Background: TRAIL can induce both apoptotic and prosurvival signaling pathways.
Results: In the apoptotic pathway, proteasome inhibition can impair caspase-8 activation, particularly at submaximal TRAIL doses.
Conclusion: TRAIL/proteasome inhibitor synergies cannot be explained by enhanced caspase-8 activation through the apoptosis signaling branch.
Significance: Inhibition of nonapoptotic signaling may be the major upstream contributor to TRAIL/proteasome inhibitor synergies.
Keywords: Apoptosis, Caspase, Cell Death, Signal Transduction, Systems Biology, Förster Resonance Energy Transfer (FRET), Tumor Necrosis Factor-related Apoptosis Inducing Ligand (TRAIL), Bortezomib, Caspase-8, Proteasome Inhibition
Abstract
Tumor necrosis factor-related apoptosis inducing ligand (TRAIL) can induce extrinsic apoptosis, resulting in caspase-8 activation, but may also initiate transcription-dependent prosurvival signaling. Proteasome inhibitors were suggested to promote TRAIL signal transduction through the death-inducing signaling complex (DISC) by modulating the relative abundance of core DISC components, thereby enhancing caspase-8 activation and apoptosis. To test this hypothesis, we quantified the changes in DISC protein levels as an early consequence of proteasome inhibition in HeLa cervical cancer cells and, based on these data, mathematically modeled the proapoptotic TRAIL signaling toward caspase-8 activation. Modeling results surprisingly suggested that caspase-8 activation might be delayed in presence of proteasome inhibitors, in particular at submaximal TRAIL doses. Subsequent FRET-based single cell time-lapse imaging at conditions where transcription dependent prosurvival signaling was blocked confirmed this hypothesis: caspase-8 activity was delayed by hours in the presence of proteasome inhibitors epoxomicin or bortezomib. Corresponding delays were detected for effector caspase processing and cell death. Contrary to current models, we therefore provide evidence that synergies between TRAIL and proteasome inhibitors do not result from changes in the levels of core DISC signaling proteins.
Introduction
The proteasome is the main cellular machinery for extralysosomal protein degradation. Together with gene transcription and translation, proteasomal activity therefore is a key determinant of cellular steady-state protein levels. Physiological modulation of proteasomal activity broadly affects the composition of the intracellular proteome and contributes to regulating numerous processes, including cell proliferation, differentiation, survival, and apoptotic cell death (1, 2). Prolonged proteasome inhibition results in the expression of BH3-only proteins such as Puma, Bim, Noxa, or Bik, which promote apoptosis via the intrinsic pathway (2–4). Proteasome inhibitors also were shown to synergistically enhance apoptosis induced by various classes of proapoptotic drugs, including tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)3 (5–7).
Apoptosis via the TRAIL pathway requires TRAIL binding to its cognate death receptors TRAIL-R1 and -R2, receptor trimerization, and cytosolic association of the adaptor protein Fas-associated death domain (FADD). Binding to FADD via death effector domains, procaspase-8 is recruited into the death-inducing signaling complex (DISC), and close proximity promotes the dimerization, autocatalytic processing, and activation of caspase-8 (8). The short splice variant of the enzymatically inactive caspase-8 homolog cFLIP (cFLIPS) as well as high expression of the long cFLIP variant (cFLIPL) compete with procaspase-8 for binding to the DISC and thereby inhibit caspase-8 activation (9, 10). Caspase-8 cleaves the BH3-only protein Bid, and truncated Bid induces activating conformational changes in Bax and Bak. If the amounts of truncated Bid and active Bax/Bak are sufficient to neutralize the pool of anti-apoptotic Bcl-2 family members, Bax/Bak pores can form and lead to mitochondrial outer membrane permeabilization (11). Mitochondrial outer membrane permeabilization is followed by cytochrome c release into the cytosol, mitochondrial depolarization, activation of effector caspases, and apoptotic cell death (11–13). Through the formation of a signaling complex secondary to the DISC, TRAIL additionally can also promote nonapoptotic, prosurvival signaling that depends on kinase signaling cascades and protein neosynthesis driven by transcription factors such as c-Jun and NFκB (14).
The mechanistic reasons for synergies between TRAIL and proteasome inhibitors are still investigated intensely. The relative abundances of signaling proteins and thereby the quantitative composition of the TRAIL signaling network are immediately subjected to complex alterations by proteasome inhibition, especially because short-lived protein species may accumulate rapidly. As extrinsic apoptosis initiation follows a mostly linear signaling sequence (15), altered quantities in any of the involved proteins have a high likelihood to affect the kinetics of signal transduction. Therefore, the reasons for synergistic responses to TRAIL/proteasome inhibitor combinations may be complex, and it is questionable whether they should or can be attributed to changes in an individual target protein, such as elevated amounts of TRAIL receptors (16, 17), without analyzing the signaling network of apoptosis initiation as a whole.
Here, we determined the immediate influence of proteasome inhibition on the quantitative composition of the apoptotic TRAIL signaling network and investigated the consequences on caspase-8 activation. To this end, we employed a multidisciplinary systems level approach that builds on biochemical measurements of protein abundances, mathematical systems modeling, and advanced quantitative single-cell time-lapse imaging.
EXPERIMENTAL PROCEDURES
Materials
Bortezomib was from Millennium Pharmaceuticals (Cambridge, MA). Epoxomicin, cycloheximide (CHX), and propidium iodide were from Sigma. Human recombinant TRAIL was prepared and provided by Carlos Ricardo Rodrigues dos Reis, University of Groningen. TMRM was from MobiTec (Göttingen, Germany).
Cell Culture and Transfection
HeLa cells were cultured in RPMI 1640 medium supplemented with penicillin (100 units/ml), streptomycin (100 μg/ml), and 10% fetal calf serum (Sigma) at 37 °C and 5% CO2. HeLa cells expressing the IETD FRET probe were described previously (18). Plasmid transfection (pRSC; pRSC-TRAIL-R2 (19); pcDNA6.1; pcDNA6.1-cFLIPL (8)) was conducted using LipofectamineTM 2000 (Invitrogen) following the manufacturer's instructions.
