Background: The microtubule-associated proteins tau and MAP2 are dephosphorylated by PP2A, a major brain Ser/Thr phosphatase.
Results: Identification of a common PP2A-binding motif in tau and MAP2.
Conclusion: Soluble tau and MAP2 can compete for binding to and dephosphorylation by PP2A/Bα.
Significance: Tau and MAP2 regulate neuronal plasticity and deregulation of tau is a hallmark of tauopathies.
Keywords: Alzheimer Disease, Gel Electrophoresis, MAPs, PP2A, Tau, Fyn, MAP2
Abstract
The predominant brain microtubule-associated proteins MAP2 and tau play a critical role in microtubule cytoskeletal organization and function. We have previously reported that PP2A/Bα, a major protein phosphatase 2A (PP2A) holoenzyme, binds to and dephosphorylates tau, and regulates microtubule stability. Here, we provide evidence that MAP2 co-purifies with and is dephosphorylated by endogenous PP2A/Bα in bovine gray matter. It co-localizes with PP2A/Bα in immature and mature human neuronal cell bodies. PP2A co-immunoprecipitates with and directly interacts with MAP2. Using in vitro binding assays, we show that PP2A/Bα binds to MAP2c isoforms through a region encompassing the microtubule-binding domain and upstream proline-rich region. Tau and MAP2 compete for binding to and dephosphorylation by PP2A/Bα. Remarkably, the protein-tyrosine kinase Fyn, which binds to the proline-rich RTPPKSP motif conserved in both MAP2 and tau, inhibits the interaction of PP2A/Bα with either tau or MAP2c. The corresponding synthetic RTPPKSP peptide, but not the phosphorylated RpTPPKSP version, competes with Tau and MAP2c for binding to PP2A/Bα. Significantly, down-regulation of PP2A/Bα and deregulation of Fyn-Tau protein interactions have been linked to enhanced tau phosphorylation in Alzheimer disease. Together, our results suggest that PP2A/Bα is part of segregated MAP2 and tau signaling scaffolds that can coordinate the action of key kinases and phosphatases involved in modulating neuronal plasticity. Deregulation of these compartmentalized multifunctional protein complexes is likely to contribute to tau deregulation, microtubule disruption, and altered signaling in tauopathies.
Introduction
MAP2 and tau are major brain microtubule-associated proteins (MAPs)2 that share amino acid sequence and structural similarities. The MAP2 and tau families regroup several heat-stable, developmentally regulated isoforms generated by alternative splicing, that primarily bind to and regulate microtubule (MT) assembly, dynamics, and function (1, 2). Besides their undisputed role in regulating cytoskeletal organization, there is increasing evidence that both MAP2 and tau serve as protein scaffolds for important adaptor and signaling molecules, including Fyn, a member of the Src family of protein-tyrosine kinases (3–5). The coordinated regulation of these protein-protein interactions is believed to affect signaling networks that modulate processes as diverse as neuronal growth, morphogenesis, polarity, and plasticity, organelle transport, and synaptic activity (1, 2, 6, 7).
Notably, tau is a key player in Alzheimer disease (AD), and deregulation of Tau-Fyn interactions has been implicated in the neurodegenerative process (8–10). Both the physiological function and MT binding activity of tau (11) and MAP2 (12) isoforms are controlled by site-specific phosphorylation through the action of many protein kinases, including glycogen synthase kinase 3β (GSK3β), protein kinase A (PKA), and ERK. In AD, tau becomes highly phosphorylated, resulting in its detachment from MTs. While normal tau is enriched in axons, hyperphosphorylated AD-tau accumulates and aggregates in the somatodendritic compartment (13), where mature high molecular weight MAP2 (HMW-MAP2) proteins are primarily concentrated (12). There, cytosolic AD-tau may sequester MAP2 from MTs, resulting in defects in MT assembly (14).
Ser/Thr protein phosphatase 2A (PP2A) is a major brain tau phosphatase (15). It is predominantly found as a heterotrimeric complex of a catalytic C, a scaffolding A, and one member of four families of regulatory B subunits. B subunit binding to the core enzyme modulates PP2A substrate specificity, intracellular targeting, and interaction with binding partners (16). Notably, Bα (or PPP2R2A)-containing PP2A isoforms (PP2A/Bα) can directly interact with tau, and this interaction is required for efficient dephosphorylation of tau by the phosphatase (17–19). Besides binding to tau, PP2A/Bα also directly interacts with (18, 20, 21) and regulates MT dynamics (22). Because neuronal PP2A/Bα expression levels are down-regulated in AD (23), we have proposed that deregulation of normal PP2A-tau and PP2A-MT interactions likely contribute to deregulation of tau and MT disruption in AD (18). Phosphorylated mature HMW-MAP2 and juvenile low molecular weight MAP2c isoforms can also be dephosphorylated by PP2A catalytic subunit in vitro (24, 25). However, the specific PP2A isoform that dephosphorylates MAP2 isoforms in cultured neurons (26, 27) and rat brain slices (28) remains to be identified. The abundance of PP2A/Bα in neuronal cell bodies and processes (23) and its association with the MT cytoskeleton (20, 21) make this particular PP2A holoenzyme a potential candidate for regulating MAP2. We show here that, as observed with tau, endogenous PP2A/Bα can co-purify with, directly bind to and dephosphorylate MAP2. Significantly, a conserved proline-rich Fyn-binding motif in MAP2 and tau plays a critical role in regulating the interaction of PP2A/Bα with both MAPs. Our data underscore the importance of both MAP2 and tau as multivalent, compartmentalized anchoring proteins for major signaling enzymes like PP2A/Bα and Fyn. We propose that deregulation of these protein-protein interactions has the potential to contribute to alterations in normal enzyme-substrate relationships and signaling pathways in AD.
EXPERIMENTAL PROCEDURES
Materials and Reagents
Unless indicated, all chemicals were from Sigma. Okadaic acid (OA) was from LC Laboratories. Purified synthetic peptides were purchased from American Peptide Company. Purified fyn kinase was from Upstate and PKA from Sigma. Previously characterized purified proteins used in this study included: Bovine brain, taxol-stabilized MTs (18); bovine brain and recombinant PP2A/Bα, AC, and C enzymes (17, 18, 29); PKA-phosphorylated bovine brain tau (18); recombinant wild-type three- (3R-Tau) and four-repeat (4R-Tau) human tau isoforms (18, 29); and recombinant wild-type and mutated rat MAP2c proteins (30, 31). Fixed and fresh human brain cortex from a 73-year-old male and fixed cortical tissue from a 22-week gestation male fetus, both of them with no neuropathological abnormalities, were obtained through the Alzheimer Disease Center Brain Bank at UT Southwestern, Dallas, TX (23).
Purification and Proteolysis of HMW-MAP2
HMW-MAP2 was purified from bovine heat-stable MAPs by gel filtration chromatography on Hiload Superdex 200 (Amersham Biosciences Pharmacia) following published procedures (32). Thrombin cleavage of MAP2 was carried out as described previously (33), by incubating purified HMW-MAP2 with thrombin (10 units/ml) in PEM buffer (0.1 m PIPES, pH 6.9, 2 mm EGTA, 5 mm MgCl2, 1 mm DTT) for 30 min at 37 °C. The mixture was then boiled for 5 min and centrifuged for 15 min at 14,000 × g in an EppendorfTM microcentrifuge to recover heat-stable HMW-MAP2.
