Background: Opioid receptor ligands provide effective pain treatment, but their use is limited by the development of tolerance and dependence.
Results: Recycling of internalized receptors is acutely regulated by the presence of their agonist.
Conclusion: Receptor recycling is actively modulated.
Significance: This acute modulation of receptor recycling provides a substrate for the rapid adaptations occurring during physiological time scales.
Keywords: G Protein-coupled Receptor (GPCR), Membrane Trafficking, Microscopic Imaging, Opiate Opioid, Receptor Recycling, Endocytosis, Mu Opioid Receptor, Live Cell Imaging, TIR-FM, Tolerance, Plasticity, Addiction, GPCR
Abstract
The μ-opioid receptor (MOR) is a member of the G protein-coupled receptor family and the main target of endogenous opioid neuropeptides and morphine. Upon activation by ligands, MORs are rapidly internalized via clathrin-coated pits in heterologous cells and dissociated striatal neurons. After initial endocytosis, resensitized receptors recycle back to the cell surface by vesicular delivery for subsequent cycles of activation. MOR trafficking has been linked to opioid tolerance after acute exposure to agonist, but it is also involved in the resensitization process. Several studies describe the regulation and mechanism of MOR endocytosis, but little is known about the recycling of resensitized receptors to the cell surface. To study this process, we induced internalization of MOR with [d-Ala2, N-Me-Phe4, Gly5-ol]-enkephalin (DAMGO) and morphine and imaged in real time single vesicles recycling receptors to the cell surface. We determined single vesicle recycling kinetics and the number of receptors contained in them. Then we demonstrated that rapid vesicular delivery of recycling MORs to the cell surface was mediated by the actin-microtubule cytoskeleton. Recycling was also dependent on Rab4, Rab11, and the Ca2+-sensitive motor protein myosin Vb. Finally, we showed that recycling is acutely modulated by the presence of agonists and the levels of cAMP. Our work identifies a novel trafficking mechanism that increases the number of cell surface MORs during acute agonist exposure, effectively reducing the development of opioid tolerance.
Introduction
The signaling of surface receptors is regulated by their functional state and cellular location. In polarized cells, receptor localization is dynamically controlled to achieve local signaling by receptor trafficking. Exocytosis is a key element controlling the number and location of functional receptors at the somatodendritic surface, hence controlling neuronal sensitivity to external stimuli. This type of modulation is especially important during synaptic plasticity and has been extensively explored with the AMPA-type glutamate receptors, providing insight to the relevance and complexity of this mechanism (1–3). The μ-opioid receptor (MOR)3 is a prototypical member of the G protein-coupled receptor (GPCR) family of signaling receptors containing seven transmembrane domains that couple to the GTP-binding proteins Gi/Go (4, 5). MOR agonists, including morphine and fentanyl, are commonly prescribed to treat pain, but their use is limited due to rapid generation of tolerance and physical dependence (6, 7).
Receptor activation by the specific agonist DAMGO induces MORs rapid internalization in heterologous and dissociated striatal neurons, producing low levels of tolerance when compared with morphine (6). After internalization, endocytosed receptors return to the cell surface for new cycles of receptor activation and signaling (6, 7). Based on the complexity of the system, it is not surprising that several studies have reported conflicting and sometimes opposite results on the role of endocytic trafficking in the development of opioid tolerance. Conflicting studies suggest that agonist-induced MOR internalization may either reduce or increase the development of opioid tolerance (6, 8–11). This apparent opposite effect of MOR trafficking reflects the difficulty of classical approaches to understand these complex events. Recycling of the MOR has been extensively studied at the biochemical, pharmacological, and electrophysiological level, but information at the single molecule level is only beginning to emerge (12–14). Furthermore, the kinetics of recycling during the initial minutes after agonist exposure and the molecular machinery involved in this process is completely unknown. Our studies sought to investigate in real time the fundamental events that mediate the delivery of resensitized receptors to the cell surface after ligand-induced endocytosis. We identified key molecular components of MOR recycling and studied whether recycling can be acutely modulated. We quantified the vesicular events at the single molecule level, investigated their cytoskeleton dependence, and described a rapid modulation of the vesicular delivery of the MOR to the cell surface by the presence of ligand and cAMP levels. Here we identified a novel mechanism that could underlie some of the complex roles of MOR trafficking during the development of opioid tolerance. Moreover, this acute modulatory mechanism could be pharmacologically exploited to prevent or impair the development of tolerance observed with opioid agonist treatments.
EXPERIMENTAL PROCEDURES
Materials
Superecliptic pHluorin-MOR (SEP-MOR) has been described previously (14). Briefly, we generated SEP-MOR by fusing the superecliptic pHluorin (15, 16), preceded by a cleavable signal sequence (17), to the murine MOR1. Mutant Rab expression constructs were generously provided by Drs. Stephen Ferguson (Robard Institute, London, Canada) and Marino Zerial (Max Planck Institute, Dresden, Germany). Myosin Vb clones were kindly provided by Dr. John Mercer (McLaughlin Research Institute, Great Falls, MT). Chemicals were purchased from Sigma unless otherwise stated. Human embryonal kidney (HEK293) cells were obtained from ATCC and maintained in Dulbecco's minimal medium supplemented with 10% fetal bovine serum (Invitrogen).
Striatal and HEK293 Culture and Transfection
Dissociated primary cultures were either dissected from embryonic day 17–18 Sprague-Dawley rat embryos (14) or purchased from BrainBits LLC (Springfield, IL). The striatum (caudate-putamen and nucleus accumbens) was dissected based on the criteria of Ventimiglia and Linsday as described elsewhere (14). Dissected tissue was dissociated in 1× trypsin/EDTA solution (Invitrogen) for 15 min before 1 ml of trypsin inhibitor was added for 5 min at room temperature. Cells were washed and triturated in DMEM plus 10% fetal calf serum (FCS; Invitrogen) using a glass pipette. Neurons were transfected with 2–3 μg of cDNA every 300,000 neurons after 5–7 days in vitro using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. HEK293 cells were transfected with Lipofectamine 2000 (Invitrogen) or Effectene following the manufacturer's protocol (Qiagen, Valencia, CA).