Proteasome Activity Assay
Chymotrypsin-like proteasome activity was measured using a fluorigenic peptide N-succinyl-Leu-Leu-Val-Tyr-7-amino-4-methyl-coumarin (suc-LLVY-AMC) (Calbiochem, Nottingham, UK). Cells were treated with proteasome inhibitors for the indicated times and were lysed using lysis buffer (10 mm HEPES, 42 mm KCl, 5 mm MgCl2, 0.1 mm EDTA, 0.1 mm EGTA, 1 mm DTT, 0.5% (w/v) CHAPS). Lysates were incubated with reaction buffer (25 mm HEPES, pH 7.4, 0.5 mm EDTA, pH 8) containing 20 μm suc-LLVY-AMC. Using 360 nm excitation, fluorescence was measured at 465 nm and 37 °C using a plate reader with the appropriate filters (GENios, Tecan, Weymouth, UK). Fluorescence signals were normalized to the protein concentrations, which were determined by Bradford assay (Thermo Fisher Scientific, Dublin, Ireland) and related to untreated, autofluorescence-corrected controls (100% activity). Fitting of data to exponential decay functions was performed using Microcal Origin software.
Western Blotting
Whole cell extracts, SDS gel electrophoresis, and blotting were prepared or conducted as described previously (20). Membranes were incubated with the following antibodies: a mouse monoclonal β-actin antibody (Sigma), a rabbit polyclonal APAF-1 antibody (BD Transduction Laboratories), a rabbit polyclonal Bax antibody (Upstate Biotechnology, Dundee, Scotland), a mouse monoclonal Bak antibody (Santa Cruz Biotechnology), a mouse monoclonal Bcl-2 antibody (Santa Cruz Biotechnology), a rabbit polyclonal Bcl-w antibody (Stressgen, Kampenhout, Belgium), a rabbit polyclonal Bcl-xL antibody (BD Transduction Laboratories), a goat polyclonal Bid antibody (R&D Systems, Abingdon, UK), a mouse monoclonal Noxa antibody (Stratagene), a rabbit polyclonal caspase-3 antibody (Cell Signaling Technology, Danvers, MA), a mouse monoclonal caspase-8 antibody (Alexis Biochemicals, San Diego, CA), a rabbit polyclonal caspase-9 antibody (Calbiochem/Merck Bioscience, Nottingham, UK), a mouse monoclonal cytochrome c antibody (BD Transduction Laboratories), a mouse monoclonal FADD antibody (BD Transduction Laboratories), a mouse monoclonal cFLIP antibody (Alexis Biochemicals), a mouse monoclonal Mcl-1 antibody (BD Transduction Laboratories), a rabbit polyclonal SMAC/Diablo antibody (R&D Systems), a rabbit polyclonal TRAIL-R1 antibody (Abcam, Cambridge, UK), a rabbit polyclonal TRAIL-R2 antibody (Abcam), a mouse monoclonal XIAP antibody (BD Transduction Laboratories), and respective IgG peroxidase-conjugated secondary antibodies (Millipore) were used. Densitometry was performed as described previously (21). Signals were corrected for background luminescence and loading and scaled against untreated controls to obtain fold changes in protein amounts.
Fluorescence Microscopy and Digital Imaging
Cells were grown on 22-mm glass-bottomed dishes (Willco BV, Amsterdam, The Netherlands) in 1 ml of medium for at least overnight to let them attach firmly. Cells were equilibrated with 30 nm TMRM in 1 ml of RPMI 1640 medium supplemented with penicillin (100 units/ml), streptomycin (100 μg/ml), and 10% fetal calf serum, buffered with HEPES (10 mm; pH 7.4) and covered with mineral oil. Cells were pretreated with 50 nm epoxomicin or 100 nm bortezomib for 2.5 h before placing the dish in a heated (37 °C) incubation chamber that was mounted on the microscope stage. After 30 min of temperature equilibration, cells were treated on stage with TRAIL plus 1 μg/ml CHX. Fluorescence was observed using an Axiovert 200 m inverted microscope (Carl Zeiss, Jena, Germany) equipped with a 40× numerical aperture 1.3 oil immersion objective, using optimized mirrors and filter sets (Semrock, Rochester, NY) for the individual fluorescence channels. The microscope was equipped with a cooled EM CCD camera (Andor Ixon BV 887-DCS, Andor Technologies, Belfast, Northern Ireland). After background subtraction, the cellular TMRM fluorescence intensities were determined, and caspase activities were detected at the single-cell level by FRET analysis as described previously (18). Images were processed using MetaMorph software (version 7.1r1, Molecular Devices, Ltd., Wokingham, UK).
Flow Cytometry
Cell death was assessed by propidium iodide staining and flow cytometry. Briefly, cells were detached and incubated with propidium iodide (2 μg/ml) at room temperature for 5 min. Propidium iodide was excited with a 561 nm laser line, and fluorescence emission was collected through a 605/40-nm band-pass filter and a 570-nm long pass filter; at least 5,000 events were acquired for each sample. Data were analyzed using Cyflogic software (CyFlo Ltd., Turku, Finland).
Statistics
Mann-Whitney U tests were used when analyzing non-normal distributed data. Bonferroni correction was employed on series of tests to yield an overall α-level of 0.05 for the test family and to reduce the probability of false positive results. Analyses were performed using SPSS 15 (Lead Technologies, Inc.).
Model Implementation
The mathematical model was implemented as a set of ordinary differential equations in MATLAB (The Mathworks) for numerical analysis. A comprehensive model description is provided as supplemental information 1. The respective MATLAB code is available as supplemental information 2.
