Abstract
Suicidal behaviors are frequent in mood disorders patients but only a subset of them ever complete suicide. Understanding predisposing factors for suicidal behaviors in high risk populations is of major importance for the prevention and treatment of suicidal behaviors. The objective of this project was to investigate gene expression changes associated with suicide in brains of mood disorder patients by microarrays (Affymetrix HG-U133 Plus2.0) in the dorsolateral prefrontal cortex (DLPFC: 6 Non-suicides, 15 suicides), the anterior cingulate cortex (ACC: 6NS, 9S) and the nucleus accumbens (NAcc: 8NS, 13S). ANCOVA was used to control for age, gender, pH and RNA degradation, with P≤0.01 and fold change±1.25 as criteria for significance. Pathway analysis revealed serotonergic signaling alterations in the DLPFC and glucocorticoid signaling alterations in the ACC and NAcc. The gene with the lowest p-value in the DLPFC was the 5-HT2A gene, previously associated both with suicide and mood disorders. In the ACC 6 metallothionein genes were down-regulated in suicide (MT1E, MT1F, MT1G, MT1H, MT1X, MT2A) and three were down-regulated in the NAcc (MT1F, MT1G, MT1H). Differential expression of selected genes was confirmed by qPCR, we confirmed the 5-HT2A alterations and the global down-regulation of members of the metallothionein subfamilies MT 1 and 2 in suicide completers. MTs 1 and 2 are neuro-protective following stress and glucocorticoid stimulations, suggesting that in suicide victims neuroprotective response to stress and cortisol may be diminished. Our results thus suggest that suicide-specific expression changes in mood disorders involve both glucocorticoids regulated metallothioneins and serotonergic signaling in different regions of the brain.
Introduction
Suicide is one of the leading causes of death in the world particularly for young males and projections of global mortality indicate that suicide rates are rising [1] and will remain a leading cause of injury and death worldwide [2]. The number of suicides is approximately 1 million per year in the world [3]. In the United States suicide is the eleventh leading cause of death for all ages and the third leading cause among adolescents [4]. One third of those suicides visited a health care professional within a week before committing suicide [4]. This suggests that better methods are needed in assessment of suicidal intent in at risk populations.
Mood disorders (major depressive disorder (MDD) and bipolar disorder (BD)) constitute prominent risk factors for suicide [5]–[7]. Accordingly, lifetime risk for suicide in mood disorders patients is particularly high when compared to the general population [8]–[12]. In BD patients suicide attempts are specifically associated with depressive episodes rather than manic episodes [13]. Despite current advances in the understanding of suicide predisposing factors, existing diagnostic algorithms are inadequate and the identification of individuals at high risk for suicide is frequently missed [14].
Suicide and suicidal behaviors have been consistently linked to monoaminergic (serotonin, norepinephrine and dopamine) neurotransmission alterations [15]–[17] and to alterations in the hypothalamic-pituitary-adrenal (HPA) axis [18]–[20]. Postmortem receptor/transporter binding studies have also confirmed alterations in the number and affinity of monoaminergic receptors in the brains of suicides [17], [21]. Interestingly, both serotonergic neurotransmission imbalance and HPA axis over-activity are related to stressful events and were proposed as risk factors for suicide [22]. Recent evidence has shown that childhood abuse alters HPA stress responses and increases the risk of suicide through epigenetic regulation of glucocorticoid receptor expression underlining the role played by the HPA-stress response in the susceptibility to suicide [23].
Recently, microarray technology has been used to investigate in post-mortem brain global gene expression alterations associated with mood disorders and suicide compared to psychiatrically normal controls [24]. A number of studies specifically explored gene expression alterations in post-mortem brains of suicides versus control subjects in prefrontal regions [25]–[30] and in the limbic system [31], [32] with very few replicated results due probably to methodological and demographic differences in the samples. These studies focused mainly on the investigation of genes differentially expressed between psychiatric cases that committed suicide and controls, but did not compare the psychiatric patients who commit suicide from those who do not. To our knowledge, only two recent articles investigated suicide specific changes in the brain by comparing suicide versus non-suicide psychiatric patients. Garbett et al. observed a prefrontal cortical down-regulation of the HTR2A receptor in subjects with schizophrenia who committed suicide using Affymetrix arrays [33]. Also, Kim et al. (2007) reanalyzed a large microarray data set to investigate gene expression in major depression and schizophrenic patients who did and did not commit suicide, in the prefrontal cortex (BA10/46) [30]. They found alterations on 2 genes across diagnoses, PLSCR4 (phospholipid scramblase 4) and EMX2 (empty spiracles homolog 2 (Drosophila)) and several members of the metallothionein subfamily 1 (MT1E, MT1X, MT1M, MT1G, MT1H, MT1F) as being specifically down-regulated among the schizophrenics who committed suicide [30].
Mental disorders and particularly mood disorders contribute significantly but not exclusively to the risk of suicide. Determining the expression changes in the brain that differentiate suicide from non-suicide mood disorder brains should help identify the genes specifically involved in suicide. In this study, we investigated a cohort of mood disorder subjects (BD and MDD) for gene expression changes specifically associated with suicide in the DLPFC, ACC, and NAcc, three regions commonly associated with suicidal behavior alterations, mood, control of impulses, motivation, reward and pleasure, behaviors know to be altered in mood disorders.
Methods
Subjects
Brain samples were obtained at the University of California, Irvine/Davis Brain Repository through a uniform process approved by the Institutional Review Board. Written informed consent was obtained from the next-of-kin of the deceased. All subjects went through an extensive review of multiple sources of information including the medical examiner’s conclusions, coroner’s investigation, medical and psychiatric records, toxicology results, and interviews of the decedents’ next-of-kin, in order to rule out as much as possible any neurological disorder. Additionally, a neuropathological examination of each brain was conducted in order to exclude cases with cerebrovascular disease (infarcts or hemorrhages), subdural hematoma, or other significant pathological features. Our psychological autopsy protocol is based largely on procedures validated by Kelly and Mann [34]. Details of death are evaluated in order to assign an Agonal Factor Score to each subject in accordance with previous guidelines hardy [35], [36]. Previous work consistently showed an association between agonal factors and tissue quality [37]–[40]. Brains are initially screened for agonal factors at the time of consent in order to exclude brains with factors potentially affecting nucleic acids and proteins. Subjects with history of drugs or alcohol dependence were also excluded from the present sample. Non-suicide subjects, including mood disorder subjects and normal controls, died rapidly from either cardiac related events or motor-vehicle accidents.
