Abstract
Purpose
We evaluated site-specific skeletal adaptation to loading during growth,comparing radius (RAD) and femoral neck (FN) DXA scans in young female gymnasts (GYM) and non-gymnasts (NON).
Methods
Subjects from an ongoing longitudinal study (8-26 yrs old) underwent annual DXA scans (proximal femur, forearm, total body) and anthropometry, completing maturity and physical activity questionnaires. This cross-sectional analysis used the most recent data meeting the following criteria: gynecological age ≤2.5 yrs post-menarche; GYM annual mean gymnastic exposure ≥5.0 h/wk in the prior year. Bone geometric and strength indices were derived from scans for 173 subjects (8-17 yrs old) via hip structural analysis (femoral narrow neck, NN) and similar radius formulae (1/3 and Ultradistal (UD)). Maturity was coded as M1 (Tanner I breast), M2 (pre-menarche, ≥Tanner II breast) or M3 (post-menarche). ANOVA and chi square compared descriptive data. Two factor ANCOVA adjusted for age, height, total body non-bone lean mass and percent body fat; significance was tested for main effects and interactions between gymnastic exposure and maturity.
Results
At the distal radius, GYM means were significantly greater than NON means for all variables (p<0.05). At the proximal femur, GYM exhibited narrower periosteal and endosteal dimensions, but greater indices of cortical thickness, BMC, aBMD and section modulus, with lower buckling ratio (p <0.05). However, significant interactions between maturity and loading were detected for the following: 1) FN bone mineral content (BMC), NN buckling ratio (GYM BMC advantages only in M1 and M3; for BMC and buckling ratio, M1 advantages were greatest; 2) 1/3 radius BMC, width, endosteal diameter, cortical cross-sectional area, section modulus (GYM advantages primarily post-menarche); 3) UD radius BMC and axial compressive strength (GYM advantages were larger with greater maturity, greatest post-menarche).
Conclusions
Maturity-specific comparisons suggested site-specific skeletal adaptation to loading during growth, with greater advantages at the radius versus the proximal femur. At the radius, GYM advantages included greater bone width, cortical cross-sectional area and cortical thickness; in contrast, at the femoral neck, GYM bone tissue cross-sectional area and cortical thickness were greater, but bone width was narrower than in NON. Future longitudinal analyses will evaluate putative maturity-specific differences.
Keywords: DXA, bone geometry, mechanical loading, bone growth, hip structural analysis, puberty
1. Introduction
The forearm and hip are major sites of osteoporotic fracture, together contributing over one third of the U.S. total (297,000 hip; 397,000 forearm)[1]. Furthermore, the distal radius is a common fracture site throughout life, with a peak in incidence occurring around the time of peak height velocity [2,3]. Thus, improvement of proximal femur and radius skeletal strength through non-pharmacological means is a vital strategy for reduction of the population fracture burden. Optimal physical activity exposure during growth may accomplish this aim, yielding long term bone strength benefits.
Supporting this premise, our group has published prospective, longitudinal evidence that gymnastics exposure during growth is associated with elevated DXA (dual X-ray absorptiometry) areal bone mineral density (aBMD), bone mineral content (BMC) and bone projected area (Area) at the distal radius/forearm, indicating persistent skeletal strength benefits at this important site [4,5]. The extreme model of artistic gymnastics is associated with uniquely exaggerated loads at both the non-dominant radius and the lower extremity [6-8]. Thus, observational studies of the gymnastic loading model provide the opportunity to evaluate skeletal adaptation to loading via site-specific DXA indices of bone geometry, density and associated theoretical skeletal strength at both the radius and the proximal femur.
Although cross-sectional studies have reported advantages in standard DXA outcomes for gymnasts (GYM) compared to non-gymnasts (NON) [9-14], few studies have evaluated indices of bone geometry and strength. Most bone geometric studies in women exposed to gymnastics have been performed at the forearm, often reporting greater indices of total bone size and strength in GYM and ex-GYM than NON [8, 15-24]. Although numerous studies have reported greater femoral neck aBMD in GYM than NON, to our knowledge, only two studies have specifically evaluated indices of femoral neck geometry and strength [25-26]. Both studies reported greater indices of bone strength but lower sub-periosteal width in GYM than NON, using hip structural analysis (HSA) [25-26]; one other study has reported lower femoral neck projected area in GYM versus NON [27]. We are not aware of any published studies that have compared indices of bone geometry and strength at these two distinct sites, evaluating the effects of loading at both the forearm and the proximal femur, while accounting for variation in physical maturity.
This paucity of evidence limits our understanding of bone properties in GYM compared to NON, as available evidence is comprised of results from a variety of different methodologies, physical maturity groupings, and skeletal sites [8]. Therefore, to address shortcomings in current knowledge, we evaluated site-specific skeletal adaptation to loading during growth, comparing radius (RAD) and proximal femur DXA scans in young female GYM versus NON, accounting for physical maturity variation from pre-puberty through post-menarche (Tanner breast stages I through V). We evaluated standard DXA outcomes of aBMD, BMC and projected area, as well as DXA-derived indices of bone geometry and strength [28-29]. We tested three related hypotheses, based upon existing literature: 1) GYM exhibit significant advantages in indices of bone mass, geometry, areal density and strength for both the radius and the femoral neck; 2) differences are of greater magnitude at the non-dominant distal radius than at the femoral neck; 3) although GYM exhibit advantages in theoretical bone strength at both sites, GYM periosteal width is greater at the radius and narrower at the femoral narrow neck.
2. Methods
2.1 Recruitment
In accordance with the Declaration of Helsinki and with the approval of our Institutional Review Board, prior to participation, subjects provided written, informed consent or assent with parental consent, as appropriate based on subject age. Subjects were enrolled in 3 separate cohorts, with GYM recruited from local gymnastic schools and NON recruited from local private schools, investigator contacts and University newletters: Cohort 1, 1997-8 (initial NON n= 25, GYM n= 55; current analysis: NON n= 25, GYM n= 50.); Cohort 2, 2002-2003 (initial NON n= 25, GYM n= 15; current analysis: NON n= 23, GYM n= 14); Cohort 3, 2008-2009 (initial NON n=30, GYM n= 50; current analysis: NON n=29, GYM n= 32). Numbers were lower in this analysis than at initial enrollment due to failure to meet gymnastic exposure criteria or incomplete data for focal variables.
2.2 Study Design
Participants from our ongoing longitudinal study (8-26 yrs old) underwent annual DXA scans (proximal femur, forearm, total body). On a semi-annual basis, height, weight and waist circumference were measured; calendar-based physical activity questionnaires and maturity questionnaires were completed [4]. The latter yielded self-reported Tanner breast and pubic stages, date of menarche, and subsequent gynecological age (years post-menarche)[4]. This cross-sectional analysis used the most recent complete data for which subjects exhibited a gynecological age no greater than 2.5 yrs. GYM were included if they had annual mean gymnastic exposure for the year prior to the DXA scan greater than or equal to 5.0 hours per week; in cases where subjects had multiple valid years of “GYM” data, the latest year with the most consistent training levels was used (to alleviate the possible influence of de-training effects related to injuries/illness).
Due to the cross-sectional nature of this analysis, we cannot determine causal relationships between factors and skeletal development within individuals across time. In this study, we will refer to the statistical “effects” of loading, estrogen exposure and their interaction as suggested by the results of cross-sectional comparisons.
2.3 Physical Activity Quantification
We used annual mean gymnastic exposure for the year prior to the DXA session as our metric of gymnastic exposure dose (GYMHRS, h/wk), in part, because GYMHRS was the most reliably acquired data. GYMHRS was either recorded prospectively on a training calendar (early years Cohort 1) or recorded at measurement sessions to summarize the preceding 6-12 months (later years, Cohort 1; Cohorts 2 and 3). In contrast, age at training initiation and long-term training history were recalled up to 15 years post-hoc. Furthermore, within and between individuals, training intensity between training initiation and the focal DXA varied markedly (e.g. < 2 h/wk for 4 years, increasing to 12 h/wk over 3 subsequent years versus >8 h/wk within the 1st year training). Thus, GYMHRS was employed to minimize recall bias.
NON were not sedentary controls; they participated in a variety of physical activities, including <2 h/wk of gymnastics training (in a few cases). Subjects who exceeded an average of 2 hours per week of gymnastics over the prior year, but did not achieve 5.0 h/wk, were excluded from analysis. This exposure contrast has been associated with significant differences in DXA and peripheral quantitative computed tomography (pQCT) outcomes in related samples [19, 20].
2.4 Physical Maturity Evaluation
Menarche status is known to be influential in skeletal development and loading associations [4, 5, 28], but distinctions between pre-pubertal, pubertal pre-menarcheal and post-menarcheal subjects have not been evaluated in a study on gymnastic loading. Accordingly, physical maturity status was evaluated in two ways: 1) maturity level (MAT) was coded as M1 (pre-menarche, Tanner breast stage I (all Tanner pubic stage I, except n=2 Tanner pubic II)), M2 (pre-menarche, Tanner breast stage II or greater) or M3 (post-menarche); 2) more standard analyses based on menarche status (MEN) contrasted differences pre-menarche versus post-menarche. In this manner, we hoped to distinguish between estrogen exposure levels without subjecting participants to invasive testing.
2.5 Densitometry
DXA scans of the left proximal femur, non-dominant forearm and total body were performed over a period from 1998 to 2010 using a single Hologic QDR4500W DXA scanner (94%), with a few exceptions performed on a cross-calibrated Discovery A scanner (6%, all from Cohort 3)(outcomes: Table 1a.-c.). The vast majority of scans were performed by the study’s main technician (C.R., 2002-2010). All scans were reanalyzed by a single investigator (JD) using Apex software (Hologic Discovery A, software v.12.7.3, Waltham, MA, USA). Proximal femur and forearm results included standard aBMD, BMC and projected area for the femoral neck (FN; NN, narrow neck) and distal radius (RAD, 1/3 and ultradistal (UD) regions of interest). Total body DXA scans provided body composition data (percent body fat (PBF), total body non-bone lean mass (nbFFM)). As published previously, for the radius, non-standard positioning placed the edge of the analysis box distal to the distal radial articular cartilage but proximal to the carpal bones [5,28]. This key practice maximizes congruence of RAD regions of interest within and between individuals, allowing comparison of data from forearm scans exhibiting different ulnar variances [5,28].