Live Cell Total Internal Reflection Fluorescent Microscopy (TIR-FM) Imaging
Imaging was performed using a fully motorized Nikon (Melville, NY) Ti-E inverted microscope with a CFI-APO ×100 1.49 numerical aperture TIR-FM objective and a motorized stage. 488- and 561-nm Coherent sapphire lasers (Coherent Inc., Santa Clara, CA) were used as a light source for total internal reflection fluorescence microscopy illumination modes, focusing the laser utilizing the back focal plane before experiments. Images were acquired at 10 Hz using an iXonEM+ DU897 back illuminated EMCCD camera (Andor, Belfast, UK) operated in the linear range during all of the imaging sessions. Sample temperature was controlled at 37 °C either using a Bioptechs Stable Z stage and objective warmer (Bioptechs, Butler, PA) or a microscope enclosure (Nikon) at 37 °C with 5% CO2. Images shown represent raw data with simple background subtraction of the averaged blank field intensity. Intensity values were corrected for photobleaching during the image collection (usually less than 5% from initial measurements). Treatments and washes were performed by perfusion of the imaged cells utilizing a custom built perfusion system. Endocytosis was induced by bath application in the incubator.
Statistical Analysis of Recycling Events
Recycling events of SEP-MORs were identified and scored blindly as described previously, images were background subtracted as described before (17–19). Analysis was performed using the public domain NIH Image program ImageJ software and AR-NIS-Elements (Nikon). To analyze the statistical significance of differences between selected treatments, we counted the number of events in each independent experiment (i.e. separate imaging session and different dishes of cultured cells were treated as independent experiments) and used the non-parametric test Mann-Whitney-Wilcoxon for single comparison. Statistical tests were calculated according to standard algorithms using GraphPad Prism software (San Diego, CA) with a significance threshold of p < 0.05.
Single Molecule Analysis
Single molecule analysis was performed as described previously (19). Single EGFP imaging was performed regularly before each recycling experiment to help compensate for day-to-day variables. The same imaging parameters were later utilized for recycling experiments (i.e. laser intensity, camera exposure, illumination angle, etc.). Imaging acquisition was performed utilizing a fully motorized Ti-E inverted Nikon microscope (Melville, NY) with a CFI-APO ×100 1.49 numerical aperture TIR-FM objective and a motorized stage at 37 °C. 488-nm Coherent sapphire lasers (Coherent Inc.) were used as a light source. Images were acquired at 10 Hz using an iXonEM+ DU897 back illuminated EMCCD camera (Andor, Belfast, UK) operated in the linear range during all of the imaging sessions. Natively folded single EGFPs purified from Escherichia coli were imaged onto poly-d-lysine-coated coverslips at pH 7.4 in the same buffer used for imaging neurons. Published data indicate that EGFP fluorescence intensity is similar to that of SEP when excited at 488 nm (19). Diffraction-limited spots of adsorbed fluorescent proteins were measured and blindly scored for maximum fluorescence intensity. Characteristic single step photobleaching was observed in all of the scored intensities.
RESULTS
Quantification and Kinetics of MOR Receptor Recycling at Single Vesicle Resolution
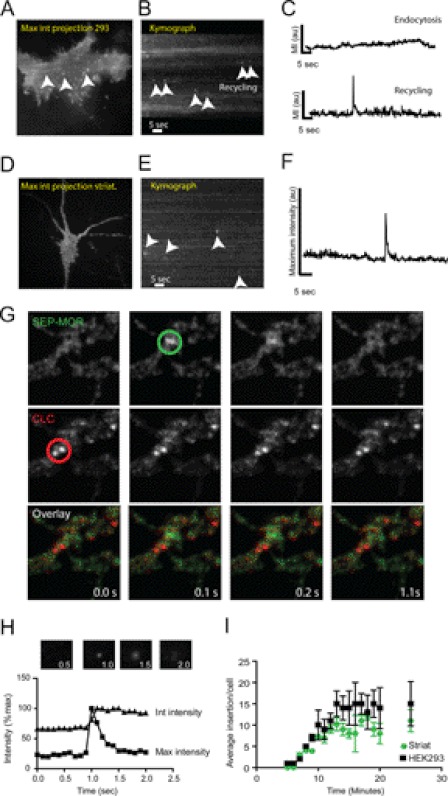
To investigate MOR recycling at the single receptor resolution, we utilized live cell TIR-FM. We combined TIR-FM, where imaging occurs close to the plasma membrane, with overexpression of MORs tagged with the pH-sensitive GFP variant SEP (15). This approach maximizes the signal from newly inserted receptors to the cell surface (17, 20). We first induced internalization of SEP-MORs expressed in HEK293 by incubation with the specific agonist 10 μm DAMGO for 10 min at 37 °C. After the incubation period, cells were transferred to a temperature-controlled imaging chamber to measure receptor recycling by TIR-FM. Single vesicles delivering recycling receptors were observed as abrupt increases of surface fluorescence in diffraction-limited spots, as depicted in maximum intensity projections (Fig. 1A). These increases in intensity were clearly visible using kymographs where a single spatial location is represented over time (Fig. 1B, arrowheads). Intensity measurements of these events presented rapid rise and decay kinetics as described previously for exocytic events of MORs (Fig. 1C, bottom) (14). Endocytic events were also present in the kymograph as prolonged streaks of receptors with lower maximum intensity when compared with recycling events (Fig. 1, B and C, top). The lifetime of GPCR endocytic events (>60 s) generally exceeds our 1-min imaging protocol, effectively reducing our capacity to observe them completely during our experiments (21). Recycling events with similar kinetics were also observed in dissociated striatal neurons transfected with SEP-MOR (10–15 days in vitro) after 10 μm DAMGO incubation. This suggests a similar mechanism of receptor delivery both in striatal neurons and HEK293 cells (Fig. 1, D–F). To further confirm that these rapid increases in intensity are indeed recycling events and not endocytic events, we co-expressed the clathrin-coated pit marker DsRed-clathrin light chain with SEP-MOR in HEK293 and striatal neurons. Clathrin-coated pits were identified by a stable (>20 s) diffraction-limited spot of DsRed-clathrin light chain (22). Clathrin pits did not colocalize with MOR insertion events, confirming that endocytosis and recycling occur at different spatial localization with different kinetics for the MOR (Fig. 1G).