The human brain dissection and freezing protocol is described in detail elsewhere [41]. Briefly, the whole brain is first inspected and photographed before the brainstem and cerebellum are separated from the cerebrum. The cerebrum is coronally sliced from anterior to posterior and slices are photographed, labeled, and bagged before being frozen. Small 5 cm squares are dissected for both pH and RNA quality measurements. Freezing is done by placing the bagged slices between super-cooled aluminum plates, then in a pre-cooled freezing rack before putting the whole rack in a −140°C freezer for a minimum of 10 minutes. The brains are stored in −80°C freezers until dissected and processed for extractions. The brain regions were dissected on dry ice from the left hemisphere of frozen tissue according to visible landmarks near the regions of interest under the direction of Dr E. G. Jones. Grey matter punches were taken from the cortical regions and from the middle of NAcc region.
Grey matter brain samples (25 mg) were used to extract total RNA with the Trizol method (Invitrogen, Carlsbad, CA), followed by a cleaning up using a silica based mini-spin column (Qiagen RNeasy Mini Kit, Valencia, CA). RNA integrity was evaluated using 18S and 28S ratios and RNA integrity numbers (RIN) using the 2100 Agilent Bioanalyzer (Santa Clara, CA). All subjects were clinically characterized with the psychological autopsy method and died suddenly without prolonged agonal state.
Microarray Analysis
Cel files from Affymetrix HG-U133 Plus2.0 chips were processed using the RMA algorithm. Statistical analysis was performed using Partek (St. Louis, MO). An ANOVA model was used to identify the differentially expressed genes between the non suicides and the suicides in a sample of mood disorder patients controlling for age, gender, RIN, and pH. Additionally, the AffyRNAdeg function was used to generate 3′/5′ slopes indicating RNA degradation and used as covariate in the ANOVA model [42]. Genes were considered as differentially expressed if they had a P-value of less than 0.01 and a fold change of ±1.25. Pathway analysis was performed using Ingenuity (Redwood City, CA) and the differentially expressed probe sets in each region.
Outlier detection was conducted in order to exclude problematic arrays using a combination of principal component analysis (PCA), and RNAdeg Slopes. In the DLPFC, 21 subjects were analyzed by microarrays, 15 suicides (3 females/12 males) and 6 non-suicides (2 females/4 males). In the ACC, a total of 15 subjects were included in the microarray analysis, 9 suicides (3 females/6 males) and 6 non-suicides (3 females/3 males). In the NAcc a total of 21 subjects were analyzed by microarrays, 13 suicides (3 females/10 males) and 8 non-suicides (2 females/6 males). For the microarray experiment sample (cohort 1), demographic and physiological variables used for the ANOVA model and to assess the RNA quality (age, pH, PMI or RIN numbers) can be found in Table 1 for the group averages and standard deviation and Table S1 for the detailed information. The entire dataset has been deposited to the Gene Expression Omnibus (GEO) with the Accession Number GSE6306 (Table S6).
Table 1. Demographic and physiological details for the subjects included in the microarray gene expression study sample (cohort 1).
| DLPFC | ACC | NAcc | ||||
| Suicide (n = 15) | NS (n = 6) | Suicide (n = 9) | NS (n = 6) | Suicide (n = 13) | NS (n = 8) | |
| Age (Mean±SD) | 44±14 | 42±17 | 43±9 | 53±10 | 43±9 | 54±8 |
| pH (Mean±SD) | 6.8±0.3 | 6.9±0.4 | 6.9±0.3 | 6.7±0.3 | 6.9±0.3 | 6.8±0.2 |
| PMI (Mean±SD) | 25±4.2 | 23±8.3 | 24±5.0 | 25±5.0 | 24±4.7 | 25±6.6 |
| Slope (Mean±SD) | 3.3±0.6 | 2.9±0.3 | 3.6±0.3 | 4.0±0.5 | 3.0±0.2 | 2.9±0.4 |
| RIN (Mean±SD) | 7.9±0.5 | 7.9±0.6 | 8.0±0.4 | 8.0±0.3 | 8.1±0.4 | 8.0±0.5 |
Mean±standard deviation values (SD) are presented. The slope was calculated using the AffyRNAdeg function. DLPFC: dorsolateral prefrontal cortex, ACC: anterior cingulate cortex, NAcc: nucleus accumbens.
Confirmation of Differential Expression
Confirmation of a subset of genes was done was done in an extended cohort including also normal controls by quantitative real-time polymerase chain reaction (qPCR) using the SYBR Green chemistry. Normalization of the qPCR results was performed using the geNorm method developed by Vandesompele et al. [43], this method consists on a factor calculation normalization based on the geometric mean of multiple endogenous control genes, in this case we used CAMK2A and SDHA. Samples were run in triplicate and confirmation of the results was determined by ANOVA controlling for age, pH, and diagnosis. Primers were designed to amplify regions close to the corresponding target sequences in the microarray experiments and spanning two exons in order to only amplify mRNA (primers are available on request). Experiments were performed in a 384 well plate in a total volume of 10 µl on a 7900 HT real time sequence detector (Applied Biosystems, Foster City, CA). The qPCR confirmation cohort (cohort 2) also included normal controls and showed no differences in terms of age, pH, gender or PMI between the groups ( Table 2 and Table S2).
Table 2. Demographic and physiological details for the subjects included in the qPCR validation sample (cohort 2).
| DLPFC | ACC | NAcc | |||||||
| Suicide (n = 20) | NS (n = 11) | Control (n = 27) | Suicide (n = 10) | NS (n = 7) | Controls (n = 18) | Suicide (n = 13) | NS (n = 7) | Control (n = 10) | |
| Age (Mean±SD) | 44±12 | 52±11 | 50±15 | 43±11 | 54±9 | 49±14 | 46±9 | 49±9 | 41±11 |
| pH (Mean±SD) | 6.9±0.2 | 6.9±0.2 | 6.9±0.3 | 7.0±0.2 | 6.9±0.2 | 7.0±0.2 | 6.9±0.2 | 6.9±0.2 | 6.9±0.2 |
| PMI (Mean± SD) | 26±4 | 24±6 | 20±7 | 24±5 | 26±5 | 20±5 | 25±5 | 24±6 | 21±5 |
| RIN (Mean ± SD) | 7.9±0.5 | 7.8±0.7 | 7.8±0.6 | 8.2±1 | 8.3±1 | 8.0±1 | NA | NA | NA |
Mean±standard deviation values (SD) are presented. DLPFC: dorsolateral prefrontal cortex, ACC: anterior cingulate cortex, NAcc: nucleus accumbens, NA: not available.
Results
No significant differences between the suicide and non-suicide groups were observed for the major demographic variables such as pH, age, RIN, PMI and RNAdeg slope ( Table 1 and Table S1). In order to control for possible confounding effects, demographic variables, such as diagnosis and age, and RNA quality measures (RIN and slope) were used as covariates.
