Abstract
Although sphingomyelin (SM) is the most abundant phospholipid in the plasma, next to phosphatidylcholine (PC), its physiological function in plasma is unclear. Here we employed plasma from various genetic models of mice which naturally differ in their plasma SM/PC ratios, to study the role of SM as a modulator of LCAT, the enzyme responsible for HDL maturation and the synthesis of cholesteryl esters (CE) in normal plasma. Serine palmitoyltransferase deficient mice, and SM synthase deficient mice, both of which have below normal SM/PC ratios, showed significantly elevated LCAT activities when assayed with the endogenous substrates. On the other hand, LDL receptor knockout mice, and apo E knockout mice, both of which have high SM/PC ratios, had markedly reduced (−80%) LCAT activities. The LCAT levels in plasma, as assayed with an exogenous substrate, were similar in all groups, except for a 45% decrease in apo E knockout mice. Plasma samples with high SM/PC ratios had lower percentage of 20:4, 22:5, and 22:6 CE all of which are formed by LCAT, and a higher percentage of the atherogenic 18:1 CE which is mainly derived from the action of liver ACAT, showing that in vivo, the contribution of LCAT to plasma CE is reduced while that of liver ACAT is increased. These results show that SM is a physiological modulator of LCAT activity as well as plasma CE composition, and this may contribute to the previously reported pro-atherogenic effect of high plasma SM levels.
Keywords: Sphingomyelin synthase 2 deficiency, Serine palmitoyl transferase deficiency, Apo E deficient mice, LDL receptor deficient mice, LCAT activity, Cholesteryl ester composition
1. Introduction
Lecithin-cholesterol acyltransferase (LCAT) plays a critical role in the reverse cholesterol transport pathway by esterifying free cholesterol (FC) derived from the peripheral tissues and driving the cholesterol efflux forward [1,2]. The majority of the cholesteryl ester (CE) present in human plasma is derived from the action of LCAT [3], and the enzyme activity is positively correlated with HDL levels [4,5]. The regulation of LCAT activity is therefore important not only in lipoprotein metabolism but also in atherogenesis. Low LCAT activity has been shown to be a strong positive marker for ischemic heart disease [6]. The complete absence of LCAT in plasma results in a drastic reduction in HDL, and in several disorders including renal disease, anemia, corneal opacity, and cardiovascular disease (CVD) [2,7]. However, high LCAT levels in plasma, as determined by the exogenous substrate assay or LCAT mass, are not necessarily protective against CVD [8], indicating that the esterification rate of the endogenous substrate is physiologically more relevant. Since the expression of LCAT in liver as well as its levels in plasma do not vary significantly in the general population [9], the regulation of cholesterol esterification occurs mainly from the modulation of the enzyme activity by its substrates, activators, and inhibitors in the plasma. We previously showed that sphingomyelin (SM), the second most abundant phospholipid in the lipoproteins, is an inhibitor of LCAT [10] and of the secretory phospholipases [11–13], and that the in vitro the activities of these enzymes are negatively correlated with the SM/phosphatidylcholine (PC) ratio in the lipoprotein substrates. Although the in vitro inhibitory effect of SM on LCAT activity has now been reported by several laboratories [14–16], the physiological relevance of this inhibition has not been established. In addition to the LCAT-derived CE which is predominantly polyunsaturated, varying amounts of CE derived from the action of acyl CoA: cholesterol acyltransferase (ACAT), which is predominantly 18:1, are present in the plasma. Since the atherogenic potential of ACAT-derived CE is much higher than that of LCAT-derived CE [17], the relative contribution of these two enzymes significantly affects the atherogenic risk.
In this study, we determined the LCAT activity and the CE composition in mouse plasma samples which have inherent differences in their SM levels due to genetic abnormalities. The SM levels are elevated naturally in apo E deficient mice because of an increased synthesis in liver and decreased degradation [18]. Similarly the plasma SM levels are elevated in LDL receptor deficient mice primarily because of the accumulation of apo B lipoproteins which are rich in SM [10]. On the other hand, there are two mouse models available where the plasma SM levels are significantly reduced because of mutations in the SM synthetic enzymes. One is the heterozygous deficiency of long chain base 2 of serine palmitoyltransferase (SPT), the rate limiting enzyme in the SM biosynthetic pathway [19]. The homozygous deficiency of SPT is embryonic lethal. The second model of lower SM level is the knockout of sphingomyelin synthase 2 (SMS2), the terminal enzyme in the SM biosynthetic pathway [20]. These mouse models with decreased plasma SM levels have been shown to have reduced risk of inflammation, insulin resistance, and atherosclerosis [19–21]. The availability of the mouse models that naturally contain varying levels of SM/PC ratios in plasma provides an opportunity to test the hypothesis that plasma SM levels directly regulate LCAT reaction in vivo, and consequently modulate the composition of plasma CE species. The results presented here show that the SM-deficient animals (with lower SM/PC ratios) indeed show a significant increase in the LCAT activity when assayed with endogenous substrates, whereas the animals with higher than normal SM/PC ratios exhibit an inhibition of the enzyme activity. The CE fatty acid composition also reflected the changes in LCAT activity, with the plasma samples with decreased LCAT activity showing lower percentage of 20:4 and 22:6, the LCAT-derived CE species, and higher percentage of 16:0 and 18:1, the ACAT-derived CE species. These results therefore support the hypothesis that plasma SM is a physiological regulator of cholesterol esterification in the lipoproteins.
