Abstract
Background
Sensitization to protease allergens, such as papain, or helminth infection is associated with basophil recruitment to draining lymph nodes. Basophils have the capacity to present antigen to naïve T cells and promote TH2 differentiation directly or indirectly through IL-4 production.
Objective
We studied how papain induces basophil migration to lymph nodes and the contribution of various leukocytes to papain-induced immune responses.
Methods
We immunized mice in the footpad with papain and studied leukocyte recruitment and inflammatory cytokine and chemokine production in the draining popliteal lymph nodes.
Results
Papain directly activated naïve T cells through protease-activated receptor 2 (PAR2) to initiate a chemokine/cytokine program that includes CCL17, CCL22, and IL-4. Papain-triggered innate immune responses were dependent on both CD4 T cells and PAR2 and were strongly reduced in the absence of CCR4, the primary receptor for CCL17/22.
Conclusion
These results elucidate a novel innate allergen recognition pathway mediated by naïve T cells through PAR2, which provide an immediate source of chemokines and IL-4 upstream of basophils and antigen-restricted TH2 differentiation. PAR2 antagonism may thus hold promise for the treatment of allergic disease.
Keywords: Chemokines, basophils, chemotaxis, TH2, allergens
Introduction
The plasticity and diversity of specific T lymphocyte populations shape the adaptive immune response. Naïve T cells differentiate into interferon-γ (IFNγ) producing T helper 1 (TH1) cells after infection with intracellular microbes including viruses and protozoa 1.T helper 2 (TH2) cells, which secrete IL-4, 5, and 13, develop in response to helminth infection and allergen exposure. The pathways linking the innate and adaptive response to allergens, in which antigen presenting cells (APCs) recognize allergens and induce antigen (Ag)-specific effector TH2 differentiation, have not been fully defined 2, 3.
Many allergens have intrinsic proteolytic activity 4, which is required for their induction of TH2-mediated allergic inflammation 3. Papain is a cysteine protease belonging to a superfamily of pathogen-derived proteases including gingipains and cruzipain of Trypanosoma cruzii 5. Papain can elicit antigen-specific IgE production, degranulation of mast cells, and is associated with occupational allergy in food industry workers and latex sensitivity due to allergen cross-reactivity 6, 7. Published work has suggested a function for basophils in the mouse TH2 immune response to allergens including papain. Subcutaneous injection of papain into mice, either in the presence 8, 9 or absence 10, 11 of “bystander” antigen such as ovalbumin (OVA), elicited basophil accumulation in draining lymph nodes (LNs). Published studies have also provided evidence that basophils regulate TH2-mediated immunity to helminths 12, tick Ags 13, and Ag-IgE complexes 14 in mice.
Basophils are short-lived leukocytes that circulate in the bloodstream and migrate transiently into the T-cell zone of draining lymph nodes after papain immunization 10, 11. The chemokines and receptors guiding basophil trafficking in mice are unknown. We examined the LN inflammatory response following subcutaneous papain immunization. Basophils were drawn to, and CCL17, and CCL22 accumulated in, pLNs of mice exposed to papain compared to PBS-treated LN, none of which was observed when Cd4−/− or Par2−/− mice were studied. Exposure of naïve T cells to papain in vitro elicited production of CCL17/22 and IL-4. These results illuminate a novel innate function of naïve T cells and define basophil trafficking pathways involved in the immune response to a protease allergen.
Methods
For additional methods, see the Methods section in this article’s Online Repository at www.jacionline.org.
Mice
Wild-type (WT) C57BL/6, Cd4−/−, Tcrα −/−, Ccr4−/−, Cd11c-Dtr-egfp, and Par2−/− mice (all on C57Bl/6 background) were purchased from Jackson Laboratories. DO11.10/4get mice on a Rag1−/− background were provided by Dr. Markus Mohrs (Trudeau Institute). All mouse experiments were performed in accordance with Animal Study Protocol LAD-3E, approved by the NIAID Animal Care and Use Committee, NIH.
Bone marrow cultures, sorting and chemotaxis
Whole bone marrow (BM) cells were obtained from mouse femurs and cultured for 10 days in RPMI medium supplemented with IL-3 (30 ng/ml), 10% FBS, 50 μM 2-mercaptoethanol and 2 mM L-glutamine. FcεRI+CD49b+c-kit− cells were sorted using a FACS Aria-Cyan followed by recovery for 24 h in culture medium prior to chemotaxis assays. In some assays, BMB were enriched from bone marrow cultures using CD49b microbeads alone (Miltenyi), which gave results equivalent to those observed with BMB sorted by flow cytometry. Chemotaxis assays were performed using 5-μm pore size 24-well ChemoTx system (Costar) per the manufacturer’s instructions. Cells were allowed to migrate in the presence of chemokine in the lower chamber for 4 h followed by quantification by hemocytometry.
Statistical analysis
Data are presented as mean ± S. E. M. where applicable and were analyzed by one- or two-way ANOVA or Student’s t-test using PRISM software (GraphPad, CA). We considered a P value < 0.05 statistically significant.
Results
The inflammatory response of lymph nodes to papain immunization
To examine papain-evoked pathways, we injected mice subcutaneously in the rear footpad with either PBS or papain then harvested the draining (popliteal, pLNs) LNs 2–4 days after immunization (see Fig. E1 in the Online Repository). Consistent with published studies 10, 11, we observed an accumulation of basophils (FcεRI+CD49b+c-kit−) in the pLNs 3 days after papain injection but not in the pLNs of mice injected with PBS (Fig. E1A). We also found an increased frequency of CD4+IL-4+ T cells following re-stimulation of pLNs of papain-injected mice 4 days post-immunization (Fig. E1B) and serum papain-specific IgE, which peaked 14 days after immunization (Fig. E1C). These results indicated a TH2-mediated immune response to papain.
