Background: “Mirrored” or complementary mammalian miRNAs have been predicted but none have yet been characterized.
Results: miR-3120 and miR-214 are produced from the same intronic locus; miR-3120 regulates heat shock cognate protein 70 (Hsc70) and auxilin expression and vesicle uncoating.
Conclusion: miR-3120 is a mirror miRNA regulating endocytic function.
Significance: Mirror miRNAs are important mammalian gene regulatory units, and many more remain to be found and characterized.
Keywords: Endocytosis, Heat Shock Protein, MicroRNA, Neurobiology, Vesicles
Abstract
We show that a single gene locus gives rise to two fully processed and functional miRNAs, i.e. that due to imperfect base pairing, two distinct microRNAs (miRNAs) can be produced from the fully complementary DNA strands. The antisense strand encodes miR-214, which is transcribed by its own promoter, whereas a novel miRNA, miR-3120, is co-expressed with its host gene mRNA. We also found that miR-3120 regulates important aspects of cellular function that are similar to that of its host gene, dynamin-3. miR-3120 was found to be located in neuronal cell bodies and to target Hsc70 and auxilin, and its lentivirus-mediated expression inhibited the uncoating of clathrin-coated vesicles. Finally, mirror miRNAs are likely to represent a new group of miRNAs with complex roles in coordinating gene expression.
Introduction
In recent years, there have been extraordinary developments in our understanding of RNA biology, including the discovery of miRNAs3 (1). miRNAs are found throughout the body, and their expression is coordinated with that of coding genes, enabling them to regulate diverse and complex cellular pathways (1, 2) For example, different families of miRNAs are expressed according to function, and they can control the expression of functionally related genes simultaneously (3, 4). Furthermore, miRNA levels can be regulated by synchronized activation (or inactivation) of miRNA promoters or by increased processing from its host gene (5, 6). The expression of miRNAs can also be directed to specific compartments; for instance, in neurons, they are found in cell bodies and/or dendrites, where they mediate activity-dependent control of spine development (4, 7). Hence, miRNAs can coordinate the expression of genes in response to neuronal activity and are being shown to be important regulators of synaptic function (4, 7). The processes governing miRNA biogenesis have yet to be fully elucidated; however, we know that most miRNA genes are transcribed from DNA in the nucleus as long double-stranded primary miRNAs, which are then processed to precursor miRNAs by Drosha and DGCR8 (1, 2). More than half of the known miRNAs are encoded within the introns of protein coding genes or noncoding transcription units, whereas ∼10% are encoded by exons of long nonprotein coding transcripts (9, 10). In some cases, the intronic (sense) miRNAs are co-expressed with their host gene mRNA, suggesting that the miRNA may help coordinate the function of the protein(s) encoded by the host mRNA (9, 10). miRNAs may be transcribed by their own promoters, and these are often found in an antisense orientation to a host gene (4, 6). Intriguingly, Drosophila genes have been predicted and shown to form functional miRNA hairpin structures on both sense and antisense strands (11). However, although many mirror miRNAs are predicted to exist in mammals, until now, fully processed and functional mirror miRNAs have not been reported. Experiments described in this study show that the sense strand of a dynamin-3 gene intron encodes a fully processed miRNA termed miR-3120, whereas the antisense strand encodes miR-214, which is produced by antisense transcription. Furthermore, we have characterized the novel miRNA miR-3120 and shown that it regulates the expression of Hsc70 and its co-chaperone-, auxilin-, and clathrin-mediated endocytosis.
EXPERIMENTAL PROCEDURES
In Vitro miRNA Processing Assays
We generated pri-miRNAs for miRNA processing assays by in vitro transcription using the MEGAshortscriptTM T7 kit (Ambion). To create the starting template, we fused the antisense sequence of the pri-miRNA to the T7 promoter (5′-antisense precursor-miRNA sequence, TATAGTGAGTCGTATTAAATT-3′), and transcription was carried out in the presence of [α-32P]UTP (3000 Ci/mmol, GE Healthcare) and at 37 °C for 30 min. In vitro processing assays using T7-labeled miRNAs have been described previously (12, 13). To generate extracts from tissues, samples were cut into small pieces, sonicated in lysis buffer (30 mm Hepes, pH 7.4, 100 mm KCL, 5 mm MgCl2, 10% glycerol, 0.5 mm DTT, 0.1 mm 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride), and centrifuged at 13,000 rpm at 4 °C. Extracts (12 mg/ml protein) were then supplemented with 100 mm KCL, 2 mm MgCl2, and 10% glycerol. 1 μl (100 nm) of the substrate was added to the reaction mixture (final concentrations of 100 mm KCL, 5 mm MgCl2, 0.5 mm DTT, 1 mm ATP, and 0.2 mm GTP), and it was then incubated for up to 2 h at 30 °C. Samples were separated on 0.4 mm 15% denaturing polyacrylamide gels and reaction products detected by using PhosphorImager screens (GE Healthcare).
