Background: Pluripotent human embryonic stem cells (hESCs) are crucial for studying the molecular processes governing human germ cell specification.
Results: Human germ cells highly expressed epithelial cell adhesion molecule (EpCAM) and the synergistic effects of BMP4/WNT3A promote hESCs toward germline differentiation.
Conclusion: BMP4/WNT3A stimulation and OCT4/EpCAM selection allow enrichment of germ cell-like cells from differentiating hESCs.
Significance: This study provides a robust system to elucidate the molecular mechanisms of human germ cell development.
Keywords: Cell Differentiation, Development, Embryonic Stem Cell, Transgenic, Wnt Signaling, BMP Signaling, Cell Biology, EpCAM, Germ Cells, OCT4
Abstract
The establishment of an effective germ cell selection/enrichment platform from in vitro differentiating human embryonic stem cells (hESCs) is crucial for studying the molecular and signaling processes governing human germ cell specification and development. In this study, we developed a germ cell-enriching system that enables us to identify signaling factors involved in germ cell-fate induction from differentiating hESCs in vitro. First, we demonstrated that selection through an OCT4-EGFP reporter system can successfully increase the percentage of meiotic-competent, germ cell-like cells from spontaneously differentiating hESCs. Furthermore, we showed that the pluripotency associated surface marker, epithelial cell adhesion molecule (EpCAM), is also expressed in human fetal gonads and can be used as an effective selection marker for germ cell enrichment from differentiating hESCs. Combining OCT4 and EpCAM selection can further enrich the meiotic-competent germ cell-like cell population. Also, with the percentage of OCT4+/EpCAM+ cells as readout, we demonstrated the synergistic effect of BMP4/pSMAD1/5/8 and WNT3A/β-CATENIN in promoting hESCs toward the germline fate. Combining BMP4/WNT3A induction and OCT4/EpCAM selection can significantly increase the putative germ cell population with meiotic competency. Co-transplantation of these cells with dissociated mouse neonatal ovary cells into SCID mice resulted in a homogenous germ cell cluster formation in vivo. The stepwise platform established in this study provides a useful tool to elucidate the molecular mechanisms of human germ cell development, which has implications not only for human fertility research but regenerative medicine in general.
Introduction
Although the broad differentiation potential of embryonic stem cells (ESC)3 has long been demonstrated in vitro, evidence of gamete generation from ESCs was hampered by the lack of appropriate markers to track and identify germline differentiation in vitro. In 2003, follicle-like structures were derived from gcOct4-Gfp mouse ESCs by Hübner et al. (1). After this pioneering study, several subsequent reports have demonstrated that primordial germ cells (PGCs), as well as sperm and oocyte-like cells, can be derived from mouse pluripotent stem cells (PSCs) (2–7). Hayashi et al. (7) have lately recreated the complex multistep pathway of germ cell development from mouse PSCs and successfully generated healthy offspring from the derived sperm. Recently, isolation of germ cells from human PSCs has been achieved using a transgenic VASA reporter (8, 9). In addition to transgenic reporter systems, surface markers such as SSEA1 and others have also been used to enrich PGCs from differentiating hESCs or induced PSCs to a certain extent (10–13). These findings demonstrated the possibility of using a simple FACS strategy for germ cell enrichment. However, given the fact that these markers are also shared by other cell types (14, 15), the combination of transgenic reporter lines and surface markers may be an alternative solution for tracking germ cell differentiation in vitro, and enriching the germ cell population for subsequent mechanistic studies.
There are two pre-requisites to the elucidation of molecular pathways governing human germ cell specification in vitro from PSCs. The first is the identification of tracking markers that can effectively label precursors for germ cells as well as early germ cells, so that transition from ESC to germ cell fate can be monitored. However, even with an optimal tracking system, the number of cells may not be sufficient to perform detailed signaling analyses. An effective induction protocol for triggering hESCs to the germ cell fate, and thus increasing the absolute number of germ cells, is therefore the second prerequisite. It has been demonstrated in mice that BMP signaling, especially BMP4 expression from the extraembryonic ectoderm is sufficient for germ cell fate specification from WNT3A-expressing epiblasts (16). In addition, during later germ cell development and migration, the SCF (KIT ligand from hindgut)-C-Kit (PGC expressing) signaling, and SDF1 (expressed alone the migratory route)-CXCR4 (PGC expressing) signaling pathways are not only important for motility of the germ cells but also for the survival and proliferation of the PGCs (17). Whether these findings in mice are similar for human germline development remains to be explored.
In this study, we aim to establish a reliable system to identify PGCs from differentiating hESCs to study the signaling pathways involved in human germline formation. According to the above mentioned criteria, we selected two pluripotent cell expressing markers, OCT4 and EpCAM, proved that they are both expressed in the germ cells of human fetal gonads, and tested the effectiveness of using individual and OCT4-EGFP/EpCAM combined selection platforms for enriching germ cell-like cells. Various combinations of cytokines for stimulating germ cell specification were tested, and the best combination, BMP4 and WNT3A, as well their downstream signaling pathways were examined. These strategies represent a significant step toward the efficient generation of early human germ cells for mechanistic studies.
EXPERIMENTAL PROCEDURES
Culture of ESC Lines
The H1 OCT4-GFP (XY), H9 (XX) (WiCell Research Institute Inc., Madison, WI), and NTU1(XX) (18) hESCs were cultured onto mitomycin C (10 μg/ml, Sigma) inactivated mouse embryonic fibroblasts as previously described (19). The medium was changed daily. ESCs were split every 6–7 days by mechanical slicing.
Construction of Human OCT4 Promoter-EGFP Lentiviral Vector, Lentiviral Production, and Generation of Transgenic hESC Line
See supplemental “Materials and Methods” for these methods.
Differentiation of Human Embryonic Stem Cells
OCT4-EGFP hESCs were detached from feeder cells by treatment with dispase (0.5 mg/ml, Invitrogen), and transferred onto Ultra Low adhesion plates (Corning Costar) for embryoid body (EB) formation. The EBs were cultured in ESC medium for 2 days, then changed to differentiation medium, consisting of 82% DMEM (Invitrogen), 15% FBS (HyClone), 1% nonessential amino acids, 1% l-glutamine, and 1% penicillin and streptomycin (Invitrogen) for another 4 days. The EBs were then transferred back onto gelatin-coated culture dishes in differentiation medium to allow attachment and spontaneous differentiation. To promote germ cell differentiation in vitro, different combinations of growth factors and cytokines/morphogenes were introduced to the induction medium. These included human BMP4, -7, and -8b (100 ng/ml) (20), WNT3A (50 ng/ml) (R&D Systems) (16), SCF (100 ng/ml), and SDF1 (20 ng/ml) (PROSPEC) (2). To confirm the germ cell induction effect of BMP4 and WNT3A, their inhibitors, NOGGIN (100 ng/ml) and DKK1 (100 ng/ml) (PeproTech), were added to the differentiation medium, respectively. For a more detailed signaling study, DMH1 (2 μm; a BMP inhibitor) (21) and FSH535 (15 μm; an inhibitor of WNT/β-CATENIN) (22) (Sigma) were supplemented in the medium.
Immunofluorescence (IF) Staining and Confocal Microscopy, Meiotic Spreads, and Meiotic Marker Staining, Flow Cytometry and Cell Sorting, Quantitative RT-PCR (Q-RT-PCR), Western Blot Analysis and DNA Content Analysis
See supplemental “Materials and Methods” and supplemental Table 1 for these methods.
Human Fetal Samples
Human fetal testes and ovaries were obtained following termination of pregnancy during the second trimester (12, 14, and 19 weeks for testes; 3 individuals from 18 to 19 weeks for ovaries collection). The developmental age was determined by recall of the last menstrual period and ultrasonographic measurement, and subtraction of 2 weeks to account for time of fetal development. Written informed consent was obtained from the parents, according to the protocol approved by the Research Ethics Committee (REC) of the National Taiwan University Hospital and the Internal Research Board (IRB) of Academia Sinica.
