Background: It is unknown whether the unfolded protein response (UPR) mediates an angiogenic response during kidney ischemia.
Results: The UPR regulates the expression of VEGFA, bFGF, and angiogenin independently of HIF-1α, during kidney ischemia.
Conclusion: PERK is a central regulator of the angiogenic response to nutrient deprivation.
Significance: Our study identifies the UPR as a potential regulator of angiogenesis, independent of HIF-1α.
Keywords: Angiogenesis, Endoplasmic Reticulum Stress, Ischemia, Kidney, Transplantation, Angiogenin, Basic Fibroblast Growth Factor, Ischemia, Unfolded Protein Response, Vascular Endothelial Growth Factor
Abstract
Ischemic injuries permanently affect kidney tissue and challenge cell viability, promoting inflammation and fibrogenesis. Ischemia results in nutrient deprivation, which triggers endoplasmic reticulum stress, ultimately resulting in the unfolded protein response (UPR). The aim of this study was to test whether the UPR could promote an angiogenic response independently of the HIF-1α pathway during ischemic stress in the human kidney epithelium. Glucose deprivation induced the secretion of vascular endothelial growth factor A (VEGFA), basic fibroblast growth factor (bFGF) and angiogenin (ANG) in human kidney epithelial cells independently of HIF-1α. Glucose deprivation, but not hypoxia, triggered endoplasmic reticulum stress and activated the UPR. RNA interference-mediated inhibition of the gene encoding the kinase PERK decreased VEGFA and bFGF expression, but neither gene was affected by the inhibition of IRE1α or ATF6. Furthermore, we show that the expression of angiogenin, which inhibits protein synthesis, is regulated by both IRE1α and PERK, which could constitute a complementary function of the UPR in the repression of translation. In a rat model of acute ischemic stress, we show that the UPR is activated in parallel with VEGFA, bFGF, and ANG expression and independently of HIF-1α.
Introduction
Chronic kidney structural deterioration progresses as a result of the dynamic combination of specific and nonspecific insults that engage cycles of cell death, inflammation, and healing, ultimately resulting in maladaptive tissue remodeling and loss of kidney function (1, 2). Ischemia occurs when the blood supply to the tissue is so restricted that it induces cellular stress and is a common factor in the progression of several, if not all, chronic kidney diseases, as it induces inflammation, epithelial-to-mesenchymal transition and fibrosis (3). A shortage of blood can produce ischemia, during which the tissues cannot fulfill their energetic demand. The cellular response to ischemia aims, in a coordinated fashion, to improve the energy input (nutrients and oxygen) and reduce the energy expenditure. This improvement in the energy input relies on angiogenesis, the cellular expression of nutrient transporters, or catabolic processes including autophagy. Alternatively, the reduction of energy expenditure can be achieved by a reduction in protein translation, which is a highly energy-consuming process. During ischemia, the inhibition of mammalian target of rapamycin (mTOR)2 signaling and the activation of the kinase PKR-like ER Kinase (PERK), mediate the general inhibition of translation. Of note, some of the proteins that are involved in the metabolic adaptation to ischemia, such as activated transcription factor 4 (ATF4), which can mediate angiogenesis (4), can be selectively translated and expressed despite the general inhibition of translation. At the cellular level, oxygen and nutrient deprivation activate adaptive mechanisms that maintain basal metabolism and vital functions. Although mainly protective, these signaling pathways also actively participate in tissue remodeling by promoting inflammation and fibrogenesis, which can result in interstitial fibrosis, tubular atrophy, capillary rarefaction, and loss of kidney function. These structural alterations cause a progressive deterioration of the vascular network and exchanging interfaces, changes that, in turn, aggravate ischemia (3, 5). Therefore, a better understanding of the biological processes activated within the cell in response to ischemia is critical both for the identification of potential therapeutic targets, and to slow structural kidney deterioration.
The best-known adaptive response to ischemia is the stabilization of the transcription factor Hypoxia Inducible Factor-1α (HIF-1α), which escapes from proteasomal degradation during hypoxia. HIF-1α drives the transcription of genes involved in adaptive responses to hypoxia, which include angiogenesis, nutrient transport, glycolysis, and inhibition of apoptosis (6). The secretion of angiogenic factors by epithelial and endothelial cells during ischemia contributes to the maintenance of an intact tubulo-interstitial compartment and slows the progression of kidney disease, although the situation is complex, as a dysregulated angiogenic response can result in deleterious effects and amplify an injury (7, 8).
Other adaptive processes implicated in the response to nutrient and oxygen deprivation involve the mTOR kinase (9, 10) and the unfolded protein response (UPR) (11, 12). Hypoxia and glucose starvation promote ATP shortage, decrease the intra-reticular calcium concentration, impair the activity of chaperone molecules, interfere with disulfide bridge formation and impair the maturation of native proteins, a process that promotes endoplasmic reticulum (ER) stress and activates the UPR (12, 13). The UPR involves three major mediators, PERK, the protein kinase/endoribonuclease Inositol-requiring Enzyme 1α (IRE-1α) and the transcription factor ATF6. The role of the UPR is to modify cellular functions in response to ER stress and to re-establish normal ER function both at the translational and transcriptional levels. The UPR is responsible for the attenuation of general mRNA translation by the phosphorylated form of elongation Initiation Factor 2α (eIF2α) and transcriptional regulation of the UPR genes encoding chaperones, folding, and proteasomal degradation enzymes, and activation of macroautophagy (13, 14).
It is currently unknown whether the UPR mediates an angiogenic response during kidney ischemia. The aim of this study was to investigate whether the UPR modulates the production of angiogenic factors by the human kidney epithelium during ischemia. Here, we demonstrate that human renal epithelial cells (HREC) subjected to glucose deprivation secrete vascular endothelial growth factor A (VEGFA), basic fibroblast growth factor (bFGF), and angiogenin (ANG) independently of HIF-1α, and we identify the PERK pathway as a central regulator of this angiogenic response. Additionally, we show that the expression of the angiogenic mediator ANG, which is also a translational repressor, is regulated by both IRE1α and PERK. This provides the basis for the characterization of other functions of the UPR in the inhibition of mRNA translation in addition to the PERK-eIF2α pathway.