miRNA Cloning and Lentiviral Vector Production
The miR-214 and -3120 genomic region was cloned from rat genomic DNA using the following primers: 5′-TCC TTC AAT CAC CAA ATC TGG and 5′-TCA CTG TGG TTG TAG CTC TTG and the FastStart high fidelity PCR system (Roche Applied Science). PCR products were cloned into the TOPO-TA vector (Invitrogen) before positive sequences were cloned into a lentiviral plasmid directly upstream of an EGFP coding sequence to be transcribed as a single unit. HIV-1-based lentiviral vectors were prepared using the HEK293T transient system previously described (14), and viral titer was determined using fluorescence-activated cell sorting (FACS) analysis of transduced HEK293T cells.
Primary Neuronal Cultures and RNA/miRNA Detection
Cortical and hippocampal neurons were cultured from embryonic day 18 Wistar rats as described previously (14). Neurons were transduced with lentiviral vectors at 3 days in vitro (DIV) and maintained up to 21 DIV. Total RNAs were isolated using the RNeasy mini kit (Qiagen), and miRNAs were isolated from cultured neurons at 4, 7, 14, and 21 DIV using the mirVana miRNA isolation kit (Ambion). miRNA expression levels were assessed using TaqMan® miRNA assays (Applied Biosystems) according to the manufacturer's protocol. Results were normalized against levels of RNU6B (as assessed by the corresponding TaqMan® assay; Applied Biosystems). For gene expression studies, total RNA was converted to cDNA according to the manufacturer's protocol (Applied Biosystems). Dynamin-3 and HSPA8 mRNA levels were assessed using the TaqMan® gene expression assays (Applied Biosystems) and analyzed using the ΔΔCt method.
In Situ Hybridization
Cultured neurons grown were fixed with 4% paraformaldehyde, washed twice in PBS-diethylpyrocarbonate, and digested with proteinase K (5 μg/ml) at room temperature for 1 min before post-fixing in 4% paraformaldehyde and incubation in acetic anhydride solution (0.09 m triethanolamine, 0.83 m HCl, 2.5 mm acetic anhydride) for 10 min. Following acetylation, neurons were washed in PBS-diethylpyrocarbonate and prehybridized in hybridization buffer (50% formamide, 5× SSC, 500 μg/ml tRNA, 1× Denhardt's solution, pH 6.0) for 3 h at 58 °C. Probe was prepared by adding 3–4 pmol of digoxigenin-labeled locked nucleic acid (LNA) oligonucleotides to detect mature miRNA (Exiqon) to 200 μl of hybridization buffer. A custom probe was designed for miR-3120, whereas a commercially available probe was used as a scrambled control. Neurons were incubated with probe for 18 h at 58 °C in a hybridization chamber. Probe was removed with a series of washes using SSC and PBS with 0.1% Tween 20 (PBT) at 60 °C. Neurons were blocked for 1 h in blocking buffer (Roche Applied Science) and incubated in a humidified chamber overnight at 4 °C in 1:500 dilution of primary antibody (mouse anti-digoxigenin HRP, Abcam). Mature miRNAs were then visualized using a tyramide signal amplification detection kit (Molecular Probes TSATM kit 5, T20915). Following TSATM substrate incubation for 15 min, neurons were washed in PBT and mounted in VECTASHIELD with DAPI (Vector Laboratories). Fluorescent images were collected with a DFC340FX Leica camera and Leica Application Suite (LAS) core software.
3′-UTR Reporter Assay
The 3′-UTRs of rat HSPA8, human HSPA8, and rat DNJC6 were obtained from genomic DNA by PCR using the following primers: forward, GTACGCGTTCAAAGTAGAGGGTATAGC, reverse, GCGAAGCTTGGTGCCAATTTAAATAG; forward, GTACTAGTGCCAACCAAGTGTAGATG, reverse, GGACGCGTTGGTGCCAATTTTAAATAG; forward, GGACGCGTTTTGTGAGCTTTTCTATGC, reverse, GCGTCGCGAATATTTTAAACAATGAC, respectively. The resulting PCR products were cloned downstream of the luciferase gene in the pmiR-reporter plasmid (Ambion). HEK293T cells were co-transfected with the luciferase reporter plasmid, a Renilla reporter plasmid, SV40 Renilla (Promega), and various miRNA sequences cloned into the HIV-1-based lentiviral shuttle plasmid pRRL-CMV-IRES-EGFP. Dual-Luciferase reporter assays (Promega) were performed following the manufacturer's instructions 48 h later.
Electron Microscopy
Neurons (10 DIV) were fixed with 2.5% glutaraldehyde in cacodylate buffer. The cells were processed for Epon embedding using a short protocol according to Parton (15). Ultrathin sections were made and analyzed using a Tecnai 12 Spirit transmission electron microscope (FEI Co.) equipped with an Eagle 4k x 4k CCD camera.