FIGURE 1.
Imaging single vesicles recycling MORs to the cell surface in living cells. A, maximum intensity projection from a HEK293 cell transfected with SEP-MOR under TIR-FM illumination. A single image represents 60 s acquired at 10 Hz. Single recycling events are indicated by arrowheads. B, kymograph from the same cell as in A depicting recycling events (arrowhead). C, maximum intensity fluorescence obtained from a representative endocytic event (top) and from a recycling event (bottom). D, maximum intensity projection of striatal neuron transfected with SEP-MOR. E, kymograph showing several insertion events from the cell in D. F, maximum fluorescence intensity measurement of a single insertion event in striatal neurons. G, simultaneous dual imaging of SEP-MOR and DsRed-clathrin light chain (CLC) utilizing TIR-FM. Sequential frames show no colocalization between a kinetically distinct recycling event (green circle) and clathrin-coated pits (red circle) in striatal neurons. H, maximum intensity measurements indicate fusion of the recycling vesicle and spreading from the insertion site. Integrated intensity measurement shows a sustained increase in intensity, indicating the delivery of new receptors to the plasma membrane (inset boxes are 10 × 10 μm). I, time course of the number of recycling events per cell observed in striatal neurons and HEK293 cells (n = 17 cells; error bars, S.E.).
Receptor delivery and lateral diffusion onto the surface membrane can be confirmed by local intensity measurements (17, 20). After an initial increase in maximum fluorescence intensity (<0.2 s), which indicates the fusion and opening of the recycling vesicle, we observed an exponential decay in the maximum intensity due to receptor diffusion onto the plasma membrane (Fig. 1C, bottom). Integrated intensity measurements of a 10 × 10-μm area surrounding the insertion site showed a persistent elevation of intensity, suggesting the delivery of tagged receptors to the plasma membrane (Fig. 1H).
Insertion events were rapidly visible 2–5 min after 10 μm DAMGO addition to the imaging medium and increased to a stable frequency of insertion 10–20 min after the agonist addition. The average number of insertion events in HEK293 cells and neurons at 10 min after incubation was 10.4 ± 3.5/min (n = 17 cells) and 7.3 ± 0.3/min (n = 17 cells), respectively (Fig. 1I). Recycling events were not observed in the absence of agonist, neither in HEK293 nor in striatal neurons, but were still observed in the presence of the protein synthesis inhibitor cyclohexamide (10 μg/ml overnight; data not shown), strongly supporting the idea that the observed events are a result of agonist-induced recycling of surface receptors.
Quantification of Receptors Contained in Recycling Vesicles
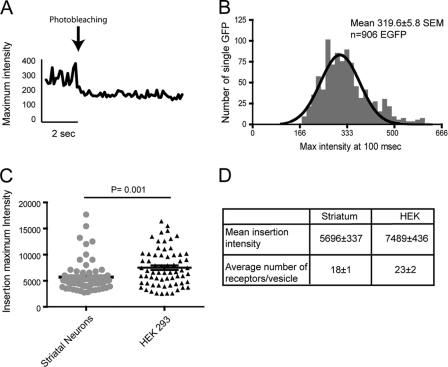
To gain molecular insight into the vesicles delivering MOR, we quantified the number of receptors per insertion event. Single EGFP and SEP molecules present similar intensities at pH 7.4 (15, 19, 23, 24). Utilizing the linear range of our EMCCD camera, we correlated the number of single EGFPs to the number of SEP-MORs observed during our imaging. To compensate for different critical angles during independent TIR-FM acquisitions, we recorded fluorescence intensity of single EGFPs imaged by TIR-FM at 488 nm prior to every recycling experiment. Intensity measurements were collected from single EGFP where characteristically single step photobleaching kinetics was observed (n = 906) (Fig. 2A). We combined all of the single measurements from multiple experiments to obtain a mean fluorescence intensity of a single EGFP under our imaging conditions. This resulted in an intensity of 319.6 ± 5.8 S.E. per single EGFP molecule (Fig. 2B). We next measured the maximum fluorescence intensity of individual insertions in HEK293 cells and striatal neurons to calculate the number of single receptors contained in a single recycling vesicle (Fig. 2, C and D). Single event intensity measurements indicated that the number of SEP-MORs per vesicle ranged from ∼8 to ∼55 with an average number of ∼18 receptors in striatal neurons and an average of ∼23 receptors in HEK293. Statistical analysis suggests that the number of receptors delivered to the cell surface in HEK293 cells is higher than those packed in striatal neurons.
FIGURE 2.
Single molecule quantification of SEP-MORs transported in recycling vesicles. A, representative maximum fluorescence intensity measurement of a single EGFP. B, single EGFP molecules were adsorbed and imaged prior to recycling experiments, utilizing identical TIR-FM imaging conditions. Isolated EGFPs were identified by their characteristic blinking and single photobleaching step; maximum intensities were binned, obtaining a mean intensity of 319.6 ± 5.8 S.E. per single EGFP molecule (n = 906). C, maximum fluorescence intensity of single recycling events was measured in striatal neurons and HEK293 cells with a mean intensity of 5696 and 7489, respectively (n = 70 for neurons, and n = 66 for HEK293 cells). D, number of SEP-MORs/vesicle ranged from ∼8 to ∼55, with an average number of ∼18 receptors in striatal neurons and an average of ∼23 receptors in HEK293.
