Background: HIV-1 Vpu enhances viral release by down-regulating the host restriction factor BST-2/Tetherin.
Results: Vpu targets BST-2 for ubiquitination, and this does not require the BST-2 cytoplasmic Lys, Ser, or Thr residues.
Conclusion: BST-2 is degraded within the lysosome as a result of Vpu-mediated ubiquitination.
Significance: Understanding how Vpu overcomes BST-2 may lead to anti-HIV-1 therapies.
Keywords: AIDS, HIV-1, Lysosomes, Protein Degradation, Ubiquitin, BST-2, Tetherin, Vpu, Viral Egress
Abstract
The cellular protein BST-2/CD317/Tetherin has been shown to inhibit the release of HIV-1 and other enveloped viruses from infected cells. The HIV-1 accessory protein Vpu binds to both BST-2 and βTrCP, a substrate-recognition subunit for the SCF (Skip1-Cullin1-F-box protein) E3 ubiquitin ligase complex. This interaction leads to both the degradation of BST-2 and the enhancement of viral egress. Recently BST-2 was shown to be ubiquitinated in this process. Here we have confirmed the Vpu- and βTrCP-dependent multi/polyubiquitination of BST-2. Ubiquitinated BST-2 accumulated in cells treated with a lysosomal inhibitor but not a proteasomal inhibitor. Additionally, we observed that a BST-2 mutant deleted for its cytosolically exposed lysine residues is also ubiquitinated. Subsequent experiments suggested that Vpu promotes BST-2 ubiquitination upon amino acid residues bearing hydroxyl- but not thiol-bearing side chains. However, a BST-2 mutant bearing substitutions for its cytoplasmically exposed Ser, Thr, and Lys residues was still down-regulated, ubiquitinated, and degraded in a Vpu-dependent manner. Our results suggest that Vpu may target either the BST-2 cytoplasmic Tyr residues or the NH2 terminus itself for ubiquitination.
Introduction
The ability to add or remove various combinations of ubiquitin (Ub)2 monomers and polymers to target proteins affords the cell an incredible degree of control and flexibility. Although ubiquitination was initially characterized as a simple mechanism to target cellular proteins for degradation within the proteasome, it is now recognized as a far more complex mechanism that functions in a wide spectrum of processes. Perhaps not surprisingly, microbial (1) and viral (2, 3) pathogens have learned to interfere with the host Ub machinery. The HIV-1 accessory proteins Vpu, Vif, and Vpr each interact with cullin E3 Ub ligase complexes, thereby allowing the virus to fine-tune and exploit the host Ub system for its own benefit (4). Our work focuses upon Vpu, which interacts with the Cullin1-Skp1 (SCF) E3 Ub ligase complex. This binding is indirect and is mediated via the β-transducin repeat containing (βTrCP) adaptor proteins 1 and 2 (5). βTrCP proteins utilize their F-box domains to bind to the SCF E3 complex and their repeating WD motifs to bind to a phosphorylated DpSGXXpS domain within Vpu. βTrCP1/2 are members of a large family of mammalian F-box proteins, many of which function as interchangeable substrate-recognition subunits for SCF (for review, see Ref. 6). The most well characterized target of the resulting Vpu-βTrCP-SCF complex is the viral receptor CD4, which interacts with Vpu in the endoplasmic reticulum (ER) (7). This leads to the ubiquitination of CD4 (8, 9) on both lysine and serine/threonine residues located within its cytoplasmic tail. CD4 is subsequently degraded by the proteasome (10) via what appears to be a non-canonical ER-associated degradation (ERAD) pathway (11). The fate of Vpu in this process is the subject of some debate, as it has been reported to either be exceptionally stable or to be ubiquitinated and degraded (12–16).
More recently, Vpu has been shown to target the host restriction factor BST-2, which is also referred to as CD317/HM1.24/Tetherin. The presence of this type II, glycosylphosphatidylinositol-anchored membrane protein at the cell surface severely impedes the egress of HIV (17) and other enveloped viruses (18, 19). HIV-1 encodes Vpu to overcome this inhibition (20), whereas other viruses appear to have devised their own means to circumvent BST-2 restriction (21–24). The Vpu-dependent down-regulation of CD4 and BST-2 share several common mechanistic features. For example, surface and total cellular levels of both proteins are diminished in the presence of Vpu (25–27). Additionally, CD4 and BST-2 have each been found in complexes with Vpu and βTrCP, and βTrCP is required for the Vpu-dependent degradation of CD4 and BST-2 (28, 29). However, unlike CD4, BST-2 and Vpu appear to colocalize within a perinuclear/trans-Golgi network compartment, and whereas several studies using exogenously expressed BST-2 have concluded that Vpu-dependent BST-2 degradation is proteasomal, studies examining endogenously expressed BST-2 weigh more heavily in favor of lysosomal degradation (30). These observations indicate that the mechanism of Vpu-dependent down-regulation for BST-2 is similar yet distinct from that of CD4. Regardless, the requirement for βTrCP in both cases suggests that ubiquitin is involved in each.
Although the conjugation of Ub moieties to proteins most often occurs via isopeptide bonds to lysine residues, ubiquitination has also been shown to take place upon cysteine, serine, threonine, and NH2-terminal methionine residues (31). Ubiquitination of membrane proteins typically takes place on cytosolically exposed target residues. Within the BST-2 cytoplasmic domain there are two lysines, two cysteines, two serines, one threonine, and the NH2-terminal methionine. The possibility that ubiquitination is involved in the Vpu-dependent degradation of BST-2 has prompted several groups to examine the effects of amino acid substitutions for the aforementioned lysine 18 and 21 residues presumably because thus far lysines have been the most commonly observed targets for ubiquitination. Mitchell et al. (32) first reported that a BST-2 mutant substituted for both of these lysine residues was down-regulated from the cell surface by Vpu, although they showed no data to illustrate this. Mangeat et al. (27) were the first to show that a BST-2 cytosolic-lysine mutant is still degraded by Vpu. They also noted that their attempts to show BST-2 ubiquitination by Vpu were unsuccessful even in the presence of a proteasomal inhibitor, but no data were presented for those experiments. Pardieu et al. (33) show what they interpret to be a monoubiquitinated form of BST-2 in bafilomycin-treated HeLa cells transfected with plasmids expressing BST-2, Vpu, and a His-tagged ubiquitin. However, because bafilomycin traps secretory proteins within the ER, which does not appear to be the cellular location wherein BST-2 interacts with Vpu, the drug likely prevented their detection of relevant species (33). Goffinet et al. (34) confirmed that a BST-2(K18R,K21R) mutant inhibits viral egress, is sensitive to inhibition by Vpu and can be down-regulated from the cell surface by Vpu. However, unlike WT BST-2, which they observed to be degraded by Vpu, the BST-2(K18R,K21R) protein remained stable in the presence of Vpu, leading them to conclude that Vpu particle release and BST-2 degradation functions are separable (34). Most recently, Tokarev et al. (35) have provided the clearest demonstration that BST-2 is ubiquitinated in the presence of Vpu. However, even after making substitutions for all of BST-2 cytoplasmically exposed lysine, cysteine, serine, and threonine residues, BST-2 was still ubiquitinated and down-regulated by Vpu, albeit to a lesser degree than WT BST-2. In their hands, mutation of the BST-2 STS sequence resulted in a largely Vpu-resistant phenotype, and they concluded that the STS sequence was important for BST-2 down-regulation by Vpu.
To confirm some of the data presented in these reports, we present here a functionally validated BST-2 expression system and its use in a sensitive assay for the detection of ubiquitinated forms of BST-2. This has allowed us to easily and reproducibly detect the Vpu- and βTrCP-dependent multi/polyubiquitination of BST-2. Because these Ub-BST-2 forms are much more readily detected when cells are treated with a lysosomal inhibitor, but not a proteasomal inhibitor, our data provide further support for the lysosomal degradation of BST-2 in the presence of Vpu. Vpu itself is similarly stabilized by lysosomal inhibitors, suggesting that Vpu and BST-2 may be destroyed simultaneously. Additionally, we show that a BST-2 protein with Lys → Arg substitutions for both cytosolically exposed lysine residues is ubiquitinated as well as WT BST-2, confirming reports that non-lysine BST-2 residues are being targeted for ubiquitination. We also present data suggesting that BST-2 residues bearing hydroxyl side chains (serine/threonine/tyrosine) are targeted for ubiquitination. However, we find that Vpu can ubiquitinate and degrade a BST-2 mutant substituted for all lysines, serines, and threonines, suggesting that Vpu may instead target either tyrosines, cysteines, or the NH2-terminal methionine residue for ubiquitination.
EXPERIMENTAL PROCEDURES
Cells, Reagents, and Antibodies
TZM-bl cells were obtained from the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH from John C. Kappes, Xiaoyun Wu, and Tranzyme Inc. (Germantown, MD) (36–38). HeLa Tet-Off cells were obtained from Clontech (Mountain View, CA). HeLa Tet-Off, HT1080, and 293T cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and penicillin-streptomycin-glutamine (Invitrogen). The following reagents were used at the indicated concentrations: concanamycin A (MP Biochemicals, Solon, OH) was used at 50 nm, and MG132 (Boston Biochem, Boston, MA) was used at 20 μm. The following antibodies were used for immunoprecipitations (IP) and immunoblots. Mouse anti-BST-2 antibody was kindly provided by Chugai Pharmaceutical Co., Ltd. (Kanagawa, Japan) and was previously characterized (39). The polyclonal anti-BST-2 antibody was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH, anti-BST-2 (catalog no. 11721) from Drs. Klaus Strebel and Amy Andrew (26). The horseradish peroxidase (HRP, EC 1.11.1.7)-conjugated anti-Myc tag and anti-hemagglutinin (HA) tag antibodies were obtained from Roche Applied Science, the anti-HA monoclonal antibody was provided by Covance (Princeton, NJ), the anti-HA mAb conjugated to agarose beads was provided by Sigma, and the actin antibody was obtained from Calbiochem.
Construction of Lentiviral Vectors and BST-2 Cell Lines
Cell lines expressing BST-2 were generated as previously described (46). 293T- and HT1080-based stable cell lines expressing BST-2, BST-2(K18R,K21R), BST-2::HA, BST-2::HA(K18R,K21R), and BST-2::HA(S3A,T4 ,S5A), were generated by lentiviral transduction and puromycin selection (2 μg/ml for 293T cells and 0.625 μg/ml for HT1080 cells). Lentivirus derived from empty pCDH-CMV-MCS-EF1-Puro vector was used to generate BST-2 null control cell lines.