RESULTS
Two Fully Processed miRNAs Are Produced from a Single Locus
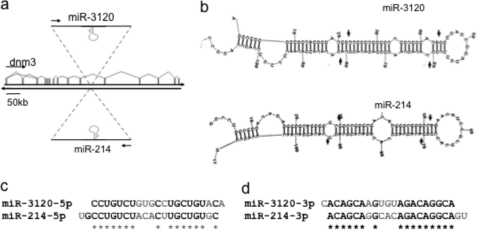
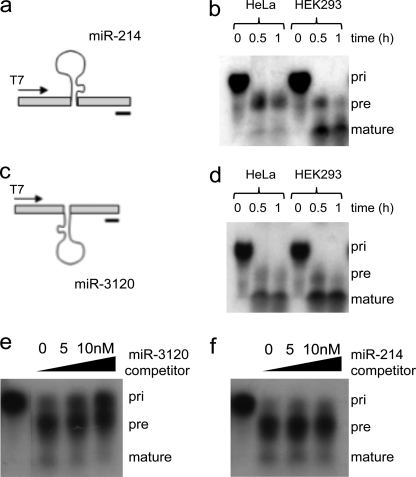
We have previously shown that during development, Twist-1 drives the expression of miR-199a and miR-214 from the dynamin-3 gene intron antisense strand (6) (Fig. 1a). Analysis using the mfold web server suggested that a functional miRNA would be formed from the pri-miR-214 (but not pri-miR-199) sense strand, termed miR-3120 (Fig. 1b). The predicted 5p and 3p mature sequences of the palindromic miRNA (miR-3120) differed from that of miR-214, with the GGCAC of miR-214-3p changing to AGUGU in miR-3120-3p and ACACU in miR-214-5p changing to GUGCC in miR-3120-5p (Fig. 1, c and d). As a result, the fully processed 22-nucleotide miR-3120 has an altered seed sequence and will consequently target a different population of mRNAs. In contrast, the antisense strand of pri-miR-199 was predicted to form a 19-nucleotide nonfunctional miRNA. To confirm that miR-3120 was processed to a mature miRNA, 32P-labeled pri-miR-214 and pri-miR-3120 were produced by T7 in vitro transcription (Fig. 2, a and b), and following incubation with (10 mg/ml) HeLa cell extracts (12, 13), they were processed to precursor and fully mature forms (Fig. 2, b and d). To further assess the specificity of this reaction, competition assays were performed with 32P-labeled pri-miR-3120 (final concentration 10 nm), with processing being measured in the presence (0, 5, and 10 nm) of cold pri-miR-3120 or pri-miR-214. In the presence of cold pri-miR-3120, 32P-labeled pri-miR-3120 accumulated, and less labeled miR-3120 was produced (Fig. 2e). However, the addition of cold pri-miR-214 did not change the processing of pri-miR-3120 (Fig. 2f). During the course of this study, the predicted mature 21-nucleotide miR-3120 was (deep) sequenced in a melanoma miRNAome (miRBase).
FIGURE 1.
Schematic of miR-214 and miR-3120 mirror miRNAs. a, the location of the mirror miRNAs, miR-3120 and miR-214, on opposite strands of the dynamin-3 intron. b, secondary structures of the miR-3120 and miR-214 pri-miRNAs as predicted by mfold showing Drosha and dicer cleavage sites (arrows). c and d, predicted sequences of the mature 5p and 3p miRNAs (complementary sequences are starred, and mismatches are in gray).
FIGURE 2.
pri-miR-3120 is transcribed, and a mature miRNA is expressed. a and c, miR-214 (a) and miR-3120 (c) were cloned into T7 constructs, and the pri-miRNAs were produced by in vitro transcription. b and d, incubation of transcribed miR-214 (b) and miR-3120 (d) with HeLa and HEK293T cell extracts show that both miRNAs are fully processed. Note the increase in the mature miRNAs over time. e, competition assays show that the processing of miR-3120 (5 nm) is inhibited (as seen by the accumulation of the pri-miR-3120) by the addition of cold (0, 5, 10 nm) pri-miR-3120. pre, precursor-miRNA. f, processing of 32P-miR-3120 was not affected by incubation with cold pri-miR-214. The first lanes in e and f are pri-miRNAs minus HeLa cell extract.