EXPERIMENTAL PROCEDURES
Cell Culture
HREC of proximal origin (HK-2) were purchased from ATCC/LGC Standards and cultured according to previously published methods (15). Hypoxia experiments were performed using the Anaerocult® P kit (Merck Chemicals). The Anaerocult® kit is a small chamber that produces an anaerobic atmosphere due to the presence of chemicals that absorb oxygen, including iron powder, citric acid, sodium carbonate, and silica (kieselguhr), which are activated by water. The Petri dishes are incubated within these chambers, in which the estimated oxygen percentage is less than 0.1% beyond 1 h. This chamber can be placed in a tissue culture incubator. HREC were incubated in glucose-free DMEM to induce ischemic stress.
siRNA Transfections
PERK, IRE1α, ATF6, and scramble (control) small interfering synthetic RNAs (siRNAs) were designed and obtained from Qiagen. Transfection was performed using HiPerFect® (Qiagen) following the manufacturer's protocol.
RNA Extraction and Real-time Quantitative Polymerase Chain Reaction (RT-qPCR)
Total RNA was extracted using the RNeasy Mini Kit® (Qiagen) following the manufacturer's protocol. The yield and purity of RNA were measured using a NanoDrop ND-1000® spectrophotometer (Nanodrop Technologies). Transcript expression levels were quantified by SYBR green real-time PCR using an ABI PRISM 7900 sequence detector system (Applied Biosystems). Vehicle-treated samples were used as the controls, and fold changes for each tested gene were normalized to housekeeping genes (Ribosomal Protein L13A for in vitro and Tata-binding protein for in vivo analyses). The relative expression levels were calculated using the 2(−ΔΔC(T)) (threshold cycle number) method (16). Primers are listed in supplemental Table S1.
Protein Extraction and Western Blot Analysis
Total protein lysate from HREC was separated by sodium-dodecyl-sulfate polyacrylamide gel electrophoresis under denaturing conditions and transferred to a PVDF membrane (GE Healthcare). Primary antibodies were visualized using horseradish peroxidase-conjugated polyclonal secondary antibodies (Sigma Aldrich) and detected by ECL reagent® (GE Healthcare). Antibody details are listed in the supplemental Table S2.
Cell Membrane Permeabilization Assessment
The level of cell necrosis was measured using the ToxiLight® BioAssay Kit (Cambrex), which is a bioluminescent assay designed to measure the release of adenylate kinase from damaged cells. Experiments were performed according to the manufacturer's protocol.
Enzyme-linked Immunosorbent Assays
Subconfluent cells were grown in 6-well plates for the indicated times under the indicated conditions. Secretion of VEGFA, bFGF, ANG, and PDGF-BB was quantified in the cell culture supernatant using the Quantikine® human VEGF immunoassay, the Quantikine® human bFGF immunoassay, the Quantikine® human ANG immunoassay and the Quantikine® human PDGF-BB immunoassay (RD Systems), respectively, according to the manufacturer's protocol. VEGFA, bFGF, ANG, and PDGF-BB concentrations in the culture medium were normalized to the number of cells in the plate.
In Vivo Experiments
Adult male Sprague-Dawley rats weighing 170–180 g were purchased from Charles River Laboratories. To characterize the acute kidney ischemia injury that occurs before kidney transplantation, we nephrectomized rats and rinsed the kidneys in IGL1® preservation solution at 4 °C for 24 h. The rats were anesthetized using intraperitoneal ketamine and xylazine. The abdomen was then opened by a midline incision, and the kidneys were perfused with cold heparinized saline. The kidneys were then washed with IGL1® and incubated with IGL1® for 24 h at 4 °C (n = 4 for each group). All procedures were strictly performed according to the Paris Descartes University Animal Care recommendations.
Statistical Analysis
All data are expressed as the mean ± S.E. of three independent experiments, unless otherwise specified. Variables were compared with the Student's t test. Statistical analyses were performed using Prism-GraphPad® software. p values of less than 0.05 were considered significant.
RESULTS
Glucose Deprivation Induces the Expression of Angiogenic Mediators
To test whether ischemia induces an angiogenic response within the human kidney epithelium, we monitored the expression of genes encoding mediators of angiogenesis in HREC exposed to glucose-deprived medium, hypoxia, or both. The combination of hypoxia and glucose starvation proved to be too cytotoxic within our cellular model to provide interpretable results (supplemental Fig. S1A). We analyzed the expression of VEGFA, bFGF, ANG, and platelet-derived growth factor β (PDGFB), as these mediators are important regulators of angiogenesis during kidney disease (7, 8, 17, 18).
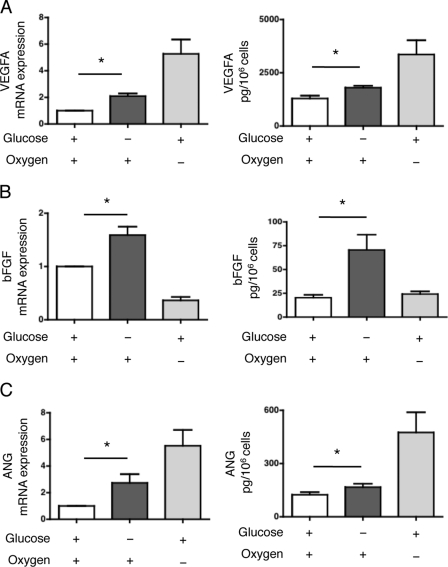
After 24 h of culture in glucose-deprived culture medium under normoxic conditions, VEGFA expression was significantly increased compared with the controls (HREC cultured in standard conditions) at both the transcriptional and protein levels (Fig. 1A). As expected, hypoxia also promoted VEGFA expression in our model (Fig. 1A). Glucose starvation for 24 h significantly increased bFGF transcript levels relative to HREC cultured in standard conditions, whereas hypoxia did not alter bFGF gene expression (Fig. 1B). Glucose starvation, but not hypoxia, also significantly increased the secretion of bFGF into the culture medium compared with basal culture conditions (Fig. 1B). Glucose starvation induced ANG expression at both the transcriptional and protein levels, as did hypoxia (Fig. 1C). PDGFB expression did not increase under glucose starvation, whereas hypoxia induced the expression of PDGFB transcripts (supplemental Fig. S1, B and C). Overall, our results suggest that the angiogenic factors VEGFA, bFGF, and ANG but not PDGFB, are expressed by HREC in response to glucose deprivation but independently of hypoxia.
FIGURE 1.