2. Materials and Methods
2.1. Plasma samples
All animal studies were approved by the Animal Care Committees of University of Illinois at Chicago, University of Chicago, and SUNY Downstate Medical Center. The apo E knockout (ApoE−/−) mice and LDL receptor knockout (Ldlr−/−) mice with C57BL/6J genetic background as well as control mice (C57BL/6J) were obtained from The Jackson Laboratory (Bar Harbor, ME) and were maintained on standard rodent chow. The generation and characterization of SMS2 homozygous knockout mice (Sms2 −/−) [20], and SPTLC2 heterozygous deficient mice (Sptlc2+/−) [22] used in these studies have been described in earlier publications. Both genotypes were backcrossed with C57BL/6J mice for five generations. Only the standard chow-fed male mice (8–20 weeks old) from all groups were used in this study. Blood was collected from anaesthetized animals in EDTA, and the plasma was prepared and frozen immediately. The plasma samples were stored at −80 °C until use. The enzyme assays were performed within 2 months of the plasma isolation. The total number of animals for each group was as follows: (WT= 24; Sptlc2−/− = 15; Sms2−/− = 17; apoE−/− = 16; Ldlr−/− = 10). Not all assays were performed in some samples because of insufficient volume of plasma. Free and total cholesterol in the plasma were determined with Amplex Red reagent kit (Invitrogen), the former by the elimination of cholesteryl ester hydrolase from the assay mixture. Cholesteryl ester (CE) content was calculated as the difference between total and free cholesterol values.
2.2. LCAT assay
LCAT activity was assayed by both the endogenous substrate and the exogenous substrate methods as described previously [23]. Briefly, for the endogenous assay, the plasma sample (30 µl) was equilibrated with human serum albumin-3H cholesterol emulsion [24] in presence of 1 mM DTNB, a specific inhibitor of LCAT. The inhibition was then released by the addition of β mercaptoethanol (5 mM) and the enzyme reaction was allowed to proceed for 30 min at 37 °C. The reaction was stopped by the addition of 1 ml ethanol containing 50 µg each of cholesterol and cholesteryl oleate. The lipids were extracted 2 times with 2 ml each hexane, and the pooled hexane extracts were concentrated and separated by TLC, using petroleum ether: ethyl acetate (85: 15 v/v) as the solvent. The radioactivity in FC and CE spots was measured by liquid scintillation counting. The enzyme activity was expressed as fractional esterification rate (% of labeled cholesterol esterified/ 30 min) and as molar esterification rate (nmol of cholesterol esterified/ h/ ml of plasma).
For the exogenous substrate assay, proteoliposomes containing 14C-labeled cholesterol , egg PC, and apo AI at molar ratios of 15:300:1 were prepared as described by Chen and Albers [25]. Plasma sample (20 µl) was incubated with proteoliposome substrate (containing 2.5 nmol cholesterol) in presence of 10 mM mercaptoethanol and 2.5 mg/ml of human serum albumin for 60 min and the % of labeled cholesterol esterified was determined as described above for the endogenous substrate. The proteoliposome assay is a direct measure of the active enzyme concentration in the plasma, and is not affected by the plasma activators and inhibitors [25].
2.3. SMase treatment of plasma
In some experiments the plasma samples were first treated with SMase to deplete the SM levels before the LCAT activity was assayed by the endogenous substrate assay. The plasma was incubated in presence of 1 mM DTNB and 1 mM Mn++ with 1.5 units of S. aureus SMase C for 60 min. The plasma was then equilibrated with labeled cholesterol by incubation with albumin-3H cholesterol complex for overnight at 4 °C, and finally incubated in presence of 5 mM mercaptoethanol. The fractional and molar esterification rates were calculated as above.
2.4. Determination of phospholipids and cholesteryl esters by mass spectroscopy
Total lipids were extracted from plasma (20 µl) using Bligh and Dyer procedure [26], after adding the internal standards (50 ng each of 15:0-15:0 PC, 17:0 LPC, and 12:0 SM and 50 µg of 17:0 CE). The solvent was evaporated and the total lipids were reconstituted in 1 ml chloroform: methanol: water (72:23:3 by vol). The identification and quantitation of CE and phospholipid species was carried out using an ABSciex QTRAP 5500 system coupled to an Agilent 1260 series liquid chromatograph. Lipids were separated on a normal phase column (Supelco Ascentis Si HPLC 3µ particle size, 10 cm × 2.1 mm) with a flow rate 0.2 ml/min using a gradient of two solvent mixtures (solvent A- chloroform: methanol: NH4OH 80:19.5:0.5 (by vol) and solvent B- chloroform: methanol: NH4OH: water 60:34:0.5:5 (by vol). The time program used was as follows: 0 –1 min, 100% A; 11 min 100% B; 21 min 100% B, 23 min 100% A, 30 min 100% A). Samples were maintained at 12 °C, and the column temperature was maintained at 27 °C. Precursor ion scans (scan rate 2000 Da/s) in the positive ionization mode were used to detect and quantify molecular species of CE (363.9 m/z, 0–6 min, range 600–800 Da), PC ( 184.1 m/z, 14–17 min, range 600–900 Da), SM (184.1 m/z, 17–20 min, range 600–900 Da) and LPC (184.1 m/z, 22–25 min, range 400–590 Da). The MS parameters used were: electrospray voltage (IS) 4500 V, collision energy 20 eV for CE species, and 50 eV for all others; collision cell exit potential (CXP) 45V for CE species, 35 V for all others, declustering potential (DP) 60V for CE species, 100V for all others; source temperature was 550 °C for CE, and 350 °C for all other lipids. The curtain and nebulizer gas (nitrogen) pressures were set at 30 psi. Quantification of lipids was performed with the help of Analyst software (AB Sciex) by the integration of peak areas, and calculation of the concentrations relative to the internal standards.
2.5. Statistical analyses
The statistical significance between WT and other groups was determined by the Student’s t test (unpaired, 2 tailed), with p<0.05 taken as the significance level. Correlation between the variables was calculated by Pearson correlation.
