chronic heart failure. J. Mol. Cell Cardiol. 33, 887–905 [DOI] [PubMed] [Google Scholar]
- 11. Winder D. G., Martin K. C., Muzzio I. A., Rohrer D., Chruscinski A., Kobilka B., Kandel E. R. (1999) ERK plays a regulatory role in induction of LTP by θ frequency stimulation and its modulation by β-adrenergic receptors. Neuron 24, 715–726 [DOI] [PubMed] [Google Scholar]
- 12. Cahill L., Prins B., Weber M., McGaugh J. L. (1994) β-Adrenergic activation and memory for emotional events. Nature 371, 702–704 [DOI] [PubMed] [Google Scholar]
- 13. Rohrer D. K., Desai K. H., Jasper J. R., Stevens M. E., Regula D. P., Jr., Barsh G. S., Bernstein D., Kobilka B. K. (1996) Targeted disruption of the mouse β1-adrenergic receptor gene. Developmental and cardiovascular effects. Proc. Natl. Acad. Sci. U.S.A. 93, 7375–7380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lohse M. J., Engelhardt S., Eschenhagen T. (2003) What is the role of β-adrenergic signaling in heart failure? Circ. Res. 93, 896–906 [DOI] [PubMed] [Google Scholar]
- 15. Limbird L. E., Vaughan D. E. (1999) Augmenting β receptors in the heart. Short-term gains offset by long-term pains? Proc. Natl. Acad. Sci. U.S.A. 96, 7125–7127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vatner S. F., Vatner D. E., Homcy C. J. (2000) β-Adrenergic receptor signaling. An acute compensatory adjustment-inappropriate for the chronic stress of heart failure? Insights from Gαs overexpression and other genetically engineered animal models. Circ. Res. 86, 502–506 [DOI] [PubMed] [Google Scholar]
- 17. Filipeanu C. M., Zhou F., Lam M. L., Kerut K. E., Claycomb W. C., Wu G. (2006) Enhancement of the recycling and activation of β-adrenergic receptor by Rab4 GTPase in cardiac myocytes. J. Biol. Chem. 281, 11097–11103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morisco C., Marrone C., Galeotti J., Shao D., Vatner D. E., Vatner S. F., Sadoshima J. (2008) Endocytosis machinery is required for β1-adrenergic receptor-induced hypertrophy in neonatal rat cardiac myocytes. Cardiovasc. Res. 78, 36–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liang W., Curran P. K., Hoang Q., Moreland R. T., Fishman P. H. (2004) Differences in endosomal targeting of human β1- and β2-adrenergic receptors following clathrin-mediated endocytosis. J. Cell Sci. 117, 723–734 [DOI] [PubMed] [Google Scholar]
- 20. Liang W., Fishman P. H. (2004) Resistance of the human β1-adrenergic receptor to agonist-induced ubiquitination. A mechanism for impaired receptor degradation. J. Biol. Chem. 279, 46882–46889 [DOI] [PubMed] [Google Scholar]
- 21. Liang W., Austin S., Hoang Q., Fishman P. H. (2003) Resistance of the human β1-adrenergic receptor to agonist-mediated down-regulation. Role of the C terminus in determining β-subtype degradation. J. Biol. Chem. 278, 39773–39781 [DOI] [PubMed] [Google Scholar]
- 22. Filardo E., Quinn J., Pang Y., Graeber C., Shaw S., Dong J., Thomas P. (2007) Activation of the novel estrogen receptor G protein-coupled receptor 30 (GPR30) at the plasma membrane. Endocrinology 148, 3236–3245 [DOI] [PubMed] [Google Scholar]
- 23. Quinn J. A., Graeber C. T., Frackelton A. R., Jr., Kim M., Schwarzbauer J. E., Filardo E. J. (2009) Coordinate regulation of estrogen-mediated fibronectin matrix assembly and epidermal growth factor receptor transactivation by the G protein-coupled receptor, GPR30. Mol. Endocrinol. 23, 1052–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fan G. H., Lapierre L. A., Goldenring J. R., Richmond A. (2003) Differential regulation of CXCR2 trafficking by Rab GTPases. Blood 101, 2115–2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kreuzer O. J., Krisch B., Déry O., Bunnett N. W., Meyerhof W. (2001) Agonist-mediated endocytosis of rat somatostatin receptor subtype 3 involves β-arrestin and clathrin-coated vesicles. J. Neuroendocrinol. 13, 279–287 [DOI] [PubMed] [Google Scholar]
- 26. Wagener B. M., Marjon N. A., Revankar C. M., Prossnitz E. R. (2009) Adaptor protein-2 interaction with arrestin regulates GPCR recycling and apoptosis. Traffic 10, 1286–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vines C. M., Revankar C. M., Maestas D. C., LaRusch L. L., Cimino D. F., Kohout T. A., Lefkowitz R. J., Prossnitz E. R. (2003) N-Formyl peptide receptors internalize but do not recycle in the absence of arrestins. J. Biol. Chem. 278, 41581–41584 [DOI] [PubMed] [Google Scholar]
- 28. Shenoy S. K., Lefkowitz R. (2011) β-Arrestin-mediated receptor trafficking and signal transduction. Trends Pharmacol. Sci. 32, 521–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Luttrell L. M., Roudabush F. L., Choy E. W., Miller W. E., Field M. E., Pierce K. L., Lefkowitz R. J. (2001) Activation and targeting of extracellular signal-regulated kinases by β-arrestin scaffolds. Proc. Natl. Acad. Sci. U.S.A. 98, 2449–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Imamura T., Huang J., Dalle S., Ugi S., Usui I., Luttrell L. M., Miller W. E., Lefkowitz R. J., Olefsky J. M. (2001) β-Arrestin-mediated recruitment of the Src family kinase Yes mediates endothelin-1-stimulated glucose transport. J. Biol. Chem. 276, 43663–43667 [DOI] [PubMed] [Google Scholar]
- 31. Oakley R. H., Laporte S. A., Holt J. A., Barak L. S., Caron M. G. (2001) Molecular determinants underlying the formation of stable intracellular G protein-coupled receptor-β-arrestin complexes after receptor endocytosis. J. Biol. Chem. 276, 19452–19460 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.