Table 1a. Subject Characteristics: Unadjusted Means (Standard Deviations).
| Variable | Total Sample | M1 | M2 | M3 | ||||
|---|---|---|---|---|---|---|---|---|
| GYM (n=96) |
NON (n=77) |
GYM (n=26) |
NON (n=20) |
GYM (n=49) |
NON (n=31) |
GYM (n=21) |
NON (n=26) |
|
| Chronological Age (yrs) | 11.9 (2.1) | 11.7 (2.2) | 9.9 (1.4) | 9.6 (1.1) | 11.8 (1.2)* | 11.1 (1.7) | 14.6 (1.2) | 14.1 (1.0) |
| Tanner Breast Stage (I-V, % Frequencies) |
I=27%; II=37%; III=24%; IV=11%; V=1% |
I=26%; II=29%; III=22%; IV=17%; V=6% |
I=100% | I=100% | II=71%; III=26%; IV=2% |
II=71%; III=23%; IV=6% |
II=0%; III=52%; IV=43%; V=5% |
II=4%; III=35%; IV=42%; V=19% |
| Height (cm) | 145.2 (12.2) | 149.7 (13.1)* | 131.7 (9.2) | 137.0 (8.5) | 147.6 (8.3) | 147.6 (10.7) | 156.3 (7.5) | 162.0 (5.8)* |
| Weight (kg) | 39.5 (10.4) | 44.2 (11.8)* | 28.8 (5.0) | 31.8 (7.1) | 40.1 (7.2) | 45.2 (10.5)* | 51.3 (8.1) | 52.5 (7.7) |
| DXA nbFFM (kg) | 29.4 (7.5) | 30.4 (7.5) | 21.6 (3.7) | 22.6 (4.2) | 29.8 (5.0) | 29.2 (5.6) | 38.3 (5.5) | 37.7 (4.0) |
| BMI (kg/cm2) | 18.4 (2.5) | 19.4 (3.3)* | 16.6 (1.8) | 16.8 (2.7) | 18.3 (1.9) | 20.6 (3.2)* | 20.9 (2.4) | 20.0 (2.7) |
| Percent Body Fat (%) | 20.4 (4.1) | 26.7 (7.2)* | 20.2 (3.4) | 24.4 (6.0)* | 20.7 (4.6) | 31.0 (7.4)* | 19.9 (3.3) | 23.4 (5.2)* |
| Waist Circumference (cm) | 62.2 (7.0) | 66.4 (9.3)* | 55.5 (3.7) | 58.2 (5.1)* | 62.9 (5.7) | 69.9 (9.5)* | 69.1 (5.4) | 68.6 (7.9) |
| Age at Menarche (yrs) | 13.6 (1.0)* | 12.8 (0.9) | --- | --- | --- | --- | 13.6 (1.0)* | 12.8 (0.9) |
| Gynecological Age (yrs) | 1.0 (0.6) | 1.3 (0.6) | --- | --- | --- | --- | 1.0 (0.6) | 1.3 (0.6) |
| Physical Activity (h/wk) | 13.3 (4.8)* | 3.7 (2.8) | 11.7 (4.8)* | 3.1 (2.5) | 12.6 (4.3)* | 2.9 (1.8) | 17.0 (4.4)* | 5.0 (3.6) |
| Gymnastics (h/wk) | 12.8 (4.6)* | 0.0 (0.2) | 11.0 (4.6)* | 0.1 (0.2) | 12.4 (4.2)* | 0.0 (0.2) | 16.1 (4.1)* | 0.0 (0.0) |
p <0.05; bold font for the significantly larger mean, comparing gymnasts vs. non-gymnasts for the total sample and within each maturity group.
M1= pre-puberty, pre-menarche; M2= puberty, pre-menarche; M3= post-menarche; DXA nbFFM= Total Body Non-bone Lean Mass; BMI= Body Mass Index; Gynecological Age= Years post-menarche; Physical Activity= Organized non-aquatic activity (includes gymnastics).
Table 1c. Subject Characteristics: Proximal Femur Unadjusted Means (Standard Deviations).
| Variable | Total Sample | M1 | M2 | M3 | ||||
|---|---|---|---|---|---|---|---|---|
| GYM (n=96) |
NON (n=77) |
GYM (n=26) |
NON (n=20) |
GYM (n=49) |
NON (n=31) |
GYM (n=21) |
NON (n=26) |
|
| FN Area (cm2) | 3.95 (0.37) | 4.18 (0.40) | 3.68 (0.28) | 3.83 (0.31) | 3.96 (0.31) | 4.18 (0.36) | 4.26 (0.34) | 4.44 (0.27) |
| FN BMC (g) | 3.24 (0.75) | 3.25 (0.83) | 2.59 (0.41) | 2.37 (0.40) | 3.18 (0.50) | 3.17 (0.62) | 4.21 (0.58) | 4.01 (0.56) |
| FN aBMD (g/cm2) | 0.815 (0.140) | 0.768 (0.141) | 0.704 (0.087) | 0.618 (0.076) | 0.802 (0.105) | 0.753 (0.094) | 0.986 (0.098) | 0.900 (0.096) |
| NN aBMD (g/cm2) | 0.977 (0.179) | 0.912 (0.181) | 0.839 (0.107) | 0.726 (0.089) | 0.957 (0.135) | 0.891 (0.135) | 1.195 (0.133) | 1.079 (0.122) |
| NN Width (cm) | 2.46 (0.24) | 2.63 (0.24) | 2.25 (0.20) | 2.45 (0.18) | 2.49 (0.19) | 2.63 (0.25) | 2.63 (0.20) | 2.78 (0.17) |
| NN CT (cm) | 0.19 (0.04) | 0.18 (0.04) | 0.17 (0.02) | 0.14 (0.02) | 0.19 (0.03) | 0.17 (0.03) | 0.24 (0.03) | 0.21 (0.03) |
| NN ED (cm) | 2.07 (0.22) | 2.28 (0.21) | 1.92 (0.20) | 2.17 (0.18) | 2.11 (0.20) | 2.28 (0.25) | 2.16 (0.22) | 2.36 (0.16) |
| NN bCSA (cm2) | 2.30 (0.54) | 2.31 (0.60) | 1.80 (0.31) | 1.70 (0.26) | 2.26 (0.35) | 2.23 (0.45) | 3.00 (0.40) | 2.87 (0.42) |
| NN Z (cm3) | 0.89 (0.28) | 0.91 (0.32) | 0.63 (0.17) | 0.60 (0.13) | 0.88 (0.18) | 0.85 (0.25) | 1.23 (0.22) | 1.21 (0.24) |
| NN BR | 6.75 (1.37) | 8.10 (1.82) | 7.20 (1.05) | 9.49 (1.60) | 6.94 (1.51) | 8.25 (1.86) | 5.76 (0.88) | 6.86 (0.89) |
| NN CSMI (cm4) | 1.15 (0.46) | 1.28 (0.54) | 0.75 (0.25) | 0.79 (0.21) | 1.13 (0.30) | 1.19 (0.43) | 1.69 (0.42) | 1.75 (0.44) |
| NN HAL (mm) | 94.4 (9.3) | 96.2 (8.6) | 85.8 (8.9) | 89.4 (7.6) | 95.5 (6.3) | 94.9 (8.0) | 102.4 (7.3) | 102.8 (4.5) |
| NN Shaft-Neck Angle (°) | 130.1 (4.7) | 132.9 (5.0) | 129.1 (5.2) | 132.6 (6.0) | 130.1 (4.5) | 134.3 (4.3) | 131.1 (4.5) | 131.2 (4.5) |
FN= Femoral Neck; Area= uncorrected bone projected area; BMC= uncorrected bone mineral content; aBMD= uncorrected areal bone mineral density; NN= narrow neck; CT= cortical thickness; ED= endosteal diameter; bCSA= bone tissue cross-sectional area (excluding soft tissue within the periosteal compartment); Z= section modulus; BR= buckling ratio; CSMI= cross-sectional moment of inertia; HAL= hip axis length; SN Angle= shaft neck angle.
FN bone geometric and strength indices were calculated using the manufacturer’s HSA program (direct computer output), with the NN box positioned at the narrowest portion of the FN. Similar formulae were used to calculate DXA-derived indices of RAD bone geometry and strength using simplified geometric models (1/3 and UD, modified for UD RAD) [28,30]. At the proximal femur, FN and NN BMC, Area and aBMD results were corrected for fan beam magnification error [31]. Uncorrected and corrected ANCOVA results were very similar; corrected data are depicted graphically and in Table 2 (uncorrected data are presented only in Table 1c and Table 3). Redundant data are not presented (Width is derived from Area, so 1/3 Area, UD Area, FN Area are not presented; NN aBMD results are nearly identical to FN aBMD results, as the regions of interest usually overlap).