3. Results
3.1. Plasma lipid composition
The genetic backgrounds of all tested groups of mice were comparable. The SM/PC ratios of plasma from various mouse models, as determined by LC/MS/MS, are shown in Fig. 1. The plasma from both Sptcl2 +/− and Sms2 −/− mice showed a significant decrease (about 30%) in SM/PC ratios compared to the wild type control (WT) mice. This decrease is due mostly to a decrease in plasma concentration of SM with no change in PC, as reported earlier [27], [19]. On the other hand, the ratios were significantly higher than WT mice in ApoE−/− mice (+74%) and Ldlr−/− mice (+28%), due to a disproportionate increase in SM compared to PC, as reported in other studies [18,28].
Figure 1.
Plasma SM/PC ratios in the various mouse models. Total lipid extracts of the plasma were analyzed by LC/MS/MS as described in the text. All values shown are mean ± SEM. (n=20 for WT, 14 for Sms2−/−, 15 for Sptlc2−/−, 16 for apoE−/−, and 10 for Ldlr−/−).
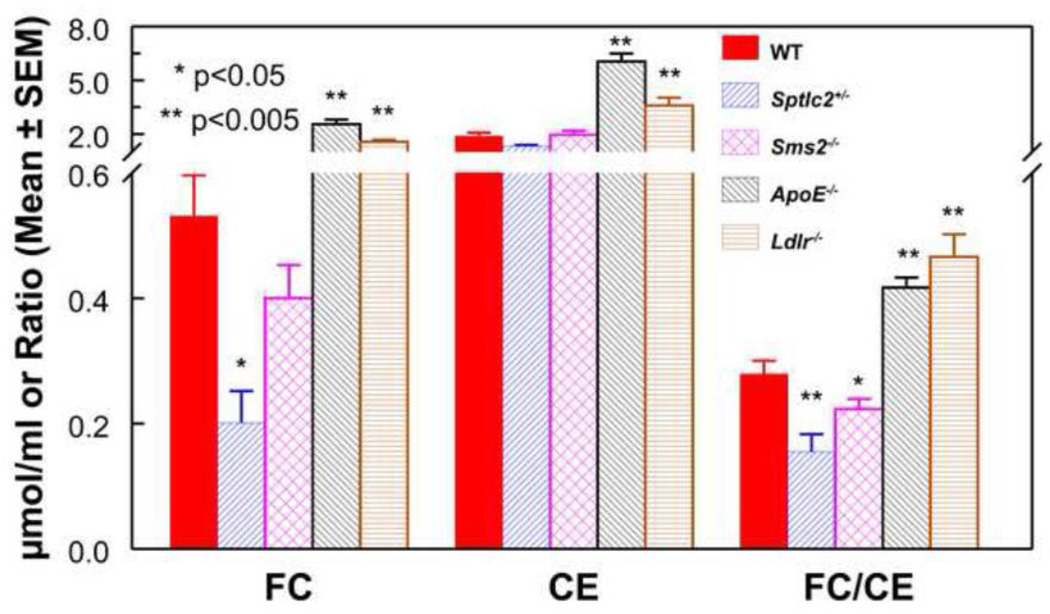
The free cholesterol (FC) and cholesteryl ester (CE) concentrations and the FC/CE ratios are shown in Fig. 2. The FC levels were lower than WT mice in Sptcl2 +/− and Sms2 −/− mice, although only the former was statistically significant. The FC values in both ApoE−/− and LDLR KO mice were significantly higher than in WT mice. The CE concentrations of Sptcl2 +/− and Sms2 −/− plasmas were in the normal range, whereas they were significantly higher in ApoE−/− and Ldlr−/− mice, as expected from the increased total cholesterol values. The FC/CE ratios, which inversely correlate with endogenous esterification efficiency, showed a significant decrease in SPT and SMS2 deficient mice, and a striking increase in the Ldlr−/− and ApoE−/− mice, indicating a higher esterification efficiency in the SM-deficient groups and a lower esterification efficiency in the high SM groups. Previous studies by Furbee et al [29] reported that the FC/CE ratio is increased in the ApoE−/− plasma although they found no significant change in the Ldlr−/− plasma.
Figure 2.
FC and CE values in plasma. Free and total cholesterol concentrations were determined by Amplex Red enzymatic kits from Invitrogen. The CE values were calculated as the difference between total and free cholesterol values.
3.2. LCAT activity
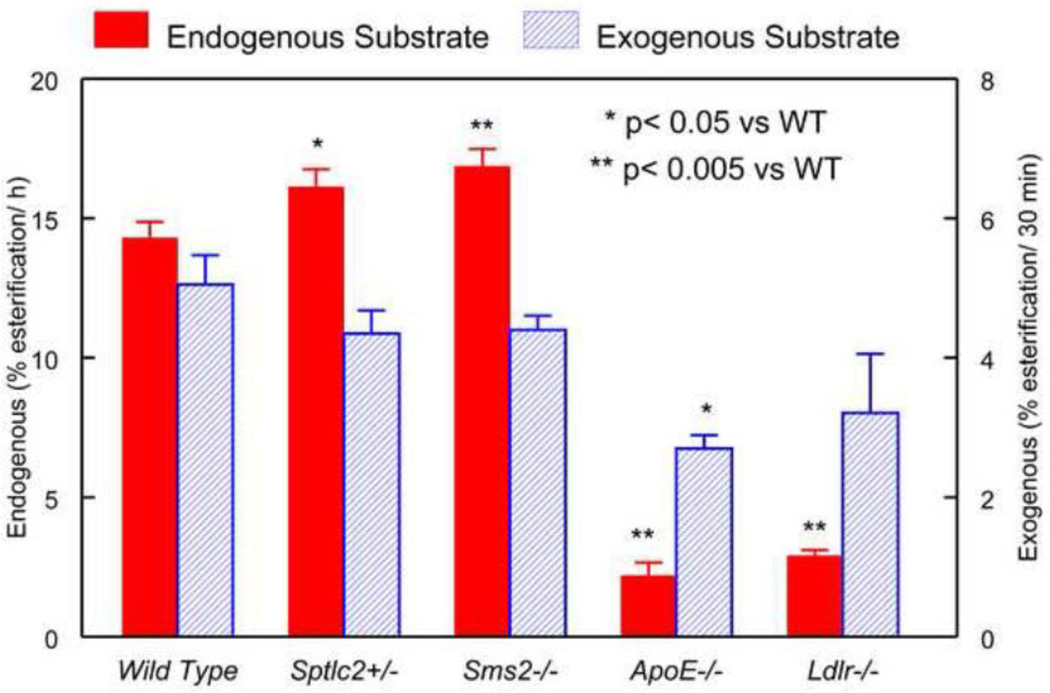
The LCAT activity in each plasma sample was assayed with both the endogenous substrates [24] and an exogenous proteoliposome substrate [25]. The endogenous activity is affected by the composition of lipoproteins, and the activators and inhibitors naturally present in the plasma, whereas the exogenous substrate assay measures the amount of active enzyme in the plasma since the substrates or activators are not limiting with respect to the amount of enzyme available [25].