Glucose deprivation induces the expression of angiogenic mediators. A, HREC were cultured in glucose-deprived medium or in standard medium with or without hypoxia for 24 h. Left: VEGFA transcript levels were measured by qRT-PCR and are presented as the mean ± S.E. relative to levels after 24 h of culture with standard conditions in four independent experiments. Right: secretion of VEGFA in the medium was quantified by ELISA. The concentration is presented as the mean ± S.E. of three independent experiments. *, p < 0.05. B, HREC were cultured in glucose-deprived medium or in standard medium with or without hypoxia for 24 h. Left: bFGF transcript levels were measured by qRT-PCR and are presented as the mean ± S.E. relative to levels after 24 h of culture with standard conditions in four independent experiments. Right: secretion of bFGF in the medium was quantified by ELISA. The concentration is presented as the mean ± S.E. of three independent experiments. *, p < 0.05. C, HREC were cultured in glucose-deprived medium or in standard medium with or without hypoxia for 24 h. Left: ANG transcript levels were measured by qRT-PCR and are presented as the mean ± S.E. relative to levels after 24 h of culture with standard conditions in four independent experiments. Right: secretion of ANG in the medium was quantified by ELISA. The concentration is presented as the mean ± S.E. of three independent experiments. *, p < 0.05.
Glucose Deprivation Does Not Activate HIF-1α Signaling
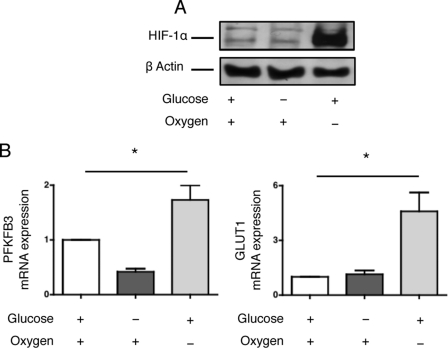
HIF-1α is a central regulator of the expression of various angiogenic molecules during ischemia (19, 20). We therefore evaluated whether glucose deprivation would activate the HIF-1α pathway. HIF-1α did not accumulate in HREC cultured in glucose-deprived medium under a normoxic atmosphere but was elevated under hypoxic conditions (Fig. 2A). Additionally, the expression of genes whose transcription is under control of HIF-1α, such as 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3) and glucose transporter 1 (GLUT1), was induced during hypoxia but not under glucose-deprived conditions (Fig. 2B). These results suggest that glucose deprivation does not activate the HIF-1α signaling pathway and that alternative pathways might be involved in the induction of the expression of angiogenic mediators.
FIGURE 2.
Glucose deprivation does not activate HIF-1α signaling. A, HREC were cultured in glucose-deprived medium or in standard medium with or without hypoxia for 24 h. HIF-1α protein expression was determined by immunoblotting. The immunoblot shown is representative of three independent experiments. B, HREC were cultured in glucose-deprived medium or in standard medium with or without hypoxia for 24 h. PFKFB3 and GLUT1 transcript levels were measured by qRT-PCR and are presented as the mean ± S.E. relative to levels after 24 h of culture with standard conditions in four independent experiments. *, p < 0.05.
Glucose Deprivation, but Not Hypoxia, Activates the UPR in HREC
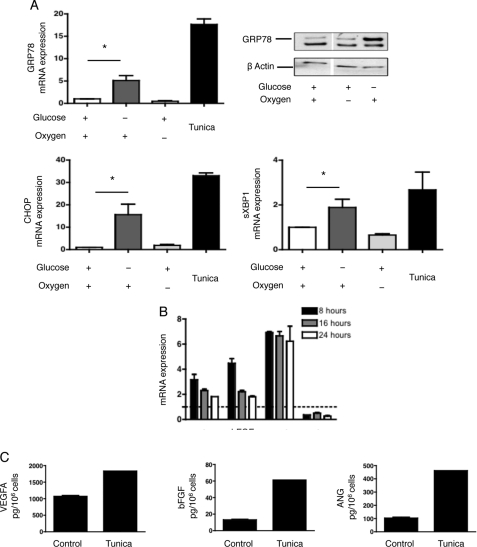
The UPR has been identified as an important conveyor of tumoral tissue resistance in response to ischemia (11, 12) and may regulate the expression of VEGFA under chemically induced ER stress (21). Given these findings, we investigated whether glucose deprivation activates the UPR in HREC. The expression of genes typically activated during the UPR, including glucose-related protein 78 (GRP78) and CCAAT/Enhancer-binding protein homologous protein (CHOP) was significantly increased during glucose deprivation, as was the spliced form of X-box-binding protein-1 (XBP1) (Fig. 3A). These genes were not expressed when HREC were cultured under hypoxic conditions, suggesting that glucose deprivation, but not hypoxia, activates the UPR within renal tubular cells. Moreover, the ER stressor tunicamycin induced the expression of VEGFA, bFGF and ANG, but not PDGFB in HREC both at the transcripts and protein levels (Fig. 3, B and C), suggesting that the UPR might promote an angiogenic response in HREC.
FIGURE 3.
Glucose deprivation, not hypoxia, activates the UPR in HREC. A, HREC were cultured in glucose-deprived medium or in standard medium with or without hypoxia for 24 h, or with 2.5 μm tunicamycin for 8 h. GRP78, CHOP, and sXBP transcript levels were measured by qRT-PCR and are presented as the mean ± S.E. relative to levels after 24 h of culture with standard conditions in four independent experiments. GRP78 protein expression was determined by immunoblotting. The immunoblot shown is representative of three independent experiments. *, p < 0.05. B, HREC were cultured with 2.5 μm tunicamycin for 8, 16, and 24 h. VEGFA, bFGF, ANG, and PDGFB transcript levels were measured by qRT-PCR and are presented as the mean ± S.E. relative to levels in non-treated cells in four independent experiments. The dotted line figures the expression level (= 1) of each gene in basal condition. C, HREC were cultured with 2.5 μm tunicamycin for 16 h, and the secretion of VEGFA, bFGF, and ANG in the medium was quantified by ELISA.
RNA Interference Directed against Three Transducers of the UPR
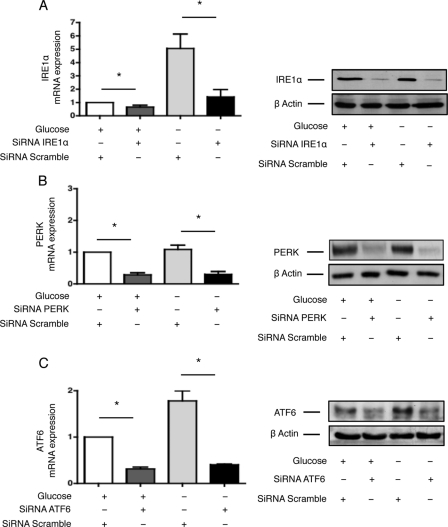
To test whether the UPR modulates the angiogenic response of HREC following glucose deprivation, we inhibited IRE1α, PERK, or ATF6 expression in HREC by siRNA-based RNA interference. The efficacy of IRE1α, PERK, or ATF6 inhibition of expression is depicted in Fig. 4. Glucose deprivation elevates the preferential expression of IRE1α and ATF6; however, the biological significance of this result, to our knowledge, is unknown. Given the known models for IRE1α and ATF6 activation during ER stress, it is unlikely that this positive regulation would interfere with the functions of the UPR.