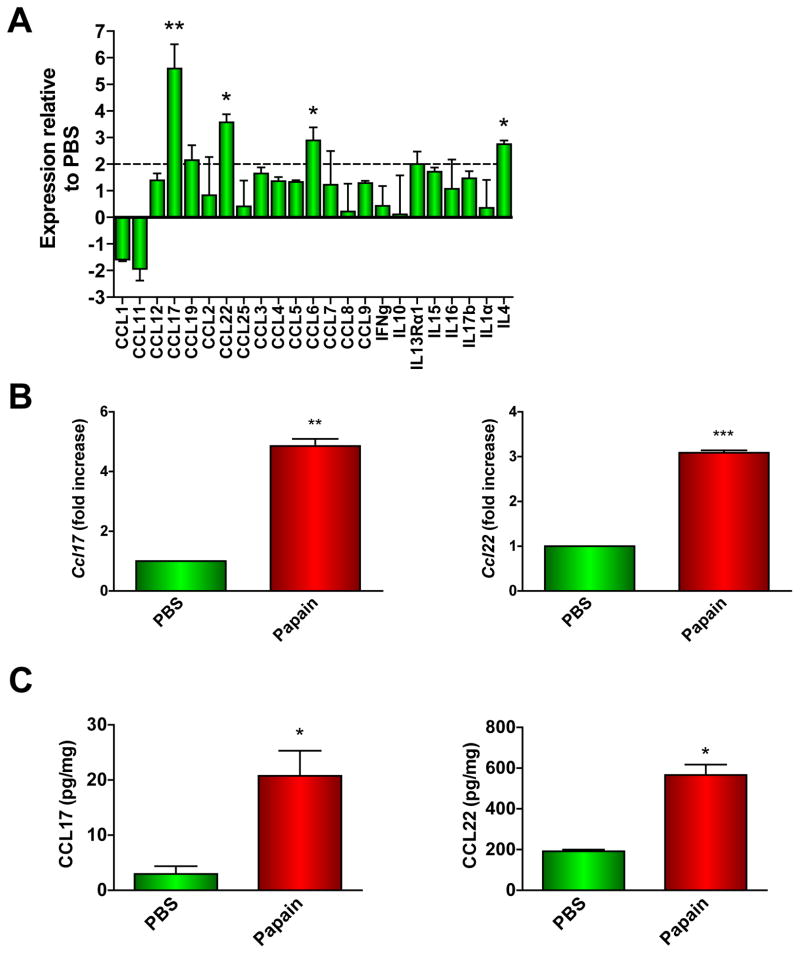
We compared chemokine/cytokine gene expression in the pLNs of PBS- or papain-immunized mice 2 days post-injection by quantitative PCR array. Several transcripts were significantly upregulated in the LNs of papain-immunized mice compared to those of PBS-treated mice, most notably Ccl17 (5-fold), Ccl22 (3.5-fold), Ccl6 (3-fold), and Il4 (3-fold) (Fig. 1A). By real-time PCR, we found that expression of both Ccl17 and Ccl22 was significantly increased in the pLNs 2 days after papain injection compared to PBS-treated pLNs (Fig. 1B). In pLN homogenates, quantities of immunoreactive CCL17 and CCL22 were increased significantly (7-fold and 3-fold, respectively) in papain-treated mice compared to PBS-immunized mice (Fig. 1C).
Figure 1. Inflammatory response of draining popliteal lymph nodes to footpad immunization with papain.
(A) qPCR array analysis of RNA from pLN RNA of PBS- or papain-treated mice (**P < 0.001; *P < 0.05; 2-way ANOVA). (B–C) Ccl17 and Ccl22 mRNA (B) or CCL17 and CCL22 protein (C) expression in LNs 2 days following PBS or papain immunization were determined by qPCR or ELISA, respectively (*P < 0.03, **P = 0.004; ***P = 0.0007, paired t-test). Data represent 3 experiments with at least 3 mice per group in each.
Basophil chemokine receptor expression and chemotaxis
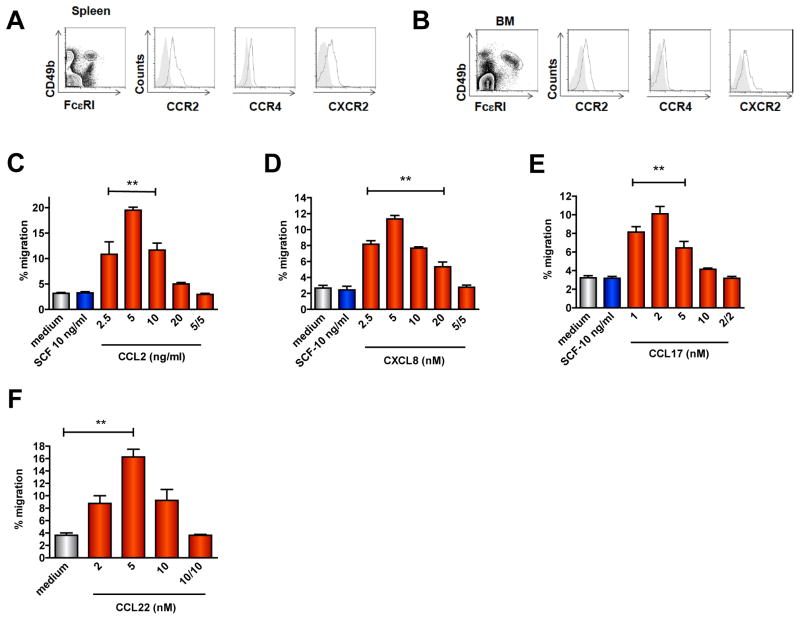
To determine relevant papain-elicited chemokines that attract basophils to the draining LNs, we analyzed chemokine receptor expression on basophils isolated from spleen and bone marrow or on basophils derived from bone marrow precursors cultured in the presence of IL-3 for 7–10 days (bone marrow-derived basophils, BMB (Fig. E2A). Freshly isolated bone marrow and splenic basophils expressed CCR2 (Figs. 2A–B) but not CCR3 (Fig. E2B). We also detected expression of CCR4 and CXCR2 (Figs. 2A–B) but no or minimal CCR1, CCR5, CCR6, CCR7, CXCR4 and CXCR5 (Fig. E2B). Although chemokine receptor expression on BMB was qualitatively similar to that of freshly isolated basophils, CCR4 and CXCR2 expression was higher in BMB by comparison (Fig. E2C).
Figure 2. Basophil chemokine receptor expression and chemotaxis.
(A–B) Percentages of basophils in live, non-T non-B spleen cells (A) or freshly isolated total bone marrow (B) (left) and chemokine receptor expression (open histograms) in basophils (right); gray shaded histograms represent isotype control. (C–F) Chemotaxis of BMB sorted from c-kit−cells in response to CCL2 (C), CXCL8 (D), CCL17 (E) or CCL22 (F). Data are mean ± S.E.M. of 2–3 experiments using BMB from 2–3 mice/experiment (**P < 0.001, indicated chemokine concentrations v. control, 1-way ANOVA).