As shown in Fig. 3, the activities with endogenous substrate were significantly higher in Sptcl2 +/− (+13%) and Sms2 −/− (+18%) plasma, compared with the wild type control. On the other hand, the enzyme activity was markedly lower in ApoE−/− (−85%) and Ldlr−/− mice (−80%), compared to the wild type. The enzyme activities observed with the exogenous substrate, however, were not significantly different from each other in 3 of the four groups. The exception was ApoE−/− plasma, which showed a 45% reduction compared to WT. This result is in variance with a report by Furbee et al [29] who reported no difference in the exogenous activity between WT, Ldlr−/− and ApoE−/− plasma. On the other hand, Forte et al [30] reported a decrease in apo E −/− plasma, but not in Ldlr−/− plasma, in agreement with the present results. The ratio of endogenous substrate/exogenous substrate activity was 30–35% higher in the SM-deficient samples, while it was 70% lower in the SM-rich samples, compared to WT. These results show that the decrease in the activity of LCAT in Ldlr−/− and ApoE−/− mice is far greater than expected from the decrease in the enzyme levels.
Figure 3.
LCAT activity in the plasma of various mouse models. The enzyme activities with the endogenous and exogenous substrates were measured as described in the text.
3.3. Molar activities
The molar esterification rates of cholesterol were calculated from the fractional esterification rates (with endogenous substrate) and the FC concentration of the plasma. The molar rates were significantly lower in Sptlc2+/− plasma, despite the higher fractional rates because of the low FC concentration in the plasma (Fig. 4). However the molar rate in the Sms2 −/− plasma was significantly higher than normal. Despite the low fractional rates in the ApoE−/− and Ldlr−/− mice their molar rates were not significantly different from the WT because of the high FC concentration in their plasma.
Figure 4.
Molar esterification rates for LCAT activity, as determined by the endogenous substrate assay. The molar activities were calculated by multiplying the fractional esterification rates (Fig. 3) with the concentration of FC in the plasma.
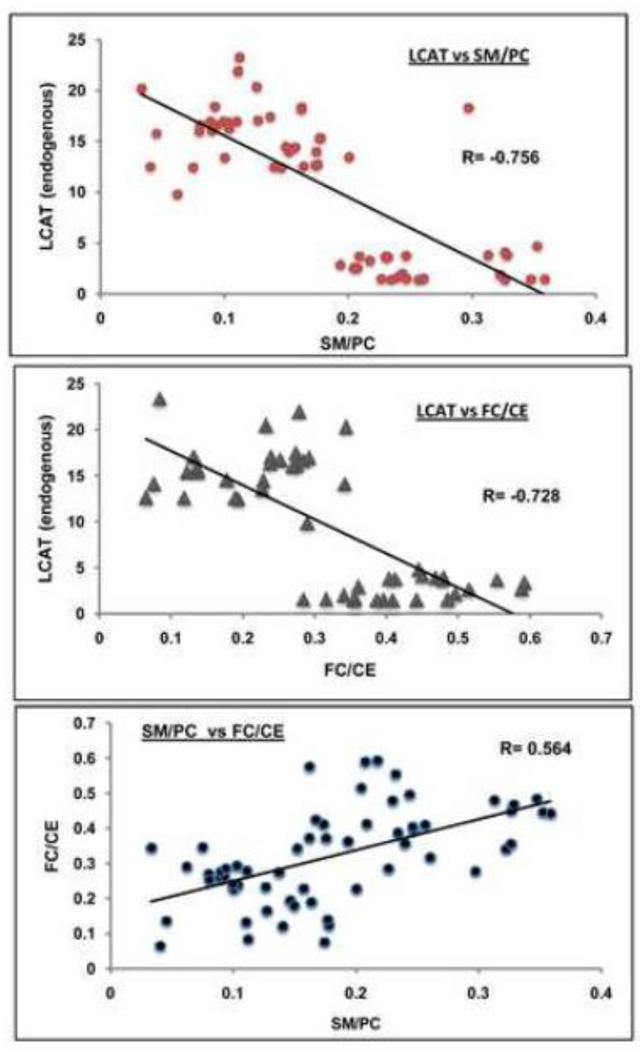
3.4. Correlations
The LCAT activity (with endogenous substrate) correlated negatively with SM/PC ratios (Fig. 5, top), as well as with FC/CE ratios (Fig. 5, middle). There was a clear segregation of SM-rich plasma samples at the lower end of LCAT activities. It should be pointed out that the graphs include only the samples where both the enzyme activity and lipid estimations were performed on the same sample, and therefore did not include samples where one of the values was missing. There was also a positive correlation between the FC/CE ratios and the SM/PC ratios (Fig. 5, bottom), again supporting the notion that plasma samples containing high SM concentration exhibit lower cholesterol esterification rate in vivo.