Vpu Plasmids and Recombinant Adenoviral Vectors
To avoid issues arising from poor transfection efficiencies and to ensure consistent protein expression levels, we utilized adenoviral clones expressing Vpu::GFP and Vpu2/6::GFP fusions, which we have previously described (40). For transductions, cells were seeded at 3 × 105 cells/well in a 6-well tissue culture plate (Costar, Corning, NY). The following day medium was removed from the cells and replaced with adenoviral vector lysates in 1.2 ml of complete medium supplemented with 8 μg/ml Polybrene (Sigma). The cells were then incubated at 37 °C with 5% CO2 for an additional 1.5 h followed by the addition of complete medium. Incubation was continued for 40–48 h. All of the adenoviral vectors contain a tetracycline-responsive promoter and therefore required the addition of the tetracycline transactivator (41), which was either provided in trans by another adenovirus (Ad-Trans) or expressed constitutively in both HeLa Tet-Off and HT1080 Tet-Off cells. Cells were transduced at a multiplicity of infection of 15.
HIV-1 Egress Assays
Viral egress assays were performed to assess the function of the BST-2 proteins expressed by our various 293T-based cell lines. Each of these cell lines, along with a 293T control cell line transduced with the empty lentiviral vector, were transfected (Effectene, Qiagen) with 300 ng of a pNL4–3-based HIV-1 proviral clone (either WT, Δvpu, or vpu2/6) and 50 ng of pEGFP (transfection control plasmid). 24 h later the transfection mixture was replaced with fresh media, and the cells were incubated for a further 24 h to allow viral production. At that time viral supernatants were collected, centrifuged to remove cells, and then transferred to fresh tubes. The relative amount of virus present in each of these samples was quantitated via titration on TZM-bl indicator cells, which are HeLa-derived cells that stably express an HIV-1/Tat-inducible firefly luciferase (EC 1.13.12.7). Briefly, TZM-bl cells were plated at a density of 104 cells/well in 96-well black, clear-bottomed tissue culture plates (Costar). The next day these cells were infected with either a 1:10 or 1:100 dilution of each viral stock in a final volume of 50 μl of complete medium supplemented with 20 μg/ml DEAE-dextran (Sigma). After 2 h, an additional 50 μl of complete medium was added to each well, and the cells were returned to the incubator. 48 h later, 100 μl of Bright-Glo reagent (Promega, Madison, WI) was added to each well, and luciferase activities were quantitated in a Veritas microplate luminometer (Turner BioSystems, Sunnyvale, CA). Egress activity is reflected in the amount of virus released by each cell line transfected with HIV-1 proviral clones and is expressed as a percentage of the virus released from control cells that do not express BST-2.
Epitope-tagged Ubiquitin Plasmids
The plasmid HA-Ubiquitin (obtained from Addgene, Cambridge, MA, catalog #18712, from Edward Yeh (42)) encodes an NH2-terminal HA-tagged ubiquitin B allele cloned into pcDNA3 (Invitrogen). From this construct (referred to below as pHA::Ub), we generated an isogenic Myc-tagged ubiquitin plasmid (pMyc::Ub) using standard PCR-based mutagenesis procedures.
Ubiquitination Assays
In vitro ubiquitination assays were performed essentially as described by Belaïdouni et al. (12). Cells expressing BST-2 (either HeLa cells or 293T-based cell lines expressing BST-2::HA) were transfected with 300 ng of a pNL4–3-based HIV-1 proviral clone (either WT, Δvpu, or vpu2/6) along with 100 ng of pMyc::Ub or pHA::Ub and 50 ng of pEGFP (as a transfection control). 24 h later either MG132 or concanamycin A (CMA) was added to the culture medium, and the cells were incubated for another 12 h. Cells were then lysed in 1% SDS, boiled for 5 min, sonicated in a cup horn using 3 × 30-s pulses with 15-s rests (Virtis Virsonic, Gardiner, NY) and analyzed for protein concentrations using a bicinchoninic acid protein assay (Thermo Fisher Scientific, Rockford, IL). 100 μg (HeLa) or 20 μg (293T) of each lysate was diluted to a final volume of 100 μl with 1% SDS and then diluted 10-fold further with 900 μl of RIPA buffer with no SDS (50 mm Tris, pH 7.5, 150 mm NaCl, 1% Nonidet P-40, 0.5% deoxycholate, 1× “Complete” inhibitors (Roche Applied Science)). BST-2 was then immunoprecipitated from these lysates using either 1 μg of BST-2 monoclonal antibody (Chugai) for HeLa lysates or 20 μl of HA-agarose beads (Sigma) for 293T::BST-2::HA lysates. Immunoprecipitates were subjected to immunoblot analysis as described below. To detect protein ubiquitination, PVDF membranes (Bio-Rad) were probed with either anti-HA antibody (Fig. 1 only, Covance), an anti-Myc antibody (Roche Applied Science), or the anti-ubiquitin antibody P4D1. The immunoblots were then stripped as described below and stained for BST-2 using either the anti-BST-2 mAb (Fig. 1 only, Chugai) or an HRP-conjugated anti-HA antibody.
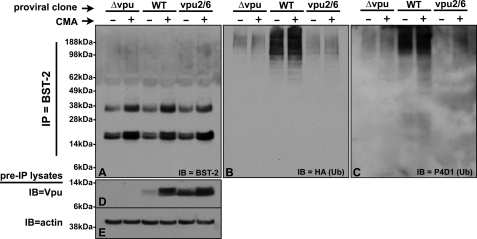
FIGURE 1.
Vpu-dependent ubiquitination of endogenously expressed BST-2. A, HeLa cells transfected with pHA::Ub and the indicated pNL4–3 proviral clones were treated ± the lysosomal inhibitor CMA. Lysates were made from these cells, BST-2 was immunoprecipitated from the lysates using an anti-BST-2 mAb (Chugai), and the final IP pellets were treated with peptide N-glycosidase F. Shown here is an immunoblot of those IPs probed with anti-BST-2 polyclonal antisera. B, the immunoblot shown in A was stripped and reprobed with an anti-HA mAb to detect the presence of HA-tagged ubiquitin conjugated to the immunoprecipitated BST-2 proteins. C, the immunoblot shown in A and B was stripped and reprobed with the anti-Ub mAb P4D1. D, an immunoblot of the input lysates used in A and B was probed with an anti-Vpu polyclonal antibody to show the level of Vpu expressed in these cells. E, the immunoblot (IB) shown in D was stripped and reprobed with an anti-actin antibody.
Chemical Cleavage of Ubiquitin Moieties from Proteins
Ubiquitinated BST-2 proteins from transfected 293T cells were isolated and prepared as described above. The resulting immunoprecipitates were then treated with either high pH or extreme reducing conditions, which have been shown to liberate Ub molecules conjugated to BST-2 via esterification to serine side chains or thioesterification to cysteine side chains, respectively. High pH treatments were accomplished essentially as described by Wang et al. (43) by resuspending immunoprecipitates in 0.5% SDS, boiling for 3 min, cooling to room temperature, adding NaOH to 100 mm, and then incubating the samples at 37 °C for 2 h. Samples were then neutralized via the addition of 0.5 m Tris, pH 6.8. Lithium dodecyl sulfate sample buffer (Invitrogen) without reducing agents was added, and the samples were boiled for 3 min. Reducing treatments were performed essentially as described by Williams et al. (44) by resuspending immunoprecipitates in 1× lithium dodecyl sulfate sample buffer supplemented with either 100 mm DTT or 10% β-mercaptoethanol (βME) followed by boiling for 3 min. All protein samples were separated via SDS-PAGE, transferred to PVDF membranes, and then immunostained for ubiquitin using an HRP-conjugated anti-Myc antibody. The immunoblots were then stripped as described below and stained for BST-2 using an HRP-conjugated anti-HA antibody.
Immunoblot Analyses and Co-immunoprecipitations
For immunoblot analyses of total cellular proteins, lysates were prepared as described above for the ubiquitination assays. After SDS-PAGE, the proteins were transferred to PVDF, incubated in blocking buffer (5% nonfat dry milk dissolved in Tris-buffered saline with 0.1% Tween 20 (TBST)) for 1 h with agitation, and then probed with one of the antibodies described above diluted in blocking buffer. Those blots that were probed with non-conjugated primary antibodies were washed 3× in TBST, incubated with the appropriate secondary antibodies in antibody buffer, then washed a final 3× in TBST, whereas blots probed with HRP-conjugated primary antibodies were washed 3× in TBST and immediately developed. Proteins reacting with the antibodies were visualized using either an ECL Plus or an ECL Advance chemiluminescent kit (Amersham Biosciences/GE Healthcare). To remove sugars from glycosylated proteins before immunoblot analysis, the protein lysates were subjected to peptide N-glycosidase F (EC 3.5.1.52) treatment as described in the manufacturer's protocol (New England Biolabs, Ipswich, MA). For those experiments in which previously stained blots were to be subsequently stained with different antibodies, the blots were incubated in strip buffer (62.5 mm Tris, pH 6.8, 2% SDS, 100 mm βME) for 30 min at 55 °C followed by 4–5 washes in TBST at room temperature. The blots were then ready for further immunostaining.