Table 2a. Gymnast Cohort Regression Results: Non-bone Lean Mass based Models for the Non-dominant Distal Radius.
| Radius Outcome |
Model Adj R2 |
Constant | Age | DXA nbFFM | PBF | MEN | GYMHRS | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| St β | PCC | St β | PCC | St β | PCC | St β | PCC | St β | PCC | |||
| 1/3 BMC | 0.84 | −0.28 | 0.27*** | 0.34 | 0.63*** | 0.68 | 0.10* | 0.24 | -- | -- | 0.12* | 0.23 |
| 1/3 aBMD | 0.74 | 0.14 | 0.41** | 0.41 | 0.42*** | 0.44 | 0.20*** | 0.36 | -- | -- | 0.11 | 0.18 |
| 1/3 width | 0.81 | 0.58 | -- | -- | 0.83*** | 0.86 | -- | -- | -- | -- | 0.14** | 0.29 |
| 1/3 ED | 0.61 | 0.43 | -- | -- | 0.75*** | 0.74 | -0.18** | -0.28 | -- | -- | 0.04 | 0.05 |
| 1/3 CT | 0.49 | 0.07 | 0.40** | 0.30 | 0.23 | 0.19 | 0.26** | 0.34 | -- | -- | 0.14 | 0.16 |
| 1/3 cCSA | 0.79 | -0.14 | -- | -- | 0.82*** | 0.85 | -- | -- | -- | -- | 0.13* | 0.25 |
| 1/3 Z | 0.78 | -0.30 | -- | -- | 0.83*** | 0.85 | -- | -- | -- | -- | 0.12* | 0.22 |
| UD BMC | 0.81 | -0.35 | -- | -- | 0.79*** | 0.86 | 0.11* | 0.24 | -- | -- | 0.21*** | 0.39 |
| UD aBMD | 0.63 | 0.10 | -- | -- | 0.63*** | 0.68 | 0.22*** | 0.33 | -- | -- | 0.28*** | 0.37 |
| UD Width | 0.83 | 0.52 | 0.24** | 0.28 | 0.78*** | 0.75 | -- | -- | -0.18** | -0.31 | 0.09 | 0.19 |
| UD IBS | 0.60 | -0.16 | -- | -- | 0.62*** | 0.67 | 0.21** | 0.32 | -- | -- | 0.26*** | 0.35 |
DXA nbFFM: Non-bone Lean Mass; PBF: % Body Fat; MEN: Pre-menarche= 0, Post-menarche=1;
GYMHRS: mean h/wk gymnastic training for the year prior to the focal DXA scan; St β: standardized beta;
PCC: partial correlation coefficient, represents square root of percent of variance explained after accounting for the effects of other variables; 1/3: 33% distal diaphysis site; UD: ultradistal (metaphysis) site; BMC: bone mineral content;
aBMD: areal bone mineral density; ED: endosteal diameter; CT: cortical thickness; cCSA: cortical tissue cross-sectional area;
Z: section modulus; IBS: index of structural strength in axial compression.
Bold type indicates that nbFFM or GYMHRS was not a significant independent predictor within the model.
Models were built as follows: age, nbFFM, PBF, MEN, GYMHRS, Interaction (GYMHRS × MEN), with forced entry for nbFFM and GYMHRS. No significant interactions were detected.
< 0.05
≤ 0.01
≤ 0.001 for t significance of β.
Table 3. Gymnast Percent Advantages Relative to Non-gymnast Means (Current study: 2 factor ANCOVA, menarche x gymnast status; all studies adjusted for height and weight).
| DXA Hip Structural Analysis Variable |
Current Study (pre and post- menarche) n= 96 GYM; 77 NON |
Faulkner et al. (pre-menarche) n= 30 GYM; 30 NON |
Maimoun et al. (pre and post-menarche) n= 23 GYM; 23 NON |
|---|---|---|---|
| FN Area (cm2) | −2.6%* | -- | -- |
| FN BMC (g) | +9.6%*** | 13.3%*** | -- |
| FN aBMD (g/cm2) | +12.9%*** | -- | +18.0%*** |
| HAL (mm) | +1.7% (0.05) | -- | +2.2% NS |
|
Shaft Neck Angle (°) |
−1.3%*; Interaction* | +1.4% NS | |
| NN aBMD (g/cm2) | +14.5%*** | +18.9%*** | -- |
| NN Width (cm) | −4.1%*** | −4.7%* | −2.3% NS |
| NN CT (cm) | +16.4%*** | -- | +26.6%*** |
| NN ED (cm) | −7.2%*** | −7.9%* | −6.2% NS |
| NN cCSA (cm2) | +9.4%*** | +12.6%** | +19.8%*** |
| NN Z (cm3) | +9.9%*** | +9.3%** | +19.2%*** |
| NN BR | −19.0%*** | -- | −21.3%** |
| NN CSMI (cm4) | +3.4% NS | +4.1% NS | +12.9% NS |
= p<0.05
= p≤0.01 (Faulkner, p<0.02)
= p≤0.001.
Results are presented for gymnasts relative to non-gymnasts as the zero reference.
Main effect for gymnast vs. non-gymnast difference is presented, as no significant interactions were detected between gymnast status and menarche status for any variable except shaft neck angle (current study, interaction p=0.02, preNON > preGYM; preNON >postNON; preGYM ≈ postGYM ≈ postNON).
Data are not corrected for fan beam magnification error.
Coefficients of variation (CVs) for RAD and FN variables were calculated using duplicate scans of 23 middle-aged females. At both 1/3 and UD RAD, CVs were <1% for projected Area (and width), BMC and aBMD, as was the CV for 1/3 cCSA. For all other variables, CVs were <3%, with 3 exceptions: NN buckling ratio (BR, 3.8%), NN section modulus (Z, 7.0%) and NN cross-sectional moment of inertia (CSMI, 8.7%). Aside from NNZ and NNCSMI, our CVs were lower than or similar to those reported in other studies using FN and HSA data [32,33].
2.6 Statistical Analysis
2.6.1 Determination of Sample Size
Based upon Tanner stage I/II comparisons [34], we projected that a minimum sample size of 17 subjects per cell would be necessary to detect significant FN differences in GYM versus NON (power ≥ 0.80). Based upon comparisons of standard and DXA-derived RAD outcomes at various maturity stages [4,18], projected minimum sample size ranged from 5 to 20. Accordingly, for all sites, we determined that 6 activity/maturity cells of at least 20 subjects should be adequate to evaluate the statistical effects of gymnastic loading exposure, maturity level (MAT) and interactions between them (latter of unknown effect size).
2.6.2 ANOVA
Analysis of variance (ANOVA) and chi square were used to compare descriptive data. As all variables were normally distributed, Pearson correlations were used to evaluate associations between potentially influential variables and bone outcomes. Two factor analysis of covariance (ANCOVA) adjusted for age, height, tbFFM and PBF. In this manner, we tested the significance of main effects for GYM status and MAT (M1, M2, M3) and evaluated the interaction between them (GYM status × MAT). Factors and covariates were kept in the model regardless of significance, to account for their potential influence upon the GYM status main effect. Additional analyses were performed for direct comparison of our FN results to the literature, adjusting for height and weight, and evaluating menarche status (MEN, pre-vs. post-menarche), GYM status and the interaction between them (GYM status × MEN) [25,26].
2.6.3 Regression
Within the GYM subgroup, we used linear regression models to evaluate the effects of gymnastics exposure dose over the year prior to the DXA scans (GYMHRS, h/wk) as a continuous variable, entering the following, in order: Model 1-age, nbFFM, PBF, MEN, GYMHRS and GYMHRS × MEN; Model 2- age, nbFFM, PBF, MAT (M1, M2, M3), GYMHRS and GYMHRS × MAT. Both nbFFM and GYMHRS were forced into the model, regardless of significance. In the event that GYMHRS was not a significant predictor using the nbFFM models, an analogous model was evaluated, substituting height for nbFFM. Aside from GYMHRS and nbFFM, variables were removed if p> 0.05 or their inclusion generated variance inflation factors (VIF) greater than 5.0. For all analyses, alpha = 0.05.
3. Results and Discussion
3.1 Subject characteristics
Data for 173 subjects were analyzed. No differences were detected for GYM versus NON in chronological age, nbFFM, arm length (not shown), Tanner breast stage, MAT or MEN (p>0.05, Table 1a). For the total sample, NON means were greater for height, weight, body mass index (BMI), PBF and waist circumference, whereas non-aquatic physical activity levels were greater in GYM (p<0.05). Within maturity levels, GYM exhibited consistently lower PBF, with higher physical activity levels. However, not all differences were consistent across maturity levels: only M3 GYM were significantly shorter; waist circumferences were smaller only in M1 and M2 GYM; and weight and BMI were significantly higher only in M2 NON. Among post-menarcheal subjects, GYM exhibited significantly higher age at menarche relative to NON, but gynecological age (years since menarche) was well-matched (Table 1a). Unadjusted values for bone outcomes are presented in Tables 1b and 1c.
Table 1b. Subject Characteristics: Distal Radius Unadjusted Means (Standard Deviations).
| Variable | Total Sample | M1 | M2 | M3 | ||||
|---|---|---|---|---|---|---|---|---|
| GYM (n=96) |
NON (n=77) |
GYM (n=26) |
NON (n=20) |
GYM (n=49) |
NON (n=31) |
GYM (n=21) |
NON (n=26) |
|
| 1/3 Area (cm2) | 2.39 (0.29) | 2.23 (0.24) | 2.11 (0.18) | 2.02 (0.19) | 2.41 (0.23) | 2.26 (0.19) | 2.71 (0.21) | 2.36 (0.23) |
| 1/3 BMC (g) | 1.41 (0.35) | 1.25 (0.27) | 1.08 (0.18) | 0.99 (0.19) | 1.41 (0.28) | 1.22 (0.19) | 1.83 (0.20) | 1.48 (0.19) |
| 1/3 aBMD (g/cm2) | 0.583 (0.084) | 0.556 (0.075) | 0.510 (0.053) | 0.489 (0.053) | 0.582 (0.070) | 0.540 (0.056) | 0.675 (0.048) | 0.627 (0.041) |
| 1/3 Width (cm) | 1.18 (0.16) | 1.10 (0.13) | 1.01 (0.10) | 0.98 (0.10) | 1.19 (0.13) | 1.11 (0.10) | 1.35 (0.11) | 1.18 (0.12) |
| 1/3 CT (cm) | 0.23 (0.03) | 0.22 (0.03) | 0.21 (0.03) | 0.20 (0.02) | 0.23 (0.03) | 0.21 (0.02) | 0.25 (0.03) | 0.24 (0.02) |
| 1/3 ED (cm) | 0.72 (0.14) | 0.67 (0.12) | 0.59 (0.09) | 0.58 (0.09) | 0.74 (0.11) | 0.70 (0.10) | 0.85 (0.12) | 0.70 (0.13) |
| 1/3 cCSA (cm2) | 1.06 (0.33) | 0.91 (0.25) | 0.74 (0.17) | 0.69 (0.18) | 1.09 (0.26) | 0.93 (0.21) | 1.42 (0.24) | 1.05 (0.25) |
| 1/3 Z (cm3) | 0.36 (0.19) | 0.27 (0.12) | 0.20 (0.08) | 0.17 (0.08) | 0.36 (0.15) | 0.27 (0.10) | 0.56 (0.15) | 0.34 (0.12) |
| UD Area (cm2) | 2.84 (0.45) | 2.70 (0.40) | 2.36 (0.28) | 2.31 (0.26) | 2.91 (0.35) | 2.66 (0.31) | 3.26 (0.29) | 3.06 (0.22) |
| UD BMC (g) | 1.18 (0.36) | 0.90 (0.23) | 0.86 (0.23) | 0.67 (0.12) | 1.17 (0.28) | 0.87 (0.17) | 1.58 (0.27) | 1.11 (0.18) |
| UD aBMD (g/cm2) | 0.406 (0.070) | 0.329 (0.047) | 0.359 (0.060) | 0.291 (0.029) | 0.399 (0.056) | 0.325 (0.033) | 0.481 (0.052) | 0.362 (0.048) |
| UD width (cm) | 1.88 (0.30) | 1.79 (0.26) | 1.56 (0.18) | 1.53 (0.17) | 1.93 (0.23) | 1.76 (0.21) | 2.16 (0.19) | 2.03 (0.15) |
| UD IBS (g2/cm4) | 0.340 (0.120) | 0.221 (0.063) | 0.265 (0.099) | 0.172 (0.035) | 0.325 (0.092) | 0.214 (0.043) | 0.468 (0.102) | 0.267 (0.068) |
Area= bone projected area; BMC= bone mineral content; aBMD= areal bone mineral density; CT= cortical thickness; ED= endosteal diameter; cCSA= cortical cross-sectional area; Z= section modulus; IBS= index of structural strength in axial compression.
