Background: There is increasing evidence for post-translational mechanisms that regulate small GTPase function.
Results: PKCα-mediated phosphorylation regulates RalB activity, subcellular localization, and effector interaction.
Conclusion: Phosphorylation is important for RalB regulation of exocyst function and trafficking.
Significance: The GTP-bound state of RalB alone does not determine activation state and activity.
Keywords: Exocytosis, Phosphorylation, Protein Kinase C (PKC), Small GTPases, Vesicles, Colorectal Cancer, RalB, Sec5, Exocyst, Vesicular Trafficking
Abstract
Ras-like (Ral) small GTPases are regulated downstream of Ras and the noncanonical Ral guanine nucleotide exchange factor (RalGEF) effector pathway. Despite RalA and RalB sharing 82% sequence identity and utilization of shared effector proteins, their roles in normal and neoplastic cell growth have been shown to be highly distinct. Here, we determined that RalB function is regulated by protein kinase Cα (PKCα) phosphorylation. We found that RalB phosphorylation on Ser-198 in the C-terminal membrane targeting sequence resulted in enhanced RalB endomembrane accumulation and decreased RalB association with its effector, the exocyst component Sec5. Additionally, RalB phosphorylation regulated vesicular trafficking and membrane fusion by regulating v- and t-SNARE interactions. RalB phosphorylation regulated vesicular traffic of α5-integrin to the cell surface and cell attachment to fibronectin. In summary, our data suggest that phosphorylation by PKCα is critical for RalB-mediated vesicle trafficking and exocytosis.
Introduction
Ral GTPases function as molecular switches that toggle between an effector-binding GTP-bound “on” state and an inactive GDP-bound “off” state. Ral GTP preferentially interacts with a spectrum of functionally distinct effectors that regulate diverse cellular processes such as actin cytoskeleton rearrangement, autophagy, migration and invasion, and vesicle trafficking (1, 2). Despite sharing 82% sequence identity and interaction with a common set of effector proteins, RalA and RalB exhibit distinct and sometimes opposing roles in normal and cancer cell growth. For example, in pancreatic ductal adenocarcinoma (PDAC)2 cells, we determined that RalA but not RalB was necessary for anchorage-independent growth and tumorigenic growth in vivo, whereas RalB was instead required for invasion and metastasis in vivo (3). In contrast, we showed that in colorectal carcinoma (CRC) cells, RalA was necessary to support anchorage-independent growth, whereas RalB antagonized anchorage-independent growth (4). Surprisingly, both RalA and RalB required binding to a common effector, RalBP1/RLIP76, to control soft agar growth but additionally needed interaction with distinct components of the exocyst to support their phenotypes. RalA required interaction with Exo84 but not Sec5 to promote anchorage-independent growth, whereas RalB required association with Sec5 but not Exo84 to antagonize anchorage-independent proliferation.
One basis for the functional differences between RalA and RalB can be attributed to their divergent C-terminal hypervariable (HV) sequences (residues 177–202). The HV sequences, together with the CAAX prenylation signal motif (where A is any aliphatic amino acid and X is any amino acid), determine the subcellular localization and specific membrane association of Ral proteins, which in turn influences specific effector interactions. Ral GTPases can be found at the plasma membrane or associated with endosomes and other endomembrane compartments. Furthermore, there are cell context differences in Ral localization and their activation state, and post-translational modifications can dynamically regulate Ral subcellular localization (5–8). For example, we determined that phosphorylation of Ser-194 regulated both RalA subcellular localization and specific effector association and was necessary for RalA to support the anchorage-independent and tumorigenic growth of PDAC cells (8). Furthermore, Hahn and co-workers (9) showed that RalA Ser-183 and Ser-194 phosphorylation was regulated by the PP2A phosphatase and that this activity is necessary for the tumor suppressor activity of PP2A.
The RalA Ser-183 and Ser-914 phosphorylation sites are not found in the RalB HV sequence. Instead, the presence of other evolutionarily conserved serine residues in the RalB C terminus suggests the possibility that RalB may be a substrate for other protein kinases. In this study, we determined that protein kinase Cα (PKCα) phosphorylated RalB on Ser-192 and Ser-198 in the HV sequence and that Ser-198 phosphorylation promoted activation of RalB and caused RalB relocalization from the plasma membrane to late endosomes. The novelty of our study is that we completed a systematic dissection of the consequences of phosphorylation, which was necessary for RalB to engage RalBP1, whereas an interaction with Sec5 and the exocyst complex was negatively regulated by Ser-198 phosphorylation. This phosphorylation-dependent regulation of RalB association with the exocyst also regulated cellular adhesion of CRC cells to fibronectin, presumably due to the regulation of vesicular trafficking. Specifically, RalB required a dynamic phosphorylation cycle for the proper delivery of α5-integrin to the cell surface by regulating SNARE engagement at the plasma membrane and ultimately vesicular fusion.
EXPERIMENTAL PROCEDURES
Cell Lines
SW480 CRC cells were obtained from the ATCC and were maintained in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS). 293T cells were obtained from the ATCC and were maintained in DMEM-H supplemented with 10% FCS. To establish stably infected mass populations of cells expressing shRNA or cDNA expression vectors, 293T cells were transfected with either pLKO.1 lentiviral or pBabe-puro retroviral expression constructs along with their respective packaging plasmids. Viral supernatants were isolated and then added to SW480 cells followed by selection in 1.0 μg/ml puromycin or 1.0 μg/ml blasticidin-S HCl. Bryostatin-1 (Calbiochem) treatment was done as indicated.
DNA Constructs
RalB retrovirus expression constructs have been described previously (4). RalB cDNA sequences encoding Ser-192 and/or Ser-198 phosphodeficient (Ser to Ala) or putative phosphomimetic (Ser to Asp) mutants were prepared by site-directed mutagenesis. A lentiviral shRNA vector targeting human RalB was prepared by subcloning a previously described shRNA sequence (4) into pLKO.1 blasticidin. An expression vector encoding RalB with an N-terminal mCherry (mCh) fluorescent reporter (mCh-RalB) was prepared by inserting the RalB cDNA sequence into pcDNA3-mCherry (10) between the EcoRI and XhoI sites where the mCh DNA sequence was inserted into the BamHI and EcoRI sites. The TfR-mCh-SEP expression vector, which encodes the transferrin receptor tagged with both mCherry and the pH-sensitive green fluorescent protein (GFP) variant superecliptic pHluorin (SEP), was provided by Michael Ehlers (Duke University) and has been described previously (11). PLKO.1 shRNA vectors targeting PKCα were obtained from the University of North Carolina, Chapel Hill, Lentiviral Core Facility, with the following TRC numbers: TRCN0000001691 and TRCN0000001692. Full-length rat PKCα has been described previously (12). Full-length human VAMP3 cDNA sequences were subcloned into pEGFP-N3 between the XhoI and HindIII sites. Full-length human Sec5 was subcloned into pEGFP-C3 between XhoI and ApaI. Full-length human RalBP1 was subcloned into pEGFP-C3 between the XhoI and BamHI sites. All sequences were verified by automated sequencing (University of North Carolina Genome Analysis Facility). α5-Integrin-GFP encodes human α5-integrin with a C-terminal GFP tag and was obtained from Addgene (Rick Horwitz; plasmid 15238) (13).