A total of 161 genes were found to be differentially expressed in the DLPFC (Table S3). The most statistically significant gene in this region was the 5-HT2A gene (P = 0.00022, FC = −1.28), previously identified as being implicated in both mood disorders and in suicide consistently. The decrease of 5-HT2A expression in the suicide group was confirmed by qPCR (data not shown). Enrichment of canonical pathways analysis using Ingenuity confirmed that the serotonin receptor signaling pathway was altered in suicide brain tissue ( Fig. 1 a). Another gene of interest previously shown to be involved in suicide [44] and that was altered in the DLPFC was the adenylate cyclase 1 (brain) (ADCY1) gene that codes for a brain specific form of adenylate cyclase responsible of the production of the second messenger cyclic AMP, ADCY was significantly down-regulated in suicide versus non-suicide patients. Finally, period homolog 1 (PER1), a major component of the circadian rhythm system, was also down-regulated in suicides compared to non-suicides. Clock genes have been in the past associated with mood disorders [45] but not specifically with suicide. In summary, the DLPFC data shows it is possible to detect significant expression alterations in human brain areas between suicides and non-suicides brains and confirms the involvement of serotonergic and second messenger system alterations in suicide.
Figure 1. Canonical pathway enrichment analysis using Ingenuity.
A In the DLPFC. B In the ACC. C In the NAcc.
In the ACC, 139 annotated probe sets were differentially expressed between the suicides and non-suicides cases (Table S4). Of these, eight probe sets corresponded to seven metallothionein family members (MT1E, MT1M, MT1F, MT1G, MT1H, MT1X, MT2A), located as a cluster on chromosome 16q13 (Table 3). All these probe sets were significantly down-regulated in suicide victims with fold changes varying from −1.46 to −1.83. Pathway analysis conducted using Ingenuity on the 139 differentially expressed probe sets revealed that the most over represented pathway was glucocorticoid receptor signaling ( Fig. 1 b). Further exploration of the probe sets altered in the glucocorticoids receptor signaling pathway revealed a global down-regulation of metallothionein subfamilies 1 and 2 genes. In fact, a closer look at the expression levels of all the metallothionein genes revealed that most of the genes in subfamilies 1 and 2 where down-regulated in the ACC, including pseudogenes co-localized in 16q13 ( Fig. 2 ). Interestingly, the brain specific MT3 gene was highly expressed, but showed no differences in gene expression like subfamilies 1 and 2.
Table 3. qPCR Confirmation of decreased expression of metallothionein subfamilies 1 and 2.
| NACC | ACC | |||
| P-value | T | P-value | T | |
| MT1E | 0.09 | 3.25 | 0.02 | 6.36 |
| MT1F | 0.02 | 7.23 | 0.05 | 4.14 |
| MT1G | 0.01 | 7.63 | 0.01 | 7.67 |
| MT1H | 0.01 | 8.96 | 0.00 | 14.19 |
| MT1X | 0.03 | 5.65 | 0.02 | 5.99 |
| MT2A | 0.03 | 5.83 | 0.04 | 4.72 |
Significant differences are in bold for one tailed t-test confirming the direction of the change.
Figure 2. Expression levels of 11 metallothionein probe sets corresponding to 9 gene as measured by microarrays (HG-U133Plus 2.0) in the ACC and NAcc of non-suicide (grey bars) and suicide (black bars) mood disorder subjects.
Significant genes/probe sets are labeled by * (P<0.01) and # (P<0.05) signs.
In the NAcc, 146 probe sets were differentially expressed between suicides and non-suicides (Table S5). Three metallothionein genes were also down-regulated in the NACC (MT1F, MT1G, MT1H) among the suicide victims and like in the ACC several others were also down-regulated although at a P<0.05 level. IPA did not reveal any canonical pathway as being over-represented, as opposed to the ACC ( Fig. 1 c), only three MT genes passed the significance criteria. However, 7 probe sets in total were differentially expressed at the 0.05 level confirming the overall dysregulation in this family of genes ( Fig. 2 ). The Wolfram syndrome gene (WFS1), previously associated with psychiatric disorders and suicide [46]–[48], was also down-regulated among the suicide victims in the NACC.
Confirmation of Results by qPCR
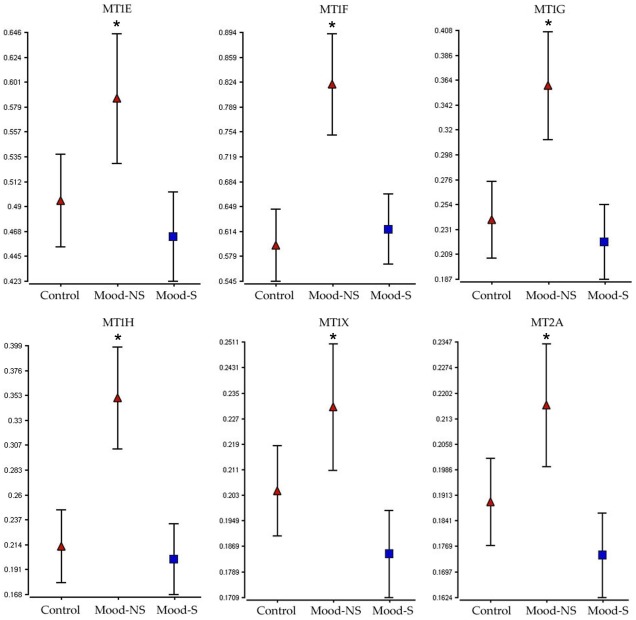
Group averages for demographic and physiological variables for the subjects (cohort 2) included in the confirmation experiments per region can be seen in Table 2 and for subjects in cohort 2 used in this analysis can be seen in Table 2 and detailed demographic data in table S2. All the subjects used the validation cohort had high pH (mean = 6.9) and RIN values (mean = 8), and an average the PMI of 23 hours (8.5 to 37 hours). Metallotionein gene expression down-regulation in both the ACC and NACC was confirmed by qPCR for all the functional variants in sub-families 1 and 2 known to be expressed in the brain (MT1E, MT1F, MT1G, MT1H, MT1X, MT2A). In summary, the pattern of metallothionein expression was confirmed to be lower in the suicides compared to the non-suicides in both the ACC and NACC for all the genes examined ( Figs. 3 and 4 ). When compared to normal controls, metallothionein levels tended to be elevated in the non-suicide mood disorder group, especially in the NAcc, while lower specifically in the suicide group.
Figure 3. Expression levels in the NACC of six metallothionein genes in as measured by qPCR in psychiatrically normal controls and mood disorder patients that did (Mood-S) or did not commit suicide (Mood-NS).
* denotes significance at the P<0.05 versus the Mood-S group.
Figure 4. qPCR confirmation in the ACC.
Expression levels in the ACC of six metallothionein genes measured by qPCR and the GE-Norm method between psychiatrically normal controls and mood disorder patients that did (Mood-S) or did not commit suicide (Mood-NS). * denotes significance at the P<0.05 versus the Mood-S group.