RESULTS
Ubiquitination of BST-2 in the Presence of Vpu
We first sought to determine if endogenously expressed BST-2 is specifically ubiquitinated in the presence of Vpu and if so, whether or not this modification is also dependent upon βTrCP. We therefore transfected HeLa cells with both a pNL4–3 derivative (either Δvpu, WT, or vpu2/6) and a plasmid expressing an HA-tagged Ub allele. It is important to note that the Vpu2/6 mutant cannot bind to βTrCP due to asparagine substitutions for serine residues 52 and 56 within the DpSGXXpS phospho-degron that comprises the Vpu BTrCP binding site. Because data from our laboratory and others have suggested that the Vpu-dependent degradation of BST-2 takes place within the lysosome, we treated these transfected cells with the lysosomal inhibitor CMA. We reasoned that if ubiquitinated forms of BST-2 are rapidly degraded, preventing their lysosomal degradation with CMA might allow us to more easily capture and detect them. After immunoprecipitating BST-2 from denatured lysates with a BST-2 mAb, the proteins were digested with peptide N-glycosidase F to remove N-linked glycans from BST-2. The samples were next subjected to immunoblot analysis for BST-2 (Fig. 1A), then stripped and reprobed for Ub (HA, Fig. 1B). Fig. 1A shows that BST-2 was consistently recovered from all samples and that CMA enhanced the recovery of BST-2 regardless of Vpu expression. The BST-2 species recovered here includes a doublet of ∼20 kDa, which is presumed to be a fully deglycosylated BST-2 protein, perhaps with and without a glycosylphosphatidylinositol anchor, along with a ∼35-kDa peptide N-glycosidase F-resistant species that we consistently detect under these conditions. When this blot was stripped and reprobed using an anti-HA antibody (Fig. 1B), we detected high molecular weight (Mr) species in all lanes, which is indicative of ubiquitinated BST-2 forms. The species that we observe may represent a mixture of ubiquitinated version of both the ∼20- and ∼35-kDa forms, thereby eliminating the typical “laddering” effect that is observed for some ubiquitinated proteins. These same products were detected by the P4D1 anti-Ub antibody (Fig. 1C), indicating that these species are indeed ubiquitinated. In the absence of Vpu, we observed low levels of these high Mr species that increased slightly when the cells were treated with CMA. This level of non-Vpu-dependent ubiquitination is likely to be a reflection of endogenous BST-2 turnover. These same high Mr species were significantly enriched in cells expressing WT Vpu, indicating that BST-2 is specifically ubiquitinated in the presence of Vpu. CMA appeared to further enhance the recovery of ubiquitinated BST-2, an observation in keeping with a lysosomal degradation mechanism. The main population of ubiquitinated products was centered around the 98-kDa molecular mass marker, and although we cannot determine whether these ubiquitinated products result from the ∼20- or ∼35-kDa BST-2 forms, given a 10-kDa Ub monomer (including the Myc tag), we predict that these ubiquitinated forms have been modified with between 6 and 15 Ub monomers and are therefore either multiply monoubiquitinated or polyubiquitinated.
To confirm that Vpu was appropriately expressed in these samples, we performed Vpu immunoblots on the pre-IP lysates (20% of input, Fig. 1D). Because we observed varying levels of Vpu, the immunoblot was reprobed for actin to confirm equal protein loading (Fig. 1E). Interestingly, like BST-2, WT Vpu was stabilized in the presence of CMA, suggesting that WT Vpu is also destroyed in the lysosome. This corroborates data reported by Dubé et al. (15), which showed that the lysomotropic agents chloroquine and ammonium chloride both prevented Vpu degradation. Not surprisingly, Vpu2/6 was stable regardless of drug treatment, suggesting that the degradation of Vpu itself depends upon its ability to interact with βTrCP. Similar results have been reported by Belaïdouni et al. (12), who have also shown that Vpu itself is ubiquitinated in a βTrCP-dependent manner.
Functional Validation of Lentiviral BST-2::HA Expression System
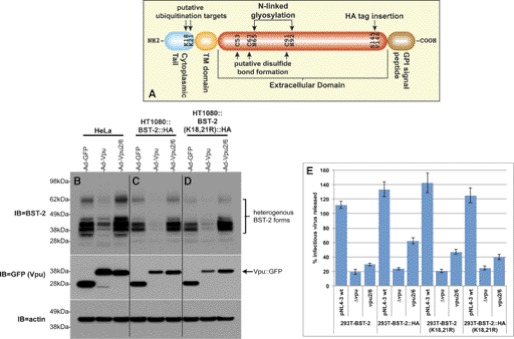
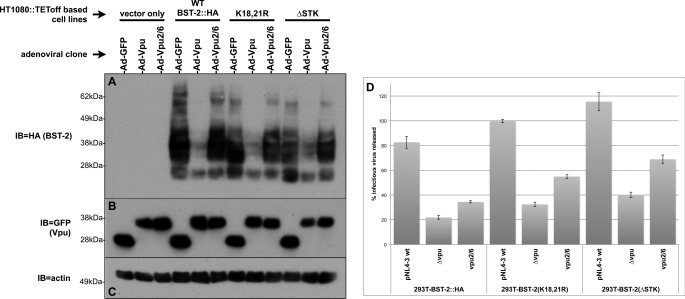
The Vpu specific interaction with both BST-2 and βTrCP (40) coupled with Vpu dependence upon βTrCP to effect the degradation of BST-2 has led many to hypothesize that ubiquitin is involved in the down-regulation of BST-2 by Vpu. We sought to develop a system that would allow us to readily generate BST-2 mutants and analyze their degree of ubiquitination by Vpu. Our initial attempts to do so utilized BST-2 expression plasmids transiently transfected into various cell lines. However, these led to large amounts of low molecular weight BST-2 protein species that were clearly distinct from those observed for endogenously expressed BST-2 (data not shown). Similar BST-2 species have been observed by Andrew et al. (45), who show that transient transfection of bst-2 constructs leads to the expression of large amounts of BST-2 that is trapped within the ER, and likely destroyed via ERAD. Unfortunately, these improperly processed species may be aberrantly ubiquitinated. In an effort to avoid these difficulties, we have instead established a lentiviral expression system that we have previously described and used for the analysis of the KSHV K5-dependent down-regulation of BST-2 (46). Lentiviral clones encoding both WT BST-2 and a variant with an HA tag inserted just upstream of the putative BST-2 glycosylphosphatidylinositol signal peptide were generated (Fig. 2A). Lentiviruses harboring these clones were then used to make HT1080- and 293T-based cell lines stably expressing BST-2::HA. HT1080 and 293T cells were chosen because in the absence of interferon stimulation they do not express detectable levels of BST-2. This makes them ideal cells in which to constitutively express various epitope-tagged and mutant BST-2 proteins. To validate this system before the generation and analysis of mutants, we tested these new cell lines for both BST-2 down-regulation by Vpu and their ability to inhibit viral egress. Fig. 2B shows a BST-2 immunoblot of HeLaTetOff cells infected with adenoviral clones expressing either GFP alone, a Vpu::GFP fusion, or a Vpu2/6 fusion, which we have previously described. This figure depicts the BST-2 down-regulation phenotype that we typically observe; WT Vpu degrades endogenously expressed BST-2, whereas Vpu2/6 does not. When these same adenoviral clones were used to infect the lentivirally transduced HT1080 cell line that constitutively expresses BST-2::HA, we observed the same phenotype in HA immunoblots (Fig. 2C), indicating that these cells express sufficiently high levels of BST-2::HA and that BST-2::HA remains susceptible to down-regulation by Vpu. To assess the function of BST-2::HA, we next performed HIV-1 egress assays in which we determined the amount of HIV-1 released from cells transfected with pNL4–3-based proviral clones (Fig. 2E). Because HT1080 cells cannot be efficiently transfected, we transduced 293T cells with our BST-2::HA lentiviral constructs to generate 293T cell lines that stably express BST-2. The resulting 293T::BST-2 cell lines can then be efficiently transfected. Conversely, because recombinant adenoviruses are toxic to 293T cells, the HT1080 cell lines were only used to demonstrate down-regulation by the Vpu adenoviral clones. Egress of the Δvpu virus was similar from 293T cells expressing both the WT and HA-tagged BST-2 proteins, indicating that the tagged variant remains functional. However, egress of both the WT and vpu2/6 viruses was higher from the BST-2::HA cell line, suggesting that insertion of the HA tag may have introduced a slight but reproducible egress defect. Note that in both cell lines, egress of the vpu2/6 virus displays a consistent intermediate phenotype that we and others have previously observed. Although BST-2 is not degraded in the presence of Vpu2/6, several labs including our own have shown that BST-2 binds to wild type Vpu and Vpu2/6 equally well (27, 40, 47). Therefore, the Vpu2/6 partial egress phenotype likely results from the transient sequestration of BST-2 within an intracellular compartment.
FIGURE 2.
Validation of BST-2::HA and BST-2(K18R,K21R)-expressing cell lines. A, shown is a schematic of BST-2 domain structure, including the location of the cytosolic lysine residues and the site at which we have inserted an HA tag. B, HeLa cells were infected with recombinant adenoviruses expressing either GFP (control), Vpu::GFP, or Vpu2/6::GFP. Immunoblots of the resulting cell lysates were then probed with BST-2 polyclonal antisera (top panel). The same blot was stripped and reprobed with an anti-GFP mAb to detect GFP or the Vpu::GFP fusion proteins (middle panel). The blot was finally stripped and reprobed with an anti-actin mAb (bottom panel). C, HT1080::BST-2::HA cells were infected with the same viruses described for B, and an immunoblot of the resulting lysates was subsequently probed with BST-2 (top), GFP (middle), and actin (bottom) antibodies. D, HT1080::BST-2::HA(K18R,K21R) cells were infected with the same viruses described for B, and immunoblots of the resulting lysates were subsequently probed with BST-2 (top), GFP (middle), and actin (bottom) antibodies. Note that B, C, and D are a single immunoblot on which all three sets of samples were run simultaneously, thereby allowing a direct comparison of BST-2 species among them. E, shown are TZM-bl viral egress assay data for supernatants collected from the various 293T-based cells lines transfected with the indicated pNL4–3-based proviral clones. Egress data are expressed as a percentage of viral release from the control 293T-based cell line that does not express BST-2.