Immunoprecipitations and Immunoblotting
Lysates were obtained from the indicated cell lines, were resolved by SDS-PAGE, transferred to PVDF filters, and blotted with anti-RalB and anti-phosphoserine (Millipore), anti-VAMP3 (Synaptic Systems), anti-SNAP-23 (Abcam), anti-Sec8 (StressGen), anti-β-actin (Sigma), anti-GFP (Clontech), anti-HA (Covance), anti-PKCα (BD Biosciences), or anti-α5-integrin (Cell Signaling) antibodies. Immunoprecipitations were performed on lysates using 3 μg of the indicated antibodies coupled to protein G Dynabeads (Invitrogen). Lysates were precleared with normal mouse IgG or normal rabbit IgG coupled to protein G Dynabeads for 2 h at 4 °C. Precleared lysates were then immunoprecipitated for 3 h to overnight at 4 °C. RalB-GTP activity assays were performed by pulldown analyses as we described previously (4).
Immunofluorescence
Cells were grown on glass coverslips and were fixed with 4% paraformaldehyde in PBS. Cells were permeabilized with 0.2% Triton X-100 followed by blocking with 5% goat serum and 5% BSA in PBS. Primary antibodies were incubated for 1 h at room temperature followed by incubation with an Alexa Fluor-conjugated secondary antibody (Invitrogen) for 2 h at room temperature. Coverslips were mounted with FluorSave (Calbiochem). Images were acquired using a Zeiss LSM 510 or Leica SP2 confocal microscope and then processed using ImageJ (National Institutes of Health).
Live Cell Imaging
Cells were grown in 35-mm glass bottom MatTek dishes to subconfluency and were transfected with the indicated fluorescent constructs using Lipofectamine 2000 (Invitrogen). To maintain pH, prior to imaging the SW480 cells were switched to DMEM/F-12 medium containing HEPES (Invitrogen) supplemented with 10% FCS. Cells were kept in an environmental chamber set to 37 °C (Zeiss TempControl 37-2 digital heater controller), and images were acquired using a Zeiss LSM 510 confocal microscope using sequential scanning for dual channel images. Cells were examined with an inverted laser scanning confocal microscope (Zeiss 510 LSM) using an oil immersion ×63 NA 1.4 objective. Images were captured by sequential scanning with the 488 nm argon and the 543 nm HeNe1 laser (488 and 543 nm for two-color staining) and the 505–530-bp (for Alexa 488) and 585–615-bp (for Alexa 568 and mCherry) emission filters. To monitor vesicle fusion events, TfR-mCh-SEP was transfected into subconfluent SW480 cells. Cells were then split onto MatTek dishes coated with 5 μg/ml fibronectin (BD Biosciences) and allowed to attach overnight. Single cells were imaged on a Zeiss LSM 510 confocal microscope, and images were collected every 2 s. Fusion events were quantitated in ImageJ by applying a grid to each cell (n = 10) and monitoring for an increase in SEP fluorescence, indicating fusion.
Statistical Analysis
Data were analyzed using an unpaired t test using Microsoft Excel, and the Pearson's correlation coefficient was determined. Values are shown as means ± S.E. A p value of <0.05 was considered significant.
RESULTS
RalB Is Phosphorylated at Ser-192 and Ser-198 by Protein Kinase Cα
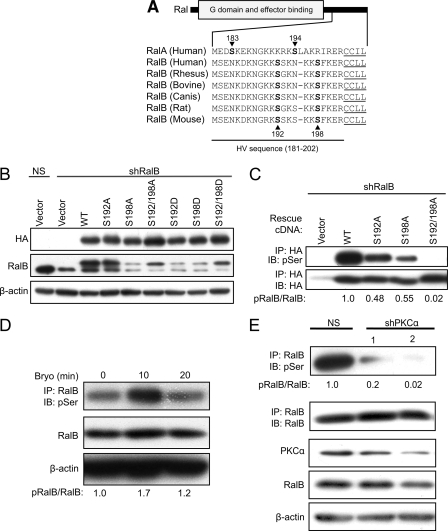
RalA and RalB share 82% overall sequence identity, with 100% sequence identity in the switch I and II sequences involved in effector interaction. However, the C-terminal HV membrane-targeting domain of RalA and RalB (Fig. 1A) contains the majority of the sequence diversity between the two proteins (∼50% identity) and dictates distinct subcellular localization and function. RalA contains a well conserved Ser-194 residue that is a target of Aurora-A kinase phosphorylation (14). Our recent studies showed that this phosphorylation site is critical for RalA to promote the anchorage-independent and tumorigenic growth of PDAC cells (8). To determine whether RalB may be similarly regulated, we performed ScanSite analyses of human RalB, which identified Ser-192 and Ser-198 (Fig. 1A) as putative targets of protein kinase C (PKC) isoforms α, β, γ, δ, and ϵ. To determine whether Ser-192 and Ser-198 of RalB are phosphorylated in vivo, we stably suppressed endogenous RalB expression in SW480 cells by retrovirus vector-expressed shRNA and ectopically expressed wild type or S192A and/or S198A point mutants of RalB from RNAi-resistant cDNA sequences (Fig. 1B). Because these mutations disrupted anti-RalB antibody recognition of these mutant proteins, we utilized HA epitope-tagged RalB proteins for these analyses. Blot analyses with anti-HA and anti-RalB antibody indicated that these ectopically expressed proteins were expressed at steady-state levels comparable with endogenous RalB levels. Mutation of Ser-192 or Ser-198 alone significantly reduced phosphorylation (∼50%), although concurrent mutation (S192A/S198A) essentially abolished RalB serine phosphorylation (Fig. 1C). Thus, both Ser-192 and Ser-198 are sites of steady-state phosphorylation in vivo.
FIGURE 1.
