Abstract
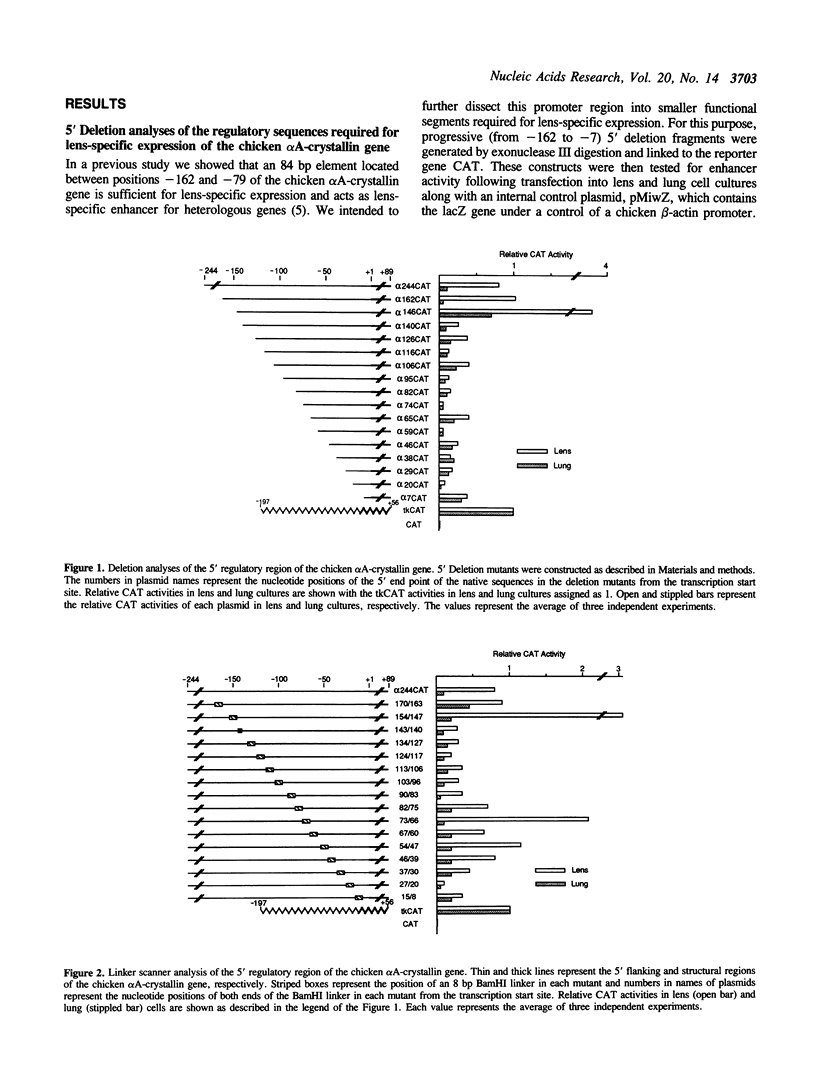
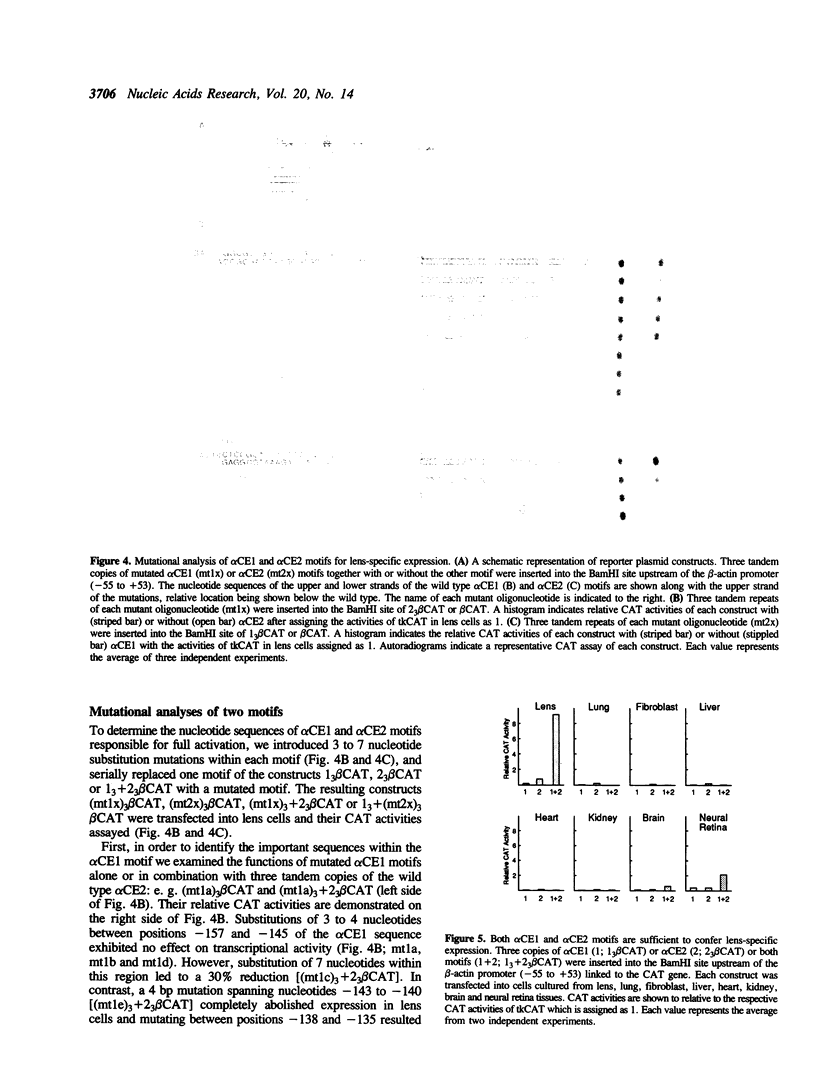
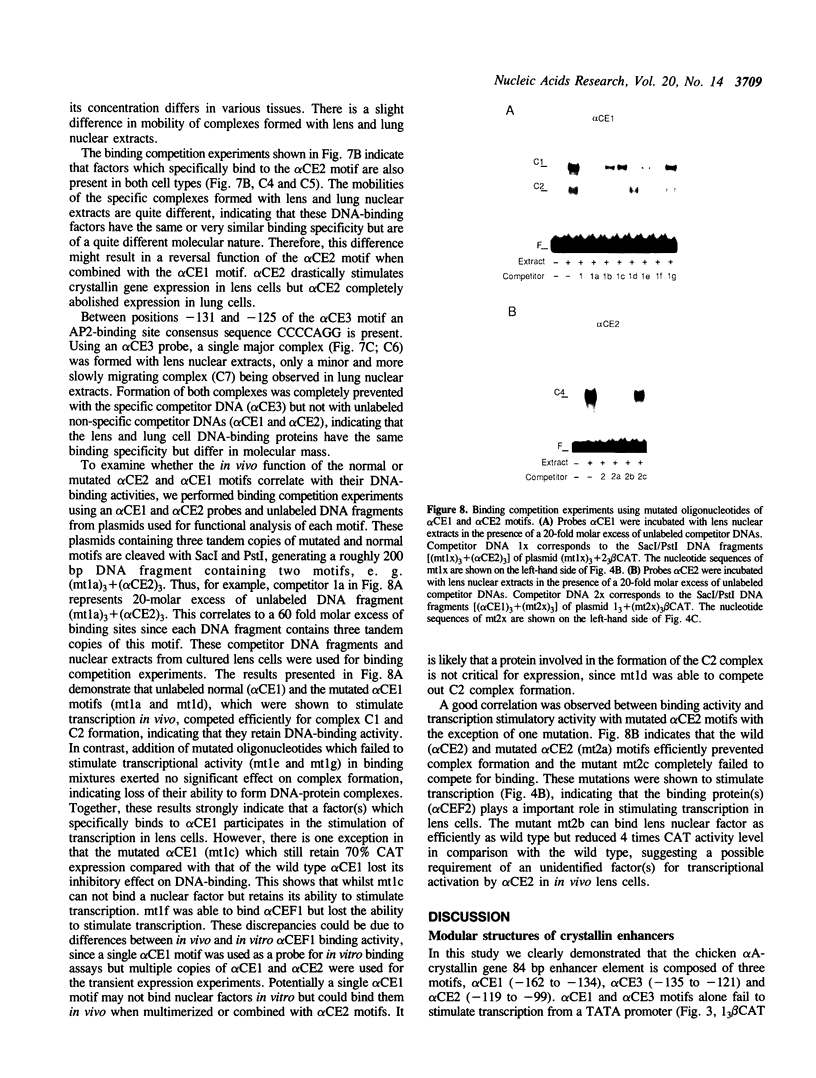
An 84 bp element located between nucleotides -162 and -79 of the chicken alpha A-crystallin gene exhibits lens-specific enhancer activity. Transient transfection experiments using 5' deletion and linker scanner mutants has indicated that the 84 bp enhancer element is composed of three motifs, alpha CE1 (-162 to -134), alpha CE3 (-135 to -121) and alpha CE2 (-119 to -99). Neither alpha CE1 or alpha CE3 motif alone can exhibit enhancer activity even when trimerized, whereas together they can direct some degree of lens-specific expression. alpha CE2 alone shows low transcriptional activity when trimerized. A combination of alpha CE1 with alpha CE2 exerts full lens-specific enhancer activity comparable with that of the 84 bp enhancer element, indicating that alpha CE1 and alpha CE2 motifs are sufficient to confer lens-specific expression. Transcriptional activation by these two motifs from a distance required the additional presence of either or both motifs adjacent to the beta-actin basal promoter. Gel shift experiments indicated that the alpha CE1, alpha CE2 and alpha CE3 motifs specifically bind nuclear proteins. alpha CE1 binds a protein predominantly present in lens cells, whereas alpha CE2- and alpha CE3-binding proteins differ between lens and lung cells. Mutations within the alpha CE1 and alpha CE2 motifs that failed to bind nuclear factors in vitro resulted in loss of transcriptional activation, indicating that these nuclear factors play a key role in controlling lens-specific expression.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chepelinsky A. B., Sommer B., Piatigorsky J. Interaction between two different regulatory elements activates the murine alpha A-crystallin gene promoter in explanted lens epithelia. Mol Cell Biol. 1987 May;7(5):1807–1814. doi: 10.1128/mcb.7.5.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin R. A., Wawrousek E. F., Piatigorsky J. Expression of the murine alpha B-crystallin gene is not restricted to the lens. Mol Cell Biol. 1989 Mar;9(3):1083–1091. doi: 10.1128/mcb.9.3.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dynan W. S. Modularity in promoters and enhancers. Cell. 1989 Jul 14;58(1):1–4. doi: 10.1016/0092-8674(89)90393-0. [DOI] [PubMed] [Google Scholar]
