Abstract
Background
Respiratory syncytial virus (RSV) and rhinovirus infections are the most common significant infant respiratory tract illnesses and are associated with increased but differential risks of childhood asthma.
Objective
We sought to determine whether maternal asthma is associated with higher odds of infant respiratory tract infection with rhinovirus versus RSV and increased infection severity.
Methods
Mother-infant dyads were enrolled from 2004-2008 during an infant respiratory tract infection (104 with rhinovirus and 279 with RSV). Mothers were classified into mutually exclusive groups (atopic asthma, nonatopic asthma, and no asthma). We determined viral cause using PCR and the severity of the infant’s respiratory tract infection using the bronchiolitis severity score. Adjusted relative odds of maternal asthma with viral cause were calculated by using logistic regression. Proportional odds models assessed the association of maternal asthma and infant infection severity.
Results
Infants with a mother with atopic asthma compared with infants whose mothers did not have asthma were more likely to have rhinovirus versus RSV infection (adjusted odds ratio, 2.42; 95% CI, 1.19-4.90). Similarly, among infants with rhinovirus, having a mother with atopic asthma was associated with increased infection severity (adjusted odds ratio, 3.10; 95% CI, 1.21-7.98). This relationship was not seen among infants with RSV.
Conclusions
Clinically significant rhinovirus infection during infancy was more strongly associated with having a mother with atopic asthma than clinically significant RSV infection. Having a mother with atopic asthma was associated with increased severity of infant rhinovirus but not RSV infections. Infants with rhinovirus were more likely to have a familial atopic predisposition, which might partly explain the subsequent increased asthma risk.
Key words: Atopic predisposition, acute respiratory tract infection, rhinovirus, respiratory syncytial virus, asthma
Abbreviations used: ARI, Acute respiratory tract infection; COAST, Childhood Origins of Asthma; HRV, Human rhinovirus; LRTI, Lower respiratory tract infection; RSV, Respiratory syncytial virus; SHS, Secondhand smoke; TCRI, Tennessee Children’s Respiratory Initiative; URTI, Upper respiratory tract infection
Bronchiolitis, a lower respiratory tract infection (LRTI) commonly caused by respiratory syncytial virus (RSV) and less commonly by human rhinovirus (HRV), affects an estimated 20% to 30% of children in the first year of life and is a leading cause of hospitalization during infancy.1, 2, 3, 4 In addition to the acute morbidity seen with bronchiolitis, infants hospitalized with bronchiolitis and young children who experience virus-induced wheezing illnesses are at increased risk of recurrent wheezing and asthma in early childhood.4, 5, 6, 7, 8 The pathogenesis of the increased wheezing after viral bronchiolitis is not fully understood.4, 5, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17 In efforts to learn whether children at risk of bronchiolitis are also at increased risk for asthma, studies have investigated whether a family history of asthma is associated with the severity or incidence of bronchiolitis during infancy, with some prior studies finding an association, whereas others did not.9, 10, 18, 19, 20, 21 Prospective birth cohorts in which all children have a familial predisposition to asthma, such as a cohort based in Perth, Australia, and the Childhood Origins of Asthma (COAST) cohort, have investigated the association of a viral cause of infections in early life and subsequent wheezing and asthma.4, 7, 22 In the COAST cohort HRV-induced wheezing illnesses in early life were found to be stronger predictors of wheezing and asthma at age 6 years than RSV-induced illnesses.4, 7, 22 However, it is not known whether infants with symptomatic HRV infections that lead to an unscheduled health care visit are more likely to have a familial predisposition to asthma than infants with symptomatic RSV infection.
In this investigation that included mother-infant dyads enrolled in the Tennessee Children’s Respiratory Initiative (TCRI), we tested the hypothesis that a familial atopic predisposition was associated with viral cause and increased severity of viral acute respiratory tract infection (ARI) during infancy.23
Methods
Study design and setting
We conducted an analysis of 383 mother-infant dyads enrolled in the TCRI to investigate the association of a familial atopic predisposition with the viral cause (HRV or RSV) and severity of infant ARIs. The rationale and methods for the TCRI cohort have been reported previously.23 The TCRI is a prospective cohort of 673 term (≥37 weeks), non–low-birth-weight (≥2250 g) infants and their mothers designed to investigate the association of characteristics of infant viral ARIs, such as severity and cause, and familial atopic predisposition on the development of early childhood asthma and atopy.23 This investigation included the 383 mother-infant dyads in which the infant presented for an unscheduled clinic or emergency department visit or hospitalization and was determined to have a sole HRV- or RSV-induced ARI. Mother-infant dyads were enrolled at the time of an infant ARI during 4 viral respiratory seasons, September through May 2004-2008.23 Dyads were recruited in the inpatient, emergency department, and clinic settings at a single academic institution, and the children are currently being followed longitudinally through age 6 years to ascertain asthma and atopy outcomes. Each woman provided written informed consent for participation of herself and her infant. The Vanderbilt University Institutional Review Board approved the study.
During study enrollment, research nurses administered an in-person structured questionnaire that included questions regarding demographics, the infant’s home environment, the index infant’s illness, previous medical history of the infant, and detailed family asthma and atopic disease history, including maternal responses to the International Study of Asthma and Allergies in Children questionnaire.23 At enrollment, research nurses obtained nasal and throat swabs from the infants for viral detection. Through a structured medical chart review, information was abstracted regarding the infant’s medical visit, including birth weight, room air pulse oximetry, requirement for supplemental oxygen, history of prior wheezing, and detailed medical information. Final discharge diagnoses were obtained through chart review after discharge.