Fluorescent in Situ Hybridization (FISH)
Sorted cells were collected and fixed with Carnoy's fixative (1:3, acetic acid:methanol), then spread onto pre-cleaned slides and air dried. The hybridization of the FISH probes against chromosome 16 (CEP 16 SpectrumGreen), X (CEP X SpectrumOrange), and Y (CEP Y SpectrumAqua) (Vysis) was performed according to the manufacturer's instruction. Nuclear DNA was stained with DAPI II counterstains (Vysis).
Kidney Capsule Transplantation
The post-FACS sorting of OCT4-EGFP/EpCAM double-positive cells from differentiation under BMP4/WNT3A conditions at in vitro differentiation (IVD) day 15 were mixed with the dissociated single cells of newborn ovaries from CD-1 female pups (The BioLasco Taiwan Co., Ltd., Taipei, Taiwan) according to the protocol generated by Nicholas et al. (2). Each graft containing at least 1–200,000 OCT4/EpCAM double-positive cells was transplanted beneath the kidney capsule of NOD-SCID mice (n = 4) using the method described from Mammary.nih.gov. 8 weeks after transplantation, host mice were sacrificed and the transplanted kidneys were retrieved, fixed, and sectioned for further histological analysis. All animal experiments were approved by the Animal Care and Use Committee of Academia Sinica and performed in accordance with the Institutional Animal Care and Use Committee guidelines of Academia Sinica.
RESULTS
In Vitro Derivation of PGCs Using OCT4-EGFP hESCs
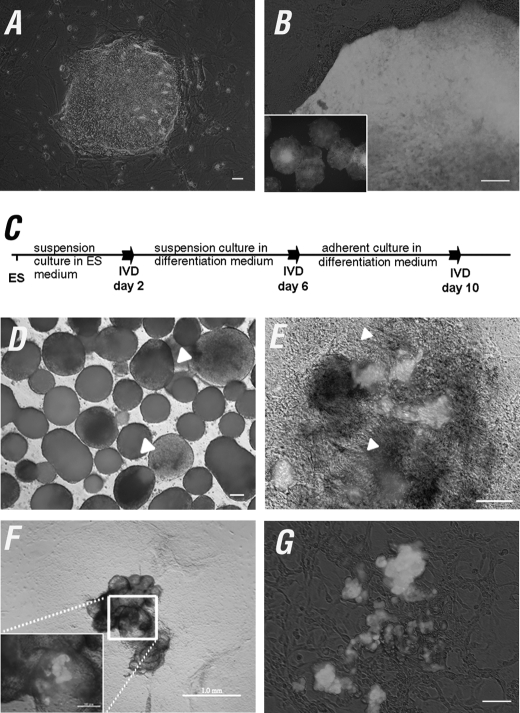
To explore the hypothesis that OCT4 is retained in the germ cell population when hESCs differentiate, we generated an OCT4 promoter-EGFP hESC line by the lentiviral system to investigate the process of PGC derivation (Fig. 1, A and B, and supplemental Fig. S1, A–C). Initially, we observed EGFP expression confined to morphologically undifferentiated hESCs (Fig. 1B). During the course of IVD (Fig. 1C), the number of OCT4-EGFP+ cells gradually decreased from IVD day 4 as evidenced by a notable reduction of EGFP+ areas in EBs (Fig. 1D). After being re-plated onto the culture surface, we noted that patches of OCT4-EGFP+ cells were frequently present within or around the multisack structures (Fig. 1F) and absent from other known differentiated cell types (Fig. 1E and supplemental Fig. 1D). The OCT4-EGFP+ cells were subsequently found mainly in floating or attached cell aggregates containing a mixture of EGFP+ and EGFP− cells at IVD day 20–30 (Fig. 1, F and G). Floating OCT4-EGFP+ cell aggregates occasionally attached to the culture surface and often formed cell colonies.
FIGURE 1.
OCT4-EGFP expression during hESC IVD. A and B, generation of the OCT4 promoter EGFP reporter hESC line. A, H9 hESCs were infected with lentiviruses 3 days post-colony splitting. B, the OCT4-EGFP+ cells were enriched by serial manual selection and re-plating of OCT4-EGFP+ cells until the majority (>90%) of the hESC population were OCT4-EGFP positive. C, schematic procedure for OCT4-EGFP hESCs IVD. D–G, the number of OCT4-EGFP+ cells gradually decreased during IVD. D, EBs expressed patchy EGFP during IVD 4 (white arrowhead). E, OCT4-EGFP expression was confined to clusters of cells at IVD day 10. The differentiated early neural rosettes as indicated by white arrowheads were absent from OCT4-EGFP expression. F, OCT4-EGFP+ signals were often found in the multisack structures at IVD 20 and onward. G, floating and attached OCT4-EGFP+ cell aggregates. Scale bars = 200 μm in A; 100 μm in B, D, E, and G; 1.0 mm in F, and 200 μm in the inset figure.
Flow cytometry analysis of the differentiating OCT4-EGFP hESCs indicated that the OCT4-EGFP+ cell population decreased along with the progress of differentiation, which resulted in less than 10% EGFP+ cells remaining at IVD day 30 (Fig. 2A). Furthermore, we found that the majority of EGFP+ cells co-expressed endogenous OCT4 in both ES (99.3%) and IVD day 20 (74.1%) cells (supplemental Fig. S2, A and B). These data demonstrated that EGFP was highly correlated to the endogenous OCT4 expression, and the reduction of EGFP reflected the down-regulation of OCT4 in hESC differentiation. To rule out the possibility that the EGFP-expressing cells observed in the IVD populations were due to contamination by undifferentiated hESCs, we compared the growth pattern of single OCT4-EGFP hESCs and their derivatives at IVD day 12 sorted by FACS. The results indicated that single OCT4-EGFP+ hESCs were able to form colonies and showed AP positive staining, whereas the day 12 differentiated OCT4-EGFP+ cells were unable to grow in ES culture conditions (supplemental Fig. 2C), indicating the OCT4-EGFP+ cells are likely to represent a germ cell population.
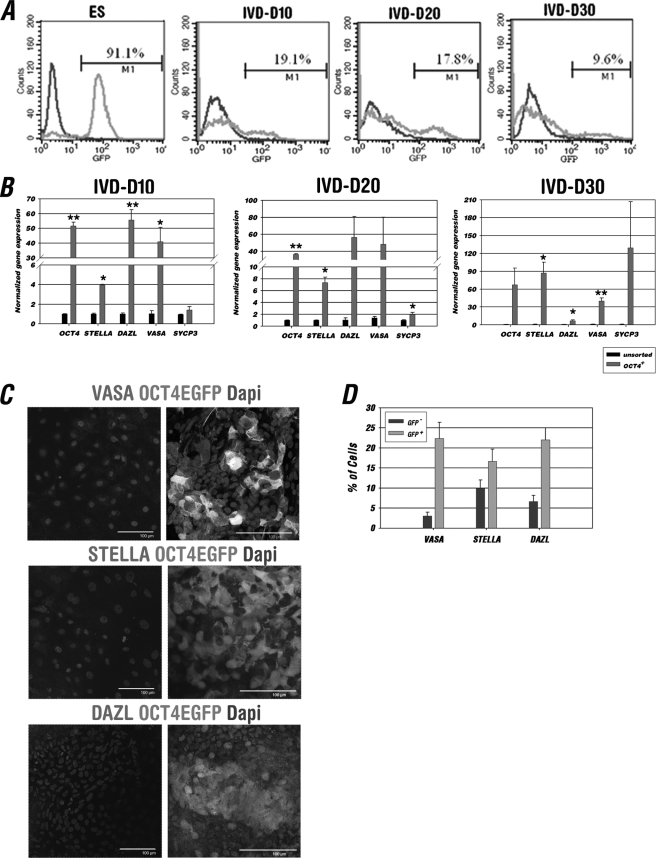
FIGURE 2.