GYM physical activity levels were 3-4 times higher than NON across maturity levels; for both GYM and NON, activity levels were higher in post-menarcheal than pre-menarcheal subjects. GYM had been participating in gymnastics for approximately 2 to 15 years (mean 6.5 yrs, sd 2.8), with starting ages ranging from 2 to 11 years (all premenarcheal: mean 5.5 yrs, sd 2.2). Although starting age did not differ significantly between maturity groups, it showed an increasing trend by MAT (M1= 4.8 yrs, sd 0.42; M2= 5.7 yrs, sd 0.31; M3= 6.1 yrs, sd 0.47; p< 0.08). GYMHRS was strongly positively correlated with years since training initiation (r= 0.62, p < 0.001), whereas there was no correlation between GYMHRS and age at training initiation (r= −0.08, p> 0.43).
3.2 Gymnastic Exposure Associations
3.2.1 Radius ANCOVA
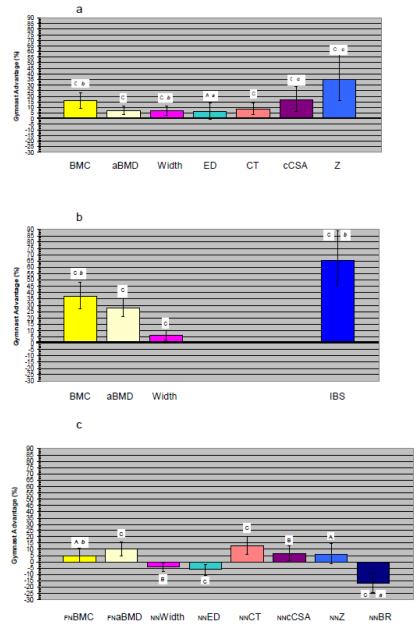
GYM adjusted means were greater than NON for all outcomes at both 1/3 and UD RAD (GYM status main effect, p<0.05; Figures 1a,1b), indicating substantial osteogenic effects from gymnastic loading exposure during growth.
Figure 1.
ANCOVA Main Effect Results for Gymnastic Exposure, displayed as Gymnast Percent Advantages. For each figure, columns represent the mean percent advantage of Gymnasts relative to Non-gymnasts as the zero reference line. Error bars represent 95% confidence intervals around the mean Gymnast percent advantage. All means are adjusted for age, height, total body non-bone lean mass and percent body fat. Figure 1a presents 1/3 Radius results; Figure 1b presents Ultradistal Radius results; Figure 1c presents Femoral Neck and Narrow Neck results. Capital letters denote significance of main effect of gymnastic exposure on bone outcome; small italic letters denote significance of maturity × activity interaction. A/a= p< 0.05; B/b= p≤ 0.01; C/c= p ≤ 0.001. Columns are aligned vertically in order to improve ease of comparison of analogous/similar results across the three skeletal sites. However, it should be noted that CSAs differ slightly (1/3 cCSA is cortical cross-sectional area only, with no trabecular tissue represented at this site; NNbCSA is total bone tissue cross-sectional area, including both cortical and trabecular tissue, but excluding non-bone tissue). Similarly, indices of bone strength (at far right) vary across sites [Z (section modulus, 1/3 and NN), IBS (axial compressive strength, UD only), BR (buckling ratio, NN only, lower value indicates lower fracture risk)]. FN BMC and FN aBMD results have been corrected for fan beam magnification error.
3.2.2 Proximal Femur ANCOVA
GYM adjusted means were greater than NON for FN BMC and FN aBMD (p<0.05) (Figure 1c). Similarly, significant differences were detected between GYM and NON for all NN HSA variables (Figure 1c), except NN CSMI and hip axis length (not depicted, raw data Table 1c). Differences included greater GYM NN cortical thickness (CT), NN bone tissue cross-sectional area (bCSA) and NN Z, accompanied by lower buckling ratio (NN BR, lower buckling ratio = greater cortical stability)(p<0.05). In contrast, GYM exhibited significantly lower NN width, endosteal diameter (ED) and shaft neck angle (last not depicted, raw data Table 1c). These findings suggest that gymnastic exposure during growth promotes FN strength via thickened cortices with narrower overall bone diameter, similar to jumping adaptations reported by Petit et al. [35].
3.3 Gymnastic Dose-GYM Regression results
3.3.1 Radius
Within GYM, GYMHRS exhibited positive, independent explanatory value for most RAD outcomes (5% to 15% of variance, p<0.05); exceptions included 1/3 aBMD, 1/3 ED, 1/3 CT and UD width (3% to 4% of variance, p≥0.09)(Table 2a). In these and all cases except CT, nbFFM was a potent, positive predictor of all RAD outcomes (19% to 74% of variance, p ≤0.001, Table 2a). When GYMHRS did not provide significant predictive value, height was substituted for nbFFM; this resulted in significant associations between GYMHRS and all RAD outcomes except ED, suggesting that a portion of the GYMHRS dose “effect” is via lean mass adaptation (Table 2b). In general, strong positive correlations with GYMHRS support the premise that gymnastic loading is the main stimulus driving GYM RAD advantages.
Table 2b. Gymnast Cohort Regression Results: Height-based Models for the Non-dominant Distal Radius.
| Radius Outcome |
Model Adj R2 |
Constant | Age | Height | PBF | MEN | GYMHRS | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| St β | PCC | St β | PCC | St β | PCC | St β | PCC | St β | PCC | |||
| 1/3 aBMD | 0.73 | -0.13 | 0.40*** | 0.36 | 0.40*** | 0.40 | 0.19*** | 0.34 | -- | -- | 0.16* | 0.24 |
| 1/3 ED | 0.54 | -0.28 | -- | -- | 0.66*** | 0.68 | -0.19** | -0.27 | -- | -- | 0.13 | 0.17 |
| 1/3 CT | 0.48 | -0.03 | -- | -- | 0.52*** | 0.56 | 0.24** | 0.31 | -- | -- | 0.29** | 0.34 |
| UD Width | 0.80 | -1.13 | -- | -- | 0.79*** | 0.86 | -- | -- | -- | -- | 0.23*** | 0.44 |
PBF: % Body Fat; MEN: Pre-menarche= 0, Post-menarche=1;
GYMHRS: mean h/wk gymnastic training for the year prior to the focal DXA scan; St β: standardized beta;
PCC: partial correlation coefficient, represents square root of percent of variance explained after accounting for the effects of other variables; 1/3: 33% distal diaphysis site; UD: ultradistal (metaphysis) site; aBMD: areal bone mineral density; ED: endosteal diameter;
CT: cortical thickness.
Bold type indicates that GYMHRS was not a significant independent predictor within the model.
Models were built as follows: age, height, PBF, MEN, GYMHRS, Interaction (GYMHRS × MEN), with forced entry for GYMHRS. No significant interactions were detected.
Note: For 1/3 aBMD and CT, in the height-based models, MEN and the interaction term were also influential, but with all variables in the model, VIFs were elevated for both MEN and interaction, also interaction p<0.06.
When the interaction term was removed, MEN p<0.06. Accordingly, MEN and the interaction term were removed.
< 0.05
≤ 0.01
≤ 0.001 for t significance of β.
3.3.2 Proximal Femur
Within GYM, GYMHRS was negatively correlated with FN width, NN CSMI, NN ED, NN BR and shaft-neck angle (p<0.05), demonstrating a strong inverse trend with NN Z (p<0.06) (Table 2c). GYM HRS was positively correlated with FN and NNaBMD, as well as NN CT. With nbFFM in the model, GYMHRS did not exhibit significant explanatory value for FNBMC, NNbCSA, NNZ or hip axis length. When height was substituted for nbFFM, the predictive value of GYMHRS improved for FNBMC (+, p<0.05) and NNbCSA (+, p<0.09), but not for NN Z or hip axis length (Table 2d). These associations support the assertion that gymnastic loading is the main stimulus driving GYM FN advantages in CT, aBMD and BR, via restriction of FN periosteal and endosteal width.
