Background: Mechanism underlying the protection of estradiol against CCl4-induced hepatic fibrosis remains unclear.
Results: miR-29 expression was differentially regulated in male/female mice during CCl4 treatment. Both estradiol and miR-29a/b-expressing adenovirus increased hepatic miR-29a/b levels and attenuated CCl4-induced hepatic fibrosis.
Conclusion: Estradiol inhibits CCl4-induced hepatic injury via inducing miR-29a/b.
Significance: Learning the role/regulation of miR-29 in hepatic fibrosis and providing novel therapeutic target.
Keywords: Estrogen, Fibrosis, Immunohistochemistry, Liver injury, MicroRNA, CCl4, qRT-PCR
Abstract
Previous studies have indicated that female animals are more resistant to carbon tetrachloride (CCl4)-induced liver fibrosis than male animals, and that estradiol (E2) treatment can inhibit CCl4-induced animal hepatic fibrosis. The underlying mechanism governing these phenomena, however, has not been fully elucidated. Here we reported the role of estrogen-induced miRNA-29 (miR-29) expression in CCl4-induced mouse liver injury. Hepatic miR-29 levels were differentially regulated in female and male mice during CCl4 treatment. Specifically, the levels of miR-29a and miR-29b expression were significantly decreased in the livers of male, but not female, mice following 4 weeks of CCl4 treatment. The down-regulation of miR-29a and miR-29b in male mouse livers correlated with the early development of liver fibrosis, as indicated by increased expressions of fibrotic markers in male mice relative to female mice. In addition, E2 was maintained at a higher level in female mice than in male mice. In contrast to TGF-β1 that decreased miR-29a/b expression in murine hepatoma IAR20 cells and normal hepatocytes, E2 enhanced the expression of miR-29a/b through suppression of the nuclear factor-κB (NF-κB) signal pathway, which negatively regulates miR-29 expression. Furthermore, both E2 treatment and intravenous injection of the recombinant adenovirus expressing miR-29a/b markedly increased the miR-29a/b level and attenuated the expression of fibrotic markers in mouse livers during CCl4 treatment, supporting the protective role of E2-induced miR-29 in CCl4-induced hepatic injury. In conclusion, our results collectively demonstrate that estrogen can inhibit CCl4-induced hepatic injury through the induction of hepatic miR-29.
Introduction
Fibrosis is characterized by the excessive deposition of components of the extracellular matrix, predominantly collagens. Liver fibrosis is a common outcome of chronic hepatic injuries or diseases and can ultimately lead to liver cirrhosis and hepatic failure. CCl4-induced fibrosis shares several characteristics with human fibrosis of various etiologies; accordingly, CCl4-induced fibrosis has been employed as a model of fibrosis (1–3). The aim of the present work was to study the differential fibrotic responses of female and male mice to CCl4 and the mechanism underlying the role of estradiol in suppressing the induction of hepatic fibrosis.
Previous investigations have observed sex differences in animals' fibrotic responses to non-alcohol fibrosis induction reagents, such as CCl4 (4), and female animals were generally described to be more resistant to CCl4-induced fibrosis than male animals. By studying these differences, several groups have identified the protective effect of estradiol in inhibiting hepatic fibrosis in CCl4-induced animal models (5–7). Yasuda et al. (5) reported that estradiol reduced the mRNA levels of type I and III procollagens and the tissue inhibitor of metalloproteinase-1. The researchers also observed that, in the castrated-female model, estradiol replacement was fibrosuppressive, and the protective effect of estradiol can be abolished by specific antibodies directed against estradiol or by the process of ovariectomy in female animals, which significantly reduced estradiol levels. Liu et al. (6) reported that treatment with 17β-estradiol reduced liver fibrosis and maintained liver function, as indicated by the levels of aspartate aminotransferase (AST)4 and alanine aminotransferase (ALT). The study by Shimizu et al. (8) in rat hepatic stellate cells suggested that the protective effect of estradiol against liver fibrosis might be related to its inhibition of hepatic stellate cell (HSC) activation. Although estradiol has been found to serve as a potent endogenous antioxidant, which suppresses hepatic fibrosis by inhibiting the generation of reactive oxygen species or by indirectly modulating the synthesis and release of cytokines and other growth factors, the mechanism underlying the inhibition of estradiol in liver fibrosis has not been thoroughly characterized.
MicroRNAs (miRNAs) are ∼22-nucleotide noncoding RNAs that post-transcriptionally regulate gene expression by undergoing imperfect base pairing with sequences in the 3′ untranslated regions (UTRs) of cellular target mRNAs (9–11). Previous studies have indicated that miRNAs play roles in almost every aspect of cellular processes, including cell proliferation, differentiation, and apoptosis. It has been widely reported that aberrant miRNA expression can lead to the development of multiple diseases. A number of reports have demonstrated that an important role is played by miRNAs during the activation of HSCs. The analysis of miRNA expression in activated rat HSCs identified a panel of up-regulated or down-regulated miRNAs (12). The overexpression of miR-16 and miR-15b was also demonstrated to inhibit the proliferation of HSCs and to induce apoptosis through the down-regulation of the mitochondrial-associated anti-apoptotic protein Bcl-2, thereby leading to the activation of caspases 3, 8, and 9 (13). These findings indicate that miRNAs play a significant role in the progression of liver fibrogenesis through the activation of HSCs. The miR-29 family, which includes the key collagen regulators miR-29a and miR-29b, has also been implicated in tissue fibrosis (14–16). The down-regulation of miR-29a and miR-29b in the border zone of murine and human hearts during myocardial infarctions has been reported by van Rooij et al. (17). Also, miR-29 serves as a major regulator of genes associated with pulmonary fibrosis (15). Most recently, after analyzing the regulation of miRNAs in a mouse model of CCl4-induced hepatic fibrogenesis via array analysis, Roderburg et al. (16) reported that all three members of the miR-29 family were significantly down-regulated in the livers of CCl4-treated mice, as well as in mice that underwent bile duct ligation; in addition, on a cellular level, overexpression of miR-29b in murine HSCs resulted in the down-regulation of collagen expression.
In the present study, we characterized the association between the protective role of estradiol in inhibiting CCl4-induced mouse hepatic fibrosis and the differential expression levels of miR-29 observed in female and male mouse livers. We determined that the levels of miR-29 (miR-29a and miR-29b) were significantly decreased in the livers of male, but not female, mice following a 4-week CCl4 treatment and that the down-regulation of miR-29a and miR-29b in male mouse livers correlated with the accelerated development of liver fibrosis, as indicated by the preferentially increased expressions of collagens, α-smooth muscle actin (α-SMA), and TGF-β1 in male mice relative to female mice. Our results also showed that E2, which enhanced the expression of miR-29a/b in cultured murine hepatoma IAR20 cells, was maintained at a higher level in female mice than in male mice. Furthermore, we observed that both E2 treatment and the elevation of miR-29a and miR-29b levels by treating mice with the recombinant adenovirus expressing miR-29a and miR-29b (Ad-miR-29a/b) markedly attenuated the expression levels of collagen I and α-SMA in the mouse liver, supporting the protective role of E2-induced miR-29a/b expression in CCl4-induced mouse hepatic fibrosis.