FIGURE 4.
RNA interference directed against three transducers of the UPR. A, HREC were transfected with siRNAs targeting IRE1α or control, non-targeted (scramble) siRNAs. Twenty-four hours post-transfection, the culture medium was replaced with glucose-free medium or with standard medium. IRE1α transcript (left) and protein (right) levels were measured after an additional 24 h and compared with levels after 24 h of culture with standard conditions. *, p < 0.05. B, HREC were transfected with siRNAs targeting PERK or control, non-targeted (scramble) siRNAs. Twenty-four hours post-transfection, the culture medium was replaced with glucose-free medium or with standard medium. PERK transcript (left) and protein (right) levels were measured after an additional 24 h and compared with levels after 24 h of culture with standard conditions. *, p < 0.05. C, HREC were transfected with siRNAs targeting ATF6 or control, non-targeted (scramble) siRNAs. Twenty-four hours post-transfection, the culture medium was replaced with glucose-free medium or with standard medium. ATF6 transcript (left) and protein (right) levels were measured after an additional 24 h and compared with levels after 24 h of culture with standard conditions. *, p < 0.05.
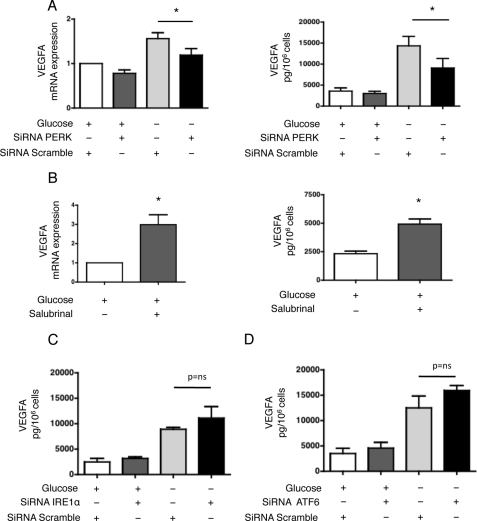
VEGFA Expression Is Regulated by PERK
We first examined the role of PERK in modulating VEGFA expression in response to glucose deprivation. Inhibition of PERK expression by RNA interference significantly reduced the expression of VEGFA at both the transcriptional and protein levels (Fig. 5A). Interestingly, HREC exposed to salubrinal, a small molecule that inhibits eIF2α dephosphorylation and maintains the PERK-eIF2α pathway activated (22), increased VEGFA expression (Fig. 5B). Conversely, inhibition of IRE1α or ATF6 expression did not alter VEGFA expression (Fig. 5, C and D and supplemental Fig. S2, A and B). These results suggest that PERK, but not IRE1α or ATF6, regulates the expression of VEGFA. However, one cannot exclude that PERK may regulate the intracellular trafficking or secretion of VEGFA in addition to its production.
FIGURE 5.
VEGFA expression is regulated by PERK. A, HREC were transfected with siRNAs targeting PERK or control, non-targeted (scramble) siRNAs. Left: VEGFA transcript levels were measured by qRT-PCR after the cells had incubated in glucose-free medium for 24 h and compared with levels after 24 h of culture with standard conditions (n = 4). Right: secretion of VEGFA in the medium was quantified by ELISA after the cells had incubated in glucose-free or standard medium for 48 h (n = 3). *, p < 0.05. B, HREC were incubated in standard medium and treated with or without 25 μm salubrinal for 48 h. Left: VEGFA transcript levels were measured by qRT-PCR and compared with levels after 48 h of culture with standard conditions (n = 3). Right: secretion of VEGFA in the medium was quantified by ELISA (n = 3). *, p < 0.05. C, HREC were transfected with siRNAs targeting IRE1α or control, non-targeted (scramble) siRNAs. The secretion of VEGFA in the medium was quantified by ELISA after the cells had incubated in glucose-free or standard medium for 48 h (n = 3). *, p < 0.05. D, HREC were transfected with siRNAs targeting ATF6 or control, non-targeted (scramble) siRNAs. The secretion of VEGFA in the medium was quantified by ELISA after the cells had incubated in glucose-free or standard medium for 48 h (n = 3). *, p < 0.05.
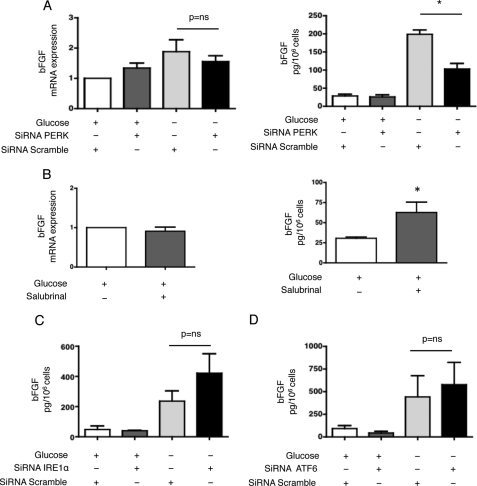
bFGF Expression Is Regulated by PERK
We next examined the role of the UPR transducers in the production of bFGF in response to glucose deprivation. Inhibition of PERK expression significantly reduced the secretion of bFGF into the culture medium but did not affect transcript levels (Fig. 6A). Salubrinal treatment of HREC led to a significant increase in bFGF secretion without alteration of transcript levels (Fig. 6B). Conversely, inhibition of IRE1α or ATF6 expression did not alter bFGF expression (Fig. 6, C and D, and supplemental Fig. S3, A and B). These results suggest that PERK, but not IRE1α or ATF6, mediates bFGF expression in renal tubular cells during glucose deprivation and that regulation may occur at both the transcriptional and post-transcriptional levels.