Purification and Analysis of Bovine Brain MAPs Fractions
White (WM) and gray (GM) matter were separated from bovine brain cortex as reported earlier (34). MAPs were purified from bovine GM or WM using taxol-dependent procedures and sedimentation, then dissociated from MTs and dialyzed, exactly as described previously (20, 34). Purified MAPs were in PEM buffer mixture of protease inhibitors (Complete miniTM, Roche). In some experiments, purified MAPs from bovine GM (∼5 mg proteins) were loaded on Hiload Superdex 200 columns, and 80 × 1.5 ml fractions were collected for further Western blot analyses. In parallel, PP2A activity was measured in 10 μl aliquots of the collected fractions.
Analysis of PP2A Activity
Endogenous PP2A activity in MAP-containing fractions was measured for 5 min at 30 °C using 32P-phosphorylated myosin light chain as a substrate, as described previously (17, 35). Duplicate aliquots were preincubated with 5 nm OA prior being assayed for phosphatase activity. Since we were unable to detect PP4 and PP6 in MAP-containing fractions, PP2A activity was defined as the myosin light chain phosphatase activity sensitive to 5 nm OA.
PP2A Binding Assays
PP2A binding assays were performed using nondenaturating gel electrophoresis as described previously (17, 18, 29, 36). Briefly, purified PP2A proteins were incubated for 20 min on ice with the indicated proteins, in a final volume of 5 μl buffer (25 mm Tris, 1 mm DTT, 1 mm EDTA, 50% glycerol, pH 7.5). After incubation, samples were applied on pre-cast 4–15% polyacrylamide nondenaturating gels (Bio-Rad), and analyzed by Western blotting.
SDS-PAGE and Western Blotting
Protein concentration was detected by the method of Bradford using the Bio-RadTM Protein Assay Kit. Proteins (∼30 μg) were resolved on 4–15% gradient SDS-polyacrylamide gels (Amersham Biosciences or Bio-Rad) and analyzed by Western blotting. Antibodies utilized included: mouse “2G9” and rabbit anti-Bα (37); mouse anti-β-tubulin (Sigma); mouse anti-C (BD Biosciences); mouse (Sigma) and rabbit (Cell Signaling Technology) anti-MAP2; anti-HA “16B12” (Covance Research Products); anti-Tau “Tau-5” (BIOSOURCE International); rabbit anti-PP4C and anti-PP6C (Bethyl Laboratories). Immunoreactive proteins were detected with SuperSignal chemiluminescent substrates (Pierce). Protein expression levels were quantified after scanning blots by densitometry using Kodak Image softwareTM.
In Vitro Phosphorylation/Dephosphorylation of MAPs and HMW-MAP2
Endogenous MAPs (100 μg aliquots) in PEM buffer were preincubated on ice for 15 min in the presence of 5 nm OA or vehicle alone, then further incubated for 30 min at 30 °C in the presence of 50 μm [γ-32P]ATP (2 μCi) in a final volume of 20 μl of PEM buffer. Purified bovine brain HMW-MAP2 (∼3 μg) in PEM buffer was phosphorylated by PKA for 30 min at 30 °C in the presence of 20 μm [γ-32P]ATP (1 μCi), 10 mm MgCl2, 1 mm DTT and 10 μm cAMP. The mixture was then boiled for 5 min and centrifuged for 15 min at 14,000 × g in an Eppendorf microcentrifuge. 32P-labeled, heat-stable HMW-MAP2 was recovered in the resulting supernatant. For dephosphorylation assays, aliquots of 32P-labeled MAPs or HMW-MAP2 were first incubated for 15 min on ice in the absence or presence of OA then incubated at 30 °C with the indicated purified PP2A enzymes. All reactions were halted by addition of 3× SDS-PAGE sample buffer. The samples were resolved on 4–16% gradient SDS-polyacrylamide gels (Amersham Biosciences) followed by autoradiography. 32P incorporation into MAP2 was directly quantified using a PhosphorImager (Molecular Dynamics).
MT Co-sedimentation Assays
MT co-sedimentation assays were performed as described previously (18). Briefly, PP2A was incubated for 15 min at room temperature in PEM buffer in the absence or presence of HMW-MAP2 then incubated for another 15 min with taxol-stabilized MTs in PEM buffer. The samples were then centrifuged at ∼50,000 × g for 20 min at 25 °C in a Beckman TLA 100.3 rotor. MT pellets were resuspended to the starting volume, and equivalent aliquots of supernatant and pellets were analyzed by SDS-PAGE followed by immunoblotting. The presence of pelleted MTs was verified by Ponceau Red staining of the immunoblots (not shown).
Cell Culture and Transfection
Control Neuro-2a (N2a, American Type Culture Collection) and N2a cells stably expressing HA-tagged PP2A Bα subunit (38) were maintained in DMEM (Invitrogen) containing 2.5 mm Hepes, pH 7.4, 10% fetal bovine serum (HyClone), and 10 μg/ml gentamycin (Invitrogen). When indicated, cells were transiently transfected with a pCMV-neo vector encoding rat MAP2b (a gift from Dr. Craig Garner, Stanford School of Medicine), using Metafectene ProTM reagent following the manufacturer's instructions (Biontex Laboratories), and processed 36 h post-transfection.
Immunoprecipitation Assays
Freshly dissected adult human cortical GM was homogenized at a ratio of 1 g tissue/10 ml IP buffer (25 mm Tris, 150 mm NaCl, 1% Nonidet P-40, 4 mm EDTA, 25 mm sodium fluoride, 1 mm sodium orthovanadate, pH 7.4) containing a mixture of protease inhibitors (Roche). MAP2b-transfected control and stable N2a cells lines were homogenized at a ratio of 1 × 100-mm dish/ml IP buffer. Tissue or cell homogenates were immunoprecipitated using either mouse anti-C antibodies, control immunoglobulins (IgG) or anti-HA monoclonal antibody-coupled affinity matrix, as described previously (29). Aliquots of immunoprecipitates were analyzed by SDS-PAGE followed by immunoblotting with anti-MAP2, anti-HA, or anti-PP2A subunit antibodies.
Immunohistochemistry
Immunohistochemical procedures were performed in the UT Southwestern Pathology Immunohistochemistry Laboratory, as described previously (23, 39). Briefly, paraffin sections (3 μm thick) from formalin-fixed brain cortices were immunostained at room temperature on an automated immunostainer using heat-induced epitope retrieval techniques. Labeling was performed using horseradish peroxidase (HRP)/diaminobenzidine (DAB) or alkaline phosphatase/new fuchsin detection. Positive reactions with DAB and new fuchsin were identified as dark brown and red reaction products, respectively. Sections were counterstained with hematoxylin, and photographed on a Nikon Optiphot-2 microscope. Positive and negative controls were included in each run.