BST-2::HA Is Ubiquitinated in Both a Vpu- and βTrCP-dependent Manner
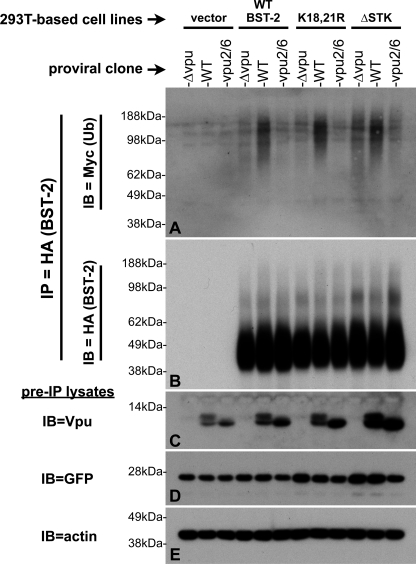
To further validate the functionality of the BST-2::HA expressed from the 293T-based cell line, we tested BST-2::HA expressed in these cells for its ability to be ubiquitinated in the presence of Vpu. These assays were performed essentially as described for Fig. 1, except that a subset of the provirally transfected cells was also treated with MG132 to evaluate the effect that proteasome inhibition has on BST-2 ubiquitination. In addition, the HA tag incorporated into BST-2 allowed us to immunopurify BST-2::HA from these lysates using an HA antibody conjugated to agarose beads, thus simplifying the procedure. At the same time, the use of an HA-tagged BST-2 precluded the use of the HA-tagged Ub construct used in Fig. 1. Instead, we generated an isogenic, Myc-tagged Ub construct for these assays (described under “Experimental Procedures”). In addition, we did not treat these samples with peptide N-glycosidase, as it did not benefit the assay in any meaningful way. Fig. 3B shows the BST-2 that was immunoprecipitated from lysates of 293T::BST-2::HA cells that were variously transfected with the WT, Δvpu, or vpu2/6 proviral clones and then treated with either CMA, MG132, or no drug. Similar to what we observed in HeLa cells (Fig. 1A), regardless of Vpu expression, the amount of BST-2 recovered from 293T::BST-2::HA cells treated with CMA was significantly higher than that recovered from untreated cells or cells treated with MG132, thus demonstrating that BST-2 is normally degraded within the lysosome. Note that the use of transfections to deliver Vpu to the cells, as opposed to the much more efficient adenoviral infections shown in Fig. 2 (B, C, and D), severely limits our ability to detect BST-2 down-regulation, as a significant fraction of cells remained untransfected. Interestingly, MG132 did not enhance the recovery of BST-2::HA from the cells and instead resulted in the detection of low Mr forms that are indicative of improper BST-2 processing. We have observed similar BST-2 species in HeLa cells treated with MG132 (data not shown). Fig. 3A shows this same blot probed with an anti-Myc antibody to detect ubiquitin. Ubiquitination of BST-2::HA was not detected for the Δvpu or vpu2/6 viruses in the absence of CMA. In the absence of drug treatment, BST-2::HA was specifically ubiquitinated in the presence of WT Vpu, although not to the degree that we observed in HeLa cells (Fig. 1B). Although CMA had little effect upon Vpu-dependent BST-2 ubiquitination in HeLa cells, CMA greatly enhanced the level of Vpu-dependent BST-2::HA ubiquitination in 293T cells. This cell type variability suggests that 293T cells may have a greater tendency or capacity for degradation than do HeLa cells. Unlike CMA, MG132 did not enhance the detection of ubiquitinated BST-2::HA (Fig. 3A, last three lanes), providing further support for a mechanism in which the Vpu-dependent degradation of BST-2 occurs in the lysosome, but not the proteasome.
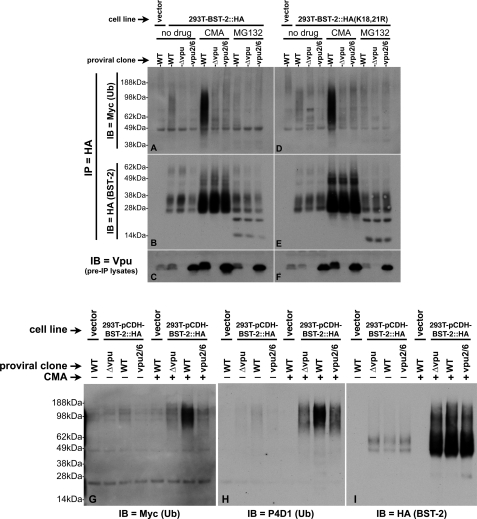
FIGURE 3.
Comparison of the ubiquitination status of WT BST-2 and BST-2(K18R,K21R). A, 293T::BST-2::HA cells that had been transfected with pMyc::Ub and the indicated pNL4–3-based proviral clones were treated with either CMA, MG132, or carrier (DMSO). A 293T cell line transduced with the empty lentiviral vector, which does not express BST-2, was transfected with both pMyc::Ub and pNL4–3 and then treated with carrier only (left lane). 100 μg each of lysates made from these cells were immunoprecipitated with an HA mAb directly conjugated to agarose beads. Shown here is an immunoblot (IB) of the IPs probed with an anti-Myc antibody to detect ubiquitin conjugated to BST-2::HA. B, the immunoblot shown in A was stripped and reprobed with an anti-BST-2 polyclonal antisera. C, an immunoblot of the input lysates used in A and B probed with an anti-Vpu polyclonal antibody shows the level of Vpu expressed in these cells. D–F, 293T::BST-2::HA(K18R,K21R) cells were subjected to the identical treatment and analyses described for the 293T::BST-2::HA cells in A–C. G, 20 μg each of a subset of the samples described in A were once again subjected to IP with HA-agarose beads followed by SDS-PAGE and immunoblotting with an anti-Myc antibody (left panel). The same blot was then sequentially stripped and reprobed, first with the anti-ubiquitin antibody P4D1 (H) and finally an HA antibody (I) to demonstrate levels of BST-2 recovered in each IP.
To confirm the expression of Vpu in these cells, an immunoblot of the same pre-IP lysates (10% of input) was probed with an anti-Vpu antibody (Fig. 3C). Like the HeLa samples described in Figs. 1, C and D, we once again observed that WT Vpu was stabilized by the lysosomal inhibitor CMA, whereas Vpu2/6 was stable regardless of drug addition. Interestingly, WT Vpu was not stabilized by the proteasome inhibitor MG132. Therefore, our data from both HeLa and 293T cells transfected with HIV-1 proviral clones suggest that both Vpu and BST-2 are degraded within the lysosome.
Vpu-dependent BST-2 Ubiquitination Correlates with Vpu-dependent BST-2 Degradation
As discussed above, our 293T-based system has advantages that allow us to demonstrate Vpu-dependent ubiquitination of BST-2, whereas our HT1080-based system is better suited for demonstrations of Vpu-dependent degradation of BST-2. To more clearly link the BST-2 ubiquitination and degradation phenotypes, we performed ubiquitin assays using only 20 μg (as opposed to 100 μg) of the CMA- and untreated 293T-based samples shown in Fig. 3A. Our goal here was to avoid saturation of the immunoprecipitating HA antibody, such that changes in total BST-2 levels could be observed in the immunoprecipitates themselves. Fig. 3G shows that, similar to the data shown in Fig. 3A, BST-2 is specifically ubiquitinated in the presence of WT Vpu. Although this can be detected in the absence of CMA, treatment of the cells with CMA greatly enhances the isolation and detection of ubiquitinated BST-2 species. Confirmation that these high Mr species are indeed ubiquitinated was obtained by re-probing this blot with the ubiquitin-specific antibody P4D1 (Fig. 3H). Importantly, when the blot was probed for BST-2 (Fig. 3I) in the absence of CMA, we can now observe a decrease (albeit slight) in BST-2 levels in cells transfected with the proviral clone expressing WT Vpu. This approach therefore allows the demonstration of the Vpu-dependent BST-2 ubiquitination and degradation phenotypes in the same sample. Note that although CMA allows for the robust detection of Vpu-dependent BST-2 ubiquitination (last three lanes of both Fig. 3, G and H), changes in BST-2 levels are undetectable in the CMA-treated samples (Fig. 3I, last three lanes), further highlighting this technical challenge.
The BST-2(K18R,K21R) Double Lysine Mutant Is Functional
Our primary goal for the lentiviral expression system was to generate cell lines that uniformly express HA-tagged, mutant BST-2 proteins. Having validated the BST-2::HA-expressing cell lines with respect to Vpu down-regulation, viral egress, and ubiquitination by Vpu, we proceeded to investigate the contribution that the BST-2 cytosolic lysine residues made in each of these assays. A K18/K21R double-substitution was created within BST-2::HA, and the resulting lentiviral clone was used to transduce both HT1080 and 293T cells. Fig. 2D shows an anti-HA-probed immunoblot of HT1080::BST-2::HA(K18R,K21R) cells infected with the control (GFP), WT Vpu::GFP, and Vpu2/6::GFP adenoviral clones. Similar to what we observed for both the HeLa and HT1080::BST-2::HA cell lines (Fig. 2, B and C), WT Vpu efficiently down-regulated total levels of the lysine-substituted BST-2 mutant compared with both the control and the Vpu2/6 mutant. This result appears to be in conflict with data reported by Goffinet et al. (34), which showed no degradation of the BST-2(K18R/K21R) mutant protein by Vpu. However, those authors also noted that their use of an NH2-terminal HA-tagged BST-2 construct transfected into 293T cells led to the accumulation of BST-2 species distinct from those observed for endogenous BST-2, suggesting that their results were perhaps a reflection of Vpu's effect on improperly localized/processed forms of BST-2. We next assayed the egress of WT, Δvpu, and vpu2/6 viruses from 293T cells expressing either BST-2(K18R,K21R) or BST-2::HA(K18R,K21R) and found them all to be similar to viral egress from 293T::BST-2::HA cells (Fig. 2E). In general, our results corroborate those reported in several recent manuscripts (27, 32, 35), which have each found that the two cytosolic lysines have no phenotype with respect to viral egress or surface down-regulation by Vpu. However, as described above, disagreement remains regarding the contribution that these lysines make toward Vpu-dependent ubiquitination. We therefore proceeded to test the lysine-substituted BST-2 mutant for its ability to be ubiquitinated in our system.
The BST-2(K18R,K21R) Double Lysine Mutant Is Still Ubiquitinated in a Vpu-dependent Manner
We next performed in vitro ubiquitination assays using the 293T::BST-2::HA(K18R,K21R) cells, exactly as described above for the 293T::BST::HA cell line. The anti-HA-probed immunoblot in Fig. 3E shows the amount of mutant BST-2 that was immunoprecipitated from these cells. Like the BST-2::HA cells, within each set of drug-treated samples, BST-2::HA(K18R,K21R) was consistently detected regardless of Vpu expression, and among the drug treatments, lysosomal inhibition with CMA greatly enhanced BST-2 recovery. Fig. 3D shows this same blot stripped and reprobed with an anti-Myc antibody to detect ubiquitin. Similar to what we observed for both endogenous and HA-tagged BST-2 proteins, the lysine mutant was specifically ubiquitinated in the presence of Vpu, and the amount of ubiquitinated BST-2::HA(K18R,K21R) recovered was significantly increased when we treated the cells with CMA. MG132 treatment did not enhance the recovery of ubiquitinated BST-2::HA(K18R,K21R) and once again led to aberrantly processed low Mr BST-2 forms. Expression of Vpu in these cells was confirmed via immunoblot of the pre-IP lysates (10% of input) with an anti-Vpu antibody, demonstrating once again that WT Vpu is stabilized in the presence of a lysosomal inhibitor but not a proteasomal inhibitor (Fig. 3F).