Ascertainment of maternal asthma and atopy
Self-reported maternal asthma status was defined as a positive response to the question “Have you ever had asthma?,” which was asked as part of the International Study of Asthma and Allergies in Children questionnaire and/or to the question “Were you diagnosed with asthma as a child?”24, 25 Maternal atopy was determined based on skin prick test results or allergen-specific IgE levels. Preferentially, women underwent skin prick tests to saline, histamine, and 8 aeroallergens: cat pelt, Alternaria species, grass mix #7, ragweed mix, oak mix, Tricophyton species, mite mix, and cockroach mix (Quintest Extract Tray; Hollister-Stier, Spokane, Wash).23 Allergen-specific IgE measurement (Phadiatop; Phadia, Kalamazoo, Mich) was performed on maternal blood samples for women who could not undergo skin prick tests or who had an inadequate skin test. Multiallergen screens for specific IgE (Phadiatop) were measured with the ImmunoCAP250 (performed by the Johns Hopkins DACI laboratory).26 A positive Phadiatop result was defined as 0.35 kUA/L or greater by using the standards in place at the time the assays were performed.27, 28 Among women for whom allergen sensitization status was determined based on skin prick test results or allergen-specific IgE levels, we classified women by whether they reported a history of asthma into 3 mutually exclusive categories: atopic asthma, nonatopic asthma, and no asthma. Women with self-reported asthma and evidence of allergen sensitization (≥1 positive skin prick test response or positive Phadiatop result) were classified as having atopic asthma, women with self-reported asthma and without evidence of allergen sensitization were classified as having nonatopic asthma, and women who did not report self-reported asthma were in the no asthma group.
Viral cause of ARIs
Nasal and throat swabs were obtained from infants at the time of enrollment, and the biospecimens were processed, placed in aliquots, and stored at −80°C. The specimens were tested in batches for RSV A and B, HRV, adenovirus, human metapneumovirus, coronaviruses, influenza A and B, and parainfluenza types 1, 2, and 3 by using real-time RT-PCR with the Cepheid Smart Cycler II, as previously described.23 PCR results were used to identify infants with a sole RSV- or HRV-induced ARI.
Infant ARIs
Infants included in this study had an HRV- or RSV-induced ARI, either viral upper respiratory tract infections (URTIs) or LRTIs. Children with a URTI had a health care provider’s diagnosis of a viral URTI and/or symptoms, including fever, cough, congestion, hoarse cry, otitis media, and/or rhinorrhea without evidence of lower respiratory tract symptoms or respiratory distress. Infants with a physician’s diagnosis of bronchiolitis or wheezing, signs and symptoms consistent with bronchiolitis on chart review, or both were considered to have a viral LRTI.23, 24 We determined the severity of the ARI by using an ordinal bronchiolitis severity score with factors including respiratory rate, room air oxygen saturation, and the presence and extent of wheezing and flaring and retractions. Scores range from 0 to 12, with higher scores indicating more severe illness.29, 30
Covariates
Other variables of interest obtained from the questionnaire administered at enrollment included self-reported maternal race/ethnicity, maternal education, secondhand smoke (SHS) exposure, infant’s insurance type (Tennessee Medicaid, private, or none), infant’s birth weight (in grams), infant’s sex, infant’s age at enrollment (in weeks), and siblings.
Statistical analysis
Descriptions of demographics and characteristics of the 383 infants with sole HRV or RSV infections are presented as frequencies and proportions for categorical variables and medians and interquartile ranges for continuous variables. Univariate analyses were conducted to compare maternal asthma factors by the infant’s HRV- or RSV-induced ARI status by using the Wilcoxon rank sum test for continuous variables or the Pearson χ2 test for categorical variables. In our analyses we first defined maternal asthma using self-reported asthma in the women. In addition, to examine the association of maternal asthma in combination with an objective measure of atopy, we repeated all analyses using a more detailed definition that classified women as having atopic asthma, nonatopic asthma, or no asthma by incorporating their skin prick test or allergen-specific IgE findings. Therefore we investigated whether (1) having a mother with self-reported asthma and (2) having a mother with atopic or nonatopic asthma was associated with an infant’s ARI with HRV or RSV. We assessed the association of measures of maternal asthma and virus type in the overall ARI group (combined URTI and LRTI) and next among the LRTI subgroup. We applied a logistic regression model with variable HRV(+) or RSV(+) as a binary outcome variable and maternal asthma as defined above, as our main factor along with covariates. Because of our limited regression power determined by the HRV(+) group, we used propensity score adjustment to prevent overfitting because the propensity score analysis adjusts for many confounding factors simultaneously while preserving analytical power.31 Variables in the propensity score model included a priori selected variables: maternal race/ethnicity, SHS exposure, infant’s insurance type, infant’s birth weight, infant’s sex, infant’s age at enrollment, and number of siblings.
In our next set of analyses we examined whether having a mother with asthma (first defined by maternal self-report and then using the atopic asthma and nonatopic asthma classifications) was associated with increased severity of the infant’s HRV- or RSV-induced ARI. We conducted a separate analysis for infants with sole HRV- or RSV-induced ARI and in the subgroups with LRTIs. We used the proportional odds model to evaluate the association of maternal asthma with infant HRV or RSV severity using the bronchiolitis severity score. For infants with RSV, a priori selected variables in the multivariable models included maternal race/ethnicity, SHS exposure, infant’s insurance type, infant’s birth weight, infant’s sex, infant’s age at enrollment, and number of siblings. Analyses among HRV-induced LRTIs were limited by small sample size for a full covariates model, and therefore we performed a propensity score–adjusted model that included infant’s sex, age, and birth weight. We performed an interaction analysis to assess whether the association between maternal asthma and severity of the infant’s ARI was different depending on the infant’s HRV or RSV status. The proportional odds model was used with a cross-product term of maternal history of asthma and virus positivity (RSV+ and HRV+) and adjustment for covariates.