Figure 5.
Correlation of the LCAT fractional esterification rates (endogenous substrate) with SM/PC (top) or FC/CE (middle), and correlation of SM/PC ratio with FC/CE ratio. Pearson correlation coefficients were calculated in Microsoft Excel for the combined samples from all groups, where both the enzyme activity and the lipid values are available for each sample (n=60 for the SM/PC vs. LCAT; n= 49 for FC/CE vs. LCAT; n=60 for SM/PC vs. FC/CE).
3.5. CE molecular species composition
LCAT is known to preferentially synthesize polyunsaturated cholesteryl esters in mouse plasma, since it predominantly transfers the sn-2 acyl group from PC. On the other hand the CE species formed by the liver ACAT (and secreted into plasma) are predominantly 18:1 species. Therefore the composition of plasma CE species can be used as a measure of the contribution of the two enzymes for the plasma CE content. As shown in Fig. 6, the concentrations of 16:0, 16:1 and 18:1 CE species were significantly higher in both Ldlr−/− and ApoE−/− mice. There was also a significant decrease in the polyunsaturated CE in these genotypes, especially the 20:4 and 22:6 CE, which are formed predominantly, if not exclusively by the LCAT reaction [29], supporting a reduced contribution of LCAT to the plasma CE species in vivo. Interestingly, both ApoE−/− and Ldlr−/− plasma samples showed a significant increase in another polyunsaturated CE, namely 18:3 CE. This is also probably a product of ACAT reaction, since it has been reported that both 18:1 and 18:3 are better substrates than either 20:4 or 20:5 for liver ACAT2 [31]. There were no significant changes in the CE composition in Sms2 −/− mice compared to the control. In Sptcl2 +/− mice, however, there was a significant decrease in 16:0 CE, and a significant increase in 20:5 CE. There was also an increase in the percentage of 18:2, 20:4, and 22:6 CE species, although the increases were not statistically significant. All these results are consistent with an increased contribution of LCAT and decreased contribution of ACAT, in SM-poor plasma samples.
Figure 6.
Molecular species of CE in plasma from various mouse models. The CE composition was analyzed by LC/MS/MS as described in the text. The x-axis shows the fatty acids of CE.
4. Discussion
Although SM is the second most abundant phospholipid in the plasma lipoproteins, its physiological function in plasma has not attracted much attention. Its concentration varies significantly between various lipoproteins and even the subfractions of lipoproteins [32], suggesting that it is not merely fulfilling a structural role. Unlike PC, however, it is not a substrate for the lipolytic enzymes of plasma, and does not exchange easily between various lipoproteins. Based on its structural similarities with PC, and its resistance to lipolytic enzymes, we suggested that it serves as a competitive inhibitor of these enzymes, thus preventing the unregulated degradation of PC [10,33]. In the past few years several of the lipolytic enzymes that utilize PC as substrate, including LCAT [10,14–16], secretory phospholipases group II, V, and X [11–13,28], as well as lipoprotein lipase [34] have been shown to be inhibited by SM in the in vitro assays. Recent results from our laboratory also showed that endothelial lipase, which is predominantly a phospholipase [35], is inhibited by SM (manuscript in preparation). It is, however, necessary to show that the inhibition by SM is physiologically relevant and that it occurs in vivo. In the present study, we took advantage of the availability of mouse models that inherently differ in their plasma SM/PC ratios to address the physiological relevance of SM inhibition of LCAT reaction. The results presented clearly show that the SM/PC ratio of the plasma is inversely correlated with the cholesterol esterification rates supporting the hypothesis that SM is a physiological modulator of LCAT reaction. The differences in the plasma CE composition among the mouse models also support the presence of in vivo differences in the LCAT activities.
Although we found a strong negative correlation between plasma SM/PC ratio and the LCAT activity, these results do not necessarily prove a direct role of SM in the inhibition of LCAT. In an attempt to test whether the LCAT activity is activated by the depletion of SM in the SM-rich plasma, we treated the plasma from ApoE−/− and Ldlr−/− mice with bacterial sphingomyelinase (SMase) C before assaying for the LCAT activity. Our previous studies showed that such treatment of the lipoprotein substrates or the proteoliposome substrates by SMase activates the LCAT [10,36], as well as the secretory phospholipase reactions [11–13]. However, when the whole plasma was treated with SMase C, the cholesterol esterification was actually inhibited (results not shown), apparently because of the inactivation of LCAT during the SMase reaction. Temporary inhibition of LCAT with DTNB during SMase treatment, followed by the release of inhibition by mercaptoethanol also did not result in reactivation of the enzyme, suggesting an irreversible effect of SMase treatment on LCAT.
The reduction of LCAT activity in the ApoE−/− mice has previously been reported by Zhao et al [37], but these authors attributed this to the deficiency of apo E itself, since apo E is one of the known apoprotein activators of the LCAT reaction [38]. However it is likely that the high SM levels in the plasma play a larger role than the absence of apo E, because of the following reasons. 1) the concentration of apo AI, the primary apoprotein activator of LCAT is not limiting in the ApoE−/− mice, and therefore it can compensate for the lack of apo E. 2) LCAT activity with the HDL substrate that contains only apo AI is 50–100 times higher than with the HDL substrate that contains only apo E [39], showing that the loss of apo E may not be significant for the esterification of cholesterol in HDL. It is, however, possible that apo E deficiency specifically affects the LCAT activity on the apo B lipoproteins.