Recycling Is Dependent on Actin-Microtubule Cytoskeleton
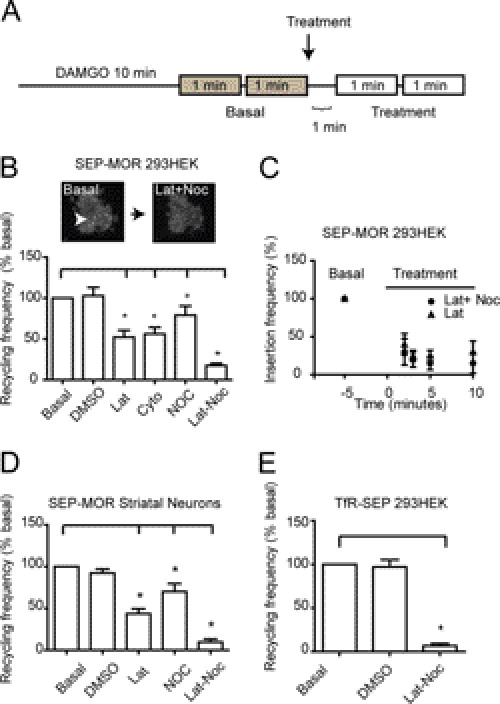
The actin and microtubule cytoskeleton, with their associated motor proteins, have a key role in vesicular and surface receptor trafficking (25–27). Microtubules have been involved in the recycling of many receptors to the cell surface, and actin has been specifically involved in the process of MOR trafficking and the recycling of the β2-adrenergic receptor (β2-AR) (25, 28, 29). Furthermore, it has been previously shown that MOR carboxyl tail binds to filamin A, an actin-binding protein that regulates MOR signaling and trafficking (30). To test whether the vesicular events delivering MOR to the cell surface were mediated by the actin-microtubule cytoskeleton, we first induced internalization of the SEP-MOR with 10 μm DAMGO for 10 min. After incubation, we measured vesicular recycling frequency as number of insertions/min. Measurements of two consecutive periods of 1 min helped decrease small variabilities observed in recycling frequency. After initial measurements (basal), cytoskeletal destabilizing agents were added to the incubation medium, and frequency was measured in the same cells in two consecutive periods of 1 min, as depicted in Fig. 3A. The addition of the actin-disrupting agents latrunculin or cytochalasin D to the incubation medium significantly reduced receptor insertion frequency to 52 and 56%, respectively, compared with the basal level (Fig. 3B). Vehicle-treated cells did not present a significant difference in frequency, suggesting that the difference observed is specific to the treatment and not an artifact of changes in the incubation medium. We next tested whether the disruption of the microtubule cytoskeleton had any effect on the recycling frequency. Recycling frequency was measured in naive cells before and after the addition of nocodazole, a microtubule-disrupting agent, to the imaging medium following the protocol shown in Fig. 3A. The addition of nocodazole reduced insertion frequency to 79% compared with basal level. We next tested whether the actin- and microtubule-disrupting drugs had an additive effect on the recycling frequency. We measured the recycling frequency of naive cells before and after the addition of both latrunculin and nocodazole to the imaging medium. Insertion frequency was significantly reduced in treated cells to 17% compared with basal level (Fig. 3B). A maximum intensity projection of a representative cell transfected with SEP-MOR and treated with DAMGO for 10 min before and after latrunculin-nocodazole depicts the reduction in recycling frequency observed in these cells (Fig. 3B, inset). To investigate if the actin and microtubule effects can be separated in different subsets of recycling events, we studied the kinetics of reinsertion in the presence of each cytoskeleton-disrupting agent. Basal levels of insertion frequency were normalized as 100% before treatment. Vesicle recycling frequency measurements were done at different time points after the addition of either latrunculin or latrunculin and nocodazole (Fig. 3C). No significant difference was observed between treatments, suggesting that the recyclings observed by TIR-FM share a similar dependence on the actin and microtubule cytoskeleton. We next asked whether this cytoskeleton dependence applied to striatal neurons where MORs are endogenously expressed. We found that the addition to the imaging medium of latrunculin or nocodazole to naive neurons reduced the frequency of insertion events to 43.5% and 70.7%, respectively, compared with basal level. The addition of both cytoskeletal disrupting agents to DAMGO-treated neurons reduced the total frequency of receptor recycling to 9.6% compared with basal level (Fig. 3D). To test whether the inhibition of vesicular recycling observed with the disruption of the cytoskeleton was a specific mechanism for the MOR or a general mechanism for all the recycling vesicles observed by TIR-FM, we measured the recycling of the transferrin receptor tagged with the superecliptic pHluorin (TfR-SEP) both in the presence and absence of latrunculin-nocodazole. TfR-SEP recycling frequency was dramatically reduced to 6.6% compared with the basal level after the addition of latrunculin-nocodazole to the imaging medium (Fig. 3E).
FIGURE 3.
MOR recycling is actin- and microtubule-dependent. A, experimental design for MOR recycling experiments. Cells were incubated with 10 μm DAMGO to induce MOR internalization and sequentially imaged by TIR-FM. Two 1-min periods were recorded and averaged for basal level and treatment to reduce small variability. B, normalized MOR recycling frequency in HEK293 cells. Recycling frequency was compared in the same cell before and after treatment. The addition of DMSO did not induce any significant changes in the recycling frequency when compared with basal level. The addition of 10 μm latrunculin or 2.5 μm cytochalasin D significantly reduced the recycling frequency compared with basal level (52 and 56%, respectively). The addition of 10 μm nocodazole reduced the frequency to 79% compared with basal level. Latrunculin (Lat) and nocodazole (Noc) had an additive effect. Maximum intensity projection of a single HEK293 cell depicts the representative reduction of recycling events observed after the addition of latrunculin and nocodazole to the imaging medium. C, normalized recycling frequency before and after the addition of either latrunculin or latrunculin and nocodazole to the imaging medium. Recycling frequency was measured 2, 3, 5, and 10 min after treatment. No significant difference in recycling kinetics was observed between conditions (n = 5 cells). D, normalized recycling frequency in dissociated striatal neurons. The latrunculin and nocodazole addition to the imaging medium significantly decreased recycling when compared with basal level (43 and 71%, respectively). The co-addition of latrunculin and nocodazole significantly reduced recycling (9%). E, TfR-SEP recycling and cytoskeletal disrupting agents were investigated by TIR-FM. The co-addition of latrunculin and nocodazole to the incubation medium had a significant reduction in the number of recycling events observed under TIR-FM illumination (6% from basal frequency).