RESULTS
Bα-containing PP2A Holoenzymes Co-purify with HMW-MAP2 from Bovine Gray Matter
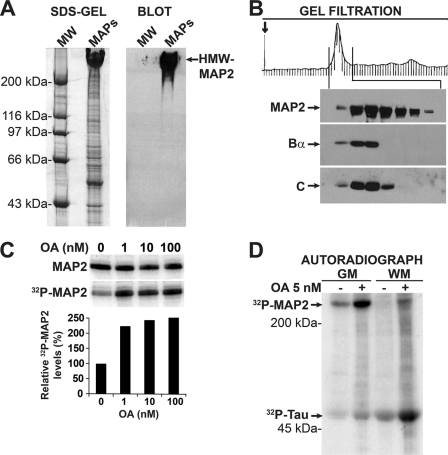
To investigate whether PP2A could co-purify with endogenous MAP2, MAPs were first prepared from adult bovine cortical GM. This approach was based on the observations that HMW-MAP2 is primarily distributed in neuronal cell bodies and dendrites, and that somatodendritic regions are concentrated in GM (34). As expected, Western blot analysis of MAP fractions prepared from adult bovine GM confirmed the presence of HMW-MAP2 proteins (Fig. 1A). Purified MAPs that had been dissociated from MTs were next resolved by gel filtration chromatography and analyzed by Western blotting for the presence of MAP2 and PP2A. HMW-MAP2-enriched fractions were detected as a major peak on the gel filtration profile. Notably, a fraction of PP2A Bα and C subunits co-eluted with HMW-MAP2 in selected gel filtration fractions (Fig. 1B). Nanomolar concentrations of OA that inhibit PP2A, PP4, and PP6 phosphatases in vitro, but not PP1, PP5, or calcineurin (41), enhanced phosphorylation of endogenous HMW-MAP2 (Fig. 1C). Indeed, HMW-MAP2 was the prevalent MAP that became phosphorylated following incubation of bovine GM MAPs with 5 nm OA (Fig. 1D). In contrast to HMW-MAP2 proteins that are largely located in the somatodendritic compartment, tau proteins are predominantly found in axons, which are concentrated in brain WM (42). Tau proteins were identified as the primary bovine WM MAP that became phosphorylated in response to OA under equivalent experimental conditions. However, we also observed minor OA-sensitive pools of phospho-tau in the GM, and phospho-MAP2 in the WM fractions. Those are consistent with the presence of low levels of tau in dendrites (10) and small amounts of MAP2 in WM (34, 43). In contrast to PP2A, we were unable to detect the presence of either PP4 or PP6 phosphatases by Western blotting in MAP fractions from either GM or WM (data not shown), indicating that PP2A enzymes were the primary phosphatases inhibited by 5 nm OA in those fractions. Interestingly, we measured similar PP2A activity levels (∼1 ± 0.2 nmol/min/mg, n = 3) in MAPs fractions purified from either GM or WM, using myosin light chain as substrate. Together, these data indicate that phosphorylated MAP2 and tau proteins are major and preferential PP2A substrates in the GM and WM, respectively.
FIGURE 1.
PP2A/Bα co-purifies with and dephosphorylates endogenous HMW-MAP2 from bovine gray matter. A, equivalent aliquots (100 μg) of MAPs purified from adult bovine brain gray matter (GM) were analyzed in parallel by SDS-PAGE (Coomassie-stained SDS-gel) and immunoblotting with anti-MAP2 antibodies (Blot). MW, molecular weight markers. B, MAPs purified from bovine GM were subjected to gel filtration chromatography. Analysis of collected fractions by Western blotting showed that PP2A Bα and C subunits were present with HMW-MAP2 in the major protein peak visible on the gel filtration profile. Note that PP2A subunits corresponding to unbound enzymes were also detected in non-MAP2 containing fractions that eluted much later in the profile (not shown). C, equivalent aliquots (∼5 μg of proteins) of HMW-MAP2-enriched fractions identified by gel filtration were pooled together and pre-incubated with okadaic acid (OA) at the indicated concentrations, or vehicle alone, then autophosphorylated in the presence of 100 μm [γ-32P]ATP (2 μCi). Samples were analyzed by gel electrophoresis followed by autoradiography. MAP2 bands were detected after staining of the gels. 32P incorporation into HMW-MAP2 was determined on dried gels using a PhosphorImager, and normalized for MAP2 protein loading. Data are expressed as the percentage of MAP2-associated 32P that was present in the absence of OA. D, equivalent aliquots (100 μg) of purified MAPs from adult bovine gray (GM) or white (WM) matter were pre-incubated for 15 min with 5 nm OA (+) or vehicle (−), then autophosphorylated in the presence of 100 μm [γ-32P]ATP (2 μCi). Samples were analyzed by SDS-gel electrophoresis followed by autoradiography. Parallel Western blot analysis of duplicate samples confirmed that the major phosphorylated bands visible on the autoradiograph corresponded to HMW-MAP2 and tau proteins, which are enriched in the GM and WM fractions, respectively (not shown). For A–D, representative data are shown. Similar results were obtained in three separate purifications.
PP2A Co-immunoprecipitates with Endogenous or Expressed MAP2
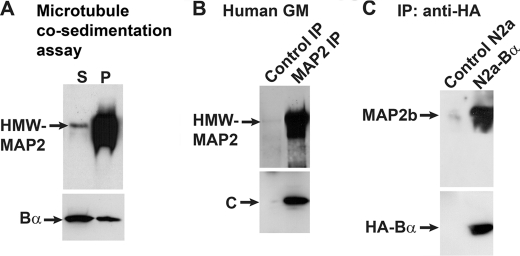
In addition to co-purify with endogenous HMW-MAP2, bovine brain PP2A/Bα co-sedimented with purified bovine brain HMW-MAP2 and MTs (Fig. 2A). PP2A C subunit was found in MAP2 immunoprecipitates prepared from adult human GM extracts (Fig. 2B), or from bovine brain (data not shown). Because of interference with tubulin, which migrates at a similar molecular weight as Bα, we were unable to confirm that Bα subunits were present in the MAP2 immunoprecipitates. Thus, to assess whether PP2A/Bα interacts with MAP2, immunoprecipitation assays were carried out in cultured neuroblastoma cells. To that end, control N2a and N2a cell lines stably expressing HA-tagged Bα (38) were transiently transfected with a plasmid encoding the HMW-MAP2b isoform. Since anti-PP2A subunit antibodies do not quantitatively immunoprecipitate PP2A/Bα heterotrimers, anti-HA monoclonal antibody-coupled affinity matrix was utilized to immunoprecipitate PP2A/Bα holoenzymes in cell homogenates, as reported previously (29, 44). MAP2b proteins were recovered in HA-Bα immunoprecipitates (Fig. 2C), in support of the existence of PP2A/Bα-MAP2 protein complexes.
FIGURE 2.
PP2A co-sediments and co-immunoprecipitates with either endogenous or transfected HMW-MAP2. A, co-sedimentation assays were performed with purified bovine brain PP2A/Bα (200 nm) that had been pre-incubated for 15 min with purified bovine brain HMW-MAP2 (2 μm) in PEM buffer alone, then incubated for 15 min at room temperature with ∼20 μm purified bovine brain taxol-stabilized MTs. The samples were centrifuged, and fractions corresponding to supernatants (S) and MT pellets (P) were collected for analysis. B, equivalent aliquots (1 ml) of total human GM homogenates were immunoprecipitated with anti-MAP2 antibody (MAP2 IP) or with control IgG (Control IP). C, total cell extracts were prepared from MAP2b-transfected N2a cells (Control N2a) and N2a cells stably expressing the HA-tagged Bα subunit (N2a-Bα), and immunoprecipitated with monoclonal HA-tag antibody-coupled affinity matrix. For A–C, samples were analyzed on 4–15% gradient polyacrylamide gels followed by immunoblotting. The top part of the blots was cut and analyzed with monoclonal anti-MAP2 antibodies. The bottom part of the blots was revealed with monoclonal anti-Bα, anti-C, or anti-HA antibodies. Representative blots are shown. Similar results were obtained in three separate assays.