Table 2c. Gymnast Cohort Regression Results: Non-bone Lean Mass based Models for the Proximal Femur.
| Proximal Femur Outcome |
Model Adj R2 |
Constant | Age | DXA nbFFM | PBF | MEN | GYMHRS | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| St β | PCC | St β | PCC | St β | PCC | St β | PCC | St β | PCC | |||
| FNBMC | 0.85 | −0.31 | 0.19* | 0.24 | 0.68*** | 0.72 | 0.11* | 0.27 | 0.13* | 0.24 | −0.01 | −0.01 |
| FNaBMD | 0.63 | 0.43 | -- | -- | 0.53*** | 0.56 | -- | -- | 0.23** | 0.28 | 0.20** | 0.29 |
| NNbCSA | 0.79 | 0.37 | 0.19* | 0.21 | 0.64*** | 0.64 | -- | -- | 0.15* | 0.24 | −0.03 | −0.05 |
| NNCSMI | 0.75 | −0.61 | 0.29** | 0.30 | 0.69*** | 0.64 | -- | -- | -- | -- | −0.17** | −0.27 |
| NN width | 0.49 | 1.90 | -- | -- | 0.78*** | 0.70 | -- | -- | -- | -- | −0.25*** | −0.30 |
| NN ED | 0.28 | 1.75 | -- | -- | 0.60*** | 0.54 | -- | -- | -- | -- | -0.33*** | -0.33 |
| NN CT | 0.63 | 0.09 | -- | -- | 0.53*** | 0.55 | -- | -- | 0.24** | 0.29 | 0.18* | 0.26 |
| NN Z | 0.79 | -0.24 | 0.29** | 0.33 | 0.69*** | 0.67 | -- | -- | -- | -- | −0.11 | −0.20 |
| NN BR | 0.25 | 9.50 | -- | -- | −0.27** | −0.28 | -- | -- | -- | -- | −0.33*** | −0.33 |
| SN Angle | 0.10 | 126.6 | -- | -- | 0.37*** | 0.34 | -- | -- | -- | -- | −0.26* | −0.24 |
| HAL | 0.69 | 64.28 | -- | -- | 0.85*** | 0.81 | -- | -- | -- | -- | −0.04 | −0.06 |
DXA nbFFM: Non-bone Lean Mass; PBF: % Body Fat; MEN: Pre-menarche= 0, Post-menarche=1;
GYMHRS: mean h/wk gymnastic training for the year prior to the focal DXA scan; St β: standardized beta; PCC: partial correlation coefficient, represents square root of percent of variance explained after accounting for the effects of other variables; FN: femoral neck; NN: narrow neck;
BMC: bone mineral content; aBMD: areal bone mineral density; bCSA: bone tissue cross-sectional area; CSMI: cross-sectional moment of inertia; ED: endosteal diameter; CT: cortical thickness; Z: section modulus; BR: buckling ratio; SN Angle: shaft neck angle;
HAL: hip axis length.
Presented FNBMC and FNBMD results use data that are corrected for fan beam magnification error.
Bold type indicates that GYMHRS was not a significant independent predictor within the model.
Models were built as follows: age, nbFFM, PBF, MEN, GYMHRS, Interaction (GYMHRS x MEN),
< 0.05
≤ 0.01
≤ 0.001 for t significance of β.
Table 2d. Gymnast Cohort Regression Results: Height based Models.
| Proximal Femur Outcome |
Model Adj R2 |
Constant | Age | Height | PBF | MEN | GYMHRS | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| St β | PCC | St β | PCC | St β | PCC | St β | PCC | St β | PCC | |||
| FNBMC | 0.80 | −4.29 | -- | -- | 0.65*** | 0.78 | 0.10* | 0.22* | 0.32*** | 0.54 | 0.10 | 0.20 |
| NNbCSA | 0.78 | −1.99 | -- | -- | 0.62*** | 0.76 | -- | -- | 0.34*** | 0.54 | 0.09 | 0.17 |
| NN Z | 0.76 | −1.41 | -- | -- | 0.67*** | 0.77 | -- | -- | 0.32*** | 0.49 | 0.03 | 0.06 |
| HAL | 0.75 | −1.82 | -- | -- | 0.86*** | 0.85 | -- | -- | -- | -- | 0.04 | 0.07 |
PBF: % Body Fat; MEN: Pre-menarche= 0, Post-menarche=1;
GYMHRS: mean h/wk gymnastic training for the year prior to the focal DXA scan; St β: standardized beta;
PCC: partial correlation coefficient, represents square root of percent of variance explained after accounting for the effects of other variables; FN: femoral neck; NN: narrow neck; BMC: bone mineral content; bCSA: bone tissue cross-sectional area;
Z: section modulus; HAL: hip axis length.
Presented FNBMC and FNBMD results use data that are corrected for fan beam magnification error.
Bold type indicates that GYMHRS was not a significant independent predictor within the model.
Models were built as follows: age, height, PBF, MEN, GYMHRS, Interaction (GYMHRS x MEN), with forced entry for GYMHRS. No significant interactions were detected.
< 0.05
≤ 0.01
≤ 0.001 for t significance of β.
3.4 Maturity-specific Differences
3.4.1 Radius ANCOVA
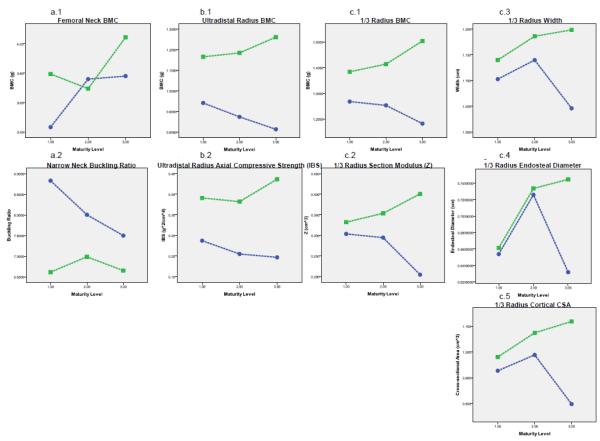
At 1/3 RAD, significant GYM status × MAT interactions were detected for BMC (GYM advantages-M2: 13%, M3: 21%), Z (GYM advantages-M2: 20%, M3: 96%), width (M3 GYM advantage: 15%), ED (M3 GYM advantage: 18%) and cortical compartment cross-sectional area (cCSA, M3 GYM advantage: 40%), with greatest overlap for GYM advantages in M1 subjects and the clearest, highest magnitude GYM advantages in M3 (Figure 2c). Specifically, no advantages were detected for 1/3 ED in M1 or M2 comparisons, but M3 GYM exhibited nearly 20% larger ED than NON. For 1/3 width and cCSA, M1 GYM and NON did not differ; strong trends were exhibited for M2 GYM advantages (4% and 9%, respectively), and M3 GYM advantages were large and significant (15% and 40%, respectively). No GYM status × MAT interactions were detected for 1/3 aBMD or CT, indicating consistent GYM advantages across maturity levels.
Figure 2.
ANCOVA Results for significant Gymnastic Exposure X Physical Maturity Interaction Terms, displayed as maturity-specific adjusted activity group means. For each figure, Gymnasts are represented as the squares and Non-gymnasts are represented as the circles. Physical maturity group increases from left to right: M1= Tanner breast stage I; M2= Pubertal/pre-menarche (Tanner breast stage II+); M3= Post-menarche. Dashed lines indicate that these graphs do not reflect repeated longitudinal measurements of growth within individuals, instead they reflect cross-sectional comparisons of maturity and activity specific group means. As the means are adjusted for age, height, non-bone lean mass and percent body fat, they are relative, not absolute (lower points in older individuals do not reflect lower raw values, but lower values after accounting for age and anthropometric characteristics). For unadjusted group means, see subject characteristics (Tables 1b and 1c). Figure 3a. presents Femoral Neck and Narrow Neck results (FN BMC data have been corrected for fan beam magnification error); Figure 3b presents Ultradistal (UD) Radius results; Figures 3.c.1 and 3.c.2 present 1/3 Radius results. Columns are aligned to improve ease of comparison of analogous/similar results across the three skeletal sites. Again, note that indices of bone strength vary across sites [Z (section modulus), IBS (axial compressive strength), BR (buckling ratio, lower value indicates lower fracture risk)].
At UD RAD, only BMC and index of structural strength in axial compression (IBS) demonstrated significant GYM status × MAT interactions (Figure 2b); GYM advantages were clear in all groups, increasing with maturity level (BMC: M1 24%, M2 35%, M3 55%; IBS: M1 44%, M2 62%, M3 96%; Figure 2b). In contrast, non-significant interactions indicated that GYM advantages in UD aBMD and width were consistent across maturity levels.
Although conclusions are limited by the cross-sectional nature of the data, ANCOVA results suggest that greater M3 GYM advantages are a function of lower RAD parameters for age and body size in M3 NON than all other groups. This pattern suggests that NON RAD bone accrual and expansion keep pace with total body growth until menarche, but are limited post-menarche. Thus, it appears that exaggerated M3 GYM RAD advantages are not due to an exaggerated post-menarcheal loading response (Figure 2b, 2c).
3.4.2 Radius Regressions
Within the GYM subset, only UD width demonstrated a significant menarche “effect” (Table 2a, 2b). Furthermore, no significant GYMHRS × MEN interactions were detected, except for 1/3 BMC and 1/3 aBMD; in both models, MEN and GYMHRS × MEN were excluded due to variance inflation > 17.0, as GYMHRS dominated. Substitution of MAT (M1-3) for MEN universally diminished explanatory value (results not presented).
All of the above suggest that physical maturation does not modulate loading “effects” or affect distal radius properties independent from age, nbFFM or loading within the GYM subset. However, as GYMHRS tended to be higher in post-menarcheal than pre-menarcheal GYM, intercorrelation of these variables may mask an underlying physical maturity effect. In fact, for many dependent variables (1/3 aBMD, 1/3 ED, 1/3 CT, UD width), PBF, age and/or MEN were significant RAD predictors, reducing the explanatory value of both nbFFM and GYMHRS. These relationships suggest that energy balance (1/3) and/or estrogen (UD) “effects” may mask dose-related loading “effects” for GYM bone geometric measures. Longitudinal analyses are necessary to evaluate within-subject change in bone parameters relative to body composition and physical maturity.
3.4.3 Proximal Femur ANCOVA
Significant GYM status × MAT interactions were detected only for FN BMC and NN BR (Figures 1c, 2a). For FN BMC, GYM advantages were greatest in M1 (11%), with overlapping confidence intervals for M3 differences (7%) and no M2 difference detected. For BR, M1 GYM had the clearest advantage, with 25% lower BR than comparable NON, whereas M2 GYM advantages were lower (-13%), and M3 confidence intervals overlapped (-11%). Findings for FN Area, BMC and aBMD were similar regardless of correction for fan beam magnification error; corrected results are presented.