MOR Recycling Is Rab4, Rab11, and Myosin Vb-dependent
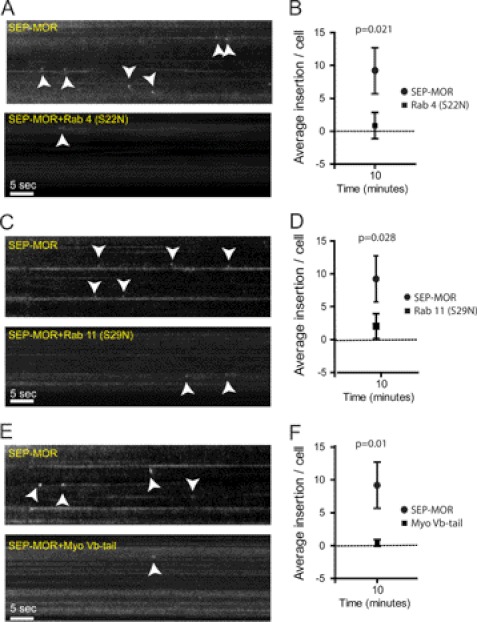
Recycling vesicles reach the cell surface through a complex sorting process (31–34). Rab4 and Rab11 are central players in the recycling of several GPCRs, including the MOR (18, 33, 35–38). However, it is not clear if the rapid recycling observed by TIR-FM is mediated by the same mechanism. To investigate the molecular mechanism involved in the acute recycling of MOR, we applied our real-time imaging approach to examine the role of Rab4 and Rab11. Internalization was induced with DAMGO in HEK293 cells co-expressing SEP-MOR with the dominant variant Rab4 (S22N). Rapid recycling measured at 10 min after agonist treatment was significantly reduced compared with control cells (Fig. 4, A and B). We next investigated the role of Rab11 by co-expressing the dominant negative Rab11 (S29N) with SEP-MOR. Rapid recycling was also impaired in these cells (Fig. 4, C and D).
FIGURE 4.
Rab 4, Rab 11, and myosin Vb mediate recycling of MORs. A, 1-min kymographs depicting recycling events in control SEP-MOR cells (top) and cells co-expressing SEP-MOR and Rab 4 (S22N) (bottom). B, statistical analysis showing the number of recycling events 10 min after agonist exposure for both groups (n = 10 cells; error bars, S.E.). C, kymograph of control cells expressing SEP-MOR (top) or SEP-MOR and Rab11 (S29N) (bottom). D, average number of recycling events in cells transfected with SEP-MOR or in cells co-transfected with the dominant negative Rab11 (S29N) (n = 10 cells; bar, S.E.). E, 1-min kymographs showing recycling events in cells expressing SEP-MOR (top) or SEP-MOR and myoVb-tail (bottom). F, average number of recycling events 10 min after agonist exposure was plotted for both groups (n = 10 cells; error bar, S.E.).
Myosin Vb binds to actin and Rab11 and is known to be extensively involved in vesicular trafficking and in receptor recycling to the cell surface (35, 39–41). To test if myosin Vb mediates the transport of MOR vesicles to the cell surface, we utilized the myosin Vb-tail as a dominant negative (35, 39, 41). Myosin Vb-tail dramatically reduced MOR recycling in cells expressing both constructs when compared with controls (Fig. 4, E and F). Taken together, these results identify part of the molecular machinery involved in the rapid recycling of MORs and provide a link between recycling vesicles and the cytoskeleton. Further studies will be needed to investigate whether myosin Vb exerts its modulation via a direct binding with the MOR.
MOR Recycling Frequency Is Regulated by Presence of Agonist
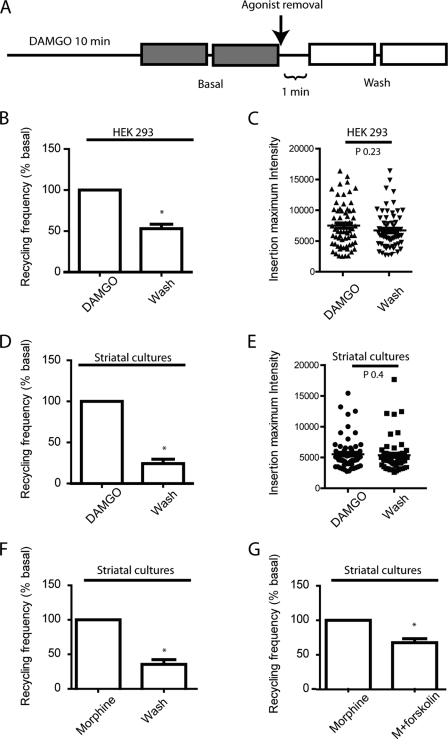
We have previously observed acute regulation of vesicular recycling of the β2-AR in HEK293 and hippocampal neurons (18). Fusion of β2-AR recycling vesicles to the plasma membrane is actively modulated by the presence of its ligands. This regulation of trafficking can potentially control the number and location of active receptors at the plasma membrane. To investigate whether the presence of the MOR ligand in the extracellular medium could regulate the delivery of the internalized receptors to the cell surface, we quantified the frequency of vesicular delivery of MOR after agonist-induced internalization. Cells were preincubated with DAMGO for 10 min to induce receptor internalization before basal measurements. After agonist removal, cells were measured again to compare frequency of vesicular insertion in the same cells before and after the presence of agonist (Fig. 5A). We first investigated the regulation of MOR recycling in HEK293 cells. After treatment, agonist removal induced a statistically significant decrease in the frequency of recycling receptors compared with basal level in HEK293 (Fig. 5B). To explore the possibility that the decrease in the number of vesicles could be associated to a change in the number of receptors delivered per vesicle, we quantified the total number of receptors for each vesicle before and after agonist washout. The number of receptors per vesicle did not significantly change before and after agonist removal, indicating that no net changes of cargo occur (Fig. 5C). We next sought to test if the regulation of the number of vesicles recycling MOR also occurs in neurons, where MORs are natively expressed. We utilized the same imaging and incubation protocol previously described (Fig. 5A) and measured recycling of MOR in dissociated striatal cultures. Recycling frequency was dramatically reduced when DAMGO was removed from the incubation medium (Fig. 5D). As observed in HEK293 cells, the number of MOR packed in recycling vesicles did not change after the removal of DAMGO (Fig. 5E). To investigate whether this type of regulation is agonist dependent or is a general mechanism, we utilized morphine, which induces robust internalization in dissociated striatal neurons (43). As measured with DAMGO, morphine removal from the incubation medium produced a rapid decrease in recycling frequency when compared with basal level (Fig. 5F). Finally, to test whether this rapid regulation is mediated by the levels of cAMP, a downstream effector of MOR activation, neurons were treated with morphine, and recycling was measured before and after the addition of forskolin to the incubation medium. Forskolin induced a rapid reduction of recycling frequency in the presence of morphine (Fig. 5G). This result mimicked the effect of agonist removal observed previously, suggesting that modulation of receptor recycling is controlled by cAMP levels.