EXPERIMENTAL PROCEDURES
Animal Model
Animal maintenance and experimental procedures were carried out in accordance with the United States National Institute of Health Guidelines for Use of Experimental Animals and approved by the Medicine Animal Care Committee of Nanjing University (Nanjing, China). Eight-week-old male and female Balb/c mice (from the Nanjing University Animal Center, Nanjing, China) were used in this study. Fifty male mice were divided into five groups of 10 mice each. For the CCl4 group, 100 ml/liter of CCl4 in olive oil was injected intraperitoneally at a dose of 50 μl twice a week (1). The estrogen group was treated intraperitoneally with estradiol (1 mg/kg) twice weekly (Shanghai Pharmaceutical Co., Shanghai, China) (5, 18) in addition to CCl4. The Ad-control and Ad-miR-29a/b groups were administered 3 × 108 infective units of adenovirus by intravenous injection into the tail vein two times per week (19) along with the CCl4 treatment described above. The control group received injections of olive oil vehicle twice weekly. Twelve female mice were divided into the control and the CCl4 groups, with each group receiving six mice. Both groups were treated identically to the corresponding group of male mice. Four or 8 weeks after treatment with CCl4, mice were sacrificed. The livers were either collected and fixed in 4% paraformaldehyde for histological examination or frozen immediately in liquid nitrogen and stored at −80 °C for RNA isolation. Levels of ALT and AST were measured by ALT and AST kits (Jiancheng Institute of Biotech, Nanjing, China). The estradiol levels were measured using an enzyme-linked immunosorbent assay kit (Adlitteram Diagnostic Lab, Wellesley, MA).
Cell Culture and Stimulation
Human embryonic kidney 293 cells (HEK293) (adenovirus E1-transformed) and murine hepatoma cell line IAR20 were obtained from the Shanghai Cell Bank (Shanghai, China). Both cell lines were cultured in DMEM supplemented with 10% fetal bovine serum and penicillin (100 units/ml)/streptomycin (100 mg/ml) at 37 °C in a humidified atmosphere of 100% air and 5% CO2. Mouse hepatocytes were isolated by collagenase perfusion of the mouse liver and primarily cultured in complete medium. IAR20 cells and isolated mouse hepatocytes were plated on six-well dishes and when the cells reached ∼90% confluence, they were cultured in serum-free medium containing vehicle controls, 10 ng/ml of recombinant human TGF-β1 (R&D Systems, Minneapolis, MN), or 100 nm E2 for 48 h (20).
Recombinant Adenovirus Construction and Cell Infection
The recombinant adenovirus expressing miR-29a/b was generated by PCR (supplemental Fig. S1). All miRNA precursor sequences were amplified using the following primers: miR-29a forward, 5′-GACGGTACCTGGTGGAGAACAACTTCG-3′; miR-29a reverse, 5′-CAGAAGCTTCATCAAACCTTCAATCCC-3′; miR-29b forward, 5′-ACCAGATCTGCGTCACGGCTCAATGTC-3′; miR-29b reverse, 5′-AGGCTCGAGCAGGCTTGATGGAGTCTGCT-3′. The fragments were cloned into the shuttle vector AdTrack-cytomegalovirus (pAdTrack-CMV) (Stratagene, La Jolla, CA) (21). The resulting recombinant shuttle vectors in which the expression of miRNAs could be driven by CMV promoter were designated pCMV-miR-29a, pCMV-miR-29b, and pCMV-control. All insertion sequences were confirmed by nucleotide sequencing. According to the procedure of He et al. (22), the recombinant shuttle vectors were linearized with PmeI and co-transformed by electroporation in combination with the adenoviral backbone plasmid pAdEasy-1 (Stratagene) into Escherichia coli BJ5183. The recombinant adenoviral plasmids were generated by homologous recombination. Positive clones were selected and confirmed by preparing DNA minipreps of amplified clones and digesting their DNA with PacI. The resulting adenoviral plasmids (pAd-miR-29a, pAd-miR-29a, and pAd-control) were linearized with PacI, purified by ethanol precipitation, and transfected into HEK293 packaging cells that had been plated into a 25-cm2 flask the previous day. The cells were transfected with 4 μg of linearized plasmid DNA using 20 μl of Lipofectamine 2000 reagent (Invitrogen) and monitored for the expression of green fluorescent protein (GFP) (23). After transfection for 12 days, the cells were collected and centrifuged 10 min at 4 °C at 500 × g to pellet the cell debris. Next, three or four cycles of freezing in a dry ice/methanol bath and thawing at 37 °C were performed to release the viruses from the cells. To generate high-titer viral stock, the adenoviruses Ad-miR-29a, Ad-miR-29b, and Ad-control were harvested, amplified, and purified using the Adeno-X Virus Purification Kit (Clontech) (22), and the levels of miR-29a/b were determined by real-time quantitative PCR. The adenovirus titer was measured by plaque formation assay following infection of HEK293 cells. The multiplicity of infection was defined as the ratio of the total number of plaque-forming units to the total number of infected cells (19). The titers of the purified viruses were as follows: 3.0 × 108 pfu/ml for Ad-control and 3.2 × 108 pfu/ml for Ad-miR-29a/b. As shown in supplemental Fig. S1, purified viruses can successfully infect cells and strongly increase miR-29a/b expression. All of the virus preparations were stored at −80 °C until use.
Histopathological Examination and Immunohistochemistry
For morphometric studies, liver tissues were preserved in 4% paraformaldehyde, embedded in paraffin, and cut into 5-μm thick sections. Next, some sections were stained with Sirius red according to the manufacturer's instructions (24, 25). The liver fibrosis was assessed histologically by quantification of the Sirius red-positive area on 10 low-power (original magnification ×100) fields per slide, with use of the NIH ImageJ software (rsbweb.nih.gov) (26). The other tissue sections were treated with xylene and rehydrated with a decreasing gradient of ethanol washes. The levels of α-SMA (Sigma), type I collagen (Col1a1), and TGF-β1 (Santa Cruz Biotechnology) were determined by immunohistochemical methods. Briefly, after blocking with goat serum at 37 °C, the treatment sections were incubated at 4 °C overnight with antibodies against three marker proteins for fibrosis. After washing three times with PBS, the sections were incubated with biotinylated anti-rabbit secondary antibody at 37 °C for 30 min. The slides were stained using 3,3′-diaminobenzidine-H2O2, counterstained with hematoxylin, and examined under a light microscope (original magnification ×200).