RalB is phosphorylated on serines 192 and 198 and PKCα is necessary for phosphorylation. A, serines 192 and 198 are evolutionarily conserved and include consensus PKC substrate motifs. Scansite high stringency analysis identified Ser-198 as a putative phosphorylation site for PKC isoforms α, β, γ, δ, and ϵ. Low stringency analysis identified Ser-192 as a putative phosphorylation site for PKC isoforms α, β, and γ and additionally Ser-192 and Ser-198 as a putative phosphorylation site for PKCζ. Ser-198 and to a lesser degree Ser-192 display similarity to the PKC consensus substrate motif of (R/K)(R/K)pSX(R/K), where pS is the phosphorylated serine residue, R/K is an arginine or lysine, and X is any amino acid. The C-terminal membrane-targeting regions of RalA and RalB were aligned using ClustalW. Phosphorylation sites are in boldface type, and the CAAX motif is underlined. B, ectopic restoration of endogenous RalB expression with putative phosphodeficient or phosphomimetic mutants of Ser-192 and Ser-198. Endogenous RalB expression was stably suppressed by pSuper retrovirus expression of control (GFP) or RalB shRNA in SW480 cells and then stably infected with empty pBabe-puro retrovirus vector or containing RNAi-insensitive cDNA sequences encoding HA epitope-tagged RalB WT or putative phosphorylation site mutants. Western blot analyses of total cell lysates were performed with anti-HA and anti-RalB and with anti-actin antibody to verify equivalent loading of protein. NS, nonspecific. C, Ser-192 and Ser-198 are the primary sites of RalB phosphorylation in vivo. HA-tagged WT or phosphodeficient RalB mutants were immunoprecipitated (IP) with an anti-HA antibody followed by separation using SDS-PAGE and by Western blot analyses with a pan-phosphoserine-specific antibody to determine RalB serine phosphorylation. IB, immunoblot. D, treatment with the bryostatin-1 (Bryo) PKC activator causes rapid and transient RalB serine phosphorylation. SW480 cells were stimulated with vehicle (DMSO) or 100 nm bryostatin-1 for the indicated times. Endogenous RalB was immunoprecipitated from harvested cell lysates, and RalB serine phosphorylation was determined by Western blotting with anti-Ser(P). Numbers represent the amount of Ser(P)-RalB normalized to time 0. Blot analyses for total RalB and β-actin were done to verify equivalent total protein loading. E, knockdown of endogenous PKCα inhibits RalB steady-state phosphorylation. Two independent shRNAs were used to knock down endogenous PKCα in SW480 cells. Endogenous RalB was immunoprecipitated; immune complexes were separated by SDS-PAGE, and Western blot analyses were performed with an anti-Ser(P) or anti-RalB antibody. Total cell lysates were blotted with anti-PKCα or anti-RalB or with anti-β-actin to verify equivalent protein loading.
We next wanted to determine the kinase(s) responsible for RalB phosphorylation. Based on our ScanSite analysis, we identified Ser-198 as a putative recognition site for conventional (α, β, and γ) and novel (δ and ϵ) PKC isoforms. To address this possibility, we treated SW480 cells with the conventional and novel PKC isoform activator bryostatin-1 to examine potential changes in endogenous RalB phosphorylation. A transient increase in RalB serine phosphorylation was observed 10 min post-stimulation with bryostatin-1 indicating that PKC activation can stimulate the phosphorylation of RalB in CRC cells (Fig. 1D). Next, treatment of SW480 cells with the broadly active PKC inhibitor Gö6983, or Gö9676 which is selective for classical PKC isoforms, reduced steady-state RalB serine phosphorylation (data not shown). These results support a PKC-dependent RalB phosphorylation mechanism in CRC cells.
We next wanted to identify the specific PKC isoform(s) involved in RalB phosphorylation. A previous study identified PKCα and PKCδ but not PKCβ, PKCγ, or PKCϵ protein expression in SW480 and other CRC cell lines (16). Another study found that PKCα antagonized colonic tumorigenesis (17), consistent with RalB antagonism of anchorage-independent growth in CRC cells. We therefore focused on addressing a role for PKCα in RalB phosphorylation. To identify whether PKCα is the isoform responsible for RalB phosphorylation, shRNA was used to stably deplete SW480 cells of PKCα. Two independent shRNAs effectively knocked down endogenous PKCα without affecting the total levels of RalB (Fig. 1E). Loss of PKCα expression resulted in a near-complete loss of RalB serine phosphorylation. We also found that the serine phosphorylation of ectopically expressed RalB was abolished in PKCα-deficient but not wild type mouse embryo fibroblasts (data not shown). Finally, our in vitro analyses showed recombinant PKCα-phosphorylated purified RalB in vitro (supplemental Fig. 1), suggesting that RalB is a direct substrate of PKCα in vivo. We conclude that PKCα is the critical isoform involved in RalB phosphorylation.
Ser-198 Phosphorylation Regulates RalB Activation, Subcellular Localization, and Effector Binding
Phosphorylation of RalA by Aurora-A (8, 14) has been shown to alter its active, GTP-loaded state. To determine whether phosphorylation could affect the activation state of RalB, we performed pulldown analyses to measure steady-state levels of RalB-GTP. Mutation of Ser-192 alone caused a limited 10% reduction in RalB-GTP levels, whereas mutation of Ser-198 alone or together with Ser-192 caused an ∼50% reduction in RalB-GTP steady-state levels. These results suggest that the phosphorylation of Ser-198 stimulates formation of active RalB-GTP (Fig. 2A).
FIGURE 2.
Ser-198 phosphorylation regulates RalB GTP-bound state, subcellular localization, and effector interaction. A, phosphodeficient S198A exhibits reduced GTP loading. SW480 cells stably infected with pSuper retrovirus vector expressing control (GFP) or RalB shRNA as well as the pBabe-puro empty vector or pBabe encoding HA-tagged RalB phosphorylation mutants were subjected to GST-Sec5-RBD pulldown analysis to monitor RalB-GTP formation. Total cell lysates were Western-blotted with anti-RalB or with anti-β-actin antibody to verify equivalent loading of protein. Numbers represent the amount of RalB-GTP normalized to WT RalB. B, bryostatin-1 treatment results in RalB endomembrane localization. SW480 cells stably expressing RalB shRNA as well as HA-tagged RalB WT or S192A/S198A were stimulated with DMSO or 100 nm bryostatin-1 for 10 min. RalB localization was visualized by confocal microscopy with anti-HA antibody. Scale bar, 20 μm. C, phosphorylation mutants of RalB exhibit different subcellular localizations. SW480 CRC cells from A were fixed, and RalB localization was visualized by confocal microscopy with anti-HA antibody. Scale bar, 20 μm. D, phosphorylation of Ser-198 regulates RalB endomembrane localization. SW480 cells transiently expressing GFP-tagged RalB WT or S198A were stimulated with DMSO (vehicle control) or 100 nm bryostatin-1 for 10 min. RalB localization was visualized by confocal microscopy. Scale bar, 20 μm. E, phosphorylation of RalB enhances RalBP1 association. SW480 cells from A were transiently transfected with GFP-RalBP1. HA-RalB was immunoprecipitated (IP), and immune complexes were separated by SDS-PAGE, and Western blot analyses were performed with anti-GFP to determine association with RalB, or with anti-HA and anti-β-actin antibodies to verify equivalent total protein loading. Data shown are representative of three independent experiments. F, phosphorylation of RalB is associated with decreased Sec5 association. SW480 cells from A were transiently transfected with GFP-Sec5. HA-RalB was immunoprecipitated, and immune complexes were separated by SDS-PAGE, and Western blot analyses were performed with anti-GFP antibody to determine co-precipitation with RalB or anti-HA and with anti-β-actin to verify equivalent total protein loading. G, phosphorylation of RalB is associated with decreased Sec8 association. HA-tagged RalB was immunoprecipitated from SW480 CRC cells from A. Immune complexes were separated by SDS-PAGE, and Western blot analyses were performed with anti-Sec8 to determine co-precipitation or anti-HA to verify equivalent protein loading. Total cell lysates were blotted with anti-HA, anti-Sec8, and anti-β-actin to verify equivalent protein expression. IB, immunoblot.