RESULTS
Consequences of Proteasome Inhibition on Steady-state Levels of Proteins Involved in TRAIL-induced Apoptosis Signaling
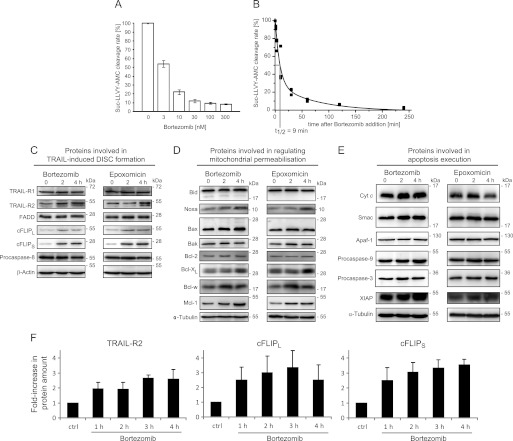
Bortezomib and epoxomicin are highly specific proteasome inhibitors and were used here to investigate the early consequences of proteasome inhibition on the relative composition of the TRAIL-induced apoptosis signaling network. Bortezomib, added at concentrations that resulted in high (>90%) and rapid (t½ = 9 min) proteasome inhibition (Fig. 1, A and B), caused a strong accumulation of TRAIL-R2 as well as cFLIPL and cFLIPS in HeLa cells within 4 h (Fig. 1C). In contrast, the amounts of TRAIL-R1, FADD, and procaspase-8 did not change notably (Fig. 1C). Similarly, the levels of Bcl-2 family members Bid, Bax, Bak, and Bcl-2 remained unchanged, with the exception of increases in Bcl-xL, Bcl-w, and, most pronouncedly, Mcl-1 and Noxa (Fig. 1D). No significant changes in the amounts of key proteins involved in apoptosis execution could be observed during this time (Fig. 1E). Comparable results were obtained with the similarly potent but irreversible proteasome inhibitor epoxomicin (Fig. 1, C–E). In additional experiments, we found that proteins that strongly increased (TRAIL-R2, cFLIP species, Mcl-1, and Noxa) are turned over rapidly in HeLa cells (supplemental Fig. 1). These results therefore indicate that short-lived proteins accumulate rapidly as an early response to proteasome inhibition. Because in the following, we were interested in investigating the consequences of proteasome inhibition on the kinetics of caspase-8 activation, we quantified the accumulation of TRAIL-R2 and cFLIP species in greater detail. We found that within 2–3 h both TRAIL-R2 and cFLIP amounts increased 3-fold in response to bortezomib (Fig. 1F). Similar responses were observed when treating cells with epoxomicin (data not shown). Because pro- and antiapoptotic proteins accumulate in parallel, it is not possible to predict whether proteasome inhibition would promote or inhibit TRAIL-induced caspase-8 activation and subsequent apoptosis signal transduction.
FIGURE 1.
Biochemical analysis of the impact of proteasome inhibition on the protein composition of the apoptotic TRAIL-signaling pathway in HeLa cells. A, HeLa cells were treated with the indicated concentrations of bortezomib for 4 h, and chymotrypsin-like proteasome activity was measured by cleavage of Suc-LLVY-AMC. Maximal inhibition was achieved with doses ≥100 nm. Remaining residual Suc-LLVY-AMC cleavage was likely due to other enzymes or substrate hydrolysis. Data are shown as mean ± S.E. from eight samples pooled from two independent experiments. B, HeLa cells were treated with 100 nm bortezomib, and Suc-LLVY-AMC cleavage rates were fitted to a biphasic exponential decay function following background subtraction. Data were pooled from n = 3 independent experiments with triplicates. C–E, whole cell extracts of HeLa cells treated with 100 nm bortezomib or 50 nm epoxomicin for the indicated times were analyzed by immunoblotting. β-Actin or α-tubulin served as loading controls. Experiments were repeated with similar results. C, proteins involved in DISC formation and caspase-8 activation. D, proteins regulating mitochondrial outer membrane permeabilization. E, proteins involved in apoptosis execution. F, quantitative analysis of TRAIL-R2 and cFLIP species accumulation in response to 100 nm bortezomib. Protein amounts were quantified by immunoblot densitometry. Data are means + S.E. from n = 4 independent experiments.
Modeling of TRAIL-induced Apoptosis Initiation Suggests That Proteasome Inhibition May Significantly Delay Caspase-8 Activation at Submaximal Stimulation of Death Receptors
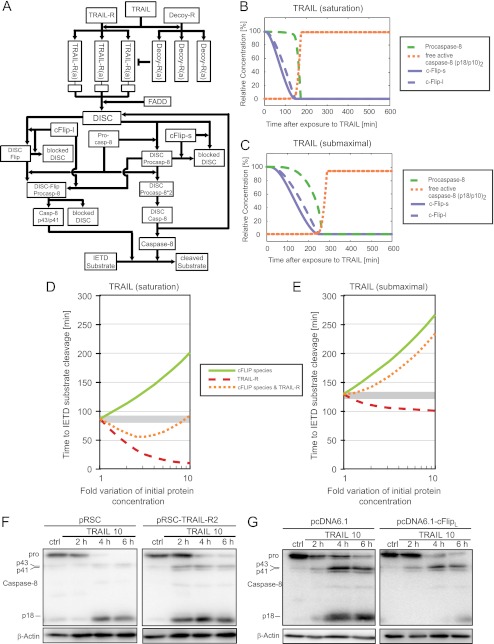
Given that both pro- and antiapoptotic DISC proteins accumulate in parallel as an early response to proteasome inhibition, it is unclear whether and how the kinetics of apoptosis initiation may be affected. In addition, TRAIL-induced signaling is complex, even when only considering the proapoptotic branch that results in DISC formation and caspase-8 activation. We therefore first investigated the signaling network of apoptosis initiation by TRAIL in silico using a system biology approach: we generated a mathematical model composed of ordinary differential equations and parameterized it with the initial concentrations of the individual reactants and the rate constants for the respective reactions. A simplified schematic of the reaction network is depicted in Fig. 2A, and a detailed biological justification for the model architecture, its parameterization, and training for control conditions in HeLa cells, and a description of the mathematical approach taken is provided as supplemental information 1. This model allowed to simulate the signaling kinetics while taking the complexity of the network into account and thereby to generate testable hypotheses on how proteasome inhibition may affect the kinetics of caspase-8 activation. Similar modeling approaches previously were used successfully to gain detailed insight into other facets of complex apoptotic signaling processes (10, 15, 21, 22).
FIGURE 2.
