Background: Macrophages represent major viral reservoirs due to their resistance to apoptosis.
Results: Resistance of differentiated macrophages to the apoptotic effects of HIV-Vpr is mediated by cIAP1/2 genes independent of Bcl-xL and Mcl-1.
Conclusion: IAPs protect against Vpr-induced apoptosis, and Bcl-xL and Mcl-1 maintain steady state survival.
Significance: IAP modulation may be a potential strategy to eliminate HIV persistence in macrophages.
Keywords: Apoptosis, Bcl2 Family Proteins, HIV, Macrophages, Signal Transduction, HIV-Vpr, IAPs
Abstract
Macrophages are resistant to HIV cytopathic effects, which contributes to viral persistence and reservoir formation. HIV viral protein R (Vpr) is a potent apoptosis-inducing agent for primary monocytes. Because the biologically active Vpr is found in serum and cerebrospinal fluid of HIV-infected patients, we investigated the apoptotic effect of Vpr on monocyte-derived macrophages and phorbol 12-myristate 13-acetate-activated THP1 macrophages. Our results show that primary monocytes and THP1 cells develop resistance to Vpr-induced apoptosis following differentiation into macrophages. To determine the effect of Vpr on the expression of antiapoptotic proteins, we show that in contrast to the undifferentiated cells, Vpr did not down-regulate the expression of antiapoptotic inhibitors of apoptosis (IAPs) and Bcl2 family members in macrophages, suggesting their involvement in resistance to Vpr-induced apoptosis. However, knocking down Bcl-xL and Mcl-1 proteins induced spontaneous apoptosis with no impact on susceptibility to Vpr-induced apoptosis. In contrast, down-regulation of cellular IAP1 (cIAP1) and cIAP2 by using siRNAs and SMAC (second mitochondria-derived activator of caspases) mimetic sensitized macrophages to Vpr-induced apoptosis. Overall, our results suggest that resistance to Vpr-induced apoptosis is specifically mediated by cIAP1/2 genes independent of Bcl-xL and Mcl-1, which play a key role in maintaining cell viability. Moreover, IAP modulation may be a potential strategy to eliminate HIV persistence in macrophages.
Introduction
Persistence of latent viral reservoirs is the main barrier that prevents eradication and cure of HIV infection. Memory CD4+ T cells and macrophages represent the two major viral reservoirs. Although CD4+ T cells ultimately decrease as a result of incessant viral replication leading to cell death and reach alarmingly low levels characteristic of AIDS-associated lymphopenia, macrophages support viral replication without any cytopathic effects (1, 2). Moreover, infected macrophages contribute to viral dissemination and bystander cell apoptosis (3). Formation of viral reservoirs in memory T cells involves a very low number of infected lymphocytes that contain integrated provirus and support viral replication only when reactivated with their cognate antigen. However, the mechanisms that enable macrophages to become viral reservoirs are poorly understood but may include an intrinsic resistance to apoptosis acquired during differentiation (4) or as an indirect result of infection or specific viral proteins (5–7).
One of the factors that contribute to HIV persistence in macrophages is their resistance to various apoptotic stimuli. This characteristic is acquired as a result of a complex differentiation process that results in increased phagocytic and secretory functions and enables macrophages to perform their function in stressful environments (8). Primary monocytes are not susceptible to in vitro HIV infection (9), but they become highly permissive after differentiation (10). Increased susceptibility to infection and a resistant phenotype acquired during differentiation are important factors that are believed to promote viral reservoir formation in macrophages (11).
Because resistance to apoptosis plays a key role in HIV persistence in cellular reservoirs, we investigated how monocyte to macrophage differentiation would impact resistance to apoptosis in the context of HIV infection. In order to circumvent the lack of productive in vitro infection of primary human monocytes, we used Vpr (viral protein R) as an apoptosis-inducing agent. Vpr is a 96-amino acid, 14-kDa accessory protein of HIV, known for its ability to cause apoptosis in several cell types (12, 13), including lymphocytes (14, 15), monocytes (16), and neurons (17). It has been shown that the two halves of Vpr are functionally distinct; the N-terminal amino acid 1–51 region of Vpr, Vpr(1–51), is responsible for nuclear localization of the preintegration complex, whereas the C-terminal region of amino acids 52–96, Vpr(52–96), induces cell cycle arrest and apoptosis (18, 19). Moreover, Vpr is required for HIV to infect macrophages (15), and extracellular Vpr can rescue replication of Vpr-deficient HIV strains in macrophages (20).
The expression of the main antiapoptotic Bcl2 and inhibitor of apoptosis (IAP)4 family members has been linked to cell survival (21). Apoptosis induced through the intrinsic pathway is initiated by proapoptotic members of the Bcl2 family, such as Bax, when not bound and thus not inactivated by antiapoptotic members. Bax forms pores into the mitochondrial membrane through which apoptogenic factors are released into the cytosol (22). The basal levels of antiapoptotic Bcl2 proteins, namely A1 (23), Mcl-1 (24), Bcl-xL (25), and Bcl2 (26), gradually increase during macrophage differentiation and contribute to the development of resistance to apoptosis in different model systems. HIV infection has been shown to induce the expression of Bcl2 and Bcl-xL in human macrophages (5). These antiapoptotic proteins were also shown to play a role in HIV Tat- and HIV Nef-mediated resistance to apoptosis (6, 27). In contrast, members of the IAP family act on caspases activated either through the extrinsic (death receptor) or the intrinsic (mitochondrial) pathway (28). X-linked IAP (XIAP) up-regulation during macrophage differentiation was linked to their enhanced survival (26, 29). However, the role of IAPs in enhanced survival of macrophages in HIV infection remains unknown.
Biologically active Vpr is released from infected cells and has been detected in the serum and cerebrospinal fluid of HIV-infected patients (30), but its effect on survival of human macrophages remains unknown. We show for the first time that differentiated macrophages, unlike primary monocytes, are resistant to the apoptotic effects of Vpr, suggesting that differentiation may render macrophages resistant to the cytopathic effects of Vpr present in the circulation. To investigate the mechanism underlying the development of this resistance to Vpr-induced apoptosis, we show that Bcl-xL and Mcl-1 play a critical role in macrophage survival in the absence of apoptotic stimuli. In contrast, down-regulation of IAPs renders macrophages susceptible to the apoptotic effects of Vpr, suggesting a critical role this family may play in acquiring resistance against Vpr-induced cell death.
EXPERIMENTAL PROCEDURES
Cells and Reagents
THP1, a promonocytic cell line derived from a human acute monocytic leukemia patient, was obtained from the American Type Culture Collection (Manassas, VA) and maintained in IMDM-10 (Sigma-Aldrich) supplemented with 10% fetal bovine serum (Invitrogen) and 100 units/ml penicillin and 100 g/ml gentamicin (both from Sigma-Aldrich). THP1 cells (5 × 105/ml) were differentiated with 20 ng/ml PMA (Sigma-Aldrich) for 2 days to obtain THP1-derived macrophages (THP1-MACs) (31). Blood was obtained from healthy volunteers according to a protocol approved by the Ethics Review Committee of Ottawa General Hospital. Donors gave written, informed consent. Human peripheral blood mononuclear cells were isolated by Ficoll Paque (GE Healthcare) density centrifugation, and the cell layer consisting mainly of mononuclear cells was collected. To generate monocyte-derived macrophages (MDMs), monocytes were isolated by the adherence method. Peripheral blood mononuclear cells were resuspended in serum-free medium (5 × 106/ml) and plated in 12-well polystyrene plates (BD Biosciences). After being allowed to adhere for 3 h, non-adherent cells were washed off, and adherent cells were cultured for 6 days in IMDM supplemented with 10% fetal bovine serum and 10 ng/ml MCSF (R&D Systems, Minneapolis, MN). MDMs thus obtained were 98% CD14+. Primary monocytes were obtained from peripheral blood mononuclear cells by using Automacs negative selection (Miltenyi Biotech, Auburn, CA) as per the manufacturer's instructions. Bcl2 inhibitor HA14-1 was purchased from Sigma-Aldrich. SMAC (second mitochondria-derived activator of caspases) mimetic AEG40730 was a generous gift from Dr. Korneluck (Children's Hospital of Eastern Ontario Research Institute, Apoptosis Research Centre).
Cell Treatment with Vpr
C-terminal Vpr (amino acids 52–96) was synthesized by Invitrogen. Mutant Vpr peptide was synthesized by Genemed Synthesis Inc. (San Francisco, CA). Peptides were obtained by automated solid-phase synthesis using 9-fluorenylmethoxycarbonyl and purified by reverse-phase high pressure liquid chromatography (>95%), followed by analysis with electrospray mass spectrometry. The amino acid sequence is as follows: Vpr(52–96), DTWAGVEAIIRILQQLLFIHFRIGCRHSRIGVTRQRRARNGASRS. Mutant Vpr (mVpr) has the same sequence as Vpr(52–96) except for Arg to Ala substitutions at positions 73, 77, and 80 (underlined in the sequence above), which are important for Vpr interaction with the mitochondria and subsequent apoptosis (32, 33). Because of the high propensity of Vpr to bind to proteins, cells were treated with Vpr peptides in an isotonic buffer for 30 min (12), followed by the addition of culture medium. Unless otherwise specified, primary monocytes were treated with Vpr for 4 h, whereas THP1-MACs and MDMs were collected after 24 h of Vpr treatment.