Sorted FcεRI+CD49b+c-Kit− basophils (Fig. E2A) displayed chemotaxis towards gradients of CCL2, CXCL8, and CCL17, and CCL22 in a concentration-dependent manner (Figs. 2C–F). BMB did not migrate in response to stem cell factor (SCF), which served as a negative control here as basophils do not express the SCF receptor (c-kit), or in response to CXCL12, CXCL13, IL-3, or papain alone (Fig. 2 and data not shown), suggesting a unique role for papain-induced chemokines in basophil recruitment to pLNs. Basophil migration in the presence of equivalent chemokine concentrations in the upper and lower chambers was similar to that observed in the absence of chemokine, indicating that chemokine gradients stimulated chemotaxis rather than chemokinesis (Figs. 2C–F). These results indicated that mouse basophils express functional CCR2, CCR4, and CXCR2 receptors.
Role of CCR4-CCL17/22 axis in papain-elicited basophil migration
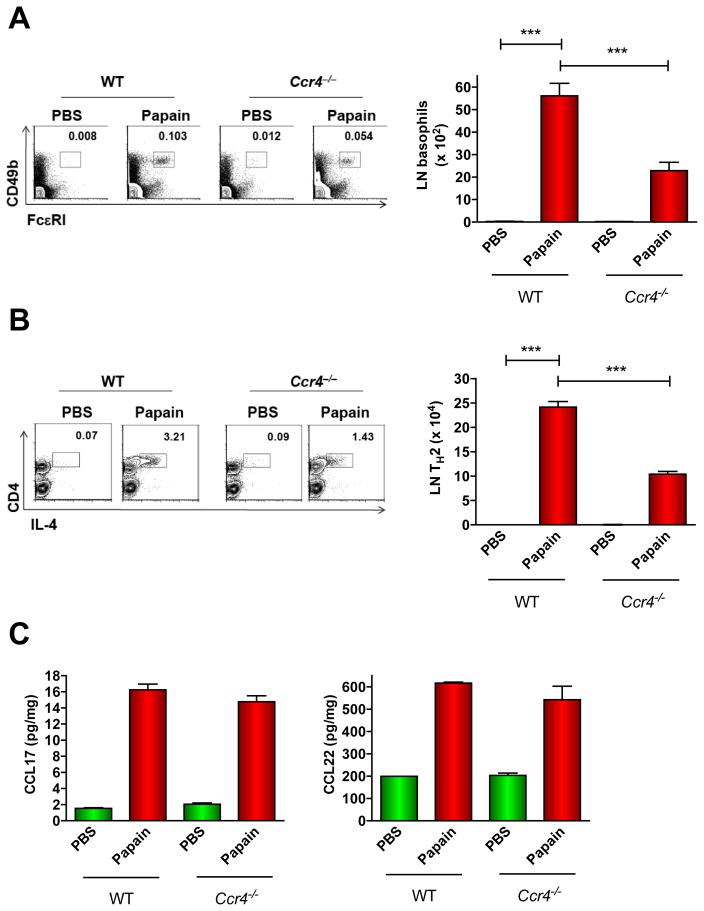
CCR4 and its ligands CCL17 and CCL22 have been linked to TH2-mediated inflammation 15. To define the role of this axis in papain responses, we immunized WT or Ccr4−/− mice with papain and quantified basophils in pLNs 3 days post-immunization. In naïve, unimmunized WT and Ccr4−/− mice, basophil frequencies were similar in spleen, and BM, and T cell, B cell, and DC frequencies in the pLNs were also comparable (Figs. E3A–C). In contrast, basophil accumulation in pLNs after papain immunization was reduced by 60% in mice lacking CCR4 compared to WT (Fig. 3A). This disparity most likely did not result from intrinsically defective pLN responses to papain as CCL17 and CCL22 concentrations were similar in pLNs from WT and Ccr4−/− papain-treated mice (Fig. 3B). Papain-induced TH2 differentiation was also reduced by CCR4 deficiency as there were 60% fewer IL-4+CD4+ T cells in the pLNs of Ccr4−/− mice compared to WT 4 days following papain injection (Fig. 3C). Collectively, these results indicate a significant role of CCR4 in the immune response to papain.
Figure 3. Role of CCR4 in papain-induced basophil trafficking to pLNs.
(A–B) WT or Ccr4−/− mice were immunized with PBS or papain followed by enumeration of pLN basophils after 3 days (A) or IL-4+ CD4 T cells (following 6 h re-stimulation with PMA/ionomycin) after 4 days (B). Numbers above outlined areas are percentages of total cells from a representative experiment. Graphs show mean ± S.E.M. of 3 experiments (***P < 0.001, 1-way ANOVA). (C) Chemokine concentrations in the pLNs 2 days after footpad immunization with papain were determined by ELISA (2 experiments with 3 mice/group).
Papain induces T cell production of basophil-attracting chemokines independently of DCs
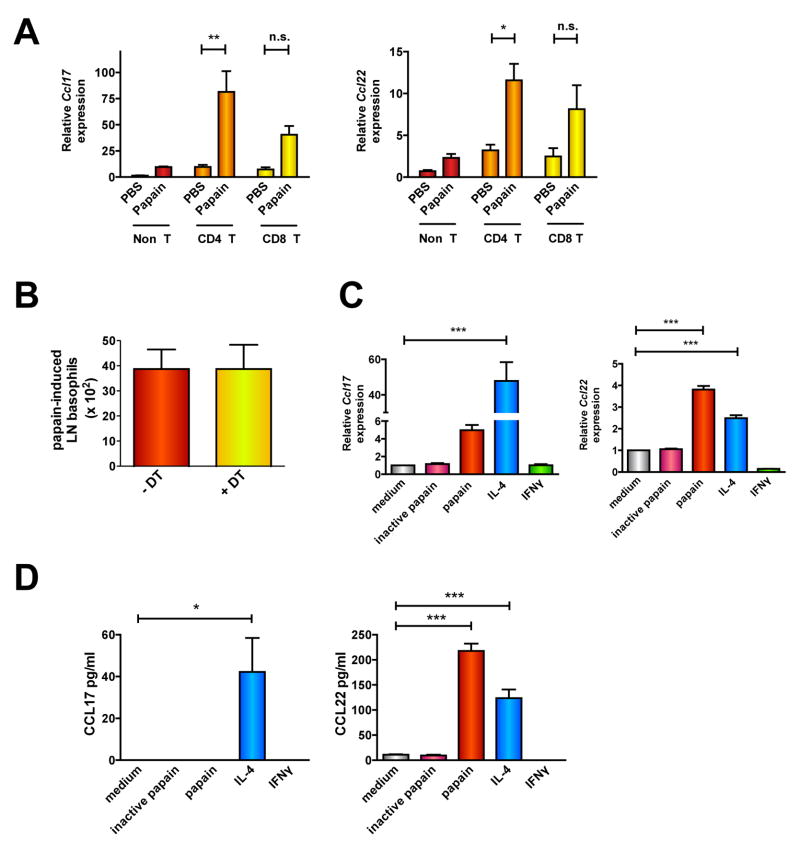
We determined the cellular source of CCL17 and CCL22 in pLNs following papain immunization by characterizing purified pLN cell populations (Fig. E4A). Papain increased Ccl17 and Ccl22 expression in LN T cells but not B cells (Fig. E4B). Ccl17 and Ccl22 were significantly upregulated in papain-treated CD4+ but not CD8+ T cell populations compared to control (Fig. 4A). Although previous studies indicated that DCs and macrophages are potent sources of these chemokines 16, 17, Ccl17 and Ccl22 expression was much higher in the pLN T cell fraction than in the non-T cell compartment, which may include B cells, macrophages, mast cells, NK cells, and DCs (Fig. 4A). Accordingly, treatment of purified splenic macrophage/DC populations with papain in vitro did not induce significant Ccl17/22 expression (Figs. E4C).