Systems modeling and sensitivity analysis of the apoptotic TRAIL signaling network in HeLa cervical cancer cells. A, the biochemical reactions depicted in the reaction network were mathematically modeled using ordinary differential equations. This allowed to calculate signal transduction kinetics and to perform analyses on the systems responsiveness to perturbations. In particular, the model was used to evaluate how the time required to translate TRAIL exposure into caspase-8 activation is influenced by single or combined perturbations in cFLIP and TRAIL-R levels. A detailed description of the model implementation is provided as supplemental information 1. B and C, signaling kinetics of TRAIL induced apoptosis initiation in response to saturating (B) or submaximal (C) TRAIL doses, respectively. D and E, response sensitivity of the signaling network when stimulated with saturating (D) or submaximal (E) TRAIL doses, respectively. Shown are the times required to initiate the cleavage of caspase-8 substrates following single perturbations in the amounts of TRAIL receptors and cFLIP species or parallel increases in both TRAIL receptors and cFLIP species. F, whole cell extracts of HeLa cells transfected with empty vector or a TRAIL-R2 expression plasmid were analyzed for procaspase-8 processing. Cells were treated with 10 ng/ml TRAIL plus 1 μg/ml CHX for the indicated times. β-Actin served as loading control. G, as in F, empty vector-transfected or cFLIPL overexpressing HeLa cells were analyzed for procaspase-8 processing.
When stimulating the systems model with high, saturating concentrations of TRAIL, the pools of free cFLIPS,L were rapidly depleted, whereas procaspase-8 was converted into fully processed, active caspase-8 only at a later time (Fig. 2B). This corresponds with the faster DISC-binding kinetics for cFLIP species that were reported previously (15). Qualitatively, these signaling features were maintained when stimulating the systems model with low doses of TRAIL, yet signaling proceeded with slower kinetics (Fig. 2B). The model therefore reflected core features of TRAIL-induced apoptosis signaling and caspase-8 activation with sufficient accuracy. We next used the model to separately assess the influence of TRAIL-R2 and cFLIP on the time required for caspase-8 activation. At high TRAIL doses, increasing the amount of TRAIL receptors resulted in an acceleration of caspase-8 activation (Fig. 2D). Reductions correspondingly resulted in delays (data not shown). Modifying cFLIP levels instead resulted in reciprocal responses (Fig. 2D). At submaximal TRAIL doses, the simulations predicted delays upon cFLIP increases that were qualitatively similar to those seen in the saturation scenario, while increasing TRAIL receptor levels slightly accelerated apoptosis initiation (Fig. 2E). The influence of altered TRAIL-R2 or cFLIP amounts on apoptosis signal transduction in HeLa cells also was confirmed experimentally. Overexpression of TRAIL-R2 accelerated, whereas elevating the amounts of cFLIPL delayed caspase-8 processing, as evidenced by earlier or later detection of the p18 caspase-8 subunit (Fig. 2, F and G).
When up-regulating TRAIL receptor and cFLIP levels in parallel, as observed experimentally in response to proteasome inhibition (see Fig. 1), our mathematical simulations instead suggested that the effects of TRAIL receptor and cFLIP level changes were reduced significantly at saturating TRAIL concentrations: the time required for caspase-8 activation and substrate cleavage deviated only mildly from that at control conditions (Fig. 2D). However, this was not the case for submaximal TRAIL doses. Here, our mathematical simulations predicted a significant delay in caspase-8 activation upon combined modulation of TRAIL receptors and cFLIP (Fig. 2E). On the systems level, this behavior is explained by the submaximal TRAIL doses that cannot efficiently take advantage of the additional amount of death receptors that accumulate upon proteasome inhibition. In the following, we therefore set out to investigate whether these in silico predictions could be validated experimentally.
Proteasome Inhibition Delays Caspase-8 Activation in Response to Submaximal TRAIL Doses in HeLa Cervical Cancer Cells
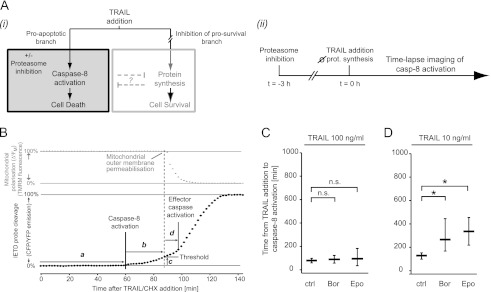
To experimentally test the predictions generated by systems modeling, we measured caspase-8 activation and activity at the single cell level using time-lapse imaging. HeLa cells were pretreated with proteasome inhibitors bortezomib or epoxomicin and were then exposed to TRAIL at high or submaximal doses. Proteasome inhibition has been described to also antagonize TRAIL-induced transcription/translation-dependent survival signaling and thereby can affect the efficiency of apoptosis initiation (23, 24). We therefore had to establish conditions where this contribution was suppressed to investigate the consequences of proteasome inhibitor-induced accumulation of TRAIL-R2 and cFLIP on DISC signaling kinetics and caspase-8 activation (see scheme in Fig. 3A). To this end, we added translation inhibitor CHX at all conditions investigated. Although CHX can reduce TRAIL-R2 and cFLIP levels in absence of proteasome inhibition (supplemental Fig. 1), this experimental design allowed to compare scenarios in which cFLIP/TRAIL-R2 levels are either normal/low (TRAIL/CHX condition) or elevated due to proteasome inhibition (TRAIL/CHX + proteasome inhibition). This permitted us to investigate the systems modeling hypothesis that caspase-8 activation kinetics are affected selectively at submaximal TRAIL doses. CHX addition also reduces active stress responses to proteasome inhibition, which may manifest at later times (e.g. the transcriptional induction of BH3-only proteins), were suppressed and did not interfere with the analysis of signal transduction in these experiments. At the concentration used (1 μm), CHX alone does not cause any detectable amount of cell death in HeLa cells within 24 h of treatment (18).
FIGURE 3.
Consequences of proteasome inhibition on the kinetics of TRAIL-induced caspase-8 activation. A, panel i, schematic of the experimental conditions that needed to be established to investigate caspase-8 (casp-8) activation without interference from parallel prosurvival signaling; panel ii, schematic of the experimental time line. Proteasome inhibition preceded TRAIL addition, and imaging of IETD FRET probe cleavage commenced upon addition of TRAIL. B, schematic presentation of parameters that can be quantified by time-lapse imaging of IETD FRET probe cleavage. Probe cleavage displays as an increase in the CFP/YFP emission ratio (black). Changes in the mitochondrial membrane potential (ΔΨM) as observed by TMRM fluorescence intensity are shown in gray. The following parameters can be determined for individual cells: the time from TRAIL addition to caspase-8/10 activation (a); the time from caspase-8/10 activation to mitochondrial outer membrane permeabilization (b); the percentage of cleaved substrate at the time of mitochondrial engagement (c); the time from mitochondrial engagement to effector caspase activation (d). C, quantification and comparison of the time required from TRAIL addition to caspase-8 activation for the shown treatment conditions. Proteasome inhibition significantly delayed caspase-8 activation at submaximal TRAIL doses. Data are from n = 33–73 cells per group and shown as median ± one quartile. An asterisk indicates significant difference (p < 0.01, Bonferroni-corrected Mann-Whitney U tests). ctrl, control; n.s., not significant. Bor, bortezomib; Epo, epoxomicin.