Apoptosis Analysis by Intracellular Propidium Iodide (PI) or Annexin-V Staining
Apoptosis in primary monocytes and THP1 cells was measured by staining cells with FITC-labeled annexin-V (Invitrogen) for 15 min at room temperature in the dark. Annexin-V-positive cells were quantified by flow cytometry. Because annexin-V staining is not appropriate for adherent cells, adherent macrophages were evaluated for cell death by using intracellular PI staining. Following Vpr treatment, cells were washed with PBS and fixed with methanol for 15 min at 4 °C. The methanol was washed away with PBS and cells were treated with 25 μl of 10 μg/ml RNase A, followed by staining with 25 μl of 1 mg/ml PI solution (Sigma-Aldrich) at 4 °C for 1 h. The DNA content was analyzed using a FACSCanto flow cytometer (BD Biosciences) and the FACSDiva software. The subdiploid DNA peak (<2N DNA), immediately adjacent to the G0/G1 peak (2N DNA), represents apoptotic cells and was quantified by histogram analyses. PI histograms figures were obtained with WinMDI version 2.8 software (J. Trotter, Scripps Institute, San Diego, CA).
Determination of Mitochondrial Membrane Permeability
Mitochondrial dysfunction was assessed by utilizing Rh123 cationic lipophilic green fluorochrome rhodamine (Invitrogen). Disruption of the mitochondrial membrane potential is associated with decreased Rh123 retention and lower fluorescence. After Vpr treatment, cells were incubated with 100 ng/ml Rh123 for 30 min at 37 °C (23), followed by flow cytometry analysis as described above.
Confocal Laser-scanning Fluorescence Microscopy
For experiments involving MDMs, monocytes were initially adhered and differentiated on round glass coverslips in 12-well plates. After Vpr treatment, cells were fixed in 4% paraformaldehyde for 15 min, rinsed in PBS, and then permeabilized with 0.1% Triton X-100 for another 10 min. The coverslips were incubated with anti-Vpr rabbit primary antibody at 4 °C overnight. On the second day, coverslips were rinsed with 5% BSA in PBS and incubated in the same buffer with Alexa Fluor 680-conjugated goat anti-rabbit secondary antibody for 1 h at room temperature. Coverslips were mounted on microscopy slides using ProLong Gold antifade mounting medium with DAPI (Invitrogen) and examined with a Zeiss LSM 510 Meta confocal microscope using a red HeNe (633-nm) laser. The objective used was Plan-Apochromat ×63. Fluorescent images were acquired with ZEN 2009 software and analyzed with ImageJ software.
Western Blot Analysis
Total cell proteins obtained after lysis of cell pellets were subjected to SDS-PAGE and transferred onto a polyvinylidene difluoride membrane (Bio-Rad). Membranes were probed with antibodies against cellular IAP1 (cIAP1), cIAP2, XIAP, Bcl2, Bcl-xL, Mcl-1, poly(ADP-ribose) polymerase (PARP), and caspase-3 (all from Cell Signaling Technology, Danvers, MA), followed by goat secondary antibodies conjugated to horseradish peroxidase (Bio-Rad). The anti-Vpr antibody was kindly provided by Dr. Eric Cohen (University of Montreal). To control for total protein loading, membranes were stripped of the primary antibodies and reprobed with anti- GAPDH (Sigma-Aldrich) or β-actin (Cell Signaling Technology) antibodies. Immunoblots were visualized using the Amersham Biosciences ECL Western blotting detection system. The images were obtained with the Chemigenius bioimaging system and the GeneSnap software (both from Syngene). Quantification of band intensities was performed using GeneTools software (Syngene).
Transfection with siRNA
THP1-MACs (5 × 105/ml) were treated with cIAP1 siRNA, cIAP2 siRNA (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), and control siRNA (Qiagen, Venlo, The Netherlands) in serum-free medium, using Fugene 6 (Roche Applied Science) as a transfection reagent, according to the manufacturer's instructions. Initially, 250 and 500 ng of siRNA were mixed with Fugene 6 in 100 μl of medium to allow formation of RNA complexes. The proportion of Fugene 6 to siRNA was 3 μl per 1 μg of siRNA. After 45 min, the siRNA mixtures were added to cells in serum-free medium, which was replaced with complete medium after 5 h. Mcl-1 and Bcl-xL siRNAs along with the transfection reagent were purchased from Santa Cruz Biotechnology, Inc., and used as per the manufacturer's instructions. Briefly, 40 pmol of siRNAs were preincubated for 30 min with 4 μl of transfection reagent in 100 μl of serum-free medium before the addition to the cells. Serum-free medium was replaced with complete medium after 5 h. Following transfection, cells were either collected after 24 h for evaluation of knockdown efficiency or treated with 2.5 μm Vpr for another 6 h (for PARP cleavage and caspase-3 cleavage) or 24 h (for apoptosis measurement).
Statistical Analyses
Data were plotted using Windows Excel 2010 (Microsoft) and GraphPad Prism 5. Significance was determined using Student's t test or analysis of variance, followed by Tukey's test. A p value of less than 0.05 (indicated by an asterisk) was considered significant. Unless otherwise specified, plotted data represent the mean ± S.D. of at least three experiments.
RESULTS
Macrophages Are Resistant to Vpr-induced Apoptosis Compared with Undifferentiated Monocytic Cells
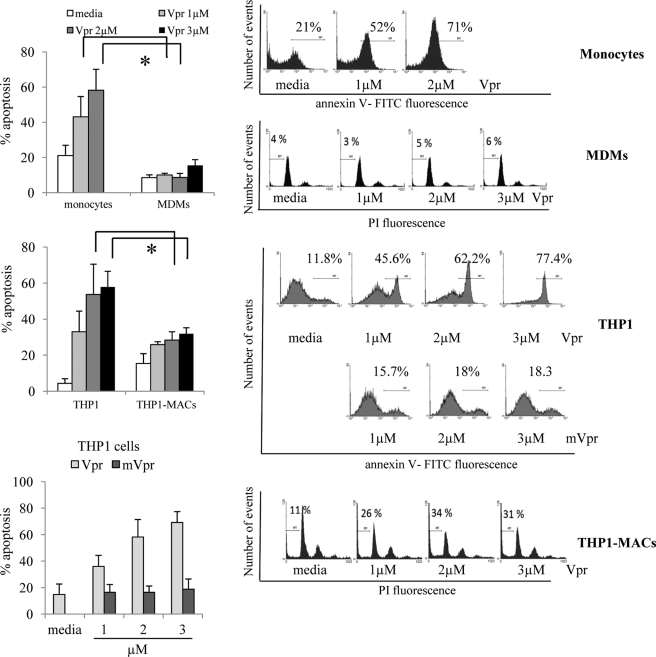
To determine whether macrophage differentiation influences susceptibility to Vpr-induced apoptosis, we measured apoptosis in response to Vpr in undifferentiated primary monocytes and THP1 cells and compared it with differentiated MDMs and THP1-MACs. We utilized synthetic C terminus Vpr(52–96) peptide because it is free of contaminating bacterial products present in recombinant Vpr proteins and other factors present in retroviral supernatants that may nonspecifically activate various signaling pathways. Moreover, Vpr(52–96) peptide mimics the apoptotic activity of full-length Vpr peptide (12). The commercial synthesis of full-length Vpr peptide (1–96) was not feasible for the purpose of apoptosis induction because its length may have caused stability and functionality issues. Unless otherwise specified, we will refer to the Vpr(52–96) peptide as Vpr. Primary monocytes, THP1 cells, THP1-MACs, and MDMs were treated with Vpr for varying periods of time followed by measurement of apoptosis by intracellular PI and annexin-V staining. Vpr induced a high level of apoptosis in THP1 cells and primary monocytes in a dose-dependent manner (Fig. 1, top and middle left). In contrast, the apoptotic effect of Vpr was completely abolished in MDMs (Fig. 1, top left). Mutant Vpr (mVpr) was used as a negative control because it does not induce apoptosis (Fig. 1, bottom left).
FIGURE 1.
Monocyte differentiation confers resistance to Vpr-induced apoptosis. THP1 cells, MDMs and THP1-MACs (0.5 × 106/ml) were treated with the indicated concentrations of Vpr or mVpr for 24 h. Primary monocytes (0.5 × 106/ml) were treated with Vpr for 4 h. Apoptosis was measured by flow cytometry using intracellular PI and/or annexin-V staining. The numbers represent the percentage of apoptotic cells with subdiploid DNA content (MDMs and THP1-MACs) or annexin-V-positive cells (THP1, monocytes). Left panels show the mean percentage of apoptotic cells ± S.D. of at least three experiments. Histograms on the right show data from one representative experiment with each cell type. *, p < 0.05. Error bars, S.D.
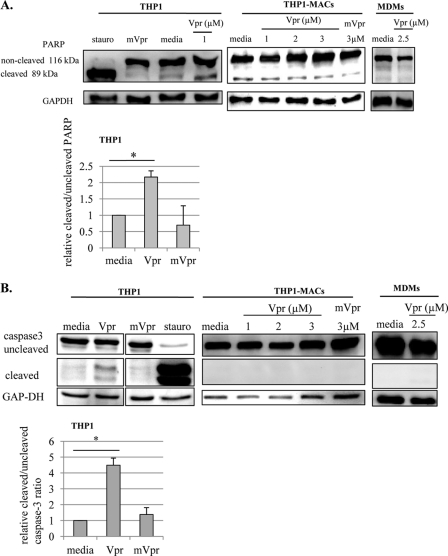
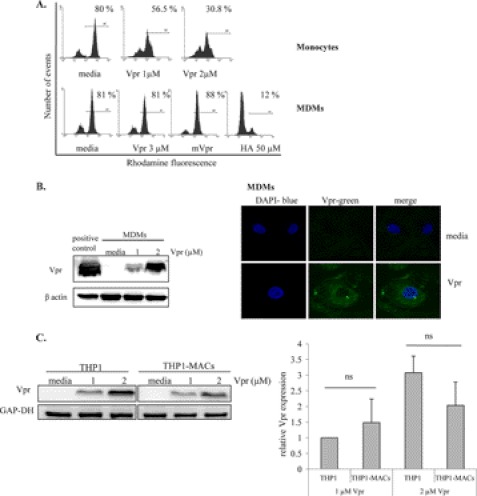
Treatment of THP1-MACs with Vpr caused apoptosis to moderately low levels of 2-fold compared with the 12 fold increase seen in THP1 cells. THP1-MACs exhibited a significant reduction in cell death, with an apoptosis level of around 30% even with the highest dose of Vpr peptide (Fig. 1, middle left). Vpr-induced apoptosis was confirmed by analyzing caspase-3 cleavage and PARP cleavage as a marker of caspase-dependent apoptosis (34). Both PARP (Fig. 2A) and caspase-3 cleavage (Fig. 2B) were observed after 6 h of Vpr treatment in THP1 cells, whereas this effect was not seen in THP1-MACs and MDMs, in agreement with their lower sensitivity to Vpr-induced apoptosis. In correlation with the lack of apoptosis, mutant Vpr did not affect either caspase-3 or PARP cleavage (Fig. 2, A and B). Because Vpr is known to induce apoptosis via the intrinsic pathway in sensitive cells (14), we determined if Vpr causes mitochondrial depolarization in MDMs by rhodamine staining. Mitochondrial depolarization was seen in Vpr-treated primary monocytes in a dose-dependent manner, whereas no difference was detected between the levels of rhodamine-positive cells in Vpr-treated MDMs compared with the untreated cells. Mitochondrial depolarization of MDMs was easily detected after a 4-h treatment with the Bcl2 family inhibitor HA14-1 as a positive control (Fig. 3A).