Oligonucleotides and Transfection
The pBD-NF-κB plasmid expressing the transcriptional activation domain of the mouse NF-κB gene was purchased from Stratagene. The siRNA oligonucleotides against mouse IκBα or NF-κB p65 were obtained from GenePharma Biotechnology. IAR20 cells were plated at a density of 1 × 106 cells 24 h prior to transfection. Cells were transfected with IκBα siRNA, NF-κB p65 siRNA, or negative control siRNA using Lipofectamine 2000 as suggested by the manufacturer (Invitrogen). For overexpression of NF-κB, 24 μg of pBD-NF-κB or pCMV-control (control) per 106 cells was used in the absence or presence of E2. Total RNA and protein were harvested at 48 h post-transfection and analyzed for IκBα, NF-κB p65, and miR-29a/b expression.
Quantitative Real-time PCR
Total RNA (2 μg) was isolated from liver tissues using the TRIzol Reagent (Invitrogen) and reverse-transcribed into cDNA in a reaction volume of 20 μl using the avian myeloblastosis virus reverse transcriptase (Takara) according to the manufacturer's instructions. The cDNA samples (2 μl) were employed for real-time PCR in a total volume of 20 μl using the SYBR Green reagent (Invitrogen) on an Applied Biosystems 7300 Sequence Detection system. The sequences of the forward and reverse primers for the quantification of TGF-β1, α-SMA, and collagen 1 (Col1a1) mRNA and murine β-actin mRNA are as indicated: TGF-β1 forward, ATCCCGCCCACTTTCTAC; TGF-β1 reverse, AGTTCAATCCGCTGCTCG; α-SMA forward, TCGGATACTTCACGTCAGGA; α-SMA reverse, GTCCCAGACATCGGGAGTAA; Col1a1 forward, GCTCCTCTTAGGGGCCACT; Col1a1 reverse, CCACGTCTCACCATTGGGG; β-actin forward, GAGACCTTCAACACCCCAGC; β-actin reverse, ATGTCACGCACGATTTCCC. The reactions were incubated in a 96-well optical plate at 95 °C for 5 min, followed by 40 cycles of 95 °C for 15 s, 58 °C for 30 s, and 72 °C for 30 s. Melting-curve analysis was performed at 90 (TGF-β1), 85 (α-SMA and Col1a1), or 88 °C (β-actin). The average of the triplicate data obtained for each sample was employed to calculate the relative change in gene expression after normalization to β-actin mRNA (42, 43). The primer sequences of miRNAs and U6 snRNA are as follows: mmu-miR-29a, UAGCACCAUCUGAAAUCGGUUA; mmu-miR-29b, UAGCACCAUUUGAAAUCAGUGUU; U6 snRNA, TGCTAATCTTCTCTGTATCGT. Quantification of U6 snRNA was performed in the same experiments as the internal control.
Western Blotting
Cell nuclear pellets and cytosolic extracts were prepared for immunoblotting analysis (27). Briefly, cell lysates were prepared by solubilizing cells in cold RIPA buffer (10 mm Tris-HCl, 1 mm EDTA, 1% SDS, 1 mm DTT, 0.1 mm PMSF, protease inhibitors, 1% Nonidet P-40, pH 8.0). After centrifugation at 10,000 × g for 5 min, the supernatant was collected as cytosolic extract. The nuclear pellets were resuspended in 30 μl of hypertonic extraction buffer, and centrifuged at 16,000 × g for 10 min. The supernatant was collected as the nuclear extract. The protein contents in cytosolic and nuclear lysates were analyzed by a Micro BCA Protein Assay kit (Pierce). Proteins (40 μg) were separated on 10% acrylamide gels and electrotransferred to polyvinylidene difluoride membranes. The membranes were blocked with 5% nonfat dry milk in TBST (TBS plus 0.1% Tween 20) for 1 h. Blots were then probed with primary antibodies against IκBα, NF-κB p65, histone H1, or GAPDH, followed by HRP-conjugated secondary antibodies. All antibodies were from Santa Cruz (Santa Cruz, CA).
Statistical Analysis
Quantitative RT-PCR was performed in triplicate, and the entire experiment was repeated several times. Data are presented as mean ± S.E. of three or more independent experiments. p values below 0.05 (*) and 0.01 (**) obtained using the Student's t test or analysis of variance analysis were considered significant.
RESULTS
Differential Expression of miR-29 and Response to CCl4-induced Liver Injury in Female and Male Mice
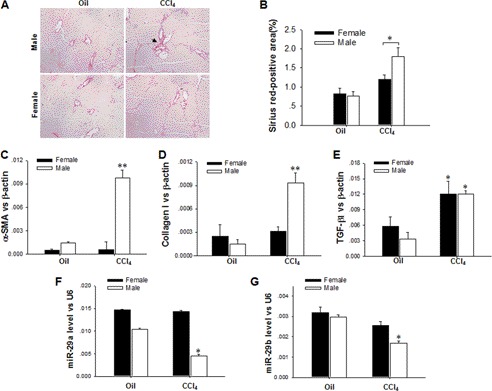
To compare the development of CCl4-induced liver fibrosis in female and male mice, we intraperitoneally injected 50 μl of CCl4 (100 ml/liter in olive oil) into male and female BALB/c mice twice a week. As depicted in Fig. 1, A and B, after 4 weeks of CCl4 treatment, most male mice exhibited significant liver fibrotic damage, as indicated by the up-regulated expression of collagen fibers (Fig. 1A, arrow). In contrast, female mice indicated no such hepatic damage. Next, we determined the levels of α-SMA (Fig. 1C), collagen I (Fig. 1D), and TGF-β1 (Fig. 1E) in mice treated with CCl4 or an oil control. In agreement with the results of tissue staining, which demonstrated that liver fibrosis occurred in male mice but not female mice, we observed that, although TGF-β1 was up-regulated in the livers of both male and female mice, α-SMA and collagen I levels were only significantly increased in the livers of male mice, but not female mice. Interestingly, by assessing microRNA expression levels in mouse liver tissues through a TaqMan probe-based quantitative RT-PCR assay, we determined that miR-29a and miR-29b, which have been reported to target collagen I (14, 16), were significantly down-regulated in the livers of male mice, but not female mice, after 4 weeks of CCl4 treatment (Fig. 1, F and G). Taken together, these results indicated the differential expression of miR-29 in response to CCl4-induced liver injury in female and male mice.
FIGURE 1.
Differential fibrotic response and expression of miR-29a/b in female and male mice treated with CCl4. A and B, tissue staining indicating liver fibrosis at the early stages of CCl4 treatment in male mice, but not in female mice (original magnification ×100). C–E, the mRNA levels observed for α-SMA (C), collagen I (D), and TGF-β1 (E) in mice treated with CCl4 or the oil control. F and G, alterations of miR-29a (F) and miR-29b (G) expression in mice treated with CCl4 or the oil control. The data are presented as mean ± S.E. for three independent experiments, and for each individual experiment, five to six mice were used. *, p < 0.05; **, p < 0.01.