For single cell imaging, we employed a FRET-based approach that allows to measure the signaling kinetics of apoptosis initiation in response to death receptor stimulation (18). The FRET probe is a recombinantly expressed cyan-yellow fluorescent fusion protein containing an IETD site (CFP-IETD-Venus). Probe cleavage results in an increase in the CFP/YFP emission ratio and time-lapse data on cellular CFP/YFP emission ratios allow multiple parameters to be determined for individual cells (Fig. 3B). Most important for our study, the time from death ligand addition until caspase-8 activation can be determined from the lag time between TRAIL addition and the onset of FRET substrate cleavage (a). In addition, we also determined the time between caspase-8 activation and apoptotic mitochondrial engagement (b) in the subsequent experiments. The amount of IETD FRET substrate cleaved at the time of mitochondrial engagement can serve as a measure for the decision threshold for subsequent apoptosis execution (c). Following a short post-mitochondrial delay (d), the cleavage rate of the IETD FRET substrate strongly increases due to activation of effector caspases, which contribute to IETD probe cleavage as well (18).
In control cells that were not pretreated with proteasome inhibitors, caspase-8 was activated on average after 88 min at high TRAIL doses. This lag time was further extended by ∼40 min at submaximal TRAIL doses (compare control groups in Fig. 3, C and D), corresponding to previous findings (18). In cells pretreated with proteasome inhibitors bortezomib or epoxomicin for 2.5 h, a higher intercellular variability was observed; however, on average, the time from TRAIL addition to caspase-8 activation did not change significantly at high TRAIL doses (Fig. 3C). In stark contrast, caspase-8 activation was significantly delayed (137 min for bortezomib; 207 min for epoxomicin) at submaximal TRAIL doses (Fig. 3C). Both experimental data and modeling predictions therefore are in agreement and have identified TRAIL dose-dependent functional consequences of proteasome inhibition on the kinetics of apoptosis initiation.
Additional experimental readouts that extend beyond the predictive capacity of the systems model are presented in supplemental Fig. 2: at high TRAIL doses, a pretreatment with proteasome inhibitors resulted in caspase-8 activity that have to persist for longer until apoptotic mitochondrial engagement, and the threshold for mitochondrial outer membrane permeabilization induction correspondingly was elevated slightly (supplemental Fig. 2A). This suggests that mild delays in apoptosis signal transduction may nevertheless occur at high TRAIL doses. However, these delays were found downstream of the DISC and caspase-8 activation. Post-DISC delays could not be observed at submaximal TRAIL doses; however, the higher cell-to-cell variability at these conditions (as indicated by bigger interquartile ranges) may have masked similar trends (supplemental Fig. 2B). Taken together, these results therefore confirm the prediction generated by systems modeling (Fig. 2) that the onset of caspase-8 activation is delayed significantly, particularly at submaximal TRAIL doses when cells are briefly exposed to proteasome inhibitors.
Delayed Caspase Processing and Cell Death at Submaximal TRAIL Doses in HeLa Cells Pretreated with Proteasome Inhibitors
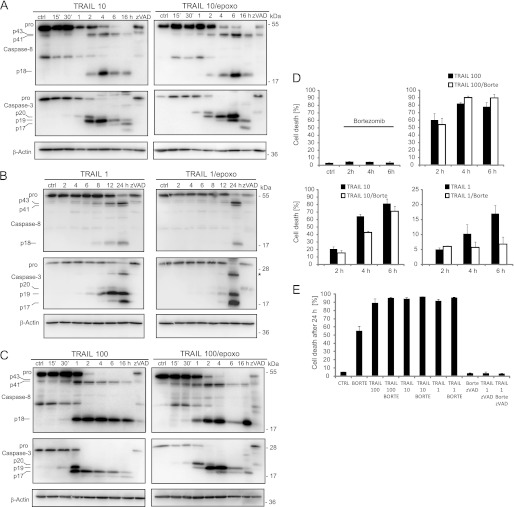
We next performed additional analyses to validate whether the results obtained by systems modeling and time-lapse imaging also correlate with delays in caspase processing and cell death. Following the treatment schedule shown in Fig. 3A, we indeed found that caspase-8 and -3 processing into active subunits was delayed significantly when HeLa cells were pretreated with epoxomicin before addition of TRAIL at submaximal doses (Fig. 4, A and B). In contrast, this effect was far less pronounced when using TRAIL at very high concentrations (Fig. 4C). Comparable results were obtained when conducting these experiments with proteasome inhibitor bortezomib (data not shown). Caspases processing by bortezomib or epoxomicin alone was negligible during the first 24 h of treatment (supplemental Fig. 3A). Delays in caspase processing at submaximal TRAIL doses correlated with alterations in cell death (Fig. 4D): in cells pretreated with bortezomib, cell death was delayed significantly at submaximal doses of TRAIL, when measuring propidium iodide uptake during the first 6 h after TRAIL addition (Fig. 4D). Bortezomib/CHX co-treatments did not result in cell death during these times (data not shown). Corresponding to the caspase processing seen late after treatment with 1 ng/ml TRAIL/epoxomicin (Fig. 4B, 24-h time point), end point measurements of cell death indicated that apoptosis eventually is induced at late times (Fig. 4E). Taken together, these findings therefore further confirm that as an early consequence of proteasome inhibition signal transduction toward caspase-8 activation in the apoptotic branch of the TRAIL pathway is impaired and delayed at submaximal TRAIL doses.
FIGURE 4.