miR-3120, miR-214, and Dynamin-3 Are Co-expressed
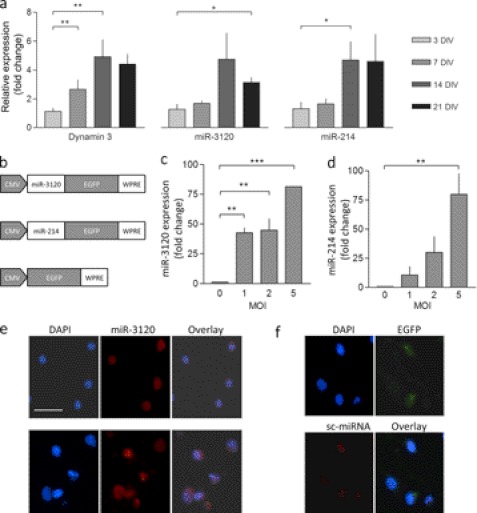
To confirm that miR-3120 was expressed endogenously, we used quantitative PCR assays specific for miR-3120 and miR-214 (Fig. 3a). We found that miR-3120 and miR-214 (and dynamin-3 mRNA) were expressed at low levels in hippocampal neurons at 3 and 7 DIV. However, at 14 and 21 DIV miR-3120, miR-214 and dynamin-3 mRNAs were all expressed at significantly higher levels (relative to day 3), suggesting they may be important for neuronal function (Fig. 2a). Northern blot analysis (not shown) also showed that miR-3120 was highly expressed in embryonic day 12.5 mouse embryos, suggesting that like miR-214, it plays a developmental role. To investigate mirror miRNA function, lentiviral vectors expressing miR-3120 and miR-214 were made (Fig. 3b), and TaqMan assays were again used to confirm expression. Fig. 3, c and d, show that transduction resulted in a multiplicity of infection-dependent increase in miR-3120 and miR-214. miR-3120 levels did not, however, increase if cells were transduced with miR-214, and miR-214 levels were not observed to increase when cells where transduced with miR-3120 (data not shown). To determine the neuronal localization of miR-3120, we performed in situ hybridization assays in the presence and absence of lentiviral vector expressing miR-3120 and a scrambled control (Fig. 3, e and f). Endogenous miR-3120 was expressed in the nuclei and cytoplasm of neuronal cell bodies (Fig. 3e). Lentivirus-mediated expression of miR-3120 increased the level of miRNA staining but did not alter the miRNA localization (Fig. 3e).
FIGURE 3.
miR-3120 and miR-214 expression in neurons. a, quantitative PCR measurements in cultured neurons showing levels of endogenous miR-3120, miR-214, and dynamin-3 mRNA relative to 3 DIV. b, schematic diagram of the lentiviral constructs used. pri-miR sequences were cloned 5′ of the EGFP coding sequence and are processed out of the mRNA to produce mature miRNAs. c and d, quantitative PCR assays show that lentiviral vectors mediate an increase in miR-3120 (c) and miR-214 (d) in cultured neurons relative to nontransduced cells. MOI, multiplicity of infection. e and f, miRNAs in hippocampal neurons were visualized by in situ hybridization. DAPI staining shows nuclei in the cells (scale bar = 50 μm). Expression of endogenous miR-3120 is localized predominantly within nuclei and cytoplasm (e, top panel), and virally overexpressed miR-3120 is found to have the same distribution (e, bottom panel). The scrambled (sc-miRNA) miR-3120 probe (f, bottom panel) did not detect miR-3120 despite overexpression of miR-3120 by lentiviral vectors (confirmed as they co-express EGFP). All experiments were conducted in triplicate, and values are means ± S.E. Statistical analyses were conducted by analysis of variance and post hoc Bonferroni's test, ***, p < 0.001, **, p < 0.01, *, p < 0.05.
miR-3120 Regulates Hsc70 and Vesicle Uncoating
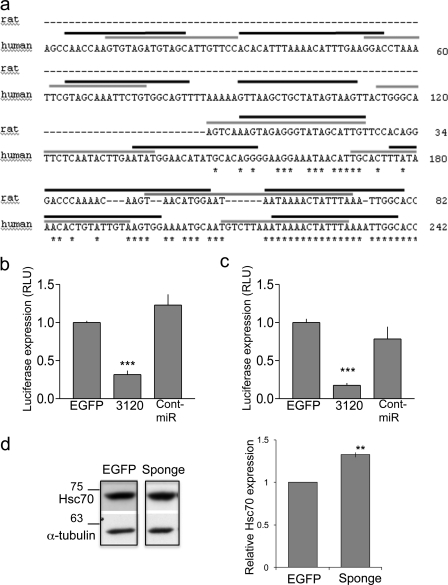
To identify miR-3120 targets, we used miRanda v1.0b (microRNA target scanning algorithm) and TargetRank and found that miR-3120 was predicted to bind the 3′-UTR of Hsc70 (Hspa8). Interestingly, both the 5p and the 3p forms of miR-3120 were predicted to interact with both the human and the rat 3′-UTR of Hsc70 (Fig. 4a). To test this predicted interaction, we co-transfected HEK293T cells with lentiviral plasmids expressing pri-miRNAs and luciferase reporter plasmids (pMIR-REPORTTM, Ambion) containing either the rat or the human Hsc70 3′-UTR downstream of the luciferase gene. Analysis of luciferase expression demonstrated that miR-3120 mediated a significant inhibition of translation that was not observed following expression of miR-214 or EGFP (Fig. 4, b and c). Transfection of neuronal cells with a vector expressing repeats of the miR-3120 recognition sequence (3120 sponge) mediated a significant increase in Hsc70 levels (Fig. 4d).