Statistical analyses were performed with R version 2.12.1 software.32
Results
A total of 383 infants with sole infection with either HRV or RSV were included in this study. Table I highlights the demographics and characteristics of the cohort by infant ARI cause, as determined by means of PCR: positive for HRV only (n = 104) or RSV only (n = 279). Compared with infants with RSV, infants with HRV were more likely to be older (20 vs 9 weeks, P < .001), have Medicaid insurance (79% vs 61%, P = .002), have mothers who were African American (32% vs 18%, P < .001), have a URTI versus an LRTI (61% vs 4%, P < .001), and have a lower median bronchiolitis severity score (2 vs 6.5, P < .001).
Table I.
Infant and maternal characteristics by type of infant ARI among dyads enrolled in the TCRI, September to May 2004-2008
| Characteristic | HRV only (n = 104) | RSV only (n = 279) | P value |
|---|---|---|---|
| Estimated gestational age (wk), median (IQR) | 39 (38-40) | 39 (38-40) | .073 |
| Infant sex, no. (%) | .046 | ||
| Male | 67 (64) | 148 (53) | |
| Female | 37 (36) | 131 (47) | |
| Birth weight (g), median (IQR) | 3345 (3062-3629) | 3260 (2984-3657) | .57 |
| Infant’s age at ARI (wk), median (IQR) | 20 (7.8-38.5) | 9 (6-17) | <.001 |
| Infant insurance, no. (%) | |||
| Private | 15 (14) | 90 (32) | .002 |
| Medicaid | 82 (79) | 171 (61) | |
| None | 7 (7) | 18 (6) | |
| Any breast-feeding, no. (%) | 64 (62) | 150 (54) | .17 |
| Prior wheezing/treatment, no. (%) | 44 (43) | 110 (40) | .43 |
| SHS, no. (%) | 53 (51) | 148 (54) | .68 |
| Maternal race/ethnicity, no. (%) | |||
| White | 46 (44) | 195 (70) | <.001 |
| Black | 33 (32) | 49 (18) | |
| Latino | 19 (18) | 29 (10) | |
| Other | 6 (6) | 6 (2) | |
| Maternal age (y), median (IQR) | 24 (21-30) | 25 (22-30) | .19 |
| Maternal asthma, no. (%) | 29 (28) | 43 (15) | .005 |
| Maternal asthma without or with allergic sensitization, no. (%) | |||
| No asthma | 71 (73) | 227 (84) | |
| Nonatopic asthma | 8 (8) | 19 (7) | .022 |
| Atopic asthma | 18 (19) | 23 (9) | |
| Maternal education (y), median (IQR), n = 303 | 12 (11-14) | 12 (12-14) | .32 |
| Siblings | 1 (0-2) | 1 (1-2) | .12 |
| Any day care, no. (%) | 34 (33) | 56 (20) | .01 |
| Enrollment season, no. (%) | |||
| 2004-2005 | 25 (24) | 71 (25) | |
| 2005-2006 | 39 (38) | 74 (27) | .18 |
| 2006-2007 | 26 (25) | 82 (29) | |
| 2007-2008 | 14 (13) | 52 (19) | |
| URTI or LRTI, no. (%) | <.001 | ||
| URTI | 63 (61) | 11 (4) | |
| LRTI | 41 (39) | 268 (96) | |
| Bronchiolitis severity score, median (IQR) | 2 (1-5.4) | 6.5 (4-9) | <.001 |
Association of infant HRV- and RSV-induced ARIs and LRTIs and maternal asthma
Among infants with ARIs and the LRTI subgroup, we determined the association of self-reported maternal asthma and infant HRV- or RSV-induced ARIs (both URTIs and LRTIs). Infants with HRV were more likely to have a mother with self-reported asthma than infants with RSV (28% vs 15%, P = .005, Table I). In adjusted analyses, compared with infants whose mothers did not have asthma, infants with a mother with self-reported asthma had an increased relative odds of having HRV-induced than RSV-induced ARI (adjusted odds ratio, 2.02; 95% CI, 1.15-3.52). In analyses limited to infants with LRTIs, infants with HRV were more likely to have a mother with self-reported asthma than infants with RSV (32% vs 15%, P = .008). In adjusted analyses infants with a mother with self-reported asthma had an increased relative odds of having HRV-induced than RSV-induced LRTI (adjusted odds ratio, 2.87; 95% CI, 1.34-6.18).
Association of infant HRV- or RSV-induced ARIs and maternal atopic and nonatopic asthma
Maternal allergen sensitization determined based on skin prick test results or allergen-specific IgE levels was available for 97% of the mothers (n = 366) and was used to identify maternal atopic asthma. A larger percentage of infants with HRV-induced ARIs had a mother with atopic asthma than infants with RSV-induced ARIs (19% vs 9%, respectively), whereas the percentages of infants with a mother with nonatopic asthma were similar (8% vs 7%, respectively). Having a mother with atopic asthma was associated with increased odds of an infant having HRV versus RSV infection when compared with having a mother without asthma (propensity score–adjusted odds ratio, 2.42; 95% CI, 1.19-4.90). Having a mother with nonatopic asthma compared with no asthma was not associated with viral cause (adjusted odds ratio, 1.26; 95% CI, 0.51-3.10).