PP2A/Bα Holoenzymes Directly Bind To and Dephosphorylate Purified MAP2 in Vitro
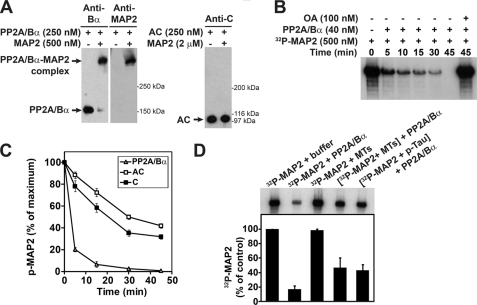
Potential direct interactions between HMW-MAP2 and PP2A were further analyzed in vitro using purified proteins and gel shift mobility assays (Fig. 3A). We have previously utilized the same experimental strategy to demonstrate that tissue-purified or recombinant PP2A/Bα enzymes specifically interact with tau (17, 18). In those assays, nearly all PP2A/Bα enzymes interacted with MAP2 at a 2:1 MAP2:PP2A molar ratio. In contrast, we were unable to detect formation of complexes between dimeric PP2A (AC) enzymes and MAP2 even when MAP2 was present at an 8-fold excess over PP2A, supporting a critical role for Bα in increasing the affinity of PP2A for MAP2. We have also reported that PP2A/Bα holoenzymes can dephosphorylate PKA-phosphorylated tau much more efficiently than dimeric or monomeric PP2A enzymes (17). Similarly, PKA-phosphorylated MAP2 was more readily dephosphorylated by PP2A/Bα than AC or C enzymes (Fig. 3, B and C). In vitro PKA-phosphorylated MAP2 and tau proteins effectively competed as substrates for PP2A/Bα (Fig. 3D). As previously observed with tau (18), the presence of assembled MTs also inhibited the MAP2 phosphatase activity of PP2A/Bα.
FIGURE 3.
PP2A/Bα binds to and dephosphorylates purified HMW-MAP2 in vitro. A, purified bovine brain PP2A heterotrimeric PP2A/Bα and dimeric AC enzymes were incubated on ice for 20 min with purified bovine brain MAP2 at the indicated concentrations, or buffer alone. The samples were subsequently analyzed by nondenaturating gel electrophoresis and Western blotting with anti-PP2A subunit and anti-MAP2 antibodies. The position of PP2A enzymes and PP2A/MAP2 complexes is shown. Similar results were observed in three separate gel shift assays, and when using either recombinant, cardiac, or bovine brain tissue-purified PP2A preparations (not shown). B, representative autoradiograph showing the time-course of dephosphorylation of 32P-labeled, PKA-phosphorylated bovine brain MAP2 by bovine brain PP2A/Bα. Incubation with OA blocks the dephosphorylation of MAP2 by PP2A. C, equivalent aliquots (200 nm) of 32P-labeled, PKA-phosphorylated bovine brain MAP2 were incubated for the indicated times with 40 nm bovine brain PP2A/Bα, AC or C enzymes. Samples were resolved by SDS-PAGE followed by autoradiography. The amount of 32P remaining in MAP2 was quantified on dried gels using a PhosphorImager. Data shown are the mean ± S.D. of results from four separate experiments performed with distinct enzymatic preparations, and are expressed as the percentage of MAP2-associated 32P that was present at time 0 before exposure to PP2A. D, equivalent aliquots of 32P-labeled, PKA-phosphorylated bovine brain MAP2 (200 nm) were pre-incubated for 15 min with either buffer alone, taxol-stabilized MTs (2 μm), or bovine brain PKA-phosphorylated tau (200 nm), then incubated for 30 min with bovine brain PP2A/Bα (40 nm) or buffer alone. The samples were then processed as described in C (n = 4). Note that dephosphorylation of MAP2 by PP2A is MT- and tau-sensitive.
Identification of a PP2A-binding Domain in MAP2
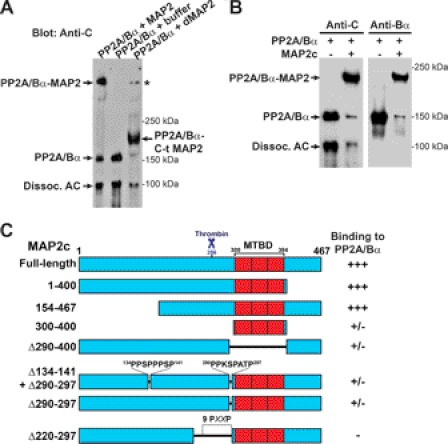
To start delineating the PP2A-binding domain in MAP2, gel shift assays were first carried out with PP2A/Bα and thrombin-digested MAP2. Cleavage of bovine brain MAP2 by thrombin yields: 1) a 28 kDa C-terminal polypeptide fragment which includes the four tandem repeats in the MT-binding domain (MTBD) and part of the upstream proline-rich region; and 2) a larger N-terminal fragment containing the projection domain (33). As shown in Fig. 4A, PP2A/Bα preferentially interacted with the C-terminal domain of thrombin-cleaved MAP2. To better narrow down the PP2A-binding sequence in MAP2, we next performed binding assays using purified PP2A/Bα and wild-type and mutated recombinant forms of MAP2c, the shortest juvenile isoform of MAP2. MAP2c is similar to adult HMW-MAP2, except that it contains only three MT-binding repeats and lacks a 1372 amino acid sequence in the projection domain (2). Gel shift assays demonstrated that PP2A/Bα efficiently interacted with wild-type rat MAP2c (Fig. 4B), and MAP2c proteins lacking the 154–467 N-terminal projection domain, or the 154–467 C-terminal region (Fig. 4C). Deletion of the MTBD and/or upstream 290–297 proline-rich motif significantly decreased the ability of MAP2c to form a complex with PP2A, but did not fully abolish PP2A binding. However, deletion of the 220–297 sequence in MAP2c containing nine proline-rich motifs prevented formation of MAP2c-PP2A complexes. Based on these data and the observation that the thrombin cleavage site in HMW-MAP2 and MAP2c is conserved (45), we propose that: 1) PP2A/Bα has independent binding sites in MAP2c that are contained within the 256–400 sequence of MAP2c; 2) Deletion of the proline-rich 290–297 motif and/or the MTBD decreases the affinity of MAP2c for PP2A; 3) at least two PXXP motifs within the 256–297 region, which contains seven PXXP motifs, are critically required for efficient PP2A-MAP2c binding.
FIGURE 4.
Identification of a PP2A-binding domain in MAP2. A, bovine brain PP2A/Bα (250 nm) was incubated on ice for 20 min with either buffer alone, intact bovine brain MAP2 (500 nm) or thrombin-digested MAP2 (dMAP2, 2 μm). The samples were subsequently analyzed by nondenaturating gel electrophoresis and Western blotting with anti-PP2A C subunit antibodies. As observed with intact MAP2, PP2A/Bα was able to form a complex with the smaller C-terminal polypeptide fragment containing the MTBD of MAP2, but not with the larger N-terminal projection domain that is generated by thrombin cleavage of MAP2 (33). The asterisk indicates PP2A/Bα in complex with small amounts of undigested MAP2. B, bovine brain PP2A/Bα (250 nm) was incubated on ice for 20 min with intact purified recombinant rat MAP2c (1 μm), prior to being analyzed by gel shift assay and Western blotting with mouse anti-C and anti-Bα subunit antibodies. Representative blots from at least 5 separate experiments are shown. For A and B, note that variable levels of AC dimers were found to dissociate from uncomplexed PP2A/Bα heterotrimers during nondenaturating electrophoresis, as reported previously (17). C, ability of bovine brain PP2A/Bα (250 nm) to form complexes with full-length and truncated recombinant MAP2c fragments (1 μm) was assessed by gel shift assays as described in B. Densitometry of the immunoblots from three separate binding assays was used to assess the binding of recombinant MAP2c proteins to PP2A/Bα: +++, >90% binding; ±, 5–20% binding; −, no detectable binding. Proline-rich PXXP motifs and the thrombin cleavage site in rat MAP2c are indicated for reference.