Figure 4. Papain induces Ccl17 and Ccl22 expression in naïve T cells independently of DCs.
(A) Chemokine expression in CD4, CD8, or non-T fractions purified from the pLNs of PBS- or papain-immunized mice (n.s.= not significant; *P < 0.05; **P < 0.005, 1-way ANOVA). (B) Cd11c-Dtr transgenic mice were injected with PBS or DT 1 day prior to immunization with papain. Basophil numbers in the pLNs were quantified 2 days after papain treatment. (D–E) Chemokine gene expression (E) or protein secretion (F) was determined in CD4+ T cells sorted from spleens of unimmunized mice and cultured in vitro with papain for 20 h (D) or 3 days (E), respectively. Data represent 2–3 experiments with 3 mice/group (*P < 0.05; ***P < 0.0005, 1-way ANOVA).
Consistent with a published study10, we also observed infiltration of migratory dermal DCs (“mDCs”, CD8α −CD11c+CD205+)18, 19 in pLNs 2 days after papain immunization (Fig. E5A). However, Ccr4−/− mice had reduced basophil migration to pLNs in response to papain compared to WT despite equivalent numbers of pLN DCs (Figs. 3A, E5A). To formally evaluate the function of DCs in papain-induced chemokine responses, we used Cd11c-Dtr-egfp transgenic mice. Consistent with previous studies11, when injected with diphtheria toxin one day prior to papain immunization and then treated with papain and examined two days later, the pLNs of these mice were largely devoid of DCs (MHChiCD11c+) (Fig. E5B). DC deficiency did not reduce either papain-induced basophil accumulation in pLNs (Fig. 4B, E5C) or upregulation of Ccl17 and Ccl22 expression (Fig. E5D). Collectively, these results indicated that DCs are neither sufficient nor required for chemokine-mediated recruitment of basophils to pLNs by papain.
To ascertain whether T cells required prior APC-mediated priming to respond to papain, we measured chemokine expression in naïve T cells purified from unimmunized mice that were pulsed with papain in vitro for 20 hours (Fig. E6A). Papain induced Ccl17 and Ccl22 mRNA expression (Fig. 4C, E6B) and CCL22 protein expression (Fig. 4D) in unprimed, naïve T cells. We detected CCL17 protein in supernatants from cells stimulated with IL-4 in vitro, which has been reported to induce strong Ccl17 expression in human CD4+ T cells through STAT6 binding sites in the Ccl17 promoter 20. We attribute our inability to detect CCL17 after papain treatment to the fact that absolute CCL17 amounts were quite lower than CCL22 in culture supernatants—most likely below assay detection limits. The proinflammatory effect of papain was dependent on its protease activity as heat-inactivation of papain abolished chemokine production by T cells (Figs. 4C–D).
Papain activates naïve T cells through PAR2-dependent mechanisms
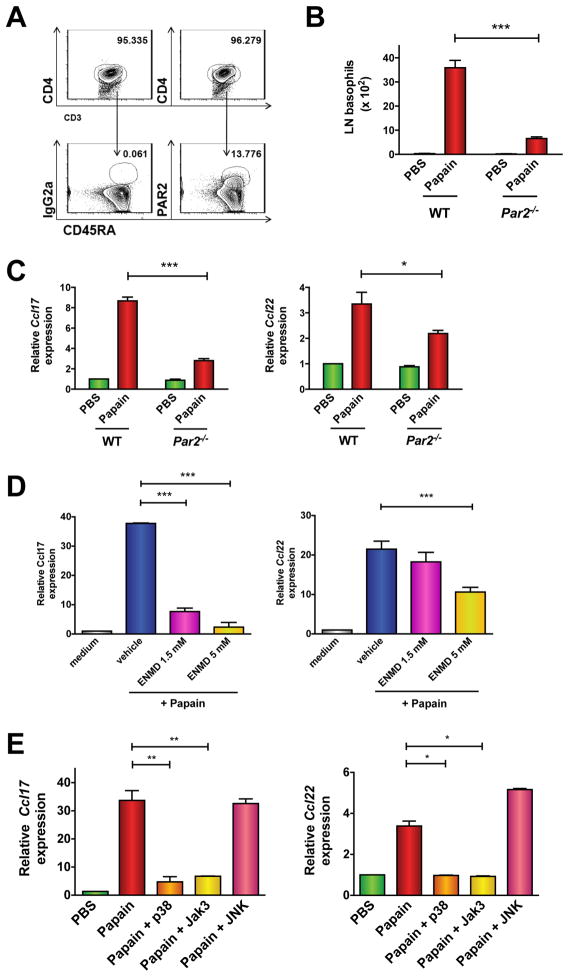
The finding that papain stimulated Ag-inexperienced T cells to produce CCL17 and CCL22 through its protease activity provoked the hypothesis that T cells may sense papain through a protease-activated receptor (PAR). Four PARs (PAR1-4) have been identified, which are activated uniquely by proteolytic cleavage at sequences within their amino-termini to expose a tethered ligand 21. As previous studies have indicated a role for PAR2 in TH2-mediated inflammation through unclear mechanisms 22, we evaluated its importance for papain-evoked T cell immunity. We detected PAR2 on 10–15% of human peripheral blood naïve T cells (Fig. 5A). Naïve and effector/memory splenic T cells from WT but not Par2−/− mice also expressed PAR2 to varying degrees (Figs. E7A–B). Papain-evoked basophil recruitment to pLNs (Fig. 5B) and Ccl17 and Ccl22 expression (Fig. 5C) were severely diminished in Par2−/− mice compared to WT although chemotaxis of purified PAR2-deficient basophils towards a CCL22 gradient in vitro was intact (Fig. E7C). Papain elicited much less Ccl17 and Ccl22 expression in purified CD4+ T cells from Par2−/− mice compared to WT (Fig. E7D), and the small molecule PAR2 antagonist ENMD-1068 significantly reduced papain-induced chemokine expression in human CD4+ T cells (Fig. 5D). Inhibitors of p38 and Jak3 activity, but not a JNK inhibitor, also strongly reduced papain-evoked Ccl17 and Ccl22 gene expression in both mouse (Fig. E7E) and human CD4+ T cells (Fig. 5E). Collectively, these results suggest that papain-elicited CCL17 and CCL22 production by T cells depends on PAR2, p38, and Jak3.