Up-regulation of miR-29 in Murine Hepatoma Cells by Estradiol Treatment
Functional analysis of mouse liver after 4 weeks of treatment with CCl4 confirmed the differential response to CCl4 treatment by female and male mice. As presented in Table 1, we determined that, compared with mice treated with oil alone, male mice treated with CCl4 exhibited strongly elevated levels of serum ALT and AST, increasing from 31.3 ± 6.90 and 32.3 ± 2.07 IU/liter, respectively, to 208.76 ± 20.6 and 207.9 ± 23.3 IU/liter, respectively. In contrast, although the levels of serum ALT and AST observed in female mice treated with CCl4 were also increased relative to mice treated with oil alone, they were significantly lower than those observed in male mice. As expected, an assay for determining estradiol (E2) levels indicated that female mice contain significantly higher levels of E2 than do male mice, regardless of treatment with CCl4 or oil (149.5 ± 23.7 pg/ml for females versus 40.1 ± 3.40 pg/ml for males in CCl4 treatment; 132.2 ± 12.3 pg/ml for females versus 40.5 ± 7.65 pg/ml for males in oil treatment). The correlation between higher levels of E2 and miR-29a/b in female mice, especially at the stage after CCl4 treatment, may suggest that E2 is involved in regulation of miR-29a/b expression.
TABLE 1.
Differential liver injury at early stage (4 weeks) in female and male mice treated with or without CCl4
| Group | n | ALT | AST | Serum estradiol level |
|---|---|---|---|---|
| IU/liter | pg/ml | |||
| Male | ||||
| Oil | 5 | 31.3 ± 6.90 | 32.3 ± 2.07 | 40.5 ± 7.65 |
| CCl4 | 5 | 208.76 ± 20.6a | 207.9 ± 23.3a | 40.1 ± 3.40 |
| Female | ||||
| Oil | 6 | 18.6 ± 3.00 | 40.2 ± 8.36 | 132.2 ± 12.3b |
| CCl4 | 6 | 94.4 ± 21.4b | 102.9 ± 27.0b | 149.5 ± 23.7b |
a Data are presented as mean ± S.E., p < 0.01.
b Data are presented as mean ± S.E., p < 0.05.
Previous studies have reported that certain miRNAs, such as miR-181a and miR-26a, are E2-sensitive (28). Accordingly, we tested whether E2 can modulate the expression of miR-29. In this experiment, we stimulated murine hepatoma IAR20 cells with E2 or TGF-β1. TGF-β1 was employed as a negative control, as it has been reported to reduce miR-29 levels (16). As shown in Fig. 2A, E2 treatment significantly increased the levels of both miR-29a and miR-29b (fold-changes control = 20 and 10, respectively; p < 0.01) compared with those exhibited by the control cells. In agreement with previous reports, we observed that TGF-β1 treatment strongly reduced miR-29a and miR-29b expression in mouse liver cells (Fig. 2B). We further tested the role of E2 and TGF-β1 in modulating miR-29 expression in primarily cultured normal murine hepatocytes (Fig. 2, C and D). As shown, E2 significantly enhanced the expression of both miR-29a and miR-29b (Fig. 2C), whereas TGF-β1 decreased the levels of miR-29a and miR-29b (Fig. 2D) in normal murine hepatocytes.
FIGURE 2.
Alterations of miR-29a and miR-29b expression levels in cells from the murine hepatoma IAR20 cells (A and B) and primarily cultured normal mouse hepatocytes (C and D) stimulated with E2 or TGF-β1. A and C, enhancement of miR-29a and miR-29b expression levels in IAR20 cells (A) and primarily cultured normal murine hepatocytes (C) by E2. B and D, reduction of miR-29a and miR-29b expression levels in IAR20 cells (B) and murine hepatocytes (D) by TGF-β1. The data are presented as mean ± S.E. for three independent experiments. *, p < 0.05; **, p < 0.01.
E2 Enhances Expression of Hepatic miR-29a/b through Blocking NF-κB Pathway
E2 regulation of inflammatory responses has broad physiological consequences. It has been well established that E2 is involved in modulation of transcriptional factors such as NF-κB and STAT-1 (29, 30). Interestingly, previous studies also showed that NF-κB served as a negative regulator for expression of miR-29a and miR-29b (31). To determine whether NF-κB is involved in the E2-mediated up-regulation of miR-29a and miR-29b, we assayed the levels of NF-κB p65 in nuclei of IAR20 cells with or without E2 treatment. As shown in Fig. 3A, E2 treatment significantly reduced the level of nuclear NF-κB p65, whereas serving as a nuclear marker protein, histone H1 was not affected by E2 treatment. In contrast, stimulation by TGF-β1 strongly increased the level of nuclear NF-κB p65 in IAR20 cells (Fig. 3B).
FIGURE 3.
E2 treatment prevents and TGF-β1 induces NF-κB protein nuclear accumulation in IAR20 cells. Western blot analysis of NF-κB p65 and histone H1 protein levels was performed using nuclear extracts from cells treated with 100 nm E2 (A) or 10 ng/ml of TGF-β1 (B) for 48 h. Blots of the Western assay were analyzed using Band scan software. The data are presented as mean ± S.E. from three independent experiments. *, p < 0.05.
Next we assessed the role of the NF-κB pathway in E2-mediated up-regulation of miR-29a/b. As shown in Fig. 4, we determined the levels of miR-29a and miR-29b in IAR20 cells when NF-κB activity was enhanced or blocked. When cells were transfected with siRNA specific for IκBα, an inhibitory protein sequesters the NF-κB as an inactive complex in the cytoplasm (32, 33), both mRNA (Fig. 4A) and protein (Fig. 4B) levels of IκBα were decreased, whereas the nuclear NF-κB p65 level was increased (Fig. 4C). Increased NF-κB activity significantly suppressed the expression of miR-29a (Fig. 4D) and miR-29b (Fig. 4E). In contrast, when we directly blocked the NF-κB activity by transfecting IAR20 cells with NF-κB p65-specific siRNA, we found that the levels of both total cellular NF-κB p65 (Fig. 4F) and nuclear NF-κB p65 (Fig. 4G) were decreased. The levels of miR-29a (Fig. 4H) and miR-29b (Fig. 4I), however, were significantly increased in NF-κB p65 siRNA-transfected cells with lower NF-κB activity. In contrast, when IAR20 cells were overexpressed with NF-κB, the nuclear NF-κB p65 level was maintained at relatively high levels even at the presence of E2 (Fig. 5, A and B), and the levels of miR-29a and miR-29b were not elevated by E2 treatment (Fig. 5, C and D). Taken together, our results suggest that E2-mediated up-regulation of miR-29a and miR-29b may be through blockade of NF-κB activity.
FIGURE 4.