Association of infant HRV- or RSV-induced LRTIs and maternal atopic and nonatopic asthma
A larger percentage of infants with HRV-induced LRTIs had a mother with atopic asthma than infants with RSV-induced LRTIs (10/39 [26%] vs 21/258 [8%]); however, the percentages of infants with a mother with nonatopic asthma were similar (3/39 [8%] vs 18/258 [7%]). In multivariable propensity score–adjusted analyses there was a statistically significant association with having a mother with atopic asthma compared with having a mother without asthma for infants with HRV-induced LRTIs compared with RSV-induced LRTIs (adjusted odds ratio, 4.12; 95% CI, 1.67-10.17). There was not a statistically significant difference between infants having a mother with nonatopic asthma compared with no asthma for infants with HRV-induced LRTIs compared with those with RSV-induced LRTIs (adjusted odds ratio, 1.40; 95% CI, 0.38-5.21).
Association of infant HRV- and RSV-induced ARI and LRTI severity and maternal asthma
In separate analyses for infants with either HRV or RSV infection, we examined whether the severity of the infant’s respiratory tract infection was associated with having a mother with self-reported asthma. In infants with HRV, the association between self-reported maternal asthma and infant infection severity was not statistically significant in the ARI group (both URTIs and LRTIs; adjusted odds ratio, 2.10; 95% CI, 0.94-4.70) or the LRTI group (adjusted odds ratio, 1.42; 95% CI, 0.44-4.60; Table II ). In infants with RSV, the association between self-reported maternal asthma and infant infection severity was not significant in the total ARI group (adjusted odds ratio, 0.73; 95% CI, 0.40-1.34) or the LRTI group (adjusted odds ratio, 0.84; 95% CI, 0.45-1.56; Table II).
Table II.
Acute respiratory illness severity in children with HRV- or RSV-induced ARIs by 2 indicators of maternal asthma among infants enrolled in the TCRI, 2004-2008
| Familial atopic predisposition |
Infants with HRV |
Infants with RSV |
|---|---|---|
| Acute respiratory tract infection (n = 383) | Odds ratio (95% CI) of increased bronchiolitis severity∗ | Odds ratio (95% CI) of increased bronchiolitis severity |
| Self-reported maternal asthma | ||
| No | 1 | 1 |
| Yes | 2.10 (0.94-4.70) | 0.73 (0.40-1.34) |
| Maternal asthma without or with allergic sensitization | ||
| No asthma | 1 | 1 |
| Nonatopic asthma | 1.61 (0.42-6.10) | 0.66 (0.31-1.40) |
| Atopic asthma | 3.10 (1.21-7.98) | 0.94 (0.38-2.34) |
| Lower respiratory tract infection subgroup (n = 309) | HRV-induced LRTI (n = 41)† | RSV-induced LRTI (n = 268) |
| Self-reported maternal asthma | ||
| No | 1 | 1 |
| Yes | 1.42 (0.44-4.60) | 0.84 (0.46-1.56) |
| Maternal asthma without or with allergic sensitization | ||
| No asthma | 1 | 1 |
| Nonatopic asthma | 1.19 (0.19-7.5) | 0.79 (0.36-1.70) |
| Atopic asthma | 1.18 (0.28-4.98) | 1.05 (0.41-2.72) |
Proportional odds ratios for increased bronchiolitis severity are presented by maternal asthma status.
Proportional odds models are adjusted for (with the exception of HRV-induced LRTIs) the following variables: maternal race/ethnicity, SHS exposure, infant’s insurance type, infant’s birth weight, infant’s sex, infant’s age at enrollment, and number of siblings.
The proportional odds propensity score–adjusted model included the following variables: infant’s age, sex, and birth weight.