MAP2 and Tau Isoforms Compete for PP2A Binding in Vitro
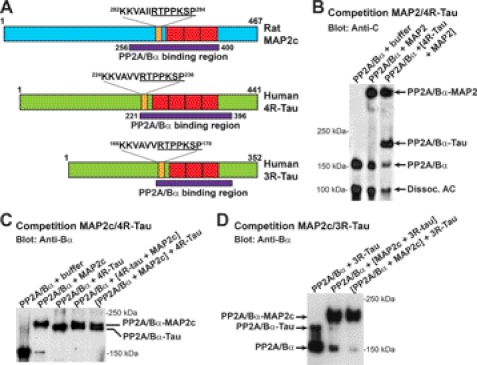
Tau isoforms contain either three (3R-Tau) or four (4R-Tau) ∼32 amino acid imperfect repeats in the MTBD (46). We have previously shown that PP2A/Bα interacts with tau via a domain encompassing the MTBD and upstream proline-rich region that is common to the shortest 3R-Tau and longest 4R-tau isoforms (18). Interestingly, MAP2 and tau isoforms share many sequence similarities in this region (1). Indeed, comparative analysis of the putative PP2A/Bα-binding domain in human tau and rat MAP2c revealed some remarkable overlap (Fig. 5A). Gel shift assays showed that Tau and MAP2 isoforms actually competed for binding to PP2A. PP2A/Bα appeared to bind equally well to human 4R-tau and either bovine adult HMW-MAP2 (Fig. 5B) or rat MAP2c (Fig. 5C). In contrast, PP2A/Bα preferentially interacted with MAP2c rather than 3R-Tau (Fig. 5D).
FIGURE 5.
Adult and fetal MAP2 and tau isofoms compete for binding to PP2A/Bα. A, schematic diagrams comparing the approximate boundaries of the PP2A-binding domain in rat MAP2c deducted from Fig. 4, and in the longest human adult 4R-Tau and fetal 3R-Tau isoforms (18). Note the presence of the conserved underlined RTPPKSP motif in all three PP2A-binding domains. B–D, gel shift assays followed by immunoblotting with anti-PP2A subunit antibodies were performed to compare the ability of bovine brain PP2A/Bα (250 nm) to preferentially form a complex with either HMW-MAP2, MAP2c, 3R-tau, or 4R-tau (B: 500 nm each, C and D: 1 μm each). Representative blots from three separate experiments are shown. Note that the bands in B were assembled from the same blot. Similar results were found using recombinant PP2A/Bα proteins (not shown).
Fyn-binding PXXP Motifs Critically Regulate the Interaction of MAP2 and Tau with PP2A/Bα
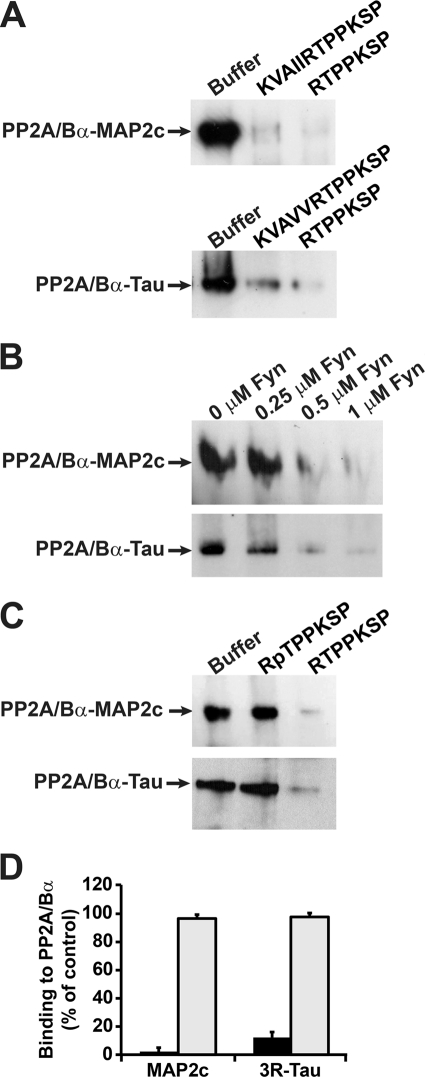
Since MAP2 and tau share the common “RTPPKSP” proline-rich sequence within their PP2A-binding domain (Fig. 5A), we hypothesized that this motif could modulate the ability of these MAPs to interact with PP2A/Bα. We have previously reported that 10 μm of the longer KKVAVVRTPPKSP peptide hinders binding of PP2A/Bα to native bovine brain tau (18). This peptide, as well as the shorter RTPPKSP version, also inhibited binding of PP2A/Bα to recombinant human 3R-Tau (Fig. 6A). Likewise, the corresponding KKVAIIRTPPKSP and RTPPKSP MAP2 peptides impaired formation of PP2A-MAP2c complexes. Interestingly, the protein-tyrosine kinase Fyn avidly binds through its SH3 domain to the “RTPPKSP” motif in MAP2c (4) and 3R-Tau (3, 5, 9). Accordingly, increasing concentrations of Fyn inhibited the association of PP2A/Bα with either MAP2c or 3R-Tau (Fig. 6B). The Ser and Thr residues in the RTPPKSP motif in MAP2 and tau are also targets for kinase-mediated phosphorylation in vivo (4, 9). Unlike the nonphosphorylated RTPPKSP peptide, the synthetic RpTPPKSP phosphopeptide failed to significantly inhibit binding of PP2A/Bα to either MAP2c or 3R-Tau (Fig. 6, C and D). Of note, we were unable to detect complex formation between PP2A and either Fyn or the RpTPPKSP peptide under the same experimental conditions (not shown). Altogether, these results support the critical importance of the RTPPKSP motif in modulating the interaction of MAP2 and tau with PP2A.
FIGURE 6.
The Fyn-SH3 binding, proline-rich RTPPKSP motif modulates the binding of PP2A/Bα to both MAP2 and Tau. Bovine brain PP2A/Bα (250 nm) was pre-incubated for 20 min on ice with the indicated peptides (10 μm) (A, C, D) or with the indicated concentrations of purified human fyn kinase (B), prior to incubation for another 15 min with either 3R-Tau or MAP2c (1 μm). The ability of the peptides or fyn to inhibit formation of PP2A-MAP2 or PP2A-Tau complexes was assessed after gel shift assays and immunoblotting with anti-Bα antibodies. Similar results were obtained in at least three separate experiments. D, ability of the RTPPKSP (black bars) or RpTPPKSP (gray bars) peptides to block formation of PP2A-MAP2 or PP2A-tau complexes was quantified after densitometry analysis of the immunoblots. Data are expressed as the percentage of maximal PP2A-MAP2 or PP2A-tau complex formation measured in absence of peptide (n = 4, mean ± S.D.).