FIGURE 5.
MOR recycling frequency is modulated by the presence of agonist. A, experimental design for MOR recycling experiments by TIR-FM. Cells are incubated with 10 μm DAMGO to induce MOR internalization and sequentially imaged by TIR-FM. Two 1-min periods are recorded for basal and wash to reduce variabilities. B, recycling frequency was measured before and after wash in HEK293 cells. Removal of DAMGO induced a significant decrease in recycling frequency to 53% compared with basal level (n = 11 cells). C, the number of receptors contained in the recycling vesicles did not change significantly between treatments (n = 64 insertions for basal and n = 66 for wash). D, MOR recycling frequency was also tested in striatal neurons. Agonist washout induced a marked reduction in the recycling frequency of MORs to 24% compared with basal level (n = 10 cells). E, the number of receptors concentrated in single recycling vesicles did not significantly change when comparing conditions (n = 69 and n = 59 insertion events for basal and wash, respectively). F, dissociated striatal cultures transfected with SEP-MOR were preincubated with 10 μm morphine. Recycling frequency was measured in the presence of or after removal of morphine. G, striatal cultures transfected with SEP-MOR were incubated with 10 μm morphine for 10 min, and recycling frequency was measured before and after the addition of 25 μm forskolin to the imaging medium in the presence of morphine.
DISCUSSION
Our work describes a novel regulatory mechanism controlling the recycling of MORs to the cell surface after agonist-induced internalization. This regulation is mediated by the presence of MOR agonists and cAMP levels suggesting acute control of receptor recycling by MOR activation. Furthermore, this type of mechanism could have direct implications for the modulation of antinociceptive tolerance to MOR agonists (7). Receptor recycling has been proposed to counteract opioid tolerance, particularly when utilizing agonists that induce strong internalization (6). To investigate the molecular mechanisms by which recycling contributes to reduced tolerance, we utilized DAMGO and morphine to induce internalization in HEK293 and striatal neurons, respectively, and measured recycling at single cell resolution by TIR-FM.
After agonist-induced activation, desensitized MORs are removed from the cell surface by clathrin-coated pits, to enter the endocytic recycling pathway. Endocytosed receptors are then resensitized and recycled to the cell surface for future rounds of receptor activation (9, 12). The recycling pathway, where internalized receptors are sorted back to the cell surface, was considered a default mechanism until recently (13, 33). New analytical approaches and a detailed study of receptor sequences involved in trafficking have shown that the recycling pathway is a complex system that can be rapidly modulated by receptor activation (17, 18, 33).
We took advantage of the increased signal/noise ratio obtained by TIR-FM to measure the fusion of recycling vesicles delivering MOR to the cell surface. This approach allowed us to perform real-time measurements of receptor recycling (44). We have previously used this technique to measure single vesicles fusing to the cell surface in HEK293 and dissociated neuronal cultures and to study the recycling kinetics of different surface receptors (17–19). Agonist-induced MOR recycling was rapidly observed 2–3 min after agonist exposure, increasing to a constant rate of vesicular fusion at ∼10 min.
Surface receptor recycling to the plasma membrane has been linked to the cytoskeleton before, and actin has been shown to have a key role during GPCR recycling to the cell surface (29, 45). We found that actin- and microtubule-disrupting agents, combined, significantly decreased recycling events. Transferrin receptor recycling was also dramatically reduced in the presence of actin- and microtubule-disrupting agents. Transferrin receptor recycling to the cell surface has not been generally associated with the microtubule cytoskeleton, and it has been proposed that TFR recycling occurs via two distinct compartments to the cell surface (28, 42).
We next investigated the molecular machinery necessary for the recycling of MOR to the cell surface. First we established that recycling observed by TIR-FM is dependent on the Rab4 and Rab11 GTPases. Second, we showed that the dominant negative myosin Vb reduced MOR surface delivery. Our work shows functional dependence but no direct interaction between myosin Vb and MOR. Further studies will be needed to determine the specific molecular mechanism by which myosin Vb controls MOR recycling. These experiments begin to identify part of the molecular mechanisms driving MOR from the recycling endosomes to the cell surface.
Our previous work described acute regulation of the β2-AR recycling pathway by the activation of the receptor (18). Here, we asked if this rapid type of regulation could be a general feature of GPCRs and be extended to MORs. We examined MOR recycling frequency in the presence of two different agonists, DAMGO and morphine, in HEK293 and striatal cultures. Within seconds of agonist removal, recycling frequency decreased in HEK293 and in striatal neurons. Next we showed that the number of MORs packed in recycling vesicles, as measured by single SEP-MOR molecules, did not change significantly whether the agonist was present or not. Finally, we showed that regulation of recycling is also observed with forskolin, suggesting that receptor activation and cAMP levels are important in this modulation (18).
Taken together, our results suggest that recycling can be actively modulated to control cellular sensitivity to opioids within minutes after exposure to agonist. This rapid modulation could be pharmacologically exploited to prevent opiate-induced tolerance.