In vitro differentiated OCT4-EGFP+ cells enriched in a PGC-like population. A, FACS analysis of OCT4-EGFP hESCs and their differentiated derivatives at IVD days 10, 20, and 30. Gating was determined independently at each time point using parental H9 ES cells and their differentiated cells at each corresponding stage in parallel as a control. The average percentage of OCT4-EGFP+ cells is indicated as M1 (n = 3). B, Q-RT-PCR analysis and comparison of germline marker gene expression between the unsorted and sorted OCT4-EGFP+ cell population at IVD 10, 20, and 30. The expression value for each gene was first normalized to GAPDH and then represented as the fold-change relative to those of the unsorted group (1-fold). Data are represented as mean ± S.D. (n = 3); *, p < 0.05, **, p < 0.01. C, IF analysis of attached cell aggregates contained OCT4-EGFP− (left panel) or OCT4-EGFP+ (right panel) cells at IVD 20–30 with germ cell markers VASA, STELLA, and DAZL. Little VASA, STELLA, and DAZL was detected in the cell aggregates contained EGFP− cells; however, VASA, STELLA, and DAZL could be seen within the cell aggregates contained EGFP+ cells. Scale bars = 100 μm. D, quantitative analysis of positive VASA, STELLA, and DAZL IF staining in the cell aggregates contained OCT4-EGFP− or OCT4-EGFP+ population. A total of 200 cells were counted in each germ cell marker staining (n = 3).
To investigate whether the germ cell population is enriched in differentiated OCT4-EGFP+ cells, we performed Q-RT-PCR and IF staining on these cells (Fig. 2, B–D, and supplemental Figs. S3 and S4). The results from Q-RT-PCR showed that germ cell markers, OCT4, STELLA, DAZL, VASA, and meiotic marker SYCP3 have higher expression levels in sorted OCT4-EGFP+ cells compared with an unsorted population at various differentiation stages (Fig. 2B). In addition, many of the OCT4-EGFP+ cells from the floating or attached cell aggregates after 20 days of differentiation were stained positively for germ cell markers, VASA, STELLA, and DAZL (Fig. 2C and supplemental Fig. S3). By quantifying the percentage of positive VASA, STELLA, and DAZL staining, we found that expression of the germ cell-specific marker VASA was around 7 times higher in OCT4-EGFP+ compared with OCT4-EGFP− cells, whereas a certain amount of enrichment was also observed in the germ cell-associated unspecific markers, STELLA and DAZL (Fig. 2D). We also noticed that the majority of germ cell marker positive but EGFP negative cells were present near the OCT4-EGFP+ cells (Fig, 2C). The expression of meiosis markers, SYCP1 and SYCP3, was also detected in the EGFP+ cell clumps (supplemental Fig. S4), indicating that some derivatives of the OCT4-EGFP+ cells entered the meiotic cell cycle in vitro. There were around 30% SYCP3-positive cells in the OCT4-EGFP+ population, compared with less than 1% in the unpurified population. Taken together, these findings demonstrated that the OCT4-EGFP selection process enriches early germ cell-like cells effectively.
EpCAM Is Expressed in Human Germ Cells in Vivo and in Vitro
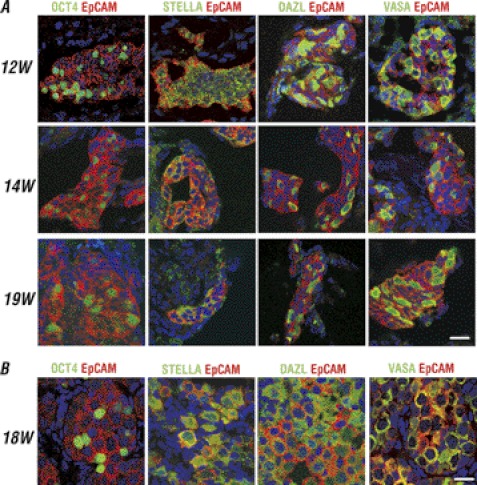
EpCAM has been found in the germ cells of mice and also positively regulates the pluripotency network including OCT4 in PSCs (19, 23, 24). Thus, we hypothesized that EpCAM is an appropriate surface marker to further enrich the germ cell population in combination with an OCT4 reporter. To confirm expression of the EpCAM in the human germ line, IF staining was performed in human fetal testes and ovaries (Fig. 3). In 12-, 14-, and 19-week old fetal testes, we found that EpCAM is expressed in germ cell clusters, indicated by their co-localization with OCT4, STELLA, DAZL, and VASA (Fig. 3A). A good correlation of EpCAM with germ cell markers, particularly VASA was also observed in three independent 18–19-week-old fetal ovarian tissues (Fig. 3B).
FIGURE 3.
EpCAM co-expression with germ cell markers in human fetal testis and ovarian tissue. A, IF staining of 12-, 14-, and 19-week-old fetal testes, and B, 18-week of fetal ovarian tissue showing EpCAM was co-expressed with OCT4, STELLA, DAZL, and VASA in germ cells. Scale bars = 20 μm.
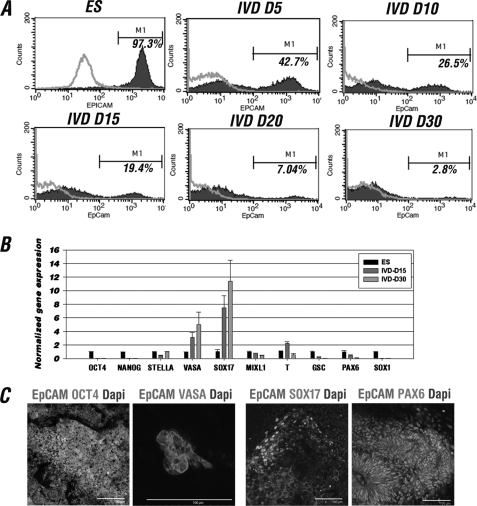
To study whether EpCAM can be used to purify the hESC-derived germ cells, we isolated the EpCAM positive population from spontaneously differentiated hESCs by FACS and examined the RNA and protein expression patterns of the EpCAM+ cells. Our data showed that EpCAM is expressed in hESCs as described in previous studies (Fig. 4A and supplemental Fig. S5A) (25). After differentiation, the percentage of EpCAM positive cells drops significantly but is still retained in a small population (19% at IVD 15, 7% at IVD 20, and around 3% at IVD 30) (Fig. 4A and supplemental Fig. S5B). The EpCAM positive cells sorted from IVD 15 and 30 of H9 hESCs were subjected to Q-RT-PCR and showed that when compared with undifferentiated hESCs, expression levels of VASA (germ line) and SOX17 (endodermal marker) were higher in both IVD 15 and 30, whereas T (BRACHYURY; mesodermal marker) was temporarily increased in IVD 15 (Fig. 4B). In differentiated hESCs, EpCAM was co-expressed with OCT4, VASA, and SOX17, but not PAX6, as demonstrated by IF staining (Fig. 4C). Together, these results indicated that the hESC-differentiated EpCAM-positive population most likely represent germ line and early endodermal lineages.
FIGURE 4.