FIGURE 2.
Vpr causes PARP and caspase-3 cleavage in monocytic cells but not in macrophages. Cells were treated with Vpr, mVpr, or staurosporine for 6 h, following which total cell proteins were subjected to Western blotting, and the membranes were probed with anti-PARP (A) or anti-caspase-3 (B) antibodies to evaluate their cleavage as markers of apoptosis. GAPDH was used as a loading control. Based on the densitometric analysis, the bar graphs in the lower panels show the mean ± S.D. (error bars) of cleaved/uncleaved ratios from three experiments in THP1 cells. *, p < 0.05.
FIGURE 3.
Vpr enters MDMs without causing mitochondrial depolarization. A, MDMs and primary monocytes were treated with the indicated concentrations of Vpr, following which cells were stained with rhodamine for mitochondrial membrane potential evaluation as described under “Experimental Procedures.” Bcl2 inhibitor HA14-1 was used as a positive control and mVpr as a negative control for MDMs. Histograms show rhodamine-positive cells, indicative of live cells. Histograms show one representative experiment for three similar results. B (left), MDMs were treated with Vpr for 24 h, following which whole cell protein extracts were subjected to Western blotting. The membrane was probed with anti-Vpr antibody and anti-β actin antibody to control for protein loading. B (right), following differentiation on coverslips, MDMs were treated with Vpr for another 24 h. Cells were stained according to the protocol described under “Experimental Procedures,” and images were acquired with a Zeiss LSM 510 Meta confocal microscope using a ×63 objective. Total magnification was ×157.5. C, THP1 cells and THP1-MACs were treated with Vpr for 6 h, following which total proteins were subjected to Western blotting. The membranes were probed with anti-Vpr antibody and anti-GAP-DH antibody to control for protein loading. Vpr expression level was quantified relative to GAPDH, and the bar graph shows the mean ± S.D. (error bars) of four experiments. ns, not significant.
Vpr enters cells by a process that occurs independently of cellular receptors (19, 20). Because MDMs exhibited resistance to Vpr-induced apoptosis, we investigated the ability of Vpr to enter human macrophages. We were able to detect Vpr in whole cell extracts prepared from Vpr-treated MDMs using an anti-Vpr antibody (Fig. 3B, left). In order to confirm the intracellular location of Vpr following treatment, we also performed confocal microscopy on Vpr-treated MDMs. Microscopy images indicate that Vpr localizes in the cytoplasm of MDMs (Fig. 3B, right), in agreement with previous findings that indicate the presence of a nuclear localization signal in the N terminus of the peptide (35). These results suggest that the lack of cell death is not due to the inability of the peptide to enter the cells because Vpr could be detected in the MDM cytoplasm.
Because THP1-MACs respond to the apoptotic effect of Vpr, albeit at lower levels compared with THP1 cells (Fig. 1, middle left), we also compared the relative levels of Vpr in THP1-MACs and THP1 cells. We did not detect significant differences in Vpr expression in whole cell extracts prepared from Vpr-treated THP1 cells and THP1-MACs (Fig. 3C), suggesting that Vpr uptake may not explain the difference in sensitivity of these cells to the apoptotic effect of Vpr.
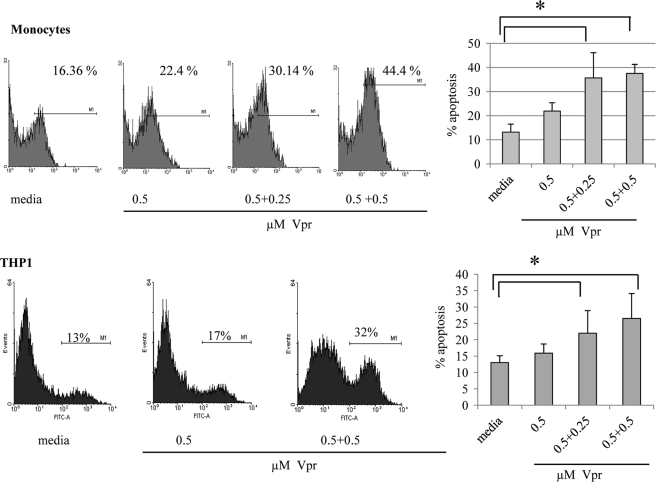
Sequential Exposure of Monocytes to Low Non-apoptogenic Concentrations of Vpr Causes Apoptosis
Although the effect of Vpr has been described as mainly apoptogenic, there are also a few reports that describe the protective effect of low concentrations of Vpr on T cells (36, 37). Moreover, because Vpr concentrations used in these in vitro experiments are higher than the ones described in the serum of HIV-infected patients (38), we wanted to determine if low concentrations of Vpr affect survival of monocytic cells. For this, cells were exposed to low concentrations of Vpr sequentially in a way that mimics the in vivo exposure to extracellular Vpr. Monocytes and THP1 cells were treated with two low, non-apoptogenic concentrations of Vpr, followed by measurement of apoptosis. Our results indicate that two sequential doses of non-apoptogenic concentrations of 0.5 or 0.25 μm Vpr will eventually cause higher apoptosis compared with a single dose (Fig. 4). These results indicate that monocytic cells are not protected from apoptosis by low doses of Vpr but, on the contrary, that repeated non-apoptogenic doses will eventually cause cell death. Furthermore, higher in vitro concentrations may mechanistically reflect in vivo continuous exposures to lower Vpr concentrations.
FIGURE 4.
Sequential exposure of undifferentiated monocytic cells to low non-apoptotic concentrations of Vpr causes apoptosis. Primary monocytes (1.0 × 106/ml) were treated first with a non-apoptotic dose of 0.5 μm Vpr for 2 h, followed by a second low dose of 0.25 or 0.5 μm Vpr for another 2 h. THP1 cells (1.0 × 106/ml) were treated first with a non-apoptotic dose of 0.5 μm Vpr for 24 h, followed by a second low dose of 0.25 or 0.5 μm Vpr for another 24 h. Cells were then analyzed by annexin-V staining and flow cytometry for the measurement of apoptosis. Right panels show the mean percentage of apoptosis ± S.D. (error bars) of three experiments. *, p < 0.05. Histograms show one representative experiment.
Analysis of Antiapoptotic Proteins Profile following Differentiation in MDMs and THP1-MACs
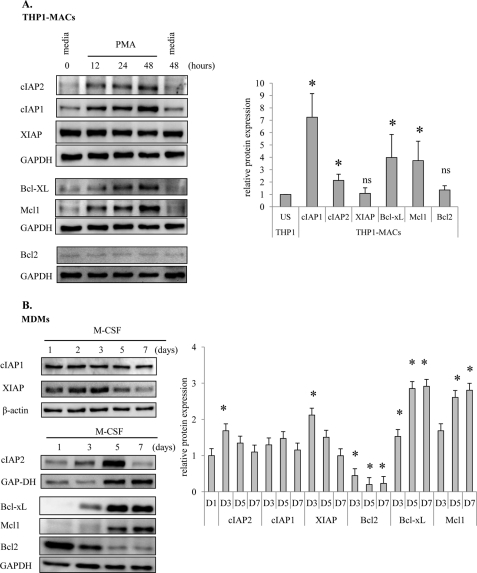
To understand the molecular mechanism governing resistance of human macrophages to Vpr-induced apoptosis, we have also employed THP1 cells as a model of differentiation because these cells are more committed toward a monocyte/macrophage lineage than other monocytic cell lines (39). In addition, the expression profile of antiapoptotic proteins in these two cell types (THP1-MACs and MDMs) is not known. Therefore, we initially determined the expression of Bcl2 and IAP antiapoptotic family members in both models of differentiation. There was a gradual increase in expression of Bcl-xL and Mcl-1 (Fig. 5A) in THP1-MACS. During monocyte differentiation with MCSF, there was a similar gradual increase in Bcl-xL and Mcl-1 expression. However, we observed a constant decrease in the levels of Bcl2, in agreement with observations from studies performed in CD34+ progenitor cells (Fig. 5B) (40). Among the IAP family members, cIAP1 and cIAP2 expression was increased, whereas XIAP levels remained unchanged following THP1 cell differentiation with PMA (Fig. 5A). In MDMs, expression of cIAP1 remained unchanged, whereas cIAP2 and XIAP levels showed a bimodal distribution, with a peak on day 3 after MCSF treatment (Fig. 5B). Overall, these results show induction of BclxL and Mcl-1 following differentiation in both models. In contrast, induction of cIAP1/2, XIAP, and Bcl2 was not similar in the two models, which may be attributed to the distinct cell types used (cell line versus primary cells) and to the agents employed for differentiation (PMA for THP1-MACs versus MCSF for MDMs). Therefore, these results suggest that both IAPs and Bcl2 family proteins may be responsible for the acquisition of a resistant phenotype toward Vpr-induced apoptosis in both models of macrophage differentiation. However, we cannot exclude the possibility that the existent basal levels of antiapoptotic proteins that did not change or decreased during differentiation in both models may be enough to protect cells from Vpr-induced cell death.