Delayed caspase processing and cell death at low TRAIL doses in HeLa cells pretreated with proteasome inhibitors. A–C, procaspase-8 and -3 processing was determined by immunoblotting of whole cell lysates of HeLa cells. Cells were treated with 10 ng/ml (A), 1 ng/ml (B), or 100 ng/ml TRAIL (C) together with 1 μg/ml CHX. Where indicated, cells were pretreated with 50 nm epoxomicin (epoxo) for 3 h. Addition of 50 μm pan-caspase inhibitor Z-VAD-fmk blocked caspase processing in all scenarios. β-Actin served as loading control. D, cell death, as determined by propidium iodide uptake, was measured by flow cytometry. Cells were treated with 1, 10, or 100 ng/ml TRAIL together with 1 μg/ml CHX for the indicated times. Cells were pretreated with bortezomib (Borte; 100 nm) for 3 h, where indicated. Error bars show S.E. from n = 3 independent samples. Similar results were obtained in repeat experiments. E, end point (24 h) measurements of cell death (propidium iodide staining) for the treatment combinations described in D. Z-VAD controls indicate caspase dependence of cell death. ctrl, control.
DISCUSSION
Proteasome inhibitors are being investigated as clinically relevant sensitizers of cancer cells to combination therapies with various other drugs, including TRAIL (6, 7). In the current study, we quantitatively and kinetically analyzed signal transduction toward caspase-8 activation in the apoptotic branch of the TRAIL-induced signaling network in HeLa cervical cancer cells and investigated how signal transduction is modulated as an early consequence of proteasome inhibition. Our study is the first to systematically analyze the changes in all related core signaling proteins of apoptosis and to combine this information with both systems modeling and a well controlled experimental approach of quantitatively and kinetically measuring signal transduction of apoptosis initiation in individual living cells. Systems modeling predicted, and subsequent experiments confirmed, that changes in the relative abundance of signaling proteins, which result from proteasome inhibition, result in a significant delay in caspase-8 activation at submaximal TRAIL receptor stimulation. Our data therefore indicate that synergies reported for co-treatments of TRAIL and proteasome inhibitors cannot be explained by an altered abundance of DISC components.
We found that TRAIL-R2 protein levels increased in response to proteasome inhibition, corresponding to earlier reports in prostate, colon, lung, and bladder cancer cells (16, 17, 25). Likewise, cFLIP species accumulated rapidly, as was previously also observed in lung cancer and HeLa cells (25, 26). Other studies interestingly reported that cFLIP levels drop in leukemia cells as well as in prostate and renal cancer as a consequence of proteasome inhibition (27, 28). However, the latter studies investigated cFLIPL levels at very late times following proteasome inhibition (18–24 h). Long term proteasome inhibition induces complex cellular responses. First, prolonged proteasome inhibition additionally induces the production of BH3-only proteins (29) that not only further sensitize cells to TRAIL but by themselves can provoke apoptotic responses through the intrinsic pathway. Effector caspase activation may therefore result in cleavage and reduction of the amounts of full-length cFLIPL. Additionally, it was shown that autophagy and lysosomal protein degradation can be induced as a compensatory stress response to proteasome inhibition (4) and thereby may reduce cFLIP levels. In addition, we recently described an autophagy-dependent process of caspase-8 activation in response to long term proteasome inhibition in human cancer cells (20). This pathway may likewise contribute to the loss in cFLIPL through its cleavage within the caspase-8 activation complex and may contribute to synergistic responses to TRAIL.
We present the first mathematical systems model of TRAIL-induced caspase-8 activation. This model was applied successfully to generate testable hypotheses on the consequences of proteasome inhibition on apoptosis initiation. It therefore extends the available repertoire of predictive, experimentally validated mathematical models that cover different aspects of apoptosis signal transduction (10, 15, 21, 22, 30, 31). The model does not yet contain components of the still insufficiently characterized prosurvival signaling branch that can be activated in TRAIL-stimulated cells. Nonapoptotic TRAIL signaling was reported to depend on a complex that may form secondary to the DISC, and this complex triggers the activation of multiple kinases (14). These include inhibitor of κB kinase (IKK), c-Jun N-terminal kinase, and p38 mitogen-activated protein kinase, and their activation provokes transcriptional prosurvival responses (14). This signaling branch therefore seems related to nonapoptotic responses induced by other tumor necrosis factor family ligands such as tumor necrosis factor α and death ligand CD95L (32, 33). As a first step toward a systems analysis of TRAIL-induced apoptosis initiation, it was therefore necessary to restrict ourselves to experimental scenarios where protein neosynthesis and therefore prosurvival responses were inhibited. This allowed direct comparability of model predictions and experimental measurements of individual cell responses. A first systems model for CD95-induced apoptosis signaling, taking into account an IKK-dependent signaling branch, has been published recently (30) and may serve as a template on which our TRAIL model could be extended in the future.
In this context, it is important to note that it was suggested that proteasome inhibition sensitizes cells to TRAIL by inhibiting prosurvival signaling through stabilizing IκBα (23). IκBα, the inhibitor of transcription factor NFκB, is phosphorylated by IKK, and phosphorylated IκBα can be degraded rapidly by the proteasome. In control experiments in which we compared cell death responses to TRAIL, TRAIL/CHX, TRAIL/bortezomib, and TRAIL/CHX/bortezomib, we found that the sensitization to TRAIL by CHX, bortezomib, or CHX/bortezomib is comparable quantitatively in HeLa cells, with 85–95% cell death achieved after 24 h of treatment (supplemental Fig. 3B). Together with our results on the effect of proteasome inhibition on apoptosis signal transduction, this indicates that the most important early contribution of proteasome inhibitors to TRAIL synergies may be the inhibition of prosurvival signaling. The stabilization of active caspases, which otherwise may be degraded rapidly by the proteasome, may be an additional contributing factor, especially at submaximal TRAIL doses that result in low amounts of apical caspase activation.
As shown here for proteasome inhibition, the power of cellular time-lapse analysis in combination with systems modeling holds the potential to decipher and quantitatively specify influences on TRAIL-induced signal transduction. In the future, this approach may also be exploited to investigate other therapeutically relevant drugs and drug combinations, such as histone deacetylase inhibitors and kinase inhibitors (34–36), whose mode of action in evoking synergies in TRAIL co-treatments is not or only poorly defined. Likewise, additional processes that have been described to co-regulate TRAIL signaling, such as the posttranslational modification of TRAIL receptors, regulation of subcellular receptor localization and their accumulation in lipid rafts, as well as the influence of noncanonical DISC-interacting regulatory proteins (reviewed in Ref. 5) can be investigated by similar research approaches.