- Edlund T., Walker M. D., Barr P. J., Rutter W. J. Cell-specific expression of the rat insulin gene: evidence for role of two distinct 5' flanking elements. Science. 1985 Nov 22;230(4728):912–916. doi: 10.1126/science.3904002. [DOI] [PubMed] [Google Scholar]
- Forsberg M., Westin G. Enhancer activation by a single type of transcription factor shows cell type dependence. EMBO J. 1991 Sep;10(9):2543–2551. doi: 10.1002/j.1460-2075.1991.tb07794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromental C., Kanno M., Nomiyama H., Chambon P. Cooperativity and hierarchical levels of functional organization in the SV40 enhancer. Cell. 1988 Sep 23;54(7):943–953. doi: 10.1016/0092-8674(88)90109-2. [DOI] [PubMed] [Google Scholar]
- Funahashi J., Kamachi Y., Goto K., Kondoh H. Identification of nuclear factor delta EF1 and its binding site essential for lens-specific activity of the delta 1-crystallin enhancer. Nucleic Acids Res. 1991 Jul 11;19(13):3543–3547. doi: 10.1093/nar/19.13.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto K., Okada T. S., Kondoh H. Functional cooperation of lens-specific and nonspecific elements in the delta 1-crystallin enhancer. Mol Cell Biol. 1990 Mar;10(3):958–964. doi: 10.1128/mcb.10.3.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S., Kondoh H., Yasuda K., Soma G., Ikawa Y., Okada T. S. Tissue-specific regulation of a chicken delta-crystallin gene in mouse cells: involvement of the 5' end region. EMBO J. 1985 Sep;4(9):2201–2207. doi: 10.1002/j.1460-2075.1985.tb03915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr W., Clarke J. The SV40 enhancer is composed of multiple functional elements that can compensate for one another. Cell. 1986 May 9;45(3):461–470. doi: 10.1016/0092-8674(86)90332-6. [DOI] [PubMed] [Google Scholar]
- Itoh Y., Eguchi G. In vitro analysis of cellular metaplasia from pigmented epithelial cells to lens phenotypes: a unique model system for studying cellular and molecular mechanisms of "transdifferentiation". Dev Biol. 1986 Jun;115(2):353–362. doi: 10.1016/0012-1606(86)90255-1. [DOI] [PubMed] [Google Scholar]
- Johnson P. F., McKnight S. L. Eukaryotic transcriptional regulatory proteins. Annu Rev Biochem. 1989;58:799–839. doi: 10.1146/annurev.bi.58.070189.004055. [DOI] [PubMed] [Google Scholar]