Figure 5. Papain induces chemokine production and basophil trafficking through PAR2-dependent mechanisms.
(A) PAR2 expression in human peripheral blood naïve CD4 T cells (CD45RA+). Numbers above outlined areas represent percentage of total live cells. (B–C) C57/Bl6 WT or Par2−/− mice were immunized in the footpad with papain followed by enumeration of or basophils (B) or Ccl17 and Ccl22 gene expression (C) in the pLNs. Data represent 3 experiments with 3 mice/group (*P < 0.05; ***P < 0.0001, 1-way ANOVA). (D–E) Chemokine expression in human peripheral blood CD4+ T cells cultured for 20 h with medium or papain following pretreatment for 1 h with vehicle (DMSO) alone, the PAR2 antagonist ENMD-1068 (D), or inhibitors of p38, JAK3, or JNK kinases (E). Data are mean ± S.E.M. of 3 experiments; *P < 0.05, **P < 0.005, 1-way ANOVA.
Requirement of T cells for the immune response to papain in vivo
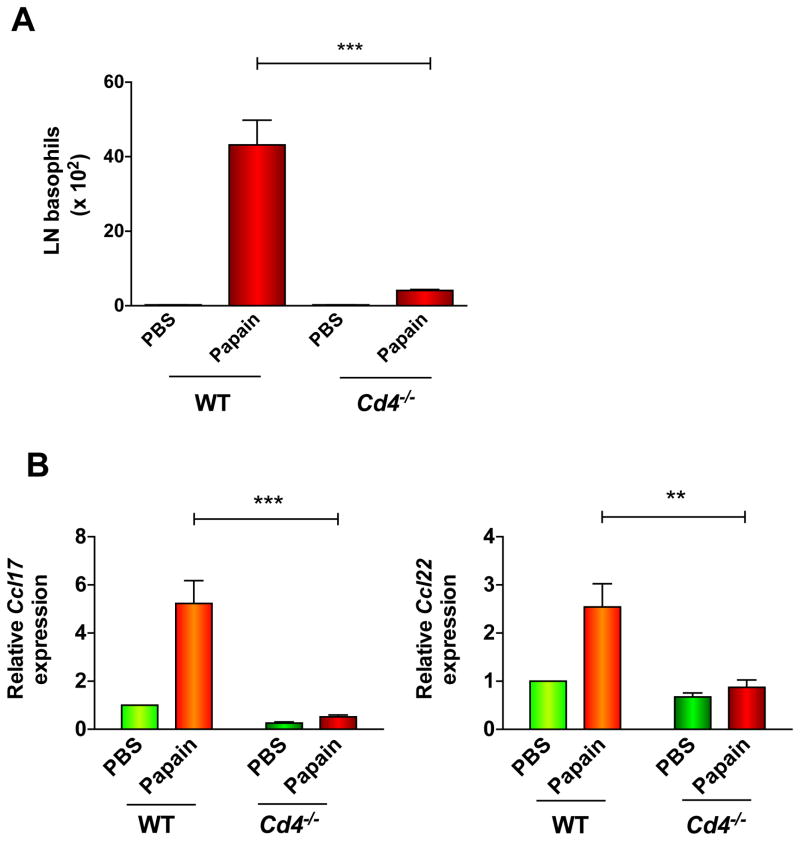
As CD4+ T cells appeared to be a major source of CCL17 and CCL22 immediately following papain injection, we evaluated their role in basophil migration to pLNs using Cd4−/− mice (Fig. E8A). Naïve WT and Cd4−/− mice had similar percentages of basophils in spleen and BM (Fig. E8B), indicating that CD4+ T cells are not required for basophil differentiation and survival in the absence of immune challenge. However, basophils did not appear in pLNs of CD4-deficient mice after papain immunization (Fig. 6A) despite equivalent numbers of total pLN DCs and influx of mDCs with papain treatment (Fig. E8C–D), and allergen-induced upregulation of pLN Ccl17 and Ccl22 was absent in these mice (Fig. 6B). We also failed to detect basophils and chemokine upregulation in pLNs of Tcrα −/− mice, which lack αβ T cells (Fig. E9A–E). To determine whether the presence of CD4+ T cells could restore papain-induced basophil migration to pLNs in Cd4−/− mice, we transferred 12.5 million CD4+ T cells into these mice, either by intravenous or direct footpad injection. However, we detected a thousand-fold fewer T cells in pLNs of the reconstituted Cd4−/− mice than were present in WT pLNs, independent of papain immunization (Fig. E10A). Increasing the number of donor CD4+ T cells did not improve engraftment (data not shown). This limitation precluded meaningful analysis of basophil numbers in pLNs of papain-treated recipients (Fig E10B).
Figure 6. Requirement of T cells for basophil migration to lymph nodes and production of chemokines in response to papain.
(A) (B–C) Basophil numbers (A) or Ccl17 and Ccl22 gene expression (B) in the pLNs of PBS- or papain-treated WT or Cd4−/− mice; **P = 0.003; ***P < 0.0001, 1-way ANOVA. Data represent 3 experiments with at least 3 mice/group.