DISCUSSION
The study of tau has attracted much attention in the past decade due to its role in AD pathogenesis, and its genetic link to frontotemporal dementia (11). Yet, MAP2 is a major neuronal MAP that regulates MT and actin cytoskeletal organization, dendritic morphogenesis and organelle trafficking (2). Like tau, the biological function of MAP2 is controlled by phosphorylation and dephosphorylation events (12). While “PP2A” has been identified as a MAP2 phosphatase in numerous studies, we provide experimental evidence that PP2A/Bα, a major brain PP2A isoform, not only dephosphorylates but also directly interacts with MAP2. The PP2A/Bα holoenzyme co-purified with MTs and MAP2 from bovine GM, and co-sedimented with purified MAP2 and MTs. Yet, it is unlikely that MAP2 serves to anchor the phosphatase to MTs since PP2A/Bα retains its ability to bind to subtilisin-cleaved MTs, which have reduced capacity to bind both tau and MAP2 (18). Moreover, as observed with tau (18), PP2A/Bα binds to a region comprising the MTBD, and the presence of MTs inhibits the MAP2 phosphatase activity of PP2A. Together with the observation that MT-associated PP2A/Bα is inactive (18, 21), it is anticipated that only MT-unbound MAP2 and tau proteins can be effectively dephosphorylated by PP2A/Bα. The prevalence of PP2A/Bα in the cytosol and MTs in close proximity with either tau or MAP2 augments the chances for fast dephosphorylation of these MAPs by PP2A in response to MT disassembly. Accordingly, Fan et al. have reported that cholesterol deficiency in cultured neurons induces the detachment of PP2A from MTs and subsequent increase of cytosolic PP2A activity and MAP2 dephosphorylation (27). In turn, this induces inhibition of dendrite outgrowth, indicating that PP2A does not need to be on dendrites per se to regulate MAP2 function in dendritic morphogenesis. Results from immunohistochemical studies comparing the distribution of PP2A/Bα and MAP2 in the developing and mature human brain (supplemental Fig. S1) also suggest that PP2A/Bα most likely associates with and dephosphorylates MAP2 in neuronal cell bodies rather than in dendrites. Other phosphatases, such as PP1 and kinase-associated phosphatase are better positioned than PP2A/Bα to dephosphorylate dendritic MAP2 (2, 12). We also do not exclude the possibility that other PP2A isoforms or phosphatases not studied here could act as somatodendritic MAP2 phosphatases.
As observed earlier for tau (17), the Bα subunit was required for optimal binding to and dephosphorylation of MAP2 by PP2A. The (AC) dimer was less active toward p-MAP2 than monomeric C, in agreement with previous studies showing that PP2A catalytic subunit activity is allosterically modulated by the regulatory A subunit (47). We were unable to detect formation of AC-MAP2 (Fig. 3) or C-MAP2 complexes (not shown) by gel shift assays, suggesting that the affinity of these PP2A enzymes for MAP2 is too low to allow formation of protein-protein complexes that can withstand the conditions of native gel electrophoresis.
We found that soluble MAP2 and tau could compete for in vitro binding to and dephosphorylation by PP2A/Bα. Binding assays indicated that PP2A/Bα associates with a domain in MAP2 encompassing the MTBD and upstream proline-rich region, as previously reported for tau (18). They suggested the existence of two peptide binding sequences for MAP2 in this domain, as described earlier for tau (19). Basic peptide sequences within this region promote the interaction of tau with the acidic groove of Bα (19). Significantly, conserved PXXP proline-rich motifs in MAP2 and tau, which bind SH3 domains of Fyn (3–5) were required for optimal PP2A-MAP2 and PP2A-Tau interactions. Of note, there is a precedent for the association of PP2A with proline-rich SH3-binding motifs in another protein, GPR54 (48). Interestingly, Fyn kinase could inhibit the in vitro binding of PP2A/Bα to both MAP2c and 3R-Tau. Fyn-SH3 has much more affinity for 3R- than 4R-Tau (9), while PP2A/Bα binds more tightly to 4R- than 3R-Tau isoforms (18). AD-like tau phosphorylation and missense tau mutations found in frontotemporal dementia dramatically increase the affinity of 4R-Tau for Fyn-SH3 (8, 9), while inhibiting its interaction with PP2A/Bα (29, 49). Furthermore, down-regulation of neuronal PP2A/Bα expression levels in AD is associated with increased tau phosphorylation (23). Based on these findings and our in vitro results (Fig. 6), it is tempting to speculate that changes in the normal composition of tau-Fyn and tau-PP2A complexes could contribute to tau deregulation and dysfunction in tauopathies.
Pseudophosphorylation of several Ser/Thr sites in the proline-rich region (residues 172–251) flanking the MTBD of tau inhibit PP2A/Bα binding, and induce functional deficiencies of tau similar to those observed in AD (49). Here, we show more specifically that the 230RTPPKSP236 peptide, but not the corresponding phosphopeptide containing the phospho-Thr231 residue, can significantly inhibit PP2A-Tau interactions. These data suggest that phosphorylation of tau at Thr-231 decreases its affinity for PP2A, and could explain why tau phosphorylated at this site is poorly dephosphorylated by PP2A/Bα (50). This is potentially physiologically significant since phosphorylation of tau at Thr-231 occurs early in AD and can further inhibit the ability of PP2A/Bα to dephosphorylate other major AD-tau phosphoepitopes (50). Likewise, phosphorylation of the RTPPKSP motif in MAP2c at the Thr site occurs during development (4) and impairs its ability to associate with PP2A/Bα.
Tau proteins were the prevalent MAPs that became phosphorylated in response to OA-mediated PP2A inhibition in the WM (Fig. 1D), in agreement with the observation that PP2A is the primary tau phosphatase (15, 51). Conversely, MAP2 was the major MAP dephosphorylated by PP2A in the GM. In AD, cytosolic hyperphosphorylated tau accumulates in the somatodendritic compartment where it sequesters MAP2 and affects MT dynamics (14). The detachment of HMWMAP2 from MTs may underlie the proposed dendritic remodeling in AD (52). The resulting enrichment of cytosolic MAP2 and Tau in neuronal cell bodies in AD could also affect normal PP2A-MAP2 interactions, thereby perturbing PP2A- and MAP2-controlled processes.
In conclusion, our results suggest that the role of PP2A/Bα is not confined to its activity as a major tau and MAP2 phosphatase, but this prevalent phosphatase also serves as an important component of localized tau and MAP2 signaling scaffolds. There is strong evidence that disruption and deregulation of tau and MAP2 multi-protein complexes can alter the phosphorylation state and localization of tau and MAP2, with severe consequences on cytoskeletal organization, stability and function. Our findings support the hypothesis that these major brain MAPs also mediate the selective and regulated sequestration of major phosphatases and kinases in discrete neuronal compartments, e.g. cell body, dendrite, or axon, allowing for exquisite control of localized signal transduction and integration. Besides regulating MAP2 (this study), tau (17), MT stability (22), and neurite outgrowth (40), PP2A/Bα is a key signaling molecule functioning in multiple signaling pathways. Thus, disruption of tau and MAP2 anchoring function has the potential to alter the compartmentalization of key signaling enzymes like PP2A/Bα and Fyn, which critically regulate neuronal plasticity and other neuronal processes. In light of the known deregulation of PP2A/Bα in AD, it will be paramount to establish the precise regulation and role of these signaling complexes in vivo.
Acknowledgments
We thank Dr. Craig Kamibayashi (UT Southwestern, TX) for the gift of purified PP2A enzymes; Drs. Mu-Yeh Bau and George Bloom (UT Southwestern, TX) for assistance in the purification of MTs and MAPs; Drs. Rita Lim and Benoit Roger (The Scripps Research Institute, CA) for the gift of purified recombinant MAP2c proteins; Drs. Roland Brand (University of Osnabrück, Germany) and Michel Goedert (Medical Research Council Laboratory of Molecular Biology, Cambridge, UK) for the gift of tau expression plasmids and purified tau proteins; Dr. Craig Garner (Stanford School of Medicine) for MAP2b expression plasmids; Dr. Egon Ogris (University of Vienna, Austria) for the gift of anti-Bα antibodies, and the immunohistochemistry laboratory at UT Southwestern for technical assistance.