- Klement J. F., Wawrousek E. F., Piatigorsky J. Tissue-specific expression of the chicken alpha A-crystallin gene in cultured lens epithelia and transgenic mice. J Biol Chem. 1989 Nov 25;264(33):19837–19844. [PubMed] [Google Scholar]
- Kondoh H., Katoh K., Takahashi Y., Fujisawa H., Yokoyama M., Kimura S., Katsuki M., Saito M., Nomura T., Hiramoto Y. Specific expression of the chicken delta-crystallin gene in the lens and the pyramidal neurons of the piriform cortex in transgenic mice. Dev Biol. 1987 Mar;120(1):177–185. doi: 10.1016/0012-1606(87)90116-3. [DOI] [PubMed] [Google Scholar]
- Kondoh H., Ueda Y., Hayashi S., Okazaki K., Yasuda K., Okada T. S. An attempt to assay the state of determination by using transfected genes as probes in transdifferentiation of neural retina into lens. Cell Differ. 1987 Mar;20(2-3):203–207. doi: 10.1016/0045-6039(87)90435-0. [DOI] [PubMed] [Google Scholar]
- Liu Q. R., Tini M., Tsui L. C., Breitman M. L. Interaction of a lens cell transcription factor with the proximal domain of the mouse gamma F-crystallin promoter. Mol Cell Biol. 1991 Mar;11(3):1531–1537. doi: 10.1128/mcb.11.3.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok S., Stevens W., Breitman M. L., Tsui L. C. Multiple regulatory elements of the murine gamma 2-crystallin promoter. Nucleic Acids Res. 1989 May 11;17(9):3563–3582. doi: 10.1093/nar/17.9.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Goodbourn S., Fischer J. A. Regulation of inducible and tissue-specific gene expression. Science. 1987 Jun 5;236(4806):1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
- Matsuo I., Kitamura M., Okazaki K., Yasuda K. Binding of a factor to an enhancer element responsible for the tissue-specific expression of the chicken alpha A-crystallin gene. Development. 1991 Oct;113(2):539–550. doi: 10.1242/dev.113.2.539. [DOI] [PubMed] [Google Scholar]
- Matsuo I., Takeuchi M., Yasuda K. Identification of the contact sites of a factor that interacts with motif I (alpha CE1) of the chicken alpha A-crystallin lens-specific enhancer. Biochem Biophys Res Commun. 1992 Apr 15;184(1):24–30. doi: 10.1016/0006-291x(92)91152-g. [DOI] [PubMed] [Google Scholar]