FIGURE 4.
miR-3120 targets conserved sites within rat and human Hsc70 UTR. a, schematic diagram of the rat and human Hsc70 3′-UTR showing species alignment and the predicted location of miR-3120 recognition sites. Black lines indicate miR-3120-3p binding, and gray lines indicate miR-3120-5p binding sites; note that the binding sites contain conserved ARE (TTTAAA) motifs. b, 3′-UTR of rat Hsc70 mRNA was co-expressed with miR-3120, control miRNA (Cont-miR) (miR-214), or an EGFP control plasmid. RLU, relative luciferase units. c, luciferase expression from reporter constructs expressing the human Hsc70 3′-UTR. d, cells transfected with an miR-3120 sponge had significantly increased Hsp70 levels. A representative Western blot is shown, and the graph represents analysis of all experiments. α-Tubulin is used as a loading control. Experiments were conducted in triplicate, and values are means ± S.E. Statistical analyses were conducted by analysis of variance and post hoc Bonferroni's test, ***, p < 0.001. Analyses were also carried out by Student's t test **, p < 0.01.
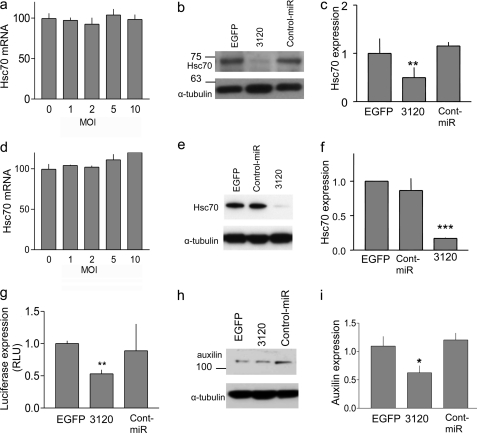
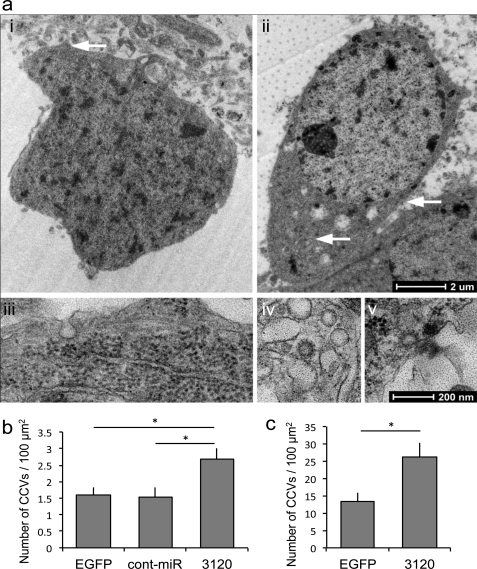
Additional experiments in hippocampal and cortical neurons supported these data, demonstrating a significant reduction in endogenous Hsc70 following transduction with miR-3120 but not miR-214 (Fig. 5, a–f). No change in Hsc70 mRNA levels was observed following transduction with miR-3120 (Fig. 5, a and d). Bioinformatics analysis predicted that auxilin (DNAJC6) may also be targeted by miR-3120, and auxilin 3′-UTR assays and Western blot analyses confirmed this (Fig. 5, g–i). Following these experiments, hippocampal and cortical neurons were transduced with miR-3120, and the number of clathrin-coated profiles (pits or vesicles) per cell surface area was measured. For quantification of clathrin-coated structures inside the cells, randomly chosen cell bodies (identified by the presence of a nucleus and surrounding cytoplasm) were taken. At low magnification (1,900× end magnification, Fig. 6a (images i and ii)), the surface area was measured, and at higher magnification (13,000×), the cytoplasm was scanned and clathrin-coated structures were counted (Fig. 6a, images iii and v). Clathrin-coated structures were identified on ultrastructural criteria by their characteristic dark coat containing rectangular extensions with “spacings” (the clathrin triskeleton) on a vesicular or membranous structure (Fig. 6a (images iv and v). Both clathrin-coated pits and vesicles were counted. They can be discerned from rough endoplasmic reticulum because ribosomes on the endoplasmic reticulum are bigger and round (Fig. 6a (images iii and v). Fig. 6, b and c, show that there was a significant increase in the number of coated particles (pits and vesicles) in hippocampal and cortical neurons following transduction with miR-3120 when compared with cells transduced with EGFP or miRNA controls.
FIGURE 5.