Because we showed that the S198A phosphodeficient mutant showed decreased GTP loading of RalB, we also determined whether bryostatin-1 stimulation of RalB phosphorylation conversely increased endogenous RalB-GTP levels. Pulldown analyses identified a rapid and transient 4-fold increase in RalB GTP-loading at 10 min post-stimulation (data not shown), similar to the kinetics of RalB phosphorylation, as shown in Fig. 1D.
We determined previously that the phosphorylation of RalA at Ser-194 in its C-terminal membrane targeting sequence stimulated loss of plasma membrane-associated RalA and increased endomembrane accumulation (8). To examine whether the phosphorylation of RalB in its C-terminal membrane-targeting sequence also affected its localization, we compared the subcellular localization of WT and phosphodeficient RalB by confocal microscopy. HA-tagged WT RalB displayed significant plasma and endomembrane staining. Phosphodeficient RalB S192A/S198A was found predominantly at the plasma membrane, whereas phosphomimetic S192D/S198D RalB was not associated with the plasma membrane and showed a punctate, perinuclear distribution (Fig. 2B). To determine whether PKC activation and stimulation of RalB phosphorylation altered RalB localization, we evaluated the consequences of bryostatin-1 treatment on RalB subcellular localization. Bryostatin-1 treatment resulted in a loss of plasma membrane association and a striking enrichment to a perinuclear region (Fig. 2C). Finally, we also addressed the importance of Ser-192 and Ser-198 phosphorylation in this translocation. Using GFP-tagged RalB, we found that WT but not the S198A single mutant translocated transiently to endomembranes upon bryostatin-1 treatment (Fig. 2D). Taken together, these observations indicate that Ser-198 phosphorylation alone regulates RalB translocation from the plasma membrane to internal membranes.
To determine the specific intracellular localization of phosphorylated RalB, we determined whether phosphomimetic S192D/S198D RalB showed co-localization with different GFP-tagged Rab GTPases that are associated with different endocytic compartments. Phosphomimetic RalB co-localization was observed with Rab5, Rab9, and Rab11 (supplemental Fig. 2) indicating that phosphorylation of RalB can result in its localization to early, late, and recycling endosomes, respectively.
RalB can interact with multiple effector proteins (2). To determine whether RalB phosphorylation and translocation to endosomes altered the association of RalB with effector proteins, we performed co-immunoprecipitation analyses with RalBP1 and Sec5 in SW480 cells transiently expressing the various RalB mutants (Fig. 2E). Similar to WT RalB, phosphomimetic RalB S192D/S198D displayed association with GFP-RalBP1. In contrast, phosphodeficient RalB S192A/S198A showed significantly reduced RalBP1 association. In sharp contrast to the RalBP1 co-immunoprecipitation analyses, we observed that WT and phosphomimetic RalB S192D/S198D showed minimal co-precipitation with GFP-Sec5, whereas phosphodeficient RalB S192A/S198A RalB displayed an enhancement in GFP-Sec5 complex formation (Fig. 2F).
Sec5 may be associated with exocyst function or an exocyst-independent function involving the TBK1 protein kinase (19). To address this issue, we determined whether RalB association with an exocyst subunit that cannot associate with RalB directly (Sec8) showed the same pattern of association. Endogenous Sec8 showed preferential co-precipitation with S192A/S198A but not S192D/S198D (Fig. 2G). Taken together, these observations are consistent with phosphorylation regulation of RalB association with and regulation of the exocyst complex function.
RalB Phosphorylation Regulates Vesicular Fusion
The exocyst is a large multisubunit complex involved in the polarized secretion of exocytic vesicles (20). Because we observed that RalB S192A/S198A and S192D/S198D showed differential association with Sec5 and the exocyst, we assessed whether the phosphorylation of RalB could regulate the vesicle trafficking machinery. VAMP3 (cellubrevin) is a vesicular SNARE protein that is involved in the membrane fusion of exocytic vesicles (21). Analysis of SW480 cells transiently co-expressing GFP-VAMP3 and mCh-RalB WT found co-localization at both the plasma membrane and on internal vesicles (Fig. 3A). When co-expressed with mCh-RalB S192A/S198A, GFP-VAMP3 displayed a more prominent co-localization with RalB at the plasma membrane. In contrast, mCh-RalB S192D/S198D showed very little co-localization with GFP-VAMP3 at the plasma membrane, and the majority of both proteins was found in a perinuclear region. These results suggest that RalB phosphorylation at Ser-192 and/or Ser-198 regulated association with VAMP3.
FIGURE 3.
RalB phosphorylation regulates vesicular fusion. A, phosphorylation regulates the co-localization of RalB with the v-SNARE VAMP3. SW480 cells were transiently transfected with GFP-RalB WT or phosphorylation mutants and mCh-VAMP3. Insets indicate co-localization of RalB with VAMP3 at the plasma membrane (1) and on internal membranes (2). Scale bar represents 20 μm. B, phosphorylation of RalB regulates the association of VAMP3 and SNAP-23. SNAP-23 was immunoprecipitated (IP) from SW480 cells expressing RalB shRNA as well as empty vector or HA-tagged RalB phosphorylation mutants. Immune complexes were separated by SDS-PAGE, and Western blot analyses were performed with anti-VAMP3 to determine SNARE complex formation and anti-SNAP-23 to verify equivalent loading. C, phosphorylation of RalB regulates vesicular fusion. SW480 cells from Fig. 2A were transiently transfected with the TfR-mCh-SEP expression construct to monitor vesicular fusion. Fusions were determined by an increase in SEP signal due to surface delivery (n = 10 cells). Images shown are representative of independent fusion events denoted by the arrows. Values shown are means ± S.E., and an unpaired t test was used to determine significance (p < 0.05). NS, nonspecific.