NF-κB negatively regulates the expression of miR-29a and miR-29b. A, IAR20 cells were transfected with either scrambled negative control (NC) siRNA or IκBα siRNA oligonucleotides, and IκBα mRNA levels were measured by quantitative RT-PCR at 48 h posttransfection. B, IκBα levels measured by immunoblot, with GAPDH served as a control. C, nuclear lysates were analyzed for NF-κB p65 and histone H1 levels by Western blot. D and E, levels of miR-29a (D) and miR-29b (E) detected by quantitative RT-PCR. F and G, levels of NF-κB p65, GAPDH, and histone H1 in cytoplasmic extracts (F) and nuclear extracts (G) from IAR20 cells transfected with either NC siRNA or NF-κB p65 siRNA oligonucleotides for 48 h. H and I, levels of miR-29a (H) and miR-29b (I) in IAR20 cells transfected with NC siRNA or NF-κB p65 siRNA. The data are expressed as mean ± S.E. from three independent experiments. *, p < 0.05.
FIGURE 5.
Overexpression of NF-κB blocks the effect of E2 on miR-29 expression in IAR20 cells. A, transfection IAR20 with pBD-NF-κB in the absence or presence of 100 nm E2 for 48 h, and nuclear lysates were analyzed for NF-κB p65 and histone H1 by Western blot. B, the quantification of the NF-κB p65 level in panel A. C and D, levels of miR-29a (C) and miR-29b (D) in IAR20 cells treated as in panel A. The data are expressed as mean ± S.E. from three independent experiments. *, p < 0.05.
Elevation of miR-29 Levels via Intravenous Injection of Ad-miR-29a/b Attenuates Fibrosis Induced by CCl4 in Male Mouse Livers
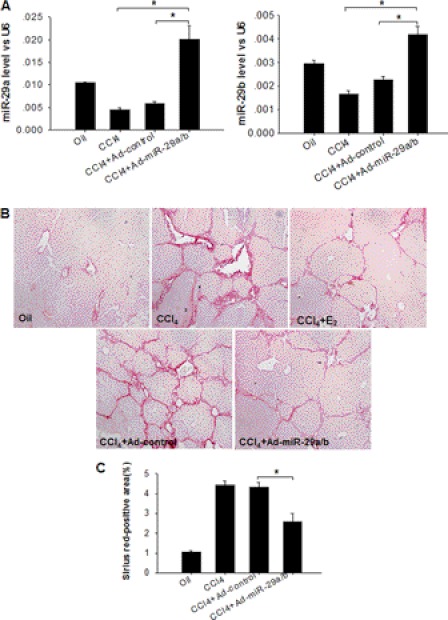
The opposing effects of estradiol and TGF-β1 on the expression levels of miR-29, suggesting suppressor and promoter functions, respectively, regarding CCl4-induced liver fibrosis, indicate a critical role for miR-29 in the modulation of animal liver fibrosis. Therefore, we tested whether elevating or maintaining the levels of miR-29 in mouse livers can prevent or attenuate CCl4-induced liver fibrosis. In this experiment, we constructed vectors expressing miR-29a and miR-29b using an adenovirus system (Ad-miR-29a/b) and intravenously injected Ad-miR-29a/b into male mice 2 weeks after CCl4 treatment (34). Ad-miR-29a/b was administered twice a week for the next 6–8 weeks of CCl4 treatment (19). As indicated in Fig. 6A, the levels of miR-29a and miR-29b in the livers of male mice treated with CCl4 plus Ad-miR-29a/b were significantly higher than those of mice treated with CCl4 only or CCl4 plus a control adenovirus vector. Histochemical examination demonstrated a reduced level of CCl4-induced liver tissue fibrotic damage in male mice treated with Ad-miR-29a/b compared with mice treated with the adenoviral control vector (Fig. 6, B and C).
FIGURE 6.
Elevation of miR-29 expression level via intravenous injection of Ad-miR-29a/b attenuates mouse liver fibrosis induced by CCl4 in males. A, elevation of miR-29a and miR-29b expression levels in male mouse livers via intravenous injection of Ad-miR-29a/b during CCl4 treatment. B and C, liver protection mediated by treatment with Ad-miR-29a/b (original magnification ×100). The data are presented as mean ± S.E. for three independent experiments, and for each individual experiment, five to six mice were employed. *, p < 0.05; **, p < 0.01.
The utility of the Ad-miR-29a/b vector in inhibiting CCl4-induced liver fibrosis was further characterized by analyzing the expression of fibrotic indicators, such as TGF-β1, α-SMA, and collagen I. As shown in Fig. 7A, the reduction of the CCl4-induced mRNA levels of α-SMA and collagen I in mouse livers was observed in male mice treated with Ad-miR-29a/b, but not in male mice treated with the Ad-control vector. Immunohistochemical results (Fig. 7B) confirmed that the CCl4-induced up-regulation of protein expression by α-SMA and collagen I in mouse livers was largely inhibited by the administration of Ad-miR-29a/b.
FIGURE 7.
Reduction of CCl4-induced α-SMA and collagen I expression levels in male mice by intravenous injection of Ad-miR-29a/b. A, the mRNA levels of α-SMA, collagen I, and TGF-β1 in mice treated with or without CCl4 or Ad-miR-29a/b. B, immunohistochemical staining of TGF-β1, α-SMA, and collagen I in mice treated with or without CCl4 or Ad-miR-29a/b (original magnification ×200). The data are presented as mean ± S.E. *, p < 0.05. **, p < 0.01.
Protective Effect of E2 in CCl4-induced Mouse Liver Injury via Enhancing miR-29 Expression
Because E2 can up-regulate miR-29 expression in mouse liver cells (Fig. 2), we next investigated whether the protection of E2 against CCl4-induced mouse liver fibrosis is working through the up-regulation of hepatic miR-29. In this experiment, male mice were treated intraperitoneally with 17β-estradiol (1 mg/kg) twice a week (5, 18). As indicated in Fig. 8A and in agreement with the data obtained from experiments in cultured cells, E2 treatment prevented the CCl4-induced down-regulation of miR-29 in mouse livers. Histochemical examination (Fig. 8, B and C) of mouse liver tissue sections indicated a reduced level of CCl4-induced liver tissue fibrotic damage in male mice treated with E2 compared with the control group.
FIGURE 8.
Protective role of E2 in attenuating CCl4-induced mouse liver fibrosis. A, up-regulation of miR-29a and miR-29b in mouse livers by E2 treatment. B and C, attenuation of CCl4-induced mouse liver fibrosis by E2 treatment (original magnification ×100). D, the mRNA levels of TGF-β1, α-SMA, and collagen I in mice treated with or without CCl4 in the presence or absence of E2. The data are presented as mean ± S.E. for three independent experiments, and five to six mice were used for each individual experiment. *, p < 0.05; **, p < 0.01.