In vitro differentiated EpCAM-positive cells represent germ cell, and early endodermal lineages. A, FACS analysis of EpCAM positive cells during in vitro H9 hESC differentiation. Gating was determined independently at each time point using parental hESCs and their differentiated cells at each corresponding stage in parallel as a control. The average percentage of EpCAM+ cells was shown as M1 (n = 3). B, lineage-specific gene expression analysis of EpCAM-sorted H9 hESCs at IVD 15 and 30. The relative expression of each gene at IVD 15 and 30 was first normalized to GAPDH and then represented as the fold-change relative to those of the ESC group (1-fold). C, IF analysis of differentiated H9 hESCs with germ cell (OCT4 and VASA), endoderm (SOX17), and ectoderm (PAX6) markers. Scale bars = 100 μm.
EpCAM and OCT4 Double Selection Enrich Germ Cell Population from Differentiating hESCs
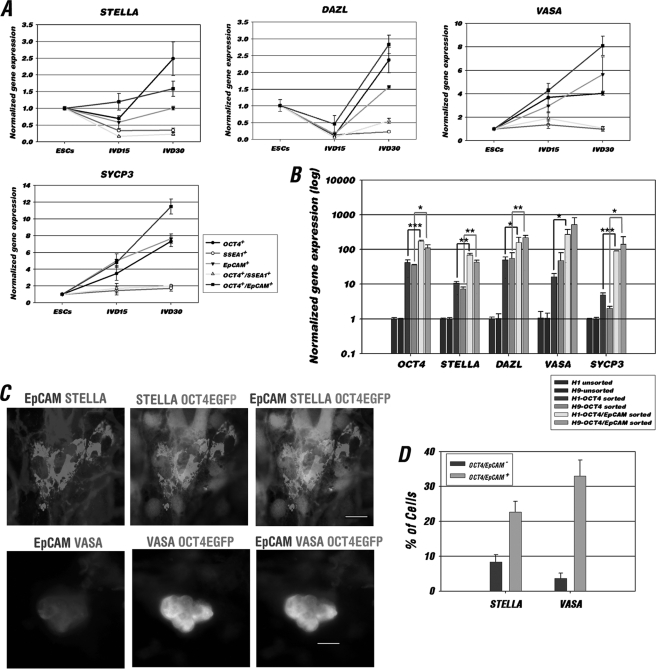
Having confirmed that EpCAM is a germ cell-enriched but not germ cell-specific marker, we further tested the effectiveness of using EpCAM alone and in combination with the OCT4-EGFP reporter to enrich a germ cell population from differentiating hESCs. As a comparison, we also included a previously published surface marker, SSEA1 (12), which has been used in enriching germ cell populations from differentiating hESCs. By comparing the gene expression of undifferentiated, IVD 15, and IVD 30 hESCs, we found that the expression level of germ cell-enriched genes STELLA, DAZL, VASA, and SYCP3 in OCT4+, EpCAM+, and double-positive cells to be higher than SSEA1+ or OCT4/SSEA1 double selected cells (Fig. 5A). Furthermore, the expression level of the analyzed germ cell marker genes and the meiosis associated marker, SYCP3, is significantly higher in the OCT4+/EpCAM+ double selected population than the OCT4 single selected population of both male (H1) and female (H9) hESC lines (Fig. 5B). Also the OCT4/EpCAM double-positive cells were validated by IF staining for co-localization with STELLA and VASA (22 versus 8% of unpurified cells for STELLA; and 33% versus 3% of unpurified cells for VASA expression) (Fig. 5, C and D). Thus, the above results demonstrate that the dual selection procedure can serve as an efficient readout for testing the effectiveness of germline induction conditions in vitro.
FIGURE 5.
OCT4/EpCAM selection improved germ cell-like cell enrichment. A, comparison of the expression levels of germ cell-enriched genes in OCT4-EGFP, SSEA1, EpCAM, OCT4-EGFP/SSEA1, and OCT4-EGFP/EpCAM-sorted H9 OCT4-EGFP hESCs at IVD 15 and 30. The relative expression of each gene at different IVD stages was first normalized to GAPDH and then represented as the fold-change relative to those of ESC group (1-fold). Data are represented as mean ± S.D. (n = 3). B, gene expression analysis in unsorted, OCT4-EGFP, and OCT4-EGFP/EpCAM-sorted cells at IVD 15 from H1 and H9-OCT4-EGFP hESCs. Each gene expression was first normalized to GAPDH and then represented as the fold-change relative to those of the unsorted group (1-fold) of each cell lines. Data are represented as mean ± S.D. (n = 3); ***, p < 0.001; **, p < 0.01; *, p < 0.05. C, IF staining showed co-expression of OCT4-EGFP (green) and EpCAM (blue) with germ cell markers, STELLA and VASA (red) in differentiated OCT4-EGFP hESCs. Scale bars = 20 μm. D, quantitative analysis of positive STELLA and VASA IF staining in OCT4-EGFP/EpCAM double-negative and -positive cells. A total 200 cells were counted in each staining (n = 3).
BMP4 and WNT3A Induction Facilitates Germ Cell Differentiation in Vitro
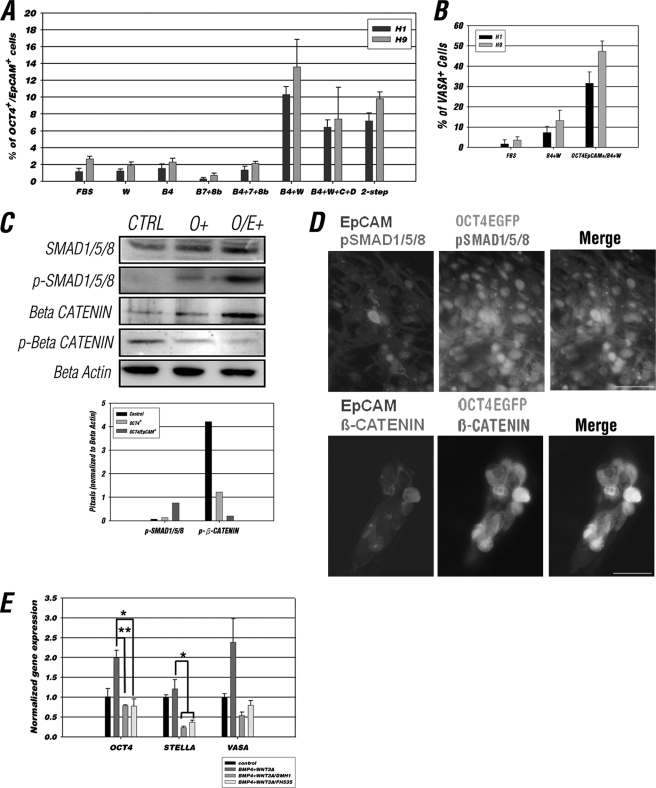
Having confirmed that OCT4/EpCAM dual selection can effectively enrich a germ cell-like population from differentiating hESCs, we sought to investigate the factors assisting germ cell induction in vitro. We tested various combinations of cytokines in the differentiation medium based on what was known for mouse germ cell development in vitro and in vivo (Fig. 6A, “Experimental Procedures”) (16, 26–32). Among the various combinations tested, WNT3A, BMP4, BMP7 + BMP8b, and BMP4 + BMP7 + 8B had no significant effect on the number of OCT4/EpCAM double-positive cells in either H1 or H9 hESC lines. In contrast, the percentage of OCT4/EpCAM double-positive cells and the expression of germ cell-enriched genes were significantly increased when BMP4 and WNT3A were administered together on the first day of the differentiation procedure (Fig. 6A, supplemental Fig. S6 and supplemental Table 2). Introduction of SCF and SDF1 into the culture medium, either from the beginning of differentiation or 5 days after the initial induction of differentiation by BMP4 and WNT3A (two-step), did not further enhance the number of OCT4+/EpCAM+ cells (Fig. 6A). The synergistic effect between BMP4 and WNT3A induction was also verified by the reversal of germ cell differentiation when adding their respective antagonists, NOGGIN and DKK1 (supplemental Fig. S7). When comparing the percentage of positive VASA-stained cells in control (FBS), BMP4 + WNT3A, and BMP4 + WNT3A-treated OCT4+/EpCAM+ cells, the data also demonstrated that addition of both cytokines can facilitate the number of VASA positive cells, especially in the OCT4/EpCAM double-enriched population (31% in H1 and 47% in H9) (Fig. 6B).