Association of infant HRV- and RSV-induced ARI and LRTI severity and atopic and nonatopic maternal asthma
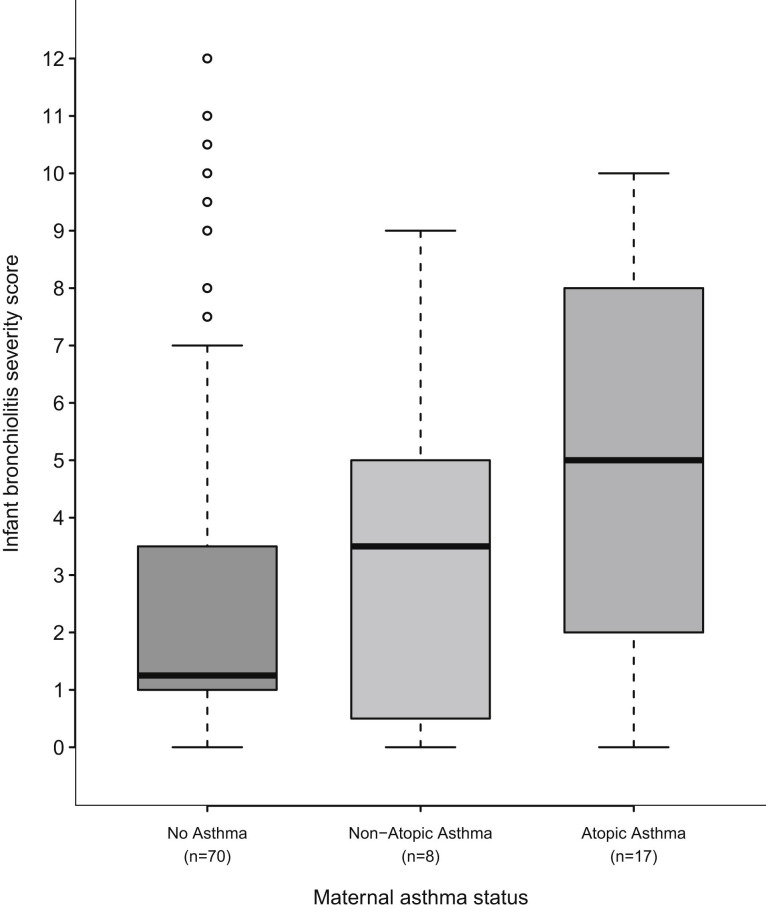
Next, in separate analyses for infants with either HRV or RSV infections, we examined whether the severity of the infant’s respiratory tract infection was associated with whether the infant’s mother had atopic or nonatopic asthma. Among infants with HRV-induced ARI, having a mother with atopic asthma was associated with increased ARI severity (Fig 1 ), and in adjusted analyses there was a more than 3-fold increased relative odds of having more severe illness compared with infants whose mothers did not have asthma (adjusted odds ratio, 3.10; 95% CI, 1.21-7.98; Table II). The interaction analysis investigating a differential effect of maternal atopic asthma by whether the infant had HRV or RSV on infection severity was also statistically significant (P = .01). This relationship was not seen when limited to the subgroup of infants with HRV-induced LRTIs; however, the number of infants with HRV-induced LRTIs was very small (n = 41) and could be adjusted through propensity scores for only the infant’s age, sex, and birth weight (Table II). There was not an association between maternal atopic asthma and infection severity in infants with RSV-induced ARIs or the RSV-induced LRTI subgroup (Table II).
Fig 1.
Severity of infant ARI by maternal asthma status in infants with rhinovirus-associated ARI enrolled in the TCRI 2004-2008. In the box plot the horizontal bold line and upper and lower hinges of the box represent medians and interquartile ranges. Whiskers extend to 1.5 times the upper and lower interquartile ranges, with outliers indicated by open circles.
Discussion
RSV and HRV are the most common viruses associated with infant ARIs, and RSV- and HRV-induced LRTIs are a leading cause of respiratory morbidity and hospitalizations in the first year of life.1, 2, 3 Viral LRTIs during infancy and early childhood are also well established to be associated with an increased risk of asthma later in childhood.5, 6, 7, 12, 22 Because of the known differential risk of early childhood asthma after RSV- and HRV-induced infant infections, we were interested in studying whether a familial predisposition to asthma and allergies was associated with the viral cause of the infant’s ARI and the severity of the ARI.4, 22, 33, 34, 35, 36, 37, 38, 39, 40, 41 Several small studies have not found an association between familial predisposition and bronchiolitis; however, in our prior large, population-based cohort investigation of infants, we found that having a mother with asthma was associated with increased risk and severity of bronchiolitis during infancy.20 In the current investigation we sought to expand on previous findings by addressing the research questions of whether infants with HRV-induced ARIs or LRTI subgroups are more likely to have a familial predisposition to asthma than those with RSV-induced infections and whether a familial predisposition to asthma is associated with more severe infant HRV- or RSV-induced ARIs.
In our previous work we found that although, as a group, infants with a history of bronchiolitis had an increased risk of early childhood asthma, infants who had bronchiolitis during HRV-predominant months had a 25% increased risk of early childhood asthma compared with infants who had bronchiolitis during RSV-predominant months.41 Furthermore, in the COAST birth cohort, in which all children have a familial predisposition to asthma,7, 14 investigators found that HRV-induced wheezing illnesses in the first 3 years of life were associated with a 9.8-fold relative odds of asthma at 6 years compared with a 2.6-fold increase among children with RSV.22 We were able to assess whether maternal asthma and atopy were associated with the viral cause and the virus-specific severity of the infant’s infection leading to an unscheduled health care visit because this cohort consisted of infants with and without a familial predisposition to asthma, and we used objective measures of maternal atopy and molecular techniques to determine the viral cause of the infant’s respiratory tract infection. We found that infants with a mother with atopic asthma had an increased relative odds of having HRV-induced ARIs than RSV-induced ARIs compared with infants whose mothers did not have asthma. It is notable that although the prevalence of self-reported maternal asthma is higher in children with HRV (28%), the 15% prevalence of maternal asthma among infants with RSV-induced ARIs is higher than the asthma prevalence in the adult population in the United States.42 These study findings suggest that for infants with HRV-induced ARIs in particular, a familial predisposition to asthma might partly explain the increased risk of asthma seen later in life and/or that a familial predisposition to asthma predisposes infants to clinically significant HRV-induced ARIs during infancy, a viral infection that, unlike RSV, can often be isolated from young children without clinically obvious respiratory symptoms.38
We also found that infants with HRV who had a mother with atopic asthma had more than a 3-fold increased relative odds of having more severe illness than infants whose mothers did not have asthma. Among those with RSV-induced ARIs, there was no relationship between maternal asthma and atopy and ARI severity. The relationship between maternal atopic asthma and the infant’s HRV infection severity is intriguing given the described altered host response to HRV, as has been found in vitro and among subjects with asthma.40, 43, 44, 45, 46, 47 These data support the notion of differential susceptibility to HRV among patients with atopic asthma. Continued follow-up of these children until the age of 6 years will further delineate whether it is the subset of infants with HRV-induced ARIs and a familial atopic predisposition who will have asthma and allergic diseases later in childhood or have more severe asthma or recurrent exacerbations.23 Ultimately, this might help us to understand whether there is an altered host response or increased susceptibility to HRV among patients with atopic asthma.