In a similar fashion, we examined the expression and localization of TGF-β1, α-SMA, and collagen I in mouse livers treated or untreated with E2. As shown in Fig. 8D, a decrease in the CCl4-induced mRNA levels of α-SMA and collagen I was observed in the livers of male mice treated with E2. Immunohistochemical results (supplemental Fig. S2) also demonstrated that CCl4-induced up-regulation of the protein expression of α-SMA and collagen I in mouse livers was largely blocked by treatment with E2.
Functional analysis of mouse livers after 8 weeks of CCl4 treatment further demonstrated the protective role of Ad-miR-29a/b and E2 in inhibiting CCl4-induced mouse fibrotic liver damage (Table 2). This study indicates that, after 8 weeks of CCl4 treatment, the liver function of male mice was severely impaired, as ALT and AST levels increased from 31.74 ± 6.91 and 32.08 ± 2.52 IU/liter, respectively, to 408.76 ± 44.8 and 407.9 ± 53.7 IU/liter, respectively. In contrast, the levels of ALT and AST in mice treated with CCl4 and either Ad-miR-29a/b or E2 (135.6 ± 26.9 and 188.3 ± 40.3 IU/liter, respectively, for Ad-miR-29a/b and 186.7 ± 35.5 and 200.95 ± 45.6 IU/liter, respectively, for E2) were significantly lower than those in mice treated with CCl4 alone.
TABLE 2.
CCl4-induced liver injury at late stage (8 weeks) in each group of male mice
| Group | n | ALT | AST | Serum estradiol level |
|---|---|---|---|---|
| IU/liter | pg/ml | |||
| Oil | 5 | 31.74 ± 6.91 | 32.08 ± 2.52 | 35.0 ± 5.77 |
| CCl4 | 5 | 408.76 ± 44.8a | 407.9 ± 53.7a | 37.2 ± 4.33 |
| CCl4 + Ad-control | 10 | 397.8 ± 27.4 | 440.3 ± 37.3 | 34.1 ± 1.71 |
| CCl4 + Ad-miR-29a/b | 10 | 135.6 ± 26.9b | 188.3 ± 40.3b | 36.7 ± 1.88 |
| CCl4 + E2 | 10 | 186.7 ± 35.5b | 200.95 ± 45.6b | 156.4 ± 10.3b |
a Data are presented as mean ± S.E., p < 0.01, CCl4 versus oil group.
b Data are presented as mean ± S.E., p < 0.05, CCl4 + Ad-miR-29a/b or CCl4 + E2 versus CCl4 group.
DISCUSSION
Liver fibrosis is a complex process modulated by a set of signaling pathways and results in the deposition of extracellular matrix proteins during fibrogenesis (35–37). The data have suggested that certain types of chronic liver diseases progress at unequal rates in males and females (38). In chronic viral hepatitis, the major sequelae, such as fibrosis or cirrhosis, are more common in men than in women (35, 38). In general, the development of cirrhosis is more common in men than in women (27, 39). In contrast, females are more susceptible to alcohol-induced liver injury than are males (40). Although the liver is not a classic sex hormone target, it contains estrogen receptors and responds to estrogen (41–43). Thus, sex hormones may play a role in the development of hepatic fibrosis (44, 45). Previous studies reported that E2 treatment inhibited hepatic fibrosis induced in rats by CCl4 or dimethylnitrosamine (5, 6). The specific mechanisms governing the gender-related differences in susceptibility to non-alcohol-induced liver fibrosis, however, have not been fully elucidated. In the present study, we demonstrated that elevation of the expression of liver miR-29 by E2 might serve as the mechanism underlying the protective role of E2 in inhibiting liver fibrosis. First, we indicated a differential rate of progression among female and male mice developing CCl4-induced liver fibrotic damage. Although long-term (8–10 weeks) treatment with CCl4 resulted in liver fibrosis in both female and male mice, at 4 weeks post-treatment with CCl4 female mice were relatively resistant to CCl4-induced liver fibrosis compared with male mice, as indicated by liver-function assays and histochemical data. Second, female mice exhibit a higher E2 level than male mice, and treatment with E2 in male mice can significantly reduce hepatic fibrosis induced by CCl4. Finally, stimulation by E2 can strongly induce the expression of miR-29a and miR-29b in IAR20 cells, indicating that the expression of miR-29a and miR-29b can be modulated by E2. Interestingly, the induction of miR-29a and miR-29b expression by E2 is opposite to the effect of TGF-β1, a well known fibrosis inducer, which reduces the levels of miR-29a and miR-29b. Together, these results demonstrate the association between the expression of miR-29 and the protective effect of E2 in inhibiting CCl4-induced mouse liver fibrosis.
Further analysis suggests that the promotion of miR-29 expression by E2 treatment may be through suppressing the NF-κB signal pathway. In contrast to TGF-β1, which enhances the level of NF-κB p65 protein in nuclei of IAR20 cells, E2 treatment significantly reduces the level of nuclear NF-κB p65 (Fig. 3). The inverse relationship between the NF-κB activity and the expression of miR-29a/b also confirms the previous report that the NF-κB pathway negatively regulates miR-29 expression (31).
The ability of miR-29 family members (mainly miR-29a and miR-29b) to target collagen (14–16), a major component of the extracellular matrix, during hepatic fibrogenesis has been widely reported. Collagen proteins are synthesized by many cell types, but primarily by mesenchymal cells, including fibroblasts. Following tissue injury, fibroblasts differentiate into myofibroblasts and, often, persistent activation of the myofibroblast phenotype, accompanied by a resistance to apoptosis, ensures the development of fibrosis (37). At the cellular level, the overexpression of miR-29 has been demonstrated to decrease the content of collagens observed in HSCs and to block the process of the development of fibrosis. Our results in a mouse model confirmed the anti-fibrogenic role of miR-29. At the early stages of CCl4 treatment, female mice exhibited a higher level of liver miR-29a and miR-29b than did male mice, which correlated with the lower degree of liver fibrosis observed in female mice than in male mice. Directly elevating miR-29 levels in mouse livers through the administration of Ad-miR-29a/b significantly attenuated the fibrotic damage observed in mouse livers during CCl4 treatment. In addition, our results suggest that miR-29 serves as a common downstream target of several profibrotic or antifibrotic mediators. As depicted in Fig. 2, treatment with E2, a fibrosis suppressor, strongly induced the expression of miR-29a and miR-29b in murine liver cells, whereas TGF-β1, a fibrosis inducer, reduced the levels of miR-29a and miR-29b. A recent study by Roderburg et al. (16) showed that various upstream signals, including TGF-β1 and LPS, reduce the levels of miR-29 expression during liver fibrosis, suggesting that the regulatory network downstream of the inflammatory signals involved in liver fibrosis is more complex than the linear TGF-β1/miR-29/collagen cascade. By describing the involvement of miR-29 in the protective role of E2 in inhibiting CCl4-induced mouse liver fibrosis, our current study demonstrated that miR-29 is an essential modulator in the dynamic process of liver fibrosis. Supporting our finding that miR-29 is sensitive to E2, a previous study by Maillot et al. (28) suggested that estrogen signaling can induce extensive alterations in miRNA expression, and these altered miRNAs, such as miR-26a and miR-181a, play key roles in estrogen-dependent functions. At present, however, the mechanism underlying the regulation of miRNAs by E2, including miR-29, remains unknown and requires further study.