FIGURE 5.
Expression of antiapoptotic proteins during macrophage differentiation. THP1 cells (0.5 × 106/ml) were differentiated with 20 ng/ml PMA (A). Monocytes isolated by adherence were differentiated with 10 ng/ml of MCSF (B). Cells were collected at the indicated times (12, 24, and 48 h for THP1-MACs and days 1, 3, 5, and 7 for MDMs) throughout differentiation, and whole cell protein extracts were subjected to Western blotting. Membranes were probed with specific antibodies against various members of the Bcl2 and IAP families, and the expression level of antiapoptotic proteins was quantified to control for equal protein loading. Right panels show the mean relative protein expression ± S.D. (error bars) of at least three experiments for each cell type at the indicated time points for MDMs (bottom right) and at 48 h for THP1-MACs (top right). *, p < 0.05; ns, not significant.
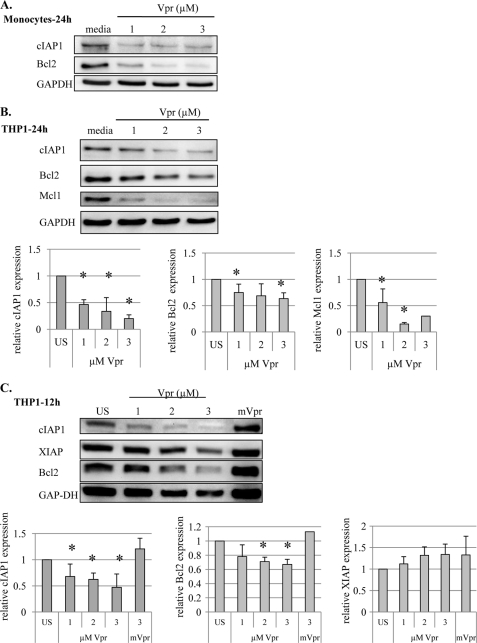
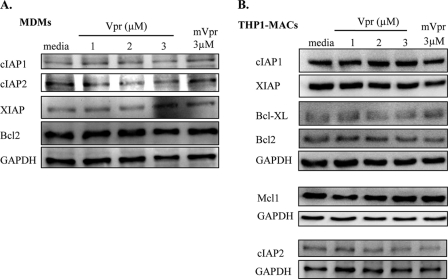
Vpr Does Not Affect Bcl2 and cIAP1 Expression in Macrophages
To elucidate the mechanisms underlying macrophage resistance to Vpr-induced apoptosis, we examined the effect of Vpr on the expression of apoptosis-related proteins of the Bcl2 and IAP families. We have recently shown that cIAP2 induction by TLR-9 ligand CpG pretreatment can protect primary monocytes from Vpr-induced apoptosis (16). Treatment with Vpr for 24 h down-regulated cIAP1 and Bcl2 expression in primary monocytes (Fig. 6A) and cIAP1, Bcl2, and Mcl-1 expression in THP1 cells (Fig. 6B). Similarly, down-regulation of cIAP1 and Bcl2 was also observed at an earlier time point, following 12 h of Vpr treatment in THP1 cells (Fig. 6C). However, there was no effect on the levels of XIAP (Fig. 6C), indicating that Vpr targets cIAP1 and Bcl2 selectively in order to induce cell death in sensitive cells. However, Vpr did not affect the expression of antiapoptotic cIAP1, cIAP2, XIAP, and Bcl2 in MDMs (Fig. 7A) or of cIAP1, cIAP2, XIAP, Bcl2, Bcl-xL, and Mcl-1 proteins in THP1-MACs (Fig. 7B), suggesting that both members of Bcl2 and IAP families may play a role in macrophage protection against Vpr-induced apoptosis.
FIGURE 6.
Vpr down-regulates the expression of Bcl2 and cIAP1 in monocytic cells. Primary monocytes (2 × 106/ml) (A) and THP1 cells (1 × 106/ml) were treated with the indicated concentrations of Vpr or mVpr for 24 h (B) and 12 h (C). Total cell proteins were subjected to Western blotting, and the membranes were probed with the indicated antibodies. In THP1 cells, the expression level of antiapoptotic proteins was quantified to control for equal protein loading. Based on the densitometric analysis, the bar graphs in the bottom panels show the mean ± S.D. (error bars) of at least three experiments. *, p < 0.05.
FIGURE 7.
Vpr does not affect the expression of Bcl2 and cIAP1 in macrophages. MDMs (A) and THP1-MACs (B) were treated with the indicated concentrations of Vpr or mVpr for 24 h. Total cell proteins were subjected to Western blotting, and the membranes were probed with specific antibodies against various members of the Bcl2 and IAP families. The results shown are representative of three separate experiments with similar results.
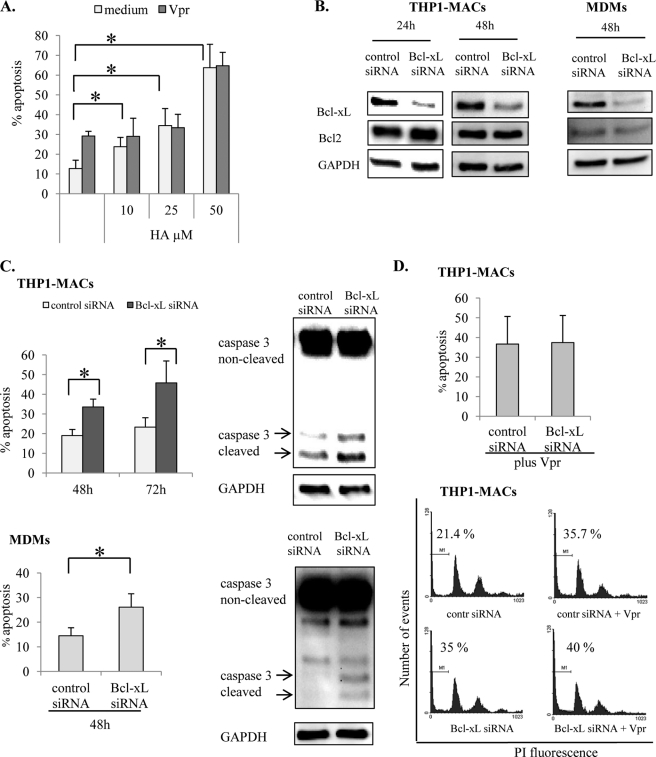
Bcl-xL and Mcl-1 Play a Role in Macrophage Survival but Not in Resistance to Vpr-induced Apoptosis
The apoptotic effect of Vpr is associated with mitochondrial depolarization and activation of the intrinsic apoptotic pathway, culminating in caspase-3 activation in T cells (41). Because the Bcl2 family members are primarily responsible for maintaining mitochondrial membrane potential (22), we investigated if Bcl2 proteins mediate resistance of macrophages against Vpr-induced cell death. To dissect the role of the Bcl2 family members, we employed HA14–1, a small molecule that mimics the Bcl2-homology 3 domain of Bcl2 proteins. The inhibitory mechanism of HA14-1 involves binding and inactivating antiapoptotic Bcl2 and Bcl-xL proteins (42). THP1-MACs were treated with various concentrations of HA14-1 for 4 h, followed by a second treatment with 2.5 μm Vpr for 24 h. The biological activity of HA14-1 was tested in NIH 3T3 cells (data not shown), where it caused caspase-dependent cell death (42). HA14-1 induced apoptosis on its own in THP1-MACs, indicating the importance of Bcl2 proteins in sustaining THP1-MAC viability (Fig. 8A). However, Vpr treatment of THP1-MACs previously exposed to HA14-1 did not enhance the levels of apoptosis beyond those observed with the HA14-1 inhibitor alone (Fig. 8A). These results suggested that blocking Bcl2 proteins does not impact susceptibility to Vpr-induced apoptosis.
FIGURE 8.
Bcl-xL contributes to macrophage survival but is not involved in resistance to Vpr-induced apoptosis. A, THP1 cells (1 × 106/ml) were treated for 2 h with the indicated concentrations of Bcl2 inhibitor HA14-1 prior to Vpr (2.5 μm) treatment for another 24 h, following which cells were stained with PI and evaluated for apoptosis by flow cytometry. The numbers represent percentage apoptotic cells with subdiploid DNA content. The bar graph shows the mean ± S.D. (error bars) of three experiments. *, p < 0.05. B, after differentiation, THP1-MACs and MDMs were treated with Bcl-xL or control siRNA as described under “Experimental Procedures.” Efficiency of protein knockdown and siRNA specificity were evaluated by Western blotting after 24 and 48 h of siRNA treatment. C, at the indicated times following transfection, cells were evaluated for apoptosis by flow cytometry (left) or for caspase-3 cleavage by Western blotting (right; 48 h post-transfection). The numbers in the left panel represent percentage of apoptotic cells with subdiploid DNA content. The bar graphs show the mean percentage of apoptosis ± S.D. of three experiments. *, p < 0.05. D, THP1-MACs were initially transfected with Bcl-xL and control siRNA for 24 h, after which Vpr (2.5 μm) was added for another 24 h. Following Vpr treatment, cells were collected and stained with PI for flow cytometry evaluation. The numbers represent percentage apoptotic cells with subdiploid DNA content. The bar graph shows the mean ± S.D. of three experiments (top). Histograms show one representative experiment for three similar results (bottom).