FIGURE 6.
bFGF expression is regulated by PERK. A, HREC were transfected with siRNAs targeting PERK or control, non-targeted (scramble) siRNAs. Left: bFGF transcript levels were measured by qRT-PCR after the cells had incubated in glucose-free medium for 48 h and compared with levels after 48 h of culture with standard conditions (n = 4). Right: secretion of bFGF in the medium was quantified by ELISA after the cells had incubated in glucose-free or standard medium for 48 h (n = 3). *, p < 0.05. B, HREC were incubated in glucose-free or standard medium and treated with or without 25 μm salubrinal for 48 h. Left: bFGF transcript levels were measured by qRT-PCR and compared with levels after 48 h of culture with standard conditions (n = 3). Right: secretion of bFGF in the medium was quantified by ELISA (n = 3). *, p < 0.05. C, HREC were transfected with siRNAs targeting IRE1α or control, non-targeted (scramble) siRNAs. The secretion of bFGF in the medium was quantified by ELISA after the cells had incubated in glucose-free or standard medium for 48 h (n = 3). D, HREC were transfected with siRNAs targeting ATF6 or control, non-targeted (scramble) siRNAs. The secretion of bFGF in the medium was quantified by ELISA after the cells had incubated in glucose-free or standard medium for 48 h (n = 3).
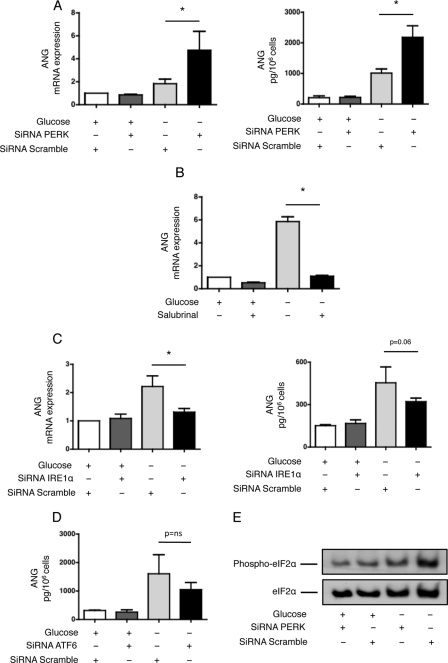
ANG Expression Is Regulated by PERK and IRE1α
We next focused on the regulation of ANG by the UPR. Inhibition of PERK expression significantly increased ANG expression at both the transcriptional and protein levels (Fig. 7A), whereas salubrinal strongly inhibited it (Fig. 7B), suggesting that PERK signaling inhibits ANG expression. Inhibition of IRE1α expression significantly reduced ANG expression at the transcriptional and protein levels (Fig. 7C), suggesting that IRE1α activates ANG expression. Inhibition of ATF6 did not alter ANG expression (Fig. 7D and supplemental Fig. S4). Together, these results suggest that ANG expression is positively regulated by IRE1α and inhibited by PERK during glucose deprivation. Given the fact that ANG promotes stress-induced translational repression (23), our findings also unravel a new mechanism of inhibition of protein synthesis activated by the UPR. This mechanism seems to occur when the activity of the PERK-eIF2α pathway is reduced because ANG expression is induced when PERK expression is inhibited (Fig. 7A) and when the phosphorylation of eIF2α is abolished (Fig. 7E).
FIGURE 7.
ANG expression is regulated by PERK and IRE1α. HREC were transfected with siRNAs targeting PERK or control, non-targeted (scramble) siRNAs. Left: ANG transcript levels were measured by qRT-PCR after the cells had incubated in glucose-free medium for 24 h and compared with levels after 24 h of culture with standard conditions (n = 4). Right: secretion of ANG in the medium was quantified by ELISA after the cells had incubated in glucose-free or standard medium for 48 h (n = 3). *, p < 0.05. B, HREC were incubated in glucose-free or standard medium and treated with or without 25 μm salubrinal for 48 h. Left: ANG transcript levels were measured by qRT-PCR and compared with levels after 48 h of culture with standard conditions (n = 3). C, HREC were transfected with siRNAs targeting IRE1α or with control, non-targeted (scramble) siRNAs. Left: ANG transcript levels were measured by qRT-PCR after the cells had incubated in glucose-free medium for 24 h and compared with levels after 24 h of culture with standard conditions (n = 4). Right: secretion of ANG in the medium was quantified by ELISA after the cells had incubated in glucose-free or standard medium for 48 h (n = 3). D, HREC were transfected with siRNAs targeting ATF6 or with control, non-targeted (scramble) siRNAs. The secretion of ANG in the medium was quantified by ELISA after the cells had incubated in glucose-free or standard medium for 48 h (n = 3). E, phosphorylation status of eIF2α. HREC were transfected with siRNAs targeting PERK or control, non-targeted (scramble) siRNAs. Phospho-eIF2α and eIF2α protein levels were measured after the cells had incubated in glucose-free or standard medium (n = 4). A representative immunoblot is shown.
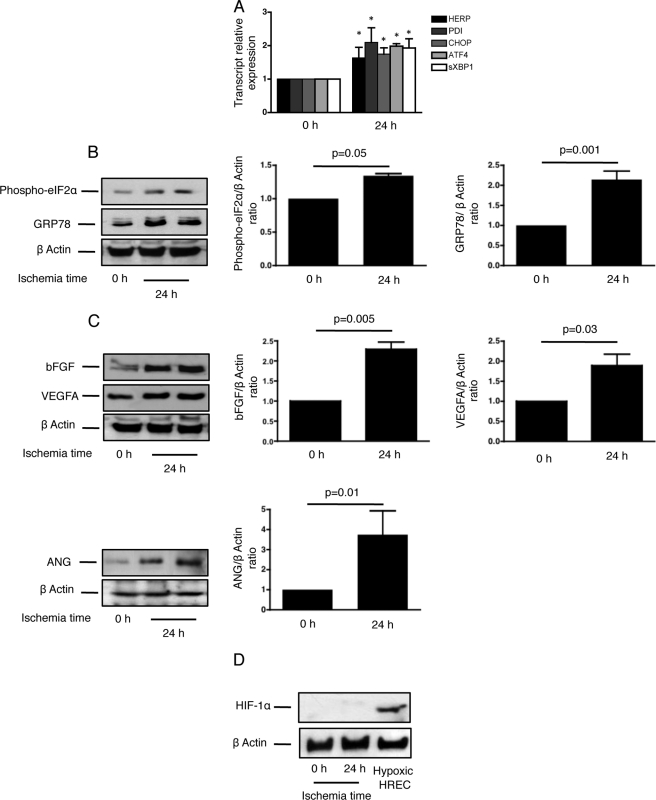
Cold Ischemia in Rat Kidneys Activates the UPR and Generates an Angiogenic Response
To translate our in vitro findings to an in vivo setting, we reproduced an ischemic kidney condition and analyzed the UPR and angiogenic responses in rat kidneys. To mimic cold ischemia, which occurs during kidney transplantation, the rats were nephrectomized, and the kidneys were stored in the IGL1® preservative solution at 4 °C for 24 h. The expression of the UPR target genes homocysteine-inducible endoplasmic reticulum stress protein (HERP), protein disulfide isomerase, (PDI), CHOP, ATF4, and sXBP1, was significantly increased in ischemic rat kidneys (Fig. 8A), the UPR surrogate marker GRP78 was expressed after 24 h and the PERK target eIF2α was phosphorylated (Fig. 8B), suggesting that the UPR is activated during acute ischemia of the kidney. We observed that bFGF, VEGFA, and ANG expression increased after 24 h and paralleled the expression of the UPR markers (Fig. 8C), whereas HIF-1α was not expressed in ischemic kidneys (Fig. 8D). Together, these results suggest that cold ischemia activates the UPR and that VEGFA, bFGF, and ANG expression also increases in parallel, independently of HIF-1α expression.