- Mitchell P. J., Tjian R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science. 1989 Jul 28;245(4916):371–378. doi: 10.1126/science.2667136. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Donovan D. M., Hamada K., Sax C. M., Norman B., Flanagan J. R., Ozato K., Westphal H., Piatigorsky J. Regulation of the mouse alpha A-crystallin gene: isolation of a cDNA encoding a protein that binds to a cis sequence motif shared with the major histocompatibility complex class I gene and other genes. Mol Cell Biol. 1990 Jul;10(7):3700–3708. doi: 10.1128/mcb.10.7.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki K., Yasuda K., Kondoh H., Okada T. S. DNA sequences responsible for tissue-specific expression of a chicken alpha-crystallin gene in mouse lens cells. EMBO J. 1985 Oct;4(10):2589–2595. doi: 10.1002/j.1460-2075.1985.tb03975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondek B., Gloss L., Herr W. The SV40 enhancer contains two distinct levels of organization. Nature. 1988 May 5;333(6168):40–45. doi: 10.1038/333040a0. [DOI] [PubMed] [Google Scholar]
- Roth H. J., Das G. C., Piatigorsky J. Chicken beta B1-crystallin gene expression: presence of conserved functional polyomavirus enhancer-like and octamer binding-like promoter elements found in non-lens genes. Mol Cell Biol. 1991 Mar;11(3):1488–1499. doi: 10.1128/mcb.11.3.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartorelli V., Webster K. A., Kedes L. Muscle-specific expression of the cardiac alpha-actin gene requires MyoD1, CArG-box binding factor, and Sp1. Genes Dev. 1990 Oct;4(10):1811–1822. doi: 10.1101/gad.4.10.1811. [DOI] [PubMed] [Google Scholar]
- Schatt M. D., Rusconi S., Schaffner W. A single DNA-binding transcription factor is sufficient for activation from a distant enhancer and/or from a promoter position. EMBO J. 1990 Feb;9(2):481–487. doi: 10.1002/j.1460-2075.1990.tb08134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber E., Matthias P., Müller M. M., Schaffner W. Rapid detection of octamer binding proteins with 'mini-extracts', prepared from a small number of cells. Nucleic Acids Res. 1989 Aug 11;17(15):6419–6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi H., Shimura Y. A rapid and efficient method for targeted random mutagenesis. Gene. 1988 Apr 29;64(2):313–319. doi: 10.1016/0378-1119(88)90346-0. [DOI] [PubMed] [Google Scholar]
- Smale S. T., Baltimore D. The "initiator" as a transcription control element. Cell. 1989 Apr 7;57(1):103–113. doi: 10.1016/0092-8674(89)90176-1. [DOI] [PubMed] [Google Scholar]