In summary, by employing a mouse model of CCl4-induced liver fibrosis, we demonstrated the protective role of miR-29 in inhibiting liver fibrosis in CCl4-treated mice. The enhanced expression of miR-29 in mouse livers by treatment with either E2 or directly administered Ad-miR-29a/b strongly attenuates the level of liver fibrotic damage, as indicated by the reduction of serum levels of ALT and AST, hepatic type I collagen content and areas observed to be positive for α-SMA. The identification of the induction of miR-29 expression in liver cells by E2 suggests a novel mechanism by which E2 serves as an antifibrotic modulator during liver fibrogenesis.
Supplementary Material
This work was supported by National Basic Research Program of China Grants 2012CB517603 and 2011CB504803863 and National Natural Science Foundation of China Grants 30871019, 30890044, and 30988003.

This article contains supplemental Figs. S1 and S2.
- AST
- aspartate aminotransferase
- ALT
- alanine aminotransferase
- HSC
- hepatic stellate cell
- miRNA
- microRNAs
- α-SMA
- α-smooth muscle actin
- Ad
- adenovirus.
REFERENCES
- 1. Domenicali M., Caraceni P., Giannone F., Baldassarre M., Lucchetti G., Quarta C., Patti C., Catani L., Nanni C., Lemoli R. M., Bernardi M. (2009) A novel model of CCl4-induced cirrhosis with ascites in the mouse. J. Hepatol. 51, 991–999 [DOI] [PubMed] [Google Scholar]
- 2. Akiyoshi H., Terada T. (1999) Centrilobular and perisinusoidal fibrosis in experimental congestive liver in the rat. J. Hepatol. 30, 433–439 [DOI] [PubMed] [Google Scholar]
- 3. Muriel P., Escobar Y. (2003) Kupffer cells are responsible for liver cirrhosis induced by carbon tetrachloride. J. Appl. Toxicol. 23, 103–108 [DOI] [PubMed] [Google Scholar]
- 4. Xu J. W., Gong J., Chang X. M., Luo J. Y., Dong L., Hao Z. M., Jia A., Xu G. P. (2002) Estrogen reduces CCL4-induced liver fibrosis in rats. World J. Gastroenterol. 8, 883–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yasuda M., Shimizu I., Shiba M., Ito S. (1999) Suppressive effects of estradiol on dimethylnitrosamine-induced fibrosis of the liver in rats. Hepatology 29, 719–727 [DOI] [PubMed] [Google Scholar]
- 6. Liu Q. H., Li D. G., Huang X., Zong C. H., Xu Q. F., Lu H. M. (2004) Suppressive effects of 17β-estradiol on hepatic fibrosis in CCl4-induced rat model. World J. Gastroenterol. 10, 1315–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shimizu I. (2003) Impact of oestrogens on the progression of liver disease. Liver Int. 23, 63–69 [DOI] [PubMed] [Google Scholar]
- 8. Shimizu I., Mizobuchi Y., Yasuda M., Shiba M., Ma Y. R., Horie T., Liu F., Ito S. (1999) Inhibitory effect of oestradiol on activation of rat hepatic stellate cells in vivo and in vitro. Gut 44, 127–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bartel D. P. (2004) MicroRNAs, genomics, biogenesis, mechanism, and function. Cell 116, 281–297 [DOI] [PubMed] [Google Scholar]
- 10. Ambros V. (2004) The functions of animal microRNAs. Nature 431, 350–355 [DOI] [PubMed] [Google Scholar]
- 11. Esquela-Kerscher A., Slack F. J. (2006) Oncomirs, microRNAs with a role in cancer. Nat. Rev. Cancer 6, 259–269 [DOI] [PubMed] [Google Scholar]
- 12. Guo C. J., Pan Q., Cheng T., Jiang B., Chen G. Y., Li D. G. (2009) Changes in microRNAs associated with hepatic stellate cell activation status identify signaling pathways. FEBS J. 276, 5163–5176 [DOI] [PubMed] [Google Scholar]
- 13. Guo C. J., Pan Q., Li D. G., Sun H., Liu B. W. (2009) miR-15b and miR-16 are implicated in activation of the rat hepatic stellate cell. An essential role for apoptosis. J. Hepatol. 50, 766–778 [DOI] [PubMed] [Google Scholar]
- 14. Maurer B., Stanczyk J., Jüngel A., Akhmetshina A., Trenkmann M., Brock M., Kowal-Bielecka O., Gay R. E., Michel B. A., Distler J. H., Gay S., Distler O. (2010) MicroRNA-29, a key regulator of collagen expression in systemic sclerosis. Arthritis Rheum. 62, 1733–1743 [DOI] [PubMed] [Google Scholar]
- 15. Cushing L., Kuang P. P., Qian J., Shao F., Wu J., Little F., Thannickal V. J., Cardoso W. V., Lu J. (2010) Am. J. Respir. Cell Mol. Biol. 45, 287–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roderburg C., Urban G. W., Bettermann K., Vucur M., Zimmermann H., Schmidt S., Janssen J., Koppe C., Knolle P., Castoldi M., Tacke F., Trautwein C., Luedde T. (2011) MicroRNA profiling reveals a role for miR-29 in human and murine liver fibrosis. Hepatology 53, 209–218 [DOI] [PubMed] [Google Scholar]
- 17. van Rooij E., Sutherland L. B., Thatcher J. E., DiMaio J. M., Naseem R. H., Marshall W. S., Hill J. A., Olson E. N. (2008) Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc. Natl. Acad. Sci. U.S.A. 105, 13027–13032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Imaoka M., Kato M., Tago S., Gotoh M., Satoh H., Manabe S. (2009) Effects of estradiol treatment and/or ovariectomy on spontaneous hemorrhagic lesions in the pancreatic islets of Sprague-Dawley rats. Toxicol. Pathol. 37, 218–226 [DOI] [PubMed] [Google Scholar]
- 19. Idogawa M., Sasaki Y., Suzuki H., Mita H., Imai K., Shinomura Y., Tokino T. (2009) A single recombinant adenovirus expressing p53 and p21-targeting artificial microRNAs efficiently induces apoptosis in human cancer cells. Clin. Cancer Res. 15, 3725–3732 [DOI] [PubMed] [Google Scholar]
- 20. Jiang X., Zhang Y., Hou D., Zhu L., Xu W., Ding L., Qi X., Sun G., Liu C., Zhang J., Zen K., Xiang Y., Zhang C. Y. (2010) 17β-Estradiol inhibits oleic acid-induced rat VSMC proliferation and migration by restoring PGC-1α expression. Mol. Cell. Endocrinol. 315, 74–80 [DOI] [PubMed] [Google Scholar]
- 21. He A., Zhu L., Gupta N., Chang Y., Fang F. (2007) Overexpression of micro ribonucleic acid 29, highly up-regulated in diabetic rats, leads to insulin resistance in 3T3-L1 adipocytes. Mol. Endocrinol. 21, 2785–2794 [DOI] [PubMed] [Google Scholar]