FIGURE 8.
Cold ischemia in rat kidneys activates ER stress and generates an angiogenic response. The rats were nephrectomized, and the kidneys were rinsed and incubated in an IGL1® solution for 24 h. A, HERP, PDI, CHOP, ATF4, and sXBP1 transcript levels were measured by qRT-PCR in the rat kidneys after 24 h of cold ischemia, and compared with levels in non ischemic kidneys (n = 4). *, p < 0.05. B, GRP78 and phospho-eIF2α protein levels were measured in the rat kidneys after 24 h of cold ischemia. Left: a representative immunoblot is shown. Right: a densitometric analysis of immunoblots. n = 4. C, VEGFA, bFGF, and ANG protein levels were measured in the rat kidneys after 24 h of cold ischemia. Left: a representative immunoblot is shown. Right: a densitometric analysis of immunoblots, n = 4. D, HIF-1α protein levels were measured in rat kidneys after 24 h of cold ischemia. A representative immunoblot is shown. Hypoxic HREC were used as control.
DISCUSSION
Survival in an ischemic microenvironment requires a cell to activate adaptive biological responses that regulate metabolic reprogramming, angiogenesis, translation inhibition, and macroautophagy. The angiogenic response generated by the ischemic kidney epithelium integrates components of these standard responses to ischemic stress to maintain the blood supply and fulfill cell's energetic demands. In addition to the HIF-1α-mediated transcriptional program that responds to hypoxic stress, other undefined adaptive pathways are also thought to be involved, but their specific functions have not been experimentally confirmed. Here, we describe an adaptive angiogenic response of the human kidney epithelium to nutrient deprivation that functions independently of hypoxia and HIF-1α. This response links ER stress and the UPR to the production of the angiogenic factors VEGFA, bFGF, and ANG. The fact that PDGFB expression is not altered by the UPR transducers implies that the UPR and HIF-1α drive angiogenic responses with distinctive profiles. In addition to the regulation of angiogenesis, our findings reveal a novel function of the UPR in the inhibition of translation. In fact, in our model, ANG, an RNase that inhibits translation in response to stressful conditions, is tightly regulated by the UPR transducers PERK and IRE1α, and its expression occurs when the PERK-eIF2α axis activity is abolished.
The observation that nutrient starvation in HREC activates the UPR, which in turn mediates the secretion of angiogenic factors, adds complexity to the consequences of ER stress in the progression of kidney disease. The secretion of angiogenesis mediators, such as VEGFA, in response to ischemia is essential for the maintenance of the tubulo-interstitial compartment, as it prevents endothelial cell death and peritubular capillary collapse, and helps to maintain adequate oxygen and nutrient supplies (24–26). Angiogenic mediators, however, also fuel inadequate tissue remodeling because they can facilitate leukocyte recruitment and promote inflammation, the epithelial-to-mesenchymal transition and fibroblast activation (8, 27, 28). Therapeutic modulation of the UPR with 4-phenyl-butyrate or salubrinal can alleviate the deleterious effects of the UPR, reduce kidney injury and delay tissue remodeling (29, 30). Further studies are required to address important questions regarding the consequences of these angiogenic responses in the context of kidney disease progression as well as the duration of the response, the level of secretion of these mediators and the nature of the initial injury.
To mimic cellular energetic failures that occur in response to ischemia, we analyzed the consequences of glucose starvation on human epithelial cells. Glucose starvation induces intracellular ATP depletion and serves as a relevant model of ischemic stress (31). Tissue ischemia, which promotes profound nutrient deprivation, including glucose deprivation, results in an energetic failure that may induce ER stress. Ischemia in rat kidneys is a far more complex process than glucose deprivation in cultured cells, and this difference is a limitation of in vitro models for the study of ischemia. As an example of this limitation, the combination of glucose starvation with hypoxia (which commonly occurs during tissue ischemia) was too toxic to be suitable for further studies in vitro (32). Our in vitro model used glucose starvation to induce the energetic failure that may occur during ischemia in vivo. Because our aim was to investigate the HIF-1α-independent involvement of the UPR in the angiogenic response to ischemia, we did not include hypoxia in our model, as it would not have been possible to delineate the respective contributions of HIF-1α signaling and the UPR. This model enabled us to characterize the role of the UPR transducers in generating an angiogenic response to energetic failure that is independent of HIF-1α.
Our findings highlight the central and complex involvement of the PERK kinase in the modulation of the angiogenic response to glucose deprivation. PERK phosphorylates the translational regulator eIF2α, resulting in a general inhibition of translation. One factor that escapes translational inhibition is the transcription factor ATF4, which regulates the expression of nutrient transporters and antioxidants, amino acid synthesis and the induction of autophagy (11, 31, 33). The mode of regulation of VEGFA expression by UPR mediators seems to depend on the cell type and the identity of ER stress factor. In mouse embryonic fibroblasts, VEGFA is transcriptionally regulated by IRE1α and PERK through their respective transcription factors, sXBP1 and ATF4, but in the HepG2 hepatoma cell line, VEGFA is regulated by ATF6 (21). A more complex regulation of VEGFA was observed in a medulloblastoma cell line, involving regulatory action at both the transcriptional and post-transcriptional levels (34). In our model, VEGFA expression depends only on PERK. Moreover, a salubrinal-induced increase in the activity of this pathway resulted in increased expression of VEGFA. Therefore, PERK might represent a potential therapeutic target for increasing VEGFA production. PERK also regulates bFGF expression at both the transcriptional and translational levels. The fact that salubrinal increases bFGF secretion and the inhibition of the PERK pathway reduces bFGF secretion without modifying transcript expression suggest that the UPR might activate bFGF secretion independently of a transcriptional activity. bFGF lacks the signal peptide and is secreted through a non-conventional pathway that does not involve the trans-Golgi network (35). Instead, bFGF secretion requires activation by caspase 1 within inflammasomes, followed by direct release from the cell through the plasma membrane (36). Recently, ER stress was shown to activate a non-conventional secretion pathway mediated by Golgi reassembly stacking proteins (GRASPs) (37). Other modes of non-conventional secretion, including autophagy, may be involved in bFGF secretion when the UPR is activated. PERK is known to activate autophagy (38), and autophagy is implicated in the non-conventional secretion of various molecules, including interleukin 1β (39), which is secreted in the same way as bFGF (36). Whether bFGF is secreted during the UPR through a similar non-conventional secretion pathway is an interesting hypothesis that remains to be tested.