FIGURE 6.
Combination of BMP4 and WNT3A increased the population of OCT4/EpCAM double-positive cells. A, percentage of OCT4-EGFP+/EpCAM+ cells from IVD day 15 of H1 and H9 OCT4-EGFP ESCs in 15% FBS differentiation medium or differentiation medium supplemented with various cytokines. 15% FBS differentiation medium (FBS); WNT3A (W); BMP4 (B4); BMP7 and 8b (B7 + 8b); BMP4, -7, and -8b (B4 + 7 + 8b); BMP4 and WNT3A (B4 + W); BMP4, WNT3A, SCF, and SDF (B4 + W + C + D); two-step, cells were cultured in differentiation medium supplemented with BMP4 and WNT3A for 4 days, then switched to medium with BMP4, WNT3A, SCF, and SDF. Data are presented as mean ± S.D., n = 3. B, percentage of VASA-positive cells from IVD day 15 of H1 and H9 OCT4-EGFP ESCs in cells cultured with FBS, BMP4 + WNT3A, and BMP4 + WNT3A-treated OCT4-EGFP/EpCAM double-positive cells. A total of 200 cells were counted in each staining (n = 3). C, Western blot analysis of SMAD-1/5/8, and phospho-SMAD-1/5/8, β-CATENIN, phospho-β-CATENIN in control (unpurified), OCT4-EGFP, and OCT4-EGFP/EpCAM-positive sorted cells (upper panel). Semi-quantification of the relative levels of phospho-SMAD-1/5/8 and phospho-β-CATENIN (corrected to the total levels of respective proteins) are shown in the lower panel. An equal amount of protein (20 μg) was loaded in each lane. The intensity of bands was normalized to that of β-ACTIN. D, IF analysis showed co-expression of OCT4-EGFP (green), EpCAM (blue), and phospho-SMAD1/5/8 or β-CATENIN (red) in differentiating OCT4-EGFP hESCs at IVD 15. Scale bars = 50 μm. E, comparing the gene expression level for cells differentiated in control (15% FBS medium contained vehicle (dimethyl sulfoxide)), BMP4 + WNT3A, DMH1, and FH535 added medium. The relative expression of each gene was normalized to the control group after normalization with GAPDH. Data are represented as mean ± S.D., n = 3; *, p < 0.05; **, p < 0.01.
To further demonstrate that the BMP4 and WNT3A signaling pathways are active in OCT4/EpCAM-positive cells, a Western blot analysis was performed to quantify total and phosphorylated SMAD 1/5/8 and β-CATENIN, respectively (Fig. 6C). We found the protein levels of pSMAD1/5/8 and β-CATENIN were higher in OCT4+ and OCT4+/EpCAM+ cells than unpurified cells (Fig. 6C). On the other hand, a decreasing level of phosphorylated β-CATENIN was observed in the OCT4+ and OCT4+/EpCAM+ group (Fig. 6C). We also demonstrated by IF staining at the cellular level that pSMAD1/5/8 and β-CATENIN were present in OCT4/EpCAM double-positive cells (Fig. 6D). Thus, these results indicate that the BMP/WNT signaling pathway is active in OCT4+ and OCT4+/EpCAM+-enriched cells. We further showed that the BMP4 and WNT3A signaling pathway directly influences the expression of germ cell marker genes by performing the Q-RT-PCR in IVD day 15 cells, which were cultured previously in BMP4 + WNT3A for 4 days, then switched to a medium containing DMH1 (a BMP inhibitor) and FH535 (an inhibitor of WNT/β-CATENIN). By comparing the gene expression of control (differentiation medium without cytokines), BMP4 + WNT3A-, DMH1-, and FH535-treated cells, we found that expression of OCT4, STELLA, and VASA increased in cells cultured with BMP4 + WNT3A (Fig. 6E). In contrast, the expression level of all germ cell markers analyzed was significantly decreased in both DMH1- and FH535-treated cells (Fig. 6E). We conclude that by blocking either BMP or the WNT/β-CATENIN pathway, the expression of genes important for germ cell identity is impaired. Together, these results suggest that the increased OCT4+/EpCAM+ population is a specific result of the synergistic effect of the BMP and WNT/β-CATENIN pathways.
Combined BMP4/WNT3A Induction and OCT4/EpCAM Selection Enriched Meiotic Competent Germ Cell-like Cells
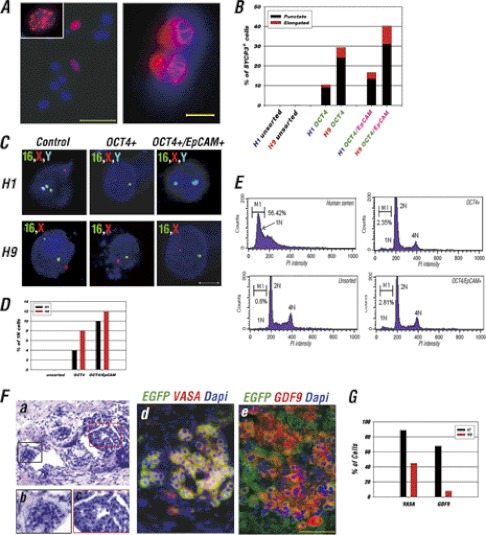
Encouraged by the significant enrichment of OCT4/EpCAM double-positive cells by BMP4 and WNT3A induction, we extended our characterization to examine the capability of these germ cell-like enriched populations to enter meiosis, the ultimate criteria to demonstrate germ cell identity. After treating cells with BMP4/WNT3A, we observed up to 40% of OCT4+/EpCAM+ cells stained positive for SYCP3 in IVD 20 of H9 ESCs (Fig. 7, A and B), representing the formation of a synaptonemal complex, a meiosis-specific structure necessary for the synapses of homologous chromosomes. The addition of EpCAM into the selection protocol increased not only the number of cells positive for SYCP3 compared with OCT4 selection alone, but also the elongated pattern of SYCP3 staining, possibly representing the pachytene stage of meiosis (Fig. 7A and B).
FIGURE 7.
OCT4/EpCAM selection in combination with BMP4 and WNT3A induction improves meiotic progression, haploid formation, and development of germ cell-like structure in kidney capsule transplantation. A, evidence of meiosis from the BMP4/WNT3A-induced in vitro differentiated germ cell-like cells as demonstrated by IF analysis with SYCP3 antibody. Meiotic nuclei with punctate (left panel) and elongated (right panel) SYCP3 staining patterns represent early and late meiosis processes, respectively. Nuclei were stained with DAPI (blue). Scale bars = 50 (left panel) and 10 μm (right panel). B, quantitative analysis of the percentage of cells with punctate or elongated SYCP3-positive signals in the unpurified, OCT4-sorted or OCT4/EpCAM-sorted cells from A. A total of 150 meiotic spread cells each were counted for H1 and H9 cell lines. C, FISH analysis on unpurified, OCT4, and OCT4/EpCAM-sorted cells at IVD day 20 of H1 and H9 OCT4-EGFP ESCs in differentiated medium containing BMP4/WNT3A with DNA probes specifically targeting chromosomes 16, X, and Y. Nuclei were stained with DAPI II (blue). Scale bars = 5 μm. D, quantitative analysis of the percentage of haploid cells with single chromosomes 16, and X or Y signal in C. A total of 100 fixed cells were counted. E, DNA content analysis of unsorted, OCT4-sorted, and OCT4/EpCAM-sorted cells in BMP4/WNT3A-induced H1 OCT4-EGFP cells. Human semen was used as a positive control to determine the gating of haploid cells (1N) by flow cytometer. The percentage of 1N cells was indicated as M1. F, characterization of hESC H9-derived OCT4+/EpCAM+ germ cells following co-transplantation with dissociated mouse fetal ovarian cells into mouse kidney capsules. a–c, H&E stain of a OCT4+/EpCAM+ cell transplanted kidney. Compact cell clusters exhibit “germ cell cluster-like” structures within the mouse kidney (b and c). d and e, IF staining of VASA (red), GDF9 (red), and EGFP (green) on cryosections of the transplanted kidney showed a uniform distribution of VASA/OCT4-EGFP or GDF9/OCT4-EGFP co-expressing cells in the germ cell-like cluster. Scale bars = 50 μm. G, quantitative comparison of the percentage of VASA+ and GDF9+ cells in the kidney capsule transplantation or IVD (day 30–40) of OCT4+/EpCAM+ H9 cells treated with BMP4/WNT3A. A total of 200 OCT4+/EpCAM+ cells were counted in the IVD group and 400 OCT4-EGFP-positive cells were counted in the kidney capsule transplantation group.