The redistribution of VAMP3 upon expression of RalB phosphorylation mutants led us to evaluate a potential change in the association of VAMP3 with its target SNARE, SNAP-23. Endogenous complexes of VAMP3 with SNAP-23 were determined by immunoprecipitation of SNAP-23 from SW480 cells stably expressing nonspecific or RalB RNAi to deplete endogenous RalB expression and also ectopically expressing WT or phosphorylation mutants of RalB. SNAP-23 was isolated by immunoprecipitation, followed by blot analysis to detect co-precipitating VAMP3 (Fig. 3B). Suppression of endogenous RalB was associated with a 40% reduction in VAMP3 association with SNAP-23 that could be restored by ectopic RalB WT or phosphodeficient RalB S192A/S198A. In contrast, phosphomimetic RalB S192D/S198D did not restore VAMP3 association with SNAP-23. Decreased association between VAMP3 and SNAP-23 would result in a defect in vesicle fusion with the plasma membrane and therefore may be the underlying cause for the increased VAMP3-positive vesicles found in cells expressing RalB S192D/S198D.
To further evaluate the role for RalB phosphorylation in vesicular traffic to the cell surface, we assessed whether the phosphorylation of RalB regulates the fusion of exocytic vesicles at the plasma membrane. For these analyses, we used a recently described optical sensor for the transferrin receptor (TfR), which is a widely used marker to monitor recycling endosomal trafficking. TfR-mCh-SEP is a dual color reporter that is composed of the TfR fused both to mCh and the superecliptic pHluorin derivative of GFP (SEP) (11). Imaging the entire cellular pool of TfR can be done in the red (mCh) channel, whereas selective detection of TfR at the plasma membrane can be determined in the green (SEP) channel. Thus, this probe allows visualization of exocytic cargo before, during, and following membrane fusion. We monitored single exocytic fusion events at the plasma membrane. Even though fusion events were observed in cells expressing WT as well as phosphorylation-deficient and phosphomimetic mutants of RalB, the number of fusion events was decreased by ∼70% upon expression of the phosphomimetic RalB S192D/S198D mutant when compared with WT (Fig. 3C). These data strongly support a role for RalB phosphorylation in regulating the fusion of exocytic vesicles at the plasma membrane, potentially by regulating the dissociation of RalB from the exocyst due to decreased RalB-Sec5 interaction.
Phosphorylation of RalB Regulates the Trafficking of α5-Integrin
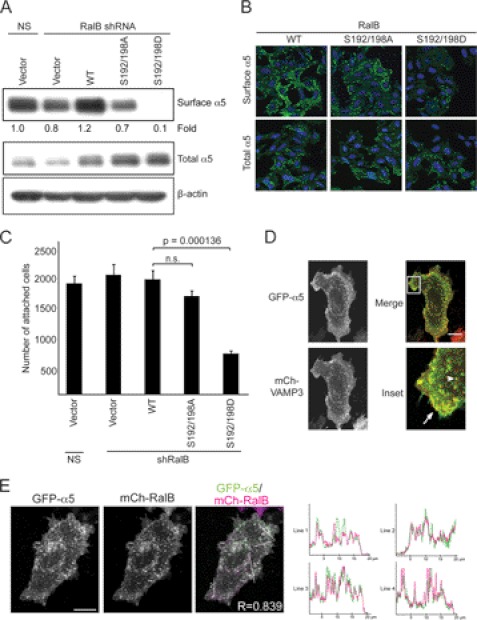
The exocyst complex facilitates the targeting of secretory vesicles and their protein cargo to specific sites of fusion on the plasma membrane (20). Recent work by Yeaman and co-workers (22) identified a role for both Sec5 and RalA/B in the delivery of the α5-integrin subunit to the plasma membrane. Because we observed decreased association of Sec5 and the exocyst complex with phosphomimetic RalB as well as diminished SNARE complex formation and vesicle fusion events in cells expressing the RalB phosphomimetic mutant, we hypothesized that RalB may regulate the delivery of proteins to the cell surface. To determine whether RalB phosphorylation is essential for the delivery and retention of α5 to the cell surface, we utilized two approaches. First, we used cell surface biotinylation to monitor the amount of cell surface-localized α5 in RalB-depleted SW480 cells stably expressing ectopic RalB WT or phosphorylation mutants. Suppression of endogenous RalB alone resulted in a limited (20%) decrease in the level of biotin-labeled α5 that was restored with ectopic RalB WT expression (Fig. 4A). Surprisingly, neither phosphodeficient nor phosphomimetic RalB could restore biotinylated α5 to levels comparable with WT RalB. In contrast, RalB S192D/S198D expression essentially abolished surface α5 expression. In a second approach, surface or total levels of α5 expression were determined by anti-α5 immunofluorescence staining of nonpermeabilized or permeabilized cells, respectively. RalB WT- and S192A/S198A-expressing cells displayed predominant surface staining of α5, whereas RalB S192D/S198D-expressing cells showed a substantial reduction in surface α5, even though total levels of expression were similar among all cells (Fig. 4B). These results indicate that RalB-mediated trafficking of α5 may require dynamic cycling between phosphorylated and nonphosphorylated states to effectively transport α5 to the cell surface. Finally, we determined whether loss of α5 at the cell surface observed in cells expressing phosphomimetic RalB has biological consequences. Cellular adhesion to fibronectin requires α5β1 integrin; therefore, we evaluated whether phosphorylation mutants of RalB cause a defect in this property. Consistent with the loss of surface-associated α5-integrin, adhesion to fibronectin of cells expressing phosphomimetic RalB was impaired as compared with WT RalB (Fig. 4C).
FIGURE 4.