The inhibitory effect of HA14–1 is limited to Bcl2 and Bcl-xL members of the Bcl2 family (42). The results of differentiation experiments indicated that only Bcl-xL expression was up-regulated, whereas Bcl2 expression remained unaffected following THP1 differentiation with PMA (Fig. 5A). Following differentiation with MCSF in MDMs, Bcl-xL expression was enhanced, and Bcl2 was down-regulated (Fig. 5B). Because Bcl-xL up-regulation was common in both models of macrophage differentiation, we confirmed the role of Bcl-xL in cell survival by using Bcl-xL-specific siRNA to inhibit its expression in both THP1-MACs and MDMs. Transfection of THP1-MACs and MDMs with Bcl-xL siRNA decreased the basal expression of Bcl-xL (Fig. 8B). In contrast, Bcl2 expression was not affected, which controlled for the siRNA specificity. Decreased Bcl-xL expression was associated with a significant increase in cell death of both cell types (Fig. 8C, left). This increase in apoptosis was associated with enhanced caspase-3 cleavage in cells transfected with Bcl-xL siRNA as compared with the cells transfected with control siRNA (Fig. 8C, right). To determine the effect of Bcl-xL inhibition on resistance to Vpr-induced apoptosis, THP1-MACs transfected with Bcl-xL siRNA for 24 h were treated with Vpr for another 24 h, following which apoptosis was measured by flow cytometry. Notably, knocking down Bcl-xL levels did not change susceptibility of macrophages to Vpr-induced apoptosis (Fig. 8D).
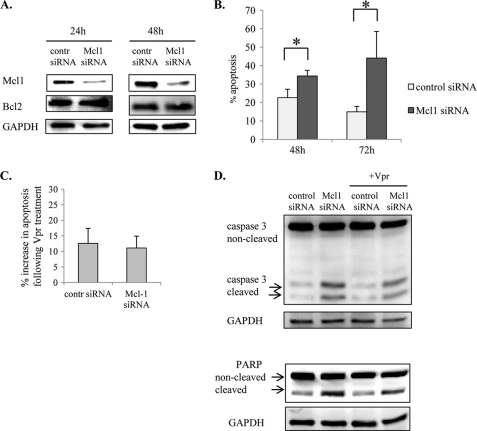
The expression of antiapoptotic Mcl-1 was also increased in THP1-MACs and MDMs (Fig. 5, A and B). Moreover, Vpr decreased the expression of Mcl-1 in THP1 cells (Fig. 6A) but not in THP1-MACs (Fig. 7B), suggesting that Mcl-1 up-regulation during differentiation may contribute to the resistant phenotype of THP1-MACs. Because the inhibitory effect of HA14–1 does not affect Mcl-1 activity (42), we analyzed the role of Mcl-1 by using specific siRNA. Mcl-1 siRNA significantly decreased Mcl-1 expression at both 24 and 48 h post-transfection of THP1-MACs, with no effect on Bcl2 (Fig. 9A). Decreased Mcl-1 expression was associated with a significant increase in apoptosis 48 h after transfection that was even more pronounced at 72 h post-transfection (Fig. 9B). In order to address the effect of Mcl-1 inhibition on susceptibility to Vpr-induced apoptosis, THP1-MACs transfected with Mcl-1 siRNA for 24 h were treated with Vpr for another 24 h, following which apoptosis was measured by flow cytometry. Similar to the Bcl-xL siRNA results, apoptosis in response to Vpr treatment did not increase following transfection of THP1-MACs with Mcl-1 siRNA (Fig. 9C). Also, Vpr did not increase PARP and caspase-3 cleavage in either control or Mcl-1 siRNA-transfected cells as compared with the control or Mcl-1 siRNA-transfected cells, respectively (Fig. 9D, lane 1 versus lane 3 and lane 2 versus lane 4). Overall, these results suggest that although Bcl2 and Mcl-1 are important in maintaining macrophage steady state viability, they do not contribute to protection against Vpr-induced apoptosis.
FIGURE 9.
Mcl-1 contributes to macrophage survival but is not involved in resistance to Vpr-induced apoptosis. A, after differentiation, THP1-MACs (5 × 105/ml) were transfected with Mcl-1 or control siRNA, as described under “Experimental Procedures.” Efficiency of protein knockdown and siRNA specificity were evaluated by Western blotting after 24 and 48 h of transfection. B, following transfection, cells were stained with PI for apoptosis assessment. The numbers represent percentage apoptotic cells with subdiploid DNA content. The bar graph shows the mean ± S.D. (error bars) of three experiments. *, p < 0.05. C, following transfection, cells were cultured for 24 h, and Vpr was added to the cells for another 24 h. Subsequently, cells were stained with PI for flow cytometry. The y axis shows the percentage increase in apoptotic cells following Vpr treatment, calculated by subtracting the percentage of apoptotic cells in transfected samples from the transfected and Vpr-treated samples. The bar graph shows the mean ± S.D. of three experiments. D, cells treated as in C were collected and evaluated for caspase-3 and PARP cleavage by Western blotting.
IAPs Protect Macrophages against Vpr-induced Apoptosis
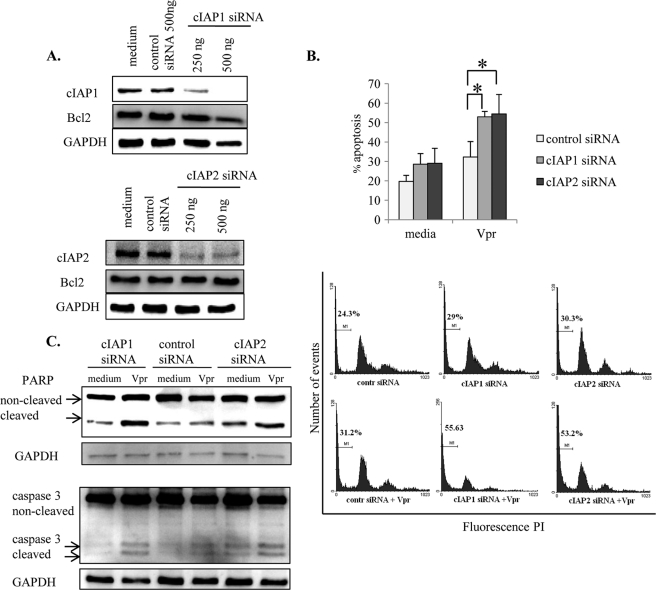
We have recently shown that cIAP2 plays a critical role in protecting undifferentiated monocytic cells from Vpr-induced apoptosis (16). The increased expression levels of cIAP1 and cIAP2 in macrophages (Fig. 5A), along with the discrepancy between the ability of Vpr to down-regulate cIAP1 in monocytes (Fig. 6) but not in macrophages (Fig. 7), prompted us to determine if cIAP1 or cIAP2 mediate decreased macrophage sensitivity to Vpr-induced apoptosis.
To study the role of cIAPs, THP1-MACs were transfected with specific siRNAs for cIAP1 and cIAP2 followed by treatment with Vpr peptide and analysis of apoptosis. The results suggest that transfection of THP1-MACs with cIAP1 and cIAP2 siRNA significantly down-regulated the endogenous expression of cIAP1 and cIAP2 proteins without affecting Bcl2 expression level (Fig. 10A). However, unlike Bcl-xL and Mcl-1 siRNA results, transfection of THP1-MACs with cIAP1 and cIAP2 siRNA did not affect the basal level of apoptosis between cells transfected with control and cIAP siRNA (Fig. 10B). In contrast, Vpr significantly increased apoptosis of cIAP1 and cIAP2 siRNA-transfected cells compared with the cells transfected with control siRNA (Fig. 10B). This increase in Vpr-induced apoptosis in IAP-transfected cells was also associated with a notable increase in PARP and caspase-3 cleavage following Vpr treatment as compared with the cells transfected with control siRNA (Fig. 10C). These results suggest that both cIAP1 and cIAP2 are involved in resistance to Vpr-induced apoptosis in THP1-MACs.
FIGURE 10.
IAPs protect macrophages against Vpr-induced apoptosis. A, after differentiation, THP1-MACs (5 × 105/ml) were transfected with cIAP1, cIAP2, or control siRNA as described under “Experimental Procedures.” Following transfection, cells were collected after 24 h for evaluation of protein knockdown. Total cell proteins were subjected to Western blotting, and the membranes were probed with antibodies specific for cIAP1, cIAP2, and Bcl2 to ensure siRNA specificity. B and C, on the second day after transfection, cells were treated with Vpr (2.5 μm) for another 24 h and stained with PI for apoptosis measurement (B) or subjected to Western blotting for PARP and caspase-3 cleavage (C). Only 500 ng of IAP siRNA and control siRNA were used for these experiments. The bar graph in the top panel of B shows the mean percentage of apoptosis ± S.D. (error bars) of four separate experiments. *, p < 0.05. Histograms in the bottom panel of B show one representative experiment indicating the percentage of cells with subdiploid DNA content.
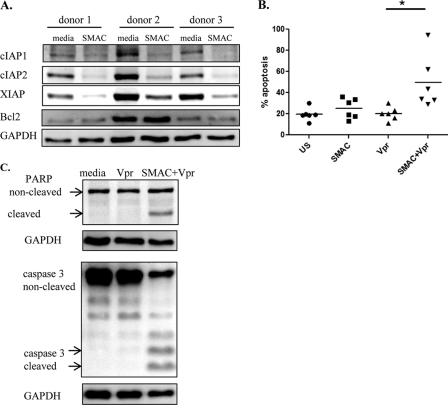
To study the involvement of IAPs in protection of MDMs, we employed SMAC mimetic AEG40730, designed to knock down IAPs protein levels by targeting them for ubiquitination and proteosomal degradation (43). Initially, we evaluated the effect of AEG40730 on the expression of various antiapoptotic proteins. Treatment of MDMs from three different donors with AEG40730 significantly decreased the expression levels of cIAP1, cIAP2, and XIAP, with no effect on Bcl2 (Fig. 11A), suggesting that the SMAC mimetic acts specifically on the IAP proteins. AEG40730 alone did not have a significant effect on apoptosis of MDMs (Fig. 11B). Similarly, Vpr alone, as expected, did not enhance apoptosis compared with untreated cells. However, pretreatment of MDMs with SMAC mimetic was able to sensitize MDMs to the apoptotic effect of Vpr. This increase in Vpr-induced apoptosis in SMAC mimetic-treated cells was associated with enhanced PARP and caspase-3 cleavage compared with the untreated cells (Fig. 11C). These results suggest that SMAC mimetic renders MDMs sensitive to caspase-3-dependent cell death. Overall, the results suggest that both cIAP1 and cIAP2 do not influence survival in the absence of an apoptotic stimulus (spontaneous apoptosis), but that these antiapoptotic proteins contribute to macrophage protection against Vpr-induced apoptosis.