Papain induces PAR2-dependent IL-4 production by naïve T cells and basophils
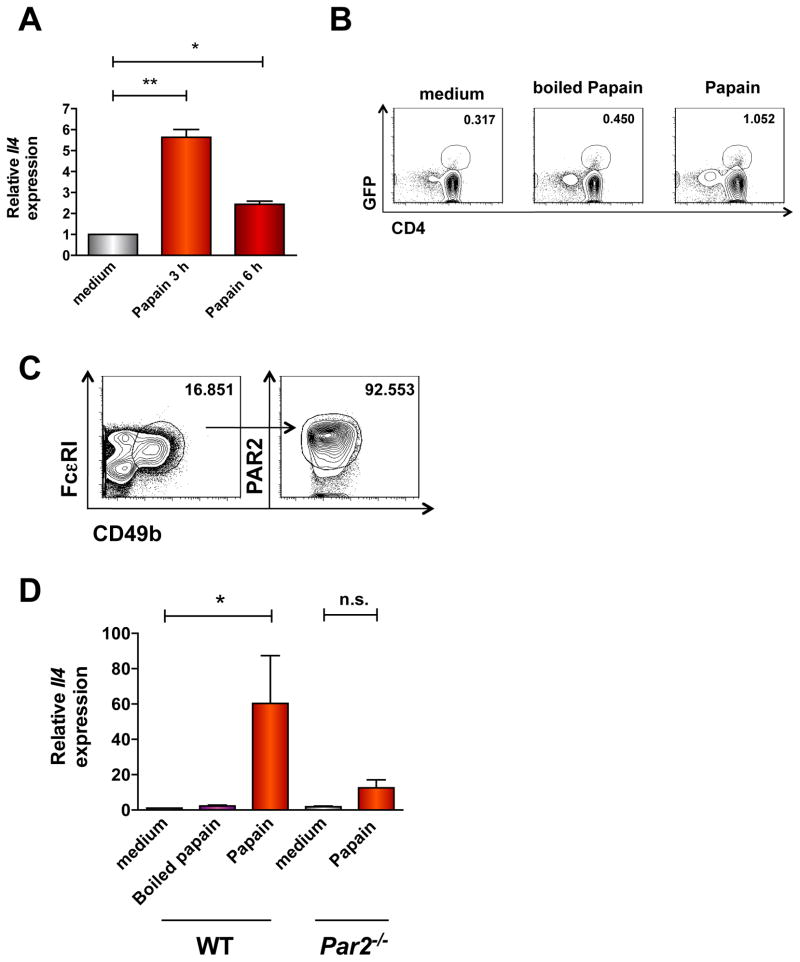
Jak3-mediated phosphorylation of STAT5 and STAT6 is integral for Il4 transcription in T cells 23. As a Jak3 inhibitor strongly reduced CCL17 and CCL22 production by naïve T cells (Fig. 5E), we hypothesized that papain also induces Il4 expression. Purified naïve CD4+ T cells from unimmunized mice upregulated Il4 mRNA within 3 hours of in vitro stimulation with papain (Fig. 7A). Naïve CD4+ T cells from unimmunized DO11.10/4get/Rag1−/− mice, which should not mount a robust Ag-restricted response to papain, also had increased GFP intensity after papain treatment in vitro, indicating IL-4 production (Fig. 7B). This result provoked the hypothesis that papain could induce an initial burst of IL-4 secretion by naïve T cells that may act in an autocrine/paracrine manner to upregulate chemokine expression and recruit basophils. To evaluate the role of IL-4 in papain-evoked T cell responses, we stimulated CD4+ T cells from Il4−/− mice with papain. Such cells had increased Ccl17/22 expression following papain treatment that was comparable to WT cells (Fig. E11). Thus, IL-4 itself is not required for papain-induced chemokine expression in T cells.
Figure 7. Papain elicits IL-4 expression in basophils and T cells through PAR2-dependent mechanisms.
(A) Il4 expression in naïve T cells from unimmunized C57Bl/6 mice stimulated with papain was determined by qPCR (*P <0.05; *P = 0.002, 1-way ANOVA). (B) IL-4 expression (GFP) in papain-treated splenocytes from DO11.10/4get/Rag1−/− mice. Data are from a single experiment with 2 mice/group representative of 2 similar experiments. (C) PAR2 on BMB was determined by flow cytometry. Numbers above outlined areas indicate percentage of total live cells. (D) Il4 gene expression in BMB from C57Bl/6 or Par2−/− mice was determined by qPCR. Data represent 2–3 experiments with 3 mice/experiment; *P < 0.05; n.s.= not significant, 1-way ANOVA.
Finally, as the receptor mediating papain-induced Il4 expression in basophils10 was unknown, we determined whether PAR2 was required for basophil respones to papain. BMB expressed abundant surface PAR2 (Fig. 7C), and Par2 gene deletion nearly abolished papain-evoked Il4 expression in BMB (Fig. 7D). These results suggest that PAR2 is the principal papain receptor on T cells and basophils and that PAR2 mediates an early cytokine/chemokine loop that drives TH2 immune responses to papain in mice.
Discussion
We have elucidated an innate allergen recognition pathway in mice mediated by conventional naïve T cells and requiring PAR2, which not only induces basophil recruitment to pLNs through chemokine production, but also provides an immediate source of IL-4 prior to induction of the antigen-specific TH2 immune response. The function(s) of PAR2 in T cells were previously unknown. Although prior work demonstrated that Par2−/− mice had reduced OVA-induced T cell differentiation and cytokine production relative to WT, PAR2-AP alone did not stimulate cytokine production by WT mouse CD4+ T cells 22. We show here that both mouse and human naïve T cells express surface PAR2 and respond directly to papain directly in a PAR2-, p38 MAP kinase-, and Jak3 kinase-dependent manner, and PAR2 appears to be crucial for the immune response to papain in vivo.
Published work had also failed to establish the primary cellular receptor for papain or other protease allergens in immune cells. Human eosinophil degranulation in response to papain required the protease activity of papain but not PAR2 24. By contrast, Kouzaki and colleagues reported that papain-evoked cytokine production by epithelial cells was partially reduced by Par2 siRNA compared to control, although the effectiveness of siRNA to reduce PAR2 expression was not demonstrated in this study 25. As we detected residual chemokine production to papain in the pLNs of Par2−/− mice, not all responses to papain may involve PAR2. Given the promiscuity of other proteases (e.g. thrombin) for multiple PARs (PAR1, 3, 4) and the ability of papain to induce alterations in membrane charge 26 or proteolytically cleave other surface receptors on T cells (e.g. CD25) 27, papain may also induce T cell and/or basophil activation through other PARs or through PAR-independent mechanisms. Alternative cellular sources of chemokines could also compensate for the reduced pLN response to papain in the absence of PAR2 in vivo.