This work was supported in part by National Institutes of Health Grants AG18883 (to E. S.), French Foundation for Alzheimer research (to E. S.), AG12300 (to E. S. and C. W.) and MH50861 (to S. H.), and NHMRC Australia grant (to E. S. and J. M. S.).

This article contains supplemental Fig. S1.
- MAP
- microtubule-associated protein
- AD
- Alzheimer disease
- GM
- grey matter
- MT
- microtubule
- MTBD
- microtubule-binding domain
- OA
- okadaic acid
- PP2A/Bα
- protein phosphatase 2A containing the Bα subunit
- 3R-Tau
- three-repeat Tau
- 4R-Tau
- four-repeat Tau
- WM
- white matter.
REFERENCES
- 1. Dehmelt L., Halpain S. (2005) The MAP2/Tau family of microtubule-associated proteins. Genome Biol. 6, 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Farah C. A., Leclerc N. (2008) HMWMAP2: new perspectives on a pathway to dendritic identity. Cell Motil. Cytoskeleton 65, 515–527 [DOI] [PubMed] [Google Scholar]
- 3. Lee G., Newman S. T., Gard D. L., Band H., Panchamoorthy G. (1998) Tau interacts with src-family non-receptor tyrosine kinases. J. Cell Sci. 111, 3167–3177 [DOI] [PubMed] [Google Scholar]
- 4. Zamora-Leon S. P., Lee G., Davies P., Shafit-Zagardo B. (2001) Binding of Fyn to MAP-2c through an SH3 binding domain. Regulation of the interaction by ERK2. J. Biol. Chem. 276, 39950–39958 [DOI] [PubMed] [Google Scholar]
- 5. Reynolds C. H., Garwood C. J., Wray S., Price C., Kellie S., Perera T., Zvelebil M., Yang A., Sheppard P. W., Varndell I. M., Hanger D. P., Anderton B. H. (2008) Phosphorylation regulates tau interactions with Src homology 3 domains of phosphatidylinositol 3-kinase, phospholipase Cγ1, Grb2, and Src family kinases. J. Biol. Chem. 283, 18177–18186 [DOI] [PubMed] [Google Scholar]
- 6. Götz J., Ittner L. M., Kins S. (2006) Do axonal defects in tau and amyloid precursor protein transgenic animals model axonopathy in Alzheimer disease? J. Neurochem. 98, 993–1006 [DOI] [PubMed] [Google Scholar]
- 7. Morris M., Maeda S., Vossel K., Mucke L. (2011) The many faces of tau. Neuron 70, 410–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee G., Thangavel R., Sharma V. M., Litersky J. M., Bhaskar K., Fang S. M., Do L. H., Andreadis A., Van Hoesen G., Ksiezak-Reding H. (2004) Phosphorylation of tau by fyn: implications for Alzheimer disease. J. Neurosci. 24, 2304–2312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bhaskar K., Yen S. H., Lee G. (2005) Disease-related modifications in tau affect the interaction between Fyn and Tau. J. Biol. Chem. 280, 35119–35125 [DOI] [PubMed] [Google Scholar]
- 10. Ittner A., Ke Y. D., Eersel J., Gladbach A., Götz J., Ittner L. M. (2011) Brief update on different roles of tau in neurodegeneration. IUBMB. Life 63, 495–502 [DOI] [PubMed] [Google Scholar]
- 11. Iqbal K., Liu F., Gong C. X., Grundke-Iqbal I. (2010) Tau in Alzheimer disease and related tauopathies. Curr. Alzheimer Res. 7, 656–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sánchez C., Díaz-Nido J., Avila J. (2000) Phosphorylation of microtubule-associated protein 2 (MAP2) and its relevance for the regulation of the neuronal cytoskeleton function. Prog. Neurobiol. 61, 133–168 [DOI] [PubMed] [Google Scholar]
- 13. Ballatore C., Lee V. M., Trojanowski J. Q. (2007) Tau-mediated neurodegeneration in Alzheimer disease and related disorders. Nat. Rev. Neurosci. 8, 663–672 [DOI] [PubMed] [Google Scholar]
- 14. Iqbal K., Alonso Adel C., Grundke-Iqbal I. (2008) Cytosolic abnormally hyperphosphorylated tau but not paired helical filaments sequester normal MAPs and inhibit microtubule assembly. J. Alzheimers. Dis. 14, 365–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu F., Grundke-Iqbal I., Iqbal K., Gong C. X. (2005) Contributions of protein phosphatases PP1, PP2A, PP2B, and PP5 to the regulation of tau phosphorylation. Eur. J. Neurosci. 22, 1942–1950 [DOI] [PubMed] [Google Scholar]
- 16. Virshup D. M., Shenolikar S. (2009) From promiscuity to precision: protein phosphatases get a makeover. Mol. Cell 33, 537–545 [DOI] [PubMed] [Google Scholar]
- 17. Sontag E., Nunbhakdi-Craig V., Lee G., Bloom G. S., Mumby M. C. (1996) Regulation of the phosphorylation state and microtubule-binding activity of Tau by protein phosphatase 2A. Neuron 17, 1201–1207 [DOI] [PubMed] [Google Scholar]
- 18. Sontag E., Nunbhakdi-Craig V., Lee G., Brandt R., Kamibayashi C., Kuret J., White C. L., 3rd, Mumby M. C., Bloom G. S. (1999) Molecular interactions among protein phosphatase 2A, tau, and microtubules. Implications for the regulation of tau phosphorylation and the development of tauopathies. J. Biol. Chem. 274, 25490–25498 [DOI] [PubMed] [Google Scholar]
- 19. Xu Y., Chen Y., Zhang P., Jeffrey P. D., Shi Y. (2008) Structure of a protein phosphatase 2A holoenzyme: insights into B55-mediated Tau dephosphorylation. Mol. Cell 31, 873–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sontag E., Nunbhakdi-Craig V., Bloom G. S., Mumby M. C. (1995) A novel pool of protein phosphatase 2A is associated with microtubules and is regulated during the cell cycle. J. Cell Biol. 128, 1131–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hiraga A., Tamura S. (2000) Protein phosphatase 2A is associated in an inactive state with microtubules through 2A1-specific interaction with tubulin. Biochem. J. 346, 433–439 [PMC free article] [PubMed] [Google Scholar]
- 22. Nunbhakdi-Craig V., Schuechner S., Sontag J. M., Montgomery L., Pallas D. C., Juno C., Mudrak I., Ogris E., Sontag E. (2007) Expression of protein phosphatase 2A mutants and silencing of the regulatory B α subunit induce a selective loss of acetylated and detyrosinated microtubules. J. Neurochem. 101, 959–971 [DOI] [PubMed] [Google Scholar]
- 23. Sontag E., Luangpirom A., Hladik C., Mudrak I., Ogris E., Speciale S., White C. L., 3rd (2004) Altered expression levels of the protein phosphatase 2A ABαC enzyme are associated with Alzheimer disease pathology. J. Neuropathol. Exp. Neurol. 63, 287–301 [DOI] [PubMed] [Google Scholar]
- 24. Sánchez C., Tompa P., Szücs K., Friedrich P., Avila J. (1996) Phosphorylation and dephosphorylation in the proline-rich C-terminal domain of microtubule-associated protein 2. Eur. J. Biochem. 241, 765–771 [DOI] [PubMed] [Google Scholar]
- 25. Alexa A., Schmidt G., Tompa P., Ogueta S., Vázquez J., Kulcsár P., Kovács J., Dombrádi V., Friedrich P. (2002) The phosphorylation state of threonine-220, a uniquely phosphatase-sensitive protein kinase A site in microtubule-associated protein MAP2c, regulates microtubule binding and stability. Biochemistry 41, 12427–12435 [DOI] [PubMed] [Google Scholar]