Discussion
A gene expression survey by microarrays in three brain regions (DLPFC, NAcc and ACC) revealed alterations in the serotonergic and second messenger systems in the DLPFC and a consistent down-regulation of several members of the metallothionein subfamilies 1 and 2 genes in the NAcc and ACC in mood disorder suicides. Our strategy consisted on using high quality brains (short PMI, high pH, high RIN) with no history of drug dependence, as well as the development of a general linear model in which factors known to influence RNA quality and expression were taken into account when exploring the suicide specific expression changes. This is to our knowledge the first study investigating suicide specific changes in mood disorders in three highly relevant areas as they are involved in the control of impulses and in mood disorders (DLPFC, ACC) and in motivation-reward mechanisms (NAcc).
We studied gene expression changes in the DLPFC, an area previously associated with both mood disorders, control of impulses and suicide. Interestingly, the most statistically significant gene differentially expressed in suicide victims was the 5-HT2A serotonergic receptor, with a1.28 fold-change reduction in mood disorder subjects that committed suicide versus non-suicide mood disorder subjects. Genetic association studies have often shown that mutations in the 5-HT2A receptor are associated with mood disorders and suicide [49], as well as with the response to antidepressants [50]. Additionally, gene expression post-mortem studies have revealed alterations 5-HT2Areceptor levels in mood disorder subject suicides when compared to controls. However the direction of the alterations in 5-HT2A is controversial, with some reports showing elevations in the 5-HT2A binding and expression [51]–[55] while others showed reduced levels or no differences [56]–[58]. Recently, a well designed study including matched suicides and controls also confirmed the absence of changes in 5-HT2 binding but reported a decrease in neuronal densities in the prefrontal cortex in a sample of predominantly major depressed suicides [59]. In another recent study designed to investigate 5-HT2A levels in major depression, Shelton et al. reported an increase in 5-HT2A expression in major depression subjects, but reported no differences when comparing suicides versus non-suicide subjects [55]. In this study, we aimed to investigate suicide specific changes in mood disorder patients and observed a significant decrease in 5-HT2A levels in suicide which can at least partially explain previous discordant findings when comparing mood disorder to controls or suicides to controls. The reduction in 5-HT2A levels is also in concordance with another study comparing suicide versus non-suicide schizophrenia subjects using also Affymetrix microarrays [33], suggesting that this reduction in 5-HT2A levels might be involved in suicide irrespective of the diagnosis. We also observed decreased expression in suicide group of the brain specific ADCY1, a brain specific form of adenylate cyclase, suggesting altered neurotransmission and cyclic AMP signaling in suicide and confirms previous observations pointing to cAMP signaling alterations in suicide and mood disorders [60]–[63] and in animal models of depression [64]. Both serotonergic alterations and second messenger systems underline global neurotransmission alterations in the DLPFC that seem to play a role in the predisposition to commit suicide.
In both the ACC and NAcc decreased expression was observed in gene members of two subfamilies (1 and 2) of the metallothionein super family in the suicides versus the non-suicides mood disorder subjects. These changes were confirmed successfully Metallothioneins are small single chain proteins with a high content of cysteine residues organized in specific sequences enabling the formation of thiolate cluster and the binding of certain metals react to oxidative stress, glucocorticoids and inflammatory mediators [65]. There are four subfamilies of metallothioneins proteins in mammals corresponding to types 1 to 4, all metallothionein genes are closely located in chromosome 16q13. MT1 and MT2 are expressed ubiquitously and highly co-expressed, whereas MT4 is not expressed in the brain and MT3 is brain specific and expressed mainly in hippocampus amygdala and cortex in zinc enriched neurons and astrocytes [66]. Consistent with this, MT3 was also observed to be expressed in the three brain areas investigated but was not differentially expressed like MT1 and 2 subfamilies. MT3, unlike MT 1 and 2 is not regulated by glucocorticoids and stress, which can explain this difference [67]–[72].
MT1 and MT2 are not only known to be responsive to several forms of stress and to glucocorticoids [73], they also play an important role in neuroprotection [66]. In animal models of stress, both MT1 and MT2 mRNA were elevated as much as 30-fold following just 12 h (one cycle) of restraint stress whereas MT3 gene expression was not altered by stress [74]. The induction occurred at the transcriptional level and was mediated by the activation of glucocorticoid receptor [74]. In a stress mouse model, treatment with a glucocorticoid receptor antagonist (RU 486) prior to restraint stress inhibited MT induction by at least 50% indication that at least in part MT induction is under direct control of the glucocorticoid receptor at the transcriptional levels [75]. Glucocorticoids induction of metallothioneins expression is done through the direct binding of the ligand activated glucocorticoid receptor to the glucocorticoid response elements (GRE) in the promoter of MTs [76], [77]. Dexamethasone has been shown for instance to increase significantly the levels of MT and immobilization stress has also been shown to increase selectively MT1 and MT2 of rat brains [70]. MT1 and 2 play a neuroprotective role against stress and glucocorticoids elevation by a mechanism of induced expression of metallothioneins in astrocytes followed by an extracellular release and internalization by neurons after binding to the receptor megalin that mediates the transport of MTs into neurons [78]. Thus, MT1 and 2 sub-families, that play an important role in neuroprotection from the effects of behavioral stress and in response to cortisol, are significantly reduced in mood disorder suicides compared to non-suicides. Interestingly, in another recent microarray study using Affymetrix Exon 1.0 ST arrays, Shelton et al. observed and confirmed by qPCR a decreased expression of MT1M in the BA10 of depressed subjects compared to controls [79].
Alterations in the hypothalamic-pituitary-adrenal (HPA) axis in depressive states and suicide have been consistently observed for more than 40 years [19], [80]. It has also been extensively shown that depressed states are specifically linked to both chronic stress and elevated cortisol levels relevant of HPA axis hyperactivity in depression [81]. Many studies have observed higher baseline cortisol values in young depressed patients [82] and cortisol hypersecretion in depression [83]. We propose in this study that decreased levels of MT1 and 2 can result in a higher sensitivity in suicide completers to the effects of depression-associated cortisol levels in the brain. If these results are confirmed in a larger independent cohort, pharmacological agents that increase the expression of MT 1 and 2 metallothionein sub-families or their transport to neurons by the megalin receptor without necessarily altering HPA axis activity can be envisioned for the treatment and prevention of suicide and suicidal behaviors in mood disorder patients.
The effect of known confounding variables in post-mortem brain gene expression studies (pH, PMI, age, gender, diagnosis and RNA quality) was controlled by conducting an ANCOVA using significant variable as covariates. One of the weaknesses of this study is the relatively small size of the microarray sample. However, we were able to confirm the observed results in a larger sample and to explore MT expression in control subjects also by qPCR in a larger sample in both the ACC and NAcc ( Figs. 3 and 4 ). Another problem observed was the difference in age between the non-suicides and the suicides in the NAcc and ACC. This was expected as suicide is particularly prevalent in younger populations [2], [2], [7,84] with about 200,000 young people that die every year in the world by suicide [85]. For that reason, we included age into the statistical model in order to exclude genes altered by age only. Ultimately however, MT subfamilies 1 and 2 down-regulation in suicide mood disorder subjects should be tested in peripheral tissue such as blood lymphocytes, where they have been shown to be expressed [86], in order to confirm these results in a clinical population and to investigate the validity of these changes as a biomarker for suicide.