Supplementary Material
Acknowledgments
We thank Dr. Carlos Ricardo Rodrigues dos Reis (University of Groningen) for providing human recombinant TRAIL and Dr. Marion MacFarlane (Medical Research Council Toxicology Unit) University of Leicester for providing expression vectors for cFLIP and TRAIL-R2.
This work was supported by grants from the Science Foundation Ireland (07/RFP/BICF601), the Health Research Board Ireland (RP/2006/258, RP/2008/7), the Royal College of Surgeons in Ireland Research Committee, the National Biophotonics and Imaging Platform (HEA PRTLI Cycle 4), and the European Union Framework Programme 7 (APO-SYS).
- TRAIL
- tumor necrosis factor-related apoptosis-inducing ligand
- DISC
- death-inducing signaling complex
- FADD
- Fas-associated death domain
- cFLIPL
- long cFLIP variant
- Suc-LLVY-AMC
- N-succinyl-Leu-Leu-Val-Tyr-7-amino-4-methyl-coumarin
- Z
- benzyloxycarbonyl
- fmk
- fluoromethyl ketone
- TMRM
- tetramethylrhodamine-methylester.
REFERENCES
- 1. Konstantinova I. M., Tsimokha A. S., Mittenberg A. G. (2008) Role of proteasomes in cellular regulation. Int. Rev. Cell Mol. Biol. 267, 59–124 [DOI] [PubMed] [Google Scholar]
- 2. Brancolini C. (2008) Inhibitors of the ubiquitin-proteasome system and the cell death machinery: How many pathways are activated? Curr. Mol. Pharmacol. 1, 24–37 [DOI] [PubMed] [Google Scholar]
- 3. Concannon C. G., Koehler B. F., Reimertz C., Murphy B. M., Bonner C., Thurow N., Ward M. W., Villunger A., Strasser A., Kögel D., Prehn J. H. (2007) Apoptosis induced by proteasome inhibition in cancer cells: Predominant role of the p53/PUMA pathway. Oncogene 26, 1681–1692 [DOI] [PubMed] [Google Scholar]
- 4. Pandey U. B., Nie Z., Batlevi Y., McCray B. A., Ritson G. P., Nedelsky N. B., Schwartz S. L., DiProspero N. A., Knight M. A., Schuldiner O., Padmanabhan R., Hild M., Berry D. L., Garza D., Hubbert C. C., Yao T. P., Baehrecke E. H., Taylor J. P. (2007) HDAC6 rescues neurodegeneration and provides an essential link between autophagy and the UPS. Nature 447, 859–863 [DOI] [PubMed] [Google Scholar]
- 5. Pennarun B., Meijer A., de Vries E. G., Kleibeuker J. H., Kruyt F., de Jong S. (2010) Playing the DISC: Turning on TRAIL death receptor-mediated apoptosis in cancer. Biochim. Biophys. Acta 1805, 123–140 [DOI] [PubMed] [Google Scholar]
- 6. Johnstone R. W., Frew A. J., Smyth M. J. (2008) The TRAIL apoptotic pathway in cancer onset, progression, and therapy. Nat. Rev. Cancer 8, 782–798 [DOI] [PubMed] [Google Scholar]
- 7. Hellwig C. T., Rehm M. (2012) TRAIL signaling and synergy mechanisms used in TRAIL-based combination therapies. Mol. Cancer Ther. 11, 3–13 [DOI] [PubMed] [Google Scholar]
- 8. Hughes M. A., Harper N., Butterworth M., Cain K., Cohen G. M., MacFarlane M. (2009) Reconstitution of the death-inducing signaling complex reveals a substrate switch that determines CD95-mediated death or survival. Mol. Cell 35, 265–279 [DOI] [PubMed] [Google Scholar]
- 9. Irmler M., Thome M., Hahne M., Schneider P., Hofmann K., Steiner V., Bodmer J. L., Schröter M., Burns K., Mattmann C., Rimoldi D., French L. E., Tschopp J. (1997) Inhibition of death receptor signals by cellular FLIP. Nature 388, 190–195 [DOI] [PubMed] [Google Scholar]
- 10. Fricker N., Beaudouin J., Richter P., Eils R., Krammer P. H., Lavrik I. N. (2010) Model-based dissection of CD95 signaling dynamics reveals both a pro- and antiapoptotic role of c-FLIPL. J. Cell Biol. 190, 377–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tait S. W., Green D. R. (2010) Mitochondria and cell death: Outer membrane permeabilization and beyond. Nat. Rev. Mol. Cell Biol. 11, 621–632 [DOI] [PubMed] [Google Scholar]
- 12. Liu X., Kim C. N., Yang J., Jemmerson R., Wang X. (1996) Induction of apoptotic program in cell-free extracts: Requirement for dATP and cytochrome c. Cell 86, 147–157 [DOI] [PubMed] [Google Scholar]
- 13. Huber H. J., Dussmann H., Kilbride S. M., Rehm M., Prehn J. H. (2011) Glucose metabolism determines resistance of cancer cells to bioenergetic crisis after cytochrome c release. Mol. Syst. Biol. 7, 470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Varfolomeev E., Maecker H., Sharp D., Lawrence D., Renz M., Vucic D., Ashkenazi A. (2005) Molecular determinants of kinase pathway activation by Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand. J. Biol. Chem. 280, 40599–40608 [DOI] [PubMed] [Google Scholar]
- 15. Bentele M., Lavrik I., Ulrich M., Stösser S., Heermann D. W., Kalthoff H., Krammer P. H., Eils R. (2004) Mathematical modeling reveals threshold mechanism in CD95-induced apoptosis. J. Cell Biol. 166, 839–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. He Q., Huang Y., Sheikh M. S. (2004) Proteasome inhibitor MG132 up-regulates death receptor 5 and cooperates with Apo2L/TRAIL to induce apoptosis in Bax-proficient and -deficient cells. Oncogene 23, 2554–2558 [DOI] [PubMed] [Google Scholar]
- 17. Johnson T. R., Stone K., Nikrad M., Yeh T., Zong W. X., Thompson C. B., Nesterov A., Kraft A. S. (2003) The proteasome inhibitor PS-341 overcomes TRAIL resistance in Bax and caspase-9-negative or Bcl-xL overexpressing cells. Oncogene 22, 4953–4963 [DOI] [PubMed] [Google Scholar]