Chemical Treatments of Ub BST-2 Suggest That Amino Acids Bearing Hydroxyl Side Chains Are Targeted for Ubiquitination
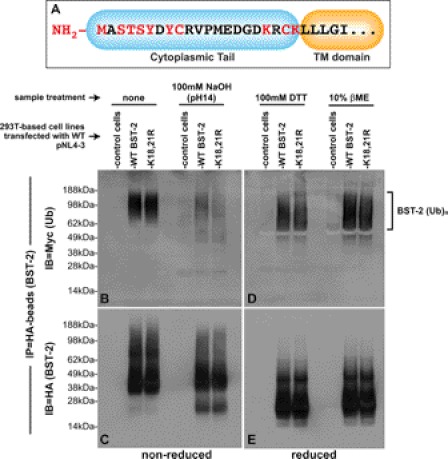
The observation that a BST-2 protein without cytosolically exposed lysine residues is ubiquitinated to a similar extent as the WT protein suggests that another residue(s) within the BST-2 cytoplasmic tail is instead targeted for this modification. Fig. 4A shows the amino acid sequence of the BST-2 cytoplasmic tail, with potential ubiquitination substrates highlighted in red. The use of the NH2-terminal methionine (48), cysteine (44, 49), and serine/threonine (50) residues as ubiquitination substrates have all been reported in the literature. Although tyrosine residues have been noted as potential substrates (48), aside from the hantavirus G1 tail protein, there are no experimental data to support this (51). Because the BST-2 cytoplasmic lysines appear to be dispensable for ubiquitination, we sought to quickly narrow the list of remaining candidate residues using a chemical approach. For these assays, 293T::BST-2::HA and control cell lines were co-transfected with both pNL43 (vpuWT) and a plasmid encoding an Myc-tagged ubiquitin followed by treatment with CMA, as described for the most highly ubiquitinated samples shown in Fig. 3. BST-2::HA proteins were then immunoprecipitated from denatured lysates made from these cells. The resulting IP pellets containing ubiquitinated BST-2 were treated with either strong reducing agents (100 mm DTT or 10% βME), which have been shown to cleave Ub chains attached to cysteine residues via thioester bonds, or strong base (100 mm NaOH), which has been shown to cleave Ub chains attached to serine or threonine residues via ester bonds. After the completion of these reactions, ubiquitination of BST-2 was determined via immunoblots as described for Fig. 3. In the absence of either NaOH or reducing agents, we detected high Mr ubiquitinated species for both WT and K18R,K21R proteins (Fig. 4B, first three lanes). The species detected here are larger and at the same time more compressed than those shown in Fig. 3 due to our use of sample buffer supplemented with DTT in Fig. 3. When a parallel set of samples was treated with 100 mm NaOH (Fig. 4B, last three lanes), the high Mr population largely dissipated into lower Mr species, indicative of serine, threonine, or tyrosine residues serving as Vpu-dependent BST-2 ubiquitination substrates. Fig. 4C shows this same immunoblot stripped and reprobed for BST-2. Interestingly, the NaOH-induced shift in size of the higher Mr ubiquitin species can be seen here in Fig. 4C as well, perhaps because of the large amount of ubiquitinated proteins recovered in our procedure, which can therefore be detected with both Myc (Ub) and HA (BST-2) antibodies. Fig. 4D shows the effect that either 100 mm DTT or 10% βME had upon WT and K18R,K21R BST-2 ubiquitination, and Fig. 4E shows same blot stripped and reprobed for BST-2 (anti-HA). Note that the high Mr population observed for reduced, ubiquitinated BST-2 proteins (Fig. 4D) migrates farther into the gel than those observed under non-reducing conditions (Fig. 4B). However, the fact that the population did not dissipate into the low Mr species observed for our NaOH-treated samples weighs against the use of cysteine residues as BST-2 ubiquitination substrates. Finally, when comparing the ubiquitinated species for both WT and K18R,K21R BST-2 proteins under both highly basic and highly reduced conditions, we see that the ubiquitinated WT and mutant proteins migrated similarly to one another (Fig. 4, B and D). This provides further support for our conclusion that the Vpu-dependent ubiquitination of the K18R,K21R mutant is similar to that of WT BST-2. In summary, these data provide evidence that Vpu promotes the ubiquitination of BST-2 upon amino acids bearing hydroxyl side chains (Ser/Thr/Tyr).
FIGURE 4.
Chemical cleavage of ubiquitin moieties from BST-2::HA: Vpu promotes the ubiquitination of BST-2 on serine/threonine/tyrosine. A, shown is a schematic of the BST-2 cytoplasmic domain, with potential ubiquitin target residues highlighted in red. B, immunoprecipitated samples of ubiquitinated BST-2::HA and BST-2::HA(K18R,K21R) were generated as described in Fig. 3, including a sample prepared from control cells that do not express BST-2::HA. Those samples were then left untreated (left three lanes) or were treated with 100 mm NaOH. The proteins were then separated via non-reducing SDS-PAGE. An immunoblot (IB) of the gel was then probed with an anti-Myc mAb to detect ubiquitinated BST-2::HA species. C, the immunoblot in B was stripped and reprobed with an anti-HA mAb to detect BST-2::HA. D, immunoprecipitated samples of ubiquitinated BST-2::HA and BST-2::HA(K18R,K21R) were generated as described in Fig. 3, including a sample prepared from control cells that do not express BST-2::HA. The immunoprecipitates were resuspended in sample buffer supplemented with either 100 mm DTT (first three lanes) or 10% βME (last three lanes). The proteins were then separated via reducing SDS-PAGE (+DTT). An immunoblot of the gel was probed with an anti-Myc mAb. E, the immunoblot in D was stripped and reprobed with an anti-HA mAb to detect BST-2::HA.
BST-2 Substituted for All Cytoplasmically Exposed Ser, Thr, and Lys Residues Is Still Degraded in Vpu-dependent Manner
The NaOH sensitivity of ubiquitinated BST-2(K18R,K21R) suggested that perhaps a BST-2 mutant entirely devoid of cytosolically exposed lysines, serines, and/or threonines might be resistant to down-regulation by Vpu. We therefore subjected our lentiviral BST-2::HA(K18R,K21R) clone to further mutagenesis, generating simultaneous Ala substitutions for Ser-2, Ser-4, and Thr-3. Although tyrosine residues are also hydroxylated, we did not make substitutions for the BST-2 cytoplasmic tyrosines, as these have been implicated in BST-2 endocytosis (30, 52, 53). The resulting clone (BST-2::HAΔSTK) was then used to create 293T- and HT1080-based cell lines stably expressing this mutant BST-2. Surprisingly, in immunoblots of the HT1080-based cell lines infected with adenoviruses expressing either WT Vpu or Vpu2/6 (Fig. 5A), we observed that like the WT and K18R,K21R BST-2 proteins, BST-2::HAΔSTK was still degraded in a Vpu-dependent manner.
FIGURE 5.
BST-2s cytoplasmically exposed serine and threonine residues are not required for down-regulation of total levels of BST-2 by Vpu nor are they required for Vpu to promote viral release. A, HT1080::TEToff-based cell lines expressing vector (control), WT, ΔK (K18R,K21R), and ΔSTK BST-2 proteins were infected with the same Vpu-expressing adenoviruses described above in Fig. 2, B–D. An immunoblot (IB) of the resulting cell lysates was successively probed with HA (panel A, to detect BST-2::HA), GFP (panel B, to detect Vpu fusions), and actin antibodies (panel C, loading control). D, shown are TZM-bl viral egress assay data for supernatants collected from 293T-based cells lines (expressing the same BST-2 mutants shown in the immunoblots) transfected with the indicated pNL4–3-based proviral clones. Egress data are expressed as a percentage of viral release from the control 293T-based cell line that does not express BST-2.
BST-2ΔSTK Functions to Limit Viral Egress, and This Can Be Overcome by Vpu
As described above for the K18R,K21R BST-2 mutant, we next assessed the functionality of BST-2ΔSTK by transfecting a 293T::BST-2ΔSTK cell line with WT, Δvpu, or vpu2/6 pNL4–3 proviral clones and then determining the amount of HIV-1 released from these cells via titration of the resulting cell supernatants in TZM-bl assays (Fig. 5D). Compared with the cells expressing WT BST-2::HA, we observed a reproducible increase in the amount of virus released from cells expressing ΔK and ΔSTK BST-2 regardless of which proviral clone was used. This suggests that the mutant BST-2 proteins have a slight defect with respect to viral egress. Nevertheless, when comparing the viral output from cells transfected with the WT and Δvpu proviral clones, it is clear that all three BST-2 proteins are overcome by Vpu to approximately the same degree. Note that this correlates well with the down-regulation data shown in Fig. 5A; careful examination of the blot indicates that WT Vpu leads to slightly less degradation of the ΔK and ΔSTK mutants compared with WT BST-2.
BST-2ΔSTK Is Ubiquitinated in Vpu-dependent Manner
The observation that BST-2ΔSTK levels were reduced by Vpu expression (Fig. 5A) suggested that BST-2ΔSTK is likely to be ubiquitinated by Vpu as well. To confirm this, we performed denaturing IP/ubiquitination assays with 293T-based cell lines expressing BST-2ΔSTK, as described for Fig. 3. Because it is difficult to detect ubiquitinated BST-2 species in the absence of CMA, for these assays all cells were treated with CMA. Fig. 6A shows that, as observed for both WT and the ΔK BST-2 proteins, BST-2ΔSTK was specifically ubiquitinated in the presence of Vpu expressed from the pNL4–3 proviral clone. Although BST-2ΔSTK appeared to have a higher degree of background ubiquitination (catalyzed in the absence of Vpu or by Vpu2/6), Vpu enhanced this to levels similar to those observed for WT or the ΔK mutant, indicating that the cytosolically exposed Ser and Thr residues are not required for Vpu-dependent BST-2 ubiquitination. The blot was reprobed for HA (Fig. 6B) to ensure that BST-2 levels were consistent among the samples. Finally, when pre-IP lysates were probed for Vpu (Fig. 6C), we noted an interesting pattern; in the absence of BST-2, we detect less Vpu and Vpu2/6 protein. In contrast, in the presence of WT, ΔK, or ΔSTK BST-2, we detect higher levels of each Vpu protein. The relevance of this observation will be discussed below.
FIGURE 6.
The BST-2 cytoplasmically exposed serine and threonine residues are not required for the specific ubiquitination of BST-2 by Vpu. BST-2 ubiquitination assays were performed essentially as described for Fig. 2, with the exception that all samples were treated with CMA. Immunoblots (IB) of HA-bead IPs from each sample were first probed for Ub (panel A, Myc) then stripped and reprobed for HA (panel B, to detect BST-2::HA). Samples of the pre-IP lysates (bottom three panels) were sequentially probed for Vpu (C), GFP (D, transfection control), and finally actin (E, loading control).