In contrast to RAD results, FN results suggest that both GYM and NON experience peri-menarcheal gains in FN BMC that are exaggerated for age and body size. This potential estrogen “effect” appears to be more extreme in peri-menarcheal NON than GYM, resulting in lower GYM BR advantages for age and body size in M3 than M2 or M1 (Figure 2a). In all cases, cross-sectional analysis limits conclusions regarding maturity-specific differences; results must be confirmed via longitudinal evaluation of growth within-subjects.
3.4.4 Proximal Femur Regressions
For FN variables, no significant GYMHRS × MEN interactions were detected. When MAT was substituted for MEN, MAT exhibited inferior explanatory value (results not presented). In contrast to RAD, FN variables demonstrated a strong positive association with MEN in many nbFFM models, explaining 6% to 8% of variance for FNBMC, FNaBMD, NNbCSA and NNCT (p<0.05)(Table 2c). MEN explanatory value was even greater in height-based models of FNBMC, NNbCSA and NNZ (24-66%, p< 0.001), suggesting that strong intercorrelation between physical maturity and nbFFM may mask maturity effects. Interestingly, PBF was less influential as a predictor of FN than RAD outcomes, suggesting that physical maturity may be more influential than energy balance at this site. Again, longitudinal analyses are necessary for more conclusive interpretation of factors in intra-individual change through time.
3.5 Site-specific comparisons
As hypothesized, for all RAD outcomes, adjusted indices of bone geometry, areal density and strength were significantly greater in GYM than NON (Figure 1a, b). Also as hypothesized, for FN, GYM demonstrated significant advantages in adjusted indices of bone mass, areal density and strength compared to NON, but adjusted indices of FN periosteal and endosteal dimensions (NN width, NN ED) were significantly lower in GYM than NON. Furthermore, for comparable outcomes, effect sizes were larger for RAD than FN differences (% difference: RAD 6% to 66%, FN 4% to 17%).
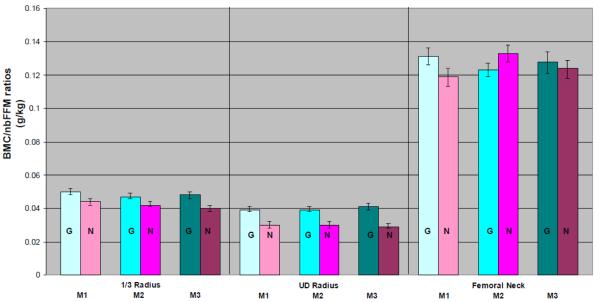
As further corroboration of site-specific differences, for ratios of BMC/nbFFM, RAD advantages for GYM versus NON were clear and consistent across maturity groups (ANOVA p< 0.001, Figure 3, panels 1 & 2). In contrast, patterns for FN BMC/nbFFM ratios were inconsistent, such that mean ratios were greater in M1 GYM than NON (p = 0.002) but greater in M2 NON than GYM (p= 0.001), with no M3 difference detected (p= 0.36) (Figure 3, 3rd panel). Across maturity levels and activity groups, ratios were much higher for FN than RAD, reflecting the role of the femur as a habitual total body mass-bearing bone during most organized and daily life activities (Figure 3, panels 1-3).
Figure 3.
Depiction of Site and Maturity-specific Ratios of Regional Bone Mineral Content (BMC, g) vs. Total Body Non-bone Lean Mass (nbFFM, kg), calculated as BMC/nbFFM (g/kg). Gymnast means (G, turquoise/dots) are presented adjacent and to the left of Non-gymnast means (N, pink/stripes). Maturity groups are presented in order of increasing maturity from left to right (darker colors for more mature individuals). Error bars indicate 95% confidence intervals around the mean. For all radius comparisons, ANOVA p<0.001. For femoral neck differences, M1 p= 0.002, M2 p=0.001 and M3 p=0.34. FN BMC has been corrected for fan beam magnification error.
Our results suggest a fundamental difference in geometric response to loading at FN versus RAD. To interpret this geometric distinction, one must consider the mathematics of the HSA formulae used to derive CSMI, Z and BR. CSMI is a function of bCSA and the sum of squared distances of each pixel of bone mass from the centroid (directly related to bone width and ED). Maximum bending strength, Z, equals CSMI/dmax(dmax= maximum distance from bending plane centroid). BR equals CT/dmax [33]. For 1/3 RAD, bCSA and cCSA are considered interchangeable.
Assuming uniform bone tissue apparent densities, CSMI may be increased in 2 ways: 1) by increasing bCSA; 2) by distributing BMC more peripherally (via increasing width and/or ED). Because Z equals CSMI/dmax, Z may increase in 2 main ways: 1) by increasing CSMI (via increasing bCSA and/or distributing BMC more peripherally); or 2) by decreasing dmax (via decreasing bone width). At 1/3 RAD, it appears that M2 and M3 GYM Z are greater due to greater CT and cCSA, coupled with more peripheral BMC distribution post-menarche (M3 GYM width and ED advantages), thereby increasing CSMI (not calculated). In contrast, at FN, GYM do not exhibit a CSMI advantage. GYM NN Z is greater than NON due only to lower dmax (smaller bone width). Thus, as observed, GYM NN Z is greater than NON, despite no GYM NN CSMI advantage and smaller NN width. Finally, because BR equals dmax/CT, GYM buckling risk is lower than NON due to smaller NN width and greater NN CT.
All bone compartment results must be confirmed using 3D imaging techniques, as HSA and RAD DXA-derivations rely on assumptions of questionable validity (see limitations). Nonetheless, DXA plane FN Area and NN width both indicate more compact FN periosteal dimensions in GYM than NON, supporting the premise that patterns of bone geometric adaptation are site-specific.
Alternatively or in addition, the lower magnitude of GYM advantages at FN than RAD may indicate diminished GYM versus NON contrast for FN. This is a reasonable hypothesis, as many of our NON were likely to exhibit FN advantages associated with non-gymnastic loading modalities [32]. Nikander and colleagues reported greater FN aBMD, NN CSA and NN Z in subjects who participated in “high and odd impact” activities compared to inactive controls, but no NN width differences were detected [32]. Our NON were not inactive controls during the study period, and many were likely to exhibit benefits associated with participation in high impact (basketball, volleyball, track and field events, etc.) and odd impact (soccer, lacrosse, field hockey, dance/aerobics, etc.) activities during growth; thus larger GYM FN advantages would be expected in comparisons against sedentary controls.
The greater magnitude of observed GYM advantages at RAD than FN supports the preferential use of a distal radius model to highlight skeletal associations with gymnastic loading. Although the proximal femur is loaded by extremely high impact forces during gymnastics, as noted above, it is also loaded by other exercise, free play and daily life. Accordingly, the proximal femur loading pattern may not clearly distinguish gymnastics-specific adaptations from adaptation to background loading. Conversely, the non-dominant radius does not bear the total body mass during most activities, but as the primary load-bearing bone in the distal forearm during many gymnastic maneuvers, it sustains repeated total body mass impacts. Furthermore, impact dampening between the foot and the hip reduces force transmission to the proximal femur to a greater extent than forces are dampened at the distal radius; this concept is supported by particularly high magnitude UD GYM advantages compared to more proximal sites (UD BMC 37% versus 1/3 BMC 17% and NNBMC 5%; 66% UD IBS versus 1/3 Z 35%, NN Z 6% and NN BR 17%).
4. Comparisons to Other Studies
We compared our FN results directly to two studies that employed ANCOVA to adjust for height and weight, accounting for menarche status [25,26] (Table 3). No significant GYM status × MEN interactions were detected except for shaft-neck angle (current study). On the whole, all three studies suggested similar qualitative benefits attributable to gymnastic training. However, our study detected significant differences where the other studies did not, possibly due to greater sample size and statistical power (Table 3). For many variables, the magnitude of GYM advantages was greatest in the cohort examined by Maimoun and colleagues, as compared to advantages in the cohorts evaluated by the current study and Faulkner and colleagues. GYM in the study by Maimoun et al. trained at a higher intensity (19.9 h/wk) than our GYM (12.8 h/wk), supporting the premise that benefit magnitude may be a function of gymnastic exposure dose. In addition, a larger percentage of GYM in the Maimoun cohort were post-menarche (48%, versus 21% (current study) and 0% (Faulkner et al.)), likely indicating both greater accumulation of estrogen exposure and training duration in the Maimoun cohort.
Petit and colleagues published the results of a randomized controlled jumping intervention in pre-pubertal and early pubertal girls [35]. Although no benefits were detected for pre-pubertal jumpers, pre-pubertal jumping exposure was associated with greater 7 month gains in NNaBMD, NNbCSA, NNZ and NNCT, with strong trends toward lower periosteal and endosteal expansion in jumpers than controls [35]. In general, our FN results corroborate these findings. However, we did not detect significant loading × maturity interactions for most FN variables. In fact, our results suggest a contradictory (or opposite) loading × maturity interaction, as FNBMC and buckling ratio benefits were greater for age and body size in pre-pubertal (M1) than early pubertal girls (M2).
Unfortunately, inter-study comparisons of radius results are not straightforward, as studies vary in sample composition, methodologies and outcomes. Accordingly, there is no consensus on adaptation in periosteal and endosteal dimensions, which may vary by site, physical maturity and/or estrogen exposure [8, 20, 36-38]. Nonetheless, our results, and those of other studies, associate gymnastic exposure during growth with RAD advantages in theoretical strength and cortical dimensions in immature and mature females [8, 20, 36-38].
Two recent studies provide specific evidence to support the premise that greater RAD loading advantages after menarche are due to continued periosteal and endosteal expansion despite estrogen exposure, whereas conditions of lower relative loading allow estrogen exposure to limit periosteal and endosteal expansion for age and body size. Ducher et al. reported that female EX-GYM exhibited greater RAD periosteal and endosteal dimensions than NON, with greater advantages in amenorrheic than eumenorrheic EX-GYM (periosteal CSA: metaphysis 34% vs. 21%, diaphysis 38% vs. 32%; endosteal CSA: diaphysis 79% vs. 51%)[38]. These results support the premise that loading promotes expansion and estrogen opposes expansion.