- 22. He T. C., Zhou S., da Costa L. T., Yu J., Kinzler K. W., Vogelstein B. (1998) A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. U.S.A. 95, 2509–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen W., Liu M., Jiao Y., Yan W., Wei X., Chen J., Fei L., Liu Y., Zuo X., Yang F., Lu Y., Zheng Z. (2006) Adenovirus-mediated RNA interference against foot-and-mouth disease virus infection both in vitro and in vivo. J. Virol. 80, 3559–3566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arias M., Sauer-Lehnen S., Treptau J., Janoschek N., Theuerkauf I., Buettner R., Gressner A. M., Weiskirchen R. (2003) Adenoviral expression of a transforming growth factor-beta1 antisense mRNA is effective in preventing liver fibrosis in bile-duct ligated rats. BMC Gastroenterol. 3, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. López-De León A., Rojkind M. (1985) A simple micromethod for collagen and total protein determination in formalin-fixed paraffin-embedded sections. J. Histochem. Cytochem. 33, 737–743 [DOI] [PubMed] [Google Scholar]
- 26. Berres M. L., Koenen R. R., Rueland A., Zaldivar M. M., Heinrichs D., Sahin H., Schmitz P., Streetz K. L., Berg T., Gassler N., Weiskirchen R., Proudfoot A., Weber C., Trautwein C., Wasmuth H. E. (2010) Antagonism of the chemokine CCl5 ameliorates experimental liver fibrosis in mice. J. Clin. Invest. 120, 4129–4140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Villa E., Baldini G. M., Pasquinelli C., Melegari M., Cariani E., Di Chirico G., Manenti F. (1988) Risk factors for hepatocellular carcinoma in Italy. Male sex, hepatitis B virus, non-A non-B infection, and alcohol. Cancer 62, 611–615 [DOI] [PubMed] [Google Scholar]
- 28. Maillot G., Lacroix-Triki M., Pierredon S., Gratadou L., Schmidt S., Bénès V., Roché H., Dalenc F., Auboeuf D., Millevoi S., Vagner S. (2009) Widespread estrogen-dependent repression of microRNAs involved in breast tumor cell growth. Cancer Res. 69, 8332–8340 [DOI] [PubMed] [Google Scholar]
- 29. Dai R., Phillips R. A., Karpuzoglu E., Khan D., Ahmed S. A. (2009) Estrogen regulates transcription factors STAT-1 and NF-κB to promote inducible nitric-oxide synthase and inflammatory responses. J. Immunol. 183, 6998–7005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Murphy A. J., Guyre P. M., Pioli P. A. (2010) Estradiol suppresses NF-κB activation through coordinated regulation of let-7a and miR-125b in primary human macrophages. J. Immunol. 184, 5029–5037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mott J. L., Kurita S., Cazanave S. C., Bronk S. F., Werneburg N. W., Fernandez-Zapico M. E. (2010) Transcriptional suppression of mir-29b-1/mir-29a promoter by c-Myc, hedgehog, and NF-κB. J. Cell. Biochem. 110, 1155–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jacobs M. D., Harrison S. C. (1998) Structure of an IκBα/NF-κB complex. Cell 95, 749–758 [DOI] [PubMed] [Google Scholar]
- 33. Yamamoto Y., Gaynor R. B. (2001) Therapeutic potential of inhibition of the NF-κB pathway in the treatment of inflammation and cancer. J. Clin. Investig. 107, 135–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kota J., Chivukula R. R., O'Donnell K. A., Wentzel E. A., Montgomery C. L., Hwang H. W., Chang T. C., Vivekanandan P., Torbenson M., Clark K. R., Mendell J. R., Mendell J. T. (2009) Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell 137, 1005–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pinzani M., Romanelli R. G., Magli S. (2001) Progression of fibrosis in chronic liver diseases. Time to tally the score. J. Hepatol. 34, 764–767 [DOI] [PubMed] [Google Scholar]
- 36. Friedman S. L. (2000) Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J. Biol. Chem. 275, 2247–2250 [DOI] [PubMed] [Google Scholar]
- 37. Bataller R., Brenner D. A. (2005) Liver fibrosis. J. Clin. Investig. 115, 209–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bissell D. M. (1999) Sex and hepatic fibrosis. Hepatology 29, 988–989 [DOI] [PubMed] [Google Scholar]
- 39. Hellerbrand C., Hartmann A., Richter G., Knöll A., Wiest R., Schölmerich J., Lock G. (2001) Hepatocellular carcinoma in southern Germany. Epidemiological and clinicopathological characteristics and risk factors. Dig. Dis. 19, 345–351 [DOI] [PubMed] [Google Scholar]
- 40. Järveläinen H. A., Lukkari T. A., Heinaro S., Sippel H., Lindros K. O. (2001) The antiestrogen toremifene protects against alcoholic liver injury in female rats. J. Hepatol. 35, 46–52 [DOI] [PubMed] [Google Scholar]
- 41. Porter L. E., Elm M. S., Van Thiel D. H., Dugas M. C., Eagon P. K. (1983) Characterization and quantitation of human hepatic estrogen receptor. Gastroenterology 84, 704–712 [PubMed] [Google Scholar]
- 42. Eagon P. K., Francavilla A., DiLeo A., Elm M. S., Gennari L., Mazzaferro V., Colella G., Van Thiel D. H., Strazl T. E. (1991) Quantitation of estrogen and androgen receptors in hepatocellular carcinoma and adjacent normal human liver. Dig. Dis. Sci. 36, 1303–1308 [DOI] [PubMed] [Google Scholar]
- 43. Villa E., Camellini L., Dugani A., Zucchi F., Grottola A., Merighi A., Buttafoco P., Losi L., Manenti F. (1995) Variant estrogen receptor messenger RNA species detected in human primary hepatocellular carcinoma. Cancer Res. 55, 498–500 [PubMed] [Google Scholar]
- 44. Giannitrapani L., Soresi M., La Spada E., Cervello M., D'Alessandro N., Montalto G. (2006) Sex hormones and risk of liver tumor. Ann. N.Y. Acad. Sci. 1089, 228–236 [DOI] [PubMed] [Google Scholar]
- 45. Becker U., Deis A., Sørensen T. I., Grønbaek M., Borch-Johnsen K., Müller C. F., Schnohr P., Jensen G. (1996) Prediction of risk of liver disease by alcohol intake, sex, and age. A prospective population study. Hepatology 23, 1025–1029 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.