Phosphorylation of RalB regulates the trafficking of α5-integrin. A, RalB phosphorylation alters α5 surface expression. Surface proteins were labeled with biotin in SW480 cells prepared as in Fig. 2A. Biotinylated proteins were isolated on neutravidin resin and resolved by SDS-PAGE. Western blot analyses were performed with anti-α5 antibody to determine the amount of biotinylated, surface-exposed α5. Total cell lysate was blotted with anti-α5 and with anti-β-actin to verify equivalent total protein loading. B, RalB phosphorylation regulates the surface expression of endogenous α5. α5 expression was determined in endogenous RalB-depleted SW480 cells as in Fig. 2A. Representative images are shown of cells that were either nonpermeabilized (for surface α5) or permeabilized (for total α5) and were stained with an Alexa 488-conjugated α5 antibody. C, cellular attachment to fibronectin is reduced in cells expressing phosphomimetic RalB. SW480 cells from Fig. 2A were allowed to attach to fibronectin. Nonadherent cells were removed, and attached cells were quantitated. Values shown are means ± S.E., and an unpaired t test was used to determine significance (p < 0.05). n.s., not significant. D, VAMP3 and α5 co-localize on endosomes and at the plasma membrane. SW480 cells were transiently transfected with mCh-VAMP3 and GFP-α5. Live cells were imaged, and co-localization of VAMP3 with α5 at the plasma membrane (arrow) and on endosomes (arrowhead) was seen. E, RalB and α5 co-localize on endosomes and at the plasma membrane. SW480 cells were transiently transfected with mCh-RalB and GFP-α5. Cells were seeded on glass coverslips for live cell imaging 48 h after transfection. Line scans of the merged image indicate co-localization of RalB with α5 on both internal vesicles and at the plasma membrane. Ten different images were quantitated and a Pearson's correlation coefficient of 0.83 ± 0.001 (S.E.) was calculated. Data are representative of at least two independent experiments. Scale bar, 20 μm.
Because we observed that RalB co-localized with VAMP3, we also investigated the potential co-localization of VAMP3 with α5. Consistent with co-localization of RalB and VAMP3 on the vesicles, we also found VAMP3 to co-localize with α5 in SW480 cells on both vesicles and at the plasma membrane (Fig. 4D). Finally, although the exocyst complex has been shown to be necessary for α5 trafficking (22), the co-trafficking of RalB with α5-containing vesicles has not been reported. To test this possibility, we co-expressed mCh-RalB together with GFP-α5 in SW480 cells (Fig. 4E) and determined co-localization by live-cell confocal microscopy. We found that the majority of mCherry-RalB vesicles also contained GFP-α5 and live-cell imaging of trafficking vesicles indicated that RalB and α5 co-traffick. Taken together, our results suggest that RalB promotes trafficking and cell surface localization of α5-integrin and that phosphorylation of RalB by PKCα is essential for RalB function in trafficking.
DISCUSSION
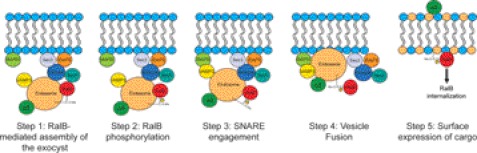
An emerging paradigm for Ras family small GTPases is the critical importance of post-translational modifications, particularly phosphorylation, for regulating their biological functions. An additional emerging concept is the striking functional differences seen in otherwise near-identical small GTPase isoforms. Our recent studies identified opposing roles for the highly related RalA and RalB small GTPases in the anchorage-independent growth of CRC tumor cells. It has been demonstrated that RalA but not RalB was a substrate for Aurora-A (14), and we found that that phosphorylation was critical for RalA support of PDAC cell anchorage-independent growth (8). In this study, we determined that RalB is a substrate for PKCα, and we demonstrated the importance of this phosphorylation for the biochemical and cellular functions of RalB. We determined that PKCα phosphorylates Ser-192 and Ser-198 in the C-terminal membrane-targeting region of RalB, regulating formation of RalB-GTP, plasma membrane-to-endosome trafficking, and differential effector association. Utilizing phosphodeficient and phosphomimetic mutants of RalB, we determined that a dynamic cycle of phosphorylation and dephosphorylation regulates RalB to disengage the Sec5 component of the exocyst, thereby allowing for proper SNARE engagement and vesicle fusion and facilitating α5-integrin surface expression and cell adhesion. Our studies thus define the mechanisms by which phosphorylation controls active RalB and its regulation of the exocyst and vesicular trafficking (Fig. 5).
FIGURE 5.
Model for RalB-regulated vesicular fusion. Based on our observations that PKC-dependent phosphorylation promotes RalB translocation from the plasma membrane to endosomes and is associated with altered association with Sec5, we propose the following model for how these changes may then alter exocytosis, as shown by our tracking of α5-integrin to the cell surface. It is known that RalB is associated with the exocyst through its interaction with Sec5. An exocyst subcomplex consisting of Sec5, Exo84, Sec6, Sec8, Sec10, and Sec15 is docked to specific sites of vesicle fusion on the plasma membrane marked by the two additional exocyst components, Sec3 and Exo70. We propose that RalB-regulated exocyst docking occurs at fusion sites (Step 1). PKCα-mediated phosphorylation of RalB is then sufficient to disengage RalB from Sec5 and presumably the exocyst complex (Step 2). Next, we speculate that the v-SNARE VAMP3 engages its t-SNARE SNAP-23 (Step 3) leading to vesicular fusion (Step 4) and delivery of cargo proteins like α5-integrin (Step 5). Phosphorylated RalB is internalized where it may be dephosphorylated to participate in a new round of vesicle delivery.
Our identification of PKCα-mediated phosphorylation of RalB also establishes another basis for the distinct functional roles of RalA and RalB. Thus, in addition to the differential membrane targeting and subcellular localization information conferred by the HV sequences of RalA and RalB, these sequences also determine their regulation by distinct protein kinases. Although the downstream consequences of Aurora-A phosphorylation of RalA and PKCα phosphorylation of RalB occur in similar categories (increased GTP loading, translocation from the plasma membrane to endomembranes, and differential effector association), the distinct mechanism of regulation and cellular roles of these protein kinases clearly add further diversity to RalA and RalB function. During the course of our studies, Theodorescu and co-workers (23) described RalB phosphorylation at Ser-198 by PKC. They additionally showed that Ser-198 phosphorylation was required for RalB support of bladder carcinoma cell line anchorage-independent growth, cell motility, and actin organization in vitro and tumorigenic growth and metastasis in vivo. However, this study did not address the consequences of Ser-198 phosphorylation to the biochemical and functional properties of RalB. Our study thus helps to explain how PKC phosphorylation of RalB can promote those biological outcomes.
Several members of the PKC family of kinases have been implicated in vesicle trafficking, particularly in neuronal systems (24). PKC isoforms have been shown to phosphorylate not only the SNARE (25) proteins but also components of the exocyst (26). In this study, we propose that PKC also phosphorylates RalB on Ser-192 and Ser-198 in the C-terminal HV membrane-targeting sequences. This phosphorylation event results in the internalization of RalB from the plasma membrane to internal membranes and a change in RalB effector utilization. Whereas phosphomimetic S192D/S198D RalB displayed preferential association with RalBP1, phosphodeficient S192A/S198A RalB showed an enhanced association with Sec5 and the exocyst complex. Although it is possible that Ser-198 phosphorylation may alter RalB intrinsic affinity of binding to effectors, we suspect that this switch in effector interaction is most likely due to the phosphorylation-mediated change in RalB subcellular localization. In our previous studies of RalA phosphorylation, we also observed that phosphorylation promoted a change both in RalA subcellular localization as well as in effector association. Finally, both RalA and RalB C-terminal phosphorylations are very similar to the PKC phosphorylation site in the C terminus of K-Ras4B, where the phosphorylation site is adjacent to the polybasic second signal for membrane association (18). Based on our findings with K-Ras4B (18), we suggest that a similar geranylgeranyl-electrostatic switch mechanism promotes RalB dissociation from the plasma membrane. Whether the translocation is also mediated by endosome-associated proteins is not clear.