Using a rat model, Leppanen and colleagues compared growth of the femoral diaphysis under conditions of: 1) control (+Loading (+L), +Estrogen (+E)); 2) ovariectomy (+L/-E); 3) hindlimb neurectomy (-L/+E); or 4) both interventions (-L/-E) [39]. Compared to -L/-E, loading increased bending strength (asymmetrical periosteal and cortical expansion); addition of estrogen to loading subtly increased bending strength advantages by thickening cortices via lower endosteal expansion [39]. In contrast, -L/+E yielded inconsistent bone strength advantages via greater cortical vBMD and endosteal contraction, without periosteal expansion. Thus, estrogen exposure may increase bone strength advantages attributed to loading via greater cortical thickening with slight limitation of periosteal and endosteal expansion. It is possible that such a loading × estrogen interaction plays a greater role at the femur than the radius, which would explain our limb-specific results.
As gymnastic training likely affects muscle and fat, adjustment for tbFFM and PBF may reduce the detected “skeletal loading effect”. Nonetheless, our analyses detected significant GYM advantages, whereas Faulkner and colleagues reported no GYM advantages after adjustment for tbFFM [25]. Still, our main results may present a conservative view of skeletal adaptation to gymnastic loading compared to studies that do not adjust for body composition.
5. Limitations
Although we did not detect significant interactions between gymnastic loading exposure and physical maturity for many variables, this does not mean that growth and adaptation processes are identical across maturity levels. Our cross-sectional analysis is not capable of detecting changes in bone parameters relative to loading exposure within individuals as they develop from one maturity phase to another. Similarly, reported maturity differences may be a function of greater cumulative and recent gymnastic loading doses with increasing maturity. Future longitudinal analyses will track intra-individual development, accounting for time-varying co-variates such as age, body size and menarche status and evaluating loading exposure as a continuous variable. Nonetheless, this preliminary cross-sectional analysis improves upon existing studies by using comparable methods to evaluate indices of bone geometry and strength at multiple sites within each individual.
Radius DXA-derived variables use simplified geometric models that assume equal cortical tissue volumetric BMD (vBMD) for GYM and NON. Our derivations attempt to account for cortical vBMD variability by applying Tanner stage-based cortical vBMDs to both activity groups [28,36]. Although a few studies have indicated lower cortical vBMD in ex-GYM versus NON [15, 37-38], it is unknown whether purported cortical vBMD differences are consistent across maturity levels. If cortical vBMD is systematically and uniformly lower in GYM than NON, GYM advantages in CT and cCSA would be underestimated by the current methods. Furthermore, because DXA-derivations appear to systematically underestimate RAD CT in larger bones [28], the current study may provide a conservative estimate of RAD CT advantages in GYM versus NON.
Similar assumptions limit HSA derivations. NN bCSA assumes uniform apparent bone tissue density of 1.05 g/cm3, relying upon: 1) similar apparent tissue density for both cortical and trabecular bone; 2) similar apparent tissue densities for GYM and NON; 3) similar apparent tissue densities across maturity groups. As discussed for RAD derivations, violation of these underlying assumptions may also influence FN HSA results. For derivation of NNCT, HSA assumes: 1) a circular NN model; 2) 60% of bCSA is cortical compartment and 40% is trabecular compartment. Just as for the radius, if there are loading-related differences in FN cortical tissue density, then our FN results represent conservative estimates of GYM advantages in NNCT, NNbCSA, NNBR and NNZ. Furthermore, relative proportions of the cortical versus trabecular compartments may differ in GYM versus NON (i.e. are not universally 60% cortical vs. 40% trabecular), yielding greater GYM advantages for NNCT and NNBR (thicker cortices and lower BR in GYM than NON).
Finally, the large number of bone outcomes evaluated increases the probability of Type I error. Significant main effects and interactions are expected based on chance alone in 5%, or 1.1/22 comparisons (excluding redundant variables reported only for context (Area, nnBMD)). As we expected differences to vary by site, it is more appropriate to evaluate significance levels by site: 1/3 α= 0.05/7= 0.007; UD α= 0.05/4= 0.0125; FN α= 0.05/11=0.005. Using these more stringent α levels and adjusting for age, height, nbFFM, PBF and MAT, all of our results remained similar except the following (no longer significant): 1/3 ED (main effect and interaction), FNBMC (main effect and interaction), NNZ (main effect) and shaft neck angle (main effect). For multiple regression analyses, any GYMHRS effects not noted as significant beyond the level of α=0.01 should be corroborated. Overall, even using more stringent alpha criteria to account for multiple testing, most of our findings are supported and corroborate those of other studies.
6. Summary
We evaluated both the distal radius and the proximal femur in a large sample of girls over a wide range of physical maturity and activity levels. Analogous methodologies were used to compare site-specific geometric adaptation within individuals. We identified site-specific differences in geometric adaptation patterns, such that GYM skeletal advantages were more consistent and of greater magnitude for distal radius than proximal femur outcomes. Specifically, loading at the distal radius diaphysis was associated with greater theoretical skeletal strength through larger periosteal and endosteal dimensions in post-menarcheal GYM than NON. In contrast, at the proximal femur, buckling ratio was more favorable due to greater gymnast bone tissue CSA and cortical thickness, despite narrower bone width and endosteal diameter. Although our analyses are cross-sectional, the results suggest: 1) accumulation of skeletal benefits with long-term loading exposure, 2) loading benefits that increase with estrogen exposure or 3) a combination of the two. Finally, we identified maturity-specific loading advantages at these sites; however, maturational processes must be elucidated in subject-specific longitudinal analyses.
Highlights.
Young, female gymnasts vs. non-gymnasts: Radius & femoral neck DXA-derived indices.
Two-factor ANCOVA tested for gymnastic exposure & maturity: effects, interactions.
Gymnastics: Greater geometry & strength benefits at radius than femoral neck.
Gymnastics: Larger cortices at both sites, wider radii vs. narrower femoral necks.
Gymnasts gain skeletal strength via maturity-& site-specific geometric adaptation.
Acknowledgments
We are grateful for the assistance of Portia Flowers and Rebecca Hickman in data management. We would also like to acknowledge the assistance of Jill Kanaley, Sue Hemingway, Rebecca Hickman, Tina Craig, Cathy Riley, Eileen Burd, Carol Sames and Kristy Kmack during data collection. Last but not least, we are very grateful for the cooperation and assistance of our study participants and their families. The project described was funded by grants from the Orthopedic Research and Education Foundation, SUNY Upstate Medical University, the National Institute of Arthritis, Musculoskeletal and Skin Diseases (R03AR047613; R01AR054145). This publication was made possible by grant number R01AR054145 from the National Institute of Arthritis, Musculoskeletal and Skin Diseases.
Abbreviations
- DXA
dual energy X-ray absorptiometry
- aBMD
areal bone mineral density
- BMC
bone mineral content
- Area
bone projected area
- HSA
DXA hip structural analysis
- RAD
radius
- GYM
gymnasts
- NON
non-gymnasts
- FN
femoral neck
- NN
femoral narrow neck
- GYMHRS
annual mean hours per week gymnastic training for the year prior to the DXA scan
- h/wk
hours per week
- pQCT
peripheral quantitative computed tomography
- MAT
maturity level
- M1
physical maturity level 1 (pre-menarche, Tanner breast stage I)
- M2
physical maturity level 2 (pre-menarche, Tanner breast stage II or greater)
- M3
physical maturity level 3 (post-menarche)
- 1/3
DXA 1/3 distal region of interest for the radial diaphysis
- MEN
menarche status
- UD
DXA ultradistal region of interest for the radial metaphysis
- nbFFM
DXA total body, non-bone, lean mass
- CVs
coefficients of variation
- ANOVA
analysis of variance
- ANCOVA
analysis of covariance
- VIF
variance inflation factor
- BMI
body mass index
- Z
section modulus
- ED
endosteal diameter
- cCSA
cortical cross-sectional area
- IBS
index of structural strength in axial compression
- CT
cortical thickness
- PBF
percent body fat
- NN bCSA
narrow neck bone tissue cross-sectional area
- NN BR
narrow neck buckling ratio
Footnotes
All authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].National Osteoporosis Foundation 2011 Sep 28; http://www.nof.org/node/40.