In the present study, we demonstrated that T cells were required for CCL17 and CCL22 production and basophil accumulation in pLNs following papain injection. Other leukocytes that have been previously reported to be reservoirs of these chemokines, including mast cells, macrophages, and DCs, apparently made minor contributions to papain-elicited immunity. These results are consistent with previous work showing that basophil migration to tissues infected with Nippostrongylus brasiliensis (Nb) is absent in Rag2−/− mice 28, 29. In contrast, a separate study reported only a modest decline in basophil recruitment to draining LNs of T-cell depleted mice (using anti-CD3 Ab) injected with papain plus OVA relative to control 9. These results suggest that an immune response to papain is distinct from that induced by simultaneous exposure to inert and protease allergens. Generation of mice with conditional deletion of PAR2 in T cells or chimeras of T cell-deficient mice containing WT or PAR2-deficient bone marrow may further define the functions of PAR2-expressing T cells in TH2 immunity.
In agreement with Sokol et al. 11 and Nakagawa et al. 30, we found that decreased pLN basophils in papain-immunized Ccr4−/− mice relative to WT led to reduced frequencies of IL-4+CD4 T cells in these same LNs. As naïve T cells do not express CCR4, these results suggest a non-redundant role of basophils in the Ag-restricted TH2 response to papain. Our work also suggests that quantification of total IL-4+ T cell numbers (as has been reported in some studies) would not distinguish differentiated, Ag-specific “TH2” cells primed to produce IL-4 from naïve CD4+ T cells stimulated through PAR2 in the absence of APC-mediated priming. Thus, the contribution of basophils to TH2 immunity overall is still unclear.
The relevance of this pathway to allergy pathogenesis in humans requires further study. We show that the protease allergen papain has the capacity to stimulate human and mouse naïve T cells and mouse basophils through PAR2-dependent mechanisms. It is unclear whether human basophils express PAR2 or become activated by papain31, 32. It is also unknown whether human mast cells (MCs) respond to papain although MCs are dispensable for immunity to papain in mice6, 10. Accordingly, we did not detect PAR2 expression on murine BMMCs (data not shown), nor did they upregulate Il4 expression in response to papain (Fig. E12).
In a previous study, naïve peripheral blood T cells from allergic asthmatics produced CCL17 whereas those from non-allergic controls did not 33. A recent population study of Korean children demonstrated significant correlation of atopy, serum IgE, and eosinophilia with a single nucleotide polymorphism (SNP) in Par2, which increased PAR2 expression in peripheral blood mononuclear cells due to enhanced mRNA stability 34. Our studies provide a molecular mechanism linking these observations—namely, that allergens could induce pathogenic T cell responses in atopic subjects as a result of increased PAR2 expression. Further elucidation of the mechanisms underlying allergen-elicited, direct programming of naïve T cells (i.e., MHC-independent) could lead to the identification of new targets for the treatment of allergies and helminth infection.
Key Messages.
Naïve T cells respond to the allergen papain through a G-protein-coupled receptor, protease activated receptor-2 (PAR2), to produce IL-4 and chemokines.
Basophils are recruited to lymph nodes by papain through chemokine- and PAR-2-dependent mechanisms and respond with a burst of IL -4 production.
PAR2 antagonists may subvert TH2 immunity by reducing the inflammatory response to allergens such as papain.
Acknowledgments
This study was supported in part by the Intramural Research Programs of the NIH, NIAID and NIAMS (grant no. AI000939 LAD to K.M.D).
Abbreviations used
- PAR2
protease activated receptor 2
- CCL
C-C-chemokine ligand
- pLN
popliteal lymph node
- MC
mast cell
- DTR
diphtheria toxin receptor
- BMMC
bone marrow-derived MCs
- BMB
bone marrow-derived basophils
- PAR2-AP
PAR2 activating peptide
- OVA
ovalbumin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28:445–89. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paul WE, Zhu J. How are T(H)2-type immune responses initiated and amplified? Nat Rev Immunol. 2010;10:225–35. doi: 10.1038/nri2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wills-Karp M, Nathan A, Page K, Karp CL. New insights into innate immune mechanisms underlying allergenicity. Mucosal Immunol. 2010;3:104–10. doi: 10.1038/mi.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapman MD, Pomes A, Breiteneder H, Ferreira F. Nomenclature and structural biology of allergens. J Allergy Clin Immunol. 2007;119:414–20. doi: 10.1016/j.jaci.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Stack C, Dalton JP, Robinson MW. The phylogeny, structure and function of trematode cysteine proteases, with particular emphasis on the fasciola hepatica cathepsin L family. Adv Exp Med Biol. 2011;712:116–35. doi: 10.1007/978-1-4419-8414-2_8. [DOI] [PubMed] [Google Scholar]
- 6.Chambers L, Brown A, Pritchard DI, Sreedharan S, Brocklehurst K, Kalsheker NA. Enzymatically active papain preferentially induces an allergic response in mice. Biochem Biophys Res Commun. 1998;253:837–40. doi: 10.1006/bbrc.1998.9862. [DOI] [PubMed] [Google Scholar]
- 7.Finkelman FD, Urban JF., Jr Cytokines: making the right choice. Parasitol Today. 1992;8:311–4. doi: 10.1016/0169-4758(92)90105-b. [DOI] [PubMed] [Google Scholar]
- 8.Pulendran B, Tang H, Manicassamy S. Programming dendritic cells to induce T(H)2 and tolerogenic responses. Nat Immunol. 11:647–55. doi: 10.1038/ni.1894. [DOI] [PubMed] [Google Scholar]