In conclusion, while expression profiling confirmed and further extended the implication of serotonergic genes in suicide in the DLPFC, the present study also reveals a significant decrease in expression of metallothioneins subfamilies1 and 2, a family of genes involved in neuroprotection and in cortisol/stress response, in suicide victims compared to non-suicide mood disorder patients. Because cortisol levels are known to be elevated in depressive states, the observed decreased in MT expression in the suicide group might point to a failed neuroprotective mechanism against cortisol in this group and point to a new marker and possible pharmacological target for suicide and suicidal behavior.
Supporting Information
Complete demographic variables for the subjects included in the microarray analysis per brain region. (RIN: Agilent 2100 RNA Integrity Number; MDD: major depressive disorder; BD: bipolar disorder; NS: non suicide; MOD: method of death).
(DOC)
Complete demographic variables for the subjects included in the confirmation qPCR analysis of the DLPFC, ACC and Nacc. (MDD: major depressive disorder; BD: bipolar disorder; NS: non suicide; PMI: post-mortem interval; MOD: method of death).
(DOC)
Differentially expressed probe sets between suicide and non-suicide mood disorder subjects in the DLPFC.
(DOC)
Differentially expressed probe sets between suicide and non-suicide mood disorder subjects in the ACC (139).
(DOC)
Differentially expressed probe sets between suicide and non-suicide mood disorder subjects in the NAcc (146).
(DOC)
Accession numbers for the chips included in the study.
(DOC)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The authors are members of the Pritzker Neuropsychiatric Disorders Research Consortium, which is supported by the Pritzker Neuropsychiatric Disorders Research Fund L.L.C. A shared intellectual property agreement exists between this philanthropic fund and the University of Michigan, Stanford University, the Weill Medical College of Cornell University, HudsonAlpha Institute of Biotechnology, the Universities of California at Davis (UCD), and at Irvine (UCI), to encourage the development of appropriate findings for research and clinical applications. The research was further supported by the William Lion Penzner Foundation (Dept of Psychiatry, UCI), Della Martin Foundation (to WEB, AS) and the National Alliance for Research on Schizophrenia and Depression (NARSAD) Young Investigator Award (to AS). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nock MK, Borges G, Bromet EJ, Cha CB, Kessler RC, et al. Suicide and suicidal behavior. Epidemiologic Reviews. 2008;30:133–154. doi: 10.1093/epirev/mxn002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO . Health Systems: Improving Performance; 2000. World Health Report 2000. [Google Scholar]
- 4.Goldsmith SK, Pellmar TC, Kleinman AM, Bunney WE. Washington, DC: The National Academy Press; 2002. Reducing Suicide: A National Imperative. [PubMed] [Google Scholar]
- 5.Robins E, Murphy GE, Wilkinson RH, Gassner S, Kayes J. Some clinical considerations in the prevention of suicide based on a study of 134 successful suicides. American Journal of Public Health. 1959;49:888–899. doi: 10.2105/ajph.49.7.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barraclough B, Bunch J, Nelson B, Sainsbury P. A hundred cases of suicide: clinical aspects. Br J Psychiatry. 1974;125:355–373. doi: 10.1192/bjp.125.4.355. [DOI] [PubMed] [Google Scholar]
- 7.Lesage AD, Boyer R, Grunberg F, Vanier C, Morisette R, et al. Suicide and mental disorders: a case-control study of young men. Am J Psychiatry. 1994;151:1063–1068. doi: 10.1176/ajp.151.7.1063. [DOI] [PubMed] [Google Scholar]
- 8.Bostwick JM, Pankratz VS. Affective disorders and suicide risk: a reexamination. Am J Psychiatry. 2000;157:1925–1932. doi: 10.1176/appi.ajp.157.12.1925. [DOI] [PubMed] [Google Scholar]
- 9.Jamison KR. Suicide and bipolar disorder. J Clin Psychiatry. 2000;61(Suppl 9):47–51. [PubMed] [Google Scholar]
- 10.Inskip HM, Harris EC, Barraclough B. Lifetime risk of suicide for affective disorder, alcoholism and schizophrenia. Br J Psychiatry. 1998;172:35–37. doi: 10.1192/bjp.172.1.35. [DOI] [PubMed] [Google Scholar]
- 11.Guze SB, Robins E. Suicide and primary affective disorders. Br J Psychiatry. 1970;117:437–438. doi: 10.1192/bjp.117.539.437. [DOI] [PubMed] [Google Scholar]
- 12.Goodwin FK, Jamison KR. Suicide, in Manic-Depressive Illness. Suicide, in Manic-Depressive Illness. 1990. pp. 227–244.
- 13.Oquendo MA, Mann JJ. The biology of impulsivity and suicidality. The Psychiatric clinics of North America. 2000;23:11–25. doi: 10.1016/s0193-953x(05)70140-4. [DOI] [PubMed] [Google Scholar]
- 14.Oquendo MA, Baca-Garcia E, Mann JJ, Giner J. Issues for DSM-V: suicidal behavior as a separate diagnosis on a separate axis. Am J Psychiatry. 2008;165:1383–1384. doi: 10.1176/appi.ajp.2008.08020281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bourne HR, Bunney WE, Colburn RW, Davis JM, Davis JN, et al. Noradrenaline, 5-hydroxytryptamine, and 5-hydroxyindoleacetic acid in hindbrains of suicidal patients. Lancet. 1968;2:805–808. doi: 10.1016/s0140-6736(68)92459-8. [DOI] [PubMed] [Google Scholar]
- 16.Bunney WE, Davis JM. Norepinephrine in depressive reactions. A review. Arch Gen Psychiat. 1965;13:483–494. doi: 10.1001/archpsyc.1965.01730060001001. [DOI] [PubMed] [Google Scholar]
- 17.Mann JJ. Neurobiology of suicidal behaviour. Nature reviews Neuroscience. 2003;4:819–828. doi: 10.1038/nrn1220. [DOI] [PubMed] [Google Scholar]
- 18.Jokinen J, Nordstrom AL, Nordstrom P. CSF 5-HIAA and DST non-suppression–orthogonal biologic risk factors for suicide in male mood disorder inpatients. Psychiatr Res. 2009;165:96–102. doi: 10.1016/j.psychres.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Bunney WE, Jr, Fawcett JA. Possibility of a Biochemical Test for Suicidal Potential: An Analysis of Endocrine Findings Prior to Three Suicides. Arch Gen Psychiat. 1965;13:232–239. doi: 10.1001/archpsyc.1965.01730030038006. [DOI] [PubMed] [Google Scholar]