Analysis of Hsc70 and auxilin expression following transduction of miR-3120. a and d, Hsc70 mRNA levels were measured in hippocampal (a) and cortical (d) neurons following the lentivirus-mediated expression of miR-3120. MOI, multiplicity of infection. b and e, Hsc70 protein in hippocampal (b) and cortical (e) neurons following transduction with lentiviral vectors expressing miR-3120 and EGFP and miRNA controls. g–i, expression of miR-3120 also mediated a reduction in luciferase activity in auxilin 3′-UTR assays (g) and in auxilin protein expression (h). RLU, relative luciferase units. c, f, and i, densitometry analysis showed a significant reduction in hippocampal (c) and cortical (f) Hsc70 protein levels in the presence of miR-3120 and a significant reduction in auxilin levels (i). Cont-miR, control miRNA. All experiments were conducted in triplicate, and values are means ± S.E. Statistical analyses were conducted by analysis of variance and post hoc Bonferroni's test, ***, p < 0.001, **, p < 0.01, *, p < 0.05.
FIGURE 6.
miR-3120 prevents uncoating of clathrin-coated vesicles. a, transmission electron microscopy image of EGFP transduced cell bodies (images i and ii) identified by the presence of a nucleus and surrounding cytoplasm (1,900 times end magnification). White arrow in image i denotes the pit shown in the (13,000 times) high magnification image (image iii), whereas the arrows in image ii show (13,000 times) high power images (images iv and v) of clathrin-coated vesicles. Image iii also shows ribosomes on the endoplasmic reticulum. b, clathrin-coated vesicle (CCV) counts in cortical neurons transduced with miR-3120 when compared with those transduced with EGFP and the miRNA control (Cont-miR). A similar increase in clathrin-coated vesicle number was seen in hippocampal neurons transduced with miR-3120. A detailed description of the procedures for counting clathrin-coated vesicles is given under “Results.” For both hippocampal and cortical neuronal cultures, a minimum total of 17 cells was analyzed in three individual experiments. Values are means ± S.E. Statistical analyses were conducted by analysis of variance and post hoc Bonferroni's test * = p > 0.01.
DISCUSSION
We have found that a single locus within intron 13 of the dynamin-3 gene encodes two mirror miRNAs, miR-3120 and miR-214. miR-3120 is produced following transcription and mRNA processing, whereas miR-214 is produced by antisense transcription. The previously uncharacterized miR-3120 was also shown to be fully processed to a functional miRNA and to be expressed with miR-214 and its host gene dynamin-3 in neurons. Intronic miRNAs are often coordinately expressed with their host gene mRNA sharing the same spatial and temporal localization patterns (4, 9). However, it is interesting that there is a coordinated expression of both mirror miRNAs (produced by different processes) in the same cells. Many similar examples of mammalian mirror or complementary miRNAs could exist. However, this is the first example characterized in mammals suggesting that it is rare for both miRNAs to have sustained beneficial effects allowing them to be retained by natural selection. We further found that miR-3120 targets the clathrin-uncoating enzyme Hsc70 (and its co-chaperone auxilin) and that an miR-3120 sponge increases Hsc70 expression significantly. This shows that miR-3120 plays a role in regulating constitutive levels of Hsc70. Interestingly, the miR-3120 (5p and 3p) binding sites were also found to exist within defined 3′-UTR AU-rich elements (ARE) motifs that were themselves conserved between human and rat. AREs interact with a number of ARE-binding proteins to modulate mRNA stability and degradation (16). For example, the displacement of double-stranded RNA-dependent protein kinase (pkr) from ARE sites in the 3′-UTR of Hsp70 has been shown to mediate the degradation of the mRNA (17). The colocalization of miR-3120 binding sites within conserved (TTTAAA) ARE binding regions suggests that a novel mechanism for the control of Hsc70 translation and rapid changes in Hsc70 translation are needed to regulate its physiological functions. Hsc70 is the ATPase that catalyzes the uncoating of clathrin-coated vesicles (18, 19), and in combination with many other chaperones, it is involved in many cellular processes including the folding and translocation of polypeptides and the activation of the glucocorticoid receptor. In neurons, clathrin uncoating by Hsc70 requires the neuron-specific co-factor auxilin or G-associated kinase (GAK) (auxilin-2) (20, 21). Hsc70 and auxilin have now been shown to have multiple roles in clathrin-mediated endocytosis (20). Inhibition of either Hsc70 or auxilin has been shown to prevent the uncoating of clathrin-coated vesicles, block receptor recycling, and impair clathrin-mediated endocytosis and transmission at hippocampal synapses (21, 22). Furthermore, auxilin and Hsc70 are shown to regulate clathrin-coated pit formation at the plasma membrane (23). It is also estimated that approximately 90% of all clathrin-coated vesicles in neurons are involved in synaptic vesicle retrieval (24). Our observations showing that the miR-3120 inhibits clathrin-mediated uncoating are in agreement with previous studies of Hsc70 and auxilin function. Importantly, they also show that miRNAs mediate a previously unknown level of control over the processes governing endosomal trafficking. It is also interesting to note that the miR-3120 host gene, dynamin-3, associates with clathrin (25) and mediates the rapid reformation of synaptic vesicles after endocytosis (26). Also, the disruption of dynamin-3 function results in reduced synaptic vesicle recycling and altered synaptic transmission (27–29). We have found that miR-3120 regulates vesicle uncoating and that miR-214 is known to target PTEN (phosphatase and tensin homolog) (30), which interacts with dynamin and regulates synaptic proteins involved in receptor cycling and synaptic plasticity (8). Hence, the miR-3120 and -214 mirror miRNAs may represent a novel genetic unit that has evolved to regulate the complex neuronal pathways associated with synaptic vesicle function and neuronal plasticity.