Our results also show that the UPR regulates ANG expression, a finding of considerable biological significance. In addition to its functions in kidney vasculogenesis (17), ANG is a general inhibitor of translation in response to stress. ANG is a secreted ribonuclease that generates stress-induced tRNA-derived fragments (tiRNAs) that contribute to the displacement of eIF4G/A from capped and uncapped mRNA and eIF4E/G/A (eIF4F) from the m7G cap, thereby inhibiting translation and inducing stress granule assembly (23). Our results suggest that, in addition to the activation of PERK and the phosphorylation of eIF2α, the UPR activates an ANG-mediated pathway that may lead to a global repression of translation. Validation of this hypothesis will require in-depth studies to precisely delineate the respective roles of ANG-induced stress-induced tiRNAs and eIF2α phosphorylation in the inhibition of translational programs during the UPR.
In conclusion, nutrient deprivation in human epithelial cells promotes an angiogenic response that involves VEGFA, bFGF, and ANG secretion. This process occurs independently of HIF-1α, with PERK acting as a central regulator, making it a promising therapeutic target. Additionally, the expression of ANG, a stress-induced translational repressor, is modulated by both IRE1α and PERK, representing an alternate pathway by which the UPR might inhibit protein synthesis in response to stress.
Supplementary Material
This work was supported by a grant from the Institut National de la Sante et de la Recherche Medicale (INSERM).

This article contains supplemental Figs. S1–S4 and Tables S1 and S2.
- mTOR
- mammalian target of rapamycin
- ATF
- activated transcription factor
- CHOP
- C/EBP homologous protein
- eIF2α
- elongation initiation factor 2α
- ER
- endoplasmic reticulum
- GRP
- glucose-related protein
- IRE1α
- inositol-requiring enzyme 1
- PERK
- protein kinase RNA (PKR)-like ER kinase
- UPR
- unfolded protein response
- PDI
- protein-disulfide isomerase
- HREC
- human renal epithelial cells
- XBP-1
- X-box-binding protein 1
- bFGF
- basic fibroblast growth factor
- ANG
- angiogenin
- VEGFA
- vascular endothelial growth factor A
- HERP
- homocysteine-induced endoplasmic reticulum stress protein
- PDI
- protein-disulfide isomerase.
REFERENCES
- 1. Racusen L. C., Regele H. (2010) Kidney Int. Suppl., S27–S32 [DOI] [PubMed] [Google Scholar]
- 2. Zeisberg M., Neilson E. G. (2010) Mechanisms of tubulointerstitial fibrosis. J. Am. Soc. Nephrol. 21, 1819–1834 [DOI] [PubMed] [Google Scholar]
- 3. Mimura I., Nangaku M. (2010) The suffocating kidney: tubulointerstitial hypoxia in end-stage renal disease. Nat. Rev. Nephrol. 6, 667–678 [DOI] [PubMed] [Google Scholar]
- 4. Oskolkova O. V., Afonyushkin T., Leitner A., von Schlieffen E., Gargalovic P. S., Lusis A. J., Binder B. R., Bochkov V. N. (2008) ATF4-dependent transcription is a key mechanism in VEGF up-regulation by oxidized phospholipids: critical role of oxidized sn-2 residues in activation of unfolded protein response. Blood 112, 330–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fine L. G., Norman J. T. (2008) Kidney Int. [Google Scholar]
- 6. Majmundar A. J., Wong W. J., Simon M. C. (2010) Hypoxia-inducible factors and the response to hypoxic stress. Mol. Cell 40, 294–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chade A. R. (2011) Renovascular disease, microcirculation, and the progression of renal injury: role of angiogenesis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 300, R783–R790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mayer G. (2011) Capillary rarefaction, hypoxia, VEGF and angiogenesis in chronic renal disease. Nephrol. Dial Transplant 26, 1132–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wouters B. G., Koritzinsky M. (2008) Hypoxia signaling through mTOR and the unfolded protein response in cancer. Nat. Rev. Cancer 8, 851–864 [DOI] [PubMed] [Google Scholar]
- 10. Sengupta S., Peterson T. R., Sabatini D. M. (2010) Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol. Cell 40, 310–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bi M., Naczki C., Koritzinsky M., Fels D., Blais J., Hu N., Harding H., Novoa I., Varia M., Raleigh J., Scheuner D., Kaufman R. J., Bell J., Ron D., Wouters B. G., Koumenis C. (2005) ER stress-regulated translation increases tolerance to extreme hypoxia and promotes tumor growth. EMBO J. 24, 3470–3481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Drogat B., Auguste P., Nguyen D. T., Bouchecareilh M., Pineau R., Nalbantoglu J., Kaufman R. J., Chevet E., Bikfalvi A., Moenner M. (2007) IRE1 signaling is essential for ischemia-induced vascular endothelial growth factor-A expression and contributes to angiogenesis and tumor growth in vivo. Cancer Res. 67, 6700–6707 [DOI] [PubMed] [Google Scholar]
- 13. Hetz C. (2012) The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 13, 89–102 [DOI] [PubMed] [Google Scholar]
- 14. Walter P., Ron D. (2011) The unfolded protein response: from stress pathway to homeostatic regulation. Science 334, 1081–1086 [DOI] [PubMed] [Google Scholar]
- 15. Pallet N., Thervet E., Le Corre D., Knebelmann B., Nusbaum P., Tomkiewicz C., Meria P., Flinois J. P., Beaune P., Legendre C., Anglicheau D. (2005) Rapamycin inhibits human renal epithelial cell proliferation: effect on cyclin D3 mRNA expression and stability. Kidney Int. 67, 2422–2433 [DOI] [PubMed] [Google Scholar]