- 26. Sánchez M. C., Díaz-Nido J., Avila J. (1998) Regulation of a site-specific phosphorylation of the microtubule-associated protein 2 during the development of cultured neurons. Neuroscience 87, 861–870 [DOI] [PubMed] [Google Scholar]
- 27. Fan Q. W., Yu W., Gong J. S., Zou K., Sawamura N., Senda T., Yanagisawa K., Michikawa M. (2002) Cholesterol-dependent modulation of dendrite outgrowth and microtubule stability in cultured neurons. J. Neurochem. 80, 178–190 [DOI] [PubMed] [Google Scholar]
- 28. Gong C. X., Wegiel J., Lidsky T., Zuck L., Avila J., Wisniewski H. M., Grundke-Iqbal I., Iqbal K. (2000) Regulation of phosphorylation of neuronal microtubule-associated proteins MAP1b and MAP2 by protein phosphatase-2A and -2B in rat brain. Brain Res. 853, 299–309 [DOI] [PubMed] [Google Scholar]
- 29. Goedert M., Satumtira S., Jakes R., Smith M. J., Kamibayashi C., White C. L., 3rd, Sontag E. (2000) Reduced binding of protein phosphatase 2A to tau protein with frontotemporal dementia and parkinsonism linked to chromosome 17 mutations. J. Neurochem. 75, 2155–2162 [DOI] [PubMed] [Google Scholar]
- 30. Lim R. W., Halpain S. (2000) Regulated association of microtubule-associated protein 2 (MAP2) with Src and Grb2: evidence for MAP2 as a scaffolding protein. J. Biol. Chem. 275, 20578–20587 [DOI] [PubMed] [Google Scholar]
- 31. Roger B., Al-Bassam J., Dehmelt L., Milligan R. A., Halpain S. (2004) MAP2c, but not tau, binds and bundles F-actin via its microtubule binding domain. Curr. Biol. 14, 363–371 [DOI] [PubMed] [Google Scholar]
- 32. Kim H., Binder L. I., Rosenbaum J. L. (1979) The periodic association of MAP2 with brain microtubules in vitro. J. Cell Biol. 80, 266–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Joly J. C., Flynn G., Purich D. L. (1989) The microtubule-binding fragment of microtubule-associated protein-2: location of the protease-accessible site and identification of an assembly-promoting peptide. J. Cell Biol. 109, 2289–2294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vallee R. B. (1982) A taxol-dependent procedure for the isolation of microtubules and microtubule-associated proteins (MAPs). J. Cell Biol. 92, 435–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mumby M. C., Russell K. L., Garrard L. J., Green D. D. (1987) Cardiac contractile protein phosphatases. Purification of two enzyme forms and their characterization with subunit-specific antibodies. J. Biol. Chem. 262, 6257–6265 [PubMed] [Google Scholar]
- 36. Eidenmüller J., Fath T., Hellwig A., Reed J., Sontag E., Brandt R. (2000) Structural and functional implications of tau hyperphosphorylation: information from phosphorylation-mimicking mutated tau proteins. Biochemistry 39, 13166–13175 [DOI] [PubMed] [Google Scholar]
- 37. Nunbhakdi-Craig V., Machleidt T., Ogris E., Bellotto D., White C. L., 3rd, Sontag E. (2002) Protein phosphatase 2A associates with and regulates atypical PKC and the epithelial tight junction complex. J. Cell Biol. 158, 967–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sontag J. M., Nunbhakdi-Craig V., Montgomery L., Arning E., Bottiglieri T., Sontag E. (2008) Folate deficiency induces in vitro and mouse brain region-specific down-regulation of leucine carboxyl methyltransferase-1 and protein phosphatase 2A B(α) subunit expression that correlate with enhanced tau phosphorylation. J. Neurosci. 28, 11477–11487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sontag E., Hladik C., Montgomery L., Luangpirom A., Mudrak I., Ogris E., White C. L., 3rd (2004) Down-regulation of protein phosphatase 2A carboxyl methylation and methyltransferase may contribute to Alzheimer disease pathogenesis. J. Neuropathol. Exp. Neurol. 63, 1080–1091 [DOI] [PubMed] [Google Scholar]
- 40. Sontag J. M., Nunbhakdi-Craig V., Mitterhuber M., Ogris E., Sontag E. (2010) Regulation of protein phosphatase 2A methylation by LCMT1 and PME-1 plays a critical role in differentiation of neuroblastoma cells. J. Neurochem. 115, 1455–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Honkanen R. E., Golden T. (2002) Regulators of serine/threonine protein phosphatases at the dawn of a clinical era? Curr. Med. Chem. 9, 2055–2075 [DOI] [PubMed] [Google Scholar]
- 42. de Ancos J. G., Avila J. (1993) Differential distribution in white and grey matter of tau phosphoisoforms containing four tubulin-binding motifs. Biochem. J. 296, 351–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Binder L. I., Frankfurter A., Rebhun L. I. (1985) The distribution of tau in the mammalian central nervous system. J. Cell Biol. 101, 1371–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ogris E., Gibson D. M., Pallas D. C. (1997) Protein phosphatase 2A subunit assembly: the catalytic subunit carboxy terminus is important for binding cellular B subunit but not polyomavirus middle tumor antigen. Oncogene 15, 911–917 [DOI] [PubMed] [Google Scholar]
- 45. Surridge C. D., Burns R. G. (1994) The difference in the binding of phosphatidylinositol distinguishes MAP2 from MAP2C and Tau. Biochemistry 33, 8051–8057 [DOI] [PubMed] [Google Scholar]
- 46. Goedert M., Spillantini M. G. (2006) A century of Alzheimer disease. Science 314, 777–781 [DOI] [PubMed] [Google Scholar]
- 47. Turowski P., Favre B., Campbell K. S., Lamb N. J., Hemmings B. A. (1997) Modulation of the enzymatic properties of protein phosphatase 2A catalytic subunit by the recombinant 65-kDa regulatory subunit PR65α. Eur. J. Biochem. 248, 200–208 [DOI] [PubMed] [Google Scholar]
- 48. Evans B. J., Wang Z., Mobley L., Khosravi D., Fujii N., Navenot J. M., Peiper S. C. (2008) Physical association of GPR54 C-terminal with protein phosphatase 2A. Biochem. Biophys. Res. Commun. 377, 1067–1071 [DOI] [PubMed] [Google Scholar]
- 49. Eidenmüller J., Fath T., Maas T., Pool M., Sontag E., Brandt R. (2001) Phosphorylation-mimicking glutamate clusters in the proline-rich region are sufficient to simulate the functional deficiencies of hyperphosphorylated tau protein. Biochem. J. 357, 759–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Landrieu I., Smet-Nocca C., Amniai L., Louis J. V., Wieruszeski J. M., Goris J., Janssens V., Lippens G. (2011) Molecular implication of PP2A and Pin1 in the Alzheimer disease specific hyperphosphorylation of Tau. PLoS. ONE. 6, e21521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Goedert M., Jakes R., Qi Z., Wang J. H., Cohen P. (1995) Protein phosphatase 2A is the major enzyme in brain that dephosphorylates tau protein phosphorylated by proline-directed protein kinases or cyclic AMP-dependent protein kinase. J. Neurochem. 65, 2804–2807 [DOI] [PubMed] [Google Scholar]
- 52. Arendt T. (2001) Alzheimer disease as a disorder of mechanisms underlying structural brain self-organization. Neuroscience 102, 723–765 [DOI] [PubMed] [Google Scholar]