- [2].Cooper C, Dennison EM, Leufkens HG, Bishop N, van Staa TP. Epidemiology of childhood fractures in Britain: A study using the general practice research database. J. Bone Miner. Res. 2004;19:1976–1981. doi: 10.1359/JBMR.040902. [DOI] [PubMed] [Google Scholar]
- [3].Ferrari SL, Chevalley T, Bonjour JP, Rizzoli R. Childhood fractures are associated with decreased bone mass gain during puberty: An early marker of persistent bone fragility? J. Bone Miner. Res. 2006;21:501–507. doi: 10.1359/jbmr.051215. [DOI] [PubMed] [Google Scholar]
- [4].Scerpella TA, Dowthwaite JN, Gero NM, Kanaley JA, Ploutz-Snyder RJ. Skeletal benefits of pre-menarcheal gymnastics are retained after activity cessation. Pediatr. Exerc. Sci. 2010;22:21–33. doi: 10.1123/pes.22.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Scerpella TA, Dowthwaite JN, Rosenbaum PF. Sustained skeletal benefit from childhood mechanical loading. Osteoporos. Int. 2010;22:2205–2210. doi: 10.1007/s00198-010-1373-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Daly RM, Rich PA, Klein R, Bass S. Effects of high-impact exercise on ultrasonic and biochemical indices of skeletal status: A prospective study in young male gymnasts. J. Bone Miner. Res. 1999;14:1222–1230. doi: 10.1359/jbmr.1999.14.7.1222. [DOI] [PubMed] [Google Scholar]
- [7].Bradshaw EJ, Le Rossignol P. Anthropometric and biomechanical field measures of floor and vault ability in 8 to 14 year old talent-selected gymnasts. Sports Biomechanics. 2004;3:249–262. doi: 10.1080/14763140408522844. [DOI] [PubMed] [Google Scholar]
- [8].Dowthwaite JN, Scerpella TA. Skeletal geometry and indices of bone strength in artistic gymnasts. J. Musculoskelet. Neuronal Interact. 2009;9:198–214. [PMC free article] [PubMed] [Google Scholar]
- [9].Cadogan J, Blumsohn A, Barker ME, Eastell R. A longitudinal study of bone gain in pubertal girls: Anthropometric and biochemical correlates. J. Bone Miner. Res. 1998;13:1602–1612. doi: 10.1359/jbmr.1998.13.10.1602. [DOI] [PubMed] [Google Scholar]
- [10].Fehling PC, Alekel L, Clasey J, Rector A, Stillman RJ. A comparison of bone mineral densities among female athletes in impact loading and active loading sports. Bone. 1995;17:205–210. doi: 10.1016/8756-3282(95)00171-9. [DOI] [PubMed] [Google Scholar]
- [11].Nurmi-Lawton JA, Baxter-Jones AD, Mirwald RL, Bishop JA, Taylor P, Cooper C, New SA. Evidence of sustained skeletal benefits from impact-loading exercise in young females: A 3-year longitudinal study. J. Bone Miner. Res. 2004;19:314–322. doi: 10.1359/JBMR.0301222. [DOI] [PubMed] [Google Scholar]
- [12].Robinson TL, Snow-Harter C, Taaffe DR, Gillis D, Shaw J, Marcus R. Gymnasts exhibit higher bone mass than runners despite similar prevalence of amenorrhea and oligomenorrhea. J. Bone Miner. Res. 1995;10:26–35. doi: 10.1002/jbmr.5650100107. [DOI] [PubMed] [Google Scholar]
- [13].Taaffe DR, Robinson TL, Snow CM, Marcus R. High-impact exercise promotes bone gain in well-trained female athletes. J. Bone Miner. Res. 1997;12:255–260. doi: 10.1359/jbmr.1997.12.2.255. [DOI] [PubMed] [Google Scholar]
- [14].Scerpella TA, Davenport M, Morganti CM, Kanaley JA, Johnson LM. Dose related association of impact activity and bone mineral density in pre-pubertal girls. Calcif. Tissue Int. 2003;72:24–31. doi: 10.1007/s00223-001-1131-x. [DOI] [PubMed] [Google Scholar]
- [15].Eser P, Hill B, Ducher G, Bass SL. Skeletal benefits after long-term retirement in former elite female gymnasts. J. Bone Miner. Res. 2009;24:1981–1988. doi: 10.1359/jbmr.090521. [DOI] [PubMed] [Google Scholar]
- [16].Nanyan P, Prouteau S, Jaffre C, Benhamou L, Courteix D. Thicker radial cortex in physically active prepubertal girls compared to controls. Int. J. Sports Med. 2005;26:110–115. doi: 10.1055/s-2004-817859. [DOI] [PubMed] [Google Scholar]
- [17].Liang MTC, Arnaud SB, Steele CR, Hatch P, Moreno A. Ulnar and tibial bending stiffness as an index of bone strength in synchronized swimmers and gymnasts. Eur. J. Appl. Physiol. 2005;94:400–407. doi: 10.1007/s00421-005-1351-2. [DOI] [PubMed] [Google Scholar]
- [18].Dowthwaite JN, Flowers PPE, Spadaro JA, Scerpella TA. Bone geometry, density and strength indices of the distal radius reflect loading via childhood gymnastic activity. J. Clin. Densitom. 2007;10:65–75. doi: 10.1016/j.jocd.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dowthwaite JN, Kanaley JA, Spadaro JA, Hickman RM, Scerpella TA. Muscle indices do not fully account for enhanced upper extremity bone mass and strength in gymnasts. J. Musculoskelet. Neuronal Interact. 2009;9:2–14. [PubMed] [Google Scholar]
- [20].Dowthwaite JN, Scerpella TA. Distal radius geometry and skeletal strength indices after peripubertal artistic gymnastics. Osteoporos. Int. 2011;22:207–216. doi: 10.1007/s00198-010-1233-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Dyson K, Blimkie CJR, Davison KS, Webber CE, Adachi JD. Gymnastic training and bone density in pre-adolescent females. Med. Sci. Sports Exerc. 1997;29:443–450. doi: 10.1097/00005768-199704000-00004. [DOI] [PubMed] [Google Scholar]
- [22].Ward KA, Roberts SA, Adams JE, Mughal MZ. Bone geometry and density in the skeleton of pre-pubertal gymnasts and school children. Bone. 2005;36:1012–1018. doi: 10.1016/j.bone.2005.03.001. [DOI] [PubMed] [Google Scholar]
- [23].Dowthwaite JN, Flowers PPE, Spadaro JA, Hickman RM, Scerpella TA. Artistic gymnastics during growth is linked to high post-menarcheal bone geometry and strength indices: Preliminary pQCT results. Bone. 2007;40:S40–S41. [Google Scholar]
- [24].Erlandson MC, Kontulainen SA, Baxter-Jones AD. Precompetitive and recreational gymnasts have greater bone density, mass, and estimated strength at the distal radius in young childhood. Osteoporos. Int. 2011;22:75–84. doi: 10.1007/s00198-010-1263-9. [DOI] [PubMed] [Google Scholar]
- [25].Faulkner RA, Forwood MR, Beck TJ, Mafukidze JC, Russell K, Wallace W. Strength indices of the proximal femur and shaft in prepubertal female gymnasts. Med. Sci. Sports Exerc. 2003;35:513–518. doi: 10.1249/01.MSS.0000053724.33480.8B. [DOI] [PubMed] [Google Scholar]
- [26].Maimoun L, Coste O, Mariano-Goulart D, Galtier F, Mura T, Philibert P, Briot K, Paris F, Sultan C. In peripubertal girls, artistic gymnastics improves areal bone mineral density and femoral bone geometry without affecting serum OPG/RANKL levels. Osteoporos. Int. doi: 10.1007/s00198-011-1541-1. (Published online 2/26/2011) DOI 10.1007/s00198-011-1541-1. [DOI] [PubMed] [Google Scholar]
- [27].Bass S, Pearce G, Bradney M, Hendrich E, Delmas PD, Harding A, Seeman E. Exercise before puberty may confer residual benefits in bone density in adulthood: Studies in active prepubertal and retired female gymnasts. J. Bone Miner. Res. 1998;13:500–507. doi: 10.1359/jbmr.1998.13.3.500. [DOI] [PubMed] [Google Scholar]
- [28].Dowthwaite JN, Flowers PPE, Scerpella TA. Agreement between pQCT and DXA-derived indices of bone geometry, density and theoretical strength in females of varying age, maturity and physical activity. J. Bone Miner. Res. 2011;26:1349–1357. doi: 10.1002/jbmr.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Beck TJ, Ruff CB, Warden KE, Scott WW, Jr, Rao GU. Predicting femoral neck strength from bone mineral data. A structural approach. Invest. Radiol. 1990;25:6–18. doi: 10.1097/00004424-199001000-00004. [DOI] [PubMed] [Google Scholar]
- [30].Sievanen H, Kannus P, Nieminen V, Heinonen A, Oja P, Vuori I. Estimation of various mechanical characteristics of human bones using dual energy X-ray absorptiometry: Methodology and precision. Bone. 1996;18:17S–27S. doi: 10.1016/8756-3282(95)00376-2. [DOI] [PubMed] [Google Scholar]
- [31].Cole JH, Dowthwaite JN, Scerpella TA, van der Meulen MC. Correcting fan-beam magnification in clinical densitometry scans of growing subjects. J. Clin. Densitom. 2009;12:322–329. doi: 10.1016/j.jocd.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Nikander Femoral Neck Structure in Adult Female Athletes Subjected to Different Loading Modalities. J Bone Miner Res. 2005;20:520–528. doi: 10.1359/JBMR.041119. 2005. [DOI] [PubMed] [Google Scholar]
- [33].Khoo BC, Beck TJ, Qiao Q-H, Parakh P, Semanick L, Prince RL, Singer KP, Price RI. In vivo short-term precision of hip structure analysis variables in comparison with bone mineral density using paired dual-energy X-ray absorptiometry scans from multi-center clinical trials. Bone. 2005;37:112–21. doi: 10.1016/j.bone.2005.03.007. [DOI] [PubMed] [Google Scholar]
- [34].Dowthwaite JN, DiStefano JG, Ploutz-Snyder RJ, Kanaley JA, Scerpella TA. Maturity and activity-related differences in bone mineral density: Tanner I vs. II and gymnasts vs. non-gymnasts. Bone. 2006;39:895–900. doi: 10.1016/j.bone.2006.04.007. [DOI] [PubMed] [Google Scholar]
- [35].Petit MA, McKay HA, MacKelvie KJ, Heinonen A, Khan KM, Beck TJ. A Randomized School-Based Jumping Intervention Confers Site and Maturity-Specific Benefits on Bone Structural Properties in Girls: A Hip Structural Analysis Study. J Bone Miner Res. 2002;17:363–372. doi: 10.1359/jbmr.2002.17.3.363. [DOI] [PubMed] [Google Scholar]
- [36].Dowthwaite JN, Hickman RM, Kanaley JA, Ploutz-Snyder RJ, Spadaro JA, Scerpella TA. Distal radius strength: A comparison of DXA-derived vs pQCT-measured parameters in adolescent females. J. Clin. Densitom. 2009;12:42–53. doi: 10.1016/j.jocd.2008.06.001. [DOI] [PubMed] [Google Scholar]
- [37].Ducher G, Hill BL, Angeli T, Bass SL, Eser P. Comparison of pQCT parameters between ulna and radius in retired elite gymnasts: The skeletal benefits associated with long-term gymnastics are bone- and site-specific. J. Musculoskelet. Neuronal Interact. 2009;9:247–255. [PubMed] [Google Scholar]
- [38].Ducher G, Eser P, Hill B, Bass S. History of amenorrhoea compromises some of the exercise-induced benefits in cortical and trabecular bone in the peripheral and axial skeleton: A study in retired elite gymnasts. Bone. 2009;45:760–767. doi: 10.1016/j.bone.2009.06.021. [DOI] [PubMed] [Google Scholar]
- [39].Leppänen OV, Sievänen H, Jokihaara J, Pajamäki I, Kannus P, Cooper DM, Järvinen TL. The effects of loading and estrogen on rat bone growth. J Appl Physiol. 2010;108:1737–1744. doi: 10.1152/japplphysiol.00989.2009. [DOI] [PubMed] [Google Scholar]