To our knowledge, the LCAT activity in Ldlr−/− mice has not been investigated using the endogenous substrate method. Two previous studies using the exogenous substrate method have reported no change in the LCAT activity [30] which is in agreement with the present studies (Fig. 3). This assay, however, would not reflect the effect of SM present in the endogenous lipoproteins. The CE fatty acid composition in these mice, however, shows a decreased contribution of LCAT for plasma cholesteryl esters in vivo [29]. It is therefore likely that the esterification of lipoprotein cholesterol is inhibited in vivo in LDL receptor deficiency.
In addition to affecting plasma HDL levels, the LCAT reaction significantly influences the plasma CE composition, which has been shown to be an independent risk factor for atherosclerosis in humans [40,41] and in animal models [42–44]. LCAT generates predominantly polyunsaturated CE species, whereas the tissue ACAT reaction generates predominantly 18:1 CE. Therefore a reduction in the contribution of LCAT to plasma CE results in a relatively more saturated CE profile, which is positively correlated with the risk of atherosclerosis and CVD [40–44]. Several studies have shown that plasma SM is an independent risk factor for CVD [45–47]. It is possible that part of this pro-atherosclerotic effect of SM is due to its inhibition of LCAT activity which not only results in lower HDL levels, but also in the production of more saturated CE profile due to an increased contribution of liver ACAT to plasma CE.
Highlights.
LCAT activity is significantly higher in SM-deficient mice (Sms2 −/−, and Sptlc2+/−)
LCAT activity is markedly inhibited in SM-rich plasma (ApoE−/−, and Ldlr−/−)
CE species composition shows a more atherogenic profile in SM-rich plasma
Acknowledgements
This research was supported by NIH grants R01 HL68585 from NHLBI, and R21 DK78165 from NIDDK and the Office of dietary supplements (to PVS), and NIH grants RO1 HL093419 and R01 HL093419-01A1 (to JXC).
Abbreviations
- ACAT
acyl CoA: cholesterol acyltransferase
- CE
cholesteryl ester
- CVD
cardiovascular disease
- DTNB
dithionitrobenzoate
- FC
free cholesterol
- LCAT
lecithin: cholesterol acyltransferase
- PC
phosphatidylcholine
- SM
sphingomyelin
- SMS
sphingomyelin synthase
- SPT
serine palmitoyltransferase
- SPTLC2
SPT long chain base 2
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jonas A. Lecithin cholesterol acyltransferase. Biochim.Biophys.Acta. 2000;1529:245–256. doi: 10.1016/s1388-1981(00)00153-0. [DOI] [PubMed] [Google Scholar]
- 2.Rousset X, Vaisman B, Amar M, Sethi AA, Remaley AT. Lecithin: cholesterol acyltransferase - from biochemistry to role in cardiovascular disease, Current Opinion in Endocrinology. Diabetes and Obesity. 2009;16 doi: 10.1097/med.0b013e328329233b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glomset JA. Lecithin: cholesterol acyltransferase. An exercise in comparative biology. Prog.Biochem.Pharmacol. 1979;15:41–66. [PubMed] [Google Scholar]
- 4.Dobiasova M. Lecithin: cholesterol acyltransferase and the regulation of endogenous cholesterol transport. Adv.Lipid Res. 1983;20:107–194. [PubMed] [Google Scholar]
- 5.Rousset X, Shamburek R, Vaisman B, Amar M, Remaley A. Lecithin Cholesterol Acyltransferase: An Anti- or Pro-atherogenic Factor? Current Atherosclerosis Reports. 2011;13:249–256. doi: 10.1007/s11883-011-0171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sethi AA, Sampson M, Warnick R, Muniz N, Vaisman B, Nordestgaard BG, Tybjaerg-Hansen A, Remaley AT. High Pre-{beta}1 HDL Concentrations and Low Lecithin: Cholesterol Acyltransferase Activities Are Strong Positive Risk Markers for Ischemic Heart Disease and Independent of HDL-Cholesterol. Clin.Chem. 2010;56:1128–1137. doi: 10.1373/clinchem.2009.139931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calabresi L, Franceschini G. Lecithin:Cholesterol Acyltransferase, High-Density Lipoproteins, and Atheroprotection in Humans. Trends in Cardiovascular Medicine. 2010;20:50–53. doi: 10.1016/j.tcm.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Dullaart RPF, Perton F, van der Klauw MM, Hillege HL, Sluiter WJ. High plasma lecithin:cholesterol acyltransferase activity does not predict low incidence of cardiovascular events: Possible attenuation of cardioprotection associated with high HDL cholesterol. Atherosclerosis. 2010;208:537–542. doi: 10.1016/j.atherosclerosis.2009.07.042. [DOI] [PubMed] [Google Scholar]
- 9.Jonas A. Regulation of lecithin cholesterol acyltransferase activity. Prog Lipid Res. 1998;37:209–234. doi: 10.1016/s0163-7827(98)00007-1. [DOI] [PubMed] [Google Scholar]
- 10.Subbaiah PV, Liu M. Role of sphingomyelin in the regulation of cholesterol esterification in the plasma lipoproteins. Inhibition of lecithin- cholesterol acyltransferase. J.Biol.Chem. 1993;268:20156–20163. [PubMed] [Google Scholar]