FIGURE 11.
SMAC mimetic AEG40730 sensitizes MDMs to Vpr-induced apoptosis. A, MDMs from three different donors were treated with SMAC mimetic AEG40730 (200 nm) for 24 h, and IAP and Bcl2 expression was evaluated by Western blotting. B and C, following a 24-h treatment with SMAC mimetic, MDMs were treated with Vpr (2.5 μm) for another 24 h. Cells were stained with PI for apoptosis evaluation (B), or they were used for Western blotting to evaluate PARP and caspase-3 cleavage (C). The dot plot in B shows flow cytometry data and the mean percentage of apoptosis ± S.D. (error bars) of six separate experiments with cells from different donors. *, p < 0.05. Western blotting results shown in C are representative of three different donors.
DISCUSSION
Macrophages represent major viral reservoirs in HIV infection because they are resistant to the viral cytopathic effects (1). Hijacking the apoptotic pathway in the initial phases of infection has been shown to promote survival of macrophages infected with either bacterial (44, 45) or viral pathogens (46). However, the mechanism underlying macrophage resistance to apoptosis and their establishment as HIV reservoirs remains unknown. In this study, we have investigated the apoptotic effect of HIV-Vpr on survival of undifferentiated and differentiated human monocytic cells. We demonstrate for the first time that although primary human monocytes are susceptible to Vpr-induced apoptosis, they develop resistance to this HIV peptide upon differentiation into macrophages, suggesting that differentiation provides a protective mechanism against the Vpr-mediated apoptotic effect. In determining the molecular mechanism governing the development of this resistance, our results suggest that resistance to Vpr-induced apoptosis is specifically mediated by cIAP1 and cIAP2 of the IAP family, independently of the Bcl-xL and Mcl-1 of the Bcl2 family, which play a critical role in maintaining viability in the absence of any apoptotic stimulus.
The mechanism by which Vpr induces apoptosis has been investigated in various cell types, including lymphocytes (14) and neurons (33). Vpr was shown to bind to the adenine nucleotide translocator of the mitochondrial membrane, resulting in membrane permeabilization and release of apoptogenic factors, such as cytochrome c, into the cytosol and culminating in caspase-3 activation and apoptosis (12). Furthermore, pretreatment of mitochondria with Bcl2 protein was able to prevent Vpr-induced cell death (12).
Because our results indicate that Vpr does not induce mitochondrial permeabilization or PARP and caspase-3 cleavage in macrophages, in contrast to primary undifferentiated monocytes, we initially investigated the role of the antiapoptotic Bcl2 family of proteins in rendering macrophages resistant to Vpr-mediated apoptosis. Differentiation of THP1 cells was associated with increased expression of Bcl-xL and Mcl-1, with no change in Bcl2. In primary monocytes, Bcl2 expression was consistently down-regulated following differentiation, whereas Bcl-xL and Mcl-1 expression were also increased. These differences are most likely due to the different stimuli used to induce differentiation (PMA and MCSF) as well as to the cell type that is being induced to differentiate (primary monocytes and leukemic THP1 cells, respectively). We also expect the apoptotic machinery to be tipped toward resistance to cell death in cell lines compared with primary cells because enhanced resistance to cell death is an intrinsic feature of cancerous cells. Therefore, chemical inhibitors and siRNA technology were employed to evaluate the role of individual antiapoptotic proteins.
Based on these observations, we initially hypothesized that increased expression of antiapoptotic Bcl2 proteins may account for the inability of Vpr to induce apoptosis in macrophages. Both Bcl-xL and Mcl-1 are important for cell viability because of their ability to maintain mitochondrial integrity. Bcl-xL has been shown to contribute to survival of bone marrow-derived macrophages in both murine (47) and human (25) models of differentiation. Mcl-1 was also implicated in survival of human macrophages, in either physiological (24) or pathological conditions (48). Our results show that inhibition of Bcl2 activity by pretreatment of THP1-MACs with HA14-1 or by siRNAs specific for Bcl-xL and Mcl-1 do not impact their susceptibility to Vpr-induced apoptosis. Instead, these antiapoptotic proteins were responsible for maintaining viability in the absence of any apoptotic stimulus.
Because members of the IAP family directly bind and inactivate both initiator and effector caspases downstream of mitochondria (28), we evaluated their involvement in macrophage resistance to Vpr-induced apoptosis. IAPs are characterized by conserved structural baculoviral IAP repeat domains that mediate protein-protein interactions. There are currently eight members of the IAP family, with cIAP1, cIAP2, and XIAP being the most studied (49). Inhibition of cIAP1 and cIAP2 by employing specific siRNAs restored susceptibility to Vpr-induced apoptosis in macrophages. Similarly, SMAC mimetic AEG40730, which induces degradation of IAPs, was also able to sensitize cells to caspase-3 cleavage and apoptosis in response to Vpr treatment. Moreover, in contrast to Bcl-xL and Mcl-1, inhibition of IAPs did not cause cell death in the absence of Vpr, making these proteins more amenable to therapeutic approaches. These results also suggest a novel, distinct role for cIAP1 and cIAP2 in conferring protection of macrophages against Vpr-induced apoptosis. To our knowledge, this is the first report implicating cIAPs in protecting cells from apoptosis in the context of HIV infection.
The precise molecular mechanism and the signaling pathways by which IAPs induce resistance to Vpr in differentiated macrophages remain to be investigated. It is possible that IAPs expressed in these cells may bind caspase-3, thereby making it inaccessible to cleavage and activation following Vpr treatment. In human peripheral blood mononuclear cells, Vpr causes release of cytochrome c from the mitochondria with subsequent activation of caspase-9 and caspase-3 (14). However, although caspase-3 was detected in macrophages pretreated with AEG40730 following Vpr treatment (Fig. 11C), we were unable to detect caspase-9 cleavage (data not shown). This result suggests that sensitization of macrophages probably occurs downstream or independently of mitochondria. This is in agreement with our observations that inhibition of Bcl2 family proteins responsible for maintaining mitochondrial integrity did not render cells susceptible to Vpr treatment.
Another possibility is that Vpr may not inhibit NF-κB activity in macrophages. Both cIAP1 and Bcl2 are known NF-κB-dependent genes (50). This mechanism may be ineffective in macrophages that have constitutively active NF-κB (24) and may explain why Vpr was unable to decrease cIAP1 and Bcl2 expression levels in macrophages. Macrophages may need additional stimuli, such as SMAC mimetic, to bring IAP levels down to a minimum threshold that would release caspase-3 from IAP blockage and make it available to Vpr cleavage in order to sensitize macrophages to Vpr-induced cell death.
The apoptotic effect of Vpr has been functionally linked to its ability to cause cell cycle arrest through the activation of the DNA damage-signaling protein ATR in T cells (51). This stress signaling pathway was defective in terminally differentiated macrophages due to the absence of key proteins, thus explaining the inability of Vpr to induce cell cycle arrest in a cell type that no longer progresses through the cell cycle (51). Although cell cycle arrest may be a requirement for Vpr-induced apoptosis in lymphocytes, our results suggest that this requirement can be bypassed by down-regulating IAPs in differentiated cells.
Although relatively low concentrations of Vpr have been detected in the blood of HIV-infected individuals (30), we and others have used relatively high concentrations of Vpr (1–3 μm) as an apoptosis-inducing agent (12, 52). It has been suggested that low levels of Vpr are protective in T cells (36). Our results show that undifferentiated monocytes exposed sequentially to low non-apoptogenic concentrations of Vpr eventually undergo apoptosis. These results suggest that primary monocytes exposed persistently to low concentrations of Vpr present in serum/lymph nodes of HIV-infected individuals under in vivo conditions may in fact undergo apoptosis.
The role of Vpr induced apoptosis in vivo has been investigated only in respect to neurons (53). Our results and previous reports suggest a complex model of Vpr effect in HIV-infected cells of the monocyte/macrophage lineage. Recently, Laforge et al. (54) reported an increased apoptosis in monocytes independently of viral replication during the early stage of SIV infection. Although the role of specific HIV peptides was not investigated, the involvement of extracellularly released Vpr in apoptosis of monocytes cannot be ruled out. We have previously shown that despite the lack of productive infection, monocytes from HIV-infected individuals displayed higher levels of both spontaneous and IFNγ-induced cell death compared with uninfected controls (55). If monocytes are also infected, once differentiated they give rise to macrophages that display impaired functioning and are prone to apoptosis (54). However, if macrophages get infected with HIV after differentiation, these cells are protected from cell death. Our results suggest that differentiated macrophages display a high degree of resistance to Vpr-induced apoptosis. Furthermore, macrophages infected in vitro with HIV were also protected from cell death (data not shown). In non-dividing cells, such as macrophages, Vpr is particularly important because it contributes to the localization of the preintegration complex to the nucleus and allows for permissive HIV infection in the absence of mitosis (56). This property along with their resistance to Vpr-induced apoptosis may promote reservoir formation in this cell type.