There are several limitations of this work. This study included a convenience sample of mother-infant dyads in which all infants presented for an unscheduled health care visit and not a cohort followed from birth. However, the study included participants recruited during viral seasons over 4 years, which should serve to strengthen the generalizability of the findings. A single episode of RSV- or rhinovirus-induced ARI was captured. It is likely that children had additional viral infections during infancy. The primary focus of this study was the association of a maternal atopic predisposition and the infant’s ARI severity, and therefore we were not able to investigate the outcome of bronchiolitis incidence as in our larger, population-based retrospective cohort of mother-infant dyads.20 In addition, maternal asthma was determined based on self-report and not based on objective criteria, such as airway reversibility testing. However, we used a validated instrument and self-reported asthma in young adults in whom there is little overlap with other diseases, and thus there is high specificity.48 Furthermore, this study included objective measures of atopy. Lastly, we cannot completely rule out the possible influence of unknown or unmeasured potential confounding factors, as with all observational studies.
In summary, infant HRV infections requiring clinical care were more strongly associated with having a mother with atopic asthma than infant RSV infections. In addition, infants with HRV-induced ARIs who had a mother with atopic asthma had more than a 3-fold increased relative odds of having a more severe ARI compared with that seen in infants with HRV whose mothers did not have asthma. This relationship was not seen in infants with RSV infections. These findings suggest that there is likely an underlying genetic basis for the risk of and response to respiratory tract infections during infancy and that the mechanisms underlying the increased asthma risk after HRV- and RSV-induced bronchiolitis might be different. Future longitudinal investigations successful at preventing or modifying the host response to infant viral infections will provide insight into the relationship of infant viral infections and early childhood asthma, as will investigations that assess the atopic host and nonatopic host response to select respiratory pathogens.
Key messages.
-
•
Infants whose mothers had atopic asthma had increased relative odds of having a rhinovirus-induced ARI than RSV infection compared with the odds in infants whose mothers did not have asthma.
-
•
In infants with rhinovirus, having a mother with atopic asthma was associated with 3-fold increased relative odds of more severe illness, a relationship not seen in infants with RSV.
-
•
For infants with rhinovirus-induced ARIs, a familial atopic predisposition might partly explain the subsequent increased risk of asthma and differential susceptibility to rhinovirus among asthmatic patients.
Footnotes
Supported by K01 AI070808 (to K.N.C.), Thrasher Research Fund Clinical Research Grant (to T.V.H.), National Institutes of Health midcareer investigator awardK24 AI 077930 (to T.V.H.), and UL1 RR024975 (Vanderbilt CTSA).
Disclosure of potential conflict of interest: K. N. Carroll receives research support from the National Institutes of Health/National Institute of Allergy and Infectious Diseases. E. K Miller receives research support from the National Institutes of Health. J. V. Williams is on the scientific advisory board for Quidel, Inc. W. D. Dupont receives research support from the National Cancer Institute. T. V. Hartert receives research support from the National Institutes of Health, the Agency for Healthcare Research and Quality, and MedImmune; has provided legal consultation services or expert witness testimony for the Merck Research Advisory Board; is a member of the American Thoracic Society; and has been an invited speaker for the American Academy of Allergy, Asthma, & Immunology and for the Pediatric Academic Societies. The rest of the authors declare that they have no relevant conflicts of interest.
References
- 1.Shay D.K., Holman R.C., Newman R.D., Liu L.L., Stout J.W., Anderson L.J. Bronchiolitis-associated hospitalizations among US children, 1980-1996. JAMA. 1999;282:1440–1446. doi: 10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- 2.Holberg C.J., Wright A.L., Martinez F.D., Ray C.G., Taussig L.M., Lebowitz M.D. Risk factors for respiratory syncytial virus-associated lower respiratory illnesses in the first year of life. Am J Epidemiol. 1991;133:1135–1151. doi: 10.1093/oxfordjournals.aje.a115826. [DOI] [PubMed] [Google Scholar]
- 3.Carroll K., Gebratsadik T., Griffin M., Wu P., Dupont W., Mitchel E. The increasing burden and risk factors for bronchiolitis-related medical visits in infants enrolled in a state healthcare insurance plan. Pediatrics. 2008;122:58–64. doi: 10.1542/peds.2007-2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kusel M.M., de Klerk N.H., Kebadze T., Vohma V., Holt P.G., Johnston S.L. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J Allergy Clin Immunol. 2007;119:1105–1110. doi: 10.1016/j.jaci.2006.12.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sigurs N., Gustafsson P.M., Bjarnason R., Lundberg F., Schmidt S., Sigurbergsson F. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med. 2005;171:137–141. doi: 10.1164/rccm.200406-730OC. [DOI] [PubMed] [Google Scholar]