To determine whether WNT3A/BMP4 induction plus OCT4/EpCAM selection also promoted the generation of haploid cells, we carried out FISH analysis. FISH with probes for chromosome 16, X, and Y satellite sequences demonstrated that up to 10 and 12% of the BMP4/WNT3A-induced OCT4/EpCAM-positive cells were derived from male (H1) and female (H9) hESCs, respectively, and had only one staining signal each for chromosome 16 and X or Y, suggesting the completion of meiosis I (Fig. 7, C and D). To further confirm the FISH results, DNA content analysis was also performed on differentiating H1 hESCs. Flow cytometric analysis revealed that 2.35 and 2.81% of the OCT4 and OCT4/EpCAM sorted cells had 1 n DNA content, respectively, whereas only 0.6% of unselected OCT4 and/or EpCAM cells had 1 n DNA content (Fig. 7E).
To investigate the in vivo developmental potential of the hESC-derived germ cell-like cells, the OCT4/EpCAM double-positive cells were sorted from differentiated H9 OCT4-EGFP ESCs (at IVD day 15), co-aggregated with dissociated newborn mouse ovaries, and then transplanted into the kidney capsule of NOD/SCID mice for 2 months. Four grafts were performed and germ cell-like clusters within the grafts were observed based on H&E staining (Fig. 7F, a-c). To confirm the origins and identity of the germ cell-like cells, the OCT4-EGFP and late germ cell markers were employed. We found that VASA and the oocyte-specific marker GDF9 were co-localized with OCT4-EGFP in clusters of cells with morphological features typical of PGCs (Fig. 7F, d and e). Furthermore, by comparing the percentage of VASA+ and GDF9+ cells in kidney capsule transplants and IVD days 30–40, we found that the OCT4+/EpCAM+ cells showed more co-localization with VASA+ and GDF9+ cells after transplantation than their IVD counterparts (89 versus 45% for VASA, and 68 versus 8% for GDF9) (Fig. 7G). The result suggested the transplanted sorted OCT4+/EpCAM+ cells could survive and reach a more homogeneous germ cell-like population in vivo, indicating a more favorable microenvironment for germ cell development.
DISCUSSION
We have presented here an OCT4-EGFP reporter system to track and enrich germ cell-like populations from spontaneously differentiated hESCs. We also found that besides the existing surface markers, SSEA1, C-Kit, and CXCR4, a new human germ cell-enriched marker, EpCAM, was an alternative selector for in vitro germ cell-like cell isolation without genetic manipulation. Furthermore, by combining the OCT4-EGFP/EpCAM selections, we identified that BMP4/WNT3A together assisted germ cell-like cell derivation from hESCs as indicated by increasing numbers of meiotic (∼40% SYCP3+) and haploid (∼12%) cells. When co-transplanting the BMP4/WNT3A-induced OCT4+/EpCAM+ early differentiating germ cell-like cells with dissociated newborn mouse ovaries, we observed a more uniform human germ cell-like population in the transplanted kidney capsule. These results demonstrate that the combination of BMP4/WNT3A induction and OCT4-EGFP/EpCAM selection can significantly enrich the germ cell-like population from differentiating hESCs.
The lack of satisfactory germ cell enrichment and induction protocols has been the major obstacle for the differentiation and study of human germ cell development from pluripotent stem cells. Recent advances include the use of hESC or induced PSC transgenic lines harboring VASA reporters, to select for a germ cell population from either spontaneous or BMPs induced differentiating cells (8, 9). Co-culture of human fetal gonadal cells with the differentiating hESCs or human induced PSCs also improved the PGCs derivation rate, judging by the C-Kit/SSEA1/PLAP triple positive cells and further epigenetic characterization (10). However, as most of the above mentioned selection platforms select for specified germ cells, it would still be beneficial to establish new enrichment platforms that cover the initiation of germ cell differentiation/specification.
The expression of OCT4, which covers the transition from pluripotent stem cells to germ cells, allowed to directly track the early events of germ cell differentiation from hESCs (33). Our results here demonstrate both the successful use of OCT4-EGFP selection for germ cell-like cell purification and the induction of meiosis. When dissecting the expression pattern of OCT4 with different germ cell markers in vitro, we noticed some correlation with documented marker expression in vivo (34–36). For example, co-localization of OCT4 and DAZL in the nuclei of IVD 20 of OCT4-EGFP ESCs resembles first trimester ovaries and testes. Furthermore, DAZL staining in IVD 20 and onwards in OCT4-EGFP positive and negative cells was similar to that observed in second trimester testes (36). OCT4 and VASA co-expression was observed between 8 and 9.5 weeks post-fertilization of human fetal ovaries (34). In the second trimester, the germ cells were usually negative for OCT4 expression, but low levels of OCT4 expression could be identified in some of the VASA-positive cells at this stage (36). Expression of germ cell markers differs at different developmental stages, which could explain why OCT4-EGFP signals from in vitro differentiated germ cell-like cells do not always colocalize with VASA, STELLA, and DAZL. When combined with single cell cDNA amplification followed by quantitative gene expression analysis (data not shown), these enriched germ cells at different developmental stages represent a great resource for studying the molecular and signaling mechanisms governing germ cell development.
OCT4-EGFP selection also provides a good tool for searching for germ cell-enriched surface markers, which could allow purification of germ cell-like cells without genetic manipulation. In this study, we showed a strong correlation between EpCAM and several germ cell markers in both fetal testes and ovaries, and by comparing EpCAM with SSEA1, which is commonly used for in vitro germ cell purification, EpCAM was shown to have a better selective effect. Moreover, the OCT4/EpCAM double selection allowed the best germ cell enrichment from hESC differentiation among the markers we used. We therefore conclude that we have provided a better readout to dissect regulatory mechanisms and an optimal induction protocol from hESCs to PGCs. Although our data demonstrates the expression of EpCAM in both human germline and hESC-derived germ cells, our IVD result, and previously published studies indicate the expression of EpCAM in other cell lineages (37–39). In our current study, we showed the contamination of nongerm cells could be minimized by combining EpCAM and OCT4-EGFP selection. In mice, EpCAM was shown in the germline as early as the migratory stage (23), however, due to the difficulty in accessing early human fetal gonads, we were unable to further stage EpCAM and dissect their relationship with OCT4 in vivo. In the future, with more information from in vivo studies, and perhaps combining the gene expression analysis from in vitro differentiated OCT4+, EpCAM+, and OCT4+/EpCAM+ cells, we might be able to shed light on this issue.