Keeping in view our results, it will be interesting to determine if macrophages infected in vitro with HIV can be killed by extracellular Vpr in the presence of agents that inhibit IAP expression or activity. We were unable to induce apoptosis in in vitro HIV-infected macrophages with SMAC mimetic as a single agent, possibly because of other accessory HIV proteins, such as Nef and Tat, which have been shown to enhance cell survival (6, 27). However, our results suggest that Vpr peptide provides an excellent experimental setting to study intrinsic cellular factors that may explain macrophage resistance and eliminate indirect effects that other accessory HIV proteins may have on cell survival.
In summary, our results suggest for the first time that by down-regulating cIAPs, macrophages could be sensitized to Vpr-induced cell death. Strategies aimed at eliminating HIV viral reservoirs have received considerable interest. Memory T cells have been reactivated with various agents that would drive them out of their resting state and thus sensitize them to conventional antiretroviral therapy (57). Because the mechanisms of viral persistence are different in memory T cells and macrophages, it is reasonable to assume that viral eradication would require different strategies in macrophages. Inducing cell death by manipulating the apoptotic machinery is a strategy currently explored in cancer treatment, where overexpression of antiapoptotic proteins is a major mechanism of chemoresistance that can be counteracted by using Bcl2 inhibitors (58) or SMAC mimetics (59). Subsequent experiments are needed to identify apoptotic stimuli to which SMAC mimetics may sensitize infected macrophages. Targeting cIAPs may thus potentially prove beneficial in purging the macrophage HIV reservoir and eventually in killing persistently infected cells.
Acknowledgments
We thank Drs. Paul MacPherson, Maya Kozlowski, and Gina Graziani for critically reading the manuscript.
This work was supported by grants from the Canadian Institutes of Health Research and the Ontario HIV Treatment Network (to A. K.).
- IAP
- inhibitor of apoptosis
- XIAP
- X-linked IAP
- cIAP
- cellular IAP
- PMA
- phorbol 12-myristate 13-acetate
- THP1-MAC
- THP1-derived macrophage
- MDM
- monocyte-derived macrophage
- mVpr
- mutant Vpr
- PI
- propidium iodide
- PARP
- poly(ADP-ribose) polymerase.
REFERENCES
- 1. Aquaro S., Bagnarelli P., Guenci T., De Luca A., Clementi M., Balestra E., Caliò R., Perno C. F. (2002) Long-term survival and virus production in human primary macrophages infected by human immunodeficiency virus. J. Med. Virol. 68, 479–488 [DOI] [PubMed] [Google Scholar]
- 2. Aquaro S., Caliò R., Balzarini J., Bellocchi M. C., Garaci E., Perno C. F. (2002) Macrophages and HIV infection. Therapeutical approaches toward this strategic virus reservoir. Antiviral Res. 55, 209–225 [DOI] [PubMed] [Google Scholar]
- 3. Badley A. D., McElhinny J. A., Leibson P. J., Lynch D. H., Alderson M. R., Paya C. V. (1996) Up-regulation of Fas ligand expression by human immunodeficiency virus in human macrophages mediates apoptosis of uninfected T lymphocytes. J. Virol. 70, 199–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kiener P. A., Davis P. M., Starling G. C., Mehlin C., Klebanoff S. J., Ledbetter J. A., Liles W. C. (1997) Differential induction of apoptosis by Fas-Fas ligand interactions in human monocytes and macrophages. J. Exp. Med. 185, 1511–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guillemard E., Jacquemot C., Aillet F., Schmitt N., Barré-Sinoussi F., Israël N. (2004) Human immunodeficiency virus 1 favors the persistence of infection by activating macrophages through TNF. Virology 329, 371–380 [DOI] [PubMed] [Google Scholar]
- 6. Zhang M., Li X., Pang X., Ding L., Wood O., Clouse K. A., Hewlett I., Dayton A. I. (2002) Bcl-2 up-regulation by HIV-1 Tat during infection of primary human macrophages in culture. J. Biomed. Sci. 9, 133–139 [DOI] [PubMed] [Google Scholar]
- 7. Olivetta E., Federico M. (2006) HIV-1 Nef protects human monocyte-derived macrophages from HIV-1-induced apoptosis. Exp. Cell Res. 312, 890–900 [DOI] [PubMed] [Google Scholar]
- 8. Gordon S., Taylor P. R. (2005) Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5, 953–964 [DOI] [PubMed] [Google Scholar]
- 9. Sonza S., Maerz A., Deacon N., Meanger J., Mills J., Crowe S. (1996) Human immunodeficiency virus type 1 replication is blocked prior to reverse transcription and integration in freshly isolated peripheral blood monocytes. J. Virol. 70, 3863–3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Peng G., Greenwell-Wild T., Nares S., Jin W., Lei K. J., Rangel Z. G., Munson P. J., Wahl S. M. (2007) Myeloid differentiation and susceptibility to HIV-1 are linked to APOBEC3 expression. Blood 110, 393–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lum J. J., Badley A. D. (2003) Resistance to apoptosis. Mechanism for the development of HIV reservoirs. Curr. HIV Res. 1, 261–274 [DOI] [PubMed] [Google Scholar]
- 12. Jacotot E., Ravagnan L., Loeffler M., Ferri K. F., Vieira H. L., Zamzami N., Costantini P., Druillennec S., Hoebeke J., Briand J. P., Irinopoulou T., Daugas E., Susin S. A., Cointe D., Xie Z. H., Reed J. C., Roques B. P., Kroemer G. (2000) The HIV-1 viral protein R induces apoptosis via a direct effect on the mitochondrial permeability transition pore. J. Exp. Med. 191, 33–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yao X. J., Mouland A. J., Subbramanian R. A., Forget J., Rougeau N., Bergeron D., Cohen E. A. (1998) Vpr stimulates viral expression and induces cell killing in human immunodeficiency virus type 1-infected dividing Jurkat T cells. J. Virol. 72, 4686–4693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Muthumani K., Hwang D. S., Desai B. M., Zhang D., Dayes N., Green D. R., Weiner D. B. (2002) HIV-1 Vpr induces apoptosis through caspase 9 in T cells and peripheral blood mononuclear cells. J. Biol. Chem. 277, 37820–37831 [DOI] [PubMed] [Google Scholar]
- 15. Ayyavoo V., Mahalingam S., Rafaeli Y., Kudchodkar S., Chang D., Nagashunmugam T., Williams W. V., Weiner D. B. (1997) HIV-1 viral protein R (Vpr) regulates viral replication and cellular proliferation in T cells and monocytoid cells in vitro. J. Leukoc. Biol. 62, 93–99 [DOI] [PubMed] [Google Scholar]
- 16. Saxena M., Busca A., Pandey S., Kryworuchko M., Kumar A. (2011) CpG protects human monocytic cells against HIV-Vpr-induced apoptosis by cellular inhibitor of apoptosis-2 through the calcium-activated JNK pathway in a TLR9-independent manner. J. Immunol. 187, 5865–5878 [DOI] [PubMed] [Google Scholar]
- 17. Patel C. A., Mukhtar M., Harley S., Kulkosky J., Pomerantz R. J. (2002) Lentiviral expression of HIV-1 Vpr induces apoptosis in human neurons. J. Neurovirol. 8, 86–99 [DOI] [PubMed] [Google Scholar]
- 18. Sawaya B. E., Khalili K., Gordon J., Srinivasan A., Richardson M., Rappaport J., Amini S. (2000) Transdominant activity of human immunodeficiency virus type 1 Vpr with a mutation at residue Arg-73. J. Virol. 74, 4877–4881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Henklein P., Bruns K., Sherman M. P., Tessmer U., Licha K., Kopp J., de Noronha C. M., Greene W. C., Wray V., Schubert U. (2000) Functional and structural characterization of synthetic HIV-1 Vpr that transduces cells, localizes to the nucleus, and induces G2 cell cycle arrest. J. Biol. Chem. 275, 32016–32026 [DOI] [PubMed] [Google Scholar]
- 20. Sherman M. P., Schubert U., Williams S. A., de Noronha C. M., Kreisberg J. F., Henklein P., Greene W. C. (2002) HIV-1 Vpr displays natural protein-transducing properties. Implications for viral pathogenesis. Virology 302, 95–105 [DOI] [PubMed] [Google Scholar]
- 21. Busca A., Saxena M., Kryworuchko M., Kumar A. (2009) Anti-apoptotic genes in the survival of monocytic cells during infection. Curr. Genomics 10, 306–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Petros A. M., Olejniczak E. T., Fesik S. W. (2004) Structural biology of the Bcl-2 family of proteins. Biochim. Biophys. Acta 1644, 83–94 [DOI] [PubMed] [Google Scholar]
- 23. Pagliari L. J., Perlman H., Liu H., Pope R. M. (2000) Macrophages require constitutive NF-κB activation to maintain A1 expression and mitochondrial homeostasis. Mol. Cell. Biol. 20, 8855–8865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu H., Perlman H., Pagliari L. J., Pope R. M. (2001) Constitutively activated Akt-1 is vital for the survival of human monocyte-differentiated macrophages. Role of Mcl-1, independent of nuclear factor (NF)-κB, Bad, or caspase activation. J. Exp. Med. 194, 113–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sanz C., Benito A., Silva M., Albella B., Richard C., Segovia J. C., Insunza A., Bueren J. A., Fernández-Luna J. L. (1997) The expression of Bcl-x is down-regulated during differentiation of human hematopoietic progenitor cells along the granulocyte but not the monocyte/macrophage lineage. Blood 89, 3199–3204 [PubMed] [Google Scholar]
- 26. Zhang J., Li Y., Yu M., Chen B., Shen B. (2003) Lineage-dependent NF-κB activation contributes to the resistance of human macrophages to apoptosis. Hematol. J. 4, 277–284 [DOI] [PubMed] [Google Scholar]