This work was supported by the Wellcome Trust, Medical Research Council (NET), and Biotechnology and Biological Sciences Research Council (BBSRC).
- miRNA
- microRNA
- miR
- microRNA
- pri-miRNA
- primary miRNA
- DIV
- days in vitro
- ARE
- AU-rich elements
- EGFP
- enhanced green fluorescent protein.
REFERENCES
- 1. Hannon G. J. (2002) RNA interference. Nature 418, 244–251 [DOI] [PubMed] [Google Scholar]
- 2. Filipowicz W., Bhattacharyya S. N., Sonenberg N. (2008) Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat. Rev. Genet. 9, 102–114 [DOI] [PubMed] [Google Scholar]
- 3. Kim J., Krichevsky A., Grad Y., Hayes G. D., Kosik K. S., Church G. M., Ruvkun G. (2004) Identification of many microRNAs that copurify with polyribosomes in mammalian neurons. Proc. Natl. Acad. Sci. U.S.A. 101, 360–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Siegel G., Obernosterer G., Fiore R., Oehmen M., Bicker S., Christensen M., Khudayberdiev S., Leuschner P. F., Busch C. J., Kane C., Hübel K., Dekker F., Hedberg C., Rengarajan B., Drepper C., Waldmann H., Kauppinen S., Greenberg M. E., Draguhn A., Rehmsmeier M., Martinez J., Schratt G. M. (2009) A functional screen implicates microRNA-138-dependent regulation of the depalmitoylation enzyme APT1 in dendritic spine morphogenesis. Nat. Cell Biol. 11, 705–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tsang J., Zhu J., van Oudenaarden A. (2007) MicroRNA-mediated feedback and feedforward loops are recurrent network motifs in mammals. Mol. Cell 26, 753–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee Y. B., Bantounas I., Lee D. Y., Phylactou L., Caldwell M. A., Uney J. B. (2009) Twist-1 regulates the miR-199a/214 cluster during development. Nucleic Acids Res. 37, 123–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schratt G. M., Tuebing F., Nigh E. A., Kane C. G., Sabatini M. E., Kiebler M., Greenberg M. E. (2006) A brain-specific microRNA regulates dendritic spine development. Nature 439, 283–289 [DOI] [PubMed] [Google Scholar]
- 8. Yang H., Kong W., He L., Zhao J. J., O'Donnell J. D., Wang J., Wenham R. M., Coppola D., Kruk P. A., Nicosia S. V., Cheng J. Q. (2008) MicroRNA expression profiling in human ovarian cancer: miR-214 induces cell survival and cisplatin resistance by targeting PTEN. Cancer Res. 68, 425–433 [DOI] [PubMed] [Google Scholar]
- 9. Rodriguez A., Griffiths-Jones S., Ashurst J. L., Bradley A. (2004) Identification of mammalian microRNA host genes and transcription units. Genome Res. 14, 1902–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baskerville S., Bartel D. (2005) Microarray profiling of microRNAs reveals frequent co-expression with neighboring miRNAs and host genes. RNA 11, 241–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tyler D. M., Okamura K., Chung W. J., Hagen J. W., Berezikov E., Hannon G. J., Lai E. C. (2008) Functionally distinct regulatory RNAs generated by bidirectional transcription and processing of microRNA loci. Genes Dev. 22, 26–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Leuschner P. J., Martinez J. (2007) In vitro analysis of microRNA processing using recombinant Dicer and cytoplasmic extracts of HeLa cells. Methods 43, 105–109 [DOI] [PubMed] [Google Scholar]
- 13. Rybak A., Fuchs H., Smirnova L., Brandt C., Pohl E. E., Nitsch R., Wulczyn F. G. (2008) A feedback loop comprising lin-28 and let-7 controls pre-let-7 maturation during neural stem cell commitment. Nat. Cell Biol. 10, 987–993 [DOI] [PubMed] [Google Scholar]
- 14. Howarth J. L., Kelly S., Keasey M. P., Glover C. P., Lee Y. B., Mitrophanous K., Chapple J. P., Gallo J. M., Cheetham M. E., Uney J. B. (2007) Hsp40 molecules that target to the ubiquitin-proteasome system decrease inclusion formation in models of polyglutamine disease. Mol. Ther. 15, 1100–1105 [DOI] [PubMed] [Google Scholar]