- Suemori H., Kadodawa Y., Goto K., Araki I., Kondoh H., Nakatsuji N. A mouse embryonic stem cell line showing pluripotency of differentiation in early embryos and ubiquitous beta-galactosidase expression. Cell Differ Dev. 1990 Mar;29(3):181–186. doi: 10.1016/0922-3371(90)90120-l. [DOI] [PubMed] [Google Scholar]
- Thompson M. A., Hawkins J. W., Piatigorsky J. Complete nucleotide sequence of the chicken alpha A-crystallin gene and its 5' flanking region. Gene. 1987;56(2-3):173–184. doi: 10.1016/0378-1119(87)90135-1. [DOI] [PubMed] [Google Scholar]
- Tronche F., Rollier A., Sourdive D., Cereghini S., Yaniv M. NFY or a related CCAAT binding factor can be replaced by other transcriptional activators for co-operation with HNF1 in driving the rat albumin promoter in vivo. J Mol Biol. 1991 Nov 5;222(1):31–43. doi: 10.1016/0022-2836(91)90735-o. [DOI] [PubMed] [Google Scholar]
- Wang W. D., Gralla J. D. Differential ability of proximal and remote element pairs to cooperate in activating RNA polymerase II transcription. Mol Cell Biol. 1991 Sep;11(9):4561–4571. doi: 10.1128/mcb.11.9.4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wawrousek E. F., Chepelinsky A. B., McDermott J. B., Piatigorsky J. Regulation of the murine alpha A-crystallin promoter in transgenic mice. Dev Biol. 1990 Jan;137(1):68–76. doi: 10.1016/0012-1606(90)90008-7. [DOI] [PubMed] [Google Scholar]
- Wingender E. Compilation of transcription regulating proteins. Nucleic Acids Res. 1988 Mar 25;16(5):1879–1902. doi: 10.1093/nar/16.5.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda K., Okada T. S. Structure and expression of the chicken crystallin genes. Oxf Surv Eukaryot Genes. 1986;3:183–209. [PubMed] [Google Scholar]
- Yasuda K. Transdifferentiation of "lentoid" structures in cultures derived from pigmented epithelium was inhibited by collagen. Dev Biol. 1979 Feb;68(2):618–623. doi: 10.1016/0012-1606(79)90231-8. [DOI] [PubMed] [Google Scholar]
- Yu C. C., Tsui L. C., Breitman M. L. Homologous and heterologous enhancers modulate spatial expression but not cell-type specificity of the murine gamma F-crystallin promoter. Development. 1990 Sep;110(1):131–139. doi: 10.1242/dev.110.1.131. [DOI] [PubMed] [Google Scholar]
- Zhou D. X., Yen T. S. The ubiquitous transcription factor Oct-1 and the liver-specific factor HNF-1 are both required to activate transcription of a hepatitis B virus promoter. Mol Cell Biol. 1991 Mar;11(3):1353–1359. doi: 10.1128/mcb.11.3.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]