- 27. Choi H. J., Smithgall T. E. (2004) HIV-1 Nef promotes survival of TF-1 macrophages by inducing Bcl-XL expression in an extracellular signal-regulated kinase-dependent manner. J. Biol. Chem. 279, 51688–51696 [DOI] [PubMed] [Google Scholar]
- 28. Riedl S. J., Shi Y. (2004) Molecular mechanisms of caspase regulation during apoptosis. Nat. Rev. Mol. Cell Biol. 5, 897–907 [DOI] [PubMed] [Google Scholar]
- 29. Lin H., Chen C., Chen B. D. (2001) Resistance of bone marrow-derived macrophages to apoptosis is associated with the expression of X-linked inhibitor of apoptosis protein in primary cultures of bone marrow cells. Biochem. J. 353, 299–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Levy D. N., Refaeli Y., MacGregor R. R., Weiner D. B. (1994) Serum Vpr regulates productive infection and latency of human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. U.S.A. 91, 10873–10877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sly L. M., Guiney D. G., Reiner N. E. (2002) Salmonella enterica serovar Typhimurium periplasmic superoxide dismutases SodCI and SodCII are required for protection against the phagocyte oxidative burst. Infect. Immun. 70, 5312–5315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lum J. J., Cohen O. J., Nie Z., Weaver J. G., Gomez T. S., Yao X. J., Lynch D., Pilon A. A., Hawley N., Kim J. E., Chen Z., Montpetit M., Sanchez-Dardon J., Cohen E. A., Badley A. D. (2003) Vpr R77Q is associated with long-term nonprogressive HIV infection and impaired induction of apoptosis. J. Clin. Invest. 111, 1547–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kitayama H., Miura Y., Ando Y., Hoshino S., Ishizaka Y., Koyanagi Y. (2008) Human immunodeficiency virus type 1 Vpr inhibits axonal outgrowth through induction of mitochondrial dysfunction. J. Virol. 82, 2528–2542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Boulares A. H., Yakovlev A. G., Ivanova V., Stoica B. A., Wang G., Iyer S., Smulson M. (1999) Role of poly(ADP-ribose) polymerase (PARP) cleavage in apoptosis. Caspase 3-resistant PARP mutant increases rates of apoptosis in transfected cells. J. Biol. Chem. 274, 22932–22940 [DOI] [PubMed] [Google Scholar]
- 35. Karni O., Friedler A., Zakai N., Gilon C., Loyter A. (1998) A peptide derived from the N-terminal region of HIV-1 Vpr promotes nuclear import in permeabilized cells. Elucidation of the NLS region of the Vpr. FEBS Lett. 429, 421–425 [DOI] [PubMed] [Google Scholar]
- 36. Conti L., Matarrese P., Varano B., Gauzzi M. C., Sato A., Malorni W., Belardelli F., Gessani S. (2000) Dual role of the HIV-1 Vpr protein in the modulation of the apoptotic response of T cells. J. Immunol. 165, 3293–3300 [DOI] [PubMed] [Google Scholar]
- 37. Fukumori T., Akari H., Iida S., Hata S., Kagawa S., Aida Y., Koyama A. H., Adachi A. (1998) The HIV-1 Vpr displays strong anti-apoptotic activity. FEBS Lett. 432, 17–20 [DOI] [PubMed] [Google Scholar]
- 38. Hoshino S., Sun B., Konishi M., Shimura M., Segawa T., Hagiwara Y., Koyanagi Y., Iwamoto A., Mimaya J., Terunuma H., Kano S., Ishizaka Y. (2007) Vpr in plasma of HIV type 1-positive patients is correlated with the HIV type 1 RNA titers. AIDS Res. Hum. Retroviruses 23, 391–397 [DOI] [PubMed] [Google Scholar]
- 39. Auwerx J. (1991) The human leukemia cell line, THP-1. A multifacetted model for the study of monocyte-macrophage differentiation. Experientia 47, 22–31 [DOI] [PubMed] [Google Scholar]
- 40. Josefsen D., Myklebust J. H., Lømo J., Sioud M., Blomhoff H. K., Smeland E. B. (2000) Differential expression of bcl-2 homologs in human CD34+ hematopoietic progenitor cells induced to differentiate into erythroid or granulocytic cells. Stem Cells 18, 261–272 [DOI] [PubMed] [Google Scholar]
- 41. Muthumani K., Choo A. Y., Hwang D. S., Chattergoon M. A., Dayes N. N., Zhang D., Lee M. D., Duvvuri U., Weiner D. B. (2003) Mechanism of HIV-1 viral protein R-induced apoptosis. Biochem. Biophys. Res. Commun. 304, 583–592 [DOI] [PubMed] [Google Scholar]
- 42. Wang J. L., Liu D., Zhang Z. J., Shan S., Han X., Srinivasula S. M., Croce C. M., Alnemri E. S., Huang Z. (2000) Structure-based discovery of an organic compound that binds Bcl-2 protein and induces apoptosis of tumor cells. Proc. Natl. Acad. Sci. U.S.A. 97, 7124–7129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cheung H. H., Mahoney D. J., Lacasse E. C., Korneluk R. G. (2009) Down-regulation of c-FLIP Enhances death of cancer cells by smac mimetic compound. Cancer Res. 69, 7729–7738 [DOI] [PubMed] [Google Scholar]
- 44. Banga S., Gao P., Shen X., Fiscus V., Zong W. X., Chen L., Luo Z. Q. (2007) Legionella pneumophila inhibits macrophage apoptosis by targeting pro-death members of the Bcl2 protein family. Proc. Natl. Acad. Sci. U.S.A. 104, 5121–5126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gross A., Terraza A., Ouahrani-Bettache S., Liautard J. P., Dornand J. (2000) In vitro Brucella suis infection prevents the programmed cell death of human monocytic cells. Infect. Immun. 68, 342–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chan G., Nogalski M. T., Bentz G. L., Smith M. S., Parmater A., Yurochko A. D. (2010) PI3K-dependent up-regulation of Mcl-1 by human cytomegalovirus is mediated by epidermal growth factor receptor and inhibits apoptosis in short-lived monocytes. J. Immunol. 184, 3213–3222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sevilla L., Zaldumbide A., Carlotti F., Dayem M. A., Pognonec P., Boulukos K. E. (2001) Bcl-XL expression correlates with primary macrophage differentiation, activation of functional competence, and survival and results from synergistic transcriptional activation by Ets2 and PU.1. J. Biol. Chem. 276, 17800–17807 [DOI] [PubMed] [Google Scholar]
- 48. Sly L. M., Hingley-Wilson S. M., Reiner N. E., McMaster W. R. (2003) Survival of Mycobacterium tuberculosis in host macrophages involves resistance to apoptosis dependent upon induction of antiapoptotic Bcl-2 family member Mcl-1. J. Immunol. 170, 430–437 [DOI] [PubMed] [Google Scholar]
- 49. Srinivasula S. M., Ashwell J. D. (2008) IAPs. What's in a name? Mol. Cell 30, 123–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Karin M., Lin A. (2002) NF-κB at the crossroads of life and death. Nat. Immunol. 3, 221–227 [DOI] [PubMed] [Google Scholar]
- 51. Zimmerman E. S., Sherman M. P., Blackett J. L., Neidleman J. A., Kreis C., Mundt P., Williams S. A., Warmerdam M., Kahn J., Hecht F. M., Grant R. M., de Noronha C. M., Weyrich A. S., Greene W. C., Planelles V. (2006) Human immunodeficiency virus type 1 Vpr induces DNA replication stress in vitro and in vivo. J. Virol. 80, 10407–10418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sabbah E. N., Roques B. P. (2005) Critical implication of the (70–96) domain of human immunodeficiency virus type 1 Vpr protein in apoptosis of primary rat cortical and striatal neurons. J. Neurovirol. 11, 489–502 [DOI] [PubMed] [Google Scholar]
- 53. Jones G. J., Barsby N. L., Cohen E. A., Holden J., Harris K., Dickie P., Jhamandas J., Power C. (2007) HIV-1 Vpr causes neuronal apoptosis and in vivo neurodegeneration. J. Neurosci. 27, 3703–3711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Laforge M., Campillo-Gimenez L., Monceaux V., Cumont M. C., Hurtrel B., Corbeil J., Zaunders J., Elbim C., Estaquier J. (2011) HIV/SIV infection primes monocytes and dendritic cells for apoptosis. PLoS Pathog. 7, e1002087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Alhetheel A., Yakubtsov Y., Abdkader K., Sant N., Diaz-Mitoma F., Kumar A., Kryworuchko M. (2008) Amplification of the signal transducer and activator of transcription I signaling pathway and its association with apoptosis in monocytes from HIV-infected patients. AIDS 22, 1137–1144 [DOI] [PubMed] [Google Scholar]
- 56. Connor R. I., Chen B. K., Choe S., Landau N. R. (1995) Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology 206, 935–944 [DOI] [PubMed] [Google Scholar]
- 57. Stellbrink H. J., van Lunzen J., Westby M., O'Sullivan E., Schneider C., Adam A., Weitner L., Kuhlmann B., Hoffmann C., Fenske S., Aries P. S., Degen O., Eggers C., Petersen H., Haag F., Horst H. A., Dalhoff K., Möcklinghoff C., Cammack N., Tenner-Racz K., Racz P. (2002) Effects of interleukin-2 plus highly active antiretroviral therapy on HIV-1 replication and proviral DNA (COSMIC trial). AIDS 16, 1479–1487 [DOI] [PubMed] [Google Scholar]
- 58. Lu M., Wang J., Li Y., Berenzon D., Wang X., Mascarenhas J., Xu M., Hoffman R. (2010) Treatment with the Bcl-xL inhibitor ABT-737 in combination with interferon α specifically targets JAK2V617F-positive polycythemia vera hematopoietic progenitor cells. Blood 116, 4284–4287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chauhan D., Neri P., Velankar M., Podar K., Hideshima T., Fulciniti M., Tassone P., Raje N., Mitsiades C., Mitsiades N., Richardson P., Zawel L., Tran M., Munshi N., Anderson K. C. (2007) Targeting mitochondrial factor Smac/DIABLO as therapy for multiple myeloma (MM). Blood 109, 1220–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]