- 16. Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-ΔΔC(T)) Method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 17. Nakamura M., Yamabe H., Osawa H., Nakamura N., Shimada M., Kumasaka R., Murakami R., Fujita T., Osanai T., Okumura K. (2006) Hypoxic conditions stimulate the production of angiogenin and vascular endothelial growth factor by human renal proximal tubular epithelial cells in culture. Nephrol. Dial Transplant 21, 1489–1495 [DOI] [PubMed] [Google Scholar]
- 18. Rajnoch J., Lodererova A., Szabo A., Honsova E., Vannay A., Bloudickova S., Matl I., Viklicky O. (2005) Regulators of angiogenesis in renal ischemia/reperfusion injury in normotensive and hypertensive rats: effect of tacrolimus. Transplant Proc. 37, 352–354 [DOI] [PubMed] [Google Scholar]
- 19. Fraisl P., Mazzone M., Schmidt T., Carmeliet P. (2009) Regulation of angiogenesis by oxygen and metabolism. Dev. Cell 16, 167–179 [DOI] [PubMed] [Google Scholar]
- 20. Aragonés J., Fraisl P., Baes M., Carmeliet P. (2009) Oxygen sensors at the crossroad of metabolism. Cell Metab. 9, 11–22 [DOI] [PubMed] [Google Scholar]
- 21. Ghosh R., Lipson K. L., Sargent K. E., Mercurio A. M., Hunt J. S., Ron D., Urano F. (2010) Transcriptional regulation of VEGF-A by the unfolded protein response pathway. PLoS One 5, e9575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Boyce M., Bryant K. F., Jousse C., Long K., Harding H. P., Scheuner D., Kaufman R. J., Ma D., Coen D. M., Ron D., Yuan J. (2005) A selective inhibitor of eIF2α dephosphorylation protects cells from ER stress. Science 307, 935–939 [DOI] [PubMed] [Google Scholar]
- 23. Ivanov P., Emara M. M., Villen J., Gygi S. P., Anderson P. (2011) Angiogenin-induced tRNA fragments inhibit translation initiation. Mol. Cell 43, 613–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leonard E. C., Friedrich J. L., Basile D. P. (2008) VEGF-121 preserves renal microvessel structure and ameliorates secondary renal disease following acute kidney injury. Am. J. Physiol. Renal Physiol. 295, F1648–F1657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Choi Y. J., Chakraborty S., Nguyen V., Nguyen C., Kim B. K., Shim S. I., Suki W. N., Truong L. D. (2000) Peritubular capillary loss is associated with chronic tubulointerstitial injury in human kidney: altered expression of vascular endothelial growth factor. Hum. Pathol. 31, 1491–1497 [DOI] [PubMed] [Google Scholar]
- 26. Rudnicki M., Perco P., Enrich J., Eder S., Heininger D., Bernthaler A., Wiesinger M., Sarközi R., Noppert S. J., Schramek H., Mayer B., Oberbauer R., Mayer G. (2009) Hypoxia response and VEGF-A expression in human proximal tubular epithelial cells in stable and progressive renal disease. Lab. Invest. 89, 337–346 [DOI] [PubMed] [Google Scholar]
- 27. Reinders M. E., Rabelink T. J., Briscoe D. M. (2006) Angiogenesis and endothelial cell repair in renal disease and allograft rejection. J. Am. Soc. Nephrol. 17, 932–942 [DOI] [PubMed] [Google Scholar]
- 28. Strutz F., Zeisberg M., Ziyadeh F. N., Yang C. Q., Kalluri R., Müller G. A., Neilson E. G. (2002) Role of basic fibroblast growth factor-2 in epithelial-mesenchymal transformation. Kidney Int. 61, 1714–1728 [DOI] [PubMed] [Google Scholar]
- 29. Inoki K., Mori H., Wang J., Suzuki T., Hong S., Yoshida S., Blattner S. M., Ikenoue T., Rüegg M. A., Hall M. N., Kwiatkowski D. J., Rastaldi M. P., Huber T. B., Kretzler M., Holzman L. B., Wiggins R. C., Guan K. L. (2011) mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J. Clin. Invest. 121, 2181–2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pallet N., Bouvier N., Bendjallabah A., Rabant M., Flinois J. P., Hertig A., Legendre C., Beaune P., Thervet E., Anglicheau D. (2008) Cyclosporine-induced endoplasmic reticulum stress triggers tubular phenotypic changes and death. Am. J. Transplant 8, 2283–2296 [DOI] [PubMed] [Google Scholar]
- 31. Ye J., Kumanova M., Hart L. S., Sloane K., Zhang H., De Panis D. N., Bobrovnikova-Marjon E., Diehl J. A., Ron D., Koumenis C. (2010) The GCN2-ATF4 pathway is critical for tumour cell survival and proliferation in response to nutrient deprivation. EMBO J. 29, 2082–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Marjon P. L., Bobrovnikova-Marjon E. V., Abcouwer S. F. (2004) Expression of the pro-angiogenic factors vascular endothelial growth factor and interleukin-8/CXCL8 by human breast carcinomas is responsive to nutrient deprivation and endoplasmic reticulum stress. Mol. Cancer 3, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kroemer G., Mariño G., Levine B. (2010) Autophagy and the integrated stress response. Mol. Cell 40, 280–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pereira E. R., Liao N., Neale G. A., Hendershot L. M. (2010) PLoS One 5, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Florkiewicz R. Z., Majack R. A., Buechler R. D., Florkiewicz E. (1995) Quantitative export of FGF-2 occurs through an alternative, energy-dependent, non-ER/Golgi pathway. J. Cell Physiol. 162, 388–399 [DOI] [PubMed] [Google Scholar]
- 36. Keller M., Rüegg A., Werner S., Beer H. D. (2008) Active caspase-1 is a regulator of unconventional protein secretion. Cell 132, 818–831 [DOI] [PubMed] [Google Scholar]
- 37. Gee H. Y., Noh S. H., Tang B. L., Kim K. H., Lee M. G. (2011) Rescue of ΔF508-CFTR trafficking via a GRASP-dependent unconventional secretion pathway. Cell 146, 746–760 [DOI] [PubMed] [Google Scholar]
- 38. Kroemer G., Mariño G., Levine B. (2010) Autophagy and the integrated stress response. Mol. Cell 40, 280–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dupont N., Jiang S., Pilli M., Ornatowski W., Bhattacharya D., Deretic V. (2011) Autophagy-based unconventional secretory pathway for extracellular delivery of IL-1β. EMBO J. 30, 4701–4711 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.