- 9.Tang H, Cao W, Kasturi SP, Ravindran R, Nakaya HI, Kundu K, et al. The T helper type 2 response to cysteine proteases requires dendritic cell-basophil cooperation via ROS-mediated signaling. Nat Immunol. 2010;11:608–17. doi: 10.1038/ni.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9:310–8. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sokol CL, Chu NQ, Yu S, Nish SA, Laufer TM, Medzhitov R. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat Immunol. 2009;10:713–20. doi: 10.1038/ni.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perrigoue JG, Saenz SA, Siracusa MC, Allenspach EJ, Taylor BC, Giacomin PR, et al. MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nat Immunol. 2009;10:697–705. doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wada T, Ishiwata K, Koseki H, Ishikura T, Ugajin T, Ohnuma N, et al. Selective ablation of basophils in mice reveals their nonredundant role in acquired immunity against ticks. J Clin Invest. 2010;120:2867–75. doi: 10.1172/JCI42680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshimoto T, Yasuda K, Tanaka H, Nakahira M, Imai Y, Fujimori Y, et al. Basophils contribute to T(H)2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nat Immunol. 2009;10:706–12. doi: 10.1038/ni.1737. [DOI] [PubMed] [Google Scholar]
- 15.Stutte S, Quast T, Gerbitzki N, Savinko T, Novak N, Reifenberger J, et al. Requirement of CCL17 for CCR7- and CXCR4-dependent migration of cutaneous dendritic cells. Proc Natl Acad Sci U S A. 2010;107:8736–41. doi: 10.1073/pnas.0906126107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alferink J, Lieberam I, Reindl W, Behrens A, Weiss S, Huser N, et al. Compartmentalized production of CCL17 in vivo: strong inducibility in peripheral dendritic cells contrasts selective absence from the spleen. J Exp Med. 2003;197:585–99. doi: 10.1084/jem.20021859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dogan RN, Long N, Forde E, Dennis K, Kohm AP, Miller SD, et al. CCL22 regulates experimental autoimmune encephalomyelitis by controlling inflammatory macrophage accumulation and effector function. J Leukoc Biol. 2011;89:93–104. doi: 10.1189/jlb.0810442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heath WR, Carbone FR. Dendritic cell subsets in primary and secondary T cell responses at body surfaces. Nat Immunol. 2009;10:1237–44. doi: 10.1038/ni.1822. [DOI] [PubMed] [Google Scholar]
- 19.Henri S, Guilliams M, Poulin LF, Tamoutounour S, Ardouin L, Dalod M, et al. Disentangling the complexity of the skin dendritic cell network. Immunol Cell Biol. 88:366–75. doi: 10.1038/icb.2010.34. [DOI] [PubMed] [Google Scholar]
- 20.Wirnsberger G, Hebenstreit D, Posselt G, Horejs-Hoeck J, Duschl A. IL-4 induces expression of TARC/CCL17 via two STAT6 binding sites. Eur J Immunol. 2006;36:1882–91. doi: 10.1002/eji.200635972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steinhoff M, Buddenkotte J, Shpacovitch V, Rattenholl A, Moormann C, Vergnolle N, et al. Proteinase-activated receptors: transducers of proteinase-mediated signaling in inflammation and immune response. Endocr Rev. 2005;26:1–43. doi: 10.1210/er.2003-0025. [DOI] [PubMed] [Google Scholar]
- 22.Shichijo M, Kondo S, Ishimori M, Watanabe S, Helin H, Yamasaki T, et al. PAR-2 deficient CD4+ T cells exhibit downregulation of IL-4 and upregulation of IFN-gamma after antigen challenge in mice. Allergol Int. 2006;55:271–8. doi: 10.2332/allergolint.55.271. [DOI] [PubMed] [Google Scholar]
- 23.Paul WE. What determines Th2 differentiation, in vitro and in vivo? Immunol Cell Biol. 2010;88:236–9. doi: 10.1038/icb.2010.2. [DOI] [PubMed] [Google Scholar]
- 24.Miike S, Kita H. Human eosinophils are activated by cysteine proteases and release inflammatory mediators. J Allergy Clin Immunol. 2003;111:704–13. doi: 10.1067/mai.2003.1332. [DOI] [PubMed] [Google Scholar]
- 25.Kouzaki H, O’Grady SM, Lawrence CB, Kita H. Proteases induce production of thymic stromal lymphopoietin by airway epithelial cells through protease-activated receptor-2. J Immunol. 2009;183:1427–34. doi: 10.4049/jimmunol.0900904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mekala DJ, Geiger TL. Functional segregation of the TCR and antigen-MHC complexes on the surface of CTL. J Immunol. 2003;171:4089–95. doi: 10.4049/jimmunol.171.8.4089. [DOI] [PubMed] [Google Scholar]
- 27.Schulz O, Laing P, Sewell HF, Shakib F. Der p I, a major allergen of the house dust mite, proteolytically cleaves the low-affinity receptor for human IgE (CD23) Eur J Immunol. 1995;25:3191–4. doi: 10.1002/eji.1830251131. [DOI] [PubMed] [Google Scholar]
- 28.Min B, Prout M, Hu-Li J, Zhu J, Jankovic D, Morgan ES, et al. Basophils produce IL-4 and accumulate in tissues after infection with a Th2-inducing parasite. J Exp Med. 2004;200:507–17. doi: 10.1084/jem.20040590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Voehringer D, Shinkai K, Locksley RM. Type 2 immunity reflects orchestrated recruitment of cells committed to IL-4 production. Immunity. 2004;20:267–77. doi: 10.1016/s1074-7613(04)00026-3. [DOI] [PubMed] [Google Scholar]
- 30.Nakagawa Y, Takamatsu H, Okuno T, Kang S, Nojima S, Kimura T, et al. Identification of semaphorin 4B as a negative regulator of basophil-mediated immune responses. J Immunol. 2011;186:2881–8. doi: 10.4049/jimmunol.1003485. [DOI] [PubMed] [Google Scholar]
- 31.Zhu W, He SH, Lin ZX, Fu YL. Histamine release properties of human basophils in response to various stimuli. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2005;21:519–21. [PubMed] [Google Scholar]
- 32.Falcone FH, Morroll S, Gibbs BF. Lack of protease activated receptor (PAR) expression in purified human basophils. Inflamm Res. 2005;54 (Suppl 1):S13–4. doi: 10.1007/s00011-004-0405-y. [DOI] [PubMed] [Google Scholar]
- 33.Hirata H, Arima M, Cheng G, Honda K, Fukushima F, Yoshida N, et al. Production of TARC and MDC by naive T cells in asthmatic patients. J Clin Immunol. 2003;23:34–45. doi: 10.1023/a:1021948214742. [DOI] [PubMed] [Google Scholar]
- 34.Lee JH, Kim KW, Gee HY, Lee J, Lee KH, Park HS, et al. A synonymous variation in protease-activated receptor-2 is associated with atopy in Korean children. J Allergy Clin Immunol. 2011 doi: 10.1016/j.jaci.2011.06.036. [DOI] [PubMed] [Google Scholar]