The genetic regulation and signaling involved in early germ cell formation has been studied extensively in mice (16, 40). It has long been documented that BMP signaling is required for germ cell specification. By successfully establishing epiblast explant culture and various knock-out lines, Ohinata et al. (16) demonstrated that only epiblast cells of a particular developmental stage (E5.5–E6.25) that express WNT3 were competent to respond to the BMP4-stimulated germ cell specification. However, little is known about the cytokines and signaling pathways governing early germline formation in humans. It was reported that the addition of BMP4, BMP7, and BMP8b during hESC differentiation could improve the efficiency of germ cell formation (20). With the OCT4/EpCAM selection system as readout, we demonstrated that only when combining BMP4 and WNT3A could we enrich the OCT4+/EpCAM+ population, and that BMP/WNT signaling is highly active in the OCT4+/EpCAM+ population, judging by the increased phosphorylation of SMAD1/5/8 and unphosphorylated β-CATENIN. Blocking either the BMP-induced SMAD 1/5/8 activation (with DMH1) or WNT/β-CATENIN pathway (with FH535) reduces the expression level of germ cell markers in differentiating hESCs. Our data suggest an evolutionarily conserved BMP4 and WNT3A signaling pathway in germ cell specification between human and mouse. However, even in mice, it is still not fully understood how WNT3A primes the epiblast to respond to BMP4. Our in vitro human germ cell differentiation model may provide a good platform to investigate whether WNT3A signaling is directly or indirectly up-regulated by the BMP4 receptor.
Indication of successful germline differentiation using OCT4/EpCAM selection was based on the fact that at least ∼30% of OCT4-EGFP positive cells were confirmed as germ cell-like cells based on their meiotic status. The double selection by OCT4/EpCAM further increased the expression level of germ cell markers. Indeed, when combining the BMP4/WNT3 induction protocol with OCT4/EpCAM selection, we demonstrate that ∼40 and ∼12% of the OCT4+/EpCAM+ cells were undergoing or completed the meiosis process, respectively, as demonstrated by the SYCP3 positive staining and haploid FISH signals. The observation that OCT4/EpCAM-selected cells formed germ a cell-like structure and expressed late germ cell-specific marker (GDF9) in kidney capsule transplants further strengthened our theory that this system may provide a useful readout for in vitro germ cell isolation.
We have demonstrated that BMP4 and WNT3A induction followed by OCT4 and EpCAM selection can successfully enrich a meiotic competent germ cell-like population. The evidence includes the positive staining of meiotic marker SYCP3 as well as a haploid karyotype, indicating the completion of meiosis. This platform is extremely useful for further dissecting the molecular and signaling mechanisms governing human germ cell specification and development.
Supplementary Material
Acknowledgments
We thank Dr. Nissim Reubinoff for the lentiviral vector; Hsiao-Jung Liu, Ling-Jen Ma, Chu-Fan Mo, Wei-Chun Chang, Luca C.W. Cheng, and Yu-Chih Wu, for technical support and discussions; Dr. Neil A. Youngson and Dr. Joanna Maldonado Youngson for critical reading and editing of this manuscript.
This work was supported by an intramural grant from Academia Sinica and Stem Cell Priority Grants 99-3111-B-001-001 and 99-3111-B-002-009 (to H. C. K.), and 99-3111-B-002-008 (to S. P. L.) from National Science Council, Taiwan.

This article contains supplemental “Materials and Methods,” Tables S1 and S2, and Figs. S1–S7.
- ESCs
- embryonic stem cells
- hESC
- human embryonic stem cells
- EpCAM
- epithelial cell adhesion molecule
- PGCs
- primordial germ cells
- PSCs
- pluripotent stem cells
- EB
- embryoid body
- IF
- immunofluorescence
- Q-RT
- quantitative reverse transcriptase
- IHC
- immunohistochemistry
- IVD
- in vitro differentiation.
REFERENCES
- 1. Hübner K., Fuhrmann G., Christenson L. K., Kehler J., Reinbold R., De La Fuente R., Wood J., Strauss J. F., 3rd, Boiani M., Schöler H. R. (2003) Derivation of oocytes from mouse embryonic stem cells. Science 300, 1251–1256 [DOI] [PubMed] [Google Scholar]
- 2. Nicholas C. R., Haston K. M., Grewall A. K., Longacre T. A., Reijo Pera R. A. (2009) Transplantation directs oocyte maturation from embryonic stem cells and provides a therapeutic strategy for female infertility. Hum. Mol. Genet. 18, 4376–4389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nayernia K., Nolte J., Michelmann H. W., Lee J. H., Rathsack K., Drusenheimer N., Dev A., Wulf G., Ehrmann I. E., Elliott D. J., Okpanyi V., Zechner U., Haaf T., Meinhardt A., Engel W. (2006) In vitro differentiated embryonic stem cells give rise to male gametes that can generate offspring mice. Dev. Cell 11, 125–132 [DOI] [PubMed] [Google Scholar]
- 4. Geijsen N., Horoschak M., Kim K., Gribnau J., Eggan K., Daley G. Q. (2004) Derivation of embryonic germ cells and male gametes from embryonic stem cells. Nature 427, 148–154 [DOI] [PubMed] [Google Scholar]
- 5. Toyooka Y., Tsunekawa N., Akasu R., Noce T. (2003) Embryonic stem cells can form germ cells in vitro. Proc. Natl. Acad. Sci. U.S.A. 100, 11457–11462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Psathaki O. E., Hubner K., Sabour D., Sebastiano V., Wu G., Sugawa F., Wieacker P., Pennekamp P., Scholer H. R. (2011) Stem. Cells Dev. 20, 2205–2215 [DOI] [PubMed] [Google Scholar]
- 7. Hayashi K., Ohta H., Kurimoto K., Aramaki S., Saitou M. (2011) Reconstitution of the mouse germ cell specification pathway in culture by pluripotent stem cells. Cell 146, 519–532 [DOI] [PubMed] [Google Scholar]
- 8. Kee K., Angeles V. T., Flores M., Nguyen H. N., Reijo Pera R. A. (2009) Human DAZL, DAZ, and BOULE genes modulate primordial germ-cell and haploid gamete formation. Nature 462, 222–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Panula S., Medrano J. V., Kee K., Bergström R., Nguyen H. N., Byers B., Wilson K. D., Wu J. C., Simon C., Hovatta O., Reijo Pera R. A. (2011) Human germ cell differentiation from fetal- and adult-derived induced pluripotent stem cells. Hum. Mol. Genet. 20, 752–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Park T. S., Galic Z., Conway A. E., Lindgren A., van Handel B. J., Magnusson M., Richter L., Teitell M. A., Mikkola H. K., Lowry W. E., Plath K., Clark A. T. (2009) Derivation of primordial germ cells from human embryonic and induced pluripotent stem cells is significantly improved by coculture with human fetal gonadal cells. Stem Cells 27, 783–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bucay N., Yebra M., Cirulli V., Afrikanova I., Kaido T., Hayek A., Montgomery A. M. (2009) A novel approach for the derivation of putative primordial germ cells and sertoli cells from human embryonic stem cells. Stem Cells 27, 68–77 [DOI] [PubMed] [Google Scholar]
- 12. Tilgner K., Atkinson S. P., Golebiewska A., Stojkovic M., Lako M., Armstrong L. (2008) Isolation of primordial germ cells from differentiating human embryonic stem cells. Stem Cells 26, 3075–3085 [DOI] [PubMed] [Google Scholar]