- 11.Gesquiere L, Cho W, Subbaiah PV. Role of group IIa and group V secretory phospholipases A2 in the metabolism of lipoproteins. Substrate specificities of the enzymes and the regulation of their activities by sphingomyelin. Biochemistry. 2002;41:4911–4920. doi: 10.1021/bi015757x. [DOI] [PubMed] [Google Scholar]
- 12.Singh DK, Gesquiere LR, Subbaiah PV. Role of sphingomyelin and ceramide in the regulation of the activity and fatty acid specificity of group V secretory phospholipase A2. Arch.Biochem.Biophys. 2007;459:280–287. doi: 10.1016/j.abb.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh DK, Subbaiah PV. Modulation of the activity and arachidonic acid selectivity of group X secretory phospholipase A2 by sphingolipids. J.Lipid Res. 2007;48:683–692. doi: 10.1194/jlr.M600421-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Bolin DJ, Jonas A. Sphingomyelin inhibits the lecithin-cholesterol acyltransferase reaction with reconstituted high density lipoproteins by decreasing enzyme binding. J.Biol.Chem. 1996;271:19152–19158. doi: 10.1074/jbc.271.32.19152. [DOI] [PubMed] [Google Scholar]
- 15.Rye KA, Hime NJ, Barter PJ. The influence of sphingomyelin on the structure and function of reconstituted high density lipoproteins. J.Biol.Chem. 1996;271:4243–4250. doi: 10.1074/jbc.271.8.4243. [DOI] [PubMed] [Google Scholar]
- 16.Sparks DL, Frank PG, Neville TAM. Effect of the surface lipid composition of reconstituted LpA-I on apolipoprotein A-I structure and lecithin: cholesterol acyltransferase activity. Biochim.Biophys.Acta. 1998;1390:160–172. doi: 10.1016/s0005-2760(97)00172-0. [DOI] [PubMed] [Google Scholar]
- 17.Lee RG, Kelley KL, Sawyer JK, Farese RV, Jr, Parks JS, Rudel LL. Plasma cholesteryl esters provided by lecithin:cholesterol acyltransferase and acyl-coenzyme a:cholesterol acyltransferase 2 have opposite atherosclerotic potential. Circ Res. 2004;95:998–1004. doi: 10.1161/01.RES.0000147558.15554.67. [DOI] [PubMed] [Google Scholar]
- 18.Jeong TS, Schissel SL, Tabas I, Pownall HJ, Tall AR, Jiang XC. Increased sphingomyelin content of plasma lipoproteins in apolipoprotein E knockout mice reflects combined production and catabolic defects and enhances reactivity with mammalian sphingomyelinase. J.Clin.Invest. 1998;101:905–912. doi: 10.1172/JCI870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Z, Zhang H, Liu J, Liang Cp, Li Y, Li Y, Teitelman G, Beyer T, Bui HH, Peake DA, Zhang Y, Sanders PE, Kuo MS, Park TS, Cao G, Jiang XC. Reducing Plasma Membrane Sphingomyelin Increases Insulin Sensitivity. Mol.Cell.Biol. 2011;31:4205–4218. doi: 10.1128/MCB.05893-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hailemariam TK, Huan C, Liu J, Li Z, Roman C, Kalbfeisch M, Bui HH, Peake DA, Kuo MS, Cao G, Wadgaonkar R, Jiang XC. Sphingomyelin Synthase 2 Deficiency Attenuates NF{kappa}B Activation. Arterioscler Thromb Vasc Biol. 2008;28:1519–1526. doi: 10.1161/ATVBAHA.108.168682. [DOI] [PubMed] [Google Scholar]
- 21.Fan Y, Shi F, Liu J, Dong J, Bui HH, Peake DA, Kuo MS, Cao G, Jiang XC. Selective Reduction in the Sphingomyelin Content of Atherogenic Lipoproteins Inhibits Their Retention in Murine Aortas and the Subsequent Development of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:2114–2120. doi: 10.1161/ATVBAHA.110.213363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hojjati MR, Li Z, Jiang XC. Serine palmitoyl-CoA transferase (SPT) deficiency and sphingolipid levels in mice. Biochim Biophys Acta. 2005;1737:44–51. doi: 10.1016/j.bbalip.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Subbaiah PV, Liu M, Witt TR. Impaired cholesterol esterification in the plasma in patients with breast cancer. Lipids. 1997;32:157–162. doi: 10.1007/s11745-997-0020-5. [DOI] [PubMed] [Google Scholar]
- 24.Stokke KT, Norum KR. Determination of lecithin:cholesterol acyltransferase in human blood plasma. Scand.J.Clin.Lab.Invest. 1971;27:21–27. doi: 10.3109/00365517109080184. [DOI] [PubMed] [Google Scholar]
- 25.Chen CH, Albers JJ. Characterization of proteoliposomes containing apoprotein A-I: a new substrate for the measurement of lecithin: cholesterol acyltransferase activity. J.Lipid Res. 1982;23:680–691. [PubMed] [Google Scholar]
- 26.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can.J.Biochem.Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 27.Liu J, Zhang H, Li Z, Hailemariam TK, Chakraborty M, Jiang K, Qiu D, Bui HH, Peake DA, Kuo MS, Wadgaonkar R, Cao G, Jiang XC. Sphingomyelin Synthase 2 Is One of the Determinants for Plasma and Liver Sphingomyelin Levels in Mice. Arterioscler Thromb Vasc Biol. 2009;29:850–856. doi: 10.1161/ATVBAHA.109.185223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boyanovsky B, Zack M, Forrest K, Webb NR. The Capacity of Group V sPLA2 to Increase Atherogenicity of ApoE−/− and LDLR−/− Mouse LDL In Vitro Predicts its Atherogenic Role In Vivo. Arterioscler Thromb Vasc Biol. 2009;29:532–538. doi: 10.1161/ATVBAHA.108.183038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Furbee JW, Francone O, Parks JS. In vivo contribution of LCAT to apolipoprotein B lipoprotein cholesteryl esters in LDL receptor and apolipoprotein E knockout mice. J.Lipid Res. 2002;43:428–437. [PubMed] [Google Scholar]