- 20.Bunney WE, Jr, Fawcett J, Davis JM, Gifford S. Further evaluation of urinary 17-hydroxycorticosteroids in suicidal patients. Arch Gen Psychiat. 1969;21:138–150. doi: 10.1001/archpsyc.1969.01740200010002. [DOI] [PubMed] [Google Scholar]
- 21.Gross-Isseroff R, Biegon A, Voet H, Weizman A. The suicide brain: a review of postmortem receptor/transporter binding studies. Neuroscience and biobehavioral reviews. 1998;22:653–661. doi: 10.1016/s0149-7634(97)00061-4. [DOI] [PubMed] [Google Scholar]
- 22.Lopez JF, Vazquez DM, Chalmers DT, Watson SJ. Regulation of 5-HT receptors and the hypothalamic-pituitary-adrenal axis. Implications for the neurobiology of suicide. Annals of the New York Academy of Sciences. 1997;836:106–134. doi: 10.1111/j.1749-6632.1997.tb52357.x. [DOI] [PubMed] [Google Scholar]
- 23.McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonte B, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nature Neuroscience. 2009;12:342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sequeira A, Turecki G. Genome wide gene expression studies in mood disorders. OMICS. 2006;10:444–454. doi: 10.1089/omi.2006.10.444. [DOI] [PubMed] [Google Scholar]
- 25.Sibille E, Arango V, Galfalvy HC, Pavlidis P, Erraji-Benchekroun L, et al. Gene expression profiling of depression and suicide in human prefrontal cortex. Neuropsychopharmacol. 2004;29:351–361. doi: 10.1038/sj.npp.1300335. [DOI] [PubMed] [Google Scholar]
- 26.Sequeira A, Gwadry FG, Ffrench-Mullen JM, Canetti L, Gingras Y, et al. Implication of SSAT by gene expression and genetic variation in suicide and major depression. Arch Gen Psychiatry. 2006;63:35–48. doi: 10.1001/archpsyc.63.1.35. [DOI] [PubMed] [Google Scholar]
- 27.Klempan TA, Sequeira A, Canetti L, Lalovic A, Ernst C, et al. Altered expression of genes involved in ATP biosynthesis and GABAergic neurotransmission in the ventral prefrontal cortex of suicides with and without major depression. Mol Psychiatr. 2007 doi: 10.1038/sj.mp.4002110. [DOI] [PubMed] [Google Scholar]
- 28.Thalmeier A, Dickmann M, Giegling I, Schneider B, Hartmann M, et al. Gene expression profiling of post-mortem orbitofrontal cortex in violent suicide victims. The international journal of neuropsychopharmacology. 2008;11:217–228. doi: 10.1017/S1461145707007894. [DOI] [PubMed] [Google Scholar]
- 29.Tochigi M, Iwamoto K, Bundo M, Sasaki T, Kato N, et al. Gene expression profiling of major depression and suicide in the prefrontal cortex of postmortem brains. Neuroscience Research. 2008;60:184–191. doi: 10.1016/j.neures.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 30.Kim S, Choi KH, Baykiz AF, Gershenfeld HK. Suicide candidate genes associated with bipolar disorder and schizophrenia: an exploratory gene expression profiling analysis of post-mortem prefrontal cortex. BMC Genomics. 2007;8:413. doi: 10.1186/1471-2164-8-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sequeira A, Klempan T, Canetti L, Ffrench-Mullen J, Benkelfat C, et al. Patterns of gene expression in the limbic system of suicides with and without major depression. Mol Psychiatr. 2007;12:640–655. doi: 10.1038/sj.mp.4001969. [DOI] [PubMed] [Google Scholar]
- 32.Yanagi M, Shirakawa O, Kitamura N, Okamura K, Sakurai K, et al. Association of 14–3-3 epsilon gene haplotype with completed suicide in Japanese. Journal of Human Genetics. 2005;50:210–216. doi: 10.1007/s10038-005-0241-0. [DOI] [PubMed] [Google Scholar]
- 33.Garbett K, Gal-Chis R, Gaszner G, Lewis DA, Mirnics K. Transcriptome alterations in the prefrontal cortex of subjects with schizophrenia who committed suicide. Neuropsychopharmacologia Hungarica. 2008;10:9–14. [PubMed] [Google Scholar]
- 34.Kelly TM, Mann JJ. Reliability of DSM-III-R diagnosis by psychological autopsy: a comparison with clinician antemortem diagnosis. Acta Psychait Scand. 1996;94:337–343. doi: 10.1111/j.1600-0447.1996.tb09869.x. [DOI] [PubMed] [Google Scholar]
- 35.Wester P, Bateman DE, Dodd PR, Edwardson JA, Hardy JA, et al. Agonal status affects the metabolic activity of nerve endings isolated from postmortem human brain. Neurochem Pathol. 1985;3:169–180. doi: 10.1007/BF02834269. [DOI] [PubMed] [Google Scholar]
- 36.Barton AJL, Pearson RCA, Najlerahim A, Harrison PJ. Pre-Postmortem influences on brain RNA. J Neurochem. 1993;61:1–11. doi: 10.1111/j.1471-4159.1993.tb03532.x. [DOI] [PubMed] [Google Scholar]
- 37.Atz M, Walsh D, Cartagena P, Li J, Evans S, et al. Methodological considerations for gene expression profiling of human brain. J Neurosci Methods. 2007;163:295–309. doi: 10.1016/j.jneumeth.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vawter MP, Tomita H, Meng F, Bolstad B, Li J, et al. Mitochondrial-related gene expression changes are sensitive to agonal-pH state: implications for brain disorders. Mol Psychiatry. 2006;615 doi: 10.1038/sj.mp.4001830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li JZ, Vawter MP, Walsh DM, Tomita H, Evans SJ, et al. Systematic changes in gene expression in postmortem human brains associated with tissue pH and terminal medical conditions. Hum Mol Genet. 2004;13:609–616. doi: 10.1093/hmg/ddh065. [DOI] [PubMed] [Google Scholar]
- 40.Tomita H, Vawter MP, Walsh DM, Evans SJ, Choudary PV, et al. Effect of agonal and postmortem factors on gene expression profile: quality control in microarray analyses of postmortem human brain. Biol Psychiatry. 2004;55:346–352. doi: 10.1016/j.biopsych.2003.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones EG, Hendry SH, Liu XB, Hodgins S, Potkin SG, et al. A method for fixation of previously fresh-frozen human adult and fetal brains that preserves histological quality and immunoreactivity. J Neurosc Methods. 1992;44:133–144. doi: 10.1016/0165-0270(92)90006-y. [DOI] [PubMed] [Google Scholar]
- 42.Gautier L, Cope L, Bolstad BM, Irizarry RA. affy–analysis of Affymetrix GeneChip data at the probe level. Bioinformatics. 2004;20:307–315. doi: 10.1093/bioinformatics/btg405. [DOI] [PubMed] [Google Scholar]