DISCUSSION
The data that we have presented here include a confirmation of the Vpu-dependent multi/polyubiquitination of BST-2, which provides a functional link between BST-2 degradation and the requirement for the βTrCP Ub ligase adaptor protein in this process. The finding that the BST-2(K18R,K21R) mutant is ubiquitinated to a similar extent as the wild type protein indicates that these two lysine residues are not absolutely required for BST-2 ubiquitination, thus providing an explanation for the observation that the K18R,K21R mutation has no impact upon Vpu-dependent BST-2 down-regulation. Although our chemical treatment experiments suggested that BST-2 cytoplasmic Ser and Thr residues are targeted by Vpu for ubiquitination, analysis of our BST-2ΔSTK mutant instead suggests that Vpu promotes BST-2 degradation by directing Ub conjugation to BST-2 tyrosyl or NH2-terminal residues. Alternatively, Vpu may relax the specificity of the SCF ligase complex such that any/all targetable residues within the BST-2 cytosolic domain can be ubiquitinated.
Our data thus far do not allow us to distinguish between the Vpu-dependent multiple-monoubiquitination and polyubiquitination of BST-2. If, as the data indicate, we are detecting 6–15 Ub monomers attached to BST-2 and neither the cytoplasmic lysine nor cysteine residues are utilized, this leaves six potential target residues (1× NH2-MET, 2× Ser, 1× Thr, 2× Tyr) within the BST-2 cytoplasmic tail. Because we cannot yet rule out any of these six residues, our data support models in which either (a) each one of these residues is simultaneously monoubiquitinated, or (b) a subset of these residues are polyubiquitinated.
Although Lys-18 and Lys-21are dispensable for BST-2 down-regulation by Vpu, these residues may still be required for BST-2 recycling or endocytosis, although our analyses of BST-2 surface levels for the K18R,K21R mutant do not support such a hypothesis (data not shown). At the very least, the location of the two lysines adjacent to the transmembrane domain would certainly support the “positive-inside rule” (54), which dictates the orientation of type II membrane proteins like BST-2 based on the abundance of positively charged residues on the NH2-terminal side of the transmembrane domain. Substituting the two lysines for arginines does not alter their charge, and so no secretory defects should be detected. By extension, we would then predict that Lys → Ala substitutions at these same two positions would lead to the topological inversion of BST-2 in the membrane, which is a hypothesis that we have not yet tested.
The esterification of Ub moieties to BST-2 via serine/threonine/tyrosine residues was indicated in experiments in which we were able to remove Ub from WT and K18R,K21R BST-2 under highly basic conditions. Targeting of non-lysine resides has been shown for the mK3 Ub ligase encoded by the mouse γ-herpesvirus 68 (43) and the K3 Ub ligase encoded by KSHV (49, 55), each of which targets MHCI for degradation. Vpu itself provides an even more relevant example here, as Vpu has been shown to lead to the ubiquitination of CD4 on serine and/or threonine residues (11). To probe this question further, we next generated a BST-2 protein with substitutions for all of the cytoplasmically exposed lysine, serine, and threonine residues and found that it was still down-regulated (surface and total cellular levels) by Vpu as well as specifically ubiquitinated in the presence of Vpu. These results suggest that either the Ser/Thr residues are not targeted by Vpu (regardless of the presence of Lys-18 and Lys-21) or that they are targeted by Vpu, but in their absence, Vpu-dependent ubiquitination/degradation of BST-2 can still take place, presumably by targeting other available residues. Tokarev et al. (35) undertook an extensive mutational analysis of the BST-2 cytoplasmic domain and found that those mutants that included substitutions for the Ser/Thr residues were the most debilitated with respect to Vpu surface down-regulation and tethering activity. Although this would appear to contradict our findings, they also showed data that the STS mutant was specifically ubiquitinated and degraded in the presence of Vpu, in agreement with our findings. In an attempt to reproduce their surface down-regulation data, we co-transfected 293T cells with both pVphu and a plasmid encoding the BST-2 KKSTS mutant and then assessed BST-2 surface levels. The results of these experiments (data not shown) were identical to what we have shown here using our cell lines; mutation of the Ser/Thr residues had little effect upon Vpu down-regulation of BST-2 from the cell surface. Our common observation that the STS mutant is both ubiquitinated and degraded in the presence of Vpu would seem to weigh in favor of a model in which BST-2 Ser/Thr targeting by Vpu is possible but not required for Vpu to overcome BST-2.
There is an ongoing debate regarding BST-2 down-regulation by Vpu that revolves around the question of BST-2 degradation. For BST-2 degradation to take place, both BST-2 and Vpu must be properly localized and expressed within the same cell. In transient transfection assays, among those cells in the population that received both Vpu and BST-2 constructs, the improperly processed, ER-bound BST-2 is unlikely to be targeted by Vpu. Among those cells receiving only the BST-2 construct, BST-2 expression is quite high. This combination of events severely limits the ability to detect BST-2 degradation in steady state lysates made from a population of such transfected cells. For these reasons we have moved to systems in which BST-2 is expressed at reasonable levels in all cells of the population. HeLa cells provide information regarding endogenously expressed BST-2, and stable lentivirus-transduced cell lines allow us to ask questions using epitope-tagged, mutant BST-2 proteins. The validation of these latter cell lines (Fig. 2) was therefore critical before proceeding with subsequent experiments. This, however, is not the final answer, as the transfection of even these cell lines with Vpu plasmids will be significantly less than 100% efficient, such that BST-2 expression in the untransfected subpopulation will once again mask the ability to detect BST-2 down-regulation in the total population. To efficiently deliver Vpu-expression constructs to the vast majority of the cells in our experiments, we therefore utilized adenoviral vectors. We have used these adenoviruses here and elsewhere (40) in well controlled experiments showing that Vpu efficiently down-regulates total levels of BST-2, whereas Vpu2/6 does not.
A second debate involves data supporting either a proteasomal versus a lysosomal degradation mechanism for Vpu-dependent BST-2 turnover. Once again, the reason that this discrepancy has arisen likely extends from the use of transfected cells. Aberrantly processed secretory proteins are typically destroyed within the ER via an ERAD-proteasomal mechanism (56). Therefore, when BST-2 is overexpressed in transiently transfected cells, processed improperly, and trapped within the ER, the observation that proteasomal inhibitors prevent its subsequent degradation is likely to be a reflection of an ER quality control mechanism and not a Vpu-dependent process. Studies that have examined Vpu action on endogenously expressed BST-2 have instead obtained data supporting the lysosomal degradation of BST-2 (30, 32, 40). Our own data in this and previous manuscripts supports this same conclusion, and here we have provided further evidence for lysosomal degradation, e.g. that CMA stabilizes ubiquitinated forms of BST-2, thereby enhancing their detection in our assays. On the other hand, we have found that the proteasomal inhibitor MG132 does not lead to the accumulation of ubiquitinated BST-2 species in the presence of Vpu and instead merely leads to defects in the processing of BST-2. Both of these results argue against a proteasome-dependent degradation mechanism.
Our demonstration that Vpu2/6 does not lead to significant BST-2 ubiquitination indicates that βTrCP is required for Vpu-dependent ubiquitination of BST-2, which in turn suggests a relatively simple mechanism whereby Vpu recruits the SCFβTrCP E3 ligase complex to transfer Ub moieties directly to BST-2. However, our data do not rule out the possibility of an indirect mechanism wherein BST-2 binding to βTrCP/Vpu results in the SCF-dependent activation of yet another Ub ligase that then adds ubiquitin moieties directly to BST-2. Similarly, binding of BST-2 to SCFβTrCP/Vpu might result in the relocation of BST-2 to a subcellular compartment in which BST-2 is acted upon by another non-SCF Ub-ligase. Thus, the direct ubiquitination of BST-2 by SCFβTrCP/Vpu remains an open question. Nevertheless, the SCF E3 complex has been shown to be responsible for the degradation of a large number of cellular substrates (57, 58), and so it is not an unreasonable expectation for SCFβTrCP/Vpu to ubiquitinate BST-2. Moreover, several of the known SCFβTrCP substrates are membrane proteins, including the interferon-α (IFN-α), prolactin, erythropoietin, and human growth hormone receptors (59–62). For each of these receptors, engagement by their cognate ligands results in their SCFβTrCP-dependent endocytosis from the cell surface. For the IFN-α, prolactin, and erythropoietin receptors, ligand engagement leads to the phosphorylation of conserved DpSGXXpS βTrCP binding motifs within their cytosolic tails. βTrCP is then recruited followed by receptor ubiquitination, uptake, and lysosomal degradation. Although there remains some controversy regarding the mechanistic details of growth hormone receptor down-regulation (63, 64), the general features appear to be similar. CD4 provides yet another example of a membrane protein targeted for ubiquitination by SCFβTrCP, with the notable exceptions that (a) CD4 is targeted at the ER membrane instead of the cell surface, (b) the CD4/SCFβTrCP interaction is mediated by Vpu, and (c) CD4 is degraded by the proteasome (10). Thus far, our data suggest that SCFβTrCP/Vpu-dependent BST-2 degradation is more similar to the mechanisms described for the IFN-α, prolactin, erythropoietin, and human growth hormone receptors than for CD4, although there remains significant disagreement as to whether Vpu acts upon BST-2 at the cell surface or within an intracellular compartment (30, 32, 47, 65, 66, 68).
It has previously been reported that Vpu is constitutively phosphorylated (69), which therefore suggests that Vpu is always associated with the SCFβTrCP E3 ubiquitin ligase. This would make Vpu itself a likely target for SCF-dependent ubiquitination and turnover. However, it has also been reported that WT Vpu is as stable as Vpu2/6 (16), that Vpu is a stable, competitive inhibitor of βTrCP (13), that Vpu is degraded in a βTrCP-independent manner (14), that Vpu is degraded by the lysosome (15), and that Vpu is both polyubiquitinated in a βTrCP-dependent mechanism and degraded by the proteasome (12). Curiously, among the reports of Vpu stability, they also show direct evidence that Vpu2/6 is more stable that WT Vpu (70), suggesting that Vpu is in fact being turned over via a βTrCP-dependent mechanism. Coupled with our own data, the consensus among these conflicting reports weighs more heavily in favor of βTrCP-dependent Vpu degradation. Because the majority of previous Vpu work has focused on the ERAD mechanism of Vpu-dependent CD4 down-regulation, many BST-2 studies have likewise focused on the proteasome, so much so that lysosomal inhibitors are frequently not tested at all. Our observations that (a) Vpu2/6 is more stable than WT Vpu and that (b) CMA stabilizes WT Vpu together suggest that Vpu is itself degraded within the lysosome in a βTrCP-dependent manner. Interestingly, we consistently find that Vpu is much more stable in cells that express BST-2 compared with cells that do not express BST-2 (see the Vpu immunoblots in Figs. 3 and 6). This may represent an example of “substrate shielding,” whereby Vpu is itself continually destroyed when its substrates (BST-2/CD4) are unavailable. Indeed, such a model has been proposed for βTrCP2 itself (71). By extension, this further suggests that once Vpu, βTrCP and BST-2 interact with one another, they may remain associated until they meet a shared fate within the lysosome.