- 18. Hellwig C. T., Kohler B. F., Lehtivarjo A. K., Dussmann H., Courtney M. J., Prehn J. H., Rehm M. (2008) Real time analysis of tumor necrosis factor-related apoptosis-inducing ligand/cycloheximide-induced caspase activities during apoptosis initiation. J. Biol. Chem. 283, 21676–21685 [DOI] [PubMed] [Google Scholar]
- 19. MacFarlane M., Ahmad M., Srinivasula S. M., Fernandes-Alnemri T., Cohen G. M., Alnemri E. S. (1997) Identification and molecular cloning of two novel receptors for the cytotoxic ligand TRAIL. J. Biol. Chem. 272, 25417–25420 [DOI] [PubMed] [Google Scholar]
- 20. Laussmann M. A., Passante E., Düssmann H., Rauen J. A., Würstle M. L., Delgado M. E., Devocelle M., Prehn J. H., Rehm M. (2011) Proteasome inhibition can induce an autophagy-dependent apical activation of caspase-8. Cell Death Differ. 18, 1584–1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rehm M., Huber H. J., Dussmann H., Prehn J. H. (2006) Systems analysis of effector caspase activation and its control by X-linked inhibitor of apoptosis protein. EMBO J. 25, 4338–4349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Albeck J. G., Burke J. M., Aldridge B. B., Zhang M., Lauffenburger D. A., Sorger P. K. (2008) Quantitative analysis of pathways controlling extrinsic apoptosis in single cells. Mol. Cell 30, 11–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cusack J. C., Jr., Liu R., Houston M., Abendroth K., Elliott P. J., Adams J., Baldwin A. S., Jr. (2001) Enhanced chemosensitivity to CPT-11 with proteasome inhibitor PS-341: Implications for systemic nuclear factor-κB inhibition. Cancer Res. 61, 3535–3540 [PubMed] [Google Scholar]
- 24. Falschlehner C., Emmerich C. H., Gerlach B., Walczak H. (2007) TRAIL signaling: Decisions between life and death. Int. J. Biochem. Cell Biol. 39, 1462–1475 [DOI] [PubMed] [Google Scholar]
- 25. Liu X., Yue P., Chen S., Hu L., Lonial S., Khuri F. R., Sun S. Y. (2007) The proteasome inhibitor PS-341 (bortezomib) up-regulates DR5 expression leading to induction of apoptosis and enhancement of TRAIL-induced apoptosis despite up-regulation of c-FLIP and survivin expression in human NSCLC cells. Cancer Res. 67, 4981–4988 [DOI] [PubMed] [Google Scholar]
- 26. Sohn D., Totzke G., Essmann F., Schulze-Osthoff K., Levkau B., Jänicke R. U. (2006) The proteasome is required for rapid initiation of death receptor-induced apoptosis. Mol. Cell Biol. 26, 1967–1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li W., Zhang X., Olumi A. F. (2007) MG-132 sensitizes TRAIL-resistant prostate cancer cells by activating c-Fos/c-Jun heterodimers and repressing c-FLIP(L). Cancer Res. 67, 2247–2255 [DOI] [PubMed] [Google Scholar]
- 28. Sayers T. J., Brooks A. D., Koh C. Y., Ma W., Seki N., Raziuddin A., Blazar B. R., Zhang X., Elliott P. J., Murphy W. J. (2003) The proteasome inhibitor PS-341 sensitizes neoplastic cells to TRAIL-mediated apoptosis by reducing levels of c-FLIP. Blood 102, 303–310 [DOI] [PubMed] [Google Scholar]
- 29. Zhang L., Lopez H., George N. M., Liu X., Pang X., Luo X. (2011) Selective involvement of BH3-only proteins and differential targets of Noxa in diverse apoptotic pathways. Cell Death Differ. 18, 864–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Neumann L., Pforr C., Beaudouin J., Pappa A., Fricker N., Krammer P. H., Lavrik I. N., Eils R. (2010) Dynamics within the CD95 death-inducing signaling complex decide life and death of cells. Mol. Syst. Biol. 6, 352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Düssmann H., Rehm M., Concannon C. G., Anguissola S., Würstle M., Kacmar S., Völler P., Huber H. J., Prehn J. H. (2010) Single-cell quantification of Bax activation and mathematical modeling suggest pore formation on minimal mitochondrial Bax accumulation. Cell Death Differ. 17, 278–290 [DOI] [PubMed] [Google Scholar]
- 32. Chen G., Goeddel D. V. (2002) TNF-R1 signaling: A beautiful pathway. Science 296, 1634–1635 [DOI] [PubMed] [Google Scholar]
- 33. Kreuz S., Siegmund D., Rumpf J. J., Samel D., Leverkus M., Janssen O., Häcker G., Dittrich-Breiholz O., Kracht M., Scheurich P., Wajant H. (2004) NF-κB activation by Fas is mediated through FADD, caspase-8, and RIP and is inhibited by FLIP. J. Cell Biol. 166, 369–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guo F., Sigua C., Tao J., Bali P., George P., Li Y., Wittmann S., Moscinski L., Atadja P., Bhalla K. (2004) Cotreatment with histone deacetylase inhibitor LAQ824 enhances Apo-2L/tumor necrosis factor-related apoptosis inducing ligand-induced death inducing signaling complex activity and apoptosis of human acute leukemia cells. Cancer Res. 64, 2580–2589 [DOI] [PubMed] [Google Scholar]
- 35. Harper N., Hughes M. A., Farrow S. N., Cohen G. M., MacFarlane M. (2003) Protein kinase C modulates tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by targeting the apical events of death receptor signaling. J. Biol. Chem. 278, 44338–44347 [DOI] [PubMed] [Google Scholar]
- 36. Söderström T. S., Poukkula M., Holmström T. H., Heiskanen K. M., Eriksson J. E. (2002) Mitogen-activated protein kinase/extracellular signal-regulated kinase signaling in activated T cells abrogates TRAIL-induced apoptosis upstream of the mitochondrial amplification loop and caspase-8. J. Immunol. 169, 2851–2860 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.