- 43.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome biology. 2002;3:RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cowburn RF, Marcusson JO, Eriksson A, Wiehager B, O’Neill C. Adenylyl cyclase activity and G-protein subunit levels in postmortem frontal cortex of suicide victims. Brain Res. 1994;633:297–304. doi: 10.1016/0006-8993(94)91552-0. [DOI] [PubMed] [Google Scholar]
- 45.Bunney WE, Bunney BG. Molecular clock genes in man and lower animals: possible implications for circadian abnormalities in depression. Neuropsychopharmacol. 2000;22:335–345. doi: 10.1016/S0893-133X(99)00145-1. [DOI] [PubMed] [Google Scholar]
- 46.Sequeira A, Kim C, Seguin M, Lesage A, Chawky N, et al. Wolfram syndrome and suicide: Evidence for a role of WFS1 in suicidal and impulsive behavior. Am J Med Genet B Neuropsychiatr Genet. 2003;119:108–113. doi: 10.1002/ajmg.b.20011. [DOI] [PubMed] [Google Scholar]
- 47.Swift M, Swift RG. Wolframin mutations and hospitalization for psychiatric illness. Mol Psychiatr. 2005;10:799–803. doi: 10.1038/sj.mp.4001681. [DOI] [PubMed] [Google Scholar]
- 48.Swift RG, Sadler DB, Swift M. Psychiatric findings in Wolfram syndrome homozygotes. Lancet. 1990;336:667–669. doi: 10.1016/0140-6736(90)92157-d. [DOI] [PubMed] [Google Scholar]
- 49.Serretti A, Drago A, De Ronchi D. HTR2A gene variants and psychiatric disorders: a review of current literature and selection of SNPs for future studies. Current medicinal chemistry. 2007;14:2053–2069. doi: 10.2174/092986707781368450. [DOI] [PubMed] [Google Scholar]
- 50.McMahon FJ, Buervenich S, Charney D, Lipsky R, Rush AJ, et al. Variation in the gene encoding the serotonin 2A receptor is associated with outcome of antidepressant treatment. American Journal of Human Genetics. 2006;78:804–814. doi: 10.1086/503820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stanley M, Mann JJ. Increase serotonin-2 binding sites in frontal cortex of suicide victims. Lancet. 1983;1:214–216. doi: 10.1016/s0140-6736(83)92590-4. [DOI] [PubMed] [Google Scholar]
- 52.Turecki G, Briere R, Dewar K, Antonetti T, Lesage AD, et al. Prediction of level of serotonin 2A receptor binding by serotonin receptor 2A genetic variation in postmortem brain samples from subjects who did or did not commit suicide. Am J Psychiatry. 1999;156:1456–1458. doi: 10.1176/ajp.156.9.1456. [DOI] [PubMed] [Google Scholar]
- 53.Hrdina PD, Du L. Levels of serotonin receptor 2A higher in suicide victims? Am J Psychiatry. 2001;158:147–148. doi: 10.1176/appi.ajp.158.1.147-a. [DOI] [PubMed] [Google Scholar]
- 54.Pandey GN, Dwivedi Y, Rizavi HS, Ren X, Pandey SC, et al. Higher expression of serotonin 5-HT(2A) receptors in the postmortem brains of teenage suicide victims. Am J Psychiatry. 2002;159:419–429. doi: 10.1176/appi.ajp.159.3.419. [DOI] [PubMed] [Google Scholar]
- 55.Shelton RC, Sanders-Bush E, Manier DH, Lewis DA. Elevated 5-HT 2A receptors in postmortem prefrontal cortex in major depression is associated with reduced activity of protein kinase A. Neuroscience. 2009;158:1406–1415. doi: 10.1016/j.neuroscience.2008.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stockmeier CA, Dilley GE, Shapiro LA, Overholser JC, Thompson PA, et al. Serotonin receptors in suicide victims with major depression. Neuropsychopharmacology. 1997;16:162–173. doi: 10.1016/S0893-133X(96)00170-4. [DOI] [PubMed] [Google Scholar]
- 57.Gross-Isseroff R, Salama D, Israeli M, Biegon A. Autoradiographic analysis of [3H]ketanserin binding in the human brain postmortem: effect of suicide. Brain Res. 1990;507:208–215. doi: 10.1016/0006-8993(90)90274-f. [DOI] [PubMed] [Google Scholar]
- 58.Rosel P, Arranz B, San L, Vallejo J, Crespo JM, et al. Altered 5-HT(2A) binding sites and second messenger inositol trisphosphate (IP(3)) levels in hippocampus but not in frontal cortex from depressed suicide victims. Psychiatry Res. 2000;99:173–181. doi: 10.1016/s0925-4927(00)00076-7. [DOI] [PubMed] [Google Scholar]
- 59.Underwood MD, Kassir SA, Bakalian MJ, Galfalvy H, Mann JJ, et al. Neuron density and serotonin receptor binding in prefrontal cortex in suicide. Int J Neuropsychopharmacol. 2011:1–13. doi: 10.1017/S1461145711000691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Young LT, Li PP, Kish SJ, Siu Po K, Kamble A, et al. Cerebral cortex Gsα protein levels and forskolin-stimulated cyclic AMP formation are increased in bipolar affective disorder. J Neurochem. 1993;61:890–898. doi: 10.1111/j.1471-4159.1993.tb03600.x. [DOI] [PubMed] [Google Scholar]
- 61.Rahman S, Li PP, Young LT, Kofman O, Kish SJ, et al. Reduced [3H]cyclic AMP binding in postmortem brain from subjects with bipolar affective disorder. J Neurochem. 1997;68:297–304. doi: 10.1046/j.1471-4159.1997.68010297.x. [DOI] [PubMed] [Google Scholar]
- 62.Dowlatshahi D, MacQueen GM, Wang JF, Reiach JS, Young LT. G Protein-coupled cyclic AMP signaling in postmortem brain of subjects with mood disorders: effects of diagnosis, suicide, and treatment at the time of death. J Neurochem. 1999;73:1121–1126. doi: 10.1046/j.1471-4159.1999.0731121.x. [DOI] [PubMed] [Google Scholar]
- 63.Dwivedi Y, Rao JS, Rizavi HS, Kotowski J, Conley RR, et al. Abnormal expression and functional characteristics of cyclic adenosine monophosphate response element binding protein in postmortem brain of suicide subjects. Arch Gen Psychiat. 2003;60:273–282. doi: 10.1001/archpsyc.60.3.273. [DOI] [PubMed] [Google Scholar]
- 64.Wann BP, D’Anjou B, Bah TM, Webster HH, Godbout R, et al. Effect of olfactory bulbectomy on adenylyl cyclase activity in the limbic system. Brain Research Bulletin. 2009;79:32–36. doi: 10.1016/j.brainresbull.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 65.Coyle P, Philcox JC, Carey LC, Rofe AM. Metallothionein: the multipurpose protein. Cellular and molecular life sciences. 2002;59:627–647. doi: 10.1007/s00018-002-8454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pedersen MO, Jensen R, Pedersen DS, Skjolding AD, Hempel C, et al. Metallothionein-I+II in neuroprotection. Biofactors. 2009;35:315–325. doi: 10.1002/biof.44. [DOI] [PubMed] [Google Scholar]