- 13. Eguizabal C., Montserrat N., Vassena R., Barragan M., Garreta E., Garcia-Quevedo L., Vidal F., Giorgetti A., Veiga A., Izpisua Belmonte J. C. (2011) Complete meiosis from human-induced pluripotent stem cells. Stem Cells 29, 1186–1195 [DOI] [PubMed] [Google Scholar]
- 14. Son M. J., Woolard K., Nam D. H., Lee J., Fine H. A. (2009) SSEA-1 is an enrichment marker for tumor-initiating cells in human glioblastoma. Cell Stem Cell 4, 440–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miettinen M., Lasota J. (2005) KIT (CD117), a review on expression in normal and neoplastic tissues, and mutations and their clinicopathologic correlation. Appl. Immunohistochem. Mol. Morphol. 13, 205–220 [DOI] [PubMed] [Google Scholar]
- 16. Ohinata Y., Ohta H., Shigeta M., Yamanaka K., Wakayama T., Saitou M. (2009) A signaling principle for the specification of the germ cell lineage in mice. Cell 137, 571–584 [DOI] [PubMed] [Google Scholar]
- 17. Nicholas C. R., Chavez S. L., Baker V. L., Reijo Pera R. A. (2009) Instructing an embryonic stem cell-derived oocyte fate. Lessons from endogenous oogenesis. Endocr. Rev. 30, 264–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen H. F., Kuo H. C., Chien C. L., Shun C. T., Yao Y. L., Ip P. L., Chuang C. Y., Wang C. C., Yang Y. S., Ho H. N. (2007) Derivation, characterization and differentiation of human embryonic stem cells. Comparing serum-containing versus serum-free media and evidence of germ cell differentiation. Hum. Reprod. 22, 567–577 [DOI] [PubMed] [Google Scholar]
- 19. Huang H. P., Chen P. H., Yu C. Y., Chuang C. Y., Stone L., Hsiao W. C., Li C. L., Tsai S. C., Chen K. Y., Chen H. F., Ho H. N., Kuo H. C. (2011) Epithelial cell adhesion molecule (EpCAM) complex proteins promote transcription factor-mediated pluripotency reprogramming. J. Biol. Chem. 286, 33520–33532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kee K., Gonsalves J. M., Clark A. T., Pera R. A. (2006) Bone morphogenetic proteins induce germ cell differentiation from human embryonic stem cells. Stem. Cells Dev. 15, 831–837 [DOI] [PubMed] [Google Scholar]
- 21. Hao J., Ho J. N., Lewis J. A., Karim K. A., Daniels R. N., Gentry P. R., Hopkins C. R., Lindsley C. W., Hong C. C. (2010) In vivo structure-activity relationship study of dorsomorphin analogues identifies selective VEGF and BMP inhibitors. ACS Chem. Biol. 5, 245–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Handeli S., Simon J. A. (2008) A small-molecule inhibitor of Tcf/β-catenin signaling down-regulates PPARγ and PPARδ activities. Mol. Cancer Ther. 7, 521–529 [DOI] [PubMed] [Google Scholar]
- 23. Anderson R., Schaible K., Heasman J., Wylie C. (1999) Expression of the homophilic adhesion molecule, EpCAM, in the mammalian germ line. J. Reprod. Fertil. 116, 379–384 [DOI] [PubMed] [Google Scholar]
- 24. Kanatsu-Shinohara M., Takashima S., Ishii K., Shinohara T. (2011) Dynamic changes in EpCAM expression during spermatogonial stem cell differentiation in the mouse testis. PLoS One 6, e23663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ng V. Y., Ang S. N., Chan J. X., Choo A. B. (2010) Characterization of epithelial cell adhesion molecule as a surface marker on undifferentiated human embryonic stem cells. Stem Cells 28, 29–35 [DOI] [PubMed] [Google Scholar]
- 26. Molyneaux K. A., Zinszner H., Kunwar P. S., Schaible K., Stebler J., Sunshine M. J., O'Brien W., Raz E., Littman D., Wylie C., Lehmann R. (2003) The chemokine SDF1/CXCL12 and its receptor CXCR4 regulate mouse germ cell migration and survival. Development 130, 4279–4286 [DOI] [PubMed] [Google Scholar]
- 27. Ara T., Nakamura Y., Egawa T., Sugiyama T., Abe K., Kishimoto T., Matsui Y., Nagasawa T. (2003) Impaired colonization of the gonads by primordial germ cells in mice lacking a chemokine, stromal cell-derived factor-1 (SDF-1). Proc. Natl. Acad. Sci. U.S.A. 100, 5319–5323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ying Y., Qi X., Zhao G. Q. (2001) Induction of primordial germ cells from murine epiblasts by synergistic action of BMP4 and BMP8B signaling pathways. Proc. Natl. Acad. Sci. U.S.A. 98, 7858–7862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ying Y., Liu X. M., Marble A., Lawson K. A., Zhao G. Q. (2000) Requirement of Bmp8b for the generation of primordial germ cells in the mouse. Mol. Endocrinol. 14, 1053–1063 [DOI] [PubMed] [Google Scholar]
- 30. Lawson K. A., Dunn N. R., Roelen B. A., Zeinstra L. M., Davis A. M., Wright C. V., Korving J. P., Hogan B. L. (1999) Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev. 13, 424–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Buehr M., McLaren A., Bartley A., Darling S. (1993) Proliferation and migration of primordial germ cells in We/We mouse embryos. Dev. Dyn 198, 182–189 [DOI] [PubMed] [Google Scholar]
- 32. Besmer P., Manova K., Duttlinger R., Huang E. J., Packer A., Gyssler C., Bachvarova R. F. (1993) The kit-ligand (steel factor) and its receptor c-kit/W. Pleiotroppic roles in gametogenesis and melanogenesis. Dev. Suppl. 125–137 [PubMed] [Google Scholar]
- 33. Yeom Y. I., Fuhrmann G., Ovitt C. E., Brehm A., Ohbo K., Gross M., Hübner K., Schöler H. R. (1996) Germline regulatory element of Oct-4 specific for the totipotent cycle of embryonal cells. Development 122, 881–894 [DOI] [PubMed] [Google Scholar]
- 34. Kerr C. L., Hill C. M., Blumenthal P. D., Gearhart J. D. (2008) Expression of pluripotent stem cell markers in the human fetal ovary. Hum. Reprod. 23, 589–599 [DOI] [PubMed] [Google Scholar]
- 35. Kerr C. L., Hill C. M., Blumenthal P. D., Gearhart J. D. (2008) Expression of pluripotent stem cell markers in the human fetal testis. Stem Cells 26, 412–421 [DOI] [PubMed] [Google Scholar]
- 36. Anderson R. A., Fulton N., Cowan G., Coutts S., Saunders P. T. (2007) Conserved and divergent patterns of expression of DAZL, VASA, and OCT4 in the germ cells of the human fetal ovary and testis. BMC Dev. Biol. 7, 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. de Boer C. J., van Krieken J. H., Janssen-van Rhijn C. M., Litvinov S. V. (1999) Expression of EpCAM in normal, regenerating, metaplastic, and neoplastic liver. J. Pathol 188, 201–206 [DOI] [PubMed] [Google Scholar]
- 38. Cirulli V., Crisa L., Beattie G. M., Mally M. I., Lopez A. D., Fannon A., Ptasznik A., Inverardi L., Ricordi C., Deerinck T., Ellisman M., Reisfeld R. A., Hayek A. (1998) KSA antigen EpCAM mediates cell-cell adhesion of pancreatic epithelial cells. Morphoregulatory roles in pancreatic islet development. J. Cell Biol. 140, 1519–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Klein C. E., Cordon-Cardo C., Soehnchen R., Cote R. J., Oettgen H. F., Eisinger M., Old L. J. (1987) Changes in cell surface glycoprotein expression during differentiation of human keratinocytes. J. Invest. Dermatol. 89, 500–506 [DOI] [PubMed] [Google Scholar]
- 40. Hayashi K., de Sousa Lopes S. M., Surani M. A. (2007) Germ cell specification in mice. Science 316, 394–396 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.