- 15. Parton R. G. (1995) Rapid processing of filter-grown cells for Epon embedding. J. Histochem. Cytochem. 43, 731–733 [DOI] [PubMed] [Google Scholar]
- 16. Barreau C., Paillard L., Osborne H. B. (2005) AU-rich elements and associated factors: are there unifying principles? Nucleic Acids Res. 33, 7138–7150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhao M., Tang D., Lechpammer S., Hoffman A., Asea A., Stevenson M. A., Calderwood S. K. (2002) Double-stranded RNA-dependent protein kinase (pkr) is essential for thermotolerance, accumulation of HSP70, and stabilization of ARE-containing HSP70 mRNA during stress. J. Biol. Chem. 277, 44539–44547 [DOI] [PubMed] [Google Scholar]
- 18. Chappell T. G., Welch W. J., Schlossman D. M., Palter K. B., Schlesinger M. J., Rothman J. E. (1986) Uncoating ATPase is a member of the 70-kilodalton family of stress proteins. Cell. 45, 3–13 [DOI] [PubMed] [Google Scholar]
- 19. Ungewickell E., Ungewickell H., Holstein S. E., Lindner R., Prasad K., Barouch W., Martin B., Greene L. E., Eisenberg E. (1995) Role of auxilin in uncoating clathrin-coated vesicles. Nature 378, 632–635 [DOI] [PubMed] [Google Scholar]
- 20. Eisenberg E., Greene L. E. (2007) Multiple roles of auxilin and hsc70 in clathrin-mediated endocytosis. Traffic 8, 640–646 [DOI] [PubMed] [Google Scholar]
- 21. Newmyer S. L., Schmid S. L. (2001) Dominant-interfering Hsc70 mutants disrupt multiple stages of the clathrin-coated vesicle cycle in vivo. J. Cell Biol. 152, 607–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yim Y. I., Sun T., Wu L. G, Raimondi A., De Camilli P., Eisenberg E., Greene L. E. (2010) Endocytosis and clathrin-uncoating defects at synapses of auxilin knockout mice. Proc. Natl. Acad. Sci. U.S.A. 107, 4412–4417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Newmyer S. L., Christensen A., Sever S. (2003) Auxilin-dynamin interactions link the uncoating ATPase chaperone machinery with vesicle formation. Dev. Cell 4, 929–940 [DOI] [PubMed] [Google Scholar]
- 24. Girard M., Allaire P. D., McPherson P. S., Blondeau F. (2005) Non-stoichiometric relationship between clathrin heavy and light chains revealed by quantitative comparative proteomics of clathrin-coated vesicles from brain and liver. Mol. Cell. Proteomics 4, 1145–1154 [DOI] [PubMed] [Google Scholar]
- 25. Raimondi A., Ferguson S. M., Lou X., Armbruster M., Paradise S., Giovedi S., Messa M., Kono N., Takasaki J., Cappello V., O'Toole E., Ryan T. A., De Camilli P. (2011) Overlapping role of dynamin isoforms in synaptic vesicle endocytosis. Neuron 70, 1100–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cao H., Garcia F., McNiven M. A. (1998) Differential distribution of dynamin isoforms in mammalian cells. Mol. Biol. Cell 9, 2595–2609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gray N. W., Fourgeaud L., Huang B., Chen J., Cao H., Oswald B. J., Hémar A., McNiven M. A. (2003) Dynamin-3 is a component of the postsynapse, where it interacts with mGluR5 and Homer. Curr. Biol. 13, 510–515 [DOI] [PubMed] [Google Scholar]
- 28. Gray N. W., Kruchten A. E., Chen J., McNiven M. A. (2005) A dynamin-3 spliced variant modulates the actin/cortactin-dependent morphogenesis of dendritic spines. J. Cell Sci. 118, 1279–1290 [DOI] [PubMed] [Google Scholar]
- 29. Lu J., Helton T. D., Blanpied T. A., Rácz B., Newpher T. M., Weinberg R. J., Ehlers M. D. (2007) Postsynaptic positioning of endocytic zones and AMPA receptor cycling by physical coupling of dynamin-3 to Homer. Neuron 55, 874–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jurado S., Benoist M., Lario A., Knafo S., Petrok C. N., Esteban J. A. (2010) PTEN is recruited to the postsynaptic terminal for NMDA receptor-dependent long-term depression. EMBO J. 29, 2827–2840 [DOI] [PMC free article] [PubMed] [Google Scholar]