- 6.Carroll K.N., Wu P., Gebretsadik T., Griffin M.R., Dupont W.D., Mitchel E.F. The severity-dependent relationship of infant bronchiolitis on the risk and morbidity of early childhood asthma. J Allergy Clin Immunol. 2009;123:1055–1061. doi: 10.1016/j.jaci.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lemanske R.F., Jr., Jackson D.J., Gangnon R.E., Evans M.D., Li Z., Shult P.A. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol. 2005;116:571–577. doi: 10.1016/j.jaci.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 8.Carroll K.N., Hartert T.V. The impact of respiratory viral infection on wheezing illnesses and asthma exacerbations. Immunol Allergy Clin North Am. 2008;28:539–561. doi: 10.1016/j.iac.2008.03.001. viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sims D.G., Gardner P.S., Weightman D., Turner M.W., Soothill J.F. Atopy does not predispose to RSV bronchiolitis or postbronchiolitic wheezing. BMJ. 1981;282:2086–2088. doi: 10.1136/bmj.282.6282.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pullan C.R., Hey E.N. Wheezing, asthma, and pulmonary dysfunction 10 years after infection with respiratory syncytial virus in infancy. BMJ. 1982;284:1665–1669. doi: 10.1136/bmj.284.6330.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez F.D., Wright A.L., Taussig L.M., Holberg C.J., Halonen M., Morgan W.J. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332:133–138. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 12.Stein R.T., Sherrill D., Morgan W.J., Holberg C.J., Halonen M., Taussig L.M. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354:541–545. doi: 10.1016/S0140-6736(98)10321-5. [DOI] [PubMed] [Google Scholar]
- 13.Peebles R.S., Jr. Viral infections, atopy, and asthma: is there a causal relationship? J Allergy Clin Immunol. 2004;113(suppl):S15–S18. doi: 10.1016/j.jaci.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 14.Lemanske R.F., Jr. The childhood origins of asthma (COAST) study. Pediatr Allergy Immunol. 2002;13(suppl 15):38–43. doi: 10.1034/j.1399-3038.13.s.15.8.x. [DOI] [PubMed] [Google Scholar]
- 15.Openshaw P.J., Yamaguchi Y., Tregoning J.S. Childhood infections, the developing immune system, and the origins of asthma. J Allergy Clin Immunol. 2004;114:1275–1277. doi: 10.1016/j.jaci.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 16.Wu P., Dupont W.D., Griffin M.R., Carroll K.N., Mitchel E.F., Gebretsadik T. Evidence of a causal role of winter virus infection during infancy in early childhood asthma. Am J Respir Crit Care Med. 2008;178:1123–1129. doi: 10.1164/rccm.200804-579OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim E.Y., Battaile J.T., Patel A.C., You Y., Agapov E., Grayson M.H. Persistent activation of an innate immune response translates respiratory viral infection into chronic lung disease. Nat Med. 2008;14:633–640. doi: 10.1038/nm1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noble V., Murray M., Webb M.S., Alexander J., Swarbrick A.S., Milner A.D. Respiratory status and allergy nine to 10 years after acute bronchiolitis. Arch Dis Child. 1997;76:315–319. doi: 10.1136/adc.76.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trefny P., Stricker T., Baerlocher C., Sennhauser F.H. Family history of atopy and clinical course of RSV infection in ambulatory and hospitalized infants. Pediatr Pulmonol. 2000;30:302–306. doi: 10.1002/1099-0496(200010)30:4<302::aid-ppul5>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 20.Carroll K.N., Gebretsadik T., Griffin M.R., Dupont W.D., Mitchel E.F., Wu P. Maternal asthma and maternal smoking are associated with increased risk of bronchiolitis during infancy. Pediatrics. 2007;119:1104–1112. doi: 10.1542/peds.2006-2837. [DOI] [PubMed] [Google Scholar]
- 21.Miller E.K., Williams J.V., Gebretsadik T., Carroll K.N., Dupont W.D., Mohamed Y.A. Host and viral factors associated with severity of human rhinovirus-associated infant respiratory tract illness. J Allergy Clin Immunol. 2011;127:883–891. doi: 10.1016/j.jaci.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson D.J., Gangnon R.E., Evans M.D., Roberg K.A., Anderson E.L., Pappas T.E. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178:667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hartert T.V., Carroll K., Gebretsadik T., Woodward K., Minton P. The Tennessee Children’s Respiratory Initiative: objectives, design and recruitment results of a prospective cohort study investigating infant viral respiratory illness and the development of asthma and allergic diseases. Respirology. 2010;15:691–699. doi: 10.1111/j.1440-1843.2010.01743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carroll K.N., Gebretsadik T., Larkin E.K., Dupont W.D., Liu Z., Van D.S. Relationship of maternal vitamin D level with maternal and infant respiratory disease. Am J Obstet Gynecol. 2011;205:215. doi: 10.1016/j.ajog.2011.04.002. e1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asher M.I., Keil U., Anderson H.R., Beasley R., Crane J., Martinez F. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J. 1995;8:483–491. doi: 10.1183/09031936.95.08030483. [DOI] [PubMed] [Google Scholar]
- 26.Hamilton R.G., Franklin A.N., Jr. In vitro assays for the diagnosis of IgE-mediated disorders. J Allergy Clin Immunol. 2004;114:213–225. doi: 10.1016/j.jaci.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 27.Gustafsson D., Danielsson D. In vitro diagnosis of atopic allergy in children. A comparison between total IgE, conventional RAST and a new multi RAST (Phadiatop) Allergy. 1988;43:105–108. doi: 10.1111/j.1398-9995.1988.tb00402.x. [DOI] [PubMed] [Google Scholar]