- 30.Forte TM, Subbanagounder G, Berliner JA, Blanche PJ, Clermont AO, Jia Z, Oda MN, Krauss RM, Bielicki JK. Altered activities of anti-atherogenic enzymes LCAT, paraoxonase, platelet-activating factor acetylhydrolase in atherosclerosis-susceptible mice. J.Lipid Res. 2002;43:477–485. [PubMed] [Google Scholar]
- 31.Seo T, Oelkers PM, Giattina MR, Worgall TS, Sturley SL, Deckelbaum RJ. Differential modulation of ACAT1 and ACAT2 transcription and activity by long chain free fatty acids in cultured cells. Biochemistry. 2001;40:4756–4762. doi: 10.1021/bi0022947. [DOI] [PubMed] [Google Scholar]
- 32.Liu M, Krul ES, Subbaiah PV. Effect of apoprotein B conformation on the activation of lysolecithin acyltransferase and lecithin:cholesterol acyltransferase. Studies with subfractions of low density lipoproteins. J.Biol.Chem. 1992;267:5139–5147. [PubMed] [Google Scholar]
- 33.Subbaiah PV, Sargis RM. Sphingomyelin: a natural modulator of membrane homeostasis and inflammation. Med Hypotheses. 2001;57:135–138. doi: 10.1054/mehy.2001.1336. [DOI] [PubMed] [Google Scholar]
- 34.Lobo LIB, Wilton DC. Combined effects of sphingomyelin and cholesterol on the hydrolysis of emulsion particle triolein by lipoprotein lipase. Biochim.Biophys.Acta. 1997;1349:122–130. doi: 10.1016/s0005-2760(97)00127-6. [DOI] [PubMed] [Google Scholar]
- 35.Broedl UC, Jin W, Rader DJ. Endothelial Lipase: A Modulator of Lipoprotein Metabolism Upregulated by Inflammation. Trend Cardiovasc Med. 2004;14:202–206. doi: 10.1016/j.tcm.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Subbaiah PV, Horvath P, Achar SB. Regulation of the Activity and Fatty Acid Specificity of Lecithin-Cholesterol Acyltransferase by Sphingomyelin and Its Metabolites, Ceramide and Ceramide Phosphate. Biochemistry. 2006;45:5029–5038. doi: 10.1021/bi0600704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao Y, Thorngate FE, Weisgraber KH, Williams DL, Parks JS. Apolipoprotein E Is the Major Physiological Activator of Lecithin Cholesterol Acyltransferase (LCAT) on Apolipoprotein B Lipoproteins. Biochemistry. 2004;44:1013–1025. doi: 10.1021/bi0481489. [DOI] [PubMed] [Google Scholar]
- 38.Chen CH, Albers JJ. Activation of lecithin: cholesterol acyltransferase by apolipoproteins E-2, E-3, and A-IV isolated from human plasma. Biochim.Biophys.Acta. 1985;836:279–285. doi: 10.1016/0005-2760(85)90131-6. [DOI] [PubMed] [Google Scholar]
- 39.Rye KA, Bright R, Psaltis M, Barter PJ. Regulation of reconstituted high density lipoprotein structure and remodeling by apolipoprotein E. J Lipid Res. 2006;47:1025–1036. doi: 10.1194/jlr.M500525-JLR200. [DOI] [PubMed] [Google Scholar]
- 40.Ma J, Folsom AR, Lewis L, Eckfeldt JH. Relation of plasma phospholipid and cholesterol ester fatty acid composition to carotid artery intima-media thickness: the Atherosclerosis Risk in Communities (ARIC) Study. Am.J.Clin.Nutr. 1997;65:551–559. doi: 10.1093/ajcn/65.2.551. [DOI] [PubMed] [Google Scholar]
- 41.Ohrvall M, Berglund L, Salminen I, Lithell H, Aro A, Vessby B. The serum cholesterol ester fatty acid composition but not the serum concentration of alpha tocopherol predicts the development of myocardial infarction in 50-year-old men: 19 years follow-up. Atherosclerosis. 1996;127:65–71. doi: 10.1016/s0021-9150(96)05936-9. [DOI] [PubMed] [Google Scholar]
- 42.Swell L, Field H, Treadwell CR. Correlation of arachidonic acid of serum cholesterol esters in different species with susceptibility to atherosclerosis. Proc.Soc.Exp.Biol.Med. 1960;104:325–328. doi: 10.3181/00379727-104-25823. [DOI] [PubMed] [Google Scholar]
- 43.Chen W, Li J. Correlation of serum cholesteryl ester fatty acids composition with susceptibility to atherosclerosis in different species. Chinese J.Med. 1994;106:163–166. [PubMed] [Google Scholar]
- 44.Rudel LL, Parks JS, Sawyer JK. Compared With Dietary Monounsaturated and Saturated Fat, Polyunsaturated Fat Protects African Green Monkeys From Coronary Artery Atherosclerosis. Arterioscler Thromb Vasc Biol. 1995;15:2101–2110. doi: 10.1161/01.atv.15.12.2101. [DOI] [PubMed] [Google Scholar]
- 45.Jiang XC, Paultre F, Pearson TA, Reed RG, Francis CK, Lin M, Berglund L, Tall AR. Plasma sphingomyelin level as a risk factor for coronary artery disease. Arterioscler.Thromb.Vasc.Biol. 2000;20:2614–2618. doi: 10.1161/01.atv.20.12.2614. [DOI] [PubMed] [Google Scholar]
- 46.Park TS, Rosebury W, Kindt EK, Kowala MC, Panek RL. Serine palmitoyltransferase inhibitor myriocin induces the regression of atherosclerotic plaques in hyperlipidemic ApoE-deficient mice. Pharmacol Res. 2008;58:45–51. doi: 10.1016/j.phrs.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 47.Schlitt A, Blankenberg S, Yan D, von Gizycki H, Buerke M, Werdan K, Bickel C, Lackner KJ, Meyer J, Rupprecht HJ, Jiang XC. Further evaluation of plasma sphingomyelin levels as a risk factor for coronary artery disease. Nutr Metab (Lond) 2006;3:5–13. doi: 10.1186/1743-7075-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]