The Vpu-dependent multi/polyubiquitination of BST-2 provides us with a more concrete direction to move in as we study the mechanism whereby Vpu enhances viral egress, e.g. a determination of which specific residues are modified, what type(s) of Ub polymer is utilized, which E2 ligase(s) is used, and whether SCFβTrCP directly ubiquitinates BST-2. At the same time, such data must be coupled with more detailed information regarding which forms of BST-2 are degraded and the location of these events within the cell. Together with our new data, the observation that Vpu and BST-2 co-localize within a perinuclear/ trans-Golgi network compartment would suggest that BST-2 ubiquitination occurs there as well. However, because BST-2 has been shown to continually recycle at the cell surface (52, 53, 67), BST-2 may encounter Vpu before reaching the surface or during recycling from the surface. Neither of these possibilities precludes the subsequent lysosomal degradation of BST-2, and so both remain equally likely.
Acknowledgment
We thank Yibing Jia at the Oregon National Primate Research Center Molecular Core facility.
This work was supported, in whole or in part, by National Institutes of Health Grants R01-AI090490-01A1 and 5-R01-AI063938-03.
- Ub
- ubiquitin
- βTrCP
- β transducin-repeat containing protein
- SCF
- Skip1-Cullin1-F-box protein
- ER
- endoplasmic reticulum
- ERAD
- ER-associated degradation
- CMA
- concanamycin A
- Tet
- tetracycline
- IP
- immunoprecipitation
- βME
- β-mercaptoethanol.
REFERENCES
- 1. Spallek T., Robatzek S., Göhre V. (2009) How microbes utilize host ubiquitination. Cell Microbiol. 11, 1425–1434 [DOI] [PubMed] [Google Scholar]
- 2. Isaacson M. K., Ploegh H. L. (2009) Ubiquitination, ubiquitin-like modifiers, and deubiquitination in viral infection. Cell Host Microbe 5, 559–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gustin J. K., Moses A. V., Früh K., Douglas J. L. (2011) Viral takeover of the host ubiquitin system. Front Microbiol. 2, 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Malim M. H., Emerman M. (2008) HIV-1 accessory proteins. Ensuring viral survival in a hostile environment. Cell Host Microbe 3, 388–398 [DOI] [PubMed] [Google Scholar]
- 5. Butticaz C., Michielin O., Wyniger J., Telenti A., Rothenberger S. (2007) Silencing of both β-TrCP1 and HOS (β-TrCP2) is required to suppress human immunodeficiency virus type 1 Vpu-mediated CD4 down-modulation. J. Virol. 81, 1502–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ho M. S., Ou C., Chan Y. R., Chien C. T., Pi H. (2008) The utility F-box for protein destruction. Cell. Mol. Life Sci. 65, 1977–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bour S., Schubert U., Strebel K. (1995) The human immunodeficiency virus type 1 Vpu protein specifically binds to the cytoplasmic domain of CD4. Implications for the mechanism of degradation. J. Virol. 69, 1510–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Meusser B., Sommer T. (2004) Vpu-mediated degradation of CD4 reconstituted in yeast reveals mechanistic differences to cellular ER-associated protein degradation. Mol. Cell 14, 247–258 [DOI] [PubMed] [Google Scholar]
- 9. Binette J., Dubé M., Mercier J., Halawani D., Latterich M., Cohen E. A. (2007) Requirements for the selective degradation of CD4 receptor molecules by the human immunodeficiency virus type 1 Vpu protein in the endoplasmic reticulum. Retrovirology 4, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schubert U., Antón L. C., Bacík I., Cox J. H., Bour S., Bennink J. R., Orlowski M., Strebel K., Yewdell J. W. (1998) CD4 glycoprotein degradation induced by human immunodeficiency virus type 1 Vpu protein requires the function of proteasomes and the ubiquitin-conjugating pathway. J. Virol. 72, 2280–2288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Magadán J. G., Pérez-Victoria F. J., Sougrat R., Ye Y., Strebel K., Bonifacino J. S. (2010) Multilayered mechanism of CD4 down-regulation by HIV-1 Vpu involving distinct ER retention and ERAD targeting steps. PLoS Pathogens 6, e1000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Belaïdouni N., Marchal C., Benarous R., Besnard-Guérin C. (2007) Involvement of the βTrCP in the ubiquitination and stability of the HIV-1 Vpu protein. Biochem. Biophys. Res. Commun. 357, 688–693 [DOI] [PubMed] [Google Scholar]
- 13. Besnard-Guerin C., Belaïdouni N., Lassot I., Segeral E., Jobart A., Marchal C., Benarous R. (2004) HIV-1 Vpu sequesters β-transducin repeat-containing protein (βTrCP) in the cytoplasm and provokes the accumulation of β-catenin and other SCFβTrCP substrates. J. Biol. Chem. 279, 788–795 [DOI] [PubMed] [Google Scholar]
- 14. Estrabaud E., Le Rouzic E., Lopez-Vergès S., Morel M., Belaïdouni N., Benarous R., Transy C., Berlioz-Torrent C., Margottin-Goguet F. (2007) Regulated degradation of the HIV-1 Vpu protein through a βTrCP-independent pathway limits the release of viral particles. PLoS Pathogens 3, e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dubé M., Roy B. B., Guiot-Guillain P., Mercier J., Binette J., Leung G., Cohen E. A. (2009) Suppression of Tetherin-restricting activity upon human immunodeficiency virus type 1 particle release correlates with localization of Vpu in the trans-Golgi network. J. Virol. 83, 4574–4590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schubert U., Strebel K. (1994) Differential activities of the human immunodeficiency virus type 1-encoded Vpu protein are regulated by phosphorylation and occur in different cellular compartments. J. Virol. 68, 2260–2271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Neil S. J., Zang T., Bieniasz P. D. (2008) Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 451, 425–430 [DOI] [PubMed] [Google Scholar]
- 18. Jouvenet N., Neil S. J., Zhadina M., Zang T., Kratovac Z., Lee Y., McNatt M., Hatziioannou T., Bieniasz P. D. (2009) Broad-spectrum inhibition of retroviral and filoviral particle release by tetherin. J. Virol. 83, 1837–1844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sakuma T., Noda T., Urata S., Kawaoka Y., Yasuda J. (2009) Inhibition of Lassa and Marburg virus production by tetherin. J. Virol. 83, 2382–2385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Neil S. J., Sandrin V., Sundquist W. I., Bieniasz P. (2007) An interferon-α-induced tethering mechanism inhibits HIV-1 and Ebola virus particle release but is counteracted by the HIV-1 Vpu protein. Cell Host Microbe 2, 193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kaletsky R. L., Francica J. R., Agrawal-Gamse C., Bates P. (2009) Tetherin-mediated restriction of filovirus budding is antagonized by the Ebola glycoprotein. Proc. Natl. Acad. Sci. U.S.A. 106, 2886–2891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bour S., Schubert U., Peden K., Strebel K. (1996) The envelope glycoprotein of human immunodeficiency virus type 2 enhances viral particle release. A Vpu-like factor? J. Virol. 70, 820–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ritter G. D., Jr., Yamshchikov G., Cohen S. J., Mulligan M. J. (1996) Human immunodeficiency virus type 2 glycoprotein enhancement of particle budding. Role of the cytoplasmic domain. J. Virol. 70, 2669–2673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang F., Wilson S. J., Landford W. C., Virgen B., Gregory D., Johnson M. C., Munch J., Kirchhoff F., Bieniasz P. D., Hatziioannou T. (2009) Nef proteins from simian immunodeficiency viruses are tetherin antagonists. Cell Host Microbe 6, 54–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bartee E., McCormack A., Früh K. (2006) Quantitative membrane proteomics reveals new cellular targets of viral immune modulators. PLoS Pathog. 2, e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miyagi E., Andrew A. J., Kao S., Strebel K. (2009) Vpu enhances HIV-1 virus release in the absence of Bst-2 cell surface down-modulation and intracellular depletion. Proc. Natl. Acad. Sci. U.S.A. 106, 2868–2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mangeat B., Gers-Huber G., Lehmann M., Zufferey M., Luban J., Piguet V. (2009) HIV-1 Vpu neutralizes the antiviral factor Tetherin/BST-2 by binding it and directing its β-TrCP2-dependent degradation. PLoS Pathogens 5, e1000574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Van Damme N., Goff D., Katsura C., Jorgenson R. L., Mitchell R., Johnson M. C., Stephens E. B., Guatelli J. (2008) The interferon-induced protein BST-2 restricts HIV-1 release and is down-regulated from the cell surface by the viral Vpu protein. Cell Host Microbe 3, 245–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goffinet C., Allespach I., Homann S., Tervo H. M., Habermann A., Rupp D., Oberbremer L., Kern C., Tibroni N., Welsch S., Krijnse-Locker J., Banting G., Kräusslich H. G., Fackler O. T., Keppler O. T. (2009) HIV-1 antagonism of CD317 is species-specific and involves Vpu-mediated proteasomal degradation of the restriction factor. Cell Host Microbe 5, 285–297 [DOI] [PubMed] [Google Scholar]