Acknowledgments
We thank Mark von Zastrow, Jacqueline Flores-Otero, and Julieta Gleiser for valuable discussion and critical comments. We thank Kurt Thorn and the UCSF Nikon Imaging Center for access to facilities used to carry out some of the live imaging experiments.
This work was supported, in whole or in part, by National Institutes of Health (NIH) Grant K99/R00; NIH, National Institute on Drug Abuse, Grant DA023444; and NIH, American Recovery and Reinvestment Act, Grant 1P30NS069258. This work was also supported by the Puerto Rico Science, Technology, and Research Trust Fund.
- MOR
- μ-opioid receptor
- GPCR
- G protein-coupled receptor
- SEP
- superecliptic pHluorin
- DAMGO
- Tyr-d-Ala-Gly-N-methyl-Phe-Gly-ol
- EGFP
- enhanced green fluorescent protein
- TfR
- transferrin receptor
- β2-AR
- β2-adrenergic receptor
- EMCCD
- electron-multiplying charge-coupled device.
REFERENCES
- 1. Malinow R., Malenka R. C. (2002) AMPA receptor trafficking and synaptic plasticity. Annu. Rev. Neurosci. 25, 103–126 [DOI] [PubMed] [Google Scholar]
- 2. Kennedy M. J., Ehlers M. D. (2011) Mechanisms and function of dendritic exocytosis. Neuron 69, 856–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Groc L., Choquet D. (2008) Measurement and characteristics of neurotransmitter receptor surface trafficking (Review). Mol. Membr. Biol. 25, 344–352 [DOI] [PubMed] [Google Scholar]
- 4. Pierce K. L., Premont R. T., Lefkowitz R. J. (2002) Seven-transmembrane receptors. Nat. Rev. Mol. Cell Biol. 3, 639–650 [DOI] [PubMed] [Google Scholar]
- 5. Gainetdinov R. R., Premont R. T., Bohn L. M., Lefkowitz R. J., Caron M. G. (2004) Desensitization of G protein-coupled receptors and neuronal functions. Annu. Rev. Neurosci. 27, 107–144 [DOI] [PubMed] [Google Scholar]
- 6. Koch T., Widera A., Bartzsch K., Schulz S. (2005) Receptor endocytosis counteracts the development of opioid tolerance. Mol. Pharmacol. 67, 280–287 [DOI] [PubMed] [Google Scholar]
- 7. Koch T., Höllt V. (2008) Role of receptor internalization in opioid tolerance and dependence. Pharmacol. Ther. 117, 199–206 [DOI] [PubMed] [Google Scholar]
- 8. Enquist J., Kim J. A., Bartlett S., Ferwerda M., Whistler J. L. (2011) A novel knock-in mouse reveals mechanistically distinct forms of morphine tolerance. J. Pharmacol. Exp. Ther. 338, 633–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koch T., Schulz S., Schröder H., Wolf R., Raulf E., Höllt V. (1998) Carboxyl-terminal splicing of the rat μ-opioid receptor modulates agonist-mediated internalization and receptor resensitization. J. Biol. Chem. 273, 13652–13657 [DOI] [PubMed] [Google Scholar]
- 10. Whistler J. L., Chuang H. H., Chu P., Jan L. Y., von Zastrow M. (1999) Functional dissociation of μ-opioid receptor signaling and endocytosis. Implications for the biology of opiate tolerance and addiction. Neuron 23, 737–746 [DOI] [PubMed] [Google Scholar]
- 11. Martini L., Whistler J. L. (2007) The role of μ-opioid receptor desensitization and endocytosis in morphine tolerance and dependence. Curr. Opin. Neurobiol. 17, 556–564 [DOI] [PubMed] [Google Scholar]
- 12. Qiu Y., Law P. Y., Loh H. H. (2003) μ-Opioid receptor desensitization. Role of receptor phosphorylation, internalization, and representation. J. Biol. Chem. 278, 36733–36739 [DOI] [PubMed] [Google Scholar]
- 13. Gage R. M., Kim K. a., Cao T. T., von Zastrow M. (2001) A transplantable sorting signal that is sufficient to mediate rapid recycling of G protein-coupled receptors. J. Biol. Chem. 276, 44712–44720 [DOI] [PubMed] [Google Scholar]
- 14. Yu Y. J., Dhavan R., Chevalier M. W., Yudowski G. A., von Zastrow M. (2010) Rapid delivery of internalized signaling receptors to the somatodendritic surface by sequence-specific local insertion. J. Neurosci. 30, 11703–11714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miesenböck G., De Angelis D. A., Rothman J. E. (1998) Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature 394, 192–195 [DOI] [PubMed] [Google Scholar]
- 16. Sankaranarayanan S., De Angelis D., Rothman J. E., Ryan T. A. (2000) The use of pHluorins for optical measurements of presynaptic activity. Biophys. J. 79, 2199–2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yudowski G. A., Puthenveedu M. A., von Zastrow M. (2006) Distinct modes of regulated receptor insertion to the somatodendritic plasma membrane. Nat. Neurosci. 9, 622–627 [DOI] [PubMed] [Google Scholar]
- 18. Yudowski G. A., Puthenveedu M. A., Henry A. G., von Zastrow M. (2009) Cargo-mediated regulation of a rapid Rab4-dependent recycling pathway. Mol. Biol. Cell 20, 2774–2784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yudowski G. A., Puthenveedu M. A., Leonoudakis D., Panicker S., Thorn K. S., Beattie E. C., von Zastrow M. (2007) Real-time imaging of discrete exocytic events mediating surface delivery of AMPA receptors. J. Neurosci. 27, 11112–11121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schmoranzer J., Goulian M., Axelrod D., Simon S. M. (2000) Imaging constitutive exocytosis with total internal reflection fluorescence microscopy. J. Cell Biol. 149, 23–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Puthenveedu M. A., von Zastrow M. (2006) Cargo regulates clathrin-coated pit dynamics. Cell 127, 113–124 [DOI] [PubMed] [Google Scholar]