This switch in effector utilization upon RalB phosphorylation represents a potential mechanism through which RalB can regulate both exocytic traffic through Sec5 and the exocyst and endocytic pathways through RalBP1. Consistent with phosphorylation of RalB regulating vesicle sorting, we found that RalB may need to cycle between phosphorylation states for proper vesicle fusion and delivery of cargo proteins to the cell surface. Nonphosphorylated S192A/S198A RalB displayed a modest reduction in SNARE engagement and vesicle fusion while phosphomimetic S192D/S198D RalB nearly abolished VAMP3/SNAP-23 SNARE complex formation and vesicle fusion. This is likely due to the internalization of S192D/S198D RalB-containing vesicles effectively sequestering the v-SNARE VAMP3 from plasma membrane where SNAP-23 resides. PKC-mediated phosphorylation of both Sec5 (26) and SNAP-23 (27) is critical in regulating proper exocytic vesicle fusion in various cell types. Our work indicates that RalB GTPase phosphorylation in addition to SNARE and exocyst phosphorylation is necessary for efficient exocytosis, thus expanding the number of PKC substrates involved in exocytosis.
A reduction in RalB-mediated delivery of exocytic vesicles prompted us to examine potential cargo proteins that may have altered expression on the cellular surface due to an impaired RalB phosphorylation cycle. To date, only a few proteins have been shown to be regulated by Ral-regulated exocyst trafficking and include E-cadherin (28), GLUT4 (29), and α5-integrin (22). Because of the known involvement of RalB in regulating cellular motility, we chose to examine α5 as a protein whose sorting may be regulated by RalB phosphorylation. We found that RalB and α5 co-trafficked on endosomes containing the v-SNARE VAMP3 and that RalB may need to undergo a dynamic phosphorylation cycling to complete the delivery of α5 to the cell surface. This impairment in α5 surface expression in turn impaired CRC cell attachment to fibronectin. We suspect that additional proteins will also show RalB phosphorylation-dependent trafficking that may contribute to the role of phosphorylation in regulating the function of RalB in CRC tumor cell behavior. Finally, we also found that phosphorylation altered RalB association with RalBP1, and we anticipate that their enhanced association also contributes to the activities of phosphorylated RalB. However, a full understanding of how phosphorylation regulates RalB function will require proteomic analyses of RalB binding partners whose interactions are controlled by phosphorylation.
Exocytosis is highly regulated in mammalian cells, with distinct cellular protein complexes necessary for vesicle trafficking, docking, and fusion at plasma membrane sites of exocytic delivery. The exocyst complex plays a key role in specifying plasma membrane sites for exocytic vesicle docking (20). At these sites, the exocyst facilitates the association of v-SNARE proteins with their t-SNARE partners present on the inner surface of the plasma membrane. This association of SNARE proteins is thought to allow for the fusion of exocytic vesicles by bringing the vesicle membrane in close proximity to the plasma membrane (31). Small GTPases of the Ras superfamily, including the Rab and Ral proteins, are required for proper exocytosis (28). Rab proteins help to tether the exocyst complex to secretory vesicles (15), whereas the Ral proteins are necessary for exocyst holocomplex formation (30). Little is known about how the Ral proteins disengage the exocyst upon vesicle docking to allow for proper vesicular fusion to occur. We propose that a phosphorylation cycle is necessary for RalB to dynamically regulate vesicle sorting and that PKCα is a key kinase involved in this process. The phosphatase that would also be necessary to dynamically regulate the RalB-phosphorylated state remains to be found. Interestingly, there is an exocyst-associated phosphatase (26), but whether or not this phosphatase acts on RalB is unknown. Overall, dynamic regulation of RalB phosphorylation serves as a mechanism through which the vesicle trafficking machinery can be recycled, thereby ensuring a proper flow of exocytic vesicles.
Supplementary Material
Acknowledgment
We thank Michael Ehlers for the TfR-mCh-SEP expression vector.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 CA042978 and P50 CA106991 (to C. J. D.) and R01 CA109550 (to A. D. C.).

This article contains supplemental Figs. 1 and 2.
- PDAC
- pancreatic ductal adenocarcinoma
- CRC
- colorectal cancer
- mCh
- mCherry
- SEP
- superecliptic pHluorin derivative of GFP
- TfR
- transferrin receptor
- HV
- hypervariable.