- 67.Kramer KK, Zoelle JT, Klaassen CD. Induction of metallothionein mRNA and protein in primary murine neuron cultures. Toxicology and Applied Pharmacology. 1996;141:1–7. doi: 10.1006/taap.1996.0253. [DOI] [PubMed] [Google Scholar]
- 68.Kramer KK, Liu J, Choudhuri S, Klaassen CD. Induction of metallothionein mRNA and protein in murine astrocyte cultures. Toxicology and Applied Pharmacology. 1996;136:94–100. doi: 10.1006/taap.1996.0011. [DOI] [PubMed] [Google Scholar]
- 69.Gasull T, Giralt M, Hernandez J, Martinez P, Bremner I, et al. Regulation of metallothionein concentrations in rat brain: effect of glucocorticoids, zinc, copper, and endotoxin. The American journal of physiology. 1994;266:E760–E767. doi: 10.1152/ajpendo.1994.266.5.E760. [DOI] [PubMed] [Google Scholar]
- 70.Hidalgo J, Belloso E, Hernandez J, Gasull T, Molinero A. Role of Glucocorticoids on Rat Brain Metallothionein-I and -III Response to Stress. Stress. 1997;1:231–240. doi: 10.3109/10253899709013743. [DOI] [PubMed] [Google Scholar]
- 71.Hidalgo J, Garcia A, Oliva AM, Giralt M, Gasull T, et al. Effect of zinc, copper and glucocorticoids on metallothionein levels of cultured neurons and astrocytes from rat brain. Chemico-biological interactions. 1994;93:197–219. doi: 10.1016/0009-2797(94)90020-5. [DOI] [PubMed] [Google Scholar]
- 72.Zheng H, Berman NE, Klaassen CD. Chemical modulation of metallothionein I and III mRNA in mouse brain. Neurochem Int. 1995;27:43–58. doi: 10.1016/0197-0186(94)00167-s. [DOI] [PubMed] [Google Scholar]
- 73.Hidalgo J, Aschner M, Zatta P, Vasak M. Roles of the metallothionein family of proteins in the central nervous system. Brain Research Bulletin. 2001;55:133–145. doi: 10.1016/s0361-9230(01)00452-x. [DOI] [PubMed] [Google Scholar]
- 74.Jacob ST, Ghoshal K, Sheridan JF. Induction of metallothionein by stress and its molecular mechanisms. Gene Expression. 1999;7:301–310. [PMC free article] [PubMed] [Google Scholar]
- 75.Ghoshal K, Wang Y, Sheridan JF, Jacob ST. Metallothionein induction in response to restraint stress. Transcriptional control, adaptation to stress, and role of glucocorticoid. The J Biolog Chem. 1998;273:27904–27910. doi: 10.1074/jbc.273.43.27904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Karin M, Haslinger A, Holtgreve H, Richards RI, Krauter P, et al. Characterization of DNA sequences through which cadmium and glucocorticoid hormones induce human metallothionein-IIA gene. Nature. 1984;308:513–519. doi: 10.1038/308513a0. [DOI] [PubMed] [Google Scholar]
- 77.Kelly EJ, Sandgren EP, Brinster RL, Palmiter RD. A pair of adjacent glucocorticoid response elements regulate expression of two mouse metallothionein genes. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:10045–10050. doi: 10.1073/pnas.94.19.10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chung RS, Penkowa M, Dittmann J, King CE, Bartlett C, et al. Redefining the role of metallothionein within the injured brain: extracellular metallothioneins play an important role in the astrocyte-neuron response to injury. The J Biolog Chem. 2008;283:15349–15358. doi: 10.1074/jbc.M708446200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shelton RC, Claiborne J, Sidoryk-Wegrzynowicz M, Reddy R, Aschner M, et al. Altered expression of genes involved in inflammation and apoptosis in frontal cortex in major depression. Mol Psychiatr. 2010 doi: 10.1038/mp.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bunney WE, Jr, Mason JW, Hamburg DA. Correlations Between Behavioral Variables and Urinary 17-Hydroxycorticosteroids in Depressed Patients. Psychosomatic Medicine. 1965;27:299–308. doi: 10.1097/00006842-196507000-00001. [DOI] [PubMed] [Google Scholar]
- 81.Vreeburg SA, Hoogendijk WJ, van PJ, DeRijk RH, Verhagen JC, et al. Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: results from a large cohort study. Arch Gen Psychiat. 2009;66:617–626. doi: 10.1001/archgenpsychiatry.2009.50. [DOI] [PubMed] [Google Scholar]
- 82.Lopez-Duran NL, Kovacs M, George CJ. Hypothalamic-pituitary-adrenal axis dysregulation in depressed children and adolescents: A meta-analysis. Psychoneuroendocrinology. 2009 doi: 10.1016/j.psyneuen.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cowen PJ. Not fade away: the HPA axis and depression. Psychological Medicine. 2009:1–4. doi: 10.1017/S0033291709005558. [DOI] [PubMed] [Google Scholar]
- 84.Brent DA, Perper JA, Goldstein CE, Kolko DJ, Allan MJ, et al. Risk factors for adolescent suicide. A comparison of adolescent suicide victims with suicidal inpatients. Arch Gen Psychiat. 1988;45:581–588. doi: 10.1001/archpsyc.1988.01800300079011. [DOI] [PubMed] [Google Scholar]
- 85.Greydanus DE, Calles J., Jr Suicide in children and adolescents. Primary care. 2007;34:259–273. doi: 10.1016/j.pop.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 86.Chang X, Jin T, Chen L, Nordberg M, Lei L. Metallothionein I isoform mRNA expression in peripheral lymphocytes as a biomarker for occupational cadmium exposure. Experimental biology and medicine. 2009;234:666–672. doi: 10.3181/0811-RM-336. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Complete demographic variables for the subjects included in the microarray analysis per brain region. (RIN: Agilent 2100 RNA Integrity Number; MDD: major depressive disorder; BD: bipolar disorder; NS: non suicide; MOD: method of death).
(DOC)
Complete demographic variables for the subjects included in the confirmation qPCR analysis of the DLPFC, ACC and Nacc. (MDD: major depressive disorder; BD: bipolar disorder; NS: non suicide; PMI: post-mortem interval; MOD: method of death).
(DOC)
Differentially expressed probe sets between suicide and non-suicide mood disorder subjects in the DLPFC.
(DOC)
Differentially expressed probe sets between suicide and non-suicide mood disorder subjects in the ACC (139).
(DOC)
Differentially expressed probe sets between suicide and non-suicide mood disorder subjects in the NAcc (146).
(DOC)
Accession numbers for the chips included in the study.
(DOC)