- 22. Ehrlich M., Boll W., Van Oijen A., Hariharan R., Chandran K., Nibert M. L., Kirchhausen T. (2004) Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell 118, 591–605 [DOI] [PubMed] [Google Scholar]
- 23. Patterson G. H., Knobel S. M., Sharif W. D., Kain S. R., Piston D. W. (1997) Use of the green fluorescent protein and its mutants in quantitative fluorescence microscopy. Biophys. J. 73, 2782–2790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stepanenko O. V., Verkhusha V. V., Kazakov V. I., Shavlovsky M. M., Kuznetsova I. M., Uversky V. N., Turoverov K. K. (2004) Comparative studies on the structure and stability of fluorescent proteins EGFP, zFP506, mRFP1, “dimer2,” and DsRed1. Biochemistry 43, 14913–14923 [DOI] [PubMed] [Google Scholar]
- 25. Hoepfner S., Severin F., Cabezas A., Habermann B., Runge A., Gillooly D., Stenmark H., Zerial M. (2005) Modulation of receptor recycling and degradation by the endosomal kinesin KIF16B. Cell 121, 437–450 [DOI] [PubMed] [Google Scholar]
- 26. Kennedy M. J., Ehlers M. D. (2006) Organelles and trafficking machinery for postsynaptic plasticity. Annu. Rev. Neurosci. 29, 325–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schonteich E., Wilson G. M., Burden J., Hopkins C. R., Anderson K., Goldenring J. R., Prekeris R. (2008) The Rip11/Rab11-FIP5 and kinesin II complex regulates endocytic protein recycling. J. Cell Sci. 121, 3824–3833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Apodaca G. (2001) Endocytic traffic in polarized epithelial cells. Role of the actin and microtubule cytoskeleton. Traffic 2, 149–159 [DOI] [PubMed] [Google Scholar]
- 29. Puthenveedu M. A., Lauffer B., Temkin P., Vistein R., Carlton P., Thorn K., Taunton J., Weiner O. D., Parton R. G., von Zastrow M. (2010) Sequence-dependent sorting of recycling proteins by actin-stabilized endosomal microdomains. Cell 143, 761–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Onoprishvili I., Andria M. L., Kramer H. K., Ancevska-Taneva N., Hiller J. M., Simon E. J. (2003) Interaction between the mu opioid receptor and filamin A is involved in receptor regulation and trafficking. Mol. Pharmacol. 64, 1092–1100 [DOI] [PubMed] [Google Scholar]
- 31. Grant B. D., Donaldson J. G. (2009) Pathways and mechanisms of endocytic recycling. Nat. Rev. Mol. Cell Biol. 10, 597–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maxfield F. R., McGraw T. E. (2004) Endocytic recycling. Nat. Rev. Mol. Cell Biol. 5, 121–132 [DOI] [PubMed] [Google Scholar]
- 33. Sorkin A., von Zastrow M. (2009) Endocytosis and signaling. Intertwining molecular networks. Nat. Rev. Mol. Cell Biol. 10, 609–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gruenberg J. (2001) The endocytic pathway. A mosaic of domains. Nat. Rev. Mol. Cell Biol. 2, 721–730 [DOI] [PubMed] [Google Scholar]
- 35. Volpicelli L. A., Lah J. J., Fang G., Goldenring J. R., Levey A. I. (2002) Rab11a and myosin Vb regulate recycling of the M4 muscarinic acetylcholine receptor. J. Neurosci. 22, 9776–9784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Leterrier C., Bonnard D., Carrel D., Rossier J., Lenkei Z. (2004) Constitutive endocytic cycle of the CB1 cannabinoid receptor. J. Biol. Chem. 279, 36013–36021 [DOI] [PubMed] [Google Scholar]
- 37. Wang F., Chen X., Zhang X., Ma L. (2008) Phosphorylation state of μ-opioid receptor determines the alternative recycling of receptor via Rab4 or Rab11 pathway. Mol. Endocrinol. 22, 1881–1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Archer-Lahlou E., Audet N., Amraei M. G., Huard K., Paquin-Gobeil M., Pineyro G. (2009) Src promotes δ-opioid receptor (DOR) desensitization by interfering with receptor recycling. J. Cell. Mol. Med. 13, 147–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lapierre L. A., Kumar R., Hales C. M., Navarre J., Bhartur S. G., Burnette J. O., Provance D. W., Jr., Mercer J. A., Bähler M., Goldenring J. R. (2001) Myosin vb is associated with plasma membrane recycling systems. Mol. Biol. Cell 12, 1843–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Evans L. L., Lee A. J., Bridgman P. C., Mooseker M. S. (1998) Vesicle-associated brain myosin-V can be activated to catalyze actin-based transport. J. Cell Sci. 111, 2055–2066 [DOI] [PubMed] [Google Scholar]
- 41. Wang Z., Edwards J. G., Riley N., Provance D. W., Jr., Karcher R., Li X. D., Davison I. G., Ikebe M., Mercer J. A., Kauer J. A., Ehlers M. D. (2008) Myosin Vb mobilizes recycling endosomes and AMPA receptors for postsynaptic plasticity. Cell 135, 535–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jin M., Snider M. D. (1993) Role of microtubules in transferrin receptor transport from the cell surface to endosomes and the Golgi complex. The J. Biol. Chem. 268, 18390–18397 [PubMed] [Google Scholar]
- 43. Haberstock-Debic H., Kim K. A., Yu Y. J., von Zastrow M. (2005) Morphine promotes rapid, arrestin-dependent endocytosis of μ-opioid receptors in striatal neurons. J. Neurosci. 25, 7847–7857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yudowski G. A., von Zastrow M. (2011) Investigating G protein-coupled receptor endocytosis and trafficking by TIR-FM. Methods Mol. Biol. 756, 325–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sheff D. R., Kroschewski R., Mellman I. (2002) Actin dependence of polarized receptor recycling in Madin-Darby canine kidney cell endosomes. Mol. Biol. Cell 13, 262–275 [DOI] [PMC free article] [PubMed] [Google Scholar]