REFERENCES
- 1. Bodemann B. O., White M. A. (2008) Ral GTPases and cancer. Linchpin support of the tumorigenic platform. Nat. Rev. Cancer 8, 133–140 [DOI] [PubMed] [Google Scholar]
- 2. Neel N. F., Martin T. D., Stratford J. K., Zand T. P., Reiner D. J., Der C. J. (2011) The RalGEF-Ral effector signaling network. The road less traveled for anti-Ras drug discovery. Genes Cancer 2, 275–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lim K. H., O'Hayer K., Adam S. J., Kendall S. D., Campbell P. M., Der C. J., Counter C. M. (2006) Divergent roles for RalA and RalB in malignant growth of human pancreatic carcinoma cells. Curr. Biol. 16, 2385–2394 [DOI] [PubMed] [Google Scholar]
- 4. Martin T. D., Samuel J. C., Routh E. D., Der C. J., Yeh J. J. (2011) Activation and involvement of Ral GTPases in colorectal cancer. Cancer Res. 71, 206–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lim K. H., Baines A. T., Fiordalisi J. J., Shipitsin M., Feig L. A., Cox A. D., Der C. J., Counter C. M. (2005) Activation of RalA is critical for Ras-induced tumorigenesis of human cells. Cancer Cell 7, 533–545 [DOI] [PubMed] [Google Scholar]
- 6. Chen X. W., Inoue M., Hsu S. C., Saltiel A. R. (2006) RalA exocyst-dependent recycling endosome trafficking is required for the completion of cytokinesis. J. Biol. Chem. 281, 38609–38616 [DOI] [PubMed] [Google Scholar]
- 7. Cascone I., Selimoglu R., Ozdemir C., Del Nery E., Yeaman C., White M., Camonis J. (2008) Distinct roles of RalA and RalB in the progression of cytokinesis are supported by distinct RalGEFs. EMBO J. 27, 2375–2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lim K. H., Brady D. C., Kashatus D. F., Ancrile B. B., Der C. J., Cox A. D., Counter C. M. (2010) Aurora-A phosphorylates, activates, and relocalizes the small GTPase RalA. Mol. Cell. Biol. 30, 508–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sablina A. A., Chen W., Arroyo J. D., Corral L., Hector M., Bulmer S. E., DeCaprio J. A., Hahn W. C. (2007) The tumor suppressor PP2A Aβ regulates the RalA GTPase. Cell 129, 969–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mitin N. Y., Ramocki M. B., Zullo A. J., Der C. J., Konieczny S. F., Taparowsky E. J. (2004) Identification and characterization of Rain, a novel Ras-interacting protein with a unique subcellular localization. J. Biol. Chem. 279, 22353–22361 [DOI] [PubMed] [Google Scholar]
- 11. Kennedy M. J., Davison I. G., Robinson C. G., Ehlers M. D. (2010) Syntaxin-4 defines a domain for activity-dependent exocytosis in dendritic spines. Cell 141, 524–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Madigan J. P., Bodemann B. O., Brady D. C., Dewar B. J., Keller P. J., Leitges M., Philips M. R., Ridley A. J., Der C. J., Cox A. D. (2009) Regulation of Rnd3 localization and function by protein kinase Cα-mediated phosphorylation. Biochem. J. 424, 153–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Laukaitis C. M., Webb D. J., Donais K., Horwitz A. F. (2001) Differential dynamics of α5-integrin, paxillin, and α-actinin during formation and disassembly of adhesions in migrating cells. J. Cell Biol. 153, 1427–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu J. C., Chen T. Y., Yu C. T., Tsai S. J., Hsu J. M., Tang M. J., Chou C. K., Lin W. J., Yuan C. J., Huang C. Y. (2005) Identification of V23RalA-Ser194 as a critical mediator for Aurora-A-induced cellular motility and transformation by small pool expression screening. J. Biol. Chem. 280, 9013–9022 [DOI] [PubMed] [Google Scholar]
- 15. Stenmark H. (2009) Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Biol. 10, 513–525 [DOI] [PubMed] [Google Scholar]
- 16. Masur K., Lang K., Niggemann B., Zanker K. S., Entschladen F. (2001) High PKCα and low E-cadherin expression contribute to high migratory activity of colon carcinoma cells. Mol. Biol. Cell 12, 1973–1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oster H., Leitges M. (2006) Protein kinase Cα but not PKCζ suppresses intestinal tumor formation in ApcMin+ mice. Cancer Res. 66, 6955–6963 [DOI] [PubMed] [Google Scholar]
- 18. Bivona T. G., Quatela S. E., Bodemann B. O., Ahearn I. M., Soskis M. J., Mor A., Miura J., Wiener H. H., Wright L., Saba S. G., Yim D., Fein A., Pérez de Castro I., Li C., Thompson C. B., Cox A. D., Philips M. R. (2006) PKC regulates a farnesyl-electrostatic switch on K-Ras that promotes its association with Bcl-XL on mitochondria and induces apoptosis. Mol. Cell 21, 481–493 [DOI] [PubMed] [Google Scholar]
- 19. Chien Y., Kim S., Bumeister R., Loo Y. M., Kwon S. W., Johnson C. L., Balakireva M. G., Romeo Y., Kopelovich L., Gale M., Jr., Yeaman C., Camonis J. H., Zhao Y., White M. A. (2006) RalB GTPase-mediated activation of the IκB family kinase TBK1 couples innate immune signaling to tumor cell survival. Cell 127, 157–170 [DOI] [PubMed] [Google Scholar]
- 20. He B., Guo W. (2009) The exocyst complex in polarized exocytosis. Curr. Opin. Cell Biol. 21, 537–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Proux-Gillardeaux V., Rudge R., Galli T. (2005) The tetanus neurotoxin-sensitive and insensitive routes to and from the plasma membrane. Fast and slow pathways? Traffic 6, 366–373 [DOI] [PubMed] [Google Scholar]
- 22. Spiczka K. S., Yeaman C. (2008) Ral-regulated interaction between Sec5 and paxillin targets Exocyst to focal complexes during cell migration. J. Cell Sci. 121, 2880–2891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang H., Owens C., Chandra N., Conaway M. R., Brautigan D. L., Theodorescu D. (2010) Phosphorylation of RalB is important for bladder cancer cell growth and metastasis. Cancer Res. 70, 8760–8769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Morgan A., Burgoyne R. D., Barclay J. W., Craig T. J., Prescott G. R., Ciufo L. F., Evans G. J., Graham M. E. (2005) Regulation of exocytosis by protein kinase C. Biochem. Soc. Trans. 33, 1341–1344 [DOI] [PubMed] [Google Scholar]
- 25. Lau C. G., Takayasu Y., Rodenas-Ruano A., Paternain A. V., Lerma J., Bennett M. V., Zukin R. S. (2010) SNAP-25 is a target of protein kinase C phosphorylation critical to NMDA receptor trafficking. J. Neurosci. 30, 242–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen X. W., Leto D., Xiao J., Goss J., Wang Q., Shavit J. A., Xiong T., Yu G., Ginsburg D., Toomre D., Xu Z., Saltiel A. R. (2011) Exocyst function is regulated by effector phosphorylation. Nat. Cell Biol. 13, 580–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hepp R., Puri N., Hohenstein A. C., Crawford G. L., Whiteheart S. W., Roche P. A. (2005) Phosphorylation of SNAP-23 regulates exocytosis from mast cells. J. Biol. Chem. 280, 6610–6620 [DOI] [PubMed] [Google Scholar]
- 28. Shipitsin M., Feig L. A. (2004) RalA but not RalB enhances polarized delivery of membrane proteins to the basolateral surface of epithelial cells. Mol. Cell. Biol. 24, 5746–5756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen X. W., Leto D., Chiang S. H., Wang Q., Saltiel A. R. (2007) Activation of RalA is required for insulin-stimulated Glut4 trafficking to the plasma membrane via the exocyst and the motor protein Myo1c. Dev. Cell 13, 391–404 [DOI] [PubMed] [Google Scholar]
- 30. Moskalenko S., Tong C., Rosse C., Mirey G., Formstecher E., Daviet L., Camonis J., White M. A. (2003) Ral GTPases regulate exocyst assembly through dual subunit interactions. J. Biol. Chem. 278, 51743–51748 [DOI] [PubMed] [Google Scholar]
- 31. Brown F. C., Pfeffer S. R. (2010) An update on transport vesicle tethering. Mol. Membr. Biol. 27, 457–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.