- 28.Garcia-Marcos L., Sanchez-Solis M., Martinez-Torres A.E., Lucas Moreno J.M., Hernando S., Gustafsson D. Phadiatop compared to skin-prick test as a tool for diagnosing atopy in epidemiological studies in schoolchildren. Pediatr Allergy Immunol. 2007;18:240–244. doi: 10.1111/j.1399-3038.2006.00508.x. [DOI] [PubMed] [Google Scholar]
- 29.Tal A., Bavilski C., Yohai D., Bearman J.E., Gorodischer R., Moses S.W. Dexamethasone and salbutamol in the treatment of acute wheezing in infants. Pediatrics. 1983;71:13–18. [PubMed] [Google Scholar]
- 30.Goebel J., Estrada B., Quinonez J., Nagji N., Sanford D., Boerth R.C. Prednisolone plus albuterol versus albuterol alone in mild to moderate bronchiolitis. Clin Pediatr (Phila) 2000;39:213–220. doi: 10.1177/000992280003900404. [DOI] [PubMed] [Google Scholar]
- 31.Rosenbaum P.R., Rubin D.B. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 32.R Foundation for Statistical Computing; Vienna (Austria): 2006. R: a language and environment for statistical computing. [Google Scholar]
- 33.Lazzaro T., Hogg G., Barnett P. Respiratory syncytial virus infection and recurrent wheeze/asthma in children under five years: an epidemiological survey. J Paediatr Child Health. 2007;43:29–33. doi: 10.1111/j.1440-1754.2007.00998.x. [DOI] [PubMed] [Google Scholar]
- 34.Johnston S.L., Pattemore P.K., Sanderson G., Smith S., Lampe F., Josephs L. Community study of role of viral infections in exacerbations of asthma in 9-11 year old children. BMJ. 1995;310:1225–1229. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McIntosh K., Ellis E.F., Hoffman L.S., Lybass T.G., Eller J.J., Fulginiti V.A. The association of viral and bacterial respiratory infections with exacerbations of wheezing in young asthmatic children. J Pediatr. 1973;82:578–590. doi: 10.1016/S0022-3476(73)80582-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Minor T.E., Baker J.W., Dick E.C., DeMeo A.N., Ouellette J.J., Cohen M. Greater frequency of viral respiratory infections in asthmatic children as compared with their nonasthmatic siblings. J Pediatr. 1974;85:472–477. doi: 10.1016/S0022-3476(74)80447-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnston N.W., Johnston S.L., Duncan J.M., Greene J.M., Kebadze T., Keith P.K. The September epidemic of asthma exacerbations in children: a search for etiology. J Allergy Clin Immunol. 2005;115:132–138. doi: 10.1016/j.jaci.2004.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rakes G.P., Arruda E., Ingram J.M., Hoover G.E., Zambrano J.C., Hayden F.G. Rhinovirus and respiratory syncytial virus in wheezing children requiring emergency care. IgE and eosinophil analyses. Am J Respir Crit Care Med. 1999;159:785–790. doi: 10.1164/ajrccm.159.3.9801052. [DOI] [PubMed] [Google Scholar]
- 39.Heymann P.W., Carper H.T., Murphy D.D., Platts-Mills T.A., Patrie J., McLaughlin A.P. Viral infections in relation to age, atopy, and season of admission among children hospitalized for wheezing. J Allergy Clin Immunol. 2004;114:239–247. doi: 10.1016/j.jaci.2004.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jartti T., Kuusipalo H., Vuorinen T., Soderlund-Venermo M., Allander T., Waris M. Allergic sensitization is associated with rhinovirus-, but not other virus-, induced wheezing in children. Pediatr Allergy Immunol. 2010;21:1008–1014. doi: 10.1111/j.1399-3038.2010.01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carroll K.N., Wu P., Gebretsadik T., Griffin M.R., Dupont W.D., Mitchel E.F. Season of infant bronchiolitis and estimates of subsequent risk and burden of early childhood asthma. J Allergy Clin Immunol. 2009;123:964–966. doi: 10.1016/j.jaci.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akinbami L.J., Moorman J.E., Liu X. Asthma prevalence, health care use, and mortality: United States, 2005-2009. Natl Health Stat Report. 2011;(32):1–14. [PubMed] [Google Scholar]
- 43.Wark P.A., Johnston S.L., Bucchieri F., Powell R., Puddicombe S., Laza-Stanca V. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005;201:937–947. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Message S.D., Laza-Stanca V., Mallia P., Parker H.L., Zhu J., Kebadze T. Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL-10 production. Proc Natl Acad Sci U S A. 2008;105:13562–13567. doi: 10.1073/pnas.0804181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bochkov Y.A., Hanson K.M., Keles S., Brockman-Schneider R.A., Jarjour N.N., Gern J.E. Rhinovirus-induced modulation of gene expression in bronchial epithelial cells from subjects with asthma. Mucosal Immunol. 2010;3:69–80. doi: 10.1038/mi.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holgate S.T. Rhinoviruses in the pathogenesis of asthma: the bronchial epithelium as a major disease target. J Allergy Clin Immunol. 2006;118:587–590. doi: 10.1016/j.jaci.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 47.Kuo C., Lim S., King N.J., Bartlett N.W., Walton R.P., Zhu J. Rhinovirus infection induces expression of airway remodelling factors in vitro and in vivo. Respirology. 2011;16:367–377. doi: 10.1111/j.1440-1843.2010.01918.x. [DOI] [PubMed] [Google Scholar]
- 48.Jenkins M.A., Clarke J.R., Carlin J.B., Robertson C.F., Hopper J.L., Dalton M.F. Validation of questionnaire and bronchial hyperresponsiveness against respiratory physician assessment in the diagnosis of asthma. Int J Epidemiol. 1996;25:609–616. doi: 10.1093/ije/25.3.609. [DOI] [PubMed] [Google Scholar]