- 30. Iwabu Y., Fujita H., Kinomoto M., Kaneko K., Ishizaka Y., Tanaka Y., Sata T., Tokunaga K. (2009) HIV-1 accessory protein Vpu internalizes cell-surface BST-2/tetherin through transmembrane interactions leading to lysosomes. J. Biol. Chem. 284, 35060–35072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Breitschopf K., Bengal E., Ziv T., Admon A., Ciechanover A. (1998) A novel site for ubiquitination. The N-terminal residue, and not internal lysines of MyoD, is essential for conjugation and degradation of the protein. EMBO J. 17, 5964–5973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mitchell R. S., Katsura C., Skasko M. A., Fitzpatrick K., Lau D., Ruiz A., Stephens E. B., Margottin-Goguet F., Benarous R., Guatelli J. C. (2009) Vpu antagonizes BST-2-mediated restriction of HIV-1 release via β-TrCP and endo-lysosomal trafficking. PLoS Pathogens 5, e1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pardieu C., Vigan R., Wilson S. J., Calvi A., Zang T., Bieniasz P., Kellam P., Towers G. J., Neil S. J. (2010) The RING-CH ligase K5 antagonizes restriction of KSHV and HIV-1 particle release by mediating ubiquitin-dependent endosomal degradation of tetherin. PLoS Pathogens 6, e1000843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Goffinet C., Homann S., Ambiel I., Tibroni N., Rupp D., Keppler O. T., Fackler O. T. (2010) Antagonism of CD317 restriction of human immunodeficiency virus type 1 (HIV-1) particle release and depletion of CD317 are separable activities of HIV-1 Vpu. J. Virol. 84, 4089–4094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tokarev A. A., Munguia J., Guatelli J. C. (2011) Serine-threonine ubiquitination mediates down-regulation of BST-2/tetherin and relief of restricted virion release by HIV-1 Vpu. J. Virology 85, 51–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Derdeyn C. A., Decker J. M., Sfakianos J. N., Zhang Z., O'Brien W. A., Ratner L., Shaw G. M., Hunter E. (2001) Sensitivity of human immunodeficiency virus type 1 to fusion inhibitors targeted to the gp41 first heptad repeat involves distinct regions of gp41 and is consistently modulated by gp120 interactions with the coreceptor. J. Virol. 75, 8605–8614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wei X., Decker J. M., Liu H., Zhang Z., Arani R. B., Kilby J. M., Saag M. S., Wu X., Shaw G. M., Kappes J. C. (2002) Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob. Agents Chemother. 46, 1896–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Platt E. J., Wehrly K., Kuhmann S. E., Chesebro B., Kabat D. (1998) Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J. Virol. 72, 2855–2864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Goto T., Kennel S. J., Abe M., Takishita M., Kosaka M., Solomon A., Saito S. (1994) A novel membrane antigen selectively expressed on terminally differentiated human B cells. Blood 84, 1922–1930 [PubMed] [Google Scholar]
- 40. Douglas J. L., Viswanathan K., McCarroll M. N., Gustin J. K., Früh K., Moses A. V. (2009) Vpu directs the degradation of the human immunodeficiency virus restriction factor BST-2/Tetherin via a βTrCP-dependent mechanism. J. Virol. 83, 7931–7947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gossen M., Bujard H. (1992) Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl. Acad. Sci. U.S.A. 89, 5547–5551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kamitani T., Kito K., Nguyen H. P., Yeh E. T. (1997) Characterization of NEDD8, a developmentally down-regulated ubiquitin-like protein. J. Biol. Chem. 272, 28557–28562 [DOI] [PubMed] [Google Scholar]
- 43. Wang X., Herr R. A., Chua W. J., Lybarger L., Wiertz E. J., Hansen T. H. (2007) Ubiquitination of serine, threonine, or lysine residues on the cytoplasmic tail can induce ERAD of MHC-I by viral E3 ligase mK3. J. Cell Biol. 177, 613–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Williams C., van den Berg M., Sprenger R. R., Distel B. (2007) A conserved cysteine is essential for Pex4p-dependent ubiquitination of the peroxisomal import receptor Pex5p. J. Biol. Chem. 282, 22534–22543 [DOI] [PubMed] [Google Scholar]
- 45. Andrew A. J., Miyagi E., Kao S., Strebel K. (2009) The formation of cysteine-linked dimers of BST-2/tetherin is important for inhibition of HIV-1 virus release but not for sensitivity to Vpu. Retrovirology 6, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mansouri M., Viswanathan K., Douglas J. L., Hines J., Gustin J., Moses A. V., Früh K. (2009) Molecular mechanism of BST2/tetherin down-regulation by K5/MIR2 of Kaposi's sarcoma-associated herpesvirus. J. Virol. 83, 9672–9681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Skasko M., Tokarev A., Chen C. C., Fischer W. B., Pillai S. K., Guatelli J. (2011) BST-2 is rapidly down-regulated from the cell surface by the HIV-1 protein Vpu. Evidence for a post-ER mechanism of Vpu-action. Virology 411, 65–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ciechanover A., Ben-Saadon R. (2004) N-terminal ubiquitination. More protein substrates join in. Trends Cell Biol. 14, 103–106 [DOI] [PubMed] [Google Scholar]
- 49. Cadwell K., Coscoy L. (2005) Ubiquitination on nonlysine residues by a viral E3 ubiquitin ligase. Science 309, 127–130 [DOI] [PubMed] [Google Scholar]
- 50. Wang X., Herr R. A., Rabelink M., Hoeben R. C., Wiertz E. J., Hansen T. H. (2009) Ube2j2 ubiquitinates hydroxylated amino acids on ER-associated degradation substrates. J. Cell Biol. 187, 655–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Geimonen E., Fernandez I., Gavrilovskaya I. N., Mackow E. R. (2003) Tyrosine residues direct the ubiquitination and degradation of the NY-1 hantavirus G1 cytoplasmic tail. J. Virol. 77, 10760–10868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rollason R., Korolchuk V., Hamilton C., Schu P., Banting G. (2007) Clathrin-mediated endocytosis of a lipid-raft-associated protein is mediated through a dual tyrosine motif. J. Cell Sci. 120, 3850–3858 [DOI] [PubMed] [Google Scholar]
- 53. Masuyama N., Kuronita T., Tanaka R., Muto T., Hirota Y., Takigawa A., Fujita H., Aso Y., Amano J., Tanaka Y. (2009) HM1.24 is internalized from lipid rafts by clathrin-mediated endocytosis through interaction with α-adaptin. J. Biol. Chem. 284, 15927–15941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Heijne G. (1986) The distribution of positively charged residues in bacterial inner membrane proteins correlates with the transmembrane topology. EMBO J. 5, 3021–3027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cadwell K., Coscoy L. (2008) The specificities of Kaposi's sarcoma-associated herpesvirus-encoded E3 ubiquitin ligases are determined by the positions of lysine or cysteine residues within the intracytoplasmic domains of their targets. J. Virol. 82, 4184–4189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Vembar S. S., Brodsky J. L. (2008) One step at a time. Endoplasmic reticulum-associated degradation. Nat. Rev. Mol. Cell Biol. 9, 944–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yen H. C., Elledge S. J. (2008) Identification of SCF ubiquitin ligase substrates by global protein stability profiling. Science 322, 923–929 [DOI] [PubMed] [Google Scholar]
- 58. Cardozo T., Pagano M. (2004) The SCF ubiquitin ligase. Insights into a molecular machine. Nat. Rev. Mol. Cell Biol. 5, 739–751 [DOI] [PubMed] [Google Scholar]
- 59. Kumar K. G., Tang W., Ravindranath A. K., Clark W. A., Croze E., Fuchs S. Y. (2003) SCF(HOS) ubiquitin ligase mediates the ligand-induced down-regulation of the interferon-α receptor. EMBO J. 22, 5480–5490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Li Y., Kumar K. G., Tang W., Spiegelman V. S., Fuchs S. Y. (2004) Negative regulation of prolactin receptor stability and signaling mediated by SCF(βTrCP) E3 ubiquitin ligase. Mol. Cell Biol. 24, 4038–4048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Meyer L., Deau B., Forejtníková H., Duménil D., Margottin-Goguet F., Lacombe C., Mayeux P., Verdier F. (2007) βTrcp mediates ubiquitination and degradation of the erythropoietin receptor and controls cell proliferation. Blood 109, 5215–5222 [DOI] [PubMed] [Google Scholar]
- 62. Strous G. J., van Kerkhof P., Govers R., Ciechanover A., Schwartz A. L. (1996) The ubiquitin conjugation system is required for ligand-induced endocytosis and degradation of the growth hormone receptor. EMBO J. 15, 3806–3812 [PMC free article] [PubMed] [Google Scholar]
- 63. van Kerkhof P., Putters J., Strous G. J. (2007) The ubiquitin ligase SCF(βTrCP) regulates the degradation of the growth hormone receptor. J. Biol. Chem. 282, 20475–20483 [DOI] [PubMed] [Google Scholar]
- 64. Deng L., He K., Wang X., Yang N., Thangavel C., Jiang J., Fuchs S. Y., Frank S. J. (2007) Determinants of growth hormone receptor down-regulation. Mol. Endocrinol. 21, 1537–1551 [DOI] [PubMed] [Google Scholar]
- 65. Dubé M., Paquay C., Roy B. B., Bego M. G., Mercier J., Cohen E. A. (2011) HIV-1 Vpu antagonizes BST-2 by interfering mainly with the trafficking of newly synthesized BST-2 to the cell surface. Traffic 12, 1714–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lau D., Kwan W., Guatelli J. (2011) Role of the endocytic pathway in the counteraction of BST-2 by human lentiviral pathogens. J. Virol. 85, 9834–9846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kupzig S., Korolchuk V., Rollason R., Sugden A., Wilde A., Banting G. (2003) Bst-2/HM1.24 is a raft-associated apical membrane protein with an unusual topology. Traffic 4, 694–709 [DOI] [PubMed] [Google Scholar]
- 68. Schmidt S., Fritz J. V., Bitzegeio J., Fackler O. T., Keppler O. T. (2011) HIV-1 Vpu blocks recycling and biosynthetic transport of the intrinsic immunity factor CD317/tetherin to overcome the virion release restriction. MBio 2, e00036–00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Schubert U., Schneider T., Henklein P., Hoffmann K., Berthold E., Hauser H., Pauli G., Porstmann T. (1992) Human immunodeficiency virus type 1-encoded Vpu protein is phosphorylated by casein kinase II. Eur. J. Biochem. 204, 875–883 [DOI] [PubMed] [Google Scholar]
- 70. Margottin F., Bour S. P., Durand H., Selig L., Benichou S., Richard V., Thomas D., Strebel K., Benarous R. (1998) A novel human WD protein, h-βTrCp, that interacts with HIV-1 Vpu connects CD4 to the ER degradation pathway through an F-box motif. Mol. Cell 1, 565–574 [DOI] [PubMed] [Google Scholar]
- 71. Li Y., Gazdoiu S., Pan Z. Q., Fuchs S. Y. (2004) Stability of homologue of Slimb F-box protein is regulated by availability of its substrate. J. Biol. Chem. 279, 11074–11080 [DOI] [PubMed] [Google Scholar]